
The First Karyological Analysis of the Sixbar Grouper Epinephelus sexfasciatus (Valenciennes, 1828) (Perciformes, Epinephelinae)
Puan PengsengPublished Date : 2020-12-17
DOI : https://doi.org/10.12982/CMUJNS.2021.005
Journal Issues : Number 1, January-March 2021
Abstract This study examines for the first time of karyotypic analysis and chromosomal characteristic of nucleolar organizer regions/NORs of Epinephelus sexfasciatus. The fish samples were collected from Andaman Sea, Phuket province, southern Thailand. The chromosomes were investigated using conventional Giemsa’s staining and Ag-NORs banding techniques. Fish chromosome preparations were conducted by squash technique from kidney. The results showed that the diploid chromosome number of E. sexfasciatus was 2n=48 and the fundamental number (NF, number of chromosome arms) was 48. The type of chromosomes included 24 large telocentric and 24 medium telocentric chromosomes. After Ag-NOR banding technique, single pair of NORs was observed on the short arm of medium telocentric chromosome pair 23. The idiogram shows gradually decreasing length of the chromosomes. A size difference of the largest and the smallest chromosomes is approximately two folds. The karyotype formula could be infered as: 2n(48) = 2n(48)= Lt24+Mt24.
Keywords: Chromosome, Epinephelus sexfasciatus, Karyotype, Sixbar grouper
Funding: This research was supported by grants funded by Walailak University. The authors thank to the Toxic Substances in Livestock and Aquatic Animals Research Group, Khon Kaen University for providing instruments.
Citation: Pengseng, P. 2021. The first karyological analysis of the sixbar grouper Epinephelus sexfasciatus (Valenciennes, 1828) (Valenciennes, 1828) (Perciformes, Epinephelinae). CMUJ. Nat. Sci. 20(1): e2021005.
INTRODUCTION
The family Serranidae is one of the most important marine fish, as many species are of commercial value and present particular biological traits. Serranids have a great size, shape and color variation; with species that are no longer than 3 cm to others more than 2 m and 300 kg. Sex determination is also peculiar; Serraninae species are synchronic hermaphrodites (genera Serranus, and Hypoplectrus), while groupers and allies Epinephelinae (genera Alpbestes, Epinephelus, Mycteroperca, and Cephalopholis) present asynchronic hermaphroditism. Among the 300 plus species, which make up the family Serranidae, about half belong to the subfamily Epinephelinae, grouped in 15 genera 159 species. These species are of considerable economic value, especially in the coastal fisheries of tropical and subtropical areas. It has been estimated that 90% of the world’s harvest of marine food is derived from artisanal fisheries and groupers are also a major component of the artisanal fisheries resource. Although groupers are usually the most expensive fishes in local markets, separate catch statistics are not reported for most species, and landings are often summarized as ‘serranids’ or ‘groupers’ (Heemstra and Randall, 1993).
The taxonomic classification of Sixbar grouper, E. sexfasciatus (Cuvier and Valenciennes, 1828) is class Actinopterygii, subclass Neopterygii, division Teleostei, subdivision Euteleostei, superorder Acanthopterygii, order Perciformes, suborder Channoidei, family Serranidae, and subfamily Epinephelinae (grouper).
Visual characteristics of E. sexfasciatus are Body robust, the depth contained 2.4 to 3.4 times in standard length (for fish 12 to 179 cm standard length), the body width contained 1.5 to 1.75 times in the depth. Head length contained 2.2 to 2.7 times in standard length; interorbital width contained 3.3 (for fish 177 cm standard length) to 6.2 (for fish 12 cm standard length) times in head length; interorbital area flat to slightly convex, the dorsal head profile convex; preopercle sub angular, finely serrate, the corner rounded; upper edge of operculum convex; eye diameter contained 5.8 to 14 times in head length; nostrils subequal; maxilla reaching past vertical at rear edge of eye; mid lateral part of lower jaw with 2 or 3 rows of teeth (specimens of 20 to 25 cm standard l length) increasing to 15 to 16 rows in a fish of 177 cm standard length; canine teeth at front of jaws small or absent. Gill rakers of juveniles 8 to 10 on upper limb, 14 to 17 on lower limb; rudiments in adults are difficult to distinguish from the bony platelets covering the gill arch. Dorsal fin with XI spines and 14 to 16 rays, the 3rd to 11th spines subequal, their length contained 3.1 to 5.7 in head length and much shorter than longest rays in adults; anal fin with III spines and 8 rays; pectoral-fin rays 18 to 20; pectoral-fin length contained 1.8 to 2.2 times in head length; pelvic fins not reaching anus, their length contained 2.1 to 2.6 times in head length: caudal fin rounded. Lateral-body scales smooth, with auxiliary scales; lateral-line scales 54 to 62, the anterior scales with branched tubules (except small juveniles); lateral-scale series 95 to 105. Colour : Small juveniles (12 cm standard length) yellow, with irregular broad black bars on body, the first from spinous dorsal fin to belly and chest and extending onto head, the second from base of soft dorsal fin to anal fin and the last at base of caudal fin; small adults (20 to 50 cm standard length) with irregular white or yellow spots on the black areas and fins with irregular black spots; adults (80 to 150 cm standard length) dark brown with faint mottling, the fins with numerous small black spots: large adults (160 to 230 cm standard length) dark brown, the fins darker (Heemstra and Randall, 1984; 1986).
In Thailand, the subfamily Epinephelinae includes 43 species which can be grouped into six genera, namely; Aethaloperca, Anyperodon, Cephalopholis, Cromileptes, Plectropomus, and Epinephelus (Heemstra and Randall, 1993). Only 30 species in the genera Alphestes, Cephalopholis, Cromileptes, Epinephelus, Mycteroperca, and Plectropomus have been cytogenetically investigated with reports that the diploid chromosome number (2n) is 48 (Pinthong et al., 2013, 2015) (Table 1).
Table 1. Review of groupers cytogenetic reports in the subfamily Epinephelinae (genera Alphestes, Cephalopholis, Cromileptes, Epinephelus, Mycteroperca, and Plectropomus).
|
Species |
2n |
Karyotype |
NF |
NORs |
Reference |
|
A. afer |
48 |
48t |
48 |
2 |
Molina et al. (2002) |
|
Ce. formosa |
48 |
2m+2a+44t |
52 |
2(TR) |
Pinthong et al. (2013) |
|
Cr. altivelis |
48 |
2a+46t |
50 |
2 |
Takai and Ojima (1995) |
|
|
|
2sm+2a+44t |
52 |
2(TR) |
Pinthong et al. (2013) |
|
E. adscencionis |
48 |
48t |
48 |
2(TR), 24(SCR) |
Molina et al. (2002) |
|
E. akaara |
48 |
5a+43t |
48 |
- |
Wang et al. (2004) |
|
E. alexandrines |
48 |
48t |
48 |
24(SCR) |
Martinez et al. (1989) |
|
E. awoara |
48 |
48t |
48 |
24(SCR) |
Hong and Yang (1988) |
|
E. bruneus |
48 |
2m+4sm+42t |
54 |
2, 9, 24 |
Guo et al. (2008) |
|
E. caninus |
48 |
48t |
48 |
24(SCR) |
Rodríguez-daga et al. (1993) |
|
E. coioides |
48 |
2sm+46t |
50 |
- |
Wang et al. (2004) |
|
|
48 |
2sm+46t |
50 |
24(SA) |
Wang et al. (2010) |
|
|
48 |
2a+46t |
50 |
2(TR) |
Pinthong et al. (2013) |
|
E. diacanthus |
48 |
2sm+46t |
50 |
- |
Natarajan and Subrahmanyan (1974) |
|
E. erythrurus |
48 |
4a+44t |
52 |
18(SA) |
Pinthong et al. (2015) |
|
E. fario |
48 |
4m+6sm+4a+34t |
62 |
- |
Zheng et al. (2005) |
|
E. fasciatomaculatus |
48 |
48t |
48 |
24(SCR) |
Li and Peng (1994) |
|
E. fasciatus |
48 |
48t |
48 |
24(SCR) |
Li and Peng (1994) |
|
E. faveatus |
48 |
2m+46t |
50 |
- |
Magtoon and Donsakul (2008) |
|
E. fuscoguttatus |
48 |
2sm+46t |
50 |
- |
Liao et al. (2006) |
|
E. guaza |
48 |
48t |
48 |
24(SCR) |
Martinez et al. (1989) |
|
E. guttatus |
48 |
48t |
48 |
24(SA) |
Medrano et al. (1988) |
|
E. lanceolatus |
48 |
8sm+40t |
56 |
- |
Jiun and Mei (2009) |
|
E. malabaricus |
48 |
48t |
48 |
5(-), 24(SCR) |
Zou et al. (2005) |
|
E. marginatus |
48 |
48t |
48 |
2(TR), 24(SCR) |
Sola et al. (2000) |
|
E. merra |
48 |
4m+6sm+4a+34t |
62 |
- |
Zheng et al. (2005) |
|
E. moara |
48 |
48t |
48 |
- |
Guo et al. (2006) |
|
E. ongus |
48 |
48t |
48 |
- |
Rishi and Haobem (1984) |
|
E. sexfasciatus |
48 |
2sm+46t |
50 |
- |
Chen et al. (1990) |
|
|
48 |
48t |
48 |
23(SA) |
Present study |
|
E. tauvina |
48 |
2sm+46t |
50 |
- |
Rodríguez-daga et al. (1993) |
|
|
48 |
48t |
48 |
24(SCR) |
Maneechot and Supiwong (2015) |
|
M. acutirostris |
48 |
48t |
48 |
- |
Galetti et al. (2006) |
|
M. rubra |
48 |
48t |
48 |
- |
Aguilar et al. (1997) |
|
P. leopardus |
48 |
48t |
48 |
2(SCR) |
Pinthong et al. (2013) |
Notes: 2n = diploid chromosome number, NF = fundamental number (number of chromosome arm), m = metacentric chromosome, sm = submetacentric chromosome, a = acrocentric chromosome, t = telocentric chromosome, NORs = nucleolar organizer regions, TR = telomeric region, SA = short arm, SCR = subcentromeric region and - = not available
In the present cytogenetic study we conducted for the first time a karyological analysis and examined chromosomal characteristics of the nucleolar organizer regions (NORs) of E.sexfasciatus .Results obtained will increase our basic knowledge of the cytogenetics of E. sexfasciatus which could be the basis for future research and provide data to ensure that we may assist in their survival.
MATERIALS AND METHODS
Sample collection
Mix sexes of coral grouper from Phuket, Andaman Sea, Thailand were collected (see Table 1 and Figure 1) and transported to the aquarium in the laboratory and kept under standard conditions for 3 days prior to the experiment.
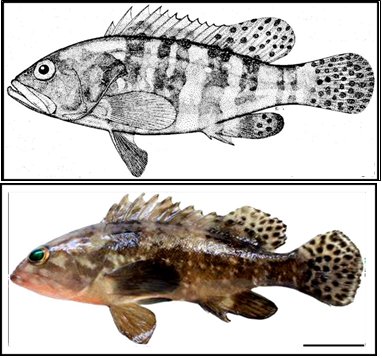
Figure 1. Morphological characters of the Six-bar grouper (Epinephelus sexfasciatus) adult 148 mm standard length (Heemstra and Randall, 1993). Scale bar indicate 2 centimeters.
Chromosome preparation
Chromosomes were directly prepared in vivo (Chen and Ehbeling; 1968, Nanda, et al., 1995). 4% phytohemagglutinin (PHA) solution was injected into the fish’s abdominal cavity at 1% of body weight. After 24 h, 0.05% of colchicine was injected into the fish’s intramuscular at 1% of body weight and/or its abdominal cavity and left for 2–4 h. The kidney was cut into small pieces then squashed and mixed with 0.075 M KCl. After discarding all large piece tissues, 15 mL of cell sediments were transferred to a centrifuge tube and incubated for 25–35 min. KCl was discarded from the supernatant after centrifugation again at 1,200 rpm for 8 min. Cells were fixed in fresh cool fixative (3 methanol:1 glacial acetic acid) gradually added up to 8 mL before centrifuged again at 1,200 rpm for 8 min, then the supernatant was discarded. The fixation was repeated until the supernatant was clear and the pellet was mixed with 1 mL fixative. The mixture was dropped onto a clean and cold slide by micropipette followed by air drying.
Chromosome staining
Conventional staining was done using 20% Giemsa’s solution for 30 min and Ag- NOR banding (Howell and Black, 1980), was performed by adding two drops of 50% silver nitrate and 2% gelatin on slides, respectively. The slides were then sealed with cover glasses and incubated at 60°C for 5 min. Subsequently, the slides were soaked in distilled water until the cover glasses separated and were then stained with 20% Giemsa’s solution for 1 min.
Chromosome checks
Chromosome counting was performed on mitotic metaphase cells under a light microscope. Twenty four clearly observable metaphase and well spread chromosomes were selected and photographed. The length of short arm chromosome (Ls) and the length of long arm chromosome (Ll) were measured to calculate the total arm length of chromosome (LT, LT = Ls+Ll). The relative length (RL), and the centrometric index (CI) were calculated (Chaiyasut, 1989). The CI (q/p+q) between 0.50–0.59, 0.60–0.69, 0.70–0.89 and 0.90–0.99 were described as metacentric, submetacentric, acrocentric, and telocentric chromosomes, respectively. The fundamental number (number of chromosome arms, NF) was obtained by assigning a value of two to metacentric, submetacentric, and acrocentric chromosomes and one to telocentric chromosome. All parameters were used in karyotyping and idiograming.
RESULTS
Chromosome number, fundamental number and karyotype of E. sexfasciatus
All fifty chromosomal metaphase spreads observed had a diploid chromosome number of 48. The fundamental number was therefore 48. All chromosomes were telocentric, 24 large- and 24 medium-sized.
Chromosome markers of E. sexfasciatus
The chromosomes of mitotic metaphase cells and the karyotypes of sexfasciatus by conventional staining and Ag-NOR banding techniques are shown in Figure 2. The lengths of chromosomes in 24 mitotic metaphase cells were measured. The mean length of short arm chromosomes (Ls), length of long arm chromosomes (Ll), total length of arm chromosomes (LT), relative length (RL), centromeric index (CI), sizes and types of chromosome are presented in Table 2. The idiogram of E. sexfasciatus shows gradually decreasing length of the chromosomes. The E. sexfasciatus demonstrate that the chromosome markers are the chromosome pairs 1 and pair 24 which are the largest telocentric chromosome and the smallest telocentric chromosome, respectively. The important karyotype feature of E. sexfasciatus is the asymmetrical karyotype, which was found in one type of chromosomes (telocentric chromosomes). The largest chromosome is two times larger than the smallest chromosome. Figure 3 shows the standardized idiogram of the E. sexfasciatus. The karyotype formula could be deduced as: 2n (48) = Lt24+Mt24. shows the standardized idiogram of the sexfasciatus. The karyotype formula could be deduced as: 2n (48) = Lt24+Mt24.
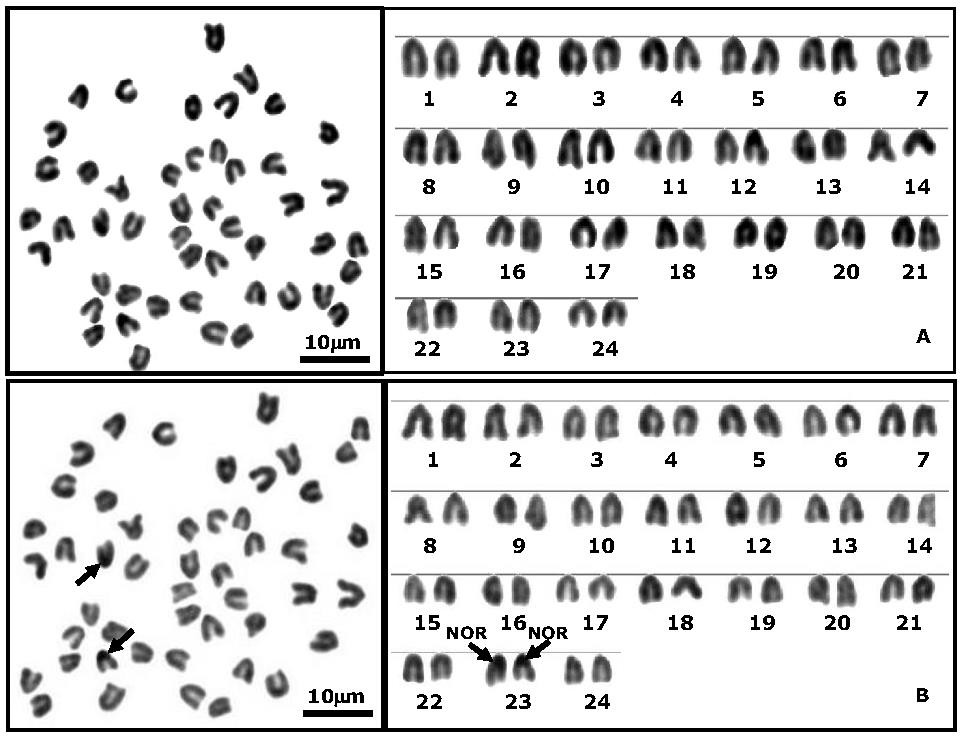
Figure 2. Metaphase chromosomes and karyotypes of the Sixbar grouper (Epinephelus sexfasciatus), 2n = 48 from Andaman Sea, Thailand by conventional staining (A.) and Ag-NOR banding techniques (B.). The arrows indicate NOR-bearing chromosomes (scale bars = 10 μm).

Figure 3. Idiogram showing lengths and shapes of chromosomes of the Sixbar grouper (Epinephelus sexfasciatus), 2n = 48 from Andaman Sea, Thailand. The region adjacent of short arm of chromosome pair 4 showed clearly observable nucleolar organizer region (NOR).
Table 2. Mean length of short arm chromosomes (Ls), long arm chromosomes (Ll), total arm chromosomes (LT), relative length (RL), centromeric index (CI), and standard deviation (SD) of RL, CI from 20 metaphase cells of male and female sixbar grouper (Epinephelus sexfasciatus), 2n = 48.
|
Chro. pair |
Ls |
Ll |
LT |
RL±SD |
CI±SD |
Chro. size |
Chro. Type |
|
1 |
0.00 |
0.81 |
0.81 |
0.03+0.00 |
1.00+0.00 |
Large |
Telocentric |
|
2 |
0.00 |
0.77 |
0.77 |
0.02+0.00 |
1.00+0.00 |
Large |
Telocentric |
|
3 |
0.00 |
0.75 |
0.75 |
0.02+0.00 |
1.00+0.00 |
Large |
Telocentric |
|
4 |
0.00 |
0.73 |
0.73 |
0.02+0.00 |
1.00+0.00 |
Large |
Telocentric |
|
5 |
0.00 |
0.72 |
0.72 |
0.02+0.00 |
1.00+0.00 |
Large |
Telocentric |
|
6 |
0.00 |
0.71 |
0.71 |
0.02+0.00 |
1.00+0.00 |
Large |
Telocentric |
|
7 |
0.00 |
0.70 |
0.70 |
0.02+0.00 |
1.00+0.00 |
Large |
Telocentric |
|
8 |
0.00 |
0.69 |
0.69 |
0.02+0.00 |
1.00+0.00 |
Large |
Telocentric |
|
9 |
0.00 |
0.68 |
0.68 |
0.02+0.00 |
1.00+0.00 |
Large |
Telocentric |
|
10 |
0.00 |
0.67 |
0.67 |
0.02+0.00 |
1.00+0.00 |
Large |
Telocentric |
|
11 |
0.00 |
0.67 |
0.67 |
0.02+0.00 |
1.00+0.00 |
Large |
Telocentric |
|
12 |
0.00 |
0.66 |
0.66 |
0.02+0.00 |
1.00+0.00 |
Large |
Telocentric |
|
13 |
0.00 |
0.64 |
0.64 |
0.02+0.00 |
1.00+0.00 |
Medium |
Telocentric |
|
14 |
0.00 |
0.63 |
0.63 |
0.02+0.00 |
1.00+0.00 |
Medium |
Telocentric |
|
15 |
0.00 |
0.62 |
0.62 |
0.02+0.00 |
1.00+0.00 |
Medium |
Telocentric |
|
16 |
0.00 |
0.62 |
0.62 |
0.02+0.00 |
1.00+0.00 |
Medium |
Telocentric |
|
17 |
0.00 |
0.61 |
0.61 |
0.02+0.00 |
1.00+0.00 |
Medium |
Telocentric |
|
18 |
0.00 |
0.60 |
0.60 |
0.02+0.00 |
1.00+0.00 |
Medium |
Telocentric |
|
19 |
0.00 |
0.59 |
0.59 |
0.02+0.00 |
1.00+0.00 |
Medium |
Telocentric |
|
20 |
0.00 |
0.57 |
0.57 |
0.02+0.00 |
1.00+0.00 |
Medium |
Telocentric |
|
21 |
0.00 |
0.56 |
0.56 |
0.02+0.00 |
1.00+0.00 |
Medium |
Telocentric |
|
22 |
0.00 |
0.55 |
0.55 |
0.02+0.00 |
1.00+0.00 |
Medium |
Telocentric |
|
23* |
0.00 |
0.53 |
0.53 |
0.02+0.00 |
1.00+0.00 |
Medium |
Telocentric |
|
24 |
0.00 |
0.50 |
0.50 |
0.02+0.00 |
1.00+0.00 |
Medium |
Telocentric |
Note: * = NOR-bearing chromosome.
DISCUSSION
To our study, this is the first record on the karyological study of E. Sexfasciatus. A comparative study with other species in the subfamily Epinephelinae gave the same chromosome number as those found in A. afer, Ce. formosa, Ce. boenak, Cr. altivelis, adscencionis, E. akaara, E. alexandrines, E. awoara, E. bruneus, E. caninus, E. coioides, E. diacanthus, E. erythrurus, E. fario, E. fasciatomaculatus, E. fasciatus, E. faveatus,E. fuscoguttatus, E. guaza, E. guttatus, E. lanceolatus, E. malabaricus, E. marginatus, E. merra, E. moara, E. ongus, E. sexfasciatus, E. tauvina, M. acutirostris, rubra, and P. leopardus (Arai, 2011; Pinthong et al., 2013, 2015; Maneechot and Supiwong, 2015). Previous cytogenetical reports on the other Serranidae species have showed a remarkable numerical, 2n = 48 (Arai, 2011) and structural chromosome homogeneity with several telocentric chromosomes and a common heterochromatin distribution at the centromeric or pericentromeric positions (Molina et al., 2002).
Although most Serranidae fish have a NF of 48, with only telocentric chromosomes (mono armed chromosomes) in the complement, the number of chromosome arms in the subfamily Epinephelinae vary from 48 to 62 (Arai, 2011). Based on the assumption that species with a large NF are more advanced in evolutionary terms (Ghigliotti et al., 2007; Wang et al., 2010), the most recent species within the genus Epinephelus would be E. bruneus, NF = 54 (Guo et al., 2008), E. lnanceolatus, NF = 56 (Jiun and Mei, 2009), E. fario, NF = 62 and E. merra, NF = 62 (Zheng et al., 2005). However, despite differences in NF, all Epinephelus species share the same diploid chromosome number (2n = 48). Such a change in the number of chromosome arms can be related to the occurrence of pericentric inversions, which are among the most common modifications contributing to karyotypic rearrangement in fish (King, 1993; Galetti et al., 2000). The presence of bi-arm chromosomes in the karyotype of E. sexfasciatus (NF = 48) represents a derived karyotypic feature that could have arisen in a common ancestor as a result of pericentric inversions in the acrocentric chromosome pairs 23.
Our cytogenetic analysis of E. sexfasciatus was accomplished by using the Ag- NOR banding technique. The objective of this technique is to detect the nucleolar organizer regions/NORs which represent the location of genes (loci) that function in ribosome synthesis (18S and 28S ribosomal RNA). Accordingly, the specific dark band (NOR-positive) is induced by the reduction of organic silver by these proteins that change silver to be dark (Sharma et al., 2002).
The region adjacent to the telomeric regions of the short arms of the chromosome pair 23 (medium acrocentric chromosomes) showed clearly observable NORs (Figure 2 and 4).
The presence of NORs, either in the terminal position or near the centromeres is considered to be a plesiomorphic character (Vitturi et al., 1993). Within the order Perciformes, the presence of a single NOR pair at interstitial position seems to be the most frequent situation, especially in species with conserved karyotypes (Galetti et al., 2006). Hence, the assumption that the presence of a single NOR is an ancestor trait of the family Serranidae is valid (Molina et al., 2002).
NORs in the family Serranidae are most frequently single and located at the interstitial position (Aguilar and Galetti, 1997). The presence of a ribosomal site on the smallest chromosome pair 24 is conserved within the genus Epinephelus (Sola et al., 2000). The presence of multiple NORs in the genus Epinephalus, as found in marginatus (Sola et al., 2000), E. adscencionis (Molina et al., 2002), E. malabaricus (Zou et al., 2005), and E. bruneus (Guo et al., 2008), would indicate that these species are more recently derived than species NOR site. The detection of extra NOR site on the large chromosome pair 2 in A. afer (Molina et al., 2002), Ce. formosa (Pinthong et al., 2013), Cr. altivelis (Takai and Ojima, 1995; Pinthong et al., 2013), and P. leopardus (Pinthong et al., 2013) is similar to the pattern described in E. marginatus (Sola et al., 2000), E. adscensionis (Molina et al., 2002), E. coioides (Pinthong et al., 2013), and E. bruneus (Guo et al., 2008), which indicates a simple isomorphic condition. Furthermore, the occurrence of a single NOR could be assumed to be an ancestral trait of Toleostei (Foresti et al., 1981).
CONCLUSION
The investigation of Six bar grouper chromosomes which were sampled from Andaman sea, Thailand. Using conventional staining and Ag-NOR staining found that the importantly karyotypic feature is the asymmetrical karyotype, which is telocentric chromosomes. No cytologically distinguishable sex chromosome was observed. In order to gain a more thorough understanding of the chromosomal evolutionary history of relationships within the groupers, further cytogenetic investigations of the remaining species in this subfamily is required. This study is the first time examines of karyotypical analysis and chromosomal characteristic of nucleolar organizer regions/NORs of the Epinephelus sexfasciatus.
REFERENCES
Aguilar, C.T., and Galetti, Jr. P.M. 1997. Chromosomal studies in South Atlantic serranids (Pisces, Perciformes). Cytobios 89: 105–114.
Arai, R. 2011. Fish karyotypes: a check list. Tokyo: Springer. p. 28–215.
Bloch, M.E. 1790. Naturgeschichte der ausländischen Fische.Vol. 4. J. Morino & Comp., Berlin. p.128.
Chaiyasut, K. 1989. Cytogenetics and cytotaxonomy of the family zephyranthes. Bangkok: Chulalongkorn University.
Chen, T.R., and Ehbeling, A.W. 1968. Karyological evidence of female heterogamety in the mosquito fish, Gambusia affinis. Copeia. 1: 70-75.
Chen, Y., Rong, S., Liu, S., Zhang, H., and Pei, M. 1990. Analysis of the karyotype of Epinephelus sexfasciatus. Journal of Zhanjiang Fisheries College. 2: 62-68.
Cuvier, G., and Valenciennes, A. 1828. Hisroire naturelle des poissons.Vol. 2. Paris: F.G. Levrault. 490 p.
Foresti, F., Almeida-Toledo, L.F., and Toledo-Filho, S.A. 1981. Polymorphic nature of nucleolus organizer regions in fishes. Cytogenetics and Cell Genetics. 31: 13- 144.
Galetti, Jr. P. M., Aguilar, C. T., and Molina, W. F. 2000. An overview of marine fish cytogenetics. Hydrobiologia. 420: 55-62.
Galetti, Jr. P.M., Molina, W.F., Affonso, P.R.A.M., and Aquilar, C.T. 2006. Assessing genetic diversity of Brazilian reef fishes by chromosomal and DNA markers. Genetica. 126: 161-177.
Ghigliotti, L., Mazzei, F., Ozouf-Costaz, C., Bonillo, C., Willams, R., Cheng, C., and Pisano, E. 2007. The two giant sister species of the Southern Ocean, Dissostichus eleginoides and Dissostichus mawsoni, differ in karyotype and chromosomal pattern of ribosomal RNA genes. Polar Biology. 30: 625-634.
Guo, F., Wang, J., Su, Y.Q., Wang, D.X., and Xu, L.N. 2006. Study on the karpyotype of Epinephelus moara. Marine Sciences. 8: 1-3.
Guo, M., Wang, S., Su, Y., and Ding, S. 2008. The cytogenetic studies of the longtooth (Epinephelus bruneus). Journal of Biotechnology. 136S: 527-540.
Heemstra, P.C., and Randall, J.E. 1984. Serranidae, without pagination. In: Fischer, W., and Bianchi, G., editors. FAO species identification sheets for fishery purposes: Western Indian Ocean.Vol. 4 Food and Agriculture Organization of the United Nations, Rome. 241 p.
Heemstra, P.C., and Randall, J.E. 1986. Serranidae, p. 509-537. In: Smiths’sea fishes. Smith M.M., and Heemstra P.C., editors. Johannesburg: Macmillan South Africa, 1047 p.
Heemstra, P.C., and Randall, J.E.1993. FAO Species Catalogue, Vol. 16: Groupers of the World. Rome: Food and Agriculture Organization of the United Nations. 413 p.
Hong, M.X., and Yang, J.H. 1988. Studies on the karyotypes of Epinephelus awoara. Journal of Xiamen University (Natural Science). 27: 714-715.
Jiun, C.S., and Mei, C.T. 2009. Karyotype identification of Epinephelus lanceolatus. In: 4th Academic Seminar, Department of Aquaculture, National Pingtung University of Science and Technology, Neipu. p. F33-37.
King, M. 1993. Species evolution: the role of chromosomes change. Cambridge: Cambridge University Press.
Li, X., and Peng, Y. 1994. Studies on karyotype of Epinephelus asciatomaculatus and Epinephelus fasciatus. Journal of Zhanjiang Fisheries College. 14: 22-26.
Liao, J.Q., Yin, S.W., Chen, G.H., Huang, H., Lei, C.G., and Lou, T.T. 2006. The karyotype of grouper Epinephelus fuscoguttatus. Fisheries Science. 25: 567-569. Magtoon, W., and Donsakul, T. 2008. Karyotype of five teleostean fishes from Thailand.
In: Technology for Global Challenges, 34th congress on science and Technology of Thailand, Bangkok. p. BO113.
Maneechot, N., and Supiwong, W. 2015. Cytogenetics of greasy grouper, Epinephelus tauvina (Perciformes, Serranidae) by conventional Staining and Ag-NOR banding techniques. Journal of Fisheries Technology Research. 9: 53-63.
Martinez, G., Thode, G., Alvarez, M.C., and Lopez, J.R. 1989. C-banding and Ag-NOR reveal a certain heterogeneity among karyotypes of serranids (Perciformes). Cytobios. 58: 53-60.
Medrano, L., Bernardi, G., Couturier, J., Dutrillaux, B., and Bernardi, G. 1988. Chromosome banding and genome compartimentalization in fishes. Chromosoma. 96: 178-183.
Molina, W.F., Maia-Lima, F.A., and Affonso, P.R.A.M. 2002. Divergence between karyotypical pattern and speciation events in Serranidae fish (Perciformes). Caryologia. 55: 299-305.
Nanda, I., Schsrtl, M., Fiechtinger, W., Schlupp, I., Parzefall, J., and Schmid, M. 1995. Chromosomal evidence for laboratory synthesis of triploid hybrid between the gynogenetic teleost Poecilia formosa and its host species. Journal of Fish Biology. 47: 619–623.
Natarajan, R., and Subrahmanyan, K. 1974. A karyotype study of some teleosts from Portonovo waters. Proceedings of the Indian Academy of Science, Section B. 79: 173–196.
Pinthong, K., Gomontean, B., Kongim, B., Khakhong, B., Sriveerachai, T., and Supiwong, W. 2013. Cytogenetic comparisons of four genera in grouper species (Pisces, Epinephelinae), and chromosome localization of the nucleolar organizer region from Thailand. Cytologia. 78: 223-234.
Pinthong, k., Maneechot, N., Tanomtong, A., Supiwong, W., Chananoon, T., and Jangsuwan, N. 2015. The first karyological analysis and chromosomal characteristic of NORs of the cloudy grouper, Epinephelus erythrurus (Perciformes, Epinephelinae) in Thailand. Cytologia. 80: 279-286.
Rishi, K.K., and Haobam, M.S. 1984. Karyological analysis of two marine fishes. Perspect. Cytology and Genetics. 4: 425-428.
Rodríguez-Daga, R., Amores, A., and Thode, G. 1993. Karyotype and nucleolus organizer regions in Epinephelus caninus (Pisces, Serranidae). Caryologia. 46: 71-76.
Sharma, O.P., Tripathi, N.K., and Sharma, K.K. 2002. A review of chromosome banding in fishes. In: Sobti, R.C., editor. some aspects of chromosome structure and functions. Delhi: New Narosa Publishing House. p. 109-122.
Sola, L., De Innocentiis, S., Gornung, E., Papalia, S., Rossi, A.R., Marino, G., De Marco, P., and Cataudella, S. 2000. Cytogenetic analysis of Epinephelus marginatus (Pisces: Serranidae), with the chromosome localization of the 18S and 5S rRNA genes and of the (TTAGGG)n telomeric sequence. Marine Biology. 137: 47-51.
Vitturi, R., Catalano, R., and Colombera, D.1993. Chromosome analysis of Bothus podas (Pisces, Pleuronectiformes) from the Mediterranean Sea. Journal of Fish Biology. 43: 221-227.
Wang, Y.X., Wang, H.D., Zhang, H.F., and Liu-Fu, Y.Z. 2004. Karyotypes of Epinephelus coioides and Epinephelus akaara. Journal of Zhanjiang Ocean University. 24: 4–8.
Wang, S., Su, Y., Ding, S., Cai, Y., and Wang, J. 2010. Cytogenetic analysis of orange- spotted grouper, Epinephelus coioides, using chromosome banding and fluorescence in situ hybridization. Hydrobiologia. 638: 1-10.
Wei, Y., Fan, T., Jiang, G., Sun, A., Xu, X., and Wang, J. 2009. Establishment of a novel fin cell line from brown-marbled grouper, Epinephelus fuscoguttatus (Forsskål), and evaluation of its viral susceptibility. Aquaculture Research. 40: 1523–1531.
Zheng, L., Liu, C.W., and Li, C.L. 2005. Studies on the karyotype of 4 groupers. Marine. Science. 29: 51–55.
Zou, J.X., Yu, Q.X., and Zhou, F. 2005. The karyotypes C-bands patterns and Ag-NORs of Epinephelus malabaricus. Journal of Fisheries of China. 29: 33–37.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand https://cmuj.cmu.ac.th
Puan Pengseng
School of Agricultural Technology, Walailak University, Nakhon Si Thammarat 80160, Thailand
Corresponding author: Puan Pengseng, E-mail: puan5956@gmail.com
Total Article Views
Editor: Wasu Pathom-aree,
Chiang Mai University, Thailand
Article history:
Received: February 11, 2020;
Revised: May 25, 2020;
Accepted: June 9, 2020

