
Toxicity of Muntingia calabura Extract on Rasbora lateristriata as Base for Biomedical Phytochemicals Screening
Hayu Swari Allimi and Bambang Retnoaji *Published Date : November 17, 2025
DOI : https://doi.org/10.12982/NLSC.2026.018
Journal Issues : Number 1, January-March 2026
Abstract Muntingia calabura is widely used in traditional medicine due to its bioactive compounds, yet its potential toxic effects remain poorly understood. This study evaluated the embryotoxicity of M. calabura extract using the Indonesian endemic fish Rasbora lateristriata as a model. The use of R. lateristriata not only reflects regional biodiversity but also provides a complementary alternative to zebrafish for future biomedical and toxicological applications. The experiment followed OECD Guideline No. 236 (Fish Embryo Acute Toxicity Test), with embryos exposed to 0, 5, 7.5, and 10 ppm extract concentrations for 96 hours. Observations at 24, 48, 72, and 96 hours included assessments of morphology, survival, heart rate, and hatching. The extract induced morphological abnormalities and significantly reduced embryo viability, indicating potential toxic effects during early development.
Keywords: Biochemicals, Biomedic, Fish embryo toxicity, Muntingia calabura, Rasbora lateristriata
Graphical Abstract:
Funding: This research was funded by the Indonesia Endowment Fund for Education (LPDP), Ministry of Finance of the Republic of Indonesia.
Citation: Allimi, H.S. and Retnoaji, B. 2026. Toxicity of Muntingia calabura Extract on Rasbora lateristriata as base for biomedical phytochemicals screening. Natural and Life Sciences Communications. 25(1): e2026018.
INTRODUCTION
Muntingia calabura is a plant widely used in traditional medicine in Peru, Colombia, Mexico, Vietnam, and the Philippines. The roots, fruits, leaves an stems of M. calabura have been untilized for medicine purpose (Mahmood et al., 2014). M. calabura contains secondary metabolite such as flavonoids, triterpenoids, tannins, alkaloids, saponins, and polyphenolic quinones (Mahmood et al., 2014; Solikhah and Solikhah, 2021). These bioactive compounds are associated with gastroprotective (Zakaria et al., 2014), anti-inflammatory (Preethi et al., 2012), anti-proliferative, antioxidant (Zakaria et al., 2011), antimicrobial (Zakaria et al., 2015), and anti-diabetic effects (Gurning and Sinaga, 2020). Despite these promising pharmacological properties, studies on the potential toxic effects of M. calabura, particularly during early vertebrate development, remain very limited. This lack of toxicity data raises concerns regarding its safety profile for broader medicinal applications.
Rasbora lateristriata, commonly known as the yellow rasbora, is an endemic freshwater fish species from Indonesia. It is distributed across Sumatra, Java, Borneo, Bali, Nusa Tenggara, and Sulawesi (Budiharjo, 2002). This species is highly sensitive to environmental changes and has been proposed as a bioindicator for water pollution (Retnoaji et al., 2016). Its natural habitat typically consists of fast-flowing streams with gravel-rich substrates (Djumanto and Setyawan, 2009).
R. lateristriata has been utilized as a model organism in several studies, including evaluations of the toxicological effects of mangosteen peel simplicia (Retnoaji et al., 2023), paracetamol (Septriani et al., 2023), and chlorpyrifos (Nita and Retnoaji, 2022). While zebrafish (Danio rerio) embryos remain the widely used model for toxicity studies, R. lateristriata belongs to the same family (Cyprinidae) and subfamily (Danioninae) as zebrafish (Tang et al., 2010), making it a taxonomically relevant alternative. However, its application in assessing the toxicity of medicinal plant extracts has not yet been reported, leaving an important gap in the use of local fish species as experimental models.
In addition to its taxonomic proximity to zebrafish, R. lateristriata offers several advantages as a potential model organism. It is cost-effective and readily available due to its abundance in natural freshwater habitats, particularly benefiting laboratories in Southeast Asia. Unlike zebrafish, which originate from temperate regions, R. lateristriata is naturally adapted to tropical freshwater ecosystems, making it highly relevant for ecotoxicological, physiological, and ecological studies in the tropics. As an endemic species, its use also supports biodiversity conservation and highlights the scientific value of underutilized tropical fauna. Therefore, R. lateristriata not only a practical and accessible alternative to zebrafish but also promotes research that is contextually grounded in tropical biodiversity and the utilization of native biological resources.
Furthermore, an individual R. lateristriata is capable of producing up to 1,000 eggs per spawning event (Djumanto and Setyawan, 2009), ensuring a sufficient supply of embryos for toxicity assays. The species' relatively small body size and high tolerance to aquarium conditions also facilitate laboratory-scale maintenance (Kusuma et al., 2016).
Accordingly, this study employs R. lateristriata embryos as a complementary model to assess the sublethal toxic effects of M. calabura leaf extract. By addressing the current lack of toxicity data for this medicinal plant and introducing the novel application of an endemic fish species, this work provides new insights into the safety evaluation of herbal medicines and underscores the importance of R. lateristriata as both a biodiversity-relevant species and a practical alternative to zebrafish in experimental research.
MATERIALS AND METHODS
Plant materials
Muntingia calabura leaves were collected in Yogyakarta, Indonesia, in 2023. The plant specimen was authenticated and deposited in the Laboratory of Plant Systematics, Faculty of Biology, Universitas Gadjah Mada, under specimen number 00678/S.Tb./VI/2024.
Plant extraction
Muntingia calabura leaves were dried and subsequently ground into powder. A total of 500 g of the powdered leaves was macerated in 96% ethanol (PT Brataco, Jakarta, Indonesia) for 5 days. The mixture was then filtered through Whatman No. 1 filter paper to obtain the filtrate. The solvent was then removed from the filtrate using a rotary evaporator at 50°C, yielding the crude extract (Gurning and Sinaga, 2020).
Animal ethics
This study utilized local R. lateristriata, bred at the Faculty of Biology, Universitas Gadjah Mada (UGM). All experimental procedures were conducted in accordance with ethical guidelines and approved by the Committee of Ethical Clearance for Pre-clinical Research of Integrated Laboratory of Research and Testing, UGM, ethical clearance number 00043/V/UN1/LPPT/EC/2023.
Maintenance of Rasbora lateristriata and egg collection
Rasbora lateristriata were maintained under standard laboratory conditions. The fish were kept under a photoperiod of 14 h light/10 h dark photoperiod, at a temperature of 27–28.5 °C, with dissolved oxygen levels of 6–8 mg/L, and were fed PF500 pellets three times daily. The broodstock were reared until ready for spawning. Spawning was conducted in stainless steel containers equipped with coconut fiber substrate and aeration. The female-to-male ratio of 1:2 was maintained, and fertilization was induced by natural light exposure in the early morning. The eggs were collected from the spawning container, transferred to petri dishes, and carefully selected, with only high-quality fertilized eggs used in the experiments.
Toxicity test
Toxicity testing on R. lateristriata embryos was conducted according to OECD Guideline No. 236 (Fish Embryo Toxicity Test), with necessary modifications. In line with the guideline, a total of 80 fertilized eggs obtained from spawning were divided into four treatment groups, each consisting of 20 embryos as replicates. The test concentrations were 0, 5, 7.5, and 10 ppm, and exposure was carried out for 96 hours. Although the number of embryos per group was relatively small, OECD 236 specifies a minimum of 20 embryos per concentration to ensure reliable statistical evaluation, and our design complied with this requirement.
Embryo observation
Fish embryos were observed for morphological alterations, survival, heartbeat rate, and hatching in all groups at 24, 48, 72, and 96 hours post-exposure to M. calabura extract using a light microscope.
Embryo morphology observation
Morphological abnormalities in embryos were observed individually with a Leica DM750 microscope at 4x magnification every 24 hours.
Fish embryo survival rate
For survival rate determination, eggs showing coagulation, lack of somite formation, non-detachment of the tail, or lack of heartbeat are considered dead. The survival rate was calculated using the following formula:
SR = Nt / No x 100%
SR = Survival rate
Nt = number of fish alive during the study
No = number of fish at the beginning of the study
Fish embryo heartbeat rate
Heartbeat rates was determined by counting the beats of individual embryos in 1 minute under a Leica DM750 microscope at 40x magnification, with observations conducted every 24 hours.
Fish embryo hatching rate
Hatching rate was determined by counting embryos that had completely hatched and were free from the chorion. The hatching rate was calculated using the following formula:
HR = Nt / No x 100%
HR = Hatching rate
Nt = number of eggs hatched during the study
No = number of eggs at the beginning of the study
Statistical analysis
Statistical analysis were performed using GraphPad Prism version 10, which was also used to generate the graphs. Since the data did not meet the assumption of homogeneity, a non-parametric Kruskal–Wallis test was applied, followed by Dunn’s post hoc test for pairwise comparisons.
RESULTS
Effect of M. calabura on embryo morphology
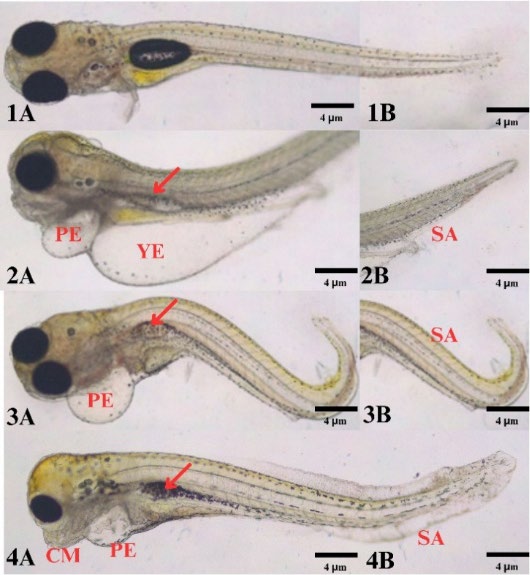
Figure 1. Morphological features of R. lateristriata embryos after exposure to M. calabura leaf extract. (1A–B) Normal embryo showing typical morphology. (2A–4A) Embryos exhibiting pericardial edema (PE), yolk edema (YE), craniofacial malformation (CM) and collapsed swim bladder (Red arrow). (2B–4B) Embryos showing spinal abnormalities (SA).
Table 1. The embryonic abnormalities of the control and treated embryo at the 96h of exposure.
|
Concentration |
The embryonic abnormalities |
|
|||||||||||
|
Coagulation |
Pericardial edema |
Yolk edema |
Spinal abnormality |
Craniofacial malformation |
Blood congestion |
Collapsed Swim bladder |
|||||||
|
0 ppm |
1 |
- |
- |
- |
- |
- |
- |
||||||
|
5 ppm |
4 |
3 |
3 |
2 |
1 |
2 |
2 |
||||||
|
7.5 ppm |
5 |
5 |
- |
5 |
- |
1 |
3 |
||||||
|
10 ppm |
7 |
6 |
4 |
6 |
- |
- |
6 |
||||||
Exposure to M. calabura leaf extract at certain concentrations induced embryonic abnormalities such as coagulation, yolk and pericardial edema, spinal abnormality, craniofacial malformations, and collapsed swim bladders (Figure 1 and Table 1). No abnormalities were observed in the control group, except for one coagulated egg. At 5 ppm, embryos showed multiple abnormalities, which became more frequent at 7.5 ppm. The 10 ppm extract resulted in more severe phenotypic abnormalities and a greater number of affected embryos. These results suggest that M. calabura extract affected fish embryonic abnormalities in dose dependent manner.
Effects of M. calabura on embryo survival rate
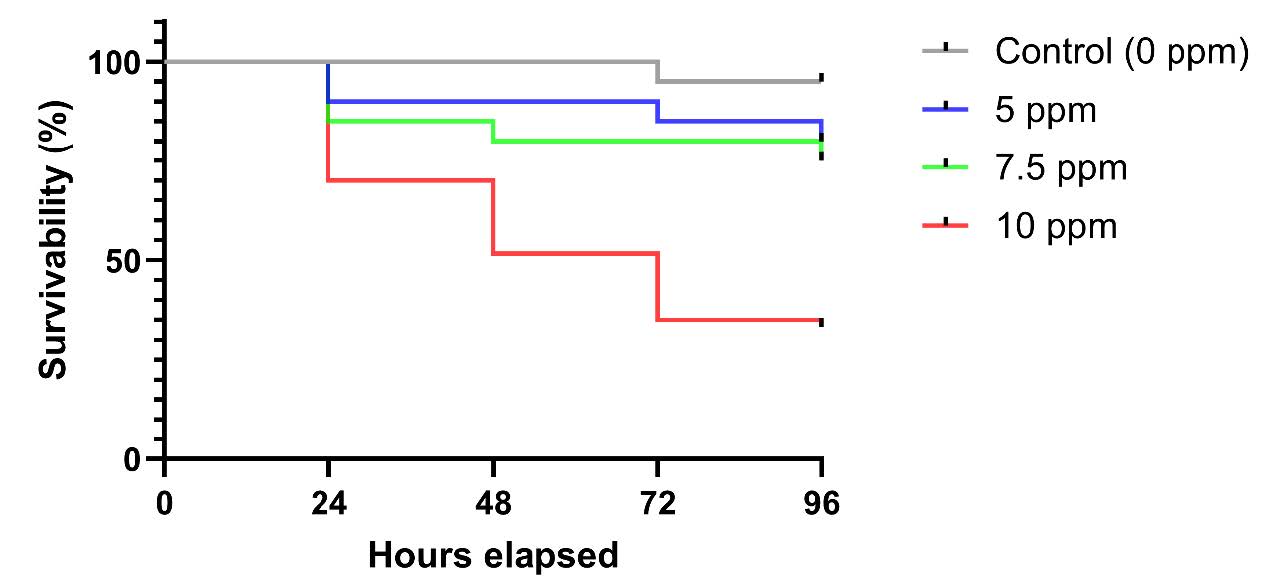
Figure 2. Survival rate of R. lateristriata embryos exposed to M. calabura leaf extract at different concentrations and time points.
The bar chart illustrates the survival rate of R. lateristriata embryos following exposure to plant extract at concentrations of 5, 7.5, and 10 ppm. Observations were recorded at 24, 48, 72, and 96 hours post-fertilization (hpf). Each bar represents the mean survival percentage for a given concentration at each time point. Survival rates were calculated based on the number of surviving embryos relative to the total number of exposed embryos. The lowest survival rate was observed in the 10 ppm group. A marked reduction in embryo survival was observed across all extract-treated groups as early as 24 hpf, suggesting a potential acute toxic effect of the extract.
Exposure to M. calabura leaf extract reduced embryo survival in both dose and time-dependent manner. At 5 ppm, mortality reached 10% by 48 hpf and increased to 20% by 96 hpf. At 7.5 ppm, mortality began at 15% at 24 hpf and gradually increased to 25% by 96 hpf. The 10 ppm resulted in the most substantial effect, with 30% embryo mortality observed as early as 24 to 48 hpf, rising to 35% by 96 hpf. In contrast, the control group maintained consistently high survival rate (Figure 2). These results demonstrate that M. calabura extract exposure compromises embryonic viability in a concentration and exposure time-dependent manner.
Effect of M. calabura on embryo heartbeat rate
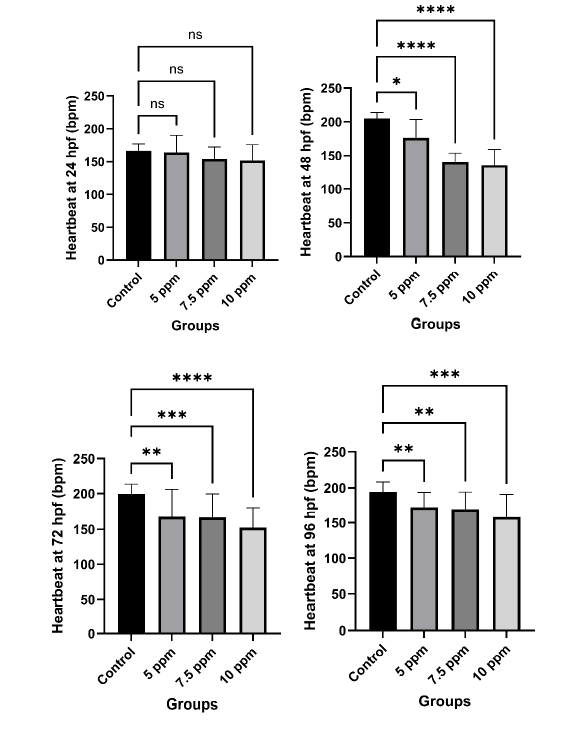
Figure 3. Heartbeat rates of R. lateristriata embryos exposed to M. calabura leaf extract.
The figure shows heartbeat rates (bpm) of embryos exposed to 5, 7.5, and 10 ppm extract at 24, 48, 72, and 96 hpf. Each panel represents one time point. Data are presented as mean ± SD. Statistical analysis was performed using the Kruskal–Wallis analysis. Asterisks (*) indicate significant differences (P<0.05), and “ns” denotes non-significance. No significant differences in heartbeat were observed at 24 hpf, but concentration-dependent reductions appeared from 48 hpf onward. The control group maintained relatively stable heartbeat rates across all time points, whereas extract-treated groups showed a decrease in heartbeat, especially at higher concentrations and longer exposures. These findings suggest potential cardiotoxic effects of M. calabura extract during early embryonic development.
Heartbeat rates of R. lateristriata embryos decreased in a dose and time dependent manner following exposure to M. calabura extract (Figure 3). At 24 hpf, no significant differences were observed between groups. However, from 48 hpf onwards, extract treated embryos showed markedly reduced heartbeat rates compared to control, with strongest suppression observed at 10 ppm. These results demonstrate a clear dose and time dependent suppression of cardiac activity in embryos exposed to M. calabura extract, indicating its potential cardiotoxic effects during early development.
Effect of M. calabura on embryo hatching rate
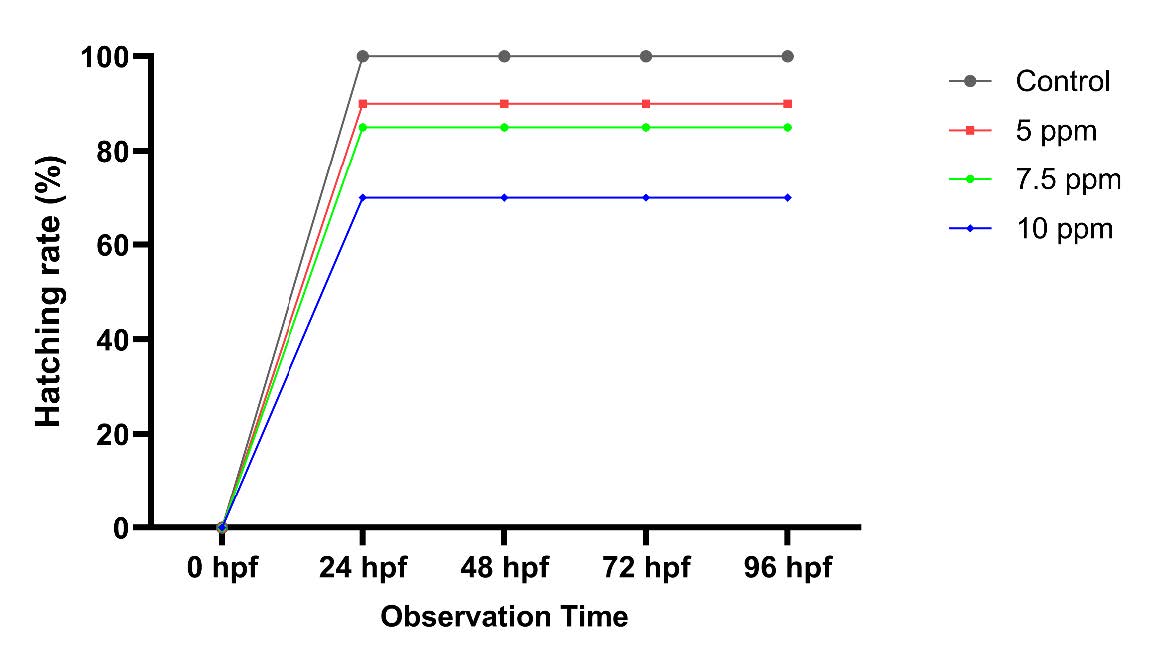
Figure 4. Hatching rates of R. lateristriata embryos exposed to M. calabura leaf extract at different concentrations and developmental stages.
Each line represents the percentage of successfully hatched embryos relative to the total number of embryos per group. The control group (no extract exposure) maintained consistently high hatching rates, while extract-treated groups showed reduced hatching percentages. A dose and time dependent decrease in hatching success was particularly evident in the 10 ppm group, indicating potential interference with normal embryogenesis.
At 24 hpf, hatching had initiated across all groups; however, hatchability decreased with increasing extract concentration (figure 4). While all control embryos hatched successfully, exposure to 5, 7.5, and 10 ppm resulted in hatching failure rates of 10%, 15%, and 30%, respectively. These results suggest that M. calabura leaf extract interferes with the hatching process of R. lateristriata embryos in a concentration-dependent manner, possibly by affecting chorion softening or embryonic movement required for hatching.
DISCUSSION
The use of fish as complementary animal models in toxicity testing has gained increasing attention due to its potential to enhance the reliability and predictivity of toxicity assessments, especially when compared to traditional rodent models such as mice and rats. One ethical and efficient approach is the use of fish embryos to observe toxic effects, including sublethal parameters such as morphological alterations, heartbeat rate, and hatching rate. This method enables early detection of the toxic potential of active compounds found in herbal preparations, including those used in traditional medicine (Khabib et al., 2022).
In this study, M. calabura extract was shown to affect the early development of R. lateristriata embryos, leading to a range of morphological abnormalities. Rather than being random defects, these abnormalities point to interference with specific developmental pathways. Pericardial edema and yolk edema are two types of edema commonly found in fish embryo toxicity studies. Edema reflects disturbances in fluid regulation and vascular permeability, while spinal curvature is consistent with altered acetylcholine receptor signaling and neuroinflammation (Wiegand et al., 2023). Spinal abnormality is one of the irreversible abnormalities. There are three types of spinal abnormalities: lordosis (inward curvature), kyphosis (outward curvature), and scoliosis (sideways curvature). Exposure to compound extracts can affect the expression of acetylcholine receptors and neuroinflammation, leading to spinal curvature (von Hellfeld et al., 2020). Craniofacial malformations are a heterogeneous group of developmental defects of the skull and face. These abnormalities are irreversible (Raterman et al., 2020). Craniofacial malformations, observed at higher extract concentrations, may result from impaired neural crest migration or disruption of retinoic acid signaling, processes essential for cranial and facial development (Liu et al., 2020; Huang et al., 2021). In other fish species, such as zebrafish, cranial malformation occurs at 48 h embryo (Pratama et al., 2022). Similarly, impaired swim bladder formation suggests disruption of organogenesis necessary for buoyancy control (Pelster, 2021). Together, these mechanistic insights suggest that secondary metabolites of M. calabura, such as flavonoids and alkaloids, may interfere with neurodevelopmental and morphogenetic processes, leading to irreversible developmental defects.
Beyond morphology, M. calabura exposure reduced embryo survival in a concentration-dependent manner. This effect is likely linked to amylase inhibitors in the extract, which act as carbohydrate blockers and disrupt glucose metabolism (Gunny et al., 2024). Amylase inhibitors act as carbohydrate blockers, limiting carbohydrate digestion and absorption (Gong et al., 2020). Since glucose availability is critical for embryonic growth and energy supply (Purcell and Moley, 2009), interference with carbohydrate digestion may compromise survival by impairing metabolic energy production.
Cardiac activity was also affected, with a significant decrease in embryo heartbeat rate following exposure to M. calabura extract. This effect may be attributed to the presence of flavonoids, which have been reported to act as natural angiotensin-converting enzyme (ACE) inhibitors (Buhian et al., 2016). Because ACE regulates arterial blood pressure and cardiovascular homeostasis (Nileeka and Vasantha, 2011), flavonoid-mediated ACE inhibition may reduce cardiac output during early development. Such interference with cardiovascular regulation underscores the potential embryotoxicity and cardiotoxicity of the extract, even at sublethal doses.
Hatching success was also compromised. Embryos exposed to the extract frequently failed to hatch due to premature mortality, which prevented both chorionase secretion by hatching gland cells and the embryonic movements necessary to break the chorion (Ługowska and Sarnowski, 2011; Korwin-Kossakowski, 2012). The average hatching period for R. daniconius embryo is 24-25 hpf (Mahapatra, 2016). This indicates that the extract disrupts critical developmental milestones beyond morphological changes alone.
Consistent with previous studies showing M. calabura leaf extract to be toxic at relatively low LC50 values (Aarthi et al., 2021), our results confirm that even sublethal concentrations (5, 7.5 and 10 ppm) can impair multiple aspects of early embryogenesis. These results indicate that, despite its widespread use in traditional medicine, M. calabura carries potential toxicity at certain levels, which should be carefully considered, particularly in the development of natural products. Therefore, this study supports the importance of integrating sublethal fish toxicity data into the safety regulation framework for biotherapeutic compounds and drug development. Furthermore, the findings highlight important biomedical implications. Although M. calabura has long been used in traditional medicine, the observed embryotoxic effects raise concerns about its safety, particularly at certain concentrations. This suggests that uncontrolled or excessive use of M. calabura preparations may pose risks to human health. From a drug discovery perspective, the toxic effects observed at sublethal levels indicate that careful dose optimization, compound isolation, and further pharmacological testing are essential before M. calabura derived compounds can be developed into safe therapeutic agents. Therefore, this study not only provides baseline evidence of potential developmental toxicity but also underscores the need for comprehensive safety evaluations to balance the therapeutic potential of M. calabura with its toxicological risks.
CONCLUSION
The ethanolic extract of M. calabura leaves exhibited dose-dependent toxic effects on the early development of R. lateristriata embryos, including morphological abnormalities, and reductions in survival, heartbeat, and hatching rates. Consistent with previous reports of its toxic potential, these findings underscore the need to evaluate sublethal concentrations in the context of biotherapeutic development. Moreover, the use of fish embryos as complementary animal models represents an ethical and reliable approach for early toxicity screening, supporting the integration of such data into safety regulations for natural product–derived therapeutics.
ACKNOWLEDGEMENTS
The authors extended their gratitude to the technician and Laboratory Head of Laboratory of Animal Structure and Development, Faculty of Biology, Universitas Gadjah Mada, and all the parties who contributed to completing this research.
AUTHOR CONTRIBUTIONS
Hayu Swari Allimi assisted in conducting the experiments, performed the statistical analysis, data visualization, and wrote the manuscript. Bambang Retnoaji designed and wrote the manuscript. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Aarthi, N., Abinaya, S., Nithyasri, R.N., Thanga Brindha, T., Sneka, P., and Vijina, C.V. 2021. Phytochemical screening and antivectoral activity of Muntingia calabura. Journal of Advanced Applied Scientific Research. 3: 43-48. https://doi.org/10.46947/joaasr352021122
Budiharjo, A. 2002. Selection and potential aquaculture of "wader" fish of the genus Rasbora. Biodiversitas. Journal of Biological Diversity. 3(2): 225-230. https://doi.org/10.13057/biodiv/d030203
Buhian, W.P.C., Rubio, R.O., Valle, D.L., and Martin-Puzon, J.J. 2016. Bioactive metabolite profiles and antimicrobial activity of ethanolic extracts from Muntingia calabura L. leaves and stems. Asian Pacific Journal of Tropical Biomedicine. 6(8): 682-685. https://doi.org/10.1016/j.apjtb.2016.06.006
Djumanto, D. and Setyawan, F. 2009. Food habits of the yellow rasbora, Rasbora lateristriata, (family: cyprinidae) broodfish during moving to spawning ground. Journal of Fisheries Sciences. 1: 107-116. https://doi.org/10.22146/jfs.3027
Gong, L., Feng, D., Wang, T., Ren, Y., Liu, Y., and Wang, J. 2020. Inhibitors of α-amylase and α-glucosidase: Potential linkage for whole cereal foods on prevention of hyperglycemia. Food Science and Nutrition. 8(12): 6320-6337. https://doi.org/10.1002/fsn3.1987
Gunny, A.A.N., Prammakumar, N.K., Ahmad, A.A., Gopinath, S.C.B., Bakar, A.R.A., Musa, H., and Mat, M.H.C. 2024. Exploring antioxidant and antidiabetic potential of Muntingia calabura (Kerukupsiam) leaf extract: In vitro analysis and molecular docking study. Results in Chemistry. 7: 101305. https://doi.org/10.1016/j.rechem.2024.101305
Gurning, K. and Sinaga, D.H. 2020. Characterization and screening of phytochemical secondary metabolite of Seri (Muntingia calabura, L) leaves which is potential as an anti-diabetic based on Indonesian herbal medicine standard. Journal of Drug Delivery and Therapeutics. 10(6): 92-94. https://doi.org/10.22270/jddt.v10i6-s.4458
Huang, W., Wu, T., Au, W.W., and Wu, K. 2021. Impact of environmental chemicals on craniofacial skeletal development: Insights from investigations using zebrafish embryos. Environmental Pollution. 286: 117541. https://doi.org/10.1016/j.envpol.2021.117541
Khabib, M.N.H., Sivasanku, Y., Lee, H.B., Kumar, S., and Kue, C.S. 2022. Alternative animal models in predictive toxicology. Toxicology. 465. https://doi.org/10.1016/j.tox.2021.153053
Korwin-Kossakowski, M. 2012. Fish hatching strategies: A review. Reviews in Fish Biology and Fisheries. 22(1): 225-240. https://doi.org/10.1007/s11160-011-9233-7
Kusuma, W.E., Ratmuangkhwang, S., and Kumazawa, Y. 2016. Molecular phylogeny and historical biogeography of the Indonesian freshwater fish Rasbora lateristriata species complex (Actinopterygii: Cyprinidae): Cryptic species and west-to-east divergences. Molecular Phylogenetics and Evolution. 105: 212-223. https://doi.org/10.1016/j.ympev.2016.08.014
Liu, S., Narumi, R., Ikeda, N., Morita, O., and Tasaki, J. 2020. Chemical-induced craniofacial anomalies caused by disruption of neural crest cell development in a zebrafish model. Developmental Dynamics. 249(7): 794-815. https://doi.org/10.1002/dvdy.179
Ługowska, K. and Sarnowski, P. 2011. Heads or tails-fish hatching. Acta Ichthyologica et Piscatoria. 4(1): 13-17. https://doi.org/10.3750/AIP2011.41.1.03
Mahapatra, B.K. 2016. Breeding and larval rearing of indigenous ornamental fish, Rashbora daniconius (Hamilton) under captivity: A potential indigenous ornamental fish of North East India. Journal of Indian Fisheries Association. 43(2): 37-41.
Mahmood, N.D., Nasir, N.L.M., Rofiee, M.S., Tohid, S.F.M., Ching, S.M., Teh, L.K., Salleh, M.Z., and Zakaria, Z.A. 2014. Muntingia calabura: A review of its traditional uses, chemical properties, and pharmacological observations. Pharmaceutical Biology. 52(12): 1598-1623. https://doi.org/10.3109/13880209.2014.908397
Nileeka Balasuriya, B.W. and Vasantha Rupasinghe, H.P. 2011. Plant flavonoids as angiotensin converting enzyme inhibitors in regulation of hypertension. Functional Foods in Health and Disease. 1(5): 172-188. https://doi.org/10.31989/ffhd.v1i5.132
Nita, J.A.F. and Retnoaji, B. 2022. The effect of chlorpyrifos insecticide on the histological structure of wader pari fish intestine (Rasbora lateristriata Bleeker, 1854). Jurnal Pengelolaan Sumberdaya Alam Dan Lingkungan. 12(1): 1-11. https://doi.org/10.29244/jpsl.12.1.1-11
Pelster, B. 2021. Using the swimbladder as a respiratory organ and/or a buoyancy structure-Benefits and consequences. Journal of Experimental Zoology Part A: Ecological and Integrative Physiology. 335(9): 831-842. https://doi.org/10.1002/jez.2460
Pratama, S.F., Retnoaji, B., and Ana, I.D. 2022. Effects of carbonate hydroxyapatite (CHA) on the development of heart and cranium cartilage of zebrafish (Danio rerio Hamilton, 1882) larvae. Chiang Mai University Journal of Natural Sciences. 21(3): e2022041. https://doi.org/10.12982/CMUJNS.2022.041.
Preethi, K., Premasudha, P., and Keerthana, K. 2012. Anti-inflammatory activity of Muntingia calabura fruits. Pharmacognosy Journal. 4(30): 51-56. https://doi.org/10.5530/pj.2012.30.10
Purcell, S.H. and Moley, K.H. 2009. Glucose transporters in gametes and preimplantation embryos. Trends in Endocrinology and Metabolism. 20(10): 483-489. https://doi.org/10.1016/j.tem.2009.06.006
Raterman, S.T., Metz, J.R., Wagener, F.A.D.T.G., and Von den Hoff, J.W. 2020. Zebrafish models of craniofacial malformations: Interactions of environmental factors. Frontiers in Cell and Developmental Biology. 8: 600926. https://doi.org/10.3389/fcell.2020.600926
Retnoaji, B., Nanda, F., Sartika, D., Eunike, N., Oktaviani, D.D., and Afriani, D. 2016. The effect of volcanic dust on the histological structure of wader pari (Rasbora lateristriata Bleeker, 1854) organs. AIP Conference Proceedings. 1744: 020007. https://doi.org/10.1063/1.4953481
Retnoaji, B., Paramita, P., and Khasanah, L.U. 2023. Mangosteen Garcinia mangostana L. simplicia effect on bone structure and behaviour of wader fish Rasbora lateristriata (Bleeker, 1854) embryo. Indonesian Journal of Pharmacy. 34(2): 182-192. https://doi.org/10.22146/ijp.6348
Septriani, N.I., Saribu, R.L.C.D., Apriliyani, T., Karlina, I., Pusparini, N.A.O., Zusrina, L.M., Sari, R.V.S., Allimi, H.S., Supraitno, M.E., Saeed, F., et al. 2023. Histopathological evaluation of hepatic tissue of yellow Rasbora (Rasbora lateristriata) exposed to paracetamol. Biological Environment and Pollution. 3(1): 8-14. https://doi.org/10.31763/bioenvipo.v3i1.595
Solikhah, T.I. and Solikhah, G.P. 2021. Effect of Muntingia calabura L. leaf extract on blood glucose levels and body weight of alloxan-induced diabetic mice. Pharmacognosy Journal. 13(6): 1450-1455. https://doi.org/10.5530/pj.2021.13.184
Tang, K.L., Agnew, M.K., Hirt, M.V., Sado, T., Schneider, L.M., Freyhof, J., Sulaiman, Z., Swartz, E., Vidthayanon, C., Miya, M., et al. 2010. Systematics of the subfamily Danioninae (Teleostei: Cypriniformes: Cyprinidae). Molecular Phylogenetics and Evolution. 57(1): 189-214. https://doi.org/10.1016/j.ympev.2010.05.021
von Hellfeld, R., Brotzmann, K., Baumann, L., Strecker, R., and Braunbeck, T. 2020. Adverse effects in the fish embryo acute toxicity (FET) test: A catalogue of unspecific morphological changes versus more specific effects in zebrafish (Danio rerio) embryos. Environmental Sciences Europe. 32: 122. https://doi.org/10.1186/s12302-020-00398-3
Wiegand, J., Avila-Barnard, S., Nemarugommula, C., Lyons, D., Zhang, S., Stapleton, H.M., and Volz, D.C. 2023. Triphenyl phosphate-induced pericardial edema in zebrafish embryos is dependent on the ionic strength of exposure media. Environment Internationa. 172: 107757. https://doi.org/10.1016/j.envint.2023.107757
Zakaria, Z.A., Balan, T., Suppaiah, V., Ahmad, S., and Jamaludin, F. 2014. Mechanism(s) of action involved in the gastroprotective activity of Muntingia calabura. Journal of Ethnopharmacology. 151(3): 1184-1193. https://doi.org/10.1016/j.jep.2013.12.045
Zakaria, Z.A., Mohamed, A.M., Jamil, N.S.M., Rofiee, M.S., Hussain, M.K., Sulaiman, M. R., Teh, L.K., and Salleh, M.Z. 2011. In vitro antiproliferative and antioxidant activities of the extracts of Muntingia calabura leaves. American Journal of Chinese Medicine. 39(1): 183-200. https://doi.org/10.1142/S0192415X11008749
Zakaria, Z.A., Ramasamy, K., Ahmat, N., and Roslan Sulaiman, M. 2015. In vitro antimicrobial activity of Muntingia calabura extracts and fractions. African Journal of Microbiology Research. 4(4): 304-308.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Hayu Swari Allimi and Bambang Retnoaji *
Faculty of Biology, Universitas Gadjah Mada, Yogyakarta, Indonesia.
Corresponding author: Bambang Retnoaji, E-mail: bambang.retnoaji@ugm.ac.id
ORCID iD:
Hayu Swari Allimi: https://orcid.org/0009-0006-7234-0305
Bambang Retnoaji: https://orcid.org/0000-0002-0290-9723
Total Article Views
Editor: Nisit Kittipongpatana,
Chiang Mai University, Thailand
Article history:
Received: August 28, 2025;
Revised: October 6, 2025;
Accepted: October 29, 2025;
Online First: November 17, 2025

