
Phytochemical Profile and Anti-inflammatory Activities of Baeckea frutescens Branches Ethanolic Extract on RAW 264.7
Nurhanani Ayub, Salfarina Ramli, Ihsan Safwan Kamarazaman, Evana Kamarudin, Pornchai Rojsitthisak, and Hasseri Halim*Published Date : November 28, 2025
DOI : https://doi.org/10.12982/NLSC.2026.021
Journal Issues : Online First
Abstract Inflammatory diseases are major contributors to global morbidity, and conventional therapies such as non-steroidal anti-inflammatory drugs are often limited by adverse effects. Baeckea frutescens (Myrtaceae), traditionally used in Southeast Asia to treat fever, cough, and rheumatism, is rich in bioactive metabolites, making it a promising candidate for safer natural therapeutics. This study aimed to investigate the phytochemical composition and anti-inflammatory properties of an ethanolic extract of B. frutescens branches (BFBE) using RAW 264.7 macrophages. LCMS/MS profiling identified quinic acid, tetrahydro formononetin sulfate, castalagin, 8-(2,4-dihydroxy-3-methyl-2-buten-1-yl)-2′-prenylquercetin 3-methyl ether, and apigenin-7-β-O-glucuronide. Cytotoxicity screening with the MTT assay showed that concentrations up to 12.5 µg/mL were non-toxic and selected for subsequent analyses. Nitric oxide (NO) production was determined using the Griess assay, where BFBE significantly suppressed NO in a concentration-dependent manner, with an IC50 value of 7.34 ± 1.03 µg/mL, calculated by nonlinear regression analysis. Western blot analysis demonstrated marked downregulation of COX-2 and iNOS at all tested concentrations, alongside significant reductions in IL-6, while IL-1β was suppressed only at 50 µg/mL. ELISA confirmed a dose-dependent inhibition of TNF-α production, with strong suppression observed at 25 and 50 µg/mL (P<0.0001). These results indicate that BFBE exhibits potent anti-inflammatory activity by inhibiting key enzymes and pro-inflammatory cytokines. Collectively, the data support B. frutescens as a promising natural therapeutic agent for inflammatory conditions, warranting further validation in in vivo models.
Keywords: Anti-inflammatory, Baeckea frutescens, Nitrite oxide, Phytochemical, RAW 264.7
Funding: This work was supported by FRGS-RACER grant by the Malaysian Ministry of Higher Education, project code RACER/1/2019/STG04/UITM//2 with project ID 16833, and UiTM grant, project code 600-RMC/GIP 5/3 (085/2022).
citation: Ayub, N., Ramli, S., Kamarazaman, I.S., Kamarudin, E., Rojsitthisak, P., and Halim, H. 2026. Phytochemical profile and anti-inflammatory activities of Baeckea frutescens branches ethanolic extract on RAW 264.7. Natural and Life Sciences Communications. 25(2): e2026021.
Graphical Abstract:

INTRODUCTION
Inflammatory diseases are a significant global health concern, contributing to various chronic conditions, including cardiovascular disease, cancer, and neurodegenerative disorders. According to the World Health Organization, chronic inflammatory conditions account for more than 50% of annual deaths worldwide, reflecting their major role in global morbidity and mortality (World Health Organization [WHO], 2022). In Southeast Asia, the prevalence of inflammation-associated disorders is increasing, driven by aging populations, environmental exposures, and lifestyle-related risk factors, which further highlights the urgent need for safer and more effective interventions (WHO, 2022; Niriella et al., 2025). These diseases collectively account for substantial morbidity and mortality worldwide, often due to their complex pathogenesis, which is underpinned by an imbalance between pro-inflammatory mediators and the body’s antioxidant defenses. This imbalance results in oxidative stress and progressive tissue damage, which exacerbate the inflammatory state and contribute to disease progression (Chaudhary et al., 2023; Jomova et al., 2023). At the molecular level, sustained activation of signaling pathways such as nuclear factor-kappa B (NF-κB), mitogen-activated protein kinases (MAPKs), and the Janus kinase/signal transducer and activator of transcription (JAK/STAT) promotes the overproduction of pro-inflammatory mediators, including nitric oxide via inducible nitric oxide synthase (iNOS), prostaglandins via cyclooxygenase-2 (COX-2), and cytokines such as TNF-α, IL-6, and IL-1β (Saleh et al., 2021; Liu et al., 2023; Nigam et al., 2025). Persistent activation of these pathways contributes to chronic tissue injury and the development of inflammation-related disorders.
Current treatments for inflammatory diseases include non-steroidal anti-inflammatory drugs (NSAIDs) and other pharmaceuticals, which act by targeting these pathways but are frequently associated with adverse side effects and reduced efficacy in long-term use. Consequently, there has been growing interest in natural products as alternative sources of anti-inflammatory agents, offering the potential for improved safety and long-term efficacy (Yahaya et al., 2024; Malcangi et al., 2025).
One promising natural source is Baeckea frutescens, a shrub from the Myrtaceae family that is indigenous to Southeast Asia and widely used in traditional medicine. Known locally by various names such as “jungrahab” and “Gang-Song-Cha” when brewed as tea, B. frutescens has been used for centuries to treat ailments including fever, cough, rheumatism, headache, and skin infections (Navanesan et al., 2015; Guo et al., 2025). Ethnobotanical studies highlight the use of B. frutescens leaves, stems, and roots, which are rich in bioactive compounds with known anti-inflammatory, antioxidant, antimicrobial, and wound-healing properties. These traditional uses have encouraged researchers to investigate the plant’s phytochemistry, identifying a range of secondary metabolites that contribute to its medicinal properties (Jiang et al., 2020; Huong et al., 2023). Approximately 130 metabolites have been isolated from this plant, with phloroglucinol derivatives and tasmanone being notable for their pharmacological activities. Our previous studies demonstrate that extracts of B. frutescens can enhance keratinocyte and fibroblast migration and proliferation, essential processes in wound healing, and upregulate the expression of key wound-healing factors, such as TGF-β, IL-1β, and VEGF (Kamarazaman et al., 2023; Kamarazaman et al., 2024a; Kamarazaman et al., 2024b). Furthermore, cytotoxic studies indicate that B. frutescens leaf and branch extracts exhibit activity against hypoxic breast cancer cells, suggesting potential applications in cancer treatment, particularly in targeting tumour hypoxia, a common feature of aggressive cancers (Shahruzaman et al., 2021).
Given its wide array of bioactive compounds and documented therapeutic activities, Baeckea frutescens is an excellent candidate for further exploration as a natural source of novel anti-inflammatory agents. This study builds on existing knowledge by examining the phytochemical profile and therapeutic potential of an ethanolic extract derived specifically from B. frutescens branches, focusing on its inhibitory effects on key inflammatory mediators in vitro. To our knowledge, this is the first study to comprehensively investigate the branch-derived phytochemical profile using LC-MS/MS and directly associate these constituents with anti-inflammatory activity in a macrophage model. By integrating phytochemical profiling with mechanistic assays targeting nitric oxide, cytokines, iNOS, and COX-2, this work provides novel insights into how branch metabolites contribute to NF-κB and MAPK modulation. Unlike prior studies that concentrated mainly on leaves or whole-plant extracts, our findings highlight the unique pharmacological relevance of branch-derived compounds, thereby expanding the therapeutic scope of B. frutescens for inflammation management. Through this research, we contribute to the growing evidence supporting natural products as viable sources of new therapeutic agents for inflammatory diseases and related conditions.
MATERIALS AND METHODS
Reagents and chemicals
Cell culture reagents included Dulbecco’s Modified Eagle Medium (DMEM) (Nacalai Tesque, Kyoto, Japan; cat. no. 08458-45), fetal bovine serum (FBS) (Tico Europe, Netherlands; cat. no. FBSEU500), penicillin-streptomycin (Nacalai Tesque, Kyoto, Japan; cat. no. 09367-34), and phosphate-buffered saline (PBS) (Sigma-Aldrich, St. Louis, MO, USA; cat. no. D8537-100ML). Chemicals used were 95% ethanol (Westlab, Australia; cat. no. 35-266), dimethyl sulfoxide (DMSO) (Sigma-Aldrich; cat. no. D2625), lipopolysaccharides (LPS) E. coli O111:B4 (Sigma-Aldrich; cat. no. L2630), potassium phosphate (KH2PO4) (Sigma-Aldrich; cat. no. P5379), ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich; cat. no. EDS-100MG), MTT (Sigma-Aldrich; cat. no. M2128), dexamethasone (Sigma-Aldrich; cat. no. D4902), and the Griess reagent system (Promega, Madison, WI, USA; cat. no. G2930). Additional reagents included formic acid (Merck, Darmstadt, Germany; cat. no. 100264), acetonitrile (Sigma-Aldrich; cat. no. 34851), phosphoric acid (Sigma-Aldrich; cat. no. 79622), protease inhibitor cocktail (Roche Applied Science, Mannheim, Germany; cat. no. 11836170001), phenylmethylsulfonyl fluoride (PMSF) (Sigma-Aldrich; cat. no. P7626), RIPA buffer (Cell Signalling Technology, Boston, MA, USA; cat. no. 9806), and bovine serum albumin (BSA) (Sigma-Aldrich; cat. no. A4737). For protein quantification and electrophoresis, the following were used: protein assay CBB solution (Nacalai Tesque; cat. no. 1617-71), 30% bis-acrylamide (Bio-Rad, Hercules, CA, USA; cat. no. 1610156), Trizma® hydrochloride (Sigma-Aldrich; cat. no. T3253), sodium dodecyl sulfate (SDS) (Sigma-Aldrich; cat. no. L4509), ammonium persulfate (APS) (Sigma-Aldrich; cat. no. A3678), TEMED (Bio-Rad; cat. no. 1610800), Trizma® base (Sigma-Aldrich; cat. no. T1503), glycine (Sigma-Aldrich; cat. no. G8898), methanol (Merck; cat. no. 179337), protein marker PM2700 ExcelBand (SMOBIO Technology, Taiwan; cat. no. PM2700), bromophenol blue (Sigma-Aldrich; cat. no. 114391), glycerol (Sigma-Aldrich; cat. no. G5516), 2-mercaptoethanol (Sigma-Aldrich; cat. no. M6250), Ponceau S (Sigma-Aldrich; cat. no. P3504), Coomassie Brilliant Blue (Bio-Rad; cat. no. 1610436), acetic acid (Sigma-Aldrich; cat. no. A6283), sodium chloride (NaCl) (Sigma-Aldrich; cat. no. S9888), nitrocellulose membrane (Bio-Rad; cat. no. 1620115), Tween-20 (Bio-Rad; cat. no. 1706531), and skim milk (Sigma-Aldrich; cat. no. M7409). The Opti 4-CN colorimetric detection kit was obtained from Bio-Rad (cat. no. 1708235). Antibodies used for Western blot analysis were as follows: anti-iNOS (Santa Cruz Biotechnology, CA, USA; cat. no. sc-7271), anti-COX-2 (Santa Cruz; cat. no. sc-166475), anti-IL-6 (Santa Cruz; cat. no. sc-57315), anti-IL-1β (Santa Cruz; cat. no. sc-52012), and secondary antibody m-IgGκ BP-HRP (Santa Cruz; cat. no. sc-516102). TNF-α levels were determined using a Mouse TNF-α ELISA kit (Elabscience, China; cat. no. E-EL M0049).
Plant material collection and extraction
Baeckea frutescens branches, sourced from Setiu, Kuala Terengganu, Terengganu, Malaysia, underwent taxonomic identification by a botanist at the Forest Research Institute Malaysia. A voucher specimen (SBID:044/20) was deposited at the Faculty of Pharmacy, Universiti Teknologi MARA, Selangor.
Following authentication, the branches were air-dried and pulverized. Extraction was performed using a method adapted from Nisa et al. (2017). Briefly, 500 g of the powdered plant material was subjected to three consecutive 72-hour extractions with 2.5 L of 95% ethanol. The combined extracts were filtered and concentrated using a rotary evaporator. The resulting crude extract was stored at -80°C until further analysis.
LCMS/MS analysis
LCMS analysis was conducted on an LTQ Orbitrap Discovery system. A Luna Omega 3 mm Polar C18 column was used for HPLC separation. The mobile phases consisted of 0.1% formic acid and acetonitrile, with a gradient starting at 95:5, linearly changing to 50:50 over 10 minutes, then to 5:95 by 15 minutes, and finally returning to 95:5 by 20 minutes, holding for an additional 5 minutes. The flow rate was 0.2 mL/min with a 10 mL injection volume. Mass spectra were collected in the m/z range of 50-1,000 using negative ion mode. The mass spectrometry parameters included a sheath gas flow rate of 18 arbitrary units, auxiliary gas flow rate of 2 arbitrary units, spray voltage of 4.40 kV, capillary temperature of 285°C, capillary voltage of 18 V, and tube lens of 100 V. Data acquisition was performed using LTQ Tune Plus software, and the data analysis was carried out using Thermo Xcalibur Roadmap software (Chunduri and Dannana, 2016; Rafińska et al., 2019).
In vitro assays
Cell culture
RAW 264.7 murine macrophages were cultured in Dulbecco’s Modified Eagle medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. Cells were maintained at 37°C in a humidified atmosphere containing 5% CO2, as described by Kim et al. (2019). Sub-culturing was performed when cells reached 80-90% confluency.
Cell viability assay
The cytotoxic effects of Baeckea frutescens branch extract (BFBE) and lipopolysaccharide on RAW 264.7 macrophages were evaluated using the MTT assay, as described (Navanesan et al., 2015; Kim et al., 2019). Cells were seeded in a 96-well plate at a density of 10,000 cells/well and incubated overnight at 37°C. Following treatment with varying concentrations of branch extract (3.13-100 µg/mL) and LPS (0.25-4 µg/mL) for 24 hours, the medium was removed, and 0.5 mg/mL MTT reagent was added. After a 3-hour incubation, the formazan crystals were dissolved in DMSO, and the absorbance was measured at 570 nm using a microplate reader. BFBE and LPS stocks were prepared by dissolving the dried extract or powder directly in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) to obtain the desired working concentrations. All treatments were freshly prepared in culture media immediately before use, and culture media without BFBE or LPS served as the vehicle control.
Nitric oxide determination
Nitric oxide production was measured according to the method described (Kim et al., 2019). RAW 264.7 cells were seeded in a 12-well plate at a density of 100,000 cells/well and incubated overnight. Cells were then pre-treated with various concentrations of Baeckea frutescens branch extract (3.125-50 µg/mL) for 1 hour before stimulation with 1 µg/mL LPS. Dexamethasone (3 µM) served as a positive control. Untreated, unstimulated cells served as the negative control. After a 24-hour incubation at 37°C, the supernatant was collected and centrifuged. 100 µL of the supernatant was then transferred to a 96-well plate and mixed with 100 µL of Griess reagent. Following a 15-minute incubation, the absorbance was measured at 540 nm using a microplate reader. IC50 values were calculated by nonlinear regression using GraphPad Prism 9.
Western blot analysis
The anti-inflammatory effects of Baeckea frutescens branch extract were evaluated by Western blot analysis, according to the method described (Kim et al., 2019). RAW 264.7 cells were pre-treated with various concentrations of branch extract (3.125-50 µg/mL) or 3 µM dexamethasone (positive control) for 1 hour, followed by stimulation with 1 µg/mL LPS for 24 hours at 37°C. Cell lysates were prepared in RIPA buffer containing protease inhibitors, and protein concentrations were determined by the Bradford assay using Coomassie brilliant blue G-250 (CBB G-250) solution. Equal amounts of protein were separated by 10% SDS-PAGE and transferred to nitrocellulose membranes. Membranes were blocked with 5% skim milk in TBS-Tween 20 and incubated overnight at 4°C with primary antibodies against iNOS, COX-2, IL-6, and IL-1β (1:5,000 dilution). Following incubation with a horseradish peroxidase-conjugated secondary antibody, protein bands were visualized using an Opti-4CN colorimetric detection kit. Band intensities were quantified by densitometry (ImageJ) and normalized to β-actin expression, which served as the internal loading control.
Enzyme-linked immunosorbent assay (ELISA)
TNF-α concentrations were determined via enzyme-linked immunosorbent assay using the Mouse TNF-α ELISA Kit from Elabscience. Sample preparation was carried out according to the methods outlined (Chang et al., 2019; Kim et al., 2019). RAW 264.7 cells were pre-treated with various concentrations of Baeckea frutescens branch extract (3.125-50 µg/mL) or 3 µM dexamethasone (positive control) for 1 hour, followed by stimulation with 1 µg/mL LPS for 24 hours at 37°C. Cell lysates were prepared and stored at -80°C until further analysis. The ELISA was performed according to the manufacturer's instructions.
Statistical analysis
All experiments were independently performed in four replicates (n = 4). Data are expressed as mean ± standard error of the mean (SEM). Statistical analysis was performed using a one-way analysis of variance followed by Tukey’s post hoc test with GraphPad Prism 9 software. A P-value less than 0.05 was considered statistically significant.
RESULTS
Phytochemical profiling of Baeckea frutescens L. branches ethanolic extract
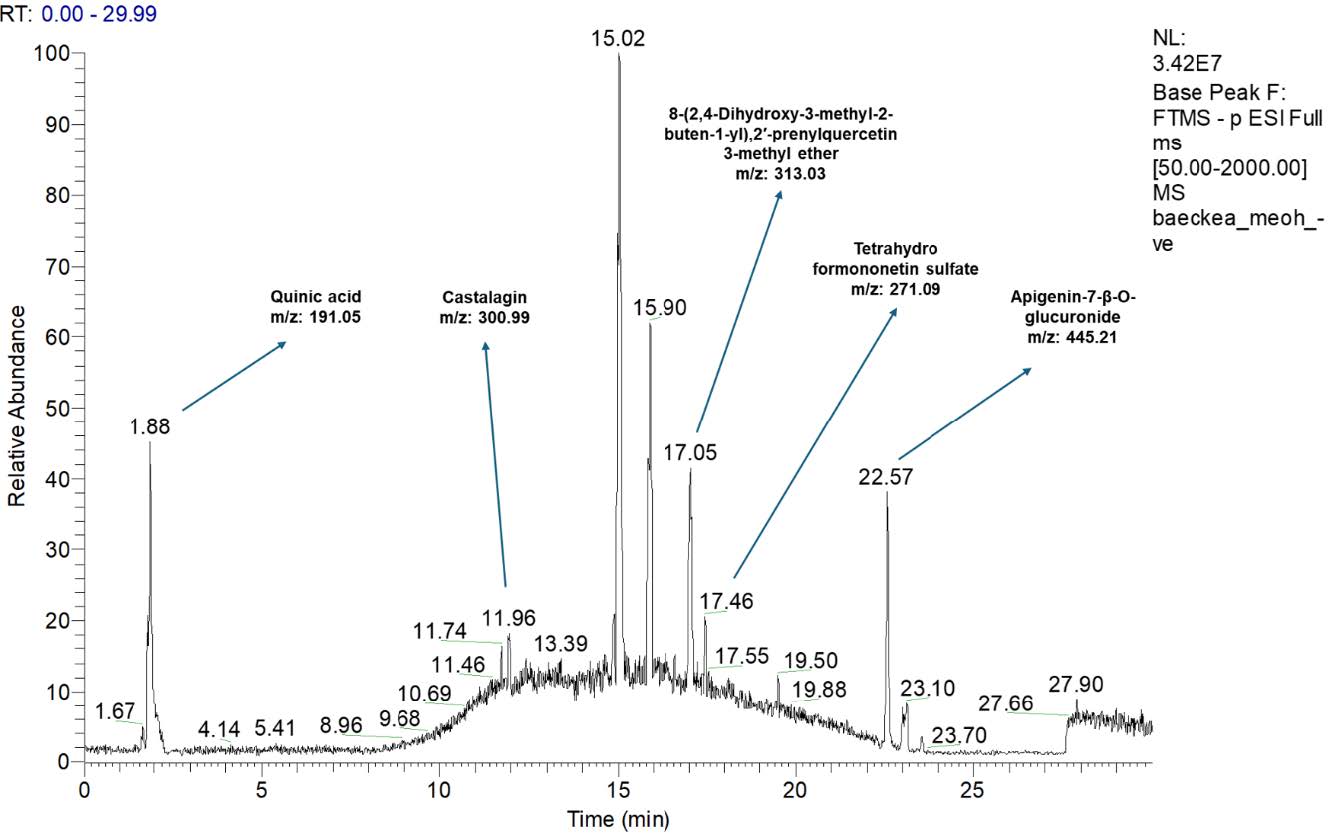
Figure 1. Total ion chromatogram of Baeckea frutescens L. branches ethanolic extract analysed by HPLC-LTQ-Orbitrap MS System.
Table 1. Mass spectral characteristics and tentative identification of compounds present in Baeckea frutescens L. branches ethanolic extract.
|
Molecular ion |
Retention time (min) |
Tentative identification |
Molecular formula |
|
191.05 |
1.88 |
Quinic acid |
C7H11O6 |
|
271.09 |
17.46 |
Tetrahydro formononetin sulfate |
C16H15O7S |
|
300.99 |
11.96 |
Castalagin (hydrolysable tannin) |
C41H26O26 |
|
313.03 |
17.05 |
8-(2,4-Dihydroxy-3-methyl-2-buten-1-yl), |
C26H28O9 |
|
445.21 |
22.57 |
Apigenin-7-β-O-glucuronide |
C21H18O12 |
The results show an LCMS/MS analysis conducted on an ethanolic extract of Baeckea frutescens L. branches. The compounds were identified by comparing the results with previous research and online databases (Figure 1 and Table 1).
Quinic acid was tentatively identified based on the detection of deprotonated molecular ions at m/z 191.05 (Sulaiman et al., 2020). The second compound that was tentatively identified was tetrahydro formononetin sulfate, from deprotonated molecular ions at m/z 271.05 (Liu et al., 2020). Next, the hydrolysable tannin castalagin was tentatively identified from deprotonated molecular ions at m/z 300.99 (Singh et al., 2016). Additionally, 8-,2′-prenylquercetin 3-methyl ether was tentatively identified from deprotonated molecular ions at m/z 313.03 and apigenin-7-β-O-glucuronide was tentatively identified from deprotonated molecular ions at m/z 445.21 (El-Askary et al., 2019).
Cell viability assay (MTT assay)
This study aimed to evaluate the impacts of Baeckea frutescens branches ethanolic extract and lipopolysaccharide on the viability of RAW 264.7 murine macrophage cells using the MTT assay. RAW 264.7 cells are a widely utilized in vitro model to investigate the effects of various compounds on inflammatory responses. The cytotoxic effects of the Baeckea frutescens extract and lipopolysaccharide on the viability of RAW 264.7 cells were assessed using the MTT assay (Razmavar et al., 2014; Navanesan et al., 2015; Nisa et al., 2017).
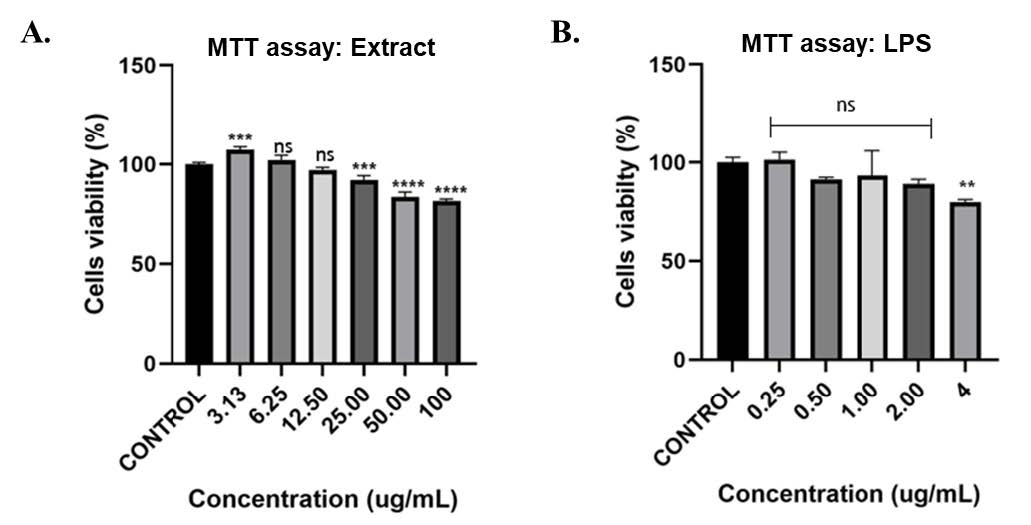
Figure 2. (A) Cell viability of RAW 264.7 macrophages following treatment with varying concentrations of Baeckea frutescens branch ethanolic extract (BFBE). (B) Cell viability of RAW 264.7 cells treated with different concentrations of lipopolysaccharide (LPS). Data are expressed as mean ± SEM (n = 4 independent experiments). Statistical analysis was conducted using one-way ANOVA with Tukey’s post hoc test. Significance: ****P< 0.0001; ***P<0.001; **P<0.01; *P<0.05; ns = not significant, compared with the control group.
The results showed that the Baeckea frutescens branches ethanolic extract exhibited a concentration-dependent effect on the viability of RAW 264.7 cells (Figure 2A). The extract at 100 μg/mL and 50 μg/mL significantly decreased cell viability, while the effect was non-significant at 12.50 μg/mL and 6.25 μg/mL. The MTT assay results for lipopolysaccharide, LPS, indicated that a significant reduction in cell viability was observed only at the highest concentration of 4 μg/mL, whereas the lower concentrations of 2 μg/mL, 1 μg/mL, 0.50 μg/mL and 0.25 μg/mL had no significant impact on the viability of RAW 264.7 cells (Figure 2B).
These findings suggest that Baeckea frutescens branches ethanolic extract, and lipopolysaccharide can modulate the viability of RAW 264.7 cells in a concentration-dependent manner. Further investigations are needed to elucidate the underlying mechanisms by which these compounds affect cell viability (Razmavar et al., 2014; Shahruzaman et al., 2019).
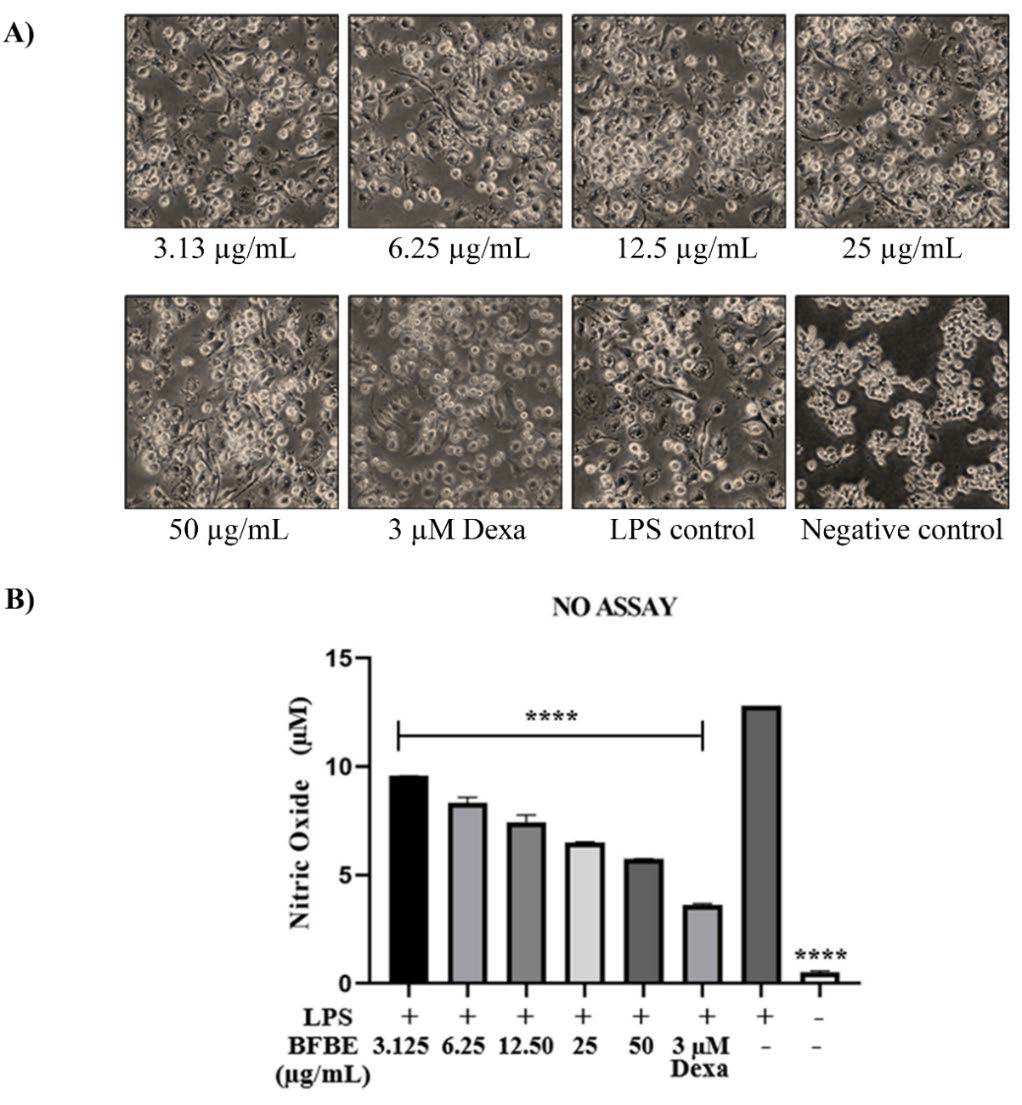
Figure 3. Effects of Baeckea frutescens branch ethanolic extract (BFBE) on LPS-induced nitric oxide (NO) production in RAW 264.7 cells. (A) Representative microscopic images (100× magnification) of RAW 264.7 cells pre-treated with the indicated concentrations of BFBE for 1 h, followed by stimulation with LPS (1 µg/mL) for 24 h. (B) Inhibitory effects of BFBE on NO production in RAW 264.7 cells. Culture supernatants were collected and analysed using the Griess reagent assay. Data are expressed as mean ± SEM (n = 4 independent experiments). Statistical analysis was performed using one-way ANOVA with Tukey’s post hoc test. Significance: ****P<0.0001; ***P<0.001; **P<0.01; *P<0.05; ns = not significant, compared with the LPS control group.
Anti-inflammatory test
Nitric oxide determination
Figure 3 provides compelling evidence for the anti-inflammatory properties of Baeckea frutescens branches ethanolic extract. Microscopic examination of RAW 264.7 macrophages. (Figure 3A) revealed that pretreatment with BFBE visibly reduced the inflammatory changes induced by lipopolysaccharide exposure, with greater protection observed at higher BFBE concentrations. This visual observation was corroborated by a significant, concentration-dependent reduction in nitric oxide production in BFBE-treated groups compared to the LPS control group (Figure 3B). Specifically, BFBE at concentrations of 3.125 µg/mL, 6.25 µg/mL, 12.50 µg/mL, 25 µg/mL, and 50 µg/mL resulted in significant reductions in NO levels, all with P-values less than 0.0001 compared to the LPS control. Notably, the highest concentration (50 µg/mL) showing an inhibitory effect approaching that of dexamethasone (3 µM), a known anti-inflammatory drug, thus demonstrating its potency at non-cytotoxic doses. We calculated the half-maximal inhibitory concentration (IC₅₀) of BFBE on nitric oxide production in LPS-stimulated RAW 264.7 cells. The IC₅₀ value was 7.34 ± 1.03 µg/mL, indicating a potent NO inhibitory effect. This quantitative parameter strengthens the pharmacological relevance of the extract. This potent inhibitory effect of BFBE on NO production, a key mediator of inflammation, underscores its potential as a therapeutic agent for inflammatory conditions. These promising results provide a strong foundation for further exploration of the specific molecular mechanisms by which BFBE inhibits NO production in macrophages, such as examining its effects on key inflammatory signalling pathways.
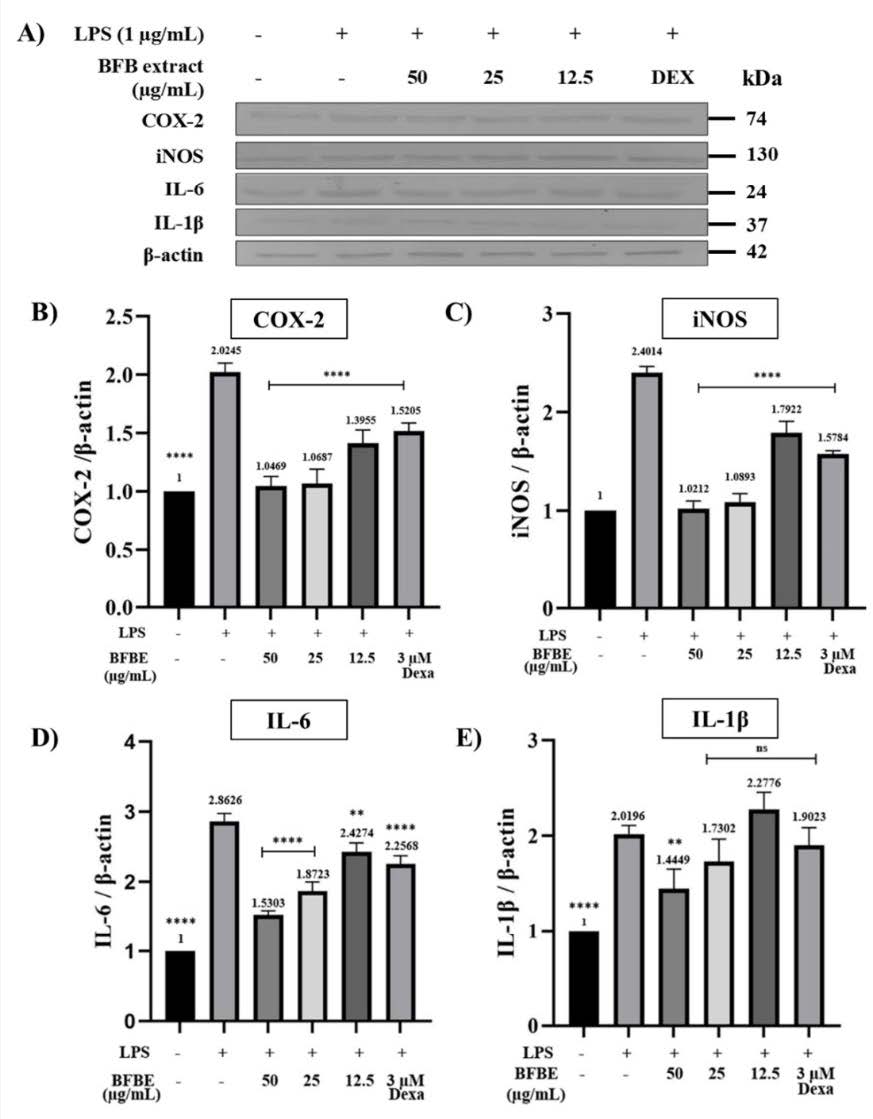
Figure 4. (A) Western blot analysis of COX-2, iNOS, IL-6, and IL-1β expression in RAW 264.7 cells. The inhibitory effects of Baeckea frutescens branch ethanolic extract (BFBE) on LPS-induced protein expression are shown for (B) COX-2, (C) iNOS, (D) IL-6, and (E) IL-1β. RAW 264.7 cells were pre-treated with the indicated concentrations of BFBE for 1 h, followed by stimulation with LPS (1 µg/mL) for 24 h. Protein levels were determined by Western blotting, with band intensities quantified by densitometry and normalized to β-actin. Data are expressed as mean ± SEM (n = 4 independent experiments). Statistical analysis was performed using one-way ANOVA with Tukey’s post hoc test. Significance: ****P<0.0001; ***P<0.001; **P<0.01; *P<0.05; ns = not significant, compared with the LPS control group.
Western blot analysis
Figure 4 illustrates the impact of Baeckea frutescens branches ethanolic extract on inflammatory mediator production in LPS-stimulated RAW 264.7 cells using western blot analysis. The figure demonstrates that pre-treatment with all concentrations of BFBE significantly reduced COX-2 and iNOS levels compared to the LPS group (****P<0.0001 for all comparisons). Furthermore, BFBE exhibited a dose-dependent reduction in IL-6 levels, with significant reductions observed at all concentrations (****P<0.0001 for the two highest concentrations, **P<0.01 for the lowest concentration).
While only the highest concentration of BFBE significantly decreased IL-1β levels compared to the LPS group (**P<0.01), the other concentrations did not show a significant difference. Dexamethasone, used as a positive control, consistently demonstrated significant reductions in COX-2, iNOS, and IL-6 levels compared to the LPS group (****P<0.0001 for all comparisons), validating the experimental setup and highlighting the potential anti-inflammatory properties of BFBE.
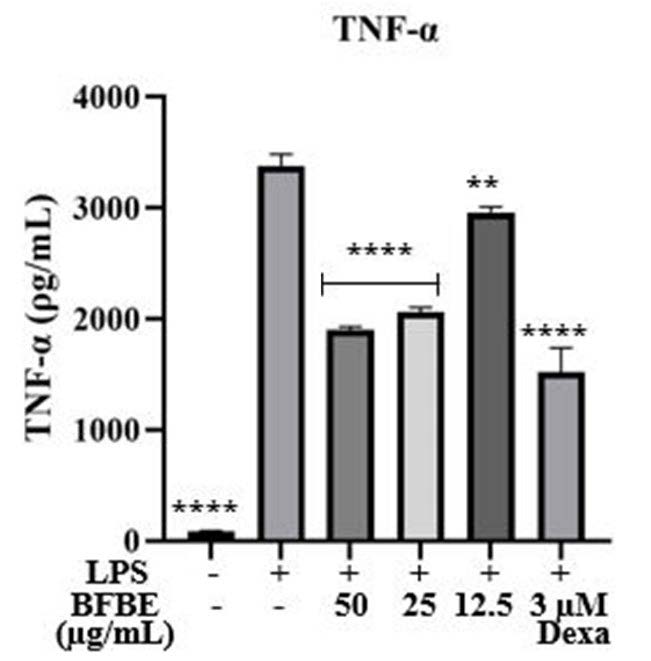
Figure 5. Inhibitory effect of Baeckea frutescens branch ethanolic extract (BFBE) on TNF-α production in RAW 264.7 cells. Cells were treated with BFBE (50, 25, or 12.5 µg/mL) in the presence or absence of LPS (1 µg/mL) for 24 h. TNF-α levels in the culture supernatants were quantified using a commercial ELISA kit (Elabscience, cat. no. E-EL-M0049). Data are expressed as mean ± SEM (n = 4 independent experiments). Statistical analysis was performed using one-way ANOVA with Tukey’s post hoc test. Significance: ****P<0.0001; ***P<0.001; **P<0.01; *P<0.05; ns = not significant, compared with the LPS-treated group.
Enzyme-linked immunosorbent assay (ELISA)
Figure 5 presents data on the effects of Baeckea frutescens branches extract on TNF-α production in LPS-stimulated RAW 264.7 macrophages. The results demonstrate that BFBE exhibits anti-inflammatory properties by significantly reducing TNF-α production. As expected, LPS stimulation dramatically increased TNF-α production compared to the unstimulated negative control (****P<0.0001). This confirms the pro-inflammatory effect of LPS. Treatment with BFBE at concentrations of 50 µg/mL and 25 µg/mL significantly reduced TNF-α production compared to the LPS group (****P<0.0001), indicating a potent anti-inflammatory effect. While a lower concentration of BFBE (12.5 µg/mL) also reduced TNF-α levels, the effect was less pronounced (**P<0.01), suggesting a possible dose-dependent response. Dexamethasone, a known anti-inflammatory agent used as a positive control, significantly suppressed TNF-α production (****P<0.0001), validating the assay's sensitivity in detecting anti-inflammatory activity. These findings suggest that BFBE possesses anti-inflammatory properties, as evidenced by its ability to suppress LPS-induced TNF-α production in macrophages. Further investigation is warranted to elucidate the underlying mechanisms of action and to evaluate the potential therapeutic benefits of BFBE in inflammatory conditions (Navanesan et al., 2015; Samtiya et al., 2021).
DISCUSSION
LCMS/MS analysis of Baeckea frutescens branch ethanolic extract (BFBE) revealed a rich phytochemical profile comprising several notable bioactive compounds, including quinic acid, tetrahydro formononetin sulfate, castalagin (a hydrolysable tannin), 8-(2,4-dihydroxy-3-methyl-2-buten-1-yl)-2′-prenylquercetin 3-methyl ether, and apigenin-7-β-O-glucuronide. These compounds are widely reported in the literature for their anti-inflammatory, antioxidant, and immunomodulatory properties. Their presence in BFBE suggests that the observed biological activities are likely driven by both individual and synergistic effects of these phytochemicals. This supports the potential of Baeckea frutescens L. as a valuable natural source for the development of novel therapeutic agents targeting inflammatory diseases.
To ensure the safety of the extract and identify suitable concentrations for subsequent anti-inflammatory assays, the cytotoxicity of BFBE was first evaluated using the MTT assay in RAW 264.7 murine macrophage cells. The MTT assay is a widely accepted colorimetric method that estimates cell viability by measuring mitochondrial metabolic activity. Metabolically active cells convert the yellow MTT salt into insoluble purple formazan, which is solubilized and quantified spectrophotometrically (Ghasemi et al., 2021). A decline in absorbance values reflects reduced cell viability, indicating cytotoxic effects. BFBE showed a concentration-dependent effect on cell viability. Significant cytotoxicity was observed at higher concentrations (50 µg/mL and above), whereas lower concentrations (3.125–12.5 µg/mL) did not produce a statistically significant impact on RAW 264.7 viability. These results suggest a threshold for cytotoxicity and allowed the selection of non-toxic doses for further experiments (Hwang et al., 2016; Carvalho and Conte-Junior, 2021; Jaradat and Hawash, 2021). Similarly, LPS, used to induce inflammation, demonstrated cytotoxicity only at its highest tested concentration (4 µg/mL), with lower doses having minimal impact. These findings demonstrate the sensitivity of the MTT assay and its utility in determining safe, effective concentrations for evaluating anti-inflammatory effects in vitro.
To investigate BFBE's anti-inflammatory potential, a multifaceted approach was employed, involving morphological evaluation, nitric oxide (NO) quantification, Western blot analysis, and ELISA (Novilla et al., 2017; Baek et al., 2020; Lin et al., 2021; Marasri et al., 2024). Morphological examination showed that LPS-stimulated macrophages developed pronounced inflammatory characteristics, such as cell swelling and altered shapes. However, pretreatment with BFBE, particularly at higher concentrations, significantly reduced these changes, suggesting a protective effect against LPS-induced inflammation. One of the key markers of inflammation in macrophages is the production of nitric oxide. Inflammatory stimuli such as LPS elevate NO levels via the induction of inducible nitric oxide synthase (iNOS) (Novilla et al., 2017; Kim et al., 2019). The Griess assay revealed a significant, concentration-dependent reduction in NO production in BFBE-treated cells.
Further mechanistic insights were provided through Western blot analysis, which showed that BFBE significantly downregulated the expression of both iNOS and cyclooxygenase-2 (COX-2), enzymes that play central roles in inflammatory pathways (Kim et al., 2019; Lin et al., 2021). This reduction in protein expression aligns with the observed decrease in NO production, further supporting BFBE’s mechanism of action in mitigating inflammatory responses. Additionally, BFBE suppressed the expression of the pro-inflammatory cytokine IL-6 at all tested concentrations. Although IL-1β was only significantly reduced at the highest BFBE dose, this finding still indicates potential for broader cytokine regulation, as similarly observed when treating macrophages with Anadenanthera colubrina extract via NF-κB/MAPK suppression (Maia et al., 2024). ELISA analysis of TNF-α levels reinforced the anti-inflammatory profile of BFBE. TNF-α, a key cytokine involved in early inflammatory signaling, was significantly reduced by BFBE in a dose-dependent manner (Navanesan et al., 2015; Kim et al., 2019; Baek et al., 2020). This finding, combined with the modulation of IL-6 and IL-1β, suggests that BFBE can interfere with upstream cytokine signaling and may modulate multiple inflammatory pathways simultaneously.
Beyond these protein-level observations, the concurrent presence of apigenin derivatives, castalagin, and quinic acid suggests synergistic interactions that may collectively underlie the broad anti-inflammatory effects of BFBE. Apigenin derivatives are well recognized for suppressing NF-κB and MAPK activation, thereby inhibiting the transcription of pro-inflammatory mediators such as iNOS, COX-2, TNF-α, IL-6, and IL-1β (Ji et al., 2023; Allemailem et al., 2024). Castalagin, a hydrolysable tannin, exerts dual actions as a potent free radical scavenger and as an inhibitor of NF-κB–dependent signaling, reducing oxidative stress that fuels chronic inflammation (Piazza et al., 2024). Quinic acid contributes by enhancing antioxidant defenses and modulating MAPK phosphorylation, with demonstrated efficacy in colitis and neuroinflammation models (Ghasemi-Dehnoo et al., 2023; Park et al., 2024). Taken together, these compounds likely act in a multi-targeted and complementary manner, consistent with the pharmacological behavior of complex plant extracts. This integrated activity aligns with the ethnopharmacological use of B. frutescens in inflammatory disorders and is further supported by recent in vivo evidence of NF-κB/MAPK pathway modulation (Huong et al., 2023; Guo et al., 2025).
Altogether, the collective findings from this study strongly support the anti-inflammatory potential of Baeckea frutescens branch extract. The ability of BFBE to suppress key inflammatory mediators such as NO, TNF-α, IL-6, COX-2, and iNOS highlights its promise as a natural therapeutic candidate for managing inflammation-related conditions. These results align with traditional uses of Baeckea frutescens in herbal medicine and contribute valuable scientific evidence supporting its medicinal application. However, while the in vitro findings are encouraging, they also highlight the need for further investigation. Future studies should explore the specific molecular signaling pathways affected by BFBE, such as NF-κB, MAPK, and JAK/STAT. Additionally, in vivo models will be essential to validate the efficacy, pharmacokinetics, and safety profile of BFBE in physiological systems. Standardization of extract composition and isolation of active constituents will also be critical for developing potential therapeutic formulations.
Nevertheless, this study has several limitations. First, gene-expression regulation of inflammatory mediators was not assessed, which would provide mechanistic confirmation at the transcriptional level. Second, assays targeting signaling cascades (e.g., NF-κB nuclear translocation, MAPK phosphorylation) were not performed. Third, findings are limited to an in vitro macrophage model; absence of in vivo validation reduces translational relevance. Addressing these gaps will be critical to strengthening the evidence base for BFBE. Future studies should therefore include in vivo models, detailed pathway analyses, and standardization of phytochemical content to ensure reproducibility. Pharmacokinetic and toxicological studies will also be essential before translation into clinical applications.
CONCLUSION
This study demonstrates that the ethanolic extract of Baeckea frutescens branches (BFBE) is rich in bioactive phytochemicals such as quinic acid, tetrahydro formononetin sulfate, castalagin, 8-(2,4-dihydroxy-3-methyl-2-buten-1-yl)-2′-prenylquercetin 3-methyl ether, and apigenin-7-β-O-glucuronide, which collectively contribute to its strong in vitro anti-inflammatory activity. BFBE significantly downregulated inflammatory mediators including NO, TNF-α, IL-6, IL-1β, iNOS, and COX-2 in LPS-stimulated RAW 264.7 macrophages. These findings provide scientific support for the ethnopharmacological use of B. frutescens and suggest its promise as a natural therapeutic agent for inflammation-related disorders. However, validation of these effects in in vivo models is essential to confirm efficacy, bioavailability, and safety under physiological conditions. Future clinical investigations will also be necessary to establish dosing, pharmacokinetics, and potential applications in human populations. Taken together, this study highlights the potential of B. frutescens as a practical source of natural anti-inflammatory agents, supporting its development into standardized botanical therapeutics for managing chronic inflammatory diseases.
ACKNOWLEDGEMENTS
The authors thank the Faculty of Pharmacy and iPROMISE, Universiti Teknologi MARA for providing instruments.
AUTHOR CONTRIBUTIONS
Nurhanani Ayub: Writing – Original Draft (Lead), Methodology (Supporting), Validation (Supporting), Formal Analysis (Supporting), Investigation (Supporting), Data Curation (Lead); Salfarina Ramli: Writing – Review & Editing (Supporting), Conceptualization (Supporting), Methodology (Supporting), Validation (Supporting); Ihsan Safwan Kamarazaman: Writing – Review & Editing (Supporting), Conceptualization (Supporting), Methodology (Supporting), Validation (Supporting); Evana Kamarudin: Writing – Review & Editing (Supporting), Conceptualization (Supporting), Methodology (Supporting), Validation (Supporting); Pornchai Rojsitthisak: Writing – Review & Editing (Supporting), Conceptualization (Supporting), Methodology (Supporting), Validation (Supporting); Hasseri Halim: Project Administration (Lead), Funding Acquisition (Lead), Formal Analysis (Lead), Writing – Review & Editing (Lead), Conceptualization (Lead), Methodology (Lead), Validation (Lead), Resources (Lead), Data Curation (Supporting), Supervision (Lead).
CONFLICT OF INTEREST
The authors hereby declare that there are no financial, personal, or professional competing interests that could have influenced the work reported in this manuscript. All aspects of the study, including its design, execution, data analysis, and interpretation, were conducted independently and without any conflicts of interest.
REFERENCES
Allemailem, K.S., Almatroudi, A., Alharbi, H.O.A., AlSuhaymi, N., Alsugoor, M.H., Aldakheel, F.M., Khan, A.A., and Rahmani, A.H. 2024. Apigenin: A bioflavonoid with a promising role in disease prevention and treatment. Biomedicines. 12(6): 1353. https://doi.org/10.3390/biomedicines12061353
Baek, S.H., Park, T., Kang, M.G., and Park, D. 2020. Anti-inflammatory activity and ROS regulation effect of sinapaldehyde in LPS-stimulated Raw 264.7 macrophages. Molecules. 25(18): 4089. https://doi.org/10.3390/molecules25184089
Carvalho, A.P.A. de and Conte-Junior, C.A. 2021. Health benefits of phytochemicals from Brazilian native foods and plants: Antioxidant, antimicrobial, anti-cancer, and risk factors of metabolic/endocrine disorders control. Trends in Food Science and Technology. 5(111): 534-548. https://doi.org/10.1016/j.tifs.2021.03.006
Chang, S.H., Lin, Y.Y., Wu, G.J., Huang, C.H., and Tsai, G.J. 2019. Effect of chitosan molecular weight on anti-inflammatory activity in the RAW 264.7 macrophage model. International Journal of Biological Macromolecules. 131: 167-175. https://doi.org/10.1016/j.ijbiomac.2019.02.066
Chaudhary, P., Janmeda, P., Docea, A.O., Yeskaliyeva, B., Abdull Razis, A.F., Modu, B., Călina, D., and Sharifi-Rad, J. 2023. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Frontiers in Chemistry. 11: 1158198. https://doi.org/10.3389/fchem.2023.1158198
Chunduri, R.H.B. and Dannana, G.S. 2016. Development and validation of a reliable and rapid LC-MS/MS method for simultaneous quantification of sacubitril and valsartan in rat plasma and its application to a pharmacokinetic study. Biomedical Chromatography. 30: 1467-1475. https://doi.org/10.1002/bmc.3707
El-Askary, H., Handoussa, H., Badria, F., El-Khatib, A.H., Alsayari, A., Linscheid, M.W., and Abdel Motaal, A. 2019. Characterization of hepatoprotective metabolites from Artemisia annua and Cleome droserifolia using HPLC/PDA/ESI/MS-MS. Revista Brasileira de Farmacognosia. 29: 213-220. https://doi.org/10.1016/j.bjp.2018.10.001
Ghasemi, M., Turnbull, T., Sebastian, S., and Kempson, I. 2021. The MTT assay: Utility, limitations, pitfalls, and interpretation in bulk and single-cell analysis. International Journal of Molecular Sciences. 22(23): 12827. https://doi.org/10.3390/ijms222312827
Ghasemi-Dehnoo, M., Lorigooini, Z., Amini-Khoei, H., Sabzevary-Ghahfarokhi, M., and Rafieian-Kopaei, M. (2023). Quinic acid ameliorates ulcerative colitis in rats, through the inhibition of two TLR4-NF-κB and NF-κB-INOS-NO signaling pathways. Immunity, Inflammation and Disease. 11(8): e926. https://doi.org/10.1002/iid3.926
Guo, X., Zhang, M., Cai, H., Jiang, X., Zhou, S., Gong, W., Wei, Z., Qiao, W., and Jia, B. 2025. Combining network pharmacology, molecular docking, and experimental verification to investigate the anti-rheumatoid arthritis effect and mechanism of Baeckea frutescens leaves. Food Science and Human Wellness. 14: 21-22. https://doi.org/10.26599/fshw.2024.9250166
Huong, D.T.L., Xuan Duc, D., and The Son, N. 2023. Baeckea frutescens L.: A review on phytochemistry, biosynthesis, synthesis, and pharmacology. Natural Product Communications. 18: 1-22. https://doi.org/10.1177/1934578X231189143
Hwang, K.A., Hwang, Y.J., and Song, J. 2016. Antioxidant activities and oxidative stress inhibitory effects of ethanol extracts from Cornus officinalis on raw 264.7 cells. BMC Complementary and Alternative Medicine. 16: 196. https://doi.org/10.1186/s12906-016-1172-3
Ji, X., Du, W., Che, W., Wang, L., and Zhao, L. 2023. Apigenin inhibits the progression of osteoarthritis by mediating macrophage polarization. Molecules. 28(7): 2915. https://doi.org/10.3390/molecules28072915
Jiang, H., Zhang, M., Qin, L., Wang, D., Yu, F., Liang, W., Song, C., and Granato, D. 2020. Chemical composition of a supercritical fluid (Sfe-CO2) extract from Baeckea frutescens L. leaves and its bioactivity against two pathogenic fungi isolated from the tea plant (Camellia sinensis (L.) O. Kuntze). Plants. 9(9): 1119. https://doi.org/10.3390/plants9091119
Jomova, K., Raptova, R., Alomar, S.Y., Lawson, M., Valko, M., and Saso, L. 2023. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Archives of Toxicology. 97(8): 2499-2574. https://doi.org/10.1007/s00204-023-03562-9
Kamarazaman, I.S., Ali, A.A., Mohamad, N.A., Saad, N.C., Ali, P.R., and Halim, H. 2023. In vitro wound healing evaluation, antioxidant and chemical profiling of Baeckea frutescens leaves ethanolic extract. Arabian Journal of Chemistry. 15: 103871. https://doi.org/10.1016/j.arabjc.2022.103871
Kamarazaman, I.S., Ling, S.K., Nik Hasan, M.K., Basherudin, N., Mohd Kasim, N.A., Ali, A.A., Ramli, S., Maniam, S., James, R.J., Rojsitthisak, P., and Halim, H. 2024a. Baeckea frutescens L. promotes wound healing by upregulating expression of TGF-β, IL-1β, VEGF and MMP-2. Saudi Pharmaceutical Journal. 32(7): 102110. https://doi.org/10.1016/j.jsps.2024.102110
Kamarazaman, I.S., Nik Hasan, M.K., Adnan, N.N., Khotib, S.S., Maniam, S., and Halim, H. 2024b. Evaluation of cytotoxicity and migratory effect of aqueous extract of Baeckea frutescens Lin. on keratinocytes. Science Letters. 18(1): 28-38. https://doi.org/10.24191/sl.v18i1.24121
Kim, M.J., Jeong, S.M., Kang, B.K., Kim, K.B.W.R., and Ahn, D.H., 2019. Anti-inflammatory effects of grasshopper ketone from Sargassum fulvellum ethanol extract on lipopolysaccharide-induced inflammatory responses in Raw 264.7 cells. Journal of Microbiology and Biotechnology. 29: 820-826. https://doi.org/10.4014/jmb.1901.01027
Lin, X., Zhang, J., Fan, D., Hou, J., Wang, H., Zhu, L., Tian, R., An, X., and Yan, M., 2021. Frutescone O from Baeckea frutescens blocked TLR4-mediated Myd88/NF-κB and MAPK signaling pathways in LPS induced RAW264.7 macrophages. Frontiers in Pharmacology. 12: 1-10. https://doi.org/10.3389/fphar.2021.643188
Liu, J., Han, X., Zhang, T., Li, Y., and Wang, Z. 2023. Reactive oxygen species (ROS) scavenging biomaterials for anti-inflammatory diseases: From mechanism to therapy. Journal of Hematology and Oncology. 16(1): 116. https://doi.org/10.1186/s13045-023-01512-7
Liu, L.J., Li, H.F., Xu, F., Wang, H.Y., Zhang, Y.F., Liu, G.X., Shang, M.Y., Wang, X., and Cai, S.Q. 2020. Exploring the in vivo existence forms (23 original constituents and 147 metabolites) of Astragali radix total flavonoids and their distributions in rats using HPLC-DAD-ESI-IT-TOF-MSn. Molecules. 25(23): 5560. https://doi.org/10.3390/molecules25235560
Maia, C.M.A., Vasconcelos, P.G.S., Pasetto, S., Verri, W.A., Júnior, A.L.R., and Azevedo, E.P. 2024. Anadenanthera colubrina regulated LPS-induced inflammation by suppressing NF-κB and p38-MAPK signaling pathways. Scientific Reports. 14: 16028. https://doi.org/10.1038/s41598-024-66590-0
Malcangi, G., Inchingolo, A.M., Casamassima, L., Trilli, I., Ferrante, L., Inchingolo, F., Palermo, A., Inchingolo, A.D., and Dipalma, G. 2025. Effectiveness of herbal medicines with anti-inflammatory, antimicrobial, and antioxidant properties in improving oral health and treating gingivitis and periodontitis: A systematic review. Nutrients. 17(5): 762. https://doi.org/10.3390/nu17050762
Marasri, P., Sookkhee, S., Wanachantararak, P., and Owittayakul, D. 2024. Anti-inflammatory activity and wound healing ability of coconut oil mouthwash on gingival fibroblast cell in vitro. Natural and Life Sciences Communications. 23(4): e2024059. https://doi.org/10.12982/NLSC.2024.059
Navanesan, S., Wahab, A.N., Manickam, S., and Sim, K.S., 2015. Evaluation of selected biological capacities of Baeckea frutescens. BMC Complementary and Alternative Medicine. 15: 186. https://doi.org/10.1186/s12906-015-0712-6
Nigam, M., Punia, B., Dimri, D.B., Mishra, A.P., Radu, A.-F., and Bungau, G. 2025. Reactive oxygen species: A double-edged sword in the modulation of cancer signaling pathway dynamics. Cells. 14(15): 1207. https://doi.org/10.3390/cells14151207
Niriella, M.A., Fernando, P.M., and Pathmeswaran, A. 2025. Epidemiological profile of inflammatory bowel disease in South Asia: A systematic review and meta-analysis. Heliyon. 11(2): e25420. https://doi.org/10.1016/j.heliyon.2025.e41840
Nisa, K., Nurhayati, S., Apriyana, W., and Indrianingsih, A.W. 2017. Investigation of total phenolic and flavonoid contents, and evaluation of antimicrobial and antioxidant activities from Baeckea frutescens extracts. IOP Conference Series: Earth and Environmental Science. 101: 012002. https://doi.org/10.1088/1755-1315/101/1/012002
Novilla, A., Djamhuri, D.S., Nurhayati, B., Rihibiha, D.D., Afifah, E., and Widowati, W. 2017. Anti-inflammatory properties of Oolong tea (Camellia sinensis) ethanol extract and epigallocatechin gallate in LPS-induced RAW 264.7 cells. Asian Pacific Journal of Tropical Biomedicine. 12: 1005-1009. https://doi.org/10.1016/j.apjtb.2017.10.002
Park, Y., Paing, Y.M.M., Cho, N., Kim, C., Yoo, J., Choi, J.W., and Lee, S.H. 2024. Quinic acid alleviates behavioral impairment by reducing neuroinflammation and MAPK activation in LPS-treated mice. Biomolecules and Therapeutics. 32(3): 309-318. https://doi.org/10.4062/biomolther.2023.184
Piazza, S., Martinelli, G., Maranta, N., Pozzoli, C., Fumagalli, M., Nicolaci, V., Sonzogni, E., Colombo, L., Sangiovanni, E., and Dell'Agli, M. 2024. Investigation into the anti-acne effects of Castanea sativa Mill leaf and its pure ellagitannin castalagin in Hacat cells infected with Cutibacterium acnes. International Journal of Molecular Sciences. 25(9): 4764. https://doi.org/10.3390/ijms25094764
Rafińska, K., Pomastowski, P., Rudnicka, J., Krakowska, A., Maruśka, A., Narkute, M., and Buszewski, B. 2019. Effect of solvent and extraction technique on composition and biological activity of Lepidium sativum extracts. Food Chemistry. 289: 16-25. https://doi.org/10.1016/j.foodchem.2019.03.025
Razmavar, S., Abdulla, M.A., Ismail, S.B., and Hassandarvish, P. 2014. Antibacterial activity of leaf extracts of Baeckea frutescens against methicillin-resistant Staphylococcus aureus. BioMed Research International. 2014: 521287. https://doi.org/10.1155/2014/521287
Saleh, H.A., Yousef, M.H., and Abdelnaser, A. 2021. The anti-inflammatory properties of phytochemicals and their effects on epigenetic mechanisms involved in TLR4/NF-κB-mediated inflammation. Frontiers in Immunology. 12: 606069. https://doi.org/10.3389/fimmu.2021.606069
Samtiya, M., Aluko, R.E., Dhewa, T., and Moreno-Rojas, J.M. 2021. Potential health benefits of plant food-derived bioactive components: An overview. Foods. 10(4): 839. https://doi.org/10.3390/foods10040839
Shahruzaman, S.H., Mustafa, M.F., Ramli, S., Fakurazi, S., and Maniam, S. 2019. The cytotoxic effect and glucose uptake modulation of Baeckea frutescens on breast cancer cells. BMC Complementary and Alternative Medicine. 19: 220. https://doi.org/10.1186/s12906-019-2628-z
Shahruzaman, S.H., Yusof, F.Z., Maniam, S., Fakurazi, S., and Maniam, S. 2021. The cytotoxic effect of Baeckea frutescens extracts in eliminating hypoxic breast cancer cells. BMC Complementary and Alternative Medicine. 21: 245. https://doi.org/10.1186/s12906-021-03417-9
Singh, A., Bajpai, V., Kumar, S., Sharma, K.R., and Kumar, B. 2016. Profiling of gallic and ellagic acid derivatives in different plant parts of Terminalia arjuna by HPLC-ESI-QTOF-MS/MS. Natural Product Communications. 11: 239-244. https://doi.org/10.1177/1934578x1601100227
Sulaiman, C.T., Ramesh P.R., Mahesh K., Madhu K.M., Anandan E.M., Praveen M., and Balachandran, I. 2020. Chemical profiling of a polyherbal formulation by tandem mass spectroscopic analysis with multiple ionization techniques. Future Journal of Pharmaceutical Sciences. 6: 4-11. https://doi.org/10.1186/s43094-020-00062-w
World Health Organization. 2022. Noncommunicable diseases. https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases
Yahaya, Z.S., Adamu, U., Iliyasu, U., Orugun, O.A., Kurfi, F.S., and Dagogot, C.N. 2024. Development of a palm kernel-based emulgel loaded with Mitracarpus scarber leaf extracts for effective anti-inflammatory activity. Natural and Life Sciences Communications. 23(1): e2024001. https://doi.org/10.12982/NLSC.2024.001
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Nurhanani Ayub1, Salfarina Ramli1,2, Ihsan Safwan Kamarazaman3, Evana Kamarudin4, Pornchai Rojsitthisak5,6, and Hasseri Halim1, 2, *
1 Faculty of Pharmacy, Universiti Teknologi MARA Selangor, 42300 Puncak Alam, Malaysia.
2 Integrative Pharmacogenomic Institute (iPROMISE), Universiti Teknologi MARA Selangor, 42300 Puncak Alam, Malaysia.
3 Forest Research Institute Malaysia, 52109 Selangor Darul Ehsan, Malaysia.
4 Centre for Medical Laboratory Technology Studies, Faculty of Health Sciences, Universiti Teknologi MARA, Puncak Alam Campus, 42300 Selangor, Malaysia.
5 Center of Excellence in Natural Products for Ageing and Chronic Diseases, Faculty of Pharmaceutical Sciences, Chulalongkorn University, Bangkok 10330, Thailand.
6 Department of Food and Pharmaceutical Chemistry, Faculty of Pharmaceutical Sciences, Chulalongkorn University, Bangkok 10330, Thailand.
Corresponding author: Hasseri Halim, E-mail: hasseri2945@uitm.edu.my
ORCID iD:
Nurhanani Ayub: https://orcid.org/0000-0002-9579-0361
Salfarina Ramli: https://orcid.org/0000-0002-4001-0751
Ihsan Safwan Kamarazaman: https://orcid.org/0000-0001-5664-2019
Evana Kamarudin: https://orcid.org/0000-0001-7116-0368
Pornchai Rojsitthisak: https://orcid.org/0000-0003-1391-6993
Hasseri Halim: https://orcid.org/0000-0002-2983-2607
Total Article Views
Editor: Nisit Kittipongpatana,
Chiang Mai University, Thailand
Article history:
Received: April 23, 2025;
Revised: August 25, 2025;
Accepted: November 14, 2025;
Online First: November 28, 2025

