
Screening of Antibacterial Activity of Goniothalamus calvicarpa Extracts against Xanthomonas axonopodis pv. citri in vitro
Juangjun Jumpathong*, Nungruthai Suphrom, Bernard Dell, Khammool Khamsuk, Thanita Boonsrangsom, and Thanatsan PoonpaiboonpipatPublished Date : 2020-03-30
DOI : https://doi.org/10.12982/CMUJNS.2020.0017
Journal Issues : Number 2, April-June 2020
ABSTRACT Citrus canker caused by Xanthomonas axonopodis pv. citri (Xac) is a serious disease in many parts of the world and is difficult to control. The use of copper-based pesticides is becoming a concern due to the accumulation of heavy metals in orchard soils and more benign treatment methods are needed. Eighteen Thai native plant extracts were screened for antibacterial activity against a Thai isolate of Xac and it was found that ethanolic extracts of Goniothalamus calvicarpa leaves showed the strongest antibacterial activity against Xac in vitro. The G. calvicarpa extracts were then sequentially dissolved with hexane, ethyl acetate and methanol and retested. A 70% aqueous ethanol extract and a methanol soluble extract produced strong inhibition zones against Xac. Although thin layer chromatographic profiles revealed the likely presence of flavonoids in the biologically active extracts of G. calvicarpa, the active compounds have yet to be identified. Work is proceeding to determine whether specific extracts of G. calvicarpa have biological activity against citrus canker in the field.
Keywords: Antibacterial activity, Plant extract, Citrus canker
INTRODUCTION
Citrus canker caused by Xanthomonas axonopodis pv. citri (Xac) is widespread in Asia and a biosecurity threat in many parts of the world affecting the global trade in citrus. The pathogen causes crater-like lesions on the fruit which are surrounded by a chlorotic halo with a water-soaked margin (Graham et al., 2004; Ngoc et al., 2007). Control is difficult. Currently, copper-based pesticides are intensively used to decrease disease incidence. However, the accumulation of Cu in orchard soils may affect root growth, nutrient uptake and allow copper-resistant strains of the causal organism to persist (Behlau et al., 2017). Lime, kaffir lime and pomelo are important orchard crops in many provinces in Thailand. Ngoc et al. (2007) first reported Xac infesting citrus in Thailand. During the monsoon season, producers spray copper-based pesticides if early stages of bacterial infection are observed. Where infestations are severe, diseased trees are destroyed to reduce crop loss in surrounding orchards. Alternative chemicals are therefore being sought to reduce copper loading into the environment. Plant based-bactericides may be one option if they can be shown to be effective.
Thailand has an abundance of plant species which possess potential bioactive compounds, some of which are effective against bacterial diseases. For example, plant extracts have been reported to be highly efficient in the control of some food-borne pathogens (Dholvitayakhun et al., 2012; Jarriyawattanachaikul et al., 2016) and against some plant pathogenic bacteria (Prakash and Karmegam, 2012; Bhagwat and Datar, 2014). Crude extracts obtained from leaves of nine Thai plant families were investigated for their herbicidal activity and allelochemical content (Poonpaiboonpipat and Jumpathong, 2019). Among these, extracts from Goniothalamus calvicarpa had the highest total phenolic content (143.7 mg gallic acid equivalent per g of crude extract). Further, Jewers et al. (1972) isolated Goniothalamin, a bioactive styryllactone, from four Goniothalamus species and Wiart (2007) reported that Goniothalamus species were a potential source of antibacterial agents.
In this study, we compared leaf extracts of G. calvicarpa with those from seventeen other Thai native plant extracts to ascertain whether there was any antibacterial activity against the causal agent of citrus canker. Among the plants selected for study, the presence of antimicrobial, anticancer and antioxidant activity have been reported in some species (Calixto et al., 2004; Uthairatsamee et al., 2011; Pukdeekumjorn et al., 2016). Also, the bark from Garcinia dulcis, Zingiber zerumbet and Cinnamomum porrectum have been used as folk medicine (Deachathai et al., 2005; Chien et al., 2008; Limsuwan et al., 2013). To study the plant extract chemical profiles, thin layer chromatography (TLC) was used to observe group of compounds. TLC is a qualitative method which can be used to aid in rapid chemical profile identification of plant extracts (Suphrom et al., 2018). As an integral part of the study, we aimed to identify a suitable solvent for leaf extraction to obtain effective bioactive compounds.
MATERIALS AND METHODS
Plant extract preparation
Samples of seventeen plant species from nine families were collected from central Thailand in November 2016 (Table 1). Leaves and other parts from these species were washed in tap water and dried at 45 °C for 48 h in a hot air oven. The dried leaves were extracted with 70% aqueous ethanol in a ratio of 1:10 w/v for 72 h in the dark. The extract solutions were filtered through Whatman No. 1 filter paper, evaporated in a rotary evaporator, lyophilized, and then stored in the dark at 4 °C until use.
Table 1. Plants used to obtain extracts.
|
Family |
Plant species |
Plant part |
Location¥ |
|
Annonaceae |
Anomianthus dulcis (Dunal) J. Sinclair |
Leaves |
1 |
|
|
Goniothalamus calvicarpa Craib |
Leaves |
1 |
|
|
Mitrephora keithii Ridl |
Leaves |
1 |
|
|
Mitrephora wangii Hu |
Leaves |
1 |
|
|
Orophea polycarpa A. DC. |
Leaves |
1 |
|
Asteraceae |
Elephantopus scaber L. |
Roots |
2 |
|
Bignoniaceae |
Pachyptera hymenaea (DC.) A. Gentry |
Leaves |
2 |
|
Clusiaceae |
Garcinia dulcis (Roxb.) Kurz |
Bark |
3 |
|
Eeicaceae |
Agapetes lobbii C.B. Clarke |
Leaves |
1 |
|
Lauraceae |
Cinnamomum loureiroi Nees |
Leaves |
1 |
|
|
Cinnamomum porrectum Kosterm. |
Leaves |
1 |
|
Magnoliaceae |
Michelia floribunda Fin. et Gagnep |
Leaves |
1 |
|
|
Manglietia garrettii Cralb |
Leaves |
1 |
|
|
Magnolia henryi Dunn. |
Leaves |
1 |
|
Moraceae |
Ficus microcarpa L.f. var. crassifolia Sheeh Liao |
Leaves |
3 |
|
Zingiberaceae |
Zingiber zerumbet (L.) Smith. |
Stems |
2 |
|
|
|
Roots |
2 |
|
|
Hedychium coronarium J. Koenig |
Leaves |
1 |
Note: ¥1 Romklao Botanical Garden under the Royal Initiative, Phitsanulok Province, Thailand.
2 Amphoe Khao Kho Phetchabun Province, Thailand.
3 Naresuan University, Phitsanulok Province, Thailand.
Antibacterial activity test
Test Microorganisms. Phytopathogenic bacterial strains of Xanthomonas axonopodis pv. citri (Xac), isolated from kaffir lime (strain number DOABC856), lime (strain number DOABC956), and pomelo (strain number DOABC992) were obtained from the Plant Protection Research and Development Office, Department of Agriculture in Thailand.
Preliminary screening for antibacterial activity. The antibacterial activity assay, that was used to test for effectiveness against Xac, used the paper disc diffusion method (Boonkaew and Camper, 2005). Bacterial suspensions, adjusted to 108 CFU/mL, were inoculated onto the surface of nutrient agar plates (Hi-media, India). The dried 70% ethanol extracts were re-dissolved in methanol and the samples were loaded onto 6 mm sterile discs (Whatman, GE) (4 mg/disc), air-dried for ca. 1 h, and placed on the surface of bioassay plates. Each experiment was carried out using three replicates, and chloramphenicol (1 mg/mL) was used as the positive control. Plates were then held in the dark for 24 h at 30 °C. The inhibition zone around the discs was measured in mm.
Secondary screening for antibacterial agents. From the preliminary screening results, crude extracts from G. calvicarpa were selected for more detailed study. Extracts were dissolved in hexane, ethyl acetate, and methanol before testing for antibacterial activity using the same paper disc diffusion method.
Chromatographic profile detected using thin layer chromatography (TLC)
Eighteen crude extracts were loaded onto a silica plate (TLC silica gel 60 F254 aluminum sheet, Merck, Damstadt, Germany). The mobile phase (5% methanol in dichloromethane) was prepared at ambient room temperature. The TLC plate was developed to a distance of 8 cm. After the development, the plate was observed under visible, short-wave UV at 254 nm and long-wave radiation at 366 nm. To detect essential oils, flavonoids, glycosides, terpenes, carbohydrates and steroids, the plate was sprayed with anisaldehyde-sulfuric acid (Nandhasri et al., 2005; Gerlacha et al., 2018). Anisaldehyde-sulfuric acid spray reagent has greater ability to detect classes of compounds than sulfuric acid alone (Gerlacha et al., 2018).
Statistical analysis
Experimental data for antibacterial activity (inhibition zone size) were compared with the control using Duncan’s new multiple range test (P < 0.05, n = 3).
RESULTS
Antibacterial activity
Primary screening used the 70% aqueous ethanol extracts from seventeen different plant species. The challenge test revealed that only the G. calvicarpa leaf extract, at a concentration of 100 mg/mL (4 mg/disc), exhibited an inhibition zone. For the secondary screening, the extracts were taken up in a range of solvents and the antibacterial activity against X. axonopodis pv. citri (Xac) was then evaluated on bioassay plates for phytopathogenic bacterial isolates isolated from kaffir lime, lime and pomelo. When chloramphenicol was used as the positive control and the different solvents as a negative control, chloramphenicol had the highest activity at a concentration of 1 mg/mL (20 µg/disc) when tested against Xac from the three citrus hosts (Figure 1).
The methanolic extract of G. calvicarpa had significantly higher antibacterial activity than other extracts when tested against Xac in lime and kaffir lime. However, the 70% aqueous ethanol extract and the methanol soluble extract both had high activity when tested against Xac from pomelo (Table 2). In contrast, the discs with added solvents alone produced no zone of inhibition. In this experiment, methanol soluble extract and 70% aqueous ethanol extract exhibited greater antibacterial activity on Xac from pomelo than Xac from pomelo from lime and kaffir lime regarding the inhibition zone presented on bioassay plate using paper disc diffusion assay. In contrast, Xac from pomelo is the least sensitive on antibacterial activity test when using chloramphenicol. The hexane soluble extract was not assayed due to the small content after evaporated to dryness.
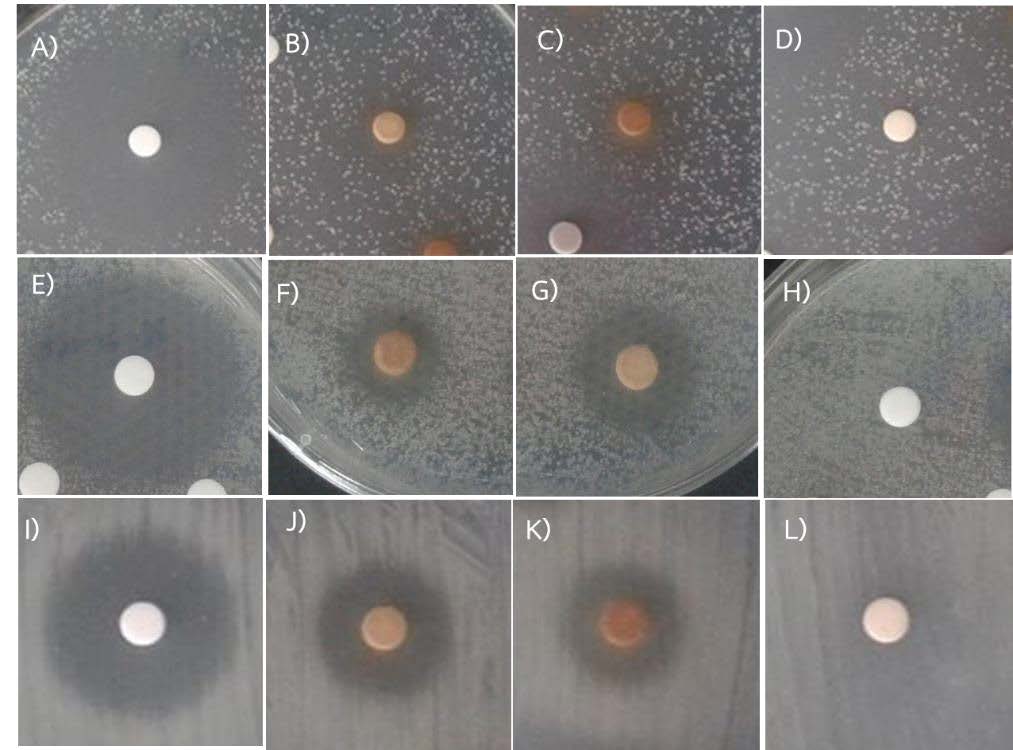
Figure 1. Growth inhibition zones of Xanthomonas axonopodis pv. citri (Xac) in challenge tests using Goniothalamus calvicarpa crude leaf extracts. The paper disc diffusion assay tested against Xac from kaffir lime using A: chloramphenicol, B: methanol soluble extract, C: 70% aqueous ethanol extract, and D: ethyl acetate soluble extract. The paper disc diffusion assay tested against Xac from lime using E: chloramphenicol, F: methanol soluble extract, G: 70% aqueous ethanol extract, and H: ethyl acetate soluble extract. The paper disc diffusion assay tested against Xac from pomelo using I: chloramphenicol, J: methanol soluble extract, K: 70% aqueous ethanol extract, and L: ethyl acetate soluble extract.
Table 2. Antibacterial screening of leaf extracts (4 mg/disc) from Goniothalamus calvicarpa against Xanthomonas axonopodis pv. citri (Xac) cultures isolated from three citrus hosts.
|
Crude leaf extract
|
Inhibition zone (mm) ¥ |
||
|
Xac (kaffir lime) |
Xac (lime) |
Xac (pomelo) |
|
|
Ethyl acetate soluble extract |
0±0.00A,d* |
0±0.00A,d |
0±0.00A,c |
|
Methanol soluble extract |
10.67±1.15B,b |
14.00±1.73B,b |
18.33±2.08A,b |
|
70% aqueous ethanol extract |
8.33±0.58C,c |
11.67±1.53B,c |
16.67±1.15A,b |
|
Chloramphenicol |
30.67±1.15A,a |
29.67±2.52A,a |
25.33±1.53B,a |
Note: ¥ Values are presented as mean ± standard deviation.
Chloramphenicol at a concentration of 20 µg/disc for Xac in lime, and at a concentration of 40 µg/disc for Xac in kaffir lime and pomelo.
* Different small letters in each column indicate significant differences from Duncan’s multiple range test (P < 0.05, n = 3).
* Different capital letters in each row indicate significant differences from Duncan’s multiple range test (P < 0.05, n = 3).
Chromatographic profile
There was a diversity of bands that were clear under UV light at 254 nm and long wave UV light at 366 nm. The developed TLC (5% methanol in dichloromethane) had a number of separate dark spots within each lane indicating that the structures of the extracts contained chromophores (i.e., C=C, C=O bonds) (Figure 2-A). Long-wave UV radiation at 366 nm revealed a number of different fluorescent compounds exhibited as bright orange, yellow, purple and blue bands. Aromatic compounds and highly conjugated systems were visualized under short-wave UV at 254 nm (Figure 2-B). After staining with p-anisaldehyde-sulfuric acid, the compounds which did not contain chromophores, appeared purple, orange and yellow (Figure 2-C). There were distinct differences between species in the TLC patterns particularly under UV.
The 70% aqueous ethanol derived extract from G. calvicarpa was dissolved in hexane, ethyl acetate and methanol, and developed on TLC (5% methanol in dichloromethane) to detect the chemical components that had antibacterial activity against gram-negative bacteria. The developed TLC showed that the different solvents used in this study produced similar chemical profiles and the most intense profile was with ethyl acetate. Components which contained chromophores were visible under UV at 254 nm (Figure 3-A), while fluorescent compounds were visible as orange, yellow and purple regions when observed under UV at 366 nm (Figure 3-B). The TLC profile also clearly exhibited a yellow compound when dissolved in ethyl acetate. Previously, the total flavonoid content in G. calvicarpa extract was found to be 3.16 mg quercetin equivalent g−1 of crude extract (Poonpaiboonpipat and Jumpathong, 2019). Moreover, the purple colored compounds dissolved very well in hexane, while the gray colored ones could be dissolved in the other solvents that were used in this study (Figure 3-C). However, structure determination of these compounds are required using more advanced analytical methods such as liquid chromatography-mass spectrometry (LC-MS), Nuclear magnetic resonance (NMR) and Infrared spectroscopy (IR spectroscopy).
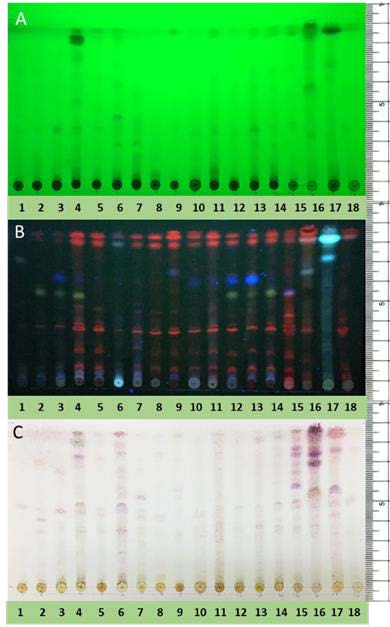
Figure 2. TLC fingerprints of 18 extracts from plant species that were investigated. The TLC plate was developed with 5% methanol in dichloromethane. A: TLC plate viewed under ultraviolet radiation at 254 nm, B: TLC plate viewed under ultraviolet radiation at 366 nm, C: TLC plate heated to 105°C and sprayed with p-anisaldehyde-sulfuric acid. Lane 1: Anomianthus dulcis, 2: Goniothalamus calvicarpa, 3: Mitrephora keithii, 4: Mitrephora wangii, 5: Orophea polycarpa, 6: Elephantopus scaber, 7: Pachyptera hymenaea, 8: Garcinia dulcis, 9: Agapetes lobbii, 10: Cinnamomum loureiroi, 11: Cinnamomum porrectum, 12: Michelia floribunda, 13: Manglietia garrettii, 14: Magnolia henryi, 15: Ficus microcarpa L.f. var. crassifolia, 16: Zingiber zerumbet (stem), 17: Zingiber zerumbet(root), 18: Hedychium coronarium
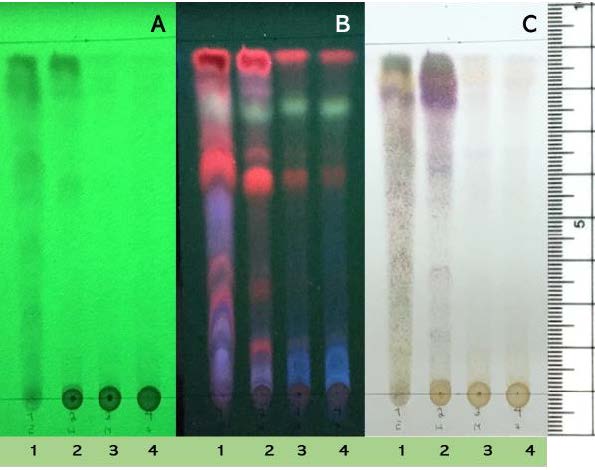
Figure 3. Goniothalamus calvicarpa extracts: the chemical profile on TLC when dissolved in ethyl acetate (1), hexane (2), methanol (3) or 70% aqueous ethanol extract (4) and developed in 5% methanol in dichloromethane. TLC visualized under UV at 254 nm (A), UV 366 nm(B) and p-anisaldehyde – sulfuric acid stain (C).
DISCUSSION
Citrus canker is a devastating disease which occurs in more than 30 countries (Gottwald et al., 2002). At present, a combination of both chemical and biological methods are applied to control this disease which indicates the need for sustainable crop protection methods. The plant kingdom has been identified as a potential source for biological active substances (Bhagwat and Datar, 2014). Plant-based extracts contain natural molecules, some of which have the capacity to inhibit the growth of bacteria. Plant extracts and plant essential oils are also alternative sources for bio-pesticide production as a number of studies have established that they are efficient in controlling phytopathogenic bacteria and help in reducing disease incidence in crops (Leksomboon et al., 2000; Leksomboon et al., 2001; Omidbeygi et al., 2007).
Plant extracts have been reported in citrus canker control (Leksomboon et al., 2000; Leksomboon et al., 2001). In Thailand, lime is one of the most important citrus plants as it has a number of different uses and is in demand year round (Issarakraisila, 2016). In the monsoon season, canker disease is wide spread in every region of the country. Several studies have been undertaken to determine the potential of plant extracts in controlling canker in various orchards. According to Leksomboon et al. (2001), an aqueous extract from Tamarindus indica Linn. reduced the number of cankers on lime leaves in a field study when compared with other plant extracts that were evaluated. In another study, antibacterial activity was tested in vitro against Xanthomonas axonopodis pv. citri (Xac) using fractions of leaf extract from Polyalthia longifolia and Achyranthes aspera. The active fractions, identified as unknown proteins, were found to have molecular weights of 19 and 30 kDa, respectively (Manonmani et al., 2009). In addition, essential oils from thyme were reported to have antibacterial activity in a greenhouse experiment and it was suggested that thyme essential oils be used as an alternate spray with copper (Samavi et al., 2009).
In general, procedures for the isolation of antibacterial agents from plants, and preliminary screening of those compounds using agar well or paper disc diffusion methods, have been used for selecting potential plant extracts that might be effective against bacterial pathogens. Further investigation, using a typical protocol such as bioassay-guided fractionation and step-by-step plant extract separation are required in order to obtain pure samples of the extracted chemical compounds before their structure can be determined.
The results obtained in this study indicate that separation of the antibacterial agents from Goniothalamus calvicarpa leaf extracts was possible using the chromatographic separation technique. Although spots were visualized readily on the TLC plates, the actual compounds remain to be characterized and identified. In the future, fractionation of compounds by HPLC and GC in association with MS would facilitate identification. The use of split columns would allow the collection of samples which could then be evaluated for their efficacy against Xac in vitro. Only after robust evaluation in greenhouse and field trials could a commercial product be developed.
CONCLUSION
Plants are important sources of antibacterial compounds. Eighteen plant extracts from this study had a diverse range of metabolites when evaluated using thin layer chromatography. A crude leaf extract from Goniothalamus calvicarpa provided promising results when tested against Xac using the paper disc diffusion method. Further study is required to identify the activity and effectiveness of antibacterial compounds.
ACKNOWLEDGEMENTS
This work was supported by Naresuan University Government budgeting Grant number R2560B125. The authors would like to thank Professor Dr. Ian James Warrington for constructive criticism and scientific proofreading of this manuscript.
REFERENCES
Behlau, F., Scandelai, L.H.M., da Silva Junior, G.J., and Lanza, F.E. 2017. Soluble and insoluble copper formulations and metallic copper rate for control of citrus canker on sweet orange trees. Crop Protection. 94:185-191. https://doi.org/10.1016/j.cropro.2017.01.003
Bhagwat, M.K., and Datar, A.G. 2014. Antibacterial activity of herbal extracts against five plant pathogenic bacteria. Archives of Phytopathology and Plant Protection. 47(7): 892-899. https://doi.org/10.1080/03235408.2013. 825398
Boonkaew, T., and Camper, N.D. 2005. Biological activities of ginkgo extracts. Phytomedicine. 12(4): 318-323. https://doi.org/10.1016/j.phymed. 2003.06.008
Calixto, J.B., Campos, M.M., Otuki, M.F., and Santos, A.R. 2004. Anti-inflammatory compounds of plant origin. Part II. Modulation of pro-inflammatory cytokines, chemokines and adhesion molecules. Planta Medica. 70(02): 93-103. https://doi.org/10.1055/s-2004-815483
Chien, T.Y., Chen, L.G., Lee, C.J., Lee, F.Y., and Wang, C.C. 2008. Anti-inflammatory constituents of Zingiber zerumbet. Food Chemistry. 110(3): 584-589. https://doi.org/10.1016/j.foodchem.2008.02.038
Deachathai, S., Mahabusarakam, W., Phongpaichit, S., and Taylor, W.C. 2005. Phenolic compounds from the fruit of Garcinia dulcis. Phytochemistry. 66(19): 2368-2375. https://doi.org/10.1016/j.phytochem.2005.06.025
Dholvitayakhun, A., Cushnie, T.T., and Trachoo, N. 2012. Antibacterial activity of three medicinal Thai plants against Campylobacter jejuni and other foodborne pathogens. Natural Product Research. 26(4): 356-363. https://doi.org/10.1080/14786419.2010.545777
Gerlacha, A.D.C.L., Gadeac, A., da Silveirab, R.M.B., Clerca, P., and Lohézic-le Dévéhatc, F. 2018. The use of Anisaldehyde Sulfuric acid as an alternative spray reagent in TLC analysis reveals three classes of compounds in the genus Usnea Adans. (Parmeliaceae, lichenized Ascomycota). https://doi.org/10.20944/preprints201802.0151.v1
Graham, J.H., Gottwald, T.R., Cubero, J., and Achor, D.S. 2004. Xanthomonas axonopodis pv. citri: factors affecting successful eradication of citrus canker. Molecular Plant Pathology. 5(1): 1-15. https://doi.org/10.1046/ j.1364-3703.2004.00197.x
Gottwald, T.R., Graham, J.H., and Schubert, T.S. 2002. Citrus canker: the pathogen and its impact. Plant Health Progress. 3(1): 15. https://doi.org/ 10.1094/PHP-2002-0812-01-RV
Jarriyawattanachaikul, W., Chaveerach, P., and Chokesajjawatee, N. 2016. Antimicrobial activity of Thai-herbal plants against food-borne pathogens E. coli, S. aureus and C. jejuni. Agriculture and Agricultural Science Procedia. 11: 20-24. https://doi.org/10.1016/j.aaspro.2016.12.004
Jewers, K., Davis, J.B., Dougan, J., Manchanda, A.H., Blunden, G., Kyi, A., and Wetchapinan, S. 1972. Goniothalamin and its distribution in four Goniothalamus species. Phytochemistry. 11(6): 2025-2030. https://doi.org /10.1016/S0031-9422(00)90168-7
Leksomboon, C., Thareechai, N. Kositratana, W., and Y. Paisooksanti. 2000. Effect of Thai medicinal plant extracts on growth of phytopathogenic bacteria. In: Proceeding of the 36th Kasetart University Annual Conferences in Plant Section, February 3 and 5, 1998 Kasetsat University Bangkok (in Thai).
Leksomboon, C., Thaveechai, N., and Kositratana, W. 2001. Potential of plant extracts for controlling citrus canker of lime. Kasetsart Journal: Natural Science. 35: 392-396.
Limsuwan, S., and Voravuthikunchai, S.P. 2013. Anti-Streptococcus pyogenes activity of selected medicinal plant extracts used in Thai Traditional Medicine. Tropical Journal of Pharmaceutical Research. 12(4): 535-540. https://doi.org/10.4314/tjpr.v12i4.14
Issarakraisila, M. 2016, November. A review of Thailand commercial success in growing lime (Citrus aurantifolia) in containers to control flowering time and canopy size. In: International Symposia on Tropical and Temperate Horticulture-ISTTH2016. 1205: 289-292. https://doi.org/10.17660/ActaHortic.2018.1205.33
Manonmani, K., Jayasekhar, M., Gailce Leo Justin, C., and Trangaselvabai, T. 2009. Identification of active principle in the herbal extracts possessing bactericidal in the herbal extracts possessing bactericidal action against citrus canker, Xanthomonas axonopodis pv. citri. Indian Journal of Agricultural Research. 43(2): 129-133.
Nandhasri, P., Pawa, K.K., Kaewtubtim, J., Jeamchanya, C., Jansom, C., and Sattaponpun, C. 2005. Nutraceutical properties of Thai ‘Yor’ Morinda citrifolia and ‘Noni’ juice extract. Songklanakarin Journal of Science and Technology. 27(2): 579-586.
Ngoc, L.B.T., Vernière, C., Pruvost, O., Kositcharoenkul, N., and Phawichit, S. 2007. First report in Thailand of Xanthomonas axonopodis pv. citri-A* causing citrus canker on lime. Plant Disease. 91(6): 771-771. https://doi.org/10.1094/PDIS-91-6-0771A.
Omidbeygi, M., Barzegar, M., Hamidi, Z., and Naghdibadi, H. 2007. Antifungal activity of thyme, summer savory and clove essential oils against Aspergillus flavus in liquid medium and tomato paste. Food Control. 18(12):1518-1523. https://doi.org/10.1016/j.foodcont. 2006.12.003
Pukdeekumjorn, P., Ruangnoo, S., and Itharat, A. 2016. Anti-inflammatory activities of extracts of Cinnamomum porrectum (Roxb.) kosterm. Wood (Thep-tha-ro). Journal of the Medical Association of Thailand= Chotmaihet thangphaet. 99: S138-43.
Poonpaiboonpipat, T., and Jumpathong, J. 2019. Evaluating herbicidal potential of aqueous-ethanol extracts of local plant species against Echinochloa crus-galli and Raphanus sativus. International Journal of Agriculture and Biology. 21(3): 648-652. https://doi.org/10.17957/IJAB/15.0940
Prakash, M., and Karmegam, N. 2012. In-vitro antibacterial activity of certain plant extracts against plant disease causing bacteria isolated from citrus plant. International Journal of Current Microbiology and Applied Sciences. 1(1): 1-11.
Samavi, S., Hassanzadeh, N., Faghihi, M.M., and Danesh, Y.R. 2009. Effects of thyme (zaatar) essential oil and some chemical compounds in the control of citrus bacterial canker in Iran. Journal of Plant Pathology. 91(3): 691- 696. https://doi.org/10.4454/jpp.v91i3.562
Suphrom, N., Insumrong, K., Sriboon, P., Sonyot, W., Ingkaninan, K., and Boonphong, S. 2018. Chromatographic fingerprint and free radical scavenging activity of Boesenbergia xiphostachya (Gagnep.) Loes Rhizome Extract. NU. International Journal of Science. 15(1): 1-8.
Uthairatsamee, S., Soonthornchareonnon, N., Wiwat, C., and Pipatwattanakul, D. 2011. Antioxidant and antibacterial activities of the extracts from different parts of Cinnamomum porrectum (Roxb.) Kosterm. In: Proceedings of the 49th Kasetsart University Annual Conference, Kasetsart University, Thailand, 1-4 February, 2011. Volume 8. Subject: Natural Resources and Environment. Bangkok: Kasetsart University. pp. 107-115.
Wiart, C. 2007. Goniothalamus species: a source of drugs for the treatment of cancers and bacterial infections. Evidence-Based Complementary and Alternative Medicine. 4(3): 299-311. https://doi.org/10.1093/ecam/ nem009
Juangjun Jumpathong1*, Nungruthai Suphrom2, Bernard Dell3, Khammool Khamsuk1, Thanita Boonsrangsom1 and Thanatsan Poonpaiboonpipat1
1Department of Agricultural Science and Center of Excellence in Research for Agricultural Biotechnology, Faculty of Agriculture, Natural Resources and Environment, Naresuan University, Phitsanulok 65000, Thailand
2Department of Chemistry, Faculty of Science and Center of Excellence for Innovation in Chemistry, Naresuan University 65000, Thailand
3Agricultural and Forestry Sciences, Murdoch University, Murdoch 6150 Australia
*Corresponding author. E-mail: juangjunj@nu.ac.th
Total Article Views
Article history:
Received: April 19, 2019;
Revised: July 9, 2019;
Accepted: July 15, 2019

