
Acid-adapted Arbuscular Mycorrhizal Fungi Promote Growth of Legumes in Phosphorus-Deficient Acid Soil
Ayut Kongpun*, Bernard Dell and Benjavan RerkasemPublished Date : 2019-08-25
DOI : 10.12982/CMUJNS.2014.0026
Journal Issues : Number 2,May-August-2014
ABSTRACT
Soil acidity is a major limiting factor for upland crops. Arbuscular mycorrhizal fungi (AMF) help improve soil fertility through a fallow enriching tree, Macaranga denticulata, and directly enhance growth of many crops, but its benefit to legumes in acid soil are not known. Three experiments evaluated the benefits from AMF on legumes growing on acidic, low phosphorus soil (pH 5, 11 mg P kg-1 by Bray II). In Experiment 1, root zone soil and root fragments of M. denticulata significantly increased cowpea (Vigna unguiculata) growth and P uptake. In experiment 2, CMU22 – a strain of Acaulospora morrowiae propagated from a single spore in the rhizosphere of mimosa (Mimosa invisa) – growing in soil with pH 5 and 11 mg P kg-1 was as effective as soil from the root zone of M. denticulata on cowpea and mimosa growth. In experiment 3, cowpea growing in soil with pH 5 and 11 mg P kg-1 was inoculated with varying rates of mimosa root zone soil containing CMU22 and CMU22 spores. Both types of inoculum promoted cowpea growth, but at a low rate of 100 spores plant-1. Root zone soil that contained infected root fragments and hyphae, as well as spores, was more effective. Arbuscular mycorrhizal fungi adapted to acidic, low P soils have been shown to be effective in alleviating acid soil stress in legumes, with CMU22, an Acaulospora morrowiae, especially well adapted to acid soil.
Keywords: Acid soil, Arbuscular mycorrhizal fungi, Legumes, Phosphorus
Introduction
Soil acidity is a major limiting factor of legume growth (Munns and Fox, 1977). In acid soil, factors such as toxicity of aluminium (Al) or manganese (Mn) or deficiencies of phosphorus (P), molybdenum (Mo), calcium (Ca) and magnesium (Mg) can limit growth and nitrogen fixation by legumes (Marschner, 1995), with P deficiency the most common (Maddox and Soileau, 1991). Lime and chemical fertilizer, especially phosphatic fertilizer, can ameliorate acid soil problems (Maddox and Soileau, 1991), but often the cost or logistics of applying these chemicals are prohibitive or surface application cannot solve subsoil acidity (Parkpian et al., 1991).
This raises a question whether the soil acidity problem in legume crops, especially in upland cropping systems where legumes provide sources of cheap protein as well as enhance soil fertility through symbiotic nitrogen fixation, can be solved in other ways. Interestingly, in an upland, shifting-cultivation system on acidic, low P soil (pH 4-6, 3-15 mg P kg-1 by Bray II) (Yimyam, 2006a) in Huai Teecha village, Mae Hong Son Province, Thailand, farmers grow legumes – such as cowpea (Vigna unguiculata), yardlong bean (V. unguicultata sesquipedalis), winged bean (Psophocarpus tetragonolobus) and other legumes – without visible symptoms of stress, while also obtaining good yields from their upland rice and other crops (Yimyam, 2006a). High densities of Macaranga denticulata, a fallow enriching tree, have been shown to be associated with accumulation of nutrients and higher yield of upland rice (Yimyam et al., 2003). M. denticulata, on the other hand, has been found to be highly dependent on arbuscular mycorrhizal fungi (AMF) for growth and nutrient accumulation on acidic, low P soil (Youpensuk et al., 2004). The local population of AMF associated with M. denticulata at Huai Teecha is especially diverse and abundant (Youpensuk et al., 2004). Moreover, they are effective in improving growth of many crop species, including rubber (Kanyasone, 2009), coffee (Yimyam, 2006b) and tangerine (Youpensuk et al., 2008). Food crops of shifting cultivation at Huai Teecha, including upland rice, Job’s tears and sorghum, have also been shown to benefit from association with AMF (Wongmo, 2008).
In spite of this, no information is available on the association between AMF and local legumes and how the symbiosis might benefit adaptation by legumes to the acidic, upland soils. Whether these AMF can directly benefit legumes in acid soil is the focus of this study. Three pot experiments were conducted to evaluate the effectiveness of acid-adapted AMF from Huai Teecha village to alleviate acid soil stress in legumes.
MATERIALS AND METHODS
Three pot experiments were conducted in a greenhouse at Chiang Mai University, Thailand in 2008 and 2009.
Growth medium preparation
The plant growth medium was prepared from a mixture of sand and soil. Sansai soil (0-30 cm depth) was collected from Mae Hia Agricultural Research Station and Training Center of Chiang Mai University. The soil had the following properties: 3 mg Bray II P kg-1, pH (1:1 H2O) 5.9, organic matter 1.98 % (w/w), total nitrogen 0.07 % (w/w), extractable K 40.6 mg kg-1, CEC 7.5 cmol kg-1. The soil was air dried, broken up and then sieved to pass a 5 mm screen. The sieved soil was mixed with washed river quartz sand in a 2:1 ratio (w/w) and placed in plastic bags at 3.6 kg bag-1. The pH of the mixture was adjusted to 5 by adding 0.909 g Al2(SO4)3 18H2O kg-1. Available soil P was adjusted to 11 mg Bray II P kg-1 by adding 55.5 mg KH2PO4 kg-1. Basal nutrients were as follows (mg kg-1): K2SO4=71, CaCl2.H2O=94, MnSO4.H2O=10, ZnSO4.7H2O=5, CuSO4.5H2O=2.1, H3BO3=0.8, CoSO4.7H2O=0.36 and Na2MoO4.2H2O=0.18.The above chemicals were mixed thoroughly through the soil. The growth medium was autoclaved at 121°C for one hour.
Inoculum preparation
1. Soil from the root zone of M. denticulata was collected from a shifting cultivation field at Teecha, Sob Moei District, Mae Hong Son Province in the rainy season of 2008. The soil, pH 5.5 and 3.8.mg P kg-1 (Bray II), contained 68 AMF spores g-1 of mixed species and genera7 (Glomus 73.5%, Acaulospora 24.7%, Gigaspora 1.1% and Scutellospora 0.7%), as well as root fragments and hyphae. This was used as the root zone soil inoculum, designated Ma. Inoculum rates were varied by adding autoclaved soil inoculum.
2. Fresh root fragments of M. denticulate in Ma were washed free of soil and used as an AMF inoculum, designated RF.
3. Single spores of the many different AMF types in Ma were multiplied in association with spineless mimosa (Mimosa invisa Mart) growing in autoclaved soil (121°C for 1 hour), pH 5 and 4.2 mg P kg-1. Although the Ma contained spores of Glomus, Acaulospora, Scutellospora and Gigaspora (in the ratio 69:28:4:2), after 3 months only one strain of Acaulospora morrowiae, CMU22, was able to multiply in abundance. The mimosa root zone soil, air dried and ground to pass a 5 mm mesh, contained 103 spores g-1, and was used as mimosa root zone soil inoculum, designated Mi. Inoculum rates were varied by adding autoclaved soil inoculum.
4. Spores of CMU22 were extracted from Mi. They were surface sterilized for 5 minutes in 70% ethanol and washed in sterilized water 3 times before being used as spore inoculum, designated Ac.
Experiment 1
Experiment 1 was designed in a randomized complete block design with 3 replications. Five treatments of AMF inoculation were conducted, with one pot as an experimental unit. The plant used was cowpea (Vigna unguiculata L. Walp.cv. Ubon Rajathanee). Seeds were surface sterilized by soaking in 70% ethanol for 5 minutes, then washed by sterilized water. Six surface sterilized seeds were sown in each 5 L drained plastic pot containing 3.6 kg of the steam-sterilized growth medium. The surface soil in each pot contained 3 holes, with 2 seeds sown in each hole. Two types of inoculum treatments were applied to the seeds in each hole: 1) 10 g of infected Macaranga denticulata root fragment (RF) and 2) 10 g of rhizosphere soil from M. denticulata (Ma) containing 100, 250 or 500 spores (Ma100, Ma250 and Ma500 respectively). Un-inoculated cowpeas were used as a control. Each hole was inoculated with 2 ml of appropriate rhizobium suspension (CP002) at 109 cells ml-1 (the suspension was provided by the Department of Soil Science, Faculty of Agriculture, Chiang Mai University). Three sets of pots with complete treatments and replications were set up for 3 harvests at 15, 35 and 46 days after emergence (V4, flowering and pod filling stage, respectively). Seedlings emerged at 3 days after sowing. Plants were thinned to 3 plants per pot (1 plant hole-1) at 1 week after emergence. At harvest, shoots were cut at ground level then oven dried at 75°C for 48 hours before weighing. Roots were washed free of soil. Root nodules were collected and oven dried before weighing. Fresh roots were weighed before cutting into pieces of approximately 1 cm in length. A root sub-sample of one-tenth total root fresh weight was randomly taken from every pot for assessment of AMF colonization. The remaining roots were oven dried. Fresh root sub-samples were cleared in 10% KOH before staining with 0.05% trypan blue in lactoglycerol. Root colonization percentage was assessed using the intercept method (Brundrett et al., 1996) under a compound microscope. Thirty-two 1-cm pieces of roots were examined for each sub-sample. Shoot and root P concentrations were measured by the molybdovanadate-phosphoric acid method (Murphy and Riley, 1962).
Experiment 2
Experiment 2 applied three types of inoculum to mimosa and cowpea (cv. Ubon Rajathanee) in 3 replications, arranged in a randomized complete block design, with one 5 L plastic pot containing 3.6 kg growth medium as an experimental unit. Seeds of the 2 legumes were surface sterilized with 70% ethanol for 5 minutes and washed 3 times with sterilized water before sowing. There was one hole in each pot. Twenty seeds of mimosa or 5 seeds of cowpea were sown in each pot. Three AMF inoculum types were applied to the seeds in each pot: 1) 22 g of soil from the root zone of Macaranga containing 1,500 spores of mixed AMF species (Ma); 2) 1500 spores pot-1 of Acaulospora morrowiae CMU22 (Ac) plus 22 g autoclaved soil inoculum and 3) 22 g pot-1 of autoclaved soil inoculum as a control treatment. Appropriate rhizobium inoculation (109 cells ml-1 suspension) was applied at the rate of 5 ml pot-1. Cowpea seedlings were thinned to 3 per pot and mimosa seedlings to 10 per pot at 11 days after sowing. At 59 days after sowing, the plants were harvested, weighed and AMF colonization and shoot and root P determined, as described in Experiment 1.
Experiment 3
Experiment 3 involved 7 AMF inoculation treatments, arranged in a randomized complete block design with 3 replications. Cowpea (cv. Ubon Rajathanee) seeds were surface sterilized and sown, six seeds in each 5 L drained plastic pot containing 3.6 kg of growth medium. The surface soil in each pot contained 3 holes, and 2 seeds were sown in each hole. Three types of inoculum treatments were applied to the seeds in each hole: 1) 10 g of mimosa root zone soil containing 100, 250 and 500 spores of A. morrowiae CMU22 (Mi100, Mi250 and Mi500, respectively); 2) 100, 250 and 500 extracted spores of A. morrowiae CMU22 (Ac100, Ac 250 and Ac500, respectively) and 3) nil-inoculation. Each hole was inoculated with 2 ml of appropriate rhizobium suspension at 109 cells ml-1. Plants were thinned to 3 plants per pot at 1 week after emergence. Plants were harvested at 46 days after emergence (pod filling stage). Biomass yield, P concentration in shoot and root, and root colonization were measured, as described in Experiment 1.
Data were analyzed using analysis of variance and difference among treatments was compared using least significant different (LSD) value (p ≤ 0.05). The data in percentage were transformed by arcsine before analysis. Data of total dry weight in Experiment 2 were log transformed before analysis. Correlation coefficients were calculated to determine relationship between parameters.
RESULTS
Experiment 1
Root colonization by AMF was evident at 15 days after emergence (Table 1). Increasing the spore rate from Ma100 to Ma500 increased root colonization, from 4.6 to 15.1% at Day 15. By Day 35, over 60% of the root system had been colonized by AMF, with no difference between the spore rates used. The RF inoculum was also very effective and the roots of plants inoculated with RF had the highest level of root colonization at Day 35. However, by Day 46 all inoculated treatments had similar levels of root colonization (Table 1). Infection of roots in the control treatment was nil or very low.
Table 1. Root colonization of cowpea inoculated with Macaranga denticulata root fragments (RF) or rhizosphere soil (Ma with three spore loads: 100, 250 and 500 spores) containing AMF inoculum at 15, 35 and 46 days after emergence (Experiment 1).
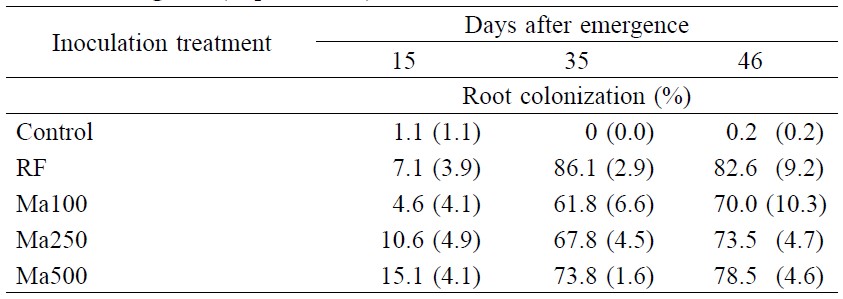
Note: Numbers in parenthesis are standard errors.
Shoot dry weight did not respond to AMF at Day 15, but the effect of AMF on shoot weight became apparent at Day 35 (Table 2). Plants inoculated with RF had the same shoot weight as those inoculated with Ma100, which was double that of the control. Shoot weight continued to increase with increasing inoculum rate to Ma500. The same response was found at Day 46 (Table 2).
In contrast to the shoot, root dry weight did not respond to AMF until Day 46 (Table 2). At this stage, all inoculated treatments had higher root weight than the control. Root weight increased with inoculum rate, from Ma100 to Ma500. Plants inoculated with RF had the same root weight as those inoculated with Ma250 (Table 2).
Table 2. Shoot and root dry weights of cowpea inoculated with Macaranga denticulata root fragments (RF) or rhizosphere soil (Ma with three spore loads: 100, 250 and 500 spores) containing AMF inoculum at 15, 35 and 46 days after emergence (Experiment 1).
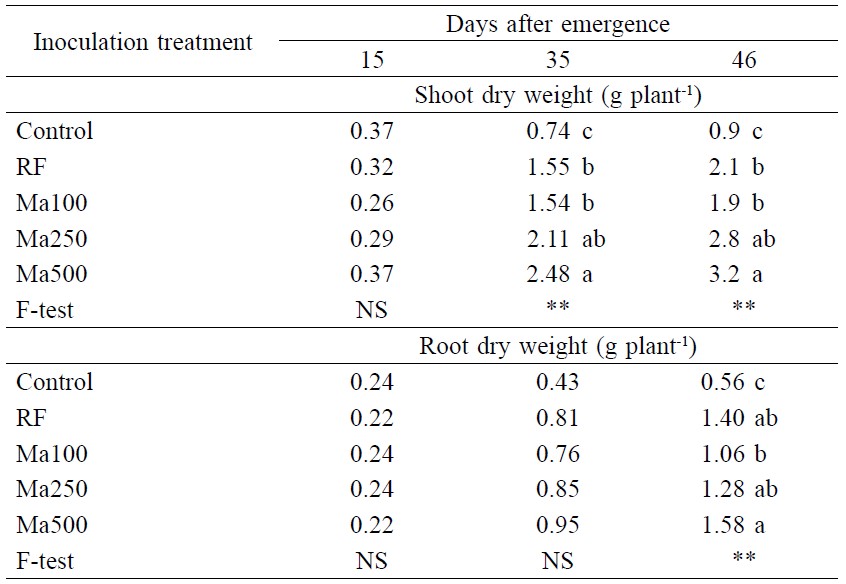
Note: NS = not significant. ** = significant at P < 0.01. Means in the same column followed by different letters indicate significant difference (P < 0.05).
At Day 15, cowpea had tiny visible nodules, but they could not be quantified. At Day 35, all inoculated plants had the same nodule dry weight (Table 3), but this changed by Day 46. At Day 46, increasing the inoculum rate from Ma100 to Ma500 increased nodule weight by 66%. Plants inoculated with RF had the same nodule weight as Ma100 plants (Table 3).
Similar to the growth response, inoculation with Ma enhanced the total plant P content from Day 35 and by Day 46 plants inoculated with the higher spore rate had accumulated the highest amount of P. Plants inoculated with RF acquired P similar to Ma100 plants (Table 3).
Table 3. Nodule dry weight and total P content of cowpea inoculated with Macaranga denticulata root fragments (RF) or rhizosphere soil (Ma with three spore loads: 100, 250 and 500 spores) containing AMF inoculum at 15, 35 and 46 days after emergence (Experiment 1).
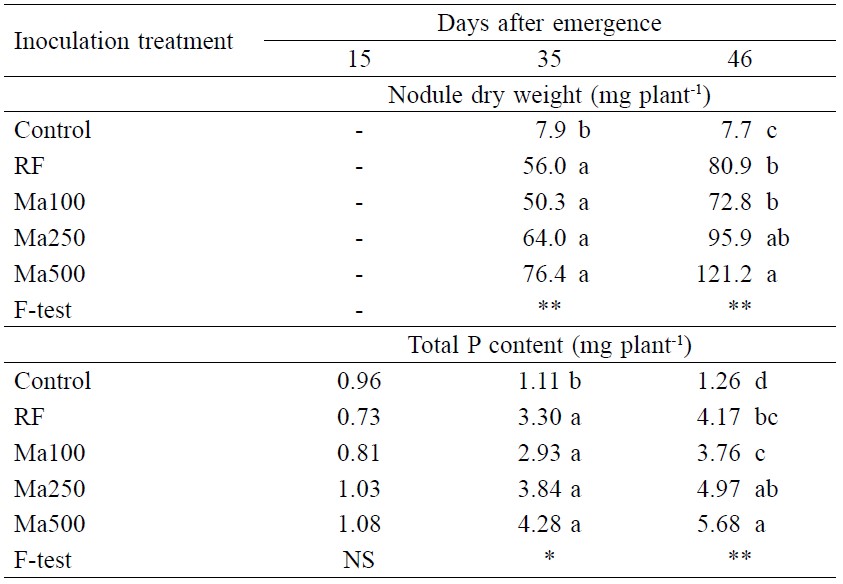
Note: NS = non-significant difference. * = significant at P < 0.05. ** = significant at P < 0.01. Means in the same column followed by different letters indicate significant difference (P < 0.05).
Experiment 2
Spores of Acaulospora morrowiae and spores of mixed AMF species were equally effective in colonizing and promoting growth of cowpea and mimosa. In cowpea, 0.4% root colonization was found in the un-inoculated control and much higher root colonization occurred in Ac and Ma (36.5 and 53.6 %, respectively, Table 4). In mimosa, root colonization was 32.8% in Ac and 46.1% in Ma plants. Inoculation with Ac or Ma increased total dry weight equally, even though in mimosa or cowpea (Table 4). Nodule dry weight of cowpea was not affected by AMF, but both Ac and Ma increased nodule dry weight of mimosa (Table 4). The total P content of both legumes was increased by inoculation (Table 4).
Table 4. Root colonization total dry weight nodule dry weight and total P content of cowpea and mimosa inoculated with spores of Acaulospora morrowiae CMU22 (Ac) or mixed species (Ma) at 59 days after sowing (Experiment 2).
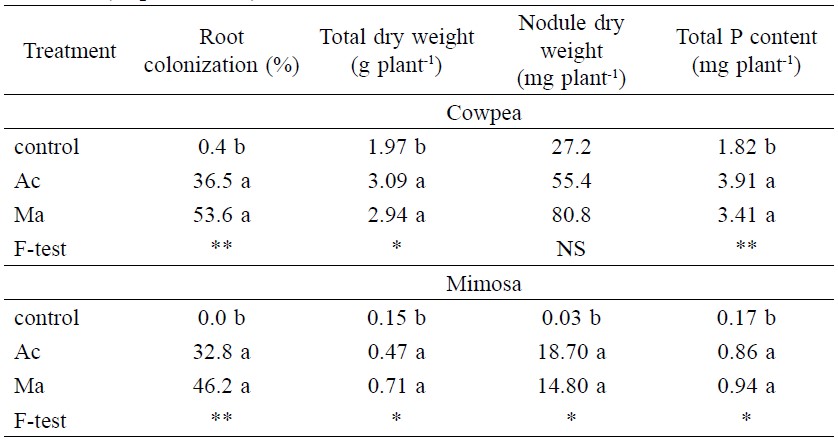
Note: * = significant at P < 0.05. ** = significant at P < 0.01. Means in the same column of each plant followed by different letters indicate significant difference (P < 0.05).
Experiment 3
Root colonization of inoculated treatments ranged between 46 and 53%, with no significant difference between inoculum types or spore rates (Figure 1A). The un-inoculated control cowpea had very low root colonization (0.2%). No effect of spore rate on total dry weight was found in Mi. By contrast, the lowest spore rate in Ac (Ac100) depressed total dry weight by 37% compared to Ac250 (Figure 1B). The nodule dry weight in all inoculated treatments was higher than in the un-inoculated plants (Figure 2A). In Mi, nodule dry weight was not affected by spore rate, whereas in Ac, nodule weight was depressed by 30% at Ac100 compared to Ac500 (Figure 2A). All types of inoculum increase the total P content in cowpea similar to the growth response. Spore rate had no effect on the P content of Mi plants, but in Ac increasing the spore rate from Ac100 to Ac250 increased the total P content by 25% (Figure 2B).
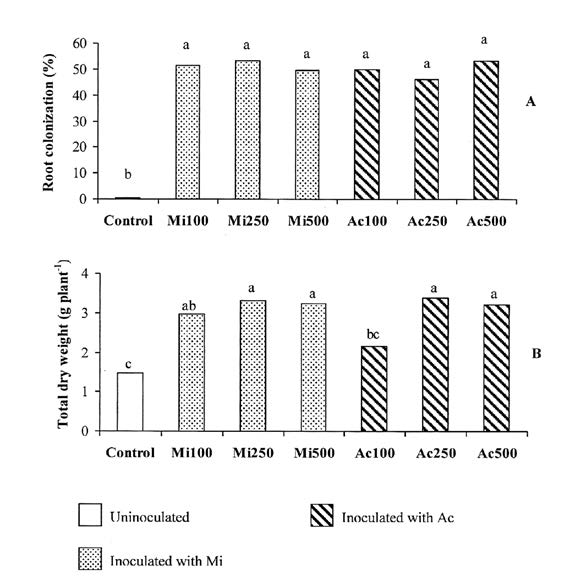
Figure 1. Root colonization (A), Total dry weight (B) of cowpea inoculated with Acaulospora morrowiae CMU22 spores (Ac) or A. morrowiae soil inoculum (Mi) at three spore rates. Each bar is the mean of 3 replications. The different lowercase letters indicate significant difference by LSD0.05 (Experiment 3).
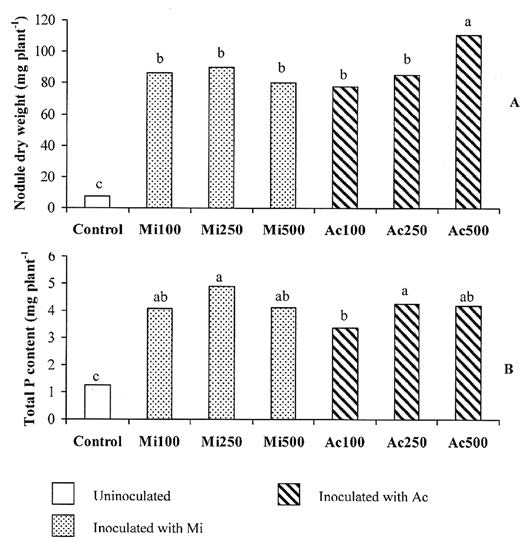
Figure 2. Total dry weight (A) and Total P content (B) of cowpea inoculated with Acaulospora morrowiae CMU22 spores (Ac) or A. morrowiae soil inoculum (Mi) at three spore rates. Each bar is the mean of 3 replications. The different lowercase letters indicate significant difference by LSD0.05 (Experiment 3).
Discussion
Growth stimulation of cowpea by Ma or RF in Experiment 1 indicated that both types of inoculum had potential to colonize and alleviate acid soil stress in cowpea (Tables 1 and 2). The effect appeared in shoot growth before root growth and the response was greater in the shoot (Table 2). This phenomenon is common in plants associated with AMF because competition for photosynthate between the root and fungi could cause root growth to have less response to AMF than shoot growth (Marschner, 1995). When shoot weight was used as a growth indicator, soil inoculum (Ma) was slightly more effective than root fragment inoculum (RF) (Table 2). This could be caused by the soil inoculum containing more infection units. Soil inoculum contains not only AMF spores but also root fragments and AMF hyphae (Brundrett et al.,1996). The higher colonizing ability of Ma showed in root colonization at Day 15 (V4 stage) (Table 1). The root colonization at V4 determined the growth of the plant at flowering and pod filling (35 and 46 days respectively) (Figure 1), although root colonization at pod filling was not different between treatments (Table 1). These results indicated that root colonization in the early stage of growth was very important and influenced growth of the host plant. This was evidenced by a strong positive correlation between root colonization at V4 and shoot dry weight at pod filling (Figure 3). The inoculated plants had more growth because they were able to uptake more P. Figure 4 shows a strong positive correlation between total P content and total dry weight.
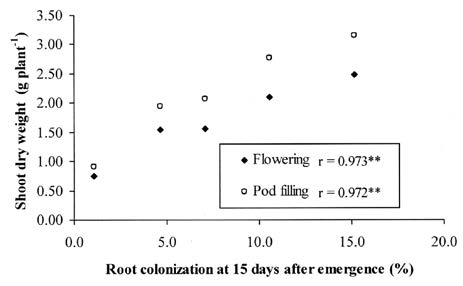
Figure 3. Correlation between root colonization at 15 days after sowing (V4) and shoot dry weight at 35 and 46 days after sowing (flowering and pod filling) r = correlation coefficient, **=significant at P<0.01 (Experiment 1).
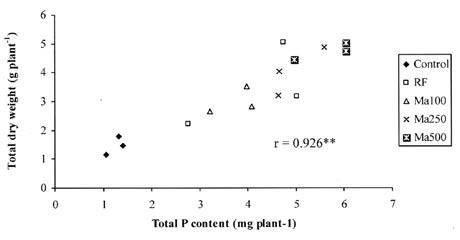
Figure 4. Correlation between total P content and total dry weight of cowpea at 46 days after emergence r = correlation coefficient, **=significant at P<0.01 (Experiment 1).
Arbuscular mycorrhizal fungi are well known for improving P uptake of the host plant (Plassard and Dell, 2010). The fungi not only increase the surface area for nutrient uptake through extensive penetration of hyphae into soil (Marschner, 1995) but also some fungi secrete siderophores (Haselwandter, 2008) that enhance P availability by reducing precipitation of phosphate in acid soil (Hu and Boyer, 1996). In acid soil, Al3+ is released from unreactive Al(OH)3 into the soil solution and binds with phosphate ions to make P unavailable to plants (Harter, 2002).
In Experiment 2, the Ma and Ac inocula had similar effectiveness in alleviating acid soil stress in cowpea and mimosa (Table 4). This indicated that the CMU22 strain of Acaulospora morrowiae was an effective AMF strain that warranted further testing. A spore of this strain was multiplied with mimosa growing in an acidic, low P soil and this inoculum was used to establish that A. morrowiae was effective not only in mimosa but also in cowpea. This result shows that CMU22 had low specificity for a plant host. This finding substantiates previous reports that AMF from the same source in Tee Cha village could stimulate the growth of many crop species, including rubber (Kanyasone, 2009), coffee (Yimyam, 2006b), tangerine (Youpensuk et al.,2008), upland rice, Job’s tears and sorghum (Wongmo, 2008). In Experiment 3, a lower inoculum dose of Mi achieved the same result as a higher inoculum dose of Ac. To stimulate legume growth, Mi needed 100 spores plant-1, while Ac needed at least 250 spores plant-1 (Figure 3).
In conclusion, arbuscular mycorrhizal fungi from acidic, low P soils of Tee Cha village have been shown to be effective in alleviating acid soil stress in cowpea, with Acaulospora morrowiae CMU22 especially well adapted to acid soil.
Acknowledgements
The first author is a recipient of a Royal Golden Jubilee Scholarship from the Thailand Research Fund. Support from the National Research University Program of Thailand’s Commission on Higher Education is gratefully acknowledged.
References
Brundrett M. N. Bougher. B. Dell, T. Grove, and N. Malajczuk. 1996. Working with Mycorrhizas in Forest and Agriculture. The Australian Centre for International
Agricultural Research (ACAIR) Monograph No. 32, Canberra, Australia.
Harter R.D. 2002. Acid Soils of the tropics [Internet]. c2002-2011. Nort Fort Myers (FL) 33917 USA: ECHO Technical Note; [cited 2011 Feb 13]. Available from: http://people.umass.edu/psoil370/Syllabus-files/Acid_Soils_of_the_Tropics.pdf
Haselwandter K. 2008. Structure and function of siderophores produced by mycorrhizal fungi. Mineral Mag 72, 61-64. 10.1180/minmag.2008.072.1.61
Hu X., and G.L. Boyer. 1996. Siderophore-Mediated Aluminum Uptake by Bacillus megaterium ATCC 19213. Appl Environ Microb 62, 4044-4048.
Kanyasone V. 2009. Arbuscular Mycorrhizal Fungi (AMF) for Rubber Seedlings and Agroforestry in Northern Lao P.D.R. MS Thesis, Chiang Mai University.
Maddox J.J., and J.M. Soileau. 1991. Effects of phosphate fertilization, lime amendments and inoculation withVA-mycorrhizal fungi on soybeans in an acid soil. Plant Soil 134, 83-93.
Marschner H. 1995. Mineral Nutrition of Higher Plant. 2nd ed, Academic Press, London, 899 p.
Munns D.N., and R.L. Fox. 1977. Comparative lime requirements of tropical and temperate legumes. Plant Soil 46, 533-548. 10.1007/BF0015912
Murphy V., and J.P. Riley. 1962. A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27, 31–36.
Parkpian P., P. Pongsakul, and P. Sangtong. 1991. Characteristics of acid soils in Thailand: A review. In Plant-Soil Interaction at Low pH. Eds. R J Wright, V C Baligar and R P Murrmann. pp 397-405. Proceeding of the Second International Symposium on Plant-Soil Interactions at Low pH. Beckley, West Virginia.
Plassard C., and B. Dell. 2010. Phosphorus nutrition of mycorrhizal trees. Tree Physiol 30, 1129-1139. 10.1093/treephys/tpq063
Wongmo J. 2008. Influences of Arbuscular Mycorrhizal Fungi on Different Food Crops. PhD Thesis, Chiang Mai University.
Yimyam N. 2006a. Fallow regeneration and upland rice yield variation in a system of shifting cultivation with Pada (Macaranga denticulataI (BL.) Muell. Arg) as the fallow enriching species in northern Thailand. PhD thesis, Chiang Mai University.
Yimyam N. 2006b. Arbuscular micorrhizal fungi on growth of arabica coffee seedling. Highland Research and Training Center, Chiang Mai University. 20 p.
Yimyam N., K. Rerkasem, and B. Rerkasem. 2003. Fallow enrichment with pada (Macaranga denticulata (Bl.) Muell. Arg.) trees in rotational shifting cultivation in Northern Thailand. Agroforest Syst. 57: 79-86. 10.1023/A:1023949628251
Youpensuk S., B. Dell, S. Lumyong, and B. Rerkasem. 2004. Arbuscular mycorrhizal fungi from the rhizosphere of a fallow enriching tree, Macaranga denticulata Muell. Arg. and their effect on the host plant. Agroforest Syst 60: 239-246. 10.1023/B:AGFO.0000024417.88703.65
Youpensuk S., S. Lordkaew, and B. Rerkasem. 2008. Arbuscular mycorrhizal fungi association with tangerine (Citrus reticulata) in northern Thailand and their effect on the host plant. Sci Asia 34, 259-264. 10.2306/scienceasia1513-1874.2008.34.259
Ayut Kongpun1*, Bernard Dell2 and Benjavan Rerkasem3
1 The Office of the Commission on Agricultural Resource Education, Chulalongkorn University, Bangkok 10330, Thailand
2 Sustainable Ecosystems Research Institute, Murdoch University, Perth, Western Australia 6150, Australia
3 Division of Agronomy, Department of Plant Science and Natural Resources, Faculty of Agriculture, Chiang Mai University, Chiang Mai 50200, Thailand
*Corresponding author. E-mail: ayutkongpun@yahoo.com
Total Article Views

