
Chemical Constituents and Antibacterial Activity of Volatile Oils of Combretum latifolium Bl.and C. quadrangulare Kurz Leaves
Wimaluk Nopsiri*, Sunee Chansakaow, Somporn Putiyanan, Surapol Natakankitkul, Khesorn Nantachit, Banyong Khantawa and Dammrong SantiarwornPublished Date : 2019-08-24
DOI : 10.12982/CMUJNS.2015.0086
Journal Issues : Number 3, September - December 2015
ABSTRACT
The volatile constituents from leaves of Combretum latifolium Bl. and Combretum quadrangulare Kurz (Combretaceae) were obtained by hydrodistillation and analyzed by GC-MS. From the leaf oil, we identified six compounds accounting for 81.6% of C. latifolium and nine compounds accounting for 68.0% of C. quadrangulare. The major compounds of the volatile oils in C. latifolium and C. quadrangulare were palmitic acid (37.05%, 17.74%), hexahydrofarnesyl acetone (11.54%, 17.36%), isophytol (13.47%, 3.71%), neophytadiene (7.71%, 3.52%) and n-nonacosane (4.68% and 5.37%), respectively. Antibacterial activity of the volatile oils was evaluated by using agar disc diffusion method. The antibacterial assay showed activity of the oils from the leaves of C. latifolium and C. quadrangulare as diameter of zones of inhibition against the gram-positive bacterium, Staphylococcus aureus (8.50±0.05, 7.50±0.05 mm, respectively), and gram-negative bacterium, Escherichia coli (9.33±0.06, 8.00±0.10 mm, respectively). The extracts showed little antibacterial activity against Pseudomonas aeruginosa. The chemistry and antibacterial activity of the volatile oils of these two plants have been studied here for the first time.
Keywords: Chemical constituents, Volatile oils, Antibacterial activity, Combretaceae, Combretum latifolium Bl., Combretum quadrangulare Kurz
INTRODUCTION
Many species of Combretum (Combretaceae) have been used as traditional medicines for many applications, including abdominal disorders, bacterial infections, diarrhea, bilharzias, malaria, respiratory infections, pneumonia, skin and venereal diseases, fevers and sore throats, especially in rural areas (Banskota et al., 2003; Eloff et al., 2008). Some Combretum species have anti-infective activities, including antibacterial, antifungal and antiparasitic activities. Antibacterial activity of Combretum extracts for ethnomedicinal uses have been confirmed in many ethnobotanical and antibacterial studies (Clarke, 1878; Fyhrquist et al., 2002; Banskota et al., 2003; Eloff et al., 2008; Lima et al., 2012). The Combretaceae is a large family, consisting of 20 genera with at least 600 species. The genus Combretum comprises about 370 species of trees, woody climbers and shrubs. Several species of this family are known in tropical and southern Africa, Madagascar, tropical America, and tropical Asia, including Thailand (Nanakorn, 1986; Eloff et al., 2008). Two Combretum species, which are common in northern Thailand, are Combretum latifolium Bl. and Combretum quadrangulare Kurz. Several reported on antibacterial activity of Combretum species: Combretum caffrum (Eckl. & Zeyh.) Kuntze bark (Masika and Afolayan, 2002), Combretum erythrophyllum (Burch.) Sond. leaves (Martini and Eloff, 1998; Eloff, 1999; Martini et al., 2004), Combretum apiculatum Sond. (McGaw et al., 2000), Combretum fruticosum (Leofl.) Stuntz. (Smith et al., 2000), Combretum imberbe Engl. & Diels. leaves (Katerere et al., 2003), Combretum woodii Dümmer leaves (Eloff et al., 2005a ; 2005b), Combretum molle R.Br. ex G. Don aerial parts (Ahmed et al., 2004; Mamidou Kone et al., 2007; Steenkamp et al., 2007; Gronhaug et al., 2008), Combretum micranthum G. Don leaves, root bark and stem bark (Karou et al., 2005; Akeem et al., 2012; Udoh et al., 2012; Osonwa et al., 2012), Combretum padoides Engl. & Diels (Angeh et al., 2007), Combretum hartmannianum Schweinf. leaves and roots (Eldeen and van Staden, 2007), Combretum adenogonium Steud ex A. Rich. leaves, stem bark and roots (Maregesi et al., 2007; Mushi et al., 2012), Combretum glandifolium (Rahman et al., 2008), Combretum niororense Aubrev. ex keay leaves (Coulidiati et al., 2009), Combretum pincianum Hook (Adejuwon et al.,2011; Kanwal and Karim, 2011), Combretum glutinosum Perr. ex DC. (Yahaya et al., 2012). Studies in Thailand on roots and seeds of C. quadrangulare reported anthelmintic activity (Euswas et al., 1988), antibacterial activity (Nantachit et al., 2006; Wungchinda, 1979) and toxicity (Nakornchai et al., 1994). While, the leaves of C. quadrangulare and C. latifolium have been reported to have antioxidant and anticancer activities (Nopsiri et al., 2014).
With many previous studies demonstrating antimicrobial activities of Combretum species, we specifically investigated the chemical constituents and antibacterial activity of the leaf volatile oils of C. quadrangulare and C. latifolium, two Combretum species from northern Thailand that have yet to be studied for these properties.
MATERIALS AND METHODS
Plant material
The plants were collected in northern Thailand. Combretum latifolium Bl. was collected in Wang Nuea District, Lampang Province in December 2009 and Combretum quadrangulare Kurz was collected in Doi Saket District, Chiang Mai Province in June 2009. Identifications were made by J. F. Maxwell at the CMU Herbarium, Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai, Thailand. Voucher specimens were deposited at the Faculty of Pharmacy and the Department of Biology, Faculty of Science, Chiang Mai University.
Isolation of the volatile oils
Fresh leaves of two Combretum species (C. latifolium 5.61 kg and C. quadrangulare 6.19 kg) were washed with distilled water, chopped into small pieces, and subjected to hydrodistillation in a Clevenger-type apparatus for 8 h, with a water-cooled oil receiver to reduce formation of artifacts due to overheating. The volatile oils were collected over water, separated and dehydrated over anhydrous sodium sulfate, and kept at 4°C for further analysis and testing.
GC-MS analysis
The volatile oils were analyzed on a Hewlett-Packard GC-7890A gas chromatograph equipped with an HP-5MS fused silica capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness), with the temperature programmed to rise from 50°C (held first for 5 min) to 200°C at 10°C/min, ending at 250°C (held for 10 min), for a total run time of 30 min; the carrier gas was He, at a constant flow rate of 1.0 ml/min; the injector temperature was 270°C; the Hewlett-Packard 5975C mass selective detector temperature was 280°C. Samples were injected by splitting mode (1:25). The GC-MS analysis was performed on a Hewlett-Packard GC-7890A coupled with a Hewlett-Packard 5975C mass selective detector using electron impact ionization (EI) and set as follows: source temperature, 230°C; interface temperature, 270°C; ionization energy, 70 eV; mass range of 30-500 m/z; and quadrupole temperature, 150°C. The components of the oils were identified by comparing their retention indices (RI) relative to the n-alkane index on an HP-5 column and by comparing the mass spectra to reference libraries (Wiely7n.1 and NIST) using corresponding data of authentic compounds or published spectra (Mancini et al., 2009; Zito et al., 2010; Radulovic et al., 2011).
Antibacterial activity
Test bacteria. Three common bacterial strains, representing human bacterial pathogens, were used for the antibacterial activity test. The gram-positive bacterium was Staphylococcus aureus (ATCC 25923). Two gram-negative bacteria were Escherichia coli (ATCC 25922) and Pseudomonas aeruginosa (ATCC 27853).
Antibacterial assay. The old National Committee for Clinical Laboratory Standards (NCCLS) method for antibacterial susceptibility testing has been modified for testing volatile oils (Hammer et al., 1998; 1999; Ferraro et al., 2000), with researchers adapting different experimental protocols to better represent future applications in their particular field. The agar disc diffusion method (Gaydos and Harrington, 1982; Pelczar et al., 1993; Hammer et al., 1998; 1999; Ferraro et al., 2000; Mubashir et al., 2008; Zafar et al., 2011) was used to determine the clear zone diameter of volatile oils from two Combretum species against the test bacteria. Sterilized Petri dishes (9 cm diameters) with 20 ml of Mueller-Hinton agar were prepared. The standardized inoculums of the test bacteria were prepared by suspending the pathogenic bacteria in trypticase soy broth to a turbidity of 0.5 McFarland standard. The innoculi were spread on the agar surface. The volatile oils were dissolved in methanol (1 mg/ml), then sterile paper discs (Whatman No.1) of 6 mm diameter were separately impregnated with 5 μl (5 μg/disc) of volatile oils and placed in appropriate position on the surface of the plate with quadrants marked at the back of the Petri dishes with the selected test bacteria. The Petri dishes were incubated at 37°C for 24 h. Three common antibiotics –ampicillin, gentamicin and ceftriaxone (1 mg/ml each) – and one standard volatile oil with known antibacterial activity – eugenol (1 mg/ml) – were used as positive controls. Methanol and sterile water were used as negative controls. The zones of inhibition were assessed by measuring the diameter of the growth-inhibition zone in millimeters (including disc diameter of 6 mm) for the test organisms compared to the controls. Each test was replicated three times. The average of the three independent determinations was recorded.
RESULTS
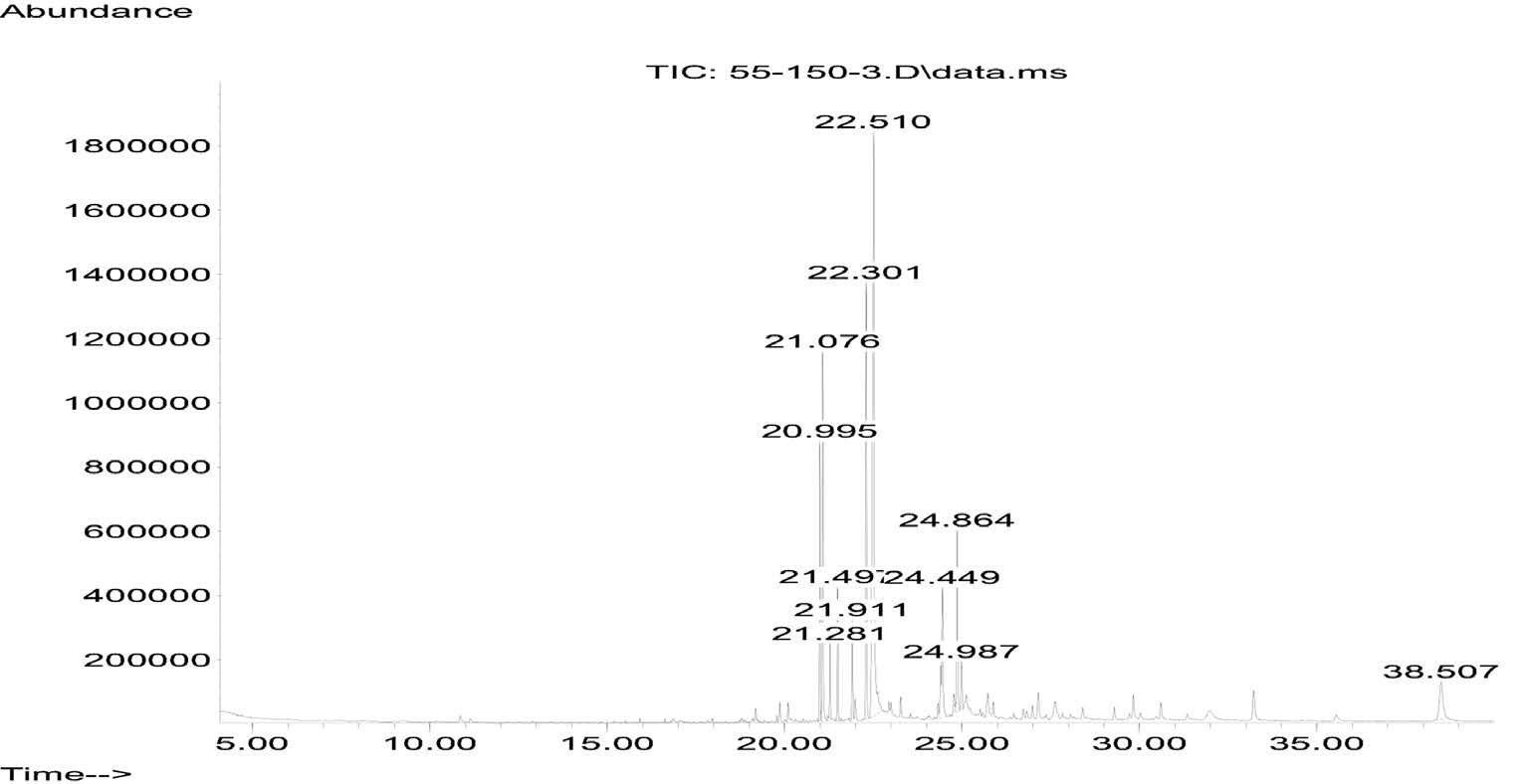
The fresh leaves of C. latifolium and C. quadrangulare were subjected to hydrodistillation in a modified Clevenger-type apparatus to yield 0.0013% and 0.0005% (w/w) of oil, respectively, both light colored. The GC chromatograms of the volatile oils from C. latifolium Bl. and C. quadrangulare Kurz are shown in Figures 1 and 2, respectively. GC-MS analysis successfully detected six components of C. latifolium Bl. and nine components of C. quadrangulare Kurz, accounting for 81.6% and 68.0%, respectively, of the chromatographic components. The components identified from the volatile oils – with their retention time (RT), percentage composition (%), and retention indices (RI) – are summarized in Table 1. Palmitic acid was the major component of the C. latifolium (37.05%) and C. quadrangulare (17.74%) oils. Other components included hexahydrofarnesyl acetone (11.54% and 17.36%, respectively), isophytol (13.47% and 3.71%, respectively), neophytadiene (7.71% and 3.52%, respectively) and n-nonacosane (4.68% and 5.37%, respectively). In addition, phytol isomer (7.17%) was found in C. latifolium oil and phytol (11.52%), n-heptacosane (4.75%), β-selinene (2.46%) and tetradecanoic acid (1.54%) were found in C. quadrangulare oil.
 3
3
Figure 1. GC chromatogram of leaf volatile oils from C. latifolium.
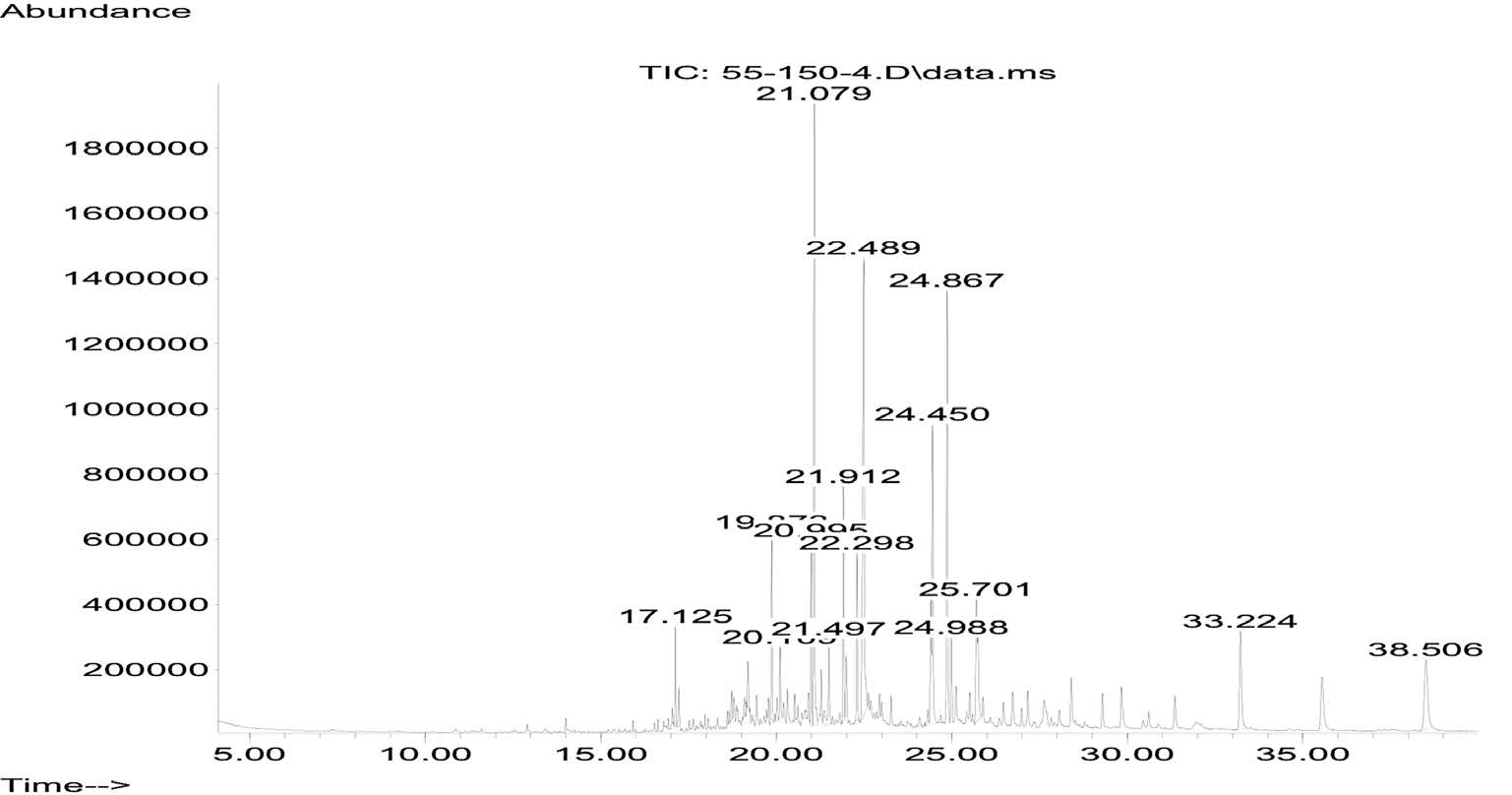
Figure 2. GC chromatogram of leaf volatile oils from C. quadrangulare.
Table 1. Chemical constituents of leaf volatile oils from C. latifolium and C. guadrangulare.
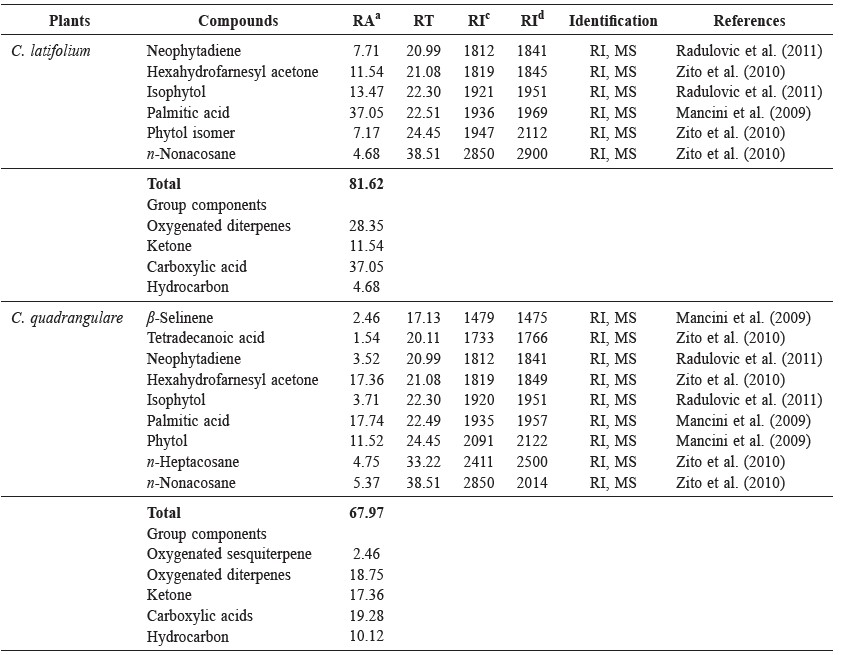
Note: aRelative area in % (peak area relative to total peak area). bRetention Time (min). cRetention Indices determined in this study. dRetention Indices of Kovat index. RI, MS: comparison of the mass spectrum with MS libraries and RI of literature.
The antibacterial test results are shown in Table 2. The oils showed antibacterial activity against both gram-positive and gram-negative bacteria. The leaf volatile oils of C. latifolium and C. guadrangulare exhibited antibacterial activity against gram-positive bacterium (S. aureus) with inhibition zones of 8.50±0.05 and 7.50±0.05 mm, respectively, and also exhibited activity against gram-negative bacterium (E. coli) with inhibition zones of 9.33±0.06 and 8.00±0.10 mm, respectively. Figure 3 and Table 2 showed antibacterial activity of the C. latifolium with larger inhibition zones (8.50±0.05 and 9.33±0.06 mm) against selected strains of bacteria (S. aureus and E. coli, respectively). And both volatile oils showed only weak antibacterial activity against P. aeruginosa. The clear zones of the methanolic blank against the three selected strains of bacteria were all 6.00±0.05 mm.
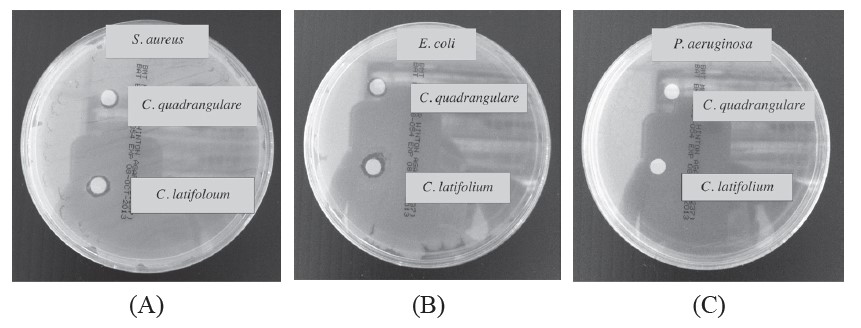
Figure 3. Antibacterial activity test of leaf volatile oils from C. latifolium and C. quadrangulare by disc diffusion method on S. aureus (A), E. coli (B) and P. aeruginosa (C).
Table 2. Antibacterial activity of leaf volatile oils from C. latifolium and C. quadrangulare.
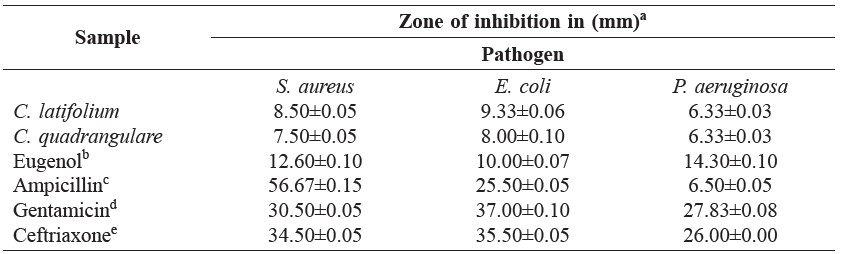
Note: aDiameter of inhibition zone (mm) values, including the diameter of disc (6 mm), are given as mean ± SD of triplicate experiments. Tested volume = 5 μg/disc. bVolatile oil and c,d,eantibiotics used as positive controls.
DISCUSSION
This is the first report that describes the chemical constituents and antibacterial activity of the leaf volatile oils of C. latifolium and C. quadrangular. The leaf volatile oils of C. latifolium and C. quadrangulare showed antibacterial activities against gram-positive bacterium (S. aureus) and gram-negative bacterium (E. coli). The oil of C. latifolium showed stronger antibacterial activity against selected strains of bacteria (S. aureus and E. coli) than C. quadrangulare. Most of the components of the volatile oils were carboxylic acids (palmitic acid and tetradecanoic acid), oxygenated diterpene (phytol and isophytol) and ketones (hexahydrofarnesyl acetone). Palmitic acid seems to be a major fatty acid. Similar results were reported in some mangroves from the Pichavaram mangrove forest (Chandrasekaran et al., 2011). Palmitic acid isolated from Schotia brachypetala Sond., Pelargonium spp. and Pentanisia prunelloides (Klotzsch ex Eckl. & Zeyh.) Walp. have been shown to have antibacterial activity (Agoramoorthy et al., 2007). Hexahydrofarnesyl acetone, a major volatile oil compound, has also been suggested as a possible antimicrobial agent (Radulovic et al., 2011). Neophytadiene, identified as a strong bactericidal compound in the red alga Centroceras clavulatum (C. agardh) Montagne, has also been reported in several plants that have been used as antipyretics, analgesics and vermifugics, including atopical application for sores and inflammation (Venkata et al., 2012). Tetradecanoic acid, which presented in the leaf volatile oil, has been reported as an antimicrobial compound (Zito et al., 2010). Phytol and isophytol are acyclic terpenoids. Phytol is a diterpene that shows antimicrobial activity and thus has become an important plant component in cosmetics, shampoos, toilet soaps and household cleaners. Interestingly, phytol shows high antimicrobial against foodborne pathogens (Venkata et al., 2012). The antibacterial activity of the oil constituents of the two Combretum species studied here seem to be in agreement with previous reports. These results suggest that the volatile oils of the leaves of C. latifolium and C. quadrangulare offer potential in treating infectious diseases caused by S. aureus and E. coli. Further research is needed on the other biological activities of these oils.
ACKNOWLEDGEMENTS
The authors wish to express their sincere thanks to the Faculty of Pharmacy and the Graduate School, Chiang Mai University for their financial support. The Department of Pharmaceutical Science, Faculty of Pharmacy, and the Microbiology Unit, Central Laboratory, Maharaj Nakorn Chiang Mai Hospital, Faculty of Medicine, Chiang Mai University, allowed us to use their scientific equipment and facilities. J. F. Maxwell, CMU Herbarium, Department of Biology, Faculty of Science, Chiang Mai University, identified our specimens.
REFERENCES
Adejuwon, A.O., M.A. Bisi-Johnson, T.M. Obuotor, and O.A. Agboola. 2011. Bioactive compounds and antimicrobial efficacy of the extracts of Combretum pincianum Hook. Journal of Medicinal Plants Research 5(15): 3561-3563.
Agoramoorthy, G., M. Chandrasekaran, V. Venkatesalu, and M.J. Hsu. 2007. Antibacterial and antifungal activities of fatty acid methyl esters of the Blind-your-eye mangrove from India. Brazilian Journal of Microbiology 38: 739-742.
Ahmed, B., T.A. Al-Howiriny, C.M. Passreiter, and J.S. Mossa. 2004. Combretene-A and B: Two new triterpene from Combretum molle. Pharmaceutical Biology 42(2): 109-113. doi: 10.1080/13880200490510883
Akeem, A.A., U.C. Ejikeme, and E.U. Okarafor. 2012. Antibacterial potentials of the ethanolic extract of the stem bark of Combretum micranthum G. Don and its fractions. Journal of Plant Studies 1(2): 75-81. doi: 10.5539/jps.v1n2p75
Angeh, J.E., X. Huang, G.E. Swan, U. Mollman, I. Sattler, and J.N. Eloff. 2007. Novel antibacterial triterpenoid from Combretum padoides (Combretaceae). Archive for Organic Chemistry 9: 113-120.
Banskota, A.H., Y. Tezuka, Q.L. Tran, and S. Kadota. 2003. Chemical constituents and biological activities of Vietnamese medicinal plants. Current Topics in Medicinal Chemistry 3: 227-248.
Chandrasekaran, M., A. Senthilkumar, and V. Venkatesalu. 2011. Antibacterial and antifungal efficacy of fatty acid methyl esters from the leaves of Sesuvium portulacastrum L. European Review for Medical and Pharmacological Sciences 15: 775-780.
Clarke, C.B. 1878. Combretaceae. p. 452-459. In Flora of British India. Vol. 2. Headley Brothers LTD, England.
Coulidiati, T.H., H. Millogo-Kone, A. Lamien-Meda, C.E. Lamien, M. Lompo, M. Kiendrebeogo, S. Bakasso, M. Yougbare-Ziebrou, J. Millogo-Rasolodimby, and O.G. Nacoulma. 2009. Antioxidant and antibacterial activities of Combretum nioroense Aubrev. Ex Keay (Combretaceae). Pakistan Journal of Biological Sciences 12(3): 264-269. doi: 10.3923/pjbs.2009.264.269
Eldeen, I.M.S., and J. van Staden. In vitro pharmacological investigation of extracts from some trees used in Sudanese traditional medicine. South African Journal of Botany 73: 435-440.
Eloff, J.N. 1999. It is possible to use herbarium specimens to screen for antibacterial components in some plants”, Journal of Ethnopharmacology 67: 355-360. doi: 10.1016/S0378-8741(99)00053-7
Eloff, J.N., D.R. Katerere, and L.J. McGaw. 2008. The biological activity and chemistry of the southern African Combretaceae. Journal of Ethnopharmacology 119: 686-699. doi: 10.1016/j.jep.2008.07.051
Eloff, J.N., J.O. Famakin, and D.R.P. Katerere. 2005a. Combretum woodii (Combretaceae) leaf extracts have high activity against gram-negative and gram-positive bacteria. African Journal of Biotechnology 4(10): 1161-1166.
Eloff, J.N., J.O. Famakin, and D.R.P. Katerere. 2005b. Isolation of antibacterial stilbene from Combretum woodii (Combretaceae) leaves. African Journal of Biotechnology 4(10): 1167-1171.
Euswas, P., S. Srirod, P. Chunthanon, and T. Chompoochant. 1988. Studies on anthelmintic activity of Sakae naa (Combretum quadrangulare Kurz) seeds on roundworms of buffalo calves. Kasetsart University Journal 22: 201-206.
Ferraro, M.J., W.A. Craig, M.N. Dudley, G.M. Eliopoulos, D.W. Hecht, J. Hindler, L.B. Reller, A.T. Sheldon, J.M. Swenson, F.C. Tenover, R.T. Testa, M.P. Weinstein, and M.A. Wikler. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically Vol. 20. No 2. (5th ed.) The National Committee for Clinical Laboratory Standards.
Fyhrquist, F., L. Mwasumbi, C. A. Haeggström, H. Vuorela, R. Hiltunen, and P. Vuorela. 2002. Ethnobotanical and antimicrobial investigation on some species of Terminalia and Combretum (Combretaceae) in Tanzania. Journal of Ethnopharmacology 79: 169-177. doi: 10.1016/S0378-8741(01)00375-0
Gaydos, J.M., and B.J. Harrington. 1982. Agar disk diffusion for the quality control testing of autobac elution disks. Antimicrobial Agents and Chemotherapy 21(3): 516-518.
Gronhaug, T.E., S. Glaeserud, M. Skogsrud, N. Ballo, S. Bah, D. Diallo, and B.S. Paulsen. 2008. Ethnopharmacological survey of six medicinal plants from Mali, West-Africa. Journal of Ethnobiology and Ethnomedicine 4(26): 1-11. doi: 10.1186/1746-4269-4-26
Hammer, K.A., C.F. Carson, and T.V. Riley. 1998. In vitro activity of essential oils, in particular Melaleuca alternifolia (tea tree) oil and tea tree oil products, against Candida spp. Journal of Antimicrobial Chemotherapy 42: 591-595.
Hammer, K.A., C.F. Carson, and T.V. Riley. 1999. Antimicrobial activity of essential oils and other plant extracts. Journal of Applied Microbiology 86: 985-990. doi: 10.1046/j.1365-2672.1999.00780.x
Kanwal, U., and A. Karim. 2011. Combretum pincianum Hook has multiple antibacterial activities. Ethnopharmacology 1(1): 1-2. doi: 10.5567/ETHNOPHARMA-IK.2011.1.2
Karou, D., M.H. Dicko, J. Simpore, and A.S. Traore. 2005. Antioxidant and antibacterial activities of polyphenols from ethomedicinal plants of Burkina Faso. African Journal of Biotechnology 4(8): 823-828.
Katerere, D.R., A.I. Gray, R.J. Nash, and R.D. Waigh. 2003. Antimicrobial activity of pentacyclictriterpenes isolated from African Combretaceae. Phytochemistry 63: 81-88. doi: 10.1016/S0031-9422(02)00726-4
Lima, G.R.M., I.R.P. Sales, M.R.D.C. Filho, N.Z.T. Jesus, H.S. Falcão, J.M. Barbosa-Filho, A.G.S. Cabral, A.L. Souto, J.F. Tavares, and L.M. Batista. 2012. Bioactivities of genus Combretum (Combretaceae): A review. Molecules 17: 9142-9206. doi: 10.3390/molecules17089142
Mamidou Kone, W., K. Kamanzi Atindehou, A. Kacou-N’Douba, and M. Dosso. 2007. Evaluation of 17 medicinal plants from Northern Cote d’Ivoire for their in vitro activity against Streptococcus pneumonia. African Journal of Traditional, Complementary and Alternative medicines 4(1): 17-22.
Mancini, E., N.A. Arnold, L.D. Martino, V.D. Feo, C. Formisano, D. Rigano, and F. Senatore. 2009. Chemical composition and phytotoxic effects of essential oils of Salvia hierosolymitana Boiss. and Salvia multicaulis Vahl. var. simplicifolia Boiss. growing wild in Lebanon. Molecules 4: 4725-4736. doi: 10.3390/molecules14114725
Maregesi, S.M., O.D. Ngassapa, L. Pieters, and A.J. Vlietinck. 2007. Ethnopharmacological survey of the Bunda district, Tanzania: Plants used to treat infectious diseases. Journal of Ethnopharmacology 113: 457-470. doi: 10.1016/j.jep.2007.07.006
Martini, N.D., D.R.P. Katerere, and J.N. Eloff. 2004. Biological activity of five antibacterial flavonoids from Combretum erythrophyllum (Combretaceae). Journal of Ethnopharmacology 93: 207-212.
Martini, N.D., and J.N. Eloff. 1998. The preliminary isolation of several antibacterial compounds from Combretum erythrophyllum (Combretaceae). Journal of Ethnopharmacology 62: 255-263.
Masika, P.J., and A.J. Afolayan. 2002. Antimicrobial activity of some plants used for the treatment of livestock disease in the Eastern Cape, South Africa. Journal of Ethnopharmacology 83: 129-134.
McGaw L.J., A.K. Jager, and J. van Staden. 2000. Antibacterial, anthelmintic and antiamoebic activity in South African medicinal plants. Journal of Ethnopharmacology 72: 247-263.
Mubashir, H.M., A. Bahar, M.Z. Iqbal, A.K. Saroor, K. Shamshir, and P. Singh. 2008. Antibacterial activity of whole plant extract of Marrubium vulgare. African Journal of Biotechnology 7(2): 86-87.
Mushi, N.F., Z.H. Mbwambo, E. Innocent, and S. Tewtrakul. 2012. Antibacterial, anti-HIV-1 protease and cytotoxic activities of aqueous ethanolic extracts from Combretum adenogonium Steud. Ex A. Rich (Combretaceae). BMC Complementary and Alternative Medicine 12(163): 1-6.
Nakornchai, S., R. Temsiririrkkul, Y. Wongkrajang, and K. Atisook. 1994. Toxicity study of Combretum quadrangulare Kurz (part II), Mahidol University Journal of Pharmaceutical Science 21(4): 118-125.
Nanakorn, W. 1986. The genus Combretum (Combretaceae) in Thailand. Thai Forest Bulletin 16: 154- 204.
Nantachit, K., D. Santiarvorn, and B. Khantawa. 2006. Antibacterial activity of the seeds of Combretum quadrangulare Kurz (Combretaceae). Chiang Mai University Journal 5: 333-339.
Nopsiri, W., S. Chansakaow, S. Putiyanan, S. Natakankitkul, and D. Santiarworn. 2014. Antioxidant and anticancer activities from leaf extracts of four Combretum species from northern Thailand. Chiang Mai University Journal of Natural Sciences 13(2): 195-205.
Osonwa, U.E., C.E. Umeyor, U.V. Okon, E.M. Uronnachi, and C.D. Nwakile. 2012. Stability studies on the aqueous extract of the fresh leaves of Combretum micranthum G. Don used as antibacterial agent. Journal of Chemistry and Chemical Engineering 6: 417-424.
Pelczar, M.J., E.C.S. Chan, and N.R. Krieg 1993. Microbiology. (Int ed.) Mcgraw Hill, New York.
Radulovic, N., M. Dekic, Z. Stojanovic Radic, and R. Palic. 2011. Chemical composition and antimicrobial activity of the essential oils of Geranium columbinum L. and G. lucidum L. (Geraniaceae). Turkish Journal of Chemistry 35: 499-512.
Rahman, M.S., M.Z. Rahman, Md.A. Wahab, R. Chowdhury, and M.A. Rashid. 2008. Antimicrobial activity of some indigenous plants of Bangladesh. Dhaka University Journal of Pharmaceutical Sciences 7(1): 23-26.
Smith, R.A., C.M. Calviello, A. DerMarderosian, and M.E. Palmer. 2000. Evaluation of antibacterial activity of Belizean plants: An improved method. Pharmaceutical Biology 38(1): 25-29.
Steenkamp, V., A.C. Fernandes, and C.E.J. van Rensburg. 2007. Antibacterial activity of Venda medicinal plants. Fitoterapia 78: 561-564.
Udoh, I.P., C.S. Nworu, C.I. Eleaza, F.N. Onyemelukwe, and C.O. Esimone. 2012. Antibacterial profile of extracts of Combretum micranthum G. Don against resistant and sensitive nosocomial isolated. Journal of Applied Pharmaceutical Science 2(4): 142-146.
Venkata, R.B., L.A. Samuel, S.M. Pardha, R.B. Narashimha, V.K.A. Naga, M. Sudhakar, and T.M. Radhakrishnan. 2012. Antibacterial, antioxidant activity and GC-MS analysis of Eupatorium odoratum. Asian Journal of Pharmaceutical and Clinical Research 5(2): 99-106.
Yahaya, O., J.A. Yabefa, and B. Usman. 2012. Phytochemical screening and antibacterial activity of Combretum glutinosum extract against some human pathogens. British Journal of Pharmacology and Toxicology 3(5): 233-236.
Zafar, S.K., N.S. Vishal, M.S. Shafikh, and N. Sahera. 2011. Antimicrobial activity of essential oils isolated from plants. Chiang Mai University Journal Natural Sciences 10(1): 159-163.
Zito, P., M. Sajeva, M. Bruno, A. Maggio, S. Rosselli, C. Formisano, and F. Senatore. 2010. Essential oil composition of stems and fruits of Caralluma europaea N.E.Br. (Apocynaceae). Molecules 15: 627-638.
Wimaluk Nopsiri1*, Sunee Chansakaow1, Somporn Putiyanan1, Surapol Natakankitkul1, Khesorn Nantachit1, Banyong Khantawa2 and Dammrong Santiarworn1
1 Department of Pharmaceutical Sciences, Faculty of Pharmacy, Chiang Mai University, Chiang Mai 50200, Thailand
2 Microbiology Unit, Central Laboratory, Maharaj Nakorn Chiang Mai Hospital, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand
*Corresponding author. E-mail: wimsiri@hotmail.com, tksiri@yahoo.com
Total Article Views

