
Expression of Osterix and SOX9 after Administration of Gourami Fish Scale Collagen in Wistar Rats
Jonathan Setiawan, Chiquita Prahasanti*, Eka Fitria Augustina, Melissa Tionardus, and Wibi RiawanPublished Date : April 3, 2023
DOI : https://doi.org/10.12982/NLSC.2023.033
Journal Issues : Number 2, April-June 2023
ABSTRACT The current goal of periodontal treatments is to regenerate periodontal tissue by adding bone grafts. Various materials are being explored as bone substitutes, including type 1 collagen. Gourami fish scales are known to be an alternative source of type 1 collagen and have potential as a substitute material for bone grafts. The objective of this study was to determine whether there is an increase in the expression of Osterix (OSX) and SOX9 after the administration of gourami (Osphronemus goramy) fish scale collagen in extracted socket Wistar rats (Rattus norvegicus). The research was conducted on 32 male Wistar rats that were classified into four groups: control groups on day 7 (K7) and day 14 (K14); and treatment groups on day 7 (P7) and day 14 (P14). The expression of OSX and SOX9 was visualized by immunohistochemical staining of bone tissue preparations by anti-OSX and SOX9 monoclonal antibodies. The ANOVA results for OSX expression had a significant difference in OSX expression between that in the control and treatment groups on day 7 and day 14. The ANOVA results for SOX9 expression on day 7 had a significant difference in SOX9 expression between that in the control and treatment groups. ANOVA results for SOX9 expression on day 14 had no significant difference in the expression of SOX9 between the treatment and control groups. The conclusion of this study was administration of Gourami scale collagen can increase the expression of OSX and SOX9 in socket’s Wistar rats.
Keywords: Bone grafts, Type 1 collagen, Gourami fish scales, Osterix, SOX9
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Citation: Setiawan, J., Prahasanti, C., Augustina, E.F., Tionardus, M., and Riawan, W. 2023. Expression of osterix and SOX9 after administration of gourami fish scale collagen in Wistar rats. Nat. Life Sci. Commun. 22(2): e2023033.
INTRODUCTION
In 2001, periodontal disease, which is an oral disease, was recorded as the most common disease among humans (Tonetti et al., 2017). The prevalence of periodontitis in Indonesia is relatively high. The 2018 RISKESDAS data showed the percentage of periodontitis cases in Indonesia to be 74.1% (Kemenkes RI, 2019). Current periodontal treatments aim to restrict the inflammatory process involved in inhibiting tissue damage and facilitate the regeneration of periodontal tissue at the site of the damage. This can be done by adding bone grafts. This method is considered to be an ideal periodontal therapy because it leads to bone regeneration, and consequently, to repair of the periodontal ligament, gingival fibers, and junctional epithelium (Newman et al., 2018).
There are three types of bone grafts: autografts, allografts, and xenografts. The concept of tissue engineering has emerged from the developments in biotechnology involving xenografts performed to assist bone regeneration because these procedures do not require additional surgery, have low morbidity, and reduce the risk of disease transmission (Rahmitasari, 2018). Type 1 collagen, the gold standard in periodontal tissue regeneration, is a xenograft material used in bone grafts (Prahasanti et al., 2018). Fish scales are an alternate supply of collagen that can be used to produce collagen scaffolds. Despite not having been previously used for this purpose, fish scales have the potential to be transformed into collagen (Prahasanti et al., 2018). Fish scale collagen derived from freshwater fish is biocompatible and has potential applications in tissue engineering (Pati et al., 2012).
Gourami (Osphronemus goramy) fish scales were selected for this research because they contain type 1 collagen and have the potential to be used as a substitute in bone grafts (Prahasanti et al., 2018). Bone grafts using collagen from extracts of fish scales, especially those of gourami, have not been reported so far. Thus, a strong case can be built for future study on the use of collagen extracted from gourami fish scales. The expression of Osterix (OSX) and SOX9 is an indicator of the regeneration of bones subjected to bone grafts. OSX, also known as the Sp7 transcription factor, is an osteoblast-specific transcription factor that activates a group of genes during the differentiation of preosteoblasts into mature osteoblasts and osteocytes (Sinha and Zhou, 2013). The SOX9 gene is a gene that is responsible for providing instructions for the formation of proteins that have an essential role in the embryonic development process (Oh et al., 2014). The purpose of this research was to determine whether there was an increase in OSX and SOX9 expression after the administration of gourami fish scale collagen by means of experiments conducted on Wistar rats (Rattus norvegicus).
MATERIALS AND METHODS
Materials
This study was conducted in a laboratory setting and used a post-test only control group research design. Collagen was extracted from gourami fish scales for this study. This research was provided ethical approval (ethics certificate number: 475/HRECC.FODM/X/2020). The sample for this research comprised 32 male Wistar rats that were aged 3 months and had a healthy physical condition and body weight in the range of 150–300 g.
Collagen extraction
Gourami fish scales were initially processed to remove non-collagen protein and fat, following which, they were subjected to a decalcification process. The collagen was subsequently extracted by enzymatic treatment using acetic acid and pepsin as well as by lyophilization. The extracted gourami fish scale collagen was sterilized before being applied to Wistar rats.
Preparation of Wistar rats
The rats were divided into four groups, with a sample size of 8 rats per group. The groups were classified as: day 7 control (K7) and treatment (P7) groups; and day 14 control (K14) and treatment (P14) groups. For the control group, extracted tooth was carried out without administration of gouramy fish scale extract. 10–30 mg collagen from gourami fish scale extract was applied to the alveolar socket in the treatment groups, following which, the socket was sutured. The rats were anesthetized on day 7 and day 14 with a 10% ether inhalation anesthetic.
Immunohistochemical
The mandibular tissues thus obtained were fixed in 70% formalin buffer solution, and the tissue fragments were immersed in paraffin by following the conventional procedure. Paraffin sections were stained with OSX and SOX9 markers and subsequently tested using immunohistochemical techniques. Quantitative observations of OSX and SOX9 expression were carried out under a light microscope at 1000x magnification with a 20x field of view.
RESULTS
The following microscope image shows osteoblast cells stained using anti-OSX monoclonal antibodies on day 7 and day 14:
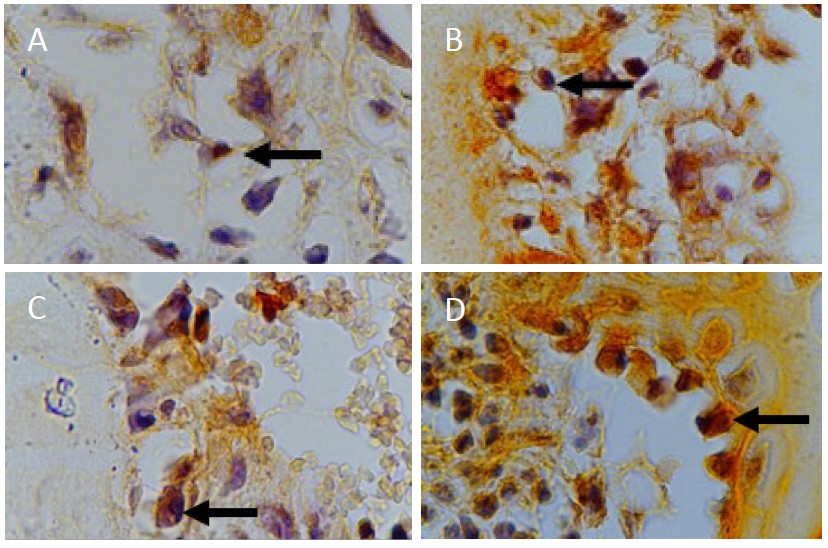
Figure 1. (A) Day 7 OSX control group (B) Day 7 OSX treatment group (C) Day 14 OSX control group (D) Day 14 OSX treatment group at 1000x magnification.
The distributions of the mean and standard deviation of OSX expression in the control and treatment groups on day 7 and day 14 were as follows:
Table 1. Mean and standard deviation of OSX expression.
|
Groups |
Day 7 |
Day 14 |
||
|
Mean |
Standard Deviation |
Mean |
Standard Deviation |
|
|
Control |
4.880 |
2.532 |
7.750 |
2.435 |
|
Treatment |
12.130 |
2.532 |
13.880 |
1.642 |
As seen in Table 1, the mean values for the treatment groups were higher than those for the control groups. In addition, the mean for the day 7 group was higher than that for the day 14 group. The day 14 treatment group had the highest mean.
Table 2. ANOVA results for OSX expression (*=P <0.05).
|
Groups |
K7 |
K14 |
P7 |
P14 |
|
K7 |
- |
0.085 |
0.000* |
0.000* |
|
K14 |
0.085 |
- |
0.004* |
0.000* |
|
P7 |
0.000* |
0.004* |
- |
0.444 |
|
P14 |
0.000* |
0.000* |
0.444 |
- |
The results of the ANOVA test are shown in Table 2 with a significance level of P-value < 0.05. These results indicate that there was a significant difference in OSX expression between the two groups (control and treatment) resulting from treatment with gourami fish scale collagen. No significant difference was found in OSX expression between the day 7 and day 14 groups (P-value = 0.05).
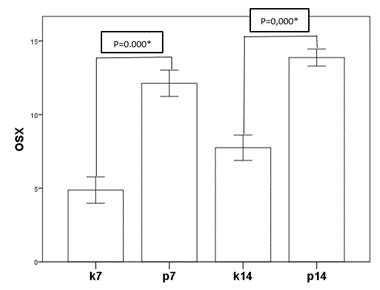
Figure 2. Mean of expression of OSX.
Figure 2 shows the OSX expression of the control and treatment groups on day 7 and day 14. The treatment groups can be seen to have significantly increased OSX expression compared to that in the respective control groups. OSX expression shows an increase when compared between the groups on day 7 and day 14. However, the increase is less significant.
The following microscope image shows osteoblast cells stained using anti-SOX9 monoclonal antibodies on day 7 and day 14:
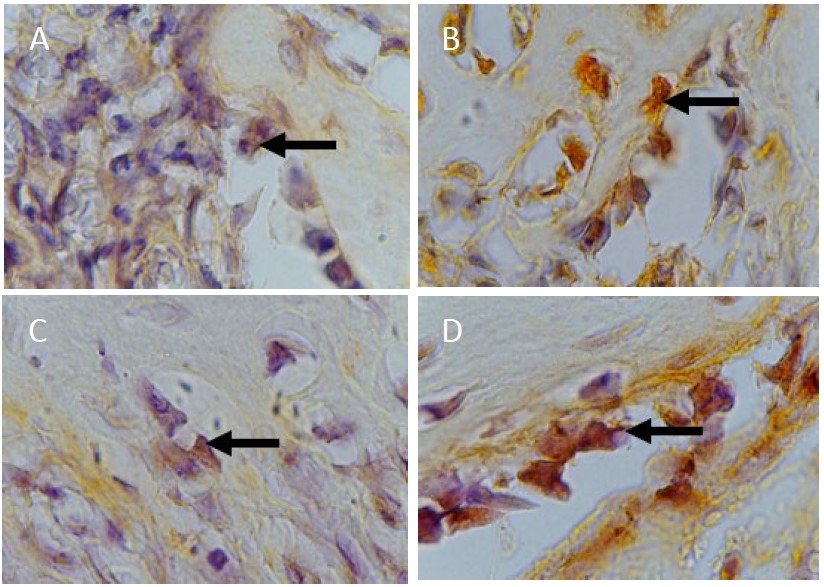
Figure 3. (A) Day 7 SOX9 control group (B) Day 7 SOX9 treatment group (C) Day 14 SOX9 control group (D) Day 14 SOX9 treatment group at 1000x magnification.
The distributions of the mean and standard deviation of SOX9 expression in the control and treatment groups on day 7 and day 14 are as follows:
Table 3. Mean and standard deviation of SOX9 expression.
|
Groups |
Day 7 |
Day 14 |
||
|
Mean |
Standard Deviation |
Mean |
Standard Deviation |
|
|
Control |
5.880 |
2.232 |
10.000 |
2.070 |
|
Treatment |
14.000 |
2.268 |
9.380 |
2.200 |
As seen in Table 3 the mean for the day 7 treatment group was higher than that for the day 7 control group. However, the mean for the day 14 treatment group was lower than that for the day 14 control group. In addition, the mean for the day 7 treatment group was higher than that for the day 14 treatment group, whereas the mean for the day 7 control group was lower than for the day 14 control group. The day 7 treatment group had the highest mean, whereas the day 7 control group had the lowest mean.
Table 4. ANOVA results for SOX9 expression (*=P <0.05).
|
Groups |
K7 |
K14 |
P7 |
P14 |
|
K7 |
- |
0.004* |
0.000* |
0.017* |
|
K14 |
0.004* |
- |
0.006* |
0.940 |
|
P7 |
0.000* |
0.006* |
- |
0.001* |
|
P14 |
0.017* |
0.940 |
0.001* |
- |
The results of the ANOVA test are shown in Table 4 with a significance level of P-value < 0.05. This indicates there to be a significant difference in the expression of SOX9 on day 7 between the two groups, namely the control group and the group treated with gourami fish scale collagen. No significant difference was seen in the expression of SOX9 on day 14 between the two groups (the control group and the treatment group) resulting from treatment with gourami fish scale collagen (P-value > 0.05). SOX9 expression in the day 7 and day 14 groups were significantly different (P-value = 0.05).
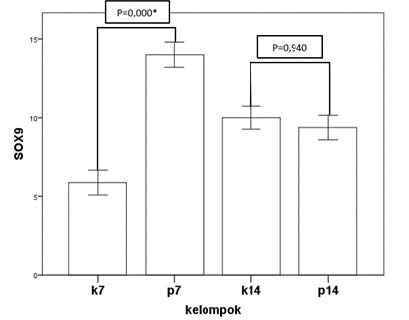
Figure 4. Mean of expression SOX9.
Figure 4 shows the SOX9 expression of the control and treatment groups on day 7 and day 14. The treatment group can be seen to have significantly increased expression of SOX9 compared to that in the control group. The day 14 treatment group has slightly decreased expression of SOX9 compared to that in the control group. In addition, SOX9 expression in the day 7 control group is significantly increased compared to that in the day 14 control group. A comparison between the day 7 treatment group and the day 14 treatment group showed a significant decrease in SOX9 expression.
DISCUSSION
Accomplishing the regeneration of alveolar bone defects remains a difficult task in dentistry (Nugraha et al., 2018; Saskianti et al., 2020). Bone regeneration is a process which need a well-coordinated cascade (Kurniawati et al., 2020). This process occurs entirely through three mechanisms: osteogenesis, osteoinduction, and osteoconduction (Poernomo et al., 2019). Regenerative treatment harnessing tissue engineering concept currently shows the most potential for treating alveolar bone defects. The following three elements of tissue engineering are collectively referred to as the tissue engineering triad: growth factors, stem cells and scaffolds (graft and platelet-rich fibrin) (Kurniawati et al., 2020; Saskianti et al., 2020).
Increased rates of bone regeneration have been achieved by various means, including the use of capable bone grafts (Nugraha et al., 2018; Saskianti et al., 2020). Bone grafts are considered to be the gold standard for bone defect therapy. Bone substitution increase the bone healing response by providing osteogenic, osteoinductive, and osteoconductive elements (Poernomo et al., 2019). Among different processes involved in the overall healing process, bone formation is a complex process that begins with the recruitment of osteoprogenitor cells that subsequently differentiate into osteoblasts, which is then followed by osteoid formation and matrix mineralization (Kurniawati et al., 2020).
Osteoblasts formation needs important transcription factors namely OSX (Chen et al., 2018). Analysis of several osteoblast signaling pathways shows that an increase in OSX transcription has a direct effect on increasing osteoblast formation (Sitasari et al., 2020). OSX is an important transcription factor in preosteoblast differentiation and bone development. In addition to osteoblast differentiation and bone formation, OSX is also required for the regulation of the expression of osteoblast genes such as osteopontin (OPN), osteocalcin, and bone sialoprotein (Ramadhani et al., 2018). Sitasari et al. (2020) showed an increase in OSX expression on day 14 compared to that on day 7 after the administration of green tea extract. This is consistent with our results showing the mean OSX expression in the treatment group to be higher than that in the control group and day 14 treatment group having the highest mean. This indicates that the administration of gourami fish scale collagen can significantly increase OSX expression in alveolar bones of Wistar rats, thus proving gourami fish scale collagen to be capable of inducing increased expression of OSX markers as bone growth markers (Sitasari et al., 2020).
SOX9 has been identified as a transcriptional master gene involved in skeletal morphogenesis (Nugraha et al., 2018). According to Stockl et al. (2013), SOX9 plays a proliferative role for rat stromal progenitor cells (SPCs), thus preventing and slowing osteogenic differentiation (Stöckl et al., 2013). However, Sumarta et al. (2016) reported that SPCs cultured in platelet-rich fibrin (PRF) increase SOX9 expression (Sumarta et al., 2016). Nugraha et al. (2018) also reported increased SOX9 expression as a result of culturing gingival stromal progenitor cells with PRF, with the highest expression of SOX9 being found in the day 7 treatment group. This is consistent with our results showing the highest mean for SOX9 expression in the day 7 treatment group (Nugraha et al., 2018). In other studies, the peak time of SOX9 of fracture healing was at day 7 and down regulated at day 10 to be dramatically decreased between days 14 and 21 (Kaback et al., 2008). In the skeletal formation process, SOX9 is expressed before RUNX-2 and OSX. SOX9 was expressed in the osteo-chondrorogenitor phase before chondrogenic and osteogenic cell lines were determined (Akiyama et al., 2005).
RUNX-2 and SOX9 are major transcription factors in the processes of osteogenesis and chondrogenesis. The temporal and spatial overlap of RUNX-2 and SOX9 expression indicates a close relationship between these two major transcription factors in bone formation. SOX9 acts as a transcriptional suppressor for osteoblast differentiation and chondrocyte hypertrophy by inhibiting RUNX-2 transactivation of its target gene (Shibata and Tamaki 2008). RUNX-2 also performs mutual inhibition of the activity of SOX9 (Cheng and Genever, 2010). Furthermore, expression of OSX in RUNX-2 expressing precursors induces these cells to differentiate into mature and functional osteoblast cells, which will eventually become osteocytes (Sinha and Zhou 2013). So it can be concluded that OSX acts as a downstream of RUNX-2 during the osteoblast differentiation process (Long, 2012). This shows that the collagen in gourami fish scales, when used as a scaffold, can bind and store growth factors (Zhang et al., 2011). Growth factors are known to stimulate and increase the expression of SOX9 from day 7 of osteogenic differentiation, which is thus useful for accelerating bone remodeling and cartilage formation (Nugraha et al., 2019).
CONCLUSION
The administration of gourami fish scale collagen with freeze drying method in extracted socket Wistar rats significantly increased OSX and SOX9 expression on day 7 compare to extracted socket without gourami fish scale collagen.
AUTHOR CONTRIBUTIONS
Jonathan Setiawan performed the analysis and wrote the paper. Chiquita Prahasanti and Eka Fitria Augustina conceived and designed the analysis. Melissa Tionardus collected data, contributed data. Wibi Riawan analysis tools. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Akiyama, H., Kim, J.E., Nakashima, K., Balmes, G., Iwai, N., Deng, J.M., Zhang, Z., Martin, J.F., Behringer, R.R., Nakamura, T., and De Crombrugghe, B. 2005. Osteo-chondroprogenitor cells are derived from SOX9 expressing precursors. Proceedings of the National Academy of Sciences of the United States of America. 102: 14665–14670.
Chen, X., Wang, Z., Duan, N., Zhu, G., Schwarz, E. M., and Xie, C. 2018. Osteoblast–osteoclast interactions. Connective Tissue Research. 59: 99–107.
Cheng, A. and Genever, P.G. 2011. SOX9 Determines RUNX2 Transactivity by directing intracellular degradation. Journal of Bone and Mineral Research. 25: 2680–2689.
Kaback, L.A., Soung, D.Y., Naik, A., Smith, N., Schwarz, E.M., O’Keefe, R.J., and Drissi, H. 2008. Osterix/Sp7 regulates mesenchymal stem cell mediated endochondral ossification. Journal of Cellular Physiology. 214: 173–182.
Kemenkes RI. 2019. Laporan nasional riskesdas 2018. Kementerian Kesehatan RI, 1: 1. https://www.kemkes.go.id/article/view/19093000001/penyakit-jantung-penyebab-kematian-terbanyak-ke-2-di-indonesia.html
Kurniawati, A., Saputra, D.R., Sari, D.Y., Amanitha, I.C., and Masruroh Hariyati, S.N. 2020. Cowhide gelatin nanoparticles and titanium-prepared platelet-rich fibrin potential in periodontitis healing process. ODONTO : Dental Journal. 7: 73.
Long, F. 2012. Building strong bones: molecular regulation of the osteoblast lineage. Nature Reviews Molecular Cell Biology. 13: 27–38.
Newman, M., Takei, H., Klokkevold, P., and Carranza, F. 2018. Newman and Carranza’s Clinical Periodontology 13th Edition. Elsevier Mosby.
Nugraha, A., Narmada, I., Savitri E.D., Hendrianto, E., Ihsan, I., Dinaryanti, A., Riawan, W., and Rantam, F. 2019. The aggrecan expression post platelet rich fibrin administration in gingival medicinal signaling cells in Wistar Rats (Rattus novergicus) during the early osteogenic differentiation (in vitro). Kafkas Universitesi Veteriner Fakultesi Dergisi. Kafkas Veterinary Journal. 25: 421-425.
Nugraha, A.P., Narmada, I.B., Ernawati, D.S., Dinaryanti, A., Hendrianto, E., Ihsan, I.S., Riawan, W., Alhasyimi, A.A., and Mada, G. 2018. Osteogenic potential of gingival stromal progenitor cells cultured in platelet rich fibrin is predicted by core-binding factor subunit-α1 / SOX9 expression ratio (in vitro). F1000Research. 7: 1134.
Oh, C., Lu, Y., Liang, S., Mori-akiyama, Y., Chen, D., Crombrugghe, B.De. and Yasuda, H. 2014. SOX9 regulates multiple genes in chondrocytes, including genes encoding ECM proteins, ECM modification enzymes, receptors, and transporters. PLoS One. 9: e107577.
Pati, F., Datta, P., Adhikari, B., Dhara, S., and Ghosh, K. 2012. Collagen scaffolds derived from fresh water fish origin and their biocompatibility. Journal of Biomedical Materials Research Part A. 100: 1068–1079.
Poernomo, H., Mulut, B.B., Gigi, F.K., and Denpasar, U.M. 2019. Teknik bone tissue engineering (BTE) untuk regenerasi jaringan periodontal dan estetik pada edentulous ridge. Interdental Jurnal Kedokteran Gigi (IJKG). 15: 56-59
Prahasanti, C., Wulandari, D.T., and Ulfa, N. 2018. Viability test of fish scale collagen (Oshpronemus gouramy) on baby hamster kidney fibroblasts-21 fibroblast cell culture. Veterinary World. 11: 506–510.
Rahmitasari, F. 2018. Scaffold 3D kitosan dan kolagen sebagai graft pada kasus kerusakan tulang. Jurnal Material Kedokteran Gigi. 5: 1.
Ramadhani, V., Narmada, I. bagus, and Hamid, T. 2018. The effect of combination of soy milk and calcium in orthodontic tooth movements on female rats with estrogen deficiency. Indonesian Journal of Dental Medicine. 1: 70–80.
Saskianti, T., Nugraha, A.P., Prahasanti, C., Ernawati, D.S., Suardita, K., and Riawan, W. 2020. Immunohistochemical analysis of stem cells from human exfoliated deciduous teeth seeded in carbonate apatite scaffold for the alveolar bone defect in Wistar rats (Rattus novergicus). F1000Research. 9: 1194.
Shibata, S., and Yokohama-tamaki, T. 2008. An in situ hybridization study of Runx2 ,Osterix , and SOX9 in the anlagen of mouse mandibular condylar cartilage in the early stages of embryogenesis. Journal of Anatomy. 213: 274–283.
Sinha, K.M. and Zhou, X. 2013. Genetic and molecular control of osterix in skeletal formation. Journal of Cellular Biochemistry. 114: 975–984.
Sitasari, P., Narmada, I., Hamid, T., Triwardhani, A., Nugraha, A., Rahmawati, D., and Garrido, G. 2020. East Java green tea methanolic extract can enhance RUNX2 and Osterix expression during orthodontic tooth movement in vivo. Journal of Pharmacy & Pharmacognosy Research. 8: 290–298.
Stöckl, S., Göttl, C., Grifka, J., and Grässel, S. 2013. SOX9 Modulates proliferation and expression of osteogenic markers of adipose-derived stem cells (ASC). Cellular Physiology and Biochemistry.31: 703–717.
Sumarta, M.N.P., D, C.P., Hendrianto, E., and Susilowati, H. 2016. Chondrogenic differentiation capacity of human umbilical cord mesenchymal stem cells with platelet rich fibrin scaffold in cartilage regeneration (In Vitro Study) 1. department of oral & maxillofacial surgery. Faculty of Dental Medicine, Airlangga Un. 5: 65–70.
Tonetti, M.S., Jepsen, S., Jin, L., and Otomo‐Corgel, J. 2017. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. Journal of Clinical Periodontology. 44: 456–462.
Zhang, F., Wang, A., Li, Z., He, S., and Shao, L. 2011. Preparation and characterisation of collagen from freshwater fish scales. Food and Nutrition Sciences. 2: 818-823.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand.
Jonathan Setiawan1, Chiquita Prahasanti2,*, Eka Fitria Augustina2, Melissa Tionardus1, and Wibi Riawan3
1 Resident of Department of Periodontics, Faculty of Dental Medicine, Airlangga University, Surabaya-Indonesia
2 Lecturer of Department of Periodontics, Faculty of Dental Medicine, Airlangga University, Surabaya-Indonesia
3 Staff of Department of Biomolecular, Faculty of Medicine, Universitas Brawijaya, Malang-Indonesia
Corresponding author: Chiquita Prahasanti, E-mail: chiquita-p-s@fkg.unair.ac.id
Total Article Views
Editor: Anak Iamaroon,
Chiang Mai University, Thailand
Article history:
Received: September 15, 2022;
Revised: March 15, 2023;
Accepted: March17, 2023;
Published online: March 27, 2023

