
The Effect of Fagonia cretica on Inflammation Induced by Hyperhomocysteinemia and Antioxidant Activities: HPLC Analysis of the Butanolic Compound
Ibtissem Bouhouche*, Sakina Zerizer*, Zahia Kabouche, and Chawki BensouiciPublished Date : August 27, 2025
DOI : https://doi.org/10.12982/NLSC.2025.069
Journal Issues : Number 4, October-December 2025
Abstract In the present study the phenolics of F. cretica extract was identified by HPLC, which revealed the presence of 3,4,5-Trimethoxycinnamic acid - 3,5-Dimethoxyphenol- 4- Ethylphenol- 4-Methylguaiacol- 3,5-Dihydroxybenzoic acid- Coniferyl alcohol- 3- Hydroxybenzoic acid- 2,4-Dihydroxybenzoic acid. The total flavonol, flavonoid and phenolis contents of the F. cretica extract were appreciated. Additionally, the antioxidant activity of the extract was checked. The protective effect of F. cretica on mice cardivacular inflammation induced by hyperhomocysteinemia was evaluated according to an experimental in vivo procedure of 21 days. The level of plasma inflammatory markers (hs-CRP, homocysteine), were measured. Additionally, the histological sections of the liver, heart and aorta tissues were examined. Our results revealed a potent antioxidant capacity of F. cretica extract. On the other hand, F. cretica considerably reduced the histopathological changes, decreased the levels of Hcy and hs-CRP, Therefore, we recommend the use of F. cretica as a natural anti hyperhomocysteinemia and anti-inflammatory therapeutic agent.
Keywords: Fagonia cretica, Antioxidant, Hyperhomocysteinemia, hs-CRP, Inflammation
Citation: Bouhouche, I., Zerizer, S., Kabouche, Z., and Bensouici, C. 2025. The effect of Fagonia cretica on inflammation induced by hyperhomocysteinemia and antioxidant activities: HPLC analysis of the butanolic compound. Natural and Life Sciences Communications. 24(4): e2025069.
INTRODUCTION
Homocysteine is a toxic nonprotein sulfur containing amino acid (Yao et al., 2019), cannot be obtained from the diet since it is produced in the body from methionine which acts as a precursor of Hcy (Rehman et al., 2020). Methionine is an essential sulfur-containing amino acid (Azzini et al., 2020). With a vital role in intermediary metabolism (Yang et al., 2020). Normal plasma concentration of methionine ranges from 13 to 45 µmol/L (Kovalska et al., 2021). The major role of Hcy in our body is it acts as a biochemical intersection between methionine metabolism and biosynthesis of cysteine which plays various important roles in our body (Rehman et al., 2020). Hcy participates in methyl, folate, and cellular thiol metabolism (Malinowska et al., 2012). The plasma levels of Hcy are controlled by two metabolic pathways: (1) remethylation of Hcy to methionine, which requires the presence of folic acid and vitamin B12 as coenzymes and occurs within the cell and in all body tissues; (2) transsulfuration of Hcy to cysteine, which requires vitamin B6 (Vezzoli et al., 2020). The level of homocysteine is considered normal between 5 and 15μmol/L, while hyperhomocysteinemia (HHcy) refers to an elevated blood Hcy level above 15 µmol/L (Rawashdeh et al., 2020). Genetic abnormalities, age, sex and various nutritional and hormonal determinants contribute to hyperhomocysteinemia (HHcy) (Yao et al., 2019). Whenever the Hcy concentration elevates in the body, whether genetic or acquired, it could lead to the generation of multiple diseases (Xiang et al., 2021). Hyperhomocysteinemia (HHcy) has been recognized as a risk factor for cardiovascular disease, stroke, vascular contributions to cognitive impairment and dementia, and Alzheimer’s disease (Chang et al., 2019). An elevated level of homocysteine induces endothelial dysfunctions, oxidative stress, or inflammation and is associated with several pathological states like neurological disorders, chronic kidney disease, osteoporosis, gastrointestinal disorders, cancer, or congenital defects (Yakovleva et al., 2020). Previous reports have revealed that homocysteine concentrations are higher in many viral infections such as human immunodeficiency virus, hepatitis virus, and human papillomavirus (Keskin et al., 2022). Experimental studies show that at the molecular level, the toxicity of Hcy includes mechanisms involving the formation of reactive oxygen species, hypomethylation, induction of unfolded protein response, and protein homocysteinylation (Šebeková et al., 2022). On the other hand the medicinal plant Fagonia cretica, a member of the family Zygophyllaceae, is a small spiny under-shrub, mostly found in dry calcareous rocks confined to warm areas of Africa and Asia (Salah Eldin et al., 2015). Fagonia cretica is distributed in tropical, subtropical and warm temerate areas. Algeria, Egypt, Morocco, Tunisia, Cyprus, Saudi Arabia and dry calcareous rocks throughout western India and Pakistan are famous for collection of this plant (Sayar and Sharma, 2019). Fagonia cretica is bitter and used for the treatment of fever, thirst, vomiting, dysentery, asthma, urinary discharges, liver trouble, typhoid, toothache, dyslipidemia, stomach troubles and skin diseases (Nagaraj, 2013; Naeem et al., 2014; Ghayas et al., 2022). Its pharmacological actions reported as antioxidant, analgesic, anti-inflammatory and wound healing, anti-allergic, neuroprotective, cytotoxic and anti-tumour, dermatological, anti-hemorrhagic, hepatoprotective, antidiabetic, antipyretic, hematological, free radical scavenging, ferric reducing and iron chelation, and anti-fungal activities. The medicinal properties of the plant were attributed due to its variety of active phytochemical constituents (Ghayas et al., 2022). It is reported that Fagonia cretica is heavenly rich in alkaloids, coumarins, phenolic compounds, flavonoid, glycosides, triterpenoids, amino acids, carbohydrates, proteins, vitamins and minerals, sterols, and saponins (Ghayas et al., 2022; Kamran et al., 2023).
The aims of the study is identifying the phenolic compounds by using HPLC, and the antioxidant activity of the butanolic extract was checked using several activities, and measuring the total Hyc, the plasma hs-CRP to estimate the anti-inflammatory effect of the F. cretica on the inflammation induced by Hyperhomocysteinemia. By the end, the histological sections of the liver, heart and aorta had been examined in order to confirm the homocysteine toxicity and the protective effect of F. cretica on tissues.
MATERIALS AND METHODS
Plant material
Fagonia cretica was collected from Ghardaia (Algerian sahara) in March 2020 and authenticated by Pr. Gérard de Bélair (University Badji-Mokhtar, Annaba). Voucher code: Lost Fc.03.20. Was stored in the Laboratory of Therapeutic Substances at University Constantine 1 brother Mentouri, Algeria.
Preparation of the extract
Air-dried and powdered aerial parts (1kg) of Fagonia cretica were macerated three times at room temperature with MeOH-H2O (8:2, v/v) for 24h. The suspensions were filtered and the filtrate obtained was concentrated under reduced pressure using rotary evaporator. For fractionation, a part of the methanol extract of each part was suspended in water and extracted with solvents of increasing polarity, petroleum ether, chloroform, ethyl acetate, n-butanol.
Determination of total phenolics
The content of total phenolics was determinant using the Folin-Ciocalteu reagent according to the method described by (Müller et al., 2010). The solution was prepared by mixing 20 µL of F. cretica with 100 µL of Folin Ciocalteu reagent (0.2N) and 75 µL of sodium carbonate (7.5%) in a 96 wells microplate. The microplate was incubated in the dark for 2h at room temperature and the absorbance was measured at 765 nm using microplate reader (Perkin Elmer, EnSpire, Singapore). The concentration of total phenolic compound was calculated from a standard curve of gallic acid.
Total flavonoid content
The total flavonoid content of plant extracts was determined using the microplate method as described by (Topçu et al., 2007). A volume of 50 µL of the extracts/fractions (1 mg/mL in methanol) was mixed with 130 µL of MeOH. This was followed by the addition of 10 µL of acetate potassium and 10 µL of aluminium nitrate. After 40 min at room temperature, the absorbance was measured at 415 nm using microplate reader.
Flavonol content
Total flavonol content was measured using the method of Kumaran and Joel Karunakaran, (2007). Briefly, a volume of 50 µL of the extract of F. cretica was mixed with 50 µL of AlCl3 (2%) prepared in methanol and 150 µL of an aqueous solution of sodium acetate (5%). The mixture was incubated in the dark for 2h and 30min, and the absorbance was measured at 440 nm.
Identification, of phenolics by high-performance liquid chromatography
The phenolics of the extract F. cretica was identified using a high-performance liquid chromatography (HPLC). The sample was prepared by diluting the plant extract in 0.2 mL of methanol then the sample solution was filtered using a syringe filter 0.45 µm and analysed by HPLC using a colon C (C18) (Agilent technologie serie 1200). The standards phenolics (caffeic acid dimethyl ether, Sinapic acid, 4-ethylguaiacol 2-tert-butyl-4- hydroxyanisole, butylated hydroxytoluene, a- methylcinnamic acid, lawson, chrysin and 4-propylphenol) were also prapared and analysed by HPLC.
Antioxidant capacity
DPPH free radical scavenging assay
The free radical-scavenging activity was determined spectrophotometrically by the method of Blois (1958). Solution of 40 μL of the sample (extracts, and standards) at various concentrations was added to 160 μL of the methanolic solution of DPPH (0.1 mM). After incubation in the dark at 37°C for 30 min, the absorbance of each solution was determined at 517 nm using a microplate reader. DPPH solution in methanol was used as a control, tocopherol, BHT, and BHA were used as antioxidant standards.
ABTS scavenging assay
The ABTS scavenging activity was determined according to the method of Re et al. (1999). ABTS•+ solution was prepared by mixing 7 mM of ABTS+ in water with 2.45 mM potassium persulfate. The mixture was left in the dark at room temperature for 12 h before use. Then, 40 μL of the extract at different concentrations was added to 160 μL of ABTS+ solution and the mixture was left at ambient temperature for 10 min and then the absorbance was measured at 734 nm using a 96-well microplate reader. ABTS+ solution in ethanol was used as a control, BHT and BHA were used as antioxidant standards.
Galvinoxyl radical scavenging activity
The trapping activity of the galvinoxyl radical was appreciated according to the method described by Shi et al. (2001). 40 μL of the extract in methanol at different concentrations was placed in 96-well microplates with 160 μL of methanolic galvinoxyl solution (4 mg of galvinoxyl in 100 mL of methanol). The plate was then incubated in the dark at room temperature for 2 h. Absorbance was measured at the wavelength of 428 nm. Galvinoxyl solution with methanol was used as a control. BHT and BHA were used as antioxidant standards.
Reducing power assays
The reducing power of the extract was evaluated according to the method described by Oyaizu (1986). Briefly, a solution of 10 μL of the extract at various concentrations was mixed with 40 μL of phosphate buffer (0.2 M, pH 6.6) and 50 μL of potassium ferricyanide (10 mg/mL). The obtained solution was incubated at 50°C for 20 min. Then, the solution was mixed with 50 μL of trichloroacetic acid solution (100 mg/mL) and 10 μL of ferric chloride solution (1.0 g/L), finally 40 μL of distilled water was added to the mixture. The absorbance was measured at 700 nm using a using a 96 well microplate reader. Ascorbic acid, tannic acid, and tocopherol were used as standards.
Hydroxyl radical scavenging assay
Hydroxyl radical scavenging assay is determined according to the method described by Smirnoff and Cumbes (1989). Briefly, a solution (24 μL) of the de FeSO4 (8 mM) was mixed with 20 μL H2O2 (20 mM), 80 μL salicylic acid (3 mM) and 40 μL of different concentration of the extracts, and standards (ascorbic acid). After incubation at 37°C for 30 min, the solution was mixed with 36 μL of H2O, the absorbance of each solution was determined at 510nm using a microplate reader.
Phenanthroline assay
The phenanthroline assay was evaluated by the method of Szydłowska-Czerniak et al. (2008). The reaction mixture containing 10 μL of different concentration of the extracts in methanol, 30 μL of 1,10-phenanthroline solution in methanol (0.5%), 50 μL ferric chloride (FeCl3) (0.2%) and 110 μL of MeOH, the reaction solution was incubated in the dark for 20 min at 30°C. The absorbance of the reaction mixture was then read at 510 nm. BHT and BHA were used as antioxidant standards.
Ethics statement
The animal exploration was performed following the procedure of the code number of the research project (F00920140076) obtained from the Ministry of Scientific Research, Algeria. The ethical principles and experiments were executed strictly with the OECD ethical principles and guidelines for monitoring and supervising animal experiments (OECD Test No. 420, 2002).
Acute oral toxicity
The present study was conducted according to the guideline proposed by the Organization for Economic Cooperation and Development (OECD) revised up-and down procedure for acute toxicity testing. This guideline is based on the procedure of Bruce et al. (1985). In the F. cretica extract group, one animal was treated with extract orally at a dose of 2,000 mg/kg and it was observed for any clinical signs or mortality during the first hour, then every hour for 3 hours then, periodically until 48h. If the animal survived, four additional mice were given the same dose sequentially at 48h intervals and again, clinical signs were inspected. In parallel the control group was treated with distilled water. The aimals were observed periodically during the first 24, once a day for 14 days and the number of mice that died over the experiment period was recorted. The lethal dose 50 (LD50) was established to be above 2,000 mg/kg, if no health disorders and nor death was registered in three or more mice. The animals used in the acute toxicity test were different from those used in the in vivo treatment experiment described later. A total of 5 mice were used in this separate test following the OECD guideline.
Animals and diets
Animals and diets adult male albino Mus musculus mice (2.5–3 months old), weighing 25–35 g, were used for the in vivo part of the study. The animals were obtained from the Central Pharmacy of Constantine (Algeria). A total of 20 mice were randomLy divided into four groups of five animals each. Group F received white flour (0.5 mg/kg/day). Group M was administered L-methionine (1 g/kg/day). Group MP received both L-methionine (1 g/kg/day) and Fagonia cretica (500 mg/kg/day). Group P was treated only with Fagonia cretica (500 mg/kg/day). Treatments were administered orally by incorporating the substances into flour balls. The experiment lasted 21 days. In this study, the sample size was determined based on standard practice, specifically, five animals were assigned to each group (n = 5), as commonly reported in similar previously published studies (Kehili et al., 2016; Slimani et al., 2020; Foughalia et al., 2024). This number was considered sufficient to ensure statistical relevance while complying with ethical standards for animal use.
Blood sampling and biochemical tests
Following the 21 day period of the treatment, mice were fasted overnight, and the blood samples were collected from the retro orbital vein into EDTA tubes by using microcapillary. The blood samples were centrifuged at 3,000 rpm for 15 min immediately, and the plasma was stored at -30°C.The values of plasma hs-CRP were measured by the immunoturbidimetric method on a Cobas Integra 400 plus analyzer (Roche). Total homocysteine (t-Hcy) was estimated by competitive solid phase chemiluminescence immunoassay.
Histological analysis
At the end of the treatment, the animals were sacrificed and liver, heart and aorta, were fixed in 10% formalin, dehydrated in ascending concentration of ethanol and cleared in xylene. The fixed tissue was embedded in paraffin wax and sectioned into 4-5 μm thick, then stained with hematoxylin and eosin (H&E) method.
Statistical analysis
Data from in vivo studies were analyzed using Statistical Package for Social Science (SPSS) program, version 20. In each study the experiment data were presented as mean ± SD, and the number of assays (n) is mentioned under every result. Results were analyzed by one way analysis of variance (ANOVA) followed by Tukey-test for multiple comparisons. The significant difference was considered at P < 0.05.
RESULTS
Total phenolic and flavonoid compounds
Evaluation of Total phenolic and flavonoid compounds contents demonstrated that F. cretica extract contains a high concentration of total phenolic and flavonoids and flavonols (343.86 ± 10.38 µg GAE/mL) (34.83 ± 3.76 µg QE/mL) (32.26 ± 1.86 µg QE/mL) respectively (Table 1).
Table 1. Total phenolic and flavonoid, flavonols contents of Fagonia cretica extract.
|
Extract |
Flavonoids content (µg QE/mL) |
Flavonols content (µg QE/mL) |
Total phenolic compounds content (µg GAE/mL) |
|
Butanol extract |
34.83 ± 3.76 |
32.26 ± 1.86 |
343.86 ± 10.38 |
Note: Results are shown as mean ± SD (n=3)
Phenolic composition of the butanolic extract of Fagonia cretica
HPLC analysis indicated that the main composition were 3, 4, 5-Trimethoxycinnamic acid - 3, 5-Dimethoxyphenol- 4 -Ethylphenol- 4-Methylguaiacol, 3,5-Dihydroxybenzoic acid- Coniferyl alcohol- 3-Hydroxybenzoic acid- 2,4-Dihydroxybenzoic acid. The chemical composition found in the F. cretica is mentioned in (Table 2; Figure 1). “3, 4, 5-Trimethoxycinnamic acid” and “3, 5-Dimethoxyphenol” are the most abundant compounds in the sample, with respective concentrations of 127.25 ppm and 433.32 ppm. “4-Ethylphenol” also presents a significant amount with 53.17 ppm. These results reveal a diversity of polyphenols in the butanolic extract of Fagonia cretica, with each compound contributing to its potential biological properties.
Table 2. Polyphenols composition of Fagonia cretica extract according to HPLC chromatography.
|
Compound name
|
Peak retention time |
Peak tail factor |
Peak area |
Compound amount |
Compound amount unit |
|
3,5 Dihydroxybenzoic acid |
2.54 |
0.71 |
0.39 |
0.02 |
ppm |
|
2,4-Dihydroxybenzoic acid |
10.06 |
1.95 |
101.77 |
3.20 |
ppm |
|
3-Hydroxybenzoic acid |
18.10 |
1.10 |
262.85 |
14.13 |
ppm |
|
Coniferyl alcohol |
27.37 |
1.12 |
2.04 |
0.03 |
ppm |
|
3,4,5-Trimethoxycinnamic |
34.16 |
0.92 |
1631.10 |
127.25 |
ppm |
|
4-Methylguaiacol |
43.78 |
4.81 |
72.36 |
13.75 |
ppm |
|
4-Ethylphenol |
44.05 |
34.49 |
476.80 |
53.17 |
ppm |
|
3,5-Dimethoxyphenol |
52.37 |
0.50 |
248.98 |
433.32 |
ppm |
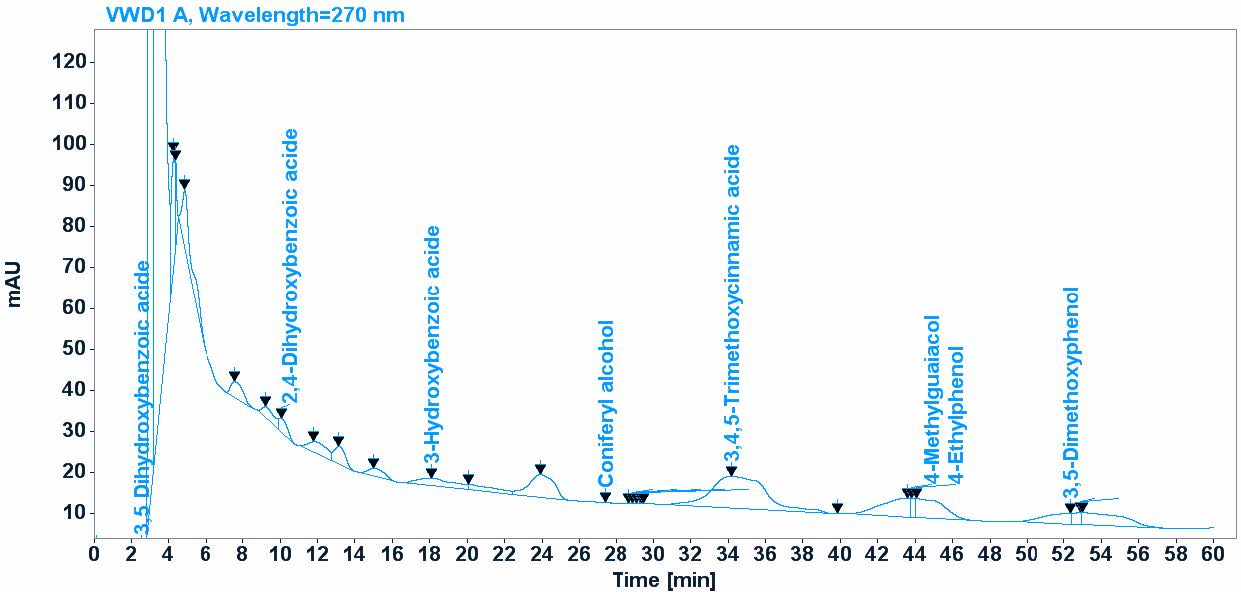
Figure 1. Polyphenols composition of Fagonia cretica extract according to HPLC chromatography.
Antioxidant activity
The F. cretica extract exhibited significant antioxidant activity, as demonstrated by the results from Table 3. It showed high scavenging potential against DPPH and ABTS and GOR radicals, with IC50 values of 110.09 ± 4.09 µg/mL and 23.06 ± 1.36 µg/mL, and 102.72 ± 7.18 respectively. Additionally, the extract was evaluated for its ability to reduce metallic ions using the Reducing Power and Hydroxyl radical and Phenanthroline assays and results revealed that the extract possessed effective reducing capabilities, with A0.50 values of 124.47 ± 19.58 µg/mL in Reducing Power assay and 63.89 ± 6.17 µg/mL in Hydroxyl radical assay, and 37.77 ± 0.94 µg/mL in Phenanthroline assays. indicating strong antioxidant activity relative to the standards.
Table 3. Antioxidant activity of Fagonia cretica extract.
|
|
DPPH• assay |
ABTS•+ |
GOR |
Reducing power assay |
Hydroxyl radical assay |
Phenanthroline |
|
Test sample |
IC50 µg/mL |
IC50 µg/mL |
IC50 µg/mL |
A0.50 µg/mL |
A0.50 µg/mL |
A0.50 µg/mL |
|
Butanol extract |
110.09 ± 4.09 |
23.06 ± 1.36 |
102.72 ± 7.18 |
124.47 ± 19.58 |
63.89 ± 6.17 |
37.77 ± 0.94 |
|
BHA |
6.14 ± 0.41 |
1.81 ± 0.10 |
5.38 ± 0.06 |
NT |
NT |
0.93 ± 0.07 |
|
BHT |
12.99 ± 0.41 |
1.29 ± 0.30 |
3.32 ± 0.18 |
NT |
NT |
2.24 ± 0.17 |
|
α-Tocopherol |
13.02 ± 5.17 |
NT |
NT |
34.93 ± 2.38 |
NT |
NT |
|
Ascorbic acid |
NT |
NT |
NT |
6.77 ± 1.15 |
32.33 ± 1.17 |
NT |
|
Tannic acid |
NT |
NT |
NT |
5.39 ± 0.91 |
NT |
NT |
Note: Results are shown as mean ± SD (n=3)
Acute oral toxicity
In the acute toxicity study, the Fagonia cretica butanolic extract at a dose of 2,000 mg/kg caused neither visible signs of toxicity or mortality, reflecting a good safety profile at a preliminary level. Thus, it can be concluded that F. cretica L. extract was safe at a dose of 2,000 mg/kg.
Effect of F.cretica on the inflammation induced by hyperhomocysteinemia
The effect of F. cretica on the homocysteine values
Hcy results for the control group (F) (22.16 ± 3.49 µmol/L), the (P) group (20.38 ± 4.75µmol/L), (M) group (50.00 ± 00.00 µmol/L), (MP) group (36.74 ± 5.86 µmol/L). Show that there is a highly significant difference between the groups in mice during 21 days of treatment (P = 0.001). The Tukey test was showed that the homocysteine concentration in mice administered with L-methionine was increased very highly significantly in group M when it was compared to the control group (P = 0,00). However the homocysteine concentration was decreased no significantly in the group of mice administered with L-methionine and treated with F. cretica when it was compared to the group (M) (P > 0.05) (Figure 2).
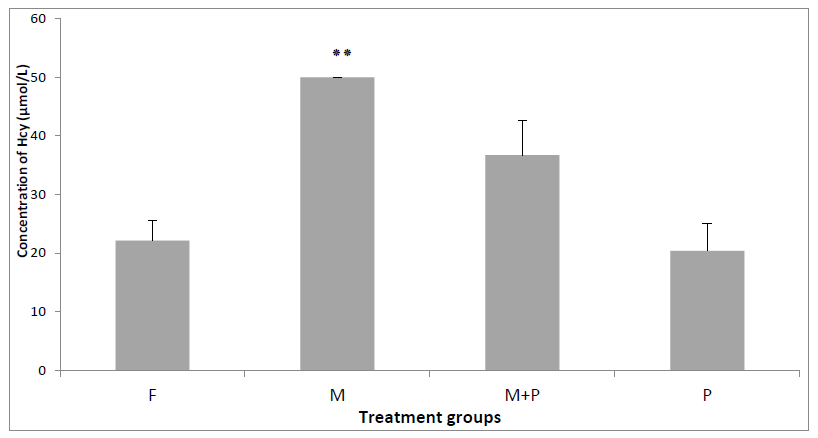
Figure 2. Total Hcy level in the groups treated during 21 days. Results are shown as mean ± SD (n=5) and significant difference from the control group is shown as *P <0.05, **P <0.01, ***P <0.001.
The effect of F. cretica on the hs-CRP values
The concentrations of hs-CRP in groups F (0.0 ± 0.0 mg/L), M (0. 046 ± 0.028 mg/L), MP (0.0 ± 0.0 mg/L) and P (0.0 ± 0.0 mg/L) were showed a significant difference between groups P ≤ 0.05. The Tukey test was revealed that the hs-CRP concentration in the group (M) was increased highly significantly when it was compared to the groups (F) and (P) P ≤ 0.01. However the concentration of hs-CRP was decreased significantly in the group (MP) when it is compared to the group (M). (Figure 3).
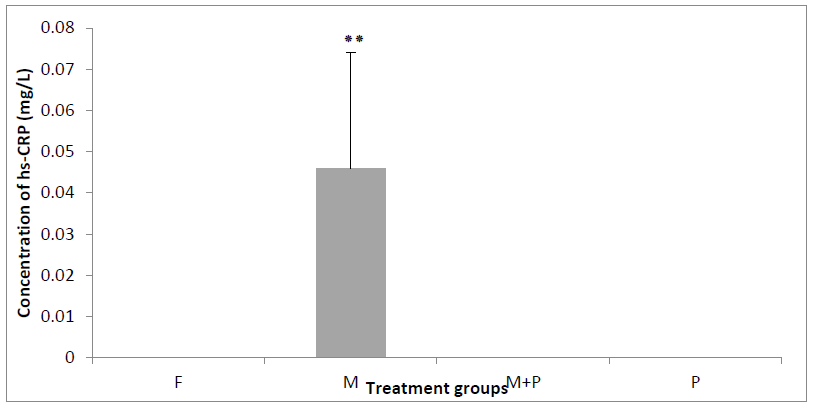
Figure 3. Concentration of hs-CRP plasma in the groups treated during 21 days. Results are shown as mean ± SD (n=5) and significant difference from the control group is shown as *P <0.05, **P <0.01, ***P <0.001.
Histological investigation
In the control group (F) and the positive control group (P), the aortic sections have intact endothelium and spindle shaped nuclei ((B, D) Figure 4). However, In the group (M), which had been fed with 1g/kg methionine, the aortic showed destruction and lysis of endothelial cells, appeared with oval nuclei of muscular fiber ((A) Figure 4).We also observed in group (M), lysis in the histological section of the cardiac muscle ((A, B) Figures 5) and hepatic necrosis in the liver histology ((A, B, C ) Figure 6), compared to the group (F) and (P) where the cardiac muscle and liver histology were intact ((D) (E) Figures 5) and ((E) (F) Figures 6) respectively. However, the aortic of the group (MP) that had been fed with 1g methionine plus F. cretica extract showed intact endothelium ((C) Figure 4). In the histological sections of the cardiac muscle and liver, no lysis and necrosis were observed ((D, E) Figures 5, 6).

Figure 4. Histological sections of the aorta of (A) treated with methionine, (B) control, (C) treated with methionine and F. cretica, and (D) treated with F. cretica for 21 days. Hematoxylin-eosin staining (A, B, C, DX 400). ENS: Endolysis, ON: Oval Nuclei, D: Destruction, IEND: Intact endothelium, SN: Spindle nuclei.

Figure 5. Histological sections of the cardiac muscle of (A) (B) treated with methionine, (C) control, (D), treated with methionine and F. cretica and (E) treated with F. cretica for 21 days. Hematoxylin-eosin staining (A, C, D and E X 400).
CMF: Cardiac Muscle Fiber, MCN: Muscle Cell Nuclei, CT: Connective Tissue, CMFL: Cardiac Muscle Fiber Lysis, L: Lysis, ICMFI: Intact Cardiac Muscle Fiber, ICMF: Intact Cardiac Muscle Fiber.
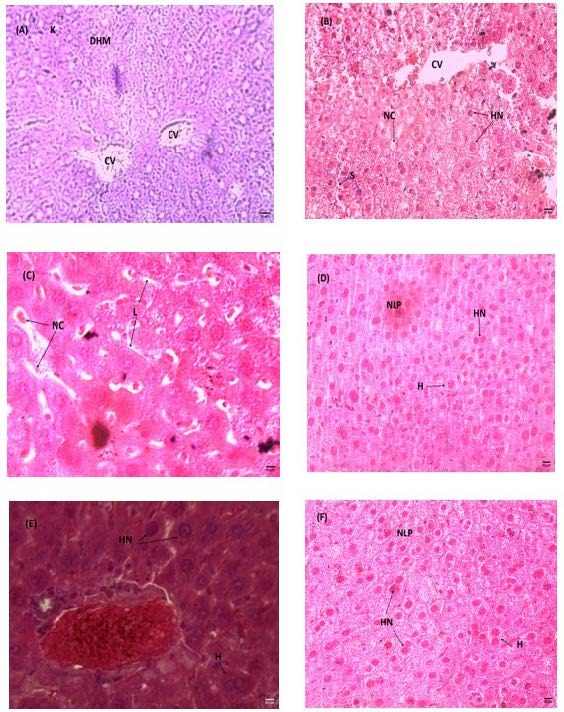
Figure 6. Histological sections of the liver of (A) (B) (C) treated with methionine, (D) control, (E) treated with methionine and F. cretica and (F) treated with F. cretica for 21 days. Hematoxylin-eosin staining (A, B, D x 100 and C, E X 400).
K: Karyolysis, DHM: Destruction Hepatocytes Membranes; S: Sinusoid, CV: Centro Lobular Vein, HN: Hepatocyte Nuclei, NC: Necrosis Cell, L: Lysis, H: Hepatocyte, NLP: Normal Liver Parenchyma.
DISCUSSION
Reactive oxygen species are generated as secondary products of aerobic metabolism and play a crucial role in various cellular signaling pathways (Sakhri et al., 2021). Phenolic compounds exhibit redox properties and function as antioxidants. Due to the hydroxyl group ability to neutralize free radicals, the phenolic content of plants can serve as an indicator of their antioxidant capacity (Malik et al., 2020). A range of studies suggests that polyphenols help reduce the risk of degenerative diseases by acting as antioxidants, thereby shielding cells from oxidative damage (Zafar et al., 2020). Flavonoids and phenolic compounds contribute significantly to antioxidant activity observed in both in vitro and in vivo studies (Malik et al., 2020). The estimation of TPC and TFC using methods described above indicates that Fagonia cretica was very rich in phenolic, flavonoid and flavonol contents. Our result is agree with Iqbal et al. (2014), other studies have reported higher findings by Yasmin et al. (2020) in methanolic extract and aqueous extract of F. cretica. These bioactive plant secondary metabolites have been an inexhaustible source of scientific interest and research (Zeljković et al., 2021). These compounds have been reported to possess important biological properties, such as anticancer, antiviral, antioxidant and antiinflammatory activities (Promraksa et al., 2021), anti-allergic (Omkar et al., 2023). Their diverse composition provides them with numerous therapeutic properties (Calvo et al., 2023). They have beneficial effects on many diseases (Moreno et al., 2022), such as cardiovascular diseases, arteriosclerosis, cancer, diabetes, cataract, disorders of cognitive function, and neurological diseases (Zeljković et al., 2021). And antithrombotic effects (Abdel Nasser et al., 2024). The compound 3,4,5-trimethoxycinnamic acid, is reported to anti-inflammatory, neuroprotective (Zhao et al., 2020), and induced anti-inflammatory cytokine (Olajide et al., 2020), anti-stress and immunocompetent actions (Olajide et al., 2020), and inhibitor of tyrosinase (Polcaro et al., 2024), and also proved the excellent antimicrobial activity of 3,4,5-trimethoxycinnamic and its derivates (Calvo et al., 2023). (3,4,5-trimethoxycinnamic acid) has been reported to have antidepressant, anticonvulsant and sedative activity (Kos et al., 2021). Gao et al. (2020) and Kang et al. (2021) reported that TMCA as a potential anti‐wrinkle agent. The compound 3,5-dimethoxyphenol has antibacterial, antifungal, antioxidant, and antiulcerogenic activities (Tabaszewska et al., 2021). The compounds 4-ethylphenol has a potent antimicrobial, regulates plant growth and has the potential to substitute antifungal agents (Ge et al., 2021). In previous studies (4-methylguaiacol) were identified in baijiu, and confirmed to possess antioxidant activity in vitro (Hong et al., 2022). The compound 3,5-dihydroxybenzoic acid has a moderate antioxidant and scavenging potential action of free radicals and inhibits lipolysis in adipocytes (Asekunowo et al., 2024). According to the scientific literature, 2,4-dihydroxybenzoic acid showed moderate antioxidant and exhibit significant antimicrobial properties against various pathogens (Popiołek et al., 2023).
The compound coniferyl alcohol is one of the four monomers that form lignin and it is widely used in the study of the synthesis mechanism and chemical structure of some natural plant products with high research and development and market value (Zhang et al., 2023). These compounds are first time reported in F. cretica, hence butanolic extract of Fagonia cretica might produce anti-inflammatory and antioxidant an effect due to presence of 3,4,5-trimethoxycinnamic acid, 3,5-dimethoxyphenol and dihydroxybenzoics acid. Recently in one study by Kamran et al. (2023) used HPLC and reported phenolic compounds including quercetin, gallic acid, vanillic acid, benzoic acid, m-coumaric acid, cinnamic acid and sinapic acid in methanol and aqueous extract. Similarly Naz et al. (2021) used HPLC and reported phenolic compounds including ferulic acid, gallic acid cinnamic acid and catechin and kaempferol, benzoic acid, coumaric acid, caffeic acid, in ethyl acetate, ethanol and methanol fractions. The protective effect observed in our study may be attributed to the high content of phenolic and flavonoid compounds in the extract, as confirmed by HPLC analysis. Several studies, despite utilizing different plant species and extract types, have similarly reported antioxidant mediated tissue protection. Such as, Zafar et al. (2020) reported that the methanolic extract of Euphorbia royleana contains several phenolic and flavonoid compounds as identified through HPLC analysis, including gallic acid, caffeic acid, chlorogenic acid, vanilic acid, quercetin, and cinnamic acid. These bioactive constituents are well documented for their potent antioxidant properties. Similarly, Malik et al. (2020) performed HPLC analysis on the methanol extract of Tamarix gallica aerial parts, idenifying quercetin, gallic acid and cinnamic acid, confirming a rich profile of phenolic and flavonoid compounds. This extract exhibited significant antioxidant capacity. Saeed et al. (2021) performed HPLC analysis on Cyperus iria aqueous-ethanol extract, identifying myercetin, quercetin, kaempferol, and ferulic acid.
F. cretica extract was found to be a high scavenging agent against DPPH and ABTS and galvinoxyl scavenging activities and reducing power assay, hydroxyl radical assay and had the strongest ability of inhibiting the hydroxyl radical as measured in phenanthroline assay. Hence, the findings obtained using DPPH are similar to those reported by Ghayas et al. (2022), who used Irochel, a polyherbal formulation comprising ethanolic extracts of Emblica officinalis (Fruit pulp), Cucurbita pepo L. (Seeds), Triticum aestivum (Grass), Fagonia cretica (Whole plant), Momordica charantia (Fruit pulp), and Tribulus terristris (Fruit) (IC50 = 106.6 ± 4.2 μg). Furthermore, El-Amier et al. (2020) reported high DPPH antioxidant activity of the F. mollis (IC50 = 0.74 mg/mL) was followed by F. arabica and F. cretica species (IC50 = 0.76 and 0.82 mg/mL, respectively). On the other hand Merchaoui et al. (2019) reported that ethanolic extract of Fagonia cretica exhibited scavenging of DPPH and ABTS activity by (IC50= 166.68 and 278.28 µg/mL, respectively). Previous studies reported that methanol and aqueous extracts of roots of F. cretica showed high antioxidant activity as compared to other solvents (Muddathir et al., 2017). Contrary to that our results showed that the butanolic extract of the aerial parts of the F. cretica exhibited high antioxidant activity, this is consistent with their high content of polyphenols, flavonoids and flavonols. The presence of powerful phenolic compounds seems to be a good reason for the antioxidant activities (Merchaoui et al., 2019). Recent studies have shown that a big number of flavonoids contribute significantly to the total antioxidant activity of many fruits and medicinal plants (Merchaoui et al., 2019). Flavonoids exhibit strong scavenging activity against oxidative molecules, including singlet oxygen, which contributes to the reduction of free radicals and the enhancement of the body antioxidant defense mechanisms (Malik et al., 2020). A high dose (1g/kg) of methionine administration to mice for 21 days was sufficient to induce hyperhomocysteinemia by high significant increase of plasma homocysteine compared to the normal group. Our results are agree with the work of Ferhi et al. (2019) who reported that there is an increase in plasma homocysteine in mice administered with high dose of L-methionine (1g ̸ kg ̸ day). Numerous findings have shown a strong association between Hcy levels and the clinical manifestations of atherosclerotic diseases such as stroke, peripheral artery disease, carotid artery disease and chronic kidney disease (Salvio et al., 2021). In addition, hyperhomocysteinemia was also reported to be associated with metabolic syndrome, obesity, insulin resistance, and inflammation (Pan et al., 2020). Homocysteine has a positive correlation with endothelin-1 (ET-1), a potent vasoconstrictor (Shirode et al., 2023). Hcy increases the oxidative stress by the generation of free radicals and inhibition of glutathione peroxidase. Free radicals can directly cause endothelial dysfunction (Sharma et al., 2015). Cao et al. (2021) reported that high-senstivity C-reactive protein (hs-CRP) plays an important role. It is a commonly used as inflammatory marker. Benmebarek et al. (2013), confirmed that methionine at dose of 200 mg/kg/day administered to mice, during the 21 days period, increased significantly the levels of plasma hs-CRP. Elevated hs-CRP levels can damage vascular endothelial cells through activation of inflammatory reactions and induction of oxidative stress (Cao et al., 2021). Our results are in agreement with the previous experimental study of Benmebarek et al. (2013) who found that hyperhomocysteinemia as angiotoxic and toxic activity explained by the loss and degeneration of the endothelium, formation of foam cells in the different sections of the aorta, change in the smooth muscle cells nuclei forms from a fusiform aspect to a rounded appearance, and the alterations of the cardiac muscle and liver necrosis. Zerizer et al. (2004) observed alterations in the aorta. In addition Vatsalya et al. (2021) reported the structural alterations in the aorta, heart and liver caused by the administration of high doses of methionine in rats during of 21 days of experimental study. Homocysteine causes hepatic injury due to its involvement in several key mechanisms. It activates necrosis factor-κB (NF-κB) and increases the production of proinflammatory cytokines, resulting in inflammatory reactions. The administration of methionine to rats can lead to morphological in liver tissue such as rare focal necrosis of hepatocytes and periportal mononuclear infiltration (Todorovic et al., 2021). Tissue damage caused by oxidative stress may trigger inflammatory responses (Zaib et al., 2020). Previous studies have indicated that herbal remedies may improve oxidative stress markers in organs such as the liver (Zafar et al., 2020). Results are in agreement with the previous experimental studies of Hemal et al. (2024) showed that treatment by crude extracts of Fagonia tenuifolia only administered and associated with methionine induced hyperhomocysteinemia in mice caused significant improvement of all oxidative stress parameters towards its normal ranges. Other experiments indicate that the administration of A. spinosa in combination with methionine in mice decreased the concentration of homocysteine, and ameliorated biomarkers changes (Aklil et al., 2017). A variety of well-known antioxidants, including polyphenolic antioxidants, have been shown to exert a protective action against Hcy toxicity (Malinowska et al., 2012). Supporting this, previous studies have shown comparable histological improvements following treatment with phenolic and flavonoid rich extracts. Zafar et al. (2020) reported antioxidant mediated tissue protection associated with reduced degeneration. Malik et al. (2020) observed significant restoration of tissue architecture post treatment. Similarly, Saeed et al. (2021) noted marked histological improvements, including reduced inflammation, minimized degeneration, and overall structural recovery, aligning with the high antioxidant potential of their plant extract.
Recently, plants have become a good source for new anti-inflammatory compounds (Mohamed et al., 2024). Crude phytochemical extracts and isolated compounds from F. cretica L have been shown to have a diverse range of biological activities including amongst others anti-haemorrhagic, anti-tumor, anti-inflammatory and neuroprotective cytotoxic anti-inflammatory and neuroprotective (Nazir et al., 2017). Active metabolites including the anti-inflammatory and anti-tumour triterpenes taraxerol and ursolic acid, and triterpenoid saponins with hederagenin and ursolic acid and oleanoic acid aglycones have been isolated (Nazir et al., 2017).
CONCLUSION
The present study concluded that, the butanol extract of the aerial parts of F. cretica is rich in phenolic compounds and exhibits excellent antioxidant activity. The HPLC (High Performance Liquid Chromatography) analysis confirms the presence 3,4,5-Trimethoxycinnamic acid - 3,5-Dimethoxyphenol- 4-Ethylphenol- 4-Methylguaiacol, 3,5-Dihydroxybenzoic acid- Coniferyl alcohol- 3-Hydroxybenzoic acid- 2,4-Dihydroxybenzoic acid in butanolic extract of Fagonia cretica. Moreover possess a significant anti hyperhomocysteinemia and anti-inflammatory effect in controlling the blood total Hyc and hs-CRP level effect at the dose of 500 mg/kg body weight. And it could be used to protect against the homocysteine mediated free oxygen radicals damages. It could be clearly depicted that selected remedies have great potential to reduce liver, heart, aorta damages. Thus further examination is expected to inspect the impact of each separated compound present in the plant extract and to explain the ultimate mechanisms of it’s therapeutic mode.
ACKNOWLEDGMENTS
The authors are grateful to the MESRS (Ministry of Scientific Research, Algeria).
AUTHOR CONTRIBUTIONS
Bouhouche I and Zerizer S: developed research methodology and analysed data. Bouhouche I wrote the manuscript, Zerizer S: supervised the study and revised and edited the manuscript, Kabouche Z: provided materials for the extraction of plants and Bensouici C helped in vitro study.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Abdel Nasser, A., Badr, A.N., Fathy, H.M., Ghareeb, M.A., Barakat, O.S., and Hathout, A.S. 2024. Antifungal, antiaflatoxigenic, and cytotoxic properties of bioactive secondary metabolites derived from Bacillus species. Scientific Reports. 14: 16590.
Aklil, B., Zerizer, S., and Kabouche, Z. 2017. The protective effects of Argania spinosa seeds against hyperhomocysteinemia induced by a high methionine diet in mice. International Journal of Pharmacy and Pharmaceutical Sciences. 9(12): 64–69.
Asekunowo, A.K., Okoro, I.S., Umoh, S.D., Siwe Noundou, X., Kraus, R.W.M., Okoh, O.O., Ashafa, A.O.T., Asekuna, O.T., and Familoni, O.B. 2024. Antioxidant evaluation and bio-guided isolation from methanol leaf extract of Acalypha godseffiana. Journal of the Nigerian Society of Physical Sciences. 6: 2038.
Azzini, E., Ruggeri, S., and Polito, A. 2020. Homocysteine: Its possible emerging role in at-risk population groups. International Journal of Molecular Sciences. 21: 1421.
Benmebarek, A., Zerizer, S., Laggoune, S., and Kabouche, Z. 2013. Effect of Stachys mialhesi de Noé on the inflammation induced by hyperhomocysteinemia in cardiovascular diseases. Der Pharmacia Lettre. 5(2): 212–223.
Blois, M.S. 1958. Antioxidant determinations by the use of a stable free radical. Nature. 4617 (181): 1119-1200.
Bruce, R.D. 1985. An up-and-down procedure for acute toxicity testing. Fundamental and Applied Toxicology. 5: 151-157.
Calvo, L.G., Castillo, A., Villarino, R., Rama, J.L.R., Abril, A.G., and Miguel, T. 2023. Study of the antibacterial activity of rich polyphenolic extracts obtained from Cytisus scoparius against foodborne pathogens. Antibiotics. 12: 1645.
Cao, L., Guo, Y., and Zhu, Z. 2021. Effects of hyperhomocysteinemia on ischemic cerebral small vessel disease and analysis of inflammatory mechanisms. International Journal of Neuroscience. 131(4): 362–369.
Chang, J., Liu, C., Liu, T., and Chang, S. 2019. Effects of intravascular laser irradiation of blood on cognitive function in a stroke survivor with hyperhomocysteinemia: Dual recuperations in thalamus and serum homocysteine. Journal of Scientific and Technical Research. 16: 2574–1241.
El-Amier, Y.A., Iman, A., and Aish, A. 2020. Phytochemical constituents of common growing Fagonia species (Zygophyllaceae) in Egyptian deserts and its biological activities. Plant Archives. 19: 2213–2219.
Ferhi, S., Zerizer, S., and Dhallewin, G. 2019. The effects of grape leaves extract on hyperhomocysteinemia-induced inflammatory endothelial damage in cardiovascular diseases. Applied Ecology and Environmental Research. 17(2): 1989–2003.
Foughalia, A., Zerizer, S., Aribi, B., Kabouche, Z., and Bensouici, C. 2024. Antioxidant, anti-inflammatory, anti-arthritic activities and acute toxicity of Calendula stellata n-butanol extract from Algeria. Asian Journal of Agriculture and Biology. 2: 1–11.
Gao, D., Cho, C.W., Kim, C.T., Jeong, W.S., and Kang, J.S. 2020. Evaluation of the antiwrinkle activity of enriched Isatidis folium extract and an HPLC–UV method for the quality control of its cream products. Plants. 9: 1586.
Ge, T., Gao, W., Liang, C., Han, C., Wang, C.Y., Xu, Q., and Wang, Q. 2021. 4-Ethylphenol, a volatile organic compound produced by disease-resistant soybean, is a potential botanical agrochemical against oomycetes. Frontiers in Plant Science. 12: 717258.
Ghayas, S., Hannan, A., and Rizwani, G. H. 2022. Phytochemical, antioxidant, toxicological, and pharmaceutical evaluation of polyherbal formulation: Irochel. Dose-response. 20(1): 1-17.
Hemal, M., Zerizer, S., Kabouche, Z., and Rouabhi, R. 2024. Protective effects of Fagonia tenuifolia methanolic extracts against hyperhomocysteinemia produced by methionine, by repeal of oxidative stress markers in mice. Journal of Bioresource Management. 11(3): 106–118.
Hong, J., Chen, L., Zhao, D., and Sun, J. 2022. Multivariate analysis on the relationship between radical scavenging activities and phenolic compounds of baijiu and its protective effect against LPS-induced inflammation in THP-1 cells. International Food Research Journal. 29(1): 116–127.
Iqbal, P., Ahmed, D., and Asghar, M.N. 2014. A comparative in vitro antioxidant potential profile of extracts from different parts of Fagonia cretica. Asian Pacific Journal of Tropical Medicine. 7(Suppl 1): S473–S480.
Kang, J., and Yadav, N.S. 2021. Special issue editorial: Isolation and analysis of characteristic compounds from herbal and plant extracts. Plants. 10: 2775.
Kamran, S., Anwar, R., Noor, A., Ihsan Ullah, M., Bagalagel, A. A., Aldurdunji, M. M., and Ishtiaq, S. 2023. Metabolic profiling and investigation of the modulatory effect of Fagonia cretica L. aerial parts on hepatic CYP3A4 and UGT2B7 enzymes in a streptozotocin-induced diabetic model. Antioxidants. 12(1): 119.
Kumaran, A. and Joel Karunakaran, R. 2007. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT- Food Science and Technology. 40(2): 344–352.
Kehili, H.E., Zerizer, S., Beladjila, A., and Kabouche, Z. 2016. Anti-inflammatory effect of Algerian date fruit (Phoenix dactylifera). Food and Agricultural Immunology. 27(6): 820–829.
Keskin, A., Ustun, G., Aci, R., and Duran, U. 2022. Homocysteine as a marker for predicting disease severity in patients with COVID-19. Biomarkers in Medicine. 16(7): 559–568.
Kos, J., Strharsky, T., Stepankova, S., Svrckova, K., Oravec, M., Hosek, J., Imramovsky, A., and Jampilek, J. 2021. Trimethoxycinnamates and their cholinesterase inhibitory activity. Applied Sciences. 11: 4691.
Kovalska, M., Baranovicova, E., Kalenska, D., Tomascova, A., Adamkov, M., Kovalska, L., and Lehotsky, J. 2021. Methionine diet-evoked hyperhomocysteinemia causes hippocampal alterations, metabolomics plasma changes and behavioral pattern in wild type rats. International Journal of Molecular Sciences. 22: 4961.
Malik, M., Sharif, A., Hassan, S.U., Muhammad, F., Khan, H.M., Akhtar, B., and Saeed, M. 2020. Amelioration of hyperglycaemia and modulation of pro-inflammatory cytokines by Tamarix gallica fractions in alloxan-induced diabetic rats. Archives of Physiology and Biochemistry. 128(6): 1666-1675.
Malinowska, J., Kolodziejczyk, J., and Olas, B. 2012. The disturbance of hemostasis induced by hyperhomocysteinemia; the role of antioxidants. Acta Biochimica Polonica. 59(2): 185–194.
Merchaoui, H., Ben Mansour, R., Oueslati, M., Medini, F., Hanana, M., and Ksouri, R. 2019. A comparative evaluation of total polyphenolic content and antioxidant potential of thirty medicinal halophytes from the Mediterranean region. Journal of Agricultural Science and Technology. 21: 1433–1446.
Mohamed, E.I.A., Elwekeel, A.H., Mohamed, D.E., Zaki, M.A., and Hassan, M.H.A. 2024. Validating anti-inflammatory and cytotoxic properties of Fagonia cretica L. through metabolic, in vitro, and in silico profiling. BMC Complementary Medicine and Therapies. 24: 411.
Moon, H., Ko, H., and Kim, A. 2020. Hyperhomocysteinemia concurrent with metabolic syndrome is independently associated with chronic kidney disease among community-dwelling adults in an urban Korean population. International Journal of Environmental Research and Public Health. 17: 6810.
Moreno, M.T., Brito, R.E., and Rodríguez Mellado, J.M.R. 2022. Structure-antioxidant capacity relationships of the dihydroxy derivatives of benzaldehyde and benzoic acid. Organic Chemistry Plus. 3(1): 1233.
Muddathir, A.M., Yamauchi, K., Batubara, I., Mohieldin, E.A.M., and Mitsunaga, T. 2017. Anti-tyrosinase, total phenolic content and antioxidant activity of selected Sudanese medicinal plants. South African Journal of Botany. 112: 9–15.
Müller, L., Gnoyke, S., Popken, A.M., and Böhm, V. 2010. Antioxidant capacity and related parameters of different fruit formulations. LWT - Food Science and Technology. 43: 992–999.
Naeem, K., Yawar, W., Muhammad, B., and Rehana, I. 2014. Assessment of macronutrients and heavy metals in Fagonia cretica linn of pakistan by atomic spectroscopy. Bulletin of the Chemical Society of Ethiopia. 28: 177-185.
Nagaraj, S. 2013. Antihyerlipidemic activity of Fagonia cretica l. Whole plant. International Journal of Pharmacotherapy. 3(2): 52-54.
Nazir, I., Rahman, N., Alvi, Z., Rahman, H., Sendker, J., Zhang, T., Frankish, N., and Sheridan, H. 2017. Antidiabetic activities of an LC/MS fingerprinted aqueous extract of Fagonia cretica L. in preclinical models. Planta Medica. 83: 1141–1148.
Naz, S., Khalid, A., and Manzoor, S. 2021. Phenolic profiling and antimicrobial studies of Fagonia cretica native to Pakistan. Journal of Advances in Nutrition Science and Technology. 1(1): 19–32.
Olajide, O.A., Akande, I.S., Bezerra Filho, C., Lepiarz-Raba, I., and de Sousa, D.P. 2020. Methyl 3,4,5-trimethoxycinnamate suppresses inflammation in RAW264.7 macrophages and blocks macrophage–adipocyte interaction. Inflammopharmacology. 28(5): 1315–1326.
Omkar, S., Sruthi, K., Rutuja, M.S., Pravin, S.G., and Praveena, B. 2023. Plant secondary metabolites: Extraction, screening, analysis and their bioactivity. International Journal of Herbal Medicine. 11(2): 1–17.
Oyaizu, M. 1986. Studies on products of browning reactions: Antioxidative activities of browning reaction prepared from glucosamine. Japanese Journal of Nutrition. 44: 307–315.
Pan, Q., Wang, Y., and Wang, G. 2020. The association between hyperhomocysteinemia and thyroid nodule prevalence in an adult population. Metabolic Syndrome and Related Disorders. 18(8): 368- 372.
Polcaro, L.M., Masullo, M., and Piacente, S. 2024. Effects of green extraction methods on the chemical profile of Schisandra chinensis fruit extracts and on their tyrosinase inhibitory activity. LWT - Food Science and Technology. 208: 116697.
Popiołek, L., Głownia-Łapińska, M., Dziduch, A., Biernasiuk, A., Pi, I., Chmiel, A., and Herbet, M. 2023. Design, synthesis, and in vitro and in vivo bioactivity studies of hydrazide–hydrazones of 2,4-dihydroxybenzoic acid. International Journal of Molecular Sciences. 24: 17481.
Promraksa, B., Katrun, P., Phetcharaburanin, J., Kittirat, Y., Namwat, N., Techasen, A., Li, J., and Loilome, W. 2021. Metabolic changes of cholangiocarcinoma cells in response to coniferyl alcohol treatment. Biomolecules. 11: 476.
Rawashdeh, S., Al-Mistarehi, A., Yassin, A., Rabab’ah, W., Skaff, H., and Ibdah, R. 2020. A concurrent ischemic stroke, myocardial infarction, and aortic thrombi in a young patient with hyperhomocysteinemia: A case report. International Medical Case Reports Journal. 13: 581–590.
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., and Rice-Evans, C. 1999. Antioxidant activity applying an improved ABTS radical cationdecolorizationassay. Free Radical Biology & Medicine. 26: 1231-1237.
Rehman, T., Shabbir, M., Ur-Raheem, M., Manzoo, M., Ahmad, N., Liu, Z., Ahmad, M., Siddeeg, A., Abid, M., and Aadil, R. 2020. Cysteine and homocysteine as biomarkers of various diseases. Food Science and Nutrition. 8(9): 4696–4707.
Saeed, M., Sharif, A., Hassan, S.U., Akhtar, B., Faqir, M., and Malik, M. 2021. Cyperus iria aqueous ethanol extract ameliorated hyperglycemia, oxidative stress, and regulated inflammatory cytokines in streptozotocin induced diabetic rats. Environmental Science and Pollution Research. 29(3): 4769–4784.
Sakhri, F.Z., Zerizer, S., and Bensouici, C. 2021. Evaluation of the antioxidant, antidiabetic and immunomodulatory activity of Cydonia oblonga fruit extract. Chiang Mai University Journal of Natural Sciences. 20(3): e2021052.
Salvio, G., Ciarloni, A., Cutini, M., and Balercia, G. 2021. Hyperhomocysteinemia: Focus on endothelial damage as a cause of erectile dysfunction. International Journal of Molecular Sciences. 22: 418.
Sayar, S. and Sharma, A. 2019. Primary metabolite profiling and potential antioxidants activity from Fagonia cretica (stem and roots). Journal of Drug Delivery and Therapeutics. 9(4-A): 376–380.
Šebeková, K., Gurecká, R., Repiská, G., Koborová, I., and Podracká, L. 2022. The presence of hyperhomocysteinemia does not aggravate the cardiometabolic risk imposed by hyperuricemia in young individuals: A retrospective analysis of a cross-sectional study. International Journal of Environmental Research and Public Health. 19: 13521.
Salah Eldin, H., Abdal Gadir, H., and Hassan, A.W. 2015. Evaluation of the hepatoprotective activity of Fagonia cretica L. Journal of Pharmacognosy and Phytochemistry. 3(3): 1- 6.
Sharma, M., Tiwari, M., and Tiwari, R. 2015. Hyperhomocysteinemia: Impact on neurodegenerative diseases. Basic and Clinical Pharmacology & Toxicology. 117: 287–296.
Shi, H., Noguchi, N., and Niki, E. 2001. Galvinoxyl method for standardizing electron and proton donation activity. Methods in Enzymology. 335: 157-166.
Shirode, P., Parekh, A., Patel, V., Vala, J., Jaimalani, A., Vora, N., Gummala, V., Patel, J., and Shriram, N. 2023. Early detection of subclinical atherosclerosis: Hyperhomocysteinemia as a promising marker in adolescents with vitamin B deficiency. Cureus. 15(7): e41571.
Slimani, W., Zerizer, S., and Kabouche, Z. 2020. Immunomodulatory and anti-arthritic activities of Stachys circinata. Jordan Journal of Biological Sciences. 13(2): 183–189.
Smirnoff, N. and Cumbes, Q.J. 1989. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry. 28: 1057–1060.
Szydlowska-Czerniaka, A., Dianoczki, C., Recseg, K., Karlovits, G., and Szlyk, E. 2008. Determination of antioxidant capacities of vegetable oils by ferric-ion spectrophotometric methods. Talanta. 76: 899-905.
Tabaszewska, M., Rutkowska, J., Skoczylas, L., Słupski, J., Antoniewska, A., Smole, S., Łukasiewicz, M., Baranowski, D., Duda, I., and Pietsch, J. 2021. Red arils of Taxus baccata L. a new source of valuable fatty acids and nutrients. Molecules. 26(3): 723.
Todorovic, D., Stojanovic, M., Medic, A., Gopcevic, K., Mutavdzin, S., Stankovic, S., and Djuric, D. 2021. Four weeks of aerobic training affects cardiac tissue matrix metalloproteinase, lactate dehydrogenase and malate dehydrogenase enzymes activities, and hepatorenal biomarkers in experimental hyperhomocysteinemia in rats. International Journal of Molecular Sciences. 22: 6792.
Topçu, G., Ay, A., Bilici, A., Sarıkürkcü, C., Öztürk, M., and Ulubelen, A. 2007. A new flavone from antioxidant extracts of Pistacia terebinthus. Food Chemistry. 103: 816–822.
Vatsalya, V., Gala, K., Hassan, A., Frimodig, J., Kong, M., Sinha, N., and Schwandt, M. 2021. Characterization of early-stage alcoholic liver disease with hyperhomocysteinemia and gut dysfunction and associated immune response in alcohol use disorder patients. Biomedicines. 9: 7.
Vezzoli, A., Dellanoce, C., Caimi, T., Vietti, D., Montorsi, M., Mrakic-Sposta, S., and Accinni, R. 2020. Influence of dietary supplementation for hyperhomocysteinemia treatments. Nutrients. 12(7): 1957.
Xiang, Y., Zhao, Q., Wang, N., Yu, Y., Wang, R., Zhang, Y., Cui, S., Wu, Y., Liu, X., Jiang, Y., et al. 2021. Association of obesity with the risk of hyperhomocysteinemia among the Chinese community residents: A prospective cohort study in Shanghai, China. Nutrients. 13: 3648.
Yakovleva, O., Bogatova, K., Mukhtarova, R., Yakovlev, A., Shakhmatova, V., Gerasimova, E., Ziyatdinova, G., Hermann, A., and Sitdikova, G. 2020. Hydrogen sulfide alleviates anxiety, motor, and cognitive dysfunctions in rats with maternal hyperhomocysteinemia via mitigation of oxidative stress. Biomolecules. 10: 995.
Yang, Z., Yang, Y., Yang, J., Wan, X., Yang, H., and Wang, Z. 2020. Hyperhomocysteinemia induced by methionine excess is effectively suppressed by betaine in geese. Animals. 10: 1642.
Yao, L., Cao, B., Cheng, Q., Cai, W., Ye, C., Liang, J., Liu, W., Tan, L., Yan, M., Li, B., et al. 2019. Inhibition of soluble epoxide hydrolase ameliorates hyperhomocysteinemia-induced hepatic steatosis by enhancing β-oxidation of fatty acids in mice. American Journal of Physiology – Gastrointestinal and Liver Physiology. 316: G527–G538.
Yasmin, T., Azam, M., Ummaimah, and Basra, M.A.R. 2020. Bioactive compounds, antioxidant, and antineoplastic activities of Asian herbs. Chulalongkorn Medical Journal. 64: 191–202.
Zafar, M., Sharif, A., Khan, D., Akhtar, B., Muhammad, F., Akhtar, M.F., and Fatima, T. 2020. Preventive effect of Euphorbia royleana Boiss. on diabetes induced by streptozotocin via modulating oxidative stress and deoxyribonucleic acid damage. Toxin Reviews. 40(4): 777-790.
Zaib, M., Sharif, A., Akhtar, B., Khan, H. M., Akhtar, M.F., Hassan, W., Razzaq, F., Nawaz, S., and Qaisar, N. 2020. Berberis lycium Royle extracts attenuate inflammation and modulates hyperglycemia in alloxan induced diabetic rats. Pakistan Journal of Pharmaceutical Sciences. 33(4): 1805–1813.
Zeljković, S., Šišková, J., Komzáková, K., Diego, N., Kaffková, K., and Tarkowski, P. 2021. Phenolic compounds and biological activity of selected Mentha species. Plants. 10(3): 550.
Zerizer, S. and Naimi, D. 2004. Homocysteine: An independent risk factor in atherogenic process. Egyptian Pharmaceutical Journal. 3: 110–114.
Zhang, Y., Wei, B., and Tang, R. 2023. Theoretical study on the mechanisms, kinetics, and toxicity evaluation of OH-initiated atmospheric oxidation reactions of coniferyl alcohol. Atmosphere. 14(6): 976.
Zhao, X., Xu, B., Wu, P., Zhao, P., Guo, C., Cui, Y., Zhang, Y., Zhang, X., and Li, H. 2020. UHPLC-MS/MS method for pharmacokinetic and bioavailability determination of five bioactive components in raw and various processed products of Polygala tenuifolia in rat plasma. Pharmaceutical Biology. 58(1): 969–978.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Ibtissem Bouhouche1, *, Sakina Zerizer1, *, Zahia Kabouche2, and Chawki Bensouici3
1 Département de Biologie Animale, Laboratoire d’Immunologie et Activités Biologiques des Substances Naturelles, Université Constantine 1 frères Mentouri, 25000 Constantine, Algeria.
2 Département de Chimie, Laboratoire d’Obtention de Substances Thérapeutiques, Université Constantine 1 frères Mentouri, 25000 Constantine, Algeria.
3 Centre de Recherche en Biotechnologie (C.R.Bt), Ali Mendjli Nouvelle Ville BPE.73 Constantine, Alegria.
Corresponding author: Ibtissem Bouhouche, E-mail: ibtissem.bouhouche@doc.umc.edu.dz
Sakina Zerizer, E-mail: zerizer.Sakina@umc.edu.dz
ORCID: Sakina Zerize: https://orcid.org/0000-0001-5414-6564
Total Article Views
Editor: Veerasak Punyapornwithaya,
Chiang Mai University, Thailand
Article history:
Received: May 9, 2025;
Revised: August 2, 2025;
Accepted: August 7, 2025;
Online First: August 27, 2025

