
Scanning Electron Microscopic Analysis and Inhibitory Effect of Oroxylum indicum Extract Against Clinical Isolated Penicillin, Aminoglycoside and Lincosamide Resistant Pasteurella multocida
Patchima Sithisarn and Pongtip Sithisarn*Published Date : September 9, 2025
DOI : https://doi.org/10.12982/NLSC.2025.070
Journal Issues : Number 4, October-December 2025
Abstract Pasteurella multocida is a pathogenic bacterium which causes severe diseases in mammals and avian including haemorrhagic septicaemia in cattle and buffaloes and respiratory disease in commercial farm pigs. Oroxylum indicum extracts have previously been reported to promote inhibitory effects against some clinical isolated pathogenic bacteria. In this study, O. indicum fruit extract was qualitatively controlled for total phenolic, total flavonoid and flavone contents determined by Folin-Ciocalteu, aluminium chloride and high performance liquid chromatography (HPLC) methods, respectively. Total phenolic and total flavonoid contents of the extract were 4%w/w gallic acid equivalent (GAE) and 5%w/w quercetin equivalent (QE) of the dried extract, respectively. The contents of the major flavones; baicalin, baicalein and chrysin were found to be 0.40, 0.36 and 1.12 %w/w of dried extract, respectively. The extract showed in vitro antibacterial effects against clinically isolated aminoglycoside and lincosamide resistant Pasteurella multocida with half-maximal inhibitory concentration (IC50) of 14.98 ± 0.35 mg/ml and the minimum inhibitory concentration (MIC) was 29.24 ± 0.05 mg/ml. Scanning electron microscopic analysis of the effects of O. indicum fruit extract against P. multocida suggests the formation of bacterial morphological changes, cell wall disruption, content leakage, abnormal groups of cell aggregations, and cell lysis within 15 h. The results suggest the inhibitory potential of O. indicum fruit extract against antibiotic resistant Pasteurella multocida. The extract could be further developed as an alternative or co-therapy in the treatments of animals and human P. multocida related diseases.
Keywords: Oroxylum indicum, Pasteurella multocida, antibacterial activity, antibiotic resistant
Citation: Sithisarn, P. and Sithisarn, P. 2025. Scanning electron microscopic analysis and inhibitory effect of Oroxylum indicum extract against clinical isolated penicillin, aminoglycoside and lincosamide resistant Pasteurella multocida. Natural and Life Sciences Communications. 24(4): e2025070.
INTRODUCTION
Pasteurella multocida, a gram-negative bacterium, is one of the most important zoonotic and nosocomial bacteria, causing severe respiratory inhibition, soft tissue infection, septicemia, and death in both humans and animals (Nath and Joshi, 2016). P. multocida can cause atrophic rhinitis in pigs when it co-infected with Bordetella bronchiseptica (Yoshimura, 2024), an infectious disease of swine, which can be characterized by serious to mucopurulent nasal discharge, shortening or twisting of the snout, atrophy of the turbinate bones and the reduction of productivity (World assembly of delegates of the OIE, 2012). Progressive atrophic rhinitis is considered a contagious respiratory disease throughout the world where modern pig husbandry is practiced. (Magyar and Lax, 2002). P. multocida can cause several other diseases impacted economic animals such as bovine mastitis in cattle as well as limited diarrhea to growth retardation in swine (Wilkie et al., 2012). Moreover, P. multocida is one of the most common poultry pathogens and a cause of fowl cholera. This fatal disease has a high economic impact on global poultry production (Christensen and Bisgaard, 2000; Herath et al., 2010). This bacterium can also cause respiratory disease complex and dysentery in humans (Reddy, 2022; Piorunek et al., 2023). Many Pasteurella species are opportunistic pathogens that associated with epizootic outbreaks. Zoonotic transmission to humans usually occurs through animal bites or contact with nasal secretions. (Wilson and Ho, 2013). Though antibiotics, vaccination and good management can reduce the severity of P. multocida complex, the use of antibiotics is now a major global public health concern in controlling bacterial infections (Jahnen et al., 2025).
Pheka or Oroxylum indicum (L.) Benth. Ex Kurz is a tree in the Bignoniaceae family. Its rootbark decoction solution has been traditionally used for the treatment of diarrhea and dysentery in India (Reddy, 2022) while the bark has been used for the treatment of gastric ulcers, oral cavity cancer, scabies and other skin diseases (Chauhan, 1999). The leaf decoction is used to treat rheumatic pain, an enlarged spleen, ulcers, coughs, and bronchitis (Singh et al., 2011). The fruits of O. indicum are sword-shaped woody capsules which promote anthelmintic and stomachic effects (Singh et al., 2011). They are also popularly consumed as vegetables while the mature seeds are components in a Chinese traditional drink. Our previous studies showed that the fruit and the seed extracts of O. indicum exhibited antibacterial activities against clinically isolated bacteria including Staphylococcus intermedius, Streptococcus suis, Pseudomonas aeruginosa, β-hemolytic Escherichia coli and Staphylococcus aureus (Sithisarn et al., 2016; 2021). Using scanning electron microscopy, the mechanism of action of the antibacterial effect was found to be from the disruption of the cell membrane and the induction of abnormal cell aggregations of O. indicum fruit extract was also reported (Sithisarn et al., 2021).
Many phytochemicals in various parts of O. indicum including the pods, the seeds, the bark and the roots with flavones such as baicalein, biochanin A, oroxylin A, chrysin, apigenin, and their glycosides have been found (Chen et al., 2003; Chen et al., 2005; Bhattacharje and Das, 2008; Jayaram and Prasad, 2008; Zaveri et al., 2008; Luitel et al., 2010; Yan et al., 2011; Tran et al., 2015; Sithisarn et al., 2016; Peng et al., 2019; Sithisarn et al., 2021). Scanning electron microscopy (SEM) has been used to visually demonstrate the antibacterial effects of plant extract. From SEM analysis, Piper betle leaf extract showed an antibacterial effect against Streptococcus mutans via the inhibition of cell adherence, cell growth, and extracellular polysaccharide formation (Rahim and Thurairajah et al., 2011), while field-emission scanning electron microscopy (FESEM) demonstrated that Parkia speciosa pod extract caused disintegration of Staphylococcus aureus due to chronic cell wall alterations with pore creation, invaginations, and morphological disorganization (Goh et al., 2025). In this study, the inhibitory effects and the mechanism of action of the fruit extract of O. indicum were investigated against a clinical strain of P. multocida using SEM technique.
MATERIAL AND METHODS
Plant material preparation
The fruits of Oroxylum indicum were collected from Nakhon Pathom and Chiang Rai provinces in 2019. The samples were botanically authenticated according to their botanical and taxonomical characteristics using the identification key described in Flora of Thailand 1987 (Smitinand et al., 1987; Santisuk, 2014) and the voucher specimens were deposited at the Department of Pharmacognosy, Faculty of Pharmacy, Mahidol University, Bangkok, Thailand. They were cleaned and dried in a hot air oven (Memmert, Schwabach, Germany) at 60°C, for 6 h then ground using an electric milling machine (Ika-Werke, Staufen, Germany) (20 mesh sieve).
Plant extract preparation and quality control
O. indicum fruit powder was extracted by maceration using 95% ethanol (plant:solvent ratio 1:20 w/v) using an electric flask shaker (Wisd Laboratory Instruments, Wertheim, Germany) for 6 h. The extraction solution was filtered after it was stored for 12 h. Each extraction process was repeated three times. The extraction solutions were then combined, filtered, and evaporated using a water bath to yield the dried extract (Sithisarn et al., 2021).
O. indicum fruit extract was quantitatively analyzed for total phenolic using the Folin-Ciocalteu method (Rojsanga et al., 2017), the total flavonoid content was analysed using the aluminium chloride method (Rojsanga et al., 2017) and flavone contents were analysed by the HPLC method (Sithisarn et al., 2021).
In vitro antibacterial activity determination
Preparation of clinical isolates, bacterial culture, and culture media
Clinical isolates of Pasteurella multocida were obtained from the Microbiological Laboratory, Veterinary Diagnostic Center, Faculty of Veterinary Medicine, Kasetsart University, Nakhon Pathom, Thailand. According to the standard method of Baron et al. (1997), the bacteria strain was isolated and characterized. P. multocida was maintained in Micro-bank Cryovials and kept at -80°C until use. Prior to any particular testing, the bacterium was maintained on a blood agar plate and incubated for 18–24 h at 37°C. Blood agar was obtained from Veterinary Diagnostic Center. The Mueller-Hinton agar and Mueller-Hinton broth were purchased from Oxoid, Thermofisher Inc. (St. Louis, MO, USA).
Confirmation of P. multocida clinical isolates by matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS)
The isolated bacterial colony was submitted to matrix-assisted laser desorption/ionization time of flight mass spectrometry, MALDI-TOF MS (Vitek® MS, Biomérieux, France) for bacterial identification and characterization according to the manufacturer’s protocol. In brief, a single colony of the isolated bacteria was deposited onto the preparation grid together with the matrix solution. The isolates were tested in duplicate on the MALDI-plate. The loaded slide was then inserted into the machine. The VITEK mass spectrometer was used to generate a spectrum of the bacterial suspension using Biotype software (version 2.0). Microbial identification was achieved by obtaining the spectra and analyzing the spectra against the reference database. The peaks from these spectra were compared with the characteristic pattern for the species, genus or family of the microorganism, leading to its identification. The results were evaluated by the cut-off of 90% reliability in each identification.
Evaluation of antimicrobial drug resistances of clinical isolated bacteria by standard antimicrobial susceptibility assays for antibiogram
Antimicrobial susceptibility testing is performed according to the Clinical and Laboratory Standards Institute (CLSI) guidelines using standard agar disc diffusion methods (CLSI, 2020). A standard antibiotic disc panel was generated appropriate to the organism, hospital formulary, and the site of infection. The sixteen standard antibiotic discs are penicillin 10 µg (P10), ampicillin 10 µg (AMP10), amoxycillin 10 µg (AML10), amoxycillin-clavulanic acid 30 µg (AMC30), cephalexin 30 µg (CL30), ceftriaxone 30 µg (CPR30), clindamycin 20 µg (DA20), doxycycline 30 µg (DO30), enrofloxacin 5 µg (ENR5), gentamicin 10 µg (CN10), kanamycin 30 µg (K30), lincomycin 15 µg (MY15), erythromycin 15 µg (E15), amikacin 30 µg (AK30), streptomycin 10 µg (S10), and sulfamethoxazole-trimethoprim 25 µg (STX25) (Oxoid, Thermo Fisher Scientific, USA).
Determination of the half-maximal inhibitory concentrations (IC50) and the minimum inhibitory concentration (MIC) by microdilution assay and bacterial enumeration
The half-maximal inhibitory concentration (IC50) and the minimum inhibitory concentration (MIC) of O. indicum young fruit extract for P. multocida were determined using the broth microdilution method combined with a drop plate technique for bacterial enumeration (Sithisarn et al., 2021). Briefly, two-fold serial dilutions of extract in Mueller-Hinton broth (MHB) at concentrations ranging from 0.39–50 mg/mL were placed in a flat-bottom transparent polystyrene 96-well plate. The bacterial inoculum was prepared from a subculture in blood agar incubated for 18–24 h at 37°C prior to the test. The bacterial culture was adjusted to 0.5 McFarland standard in MHB using a turbidimeter (Biosan, Riga, Latvia). A diluted bacterial suspension was added into the 96-well plate containing the serially diluted extracts until it reached the final 1 x 106 CFU/mL. The final volume of 200 µL suspension per well was thus set. Negative and positive growth controls were performed by adding only MHB and each bacterium together with MHB to the wells, respectively. Amoxycillin and doxycycline 5 µg/mL (Sigma-Aldrich, St. Louis, MO, USA) was used as antibiotic control. The plates were incubated at 37°C for 18–24 h. Then, the optical density (OD) at 600 nm was determined using a spectrophotometer (SPECTROstar® Nano, BMG LABTECH, Cary, NC, USA) and the concentration which had no bacterial growth comparing to the controls was examined, due to the turbidity interfering with the extracts at a certain concentration range. The capability of different extracts to inhibit bacterial growth was confirmed with quantification by a drop plate technique for the bacterial enumeration.
After the incubation time was complete, the bacterial suspension was adjusted to approximate to the desired log10 CFU/mL using a standard serial 10-fold dilution in MHB, and 20 µL were transferred for drop plating on Mueller-Hinton agar. The drops were absorbed into the agar in less than half an hour. Then, the plates were incubated at 37°C for 18–24 h and the viable pathogens were enumerated. At least 3 to 30 colonies of the bacteria grew from 10 µL of drop and 30 to 300 CFU per 100 µL of the bacteria as a confidence technique were chosen for counting. The total count of CFU from at least three drops at the countable dilution were determined and averaged. Finally, the total count was scaled up and the viable bacterial cells were determined.
The bacterial cell viability, the IC50 and MIC data were analyzed by dose-response non-linear regression best curve fit for inhibition activity using GraphPad Prism v.9.0.1 (GraphPad Software, CA, USA) and Calcusyn v.1.1 (Biosoft, Cambridge UK) programs. The data are presented as mean ± SD. The concentration at which no bacterial growth compared to controls was examined, due to the turbidity interfering with the extracts at a certain concentration range.
Evaluation of antibacterial mechanism of action by scanning electron microscopic technique
The Scanning Electron Microcopy (SEM) method was used to observe bacterial cell Morphology (Hartmann et al., 2010; Sithisarn et al., 2021; Hisada et al., 2023). Each bacterial culture suspension was adjusted to a final concentration of 3 x 106 CFU/mL in Mueller-Hinton broth with the O. indicum fruit extract at a concentration of 15 mg/mL and without extract (control culture) in parallel. The cell suspensions were incubated at 37°C for 15 h and were harvested for Scanning Electron Microscopy sample preparations. The bacterial cells were washed three times with sterile deionized water at 2,000 µg centrifugation for 10 min. The cell pellet was collected and fixed with 10% paraformaldehyde (Sigma-Aldrich, St. Louis, MO, USA) and 2.5% glutaraldehyde (Sigma-Aldrich, St. Louis, MO, USA) solution for 30–60 min. The samples were further dehydrated by serial concentration gradients of 30%, 50%, 70%, 80%, 90%, and 100% ethanol solutions and acetone. The dehydrated cells were transferred to a clean glass coverslip, dried and coated by a sputter coater Q150R ES (Quorum, Tokyo, Japan). All samples were kept, dried, in a CO2 critical point dryer until they were examined using a ultrahigh Resolution Scanning Electron Microscope Hitashi SEM SU8020 (Hitachi High-Tech, Tokyo, Japan). Bacterial cell morphological changes were then observed.
RESULTS
Plant extract preparation and quality control
O. indicum fruit extract appeared as dark brown semi-solid extract which contained total phenolic and total flavonoid contents of 4% and 5% w/w of dried extract, respectively. Baicalin, baicalein and chrysin were the major flavones in the extract with the amount of 0.40, 0.36 and 1.12% w/w of dried extract, respectively. HPLC chromatogram of O. indicum fruit extract is shown in Figure 1.

Figure 1. HPLC chromatogram of O. indicum fruit extract; 1 = baicalin (RT = 6.17 min), 2 = baicalein (RT = 9.92 min), 3 = chrysin (RT = 12.98 min).
Characterization of clinical isolated bacteria by matrix-assisted laser desorption/ ionization time of flight mass spectrometry (MALDI-TOF MS)
The bacterium was confirmed to be Pasteurella multocida at 99% reliability using MALDI-TOF MS, according to the characterization of the mass to charge ratio of the bacterial ribosomal proteins to provide a specific pattern of a mass spectrum compared to the reference library. The result corresponded to the standard method of clinical veterinary bacterial identification and characterization.
Evaluation of antibiotic resistances of clinical isolated bacteria by antibiogram method
The antibiogram of P. multocida strain to 16 standard antibiotics practically used in clinical veterinary medicine was characterized. The profile showed penicillin G, streptomycin, kanamycin, clindamycin, and lincomycin resistance. In contrast, to the five antibiotics P. multocida showed susceptibility to other standard antibiotics (Table 1). The result showed the phenotypic resistance particularly to penicillin, aminoglycoside and lincosamide antibiotic groups.
Table 1. Antibiogram of clinically isolated Pasteurella multocida.
|
Antibiotic |
Disc potency (ug) |
Abbreviation |
Inhibitory zone diameter (mm) |
susceptibility (SIR) |
|
penicillin |
10 |
P10 |
10 |
R |
|
ampicillin |
10 |
AMP10 |
36 |
S |
|
amoxycillin |
10 |
AML10 |
35 |
S |
|
amoxycillin-clavulanic acid |
30 |
AMC30 |
37 |
S |
|
cephalexin |
30 |
CL30 |
32 |
S |
|
ceftriaxone |
30 |
CPR30 |
40 |
S |
|
clindamycin |
20 |
DA20 |
0 |
R |
|
doxycycline |
30 |
DO30 |
24 |
S |
|
enrofloxacin |
5 |
ENR5 |
24 |
S |
|
gentamicin |
10 |
CN10 |
21 |
S |
|
kanamycin |
30 |
K30 |
0 |
R |
|
lincomycin |
15 |
MY15 |
11 |
R |
|
erythromycin |
15 |
E15 |
25 |
S |
|
amikacin |
30 |
AK30 |
25 |
S |
|
streptomycin |
10 |
S10 |
0 |
R |
|
sulfamethoxazole-trimethoprim |
25 |
STX25 |
25 |
S |
Determination of the half-maximal inhibitory concentrations (IC50) and the minimum inhibitory concentration (MIC) by microdilution assay and bacterial enumeration
The half-maximal inhibitory concentration (IC50) of Oroxylum indicum fruit extract to P. multocida was shown at 14.98 ± 0.35 mg/ml and the minimum inhibitory concentration (MIC) was exhibited at 29.24 ± 0.05 mg/ml. The results of IC50 and MIC of O. indicum fruit extract and some standard antibiotics to P. multocida are shown in Table 2.
Table 2. The half-maximal inhibitory concentration (IC50) and the minimum inhibitory concentration (MIC) of O. indicum fruit extract and some standard antibiotics to P. multocida.
|
Sample |
IC50 |
MIC |
|
O. indicum fruit extract (mg/ml) |
14.98 ± 0.35 |
29.24 ± 0.05 |
|
Gentamicin (µg/ml) |
5.62 ± 2.13 |
7.81 ± 0.00 |
|
Doxycycline (µg/ml) |
10.45 ± 1.19 |
15.63 ± 0.00 |
|
Cefotaxime (µg/ml) |
13.52 ± 1.49 |
31.25 ± 0.00 |
Evaluation of antibacterial mechanism of action by scanning electron microscopic technique
The scanning electron microscopy of the bacteria incubated with 15 mg/ml O. indicum fruit extract revealed the evident bacterial morphological changes, cell wall disruption, content leakage, abnormal groups of cell aggregations, cell fusion, and cell lysis (Figure 2).
Dynamics of cell morphological changes from bacilli (rod) to cocci (round) as indicators of induced cell lysis were shown. Figure 2C arrows showed cell disruption and leakage of cell contents. Figure 2D arrows showed bulge formation and bulge stagnation of bacilli cell-shaped P. multocida.
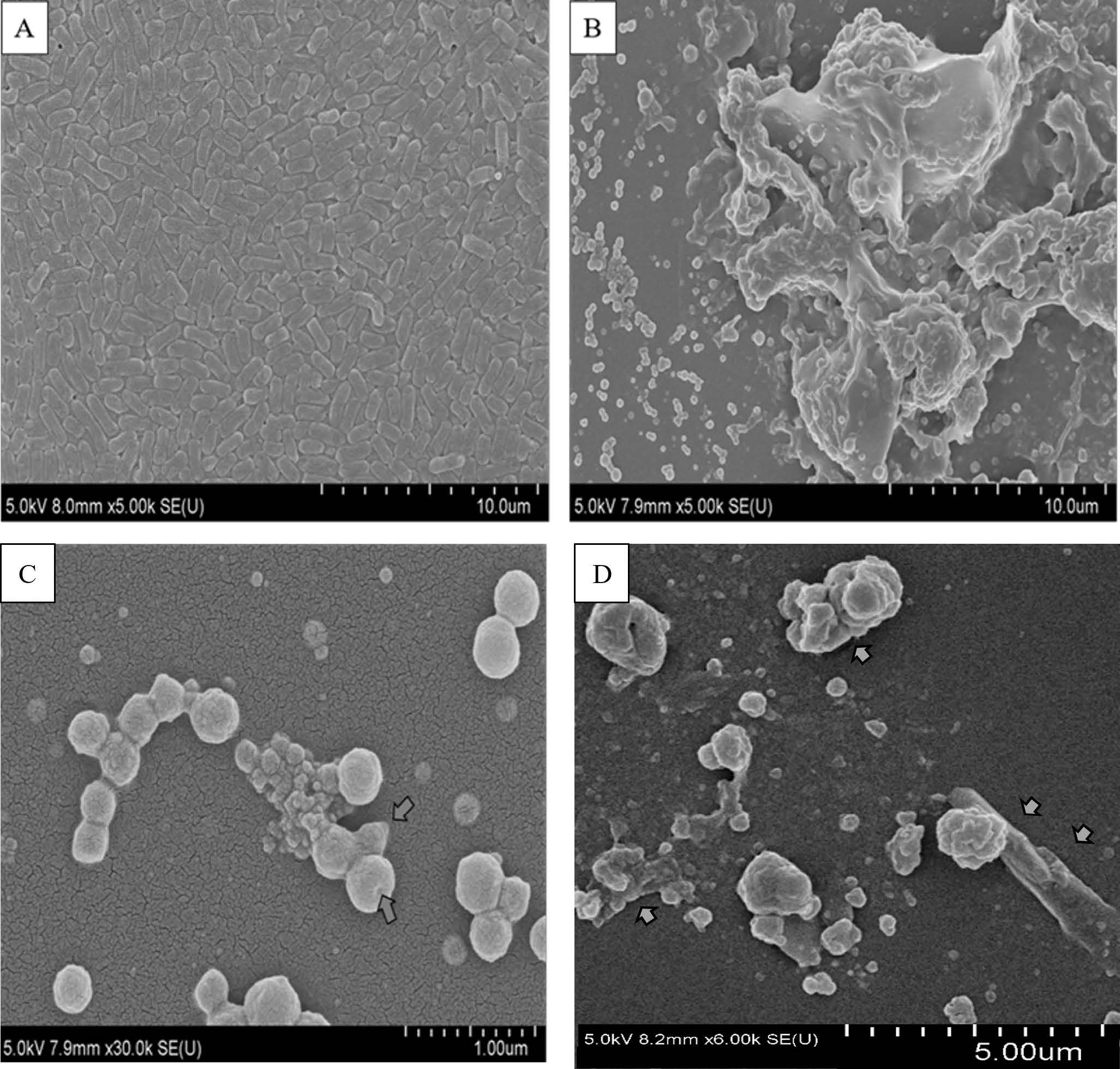
Figure 2. Scanning electron microscopy (SEM) showed evident effect of O. indicum fruit extract to clinical drug resistant Pasteurella multocida; A = P. multocida, B, C, D = P. multocida + O. indicum fruit extract at the concentration of 15 mg/ml.
DISCUSSION
Previous reports suggested that extracts from various parts of Oroxylum indicum, especially the fruit and the seed extracts, showed inhibitory effects against various zoonotic clinically isolated bacteria. The mechanisms of action for the antibacterial effects of O. indicum fruit extract were reported to disrupt the cell membrane and induce abnormal cell aggregations in S. intermedius, S. suis, P. aeruginosa, β-hemolytic E. coli, and S. aureus (Sithisarn et al., 2021). In this study, O. indicum fruit extract showed significant inhibitory effects against multiple drug resistant Pasteurella multocida with the mechanisms of action of induction of cell integrity loss and cell lysis via key membrane protein dysfunction and protein synthesis interference. It is described previously as the mechanism of cell-wall synthesis inhibition and induced lysis of prokaryotic cells by antimicrobial substances, called a bulge-mediated mechanism (Yao et al., 2012). The mechanism of inhibition proceeds through four stages: elongation, bulge formation, bulge stagnation, and lysis. Each compound affected the bacteria's cell wall and outer membrane (OM) differently. It is suggested being in the bulge stagnation period and lysis after 15 h of the experimental setting. So, the bacteria would show the bulge circular/ round cells and much cell fusion, cell aggregation, and cell lysis.
Bacteria have counterparts of all three eukaryotic cytoskeletal protein classes: FtsZ (Filamenting temperature-sensitive mutant Z) for tubulin, MreB (cell shape-determining protein MreB) for actin, and crescentin for intermediate filament proteins. As a major component of the bacterial cell wall, the peptidoglycan layer has a primary role in maintaining cell shape. One of the important proteins, such as penicillin-binding proteins (PBPs) carry out the reactions for synthesis and remodeling of peptidoglycan. Different PBPs have specific roles in cell division and elongation, and therefore in cell-shape determination. Moreover, growth of the cell wall is not uniform. It is localized to specific regions. These regions of localized peptidoglycan synthesis vary among bacteria and often change during the cell cycle, reflecting different modes of cell growth (longitudinal, septal, or polar). Therefore, the mode of cell inhibitions varies by different chemical structures (Cabeen and Jacobs-Wagner, 2005).
P. multocida is one of the most common pathogens of livestock. It can cause hemorrhagic septicemia in cattle (Hirsh et al., 2004) and pasteurellosis in rabbits (Boyce et al., 2010). The polysaccharide capsule and lipopolysaccharides (LPS) are major virulence factors involved in the pathogenesis of P. multocida in the host (Katsuda et al., 2013), while the other factors include fimbriae, adherence and colonization factors, iron-regulating and acquisition proteins, extracellular enzymes, exotoxins, and a variety of outer membrane proteins (Katoch et al., 2014). To control the infectious diseases caused by P. multocida, the use of antibiotics was suggested to be the most effective way (El-Hamid et al., 2019). However, because of the multidrug-resistant (MDR) strains of bacteria, antibiotic treatments could not completely control many infections, which could lead to the problem of ineffective treatment and the lack of prevention of bacterial infections in humans and animals (Khamesipour et al., 2014). The recommended antibiotics for the treatment of pasteurellosis are cefotaxime, ciprofloxacin, tetracyclines, and chloramphenicol (Kehrenberg et al., 2001; Yoshimura et al., 2001; Sellyei et al., 2009).
A previous study reported that 100% resistance to doxycycline, tetracycline, and colistin was a common feature among the 16 tested P. multocida clinical isolates, while 93% of them were found to be resistant to erythromycin, trimethoprim/sulfamethoxazole, chloramphenicol, ciprofloxacin, and cefotaxime (El-Hamid et al., 2019). In this study, the isolated P. multocida strain was found to resist penicillin, streptomycin, gentamicin, and clindamycin, but O. indicum fruit extract could still inhibit it.
There are only a few reports regarding the antibacterial effects of plant extracts and phytochemicals. Five herbal extracts, including marjoram, cinnamon, black seed, onion, and garlic, were reported to show antimicrobial activities against 16 P. multocida isolates from diseased rabbits, with the inhibition zones ranging from 13 to 26 mm, MIC values ranging from 8 to 96 µg/ml, and MBC values ranging from 16 to 192 µg/ml, in which majoram extract showed the best antibacterial activities (El-Hamid et al., 2019). Santalum album aqueous methanol extracts showed inhibitory effects against P. multocida with MIC values of 3.12 and 6.25 mg/ml, as tested by agar dilution and well diffusion methods, respectively (Hussain et al., 2011). Blueberry pomace and blackberry extracts were also reported to inhibit P. multocida growth with MBC at concentrations of 0.4 and 0.3 mg/ml gallic acid equivalent, respectively, while citrus oil at the concentration of 0.05% v/v can also completely inhibit P. multocida growth (Salaheen et al., 2014). The endophytic fungus, Glomerella magna, isolated from the plant Garcinia xanthochymus, showed inhibitory effects to P. multocida with MIC and MBC values of 46.9 and 750 μg/ml (Nath and Joshi, 2016). The fungal extract at the doses of 100 and 250 mg/kg body weight showed inhibitory effects on P. multocida growth in the liver and spleen after the oral administration in infected mice for 8 consecutive days (Nath and Joshi, 2016). The possible mechanism of action in inhibiting P. multocida was reported in the case of majoram extract, which was the down-regulation of the virulence genes of this bacterium that facilitate bacterium colonization and invasion into the host (El-Hamid et al., 2019). The mechanism of avoidance or disruption of host defense mechanisms, promotion of injury to host tissues, and/or stimulation of a noxious host inflammatory response were also reported (El-Hamid et al., 2019). In this study, the result suggests a potential use of rich flavonoid-containing O. indicum extracts as an alternative or complementary treatment of important zoonotic bacteria, especially those clinical strains with drug resistant profiles. This focuses on one of the key tools in both veterinary and human medicine for the future sustainability of antimicrobial drug use and infectious disease management and control.
CONCLUSION
Oroxylum indicum fruit extract, which was qualitatively controlled for total phenolic, total flavonoid, and flavone contents, showed in vitro antibacterial effects against clinically isolated penicillin, aminoglycoside and lincosamide resistant Pasteurella multocida determined by microdilution assay and bacterial enumeration methods. Using a scanning electron microscopic technique, the mechanism of action was found to be the formation of bacterial morphological changes, cell wall disruption, content leakage, abnormal groupings of cell aggregations, and cell lysis. For further applications, a study using an animal model should be performed.
ACKNOWLEDGMENTS
The authors acknowledge Miss Juree Tuangruthaivanich for her kind scientific assistance.
AUTHOR CONTRIBUTIONS
Patchima Sithisarn designed and conducted the experiments, performed the statistical analysis and data visualization, wrote and revised the manuscript. Pongtip Sithisarn designed and conducted the experiments, supervised, data analysis, wrote and revised the manuscript. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Bhattacharje, A.K. and Das, A.K. 2008. Phytochemical screening of some Indian plants. Pharmaceutical Biology. 9(3): 1408-1412.
Boyce, J.D., Harper, M., Wilkie, I.W., and Adler, B. 2010. Pasteurella: Pathogenesis of bacterial infections in animals. Wiley-Blackwell: Oxford, UK.
Cabeen, M. and Jacobs-Wagner, C. 2005. Bacterial cell shape. Nature Reviews Microbiology. 3: 601-610.
Chauhan, S.N. 1999. Medicinal and aromatic plants of Himachal Pradesh. Indus Publishing: Totnes, UK.
Chen, L.J., Games, D.E., and Jones, J. 2003. Isolation and identification of four flavonoid constituents from the seeds of Oroxylum indicum by high-speed counter-current chromatography. Journal of Chromatography A. 988(1): 95-105.
Chen, L.J., Song, H., Lan, X.Q., Games, D.E., and Sutherland, I.A. 2005. Comparison of high-speed counter-current chromatography instruments for the separation of the extracts of the seeds of Oroxylum indicum. Journal of Chromatography A. 1063(1-2): 241-245.
Christensen, J.P. and Bisgaard, M. 2000. Fowl cholera. Revue Scientifique et Technique / Office International des Epizooties 19: 626-637.
CLSI. 2020. Performance standards for antimicrobial susceptibility testing, 30th ed. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute. USA.
El-Hamid, M.I.A., El-Sayed, M.E., Alic, A.R., Abdallah, H.M., Arnaout, M.I., and El-mowalid, G.A. 2019. Marjoram extract down-regulates the expression of Pasteurella multocida adhesion, colonization and toxin genes: A potential mechanism for its antimicrobial activity. Comparative Immunology, Microbiology and Infectious Diseases. 62: 101-108.
Goh, S.M., Dassanayake, M.K., Foan, C.C., Wiart, C., Symonds, R., Khoo, T.J., Chong, C.H., and O Elfar, O.A. 2025. Antibacterial potency of mid-polar extracts obtained from Malaysian plant Parkia speciosa against human pathogenic bacteria. Microbial Pathogenesis. 198: 107134.
Hartmann, M., Berditsch, M., Hawecker, J., Ardakani, M.F., Gerthsen, D., Ulrich, A.S. 2010. Damage of the bacterial cell envelope by antimicrobial peptides gramicidin S and PGLa as revealed by transmission and scanning electron microscopy. Antimicrobial Agents and Chemotherapy. 54(8): 3132-3142.
Herath, C., Kumar, P., Singh, M., Kumar, D., Ramakrishnan, S., Goswami, T.K., and Ram, G.C. 2010. Experimental iron-inactivated Pasteurella multocida A: 1 vaccine adjuvanted with bacterial DNA is safe and protects chickens from fowl cholera. Vaccine. 28(11): 2284-2289.
Hirsh, D.C., MacLachian, N.J., and Walker, R.L. 2004. Veterinary Microbiology. 2nd ed. Blackwell Publishing: Oxford, UK.
Hisada, A., Matsumoto, E., Hirano, R., Konomi, M., Khalil, J.Y.B., Raoult, D., and Ominami, Y. 2023. Detection of antimicrobial impact on Gram-negative bacterial cell envelope based on single-cell imaging by scanning electron microscopy. Scientific Reports. 13: 11258.
Hussain, T., Arshad, M., Khan, S., Sattar, H., and Qureshi, M.S. 2011. In vitro screening of methanol plant extracts for their antibacterial activity. Pakistan Journal of Botany. 43(1): 531-538.
Jahnen, J., Hanke, D., Kadlec, K., Schwarz, S., and Krüger-Haker, H. 2025. Antimicrobial resistance in Pasteurella multocida isolates from bovine mastitis can be associated with multidrug-resistance-mediating integrative and conjugative elements (ICEs). Antibiotics. 14(2): 153.
Jayaram, K. and Prasad, M.N. 2008. Genetic diversity in Oroxylum indicum (L.) Vent. (Bignoniaceae), a vulnerable medicinal plant by random amplified polymorphic DNA marker. African Journal of Biotechnology. 7(3): 254-262.
Katoch, S., Sharma, M., Patil, R.D., Kumar, S., and Verma, S. 2014. In vitro and in vivo pathogenicity studies of Pasteurella multocida strains harbouring different ompA. Veterinary Research Communications. 38(3): 183-191.
Katsuda, K., Hoshinoo, K., Ueno, Y., Kohmoto, M., and Mikami, O. 2013. Virulence genes and antimicrobial susceptibility in Pasteurella multocida isolates from calves. Veterinary Microbiology. 167(3-4): 737-741.
Kehrenberg, C., Schulze-Tanzil, G., Martel, J.L., Chaslus-Dancla, E., and Schwarz, S. 2001. Antimicrobial resistance in Pasteurella and Mannheimia: Epidemiology and genetic basis. Veterinary Research. 32(3-4): 323-339.
Khamesipour, F., Momtaz, H., and Mamoreh, M.A. 2014. Occurrence of virulence factors and antimicrobial resistance in Pasteurella multocida strains isolated from slaughter cattle in Iran. Frontiers in Microbiology. 5: 536.
Luitel, H.N., Rajbhandari, M., Kalauni, S.K., Awale, S., Masuda, K., and Gewali, M.B. 2010. Chemical constituents from Oroxylum indicum (L.) Kurz of Nepalese origin. Scientific World. 8(8): 66-68.
Magyar, T. and Lax, A.J. 2002. Atrophic rhinitis. In Brogden, K.A., and Guthmiller, J.M., editors. Polymicrobial Diseases. Chapter 10. ASM Press: Washington (DC), USA.
Nath, A. and Joshi, S.R. 2016. Endophytic fungi from tropical ethnoveterinary plants and their antibacterial efficacy against Pasteurella multocida capsular type A strain. Revista de Biología Tropical. 64(2): 733-745.
Peng, Q., Shang, X., Zhu, C., Qin, S., Zhou, Y., Liao, Q., Zhang, R., Zhao, Z., and Zhang, L. 2019. Qualitative and quantitative evaluation of Oroxylum indicum (L.) Kurz by HPLC and LC‐qTOF‐MS/MS. Biomedical Chromatography. 33(11): 1-16.
Piorunek, M., Brajer-Luftmann, B., and Walkowiak, J. 2023. Pasteurella multocida infection in humans. Pathogens. 12(10): 1210.
Rahim, Z.H.A. and Thurairajah, N. 2011. Scanning electron microscopic study of Piper betle L. leaves extract effect against Streptococcus mutans ATCC 25175. Journal of Applied Oral Science. 19(2): 137-146.
Reddy, I.V.S. 2022. Traditional medicinal plants used by the tribes of Bhadradri Kothagudem district, Telangana - for dysentery and diarrhea. The Pharma Innovation Journal. SP-11(4): 887-891.
Rojsanga, P., Bunsupa, S., Brantner, A.H., and Sithisarn, P. 2017. Comparative phytochemical profiling and in vitro antioxidant activity of extracts from raw materials, tissue-cultured plants, and callus of Oroxylum indicum (L.) Vent. Evidence-Based Complementary and Alternative Medicine. eCAM, 2017: 6853212.
Sellyei, B., Varga, Z., Szentesi-Samu, K., Kaszanyitzky, E., and Magyar, T. 2009. Antimicrobial susceptibility of Pasteurella multocida isolated from swine and poultry. Acta Veterinaria Hungarica. 57(3): 357-367.
Salaheen, S., Almario, J.A., and Biswas, D. 2014. Inhibition of growth and alteration of host cell interactions of Pasteurella multocida with natural byproducts. Poultry Science. 93(6): 1375-1382.
Santisuk, T. 2014. Studies in flora of Thailand: Bignoniaceae. Thai Forest Bulletin (Botany). 8: 1-48.
Singh, H.V. and Chaudhary, A.K. 2011. A review on the taxonomy, ethnobotany, chemistry and pharmacology of Oroxylum indicum Vent. Indian Journal of Pharmaceutical Sciences. 73(5): 483-490.
Sithisarn, P., Nantateerapong, P., Rojsanga, P., and Sithisarn, P. 2016. Screening for antibacterial and antioxidant activities and phytochemical analysis of Oroxylum indicum fruit extracts. Molecules. 21(4): 446.
Sithisarn, P., Rojsanga, P., and Sithisarn, P. 2021. Flavone-rich fractions and extracts from Oroxylum indicum and their antibacterial activities against clinically isolated zoonotic bacteria and free radical scavenging effects. Molecules. 26(6): 1773.
Smitinand, T., Larsen, K., and Nielsen, I. 1987. Flora of Thailand, vol. 5, part 1. The Forest Herbarium. Department of National Parks. Wildlife and Plant Conservation: Bangkok, Thailand.
Tran, T.V.A., Malainer, C., Schwaiger, S., Hung, T., Atanasov, A.G., Heiss, E.K., Dirsch, V.M., and Stuppner, H. 2015. Screening of Vietnamese medicinal plants for NF-κB signaling inhibitors: Assessing the activity of flavonoids from the stem bark of Oroxylum indicum. Journal of Ethnopharmacology. 159: 36-42.
Wilkie, I.W., Harper, M., Boyce, J.D., and Adler, B. 2012. Pasteurella multocida: Diseases and pathogenesis. Current Topics in Microbiology and Immunology. 361: 1-22.
Wilson, B.A. and Ho, M. 2013. Pasteurella multocida: From zoonosis to cellular microbiology. Clinical Microbiology Reviews. 26(3): 631-655.
World Assembly of Delegates of the OIE. 2012. Atrophic rhinitis of swine OIE Terrestrial Manual (Chapter 2.8.2). Paris: OIE.
Yan, R.Y., Cao, Y.Y., Chen, C.Y., Dai, H.Q., Yu, S.X., Wei, J.L., Li, H., and Yang, B. 2011. Antioxidant flavonoids from the seed of Oroxylum indicum. Fitoterapia. 82(6): 841-848.
Yao, Z., Kahne, D., and Kishony, R. 2012. Distinct single-cell morphological dynamics under beta-lactam antibiotics. Molecular Cell. 48(5):705-712.
Yoshimura, M., Honda, Y., Yonemitsu, E., Kuraoka, K., and Suenaga, K. 2024. Method for quantifying the Pasteurella multocida antigen adsorbed on aluminum hydroxide adjuvant in swine atrophic rhinitis vaccine. PLoS One. 19(5): e0301688.
Yoshimura, H., Ishimaru, M., Endoh, Y.S., and Kojima, A. 2001. Antimicrobial susceptibility of Pasteurella multocida isolated from cattle and pigs. Journal of Veterinary Medicine. Series B. 48(7): 555-560.
Zaveri, M., Khandhar, A., and Jain, S. 2008. Quantification of baicalein, chrysin, biochanin-A and ellagic acid in root bark of Oroxylum indicum by RP-HPLC with UV detection. Eurasian Journal of Analytical Chemistry. 3(2): 245-257.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Patchima Sithisarn1 and Pongtip Sithisarn2, *
1 Faculty of Veterinary Medicine, Kasetsart University, Kampangsaen campus, Nakhon Pathom 73140, Thailand
2 Faculty of Pharmacy, Mahidol University, Bangkok 10400, Thailand.
Corresponding author: Pongtip Sithisarn, E-mail: pongtip.sit@mahidol.ac.th
ORCID: Pongtip Sithisarn: https://orcid.org/0000-0002-0199-3644
Total Article Views
Editor: Nisit Kittipongpatana,
Chiang Mai University, Thailand
Article history:
Received: May 26, 2024;
Revised: August 4, 2025;
Accepted: August 13, 2025;
Online First: September 9, 2025

