
Evaluation of Emulsion Stability and Skin Healing Efficacy of Topical Formulations Containing Viable Lacticaseibacillus rhamnosus GG
Ketrawee Pantub, Ekachai Dumkliang, Somboon Tanasupawat, and Tanatip Thamacharoensuk*Published Date : August 15, 2025
DOI : https://doi.org/10.12982/NLSC.2025.066
Journal Issues : Number 4, October-December 2025
Abstract Live microorganisms such as probiotics play a key role in maintaining skin barrier function by helping to balance the microbiome. Functionally, preparing probiotic skincare products is challenging because live cultures can be unstable in conventional packaging. This study aimed to formulate and evaluate emulsions containing Lacticaseibacillus rhamnosus GG (LGG), focusing on its viability, stability, and bioactivity. Emulsions were prepared using either live LGG strains or spray-dried microencapsulated LGG, and grouped into control bases, pH-adjusted, and preservative-added formulations. All formulations were assessed for LGG viability, appearance, pH, and viscosity. The favorable characteristic formula was further selected by microbial contamination test, antioxidant activity, ability to promote skin fibroblast proliferation, and wound healing efficacy. The results showed that the spray drying technique gives a 78.02% process yield with 96.52% LGG survival. The results from the viability test suggest that microencapsulation can support LGG survival; however, it did not improve viability in emulsion, likely due to potential nutrient effects from the wall material during storage. Further studies revealed that the LGG-loaded emulsions formulated with a pH-adjusted (F6) and a preservative-added (F9) formulation were promising. Among all formulations, the pH-adjusted emulsion containing live LGG (F6) demonstrated the highest stability, retained viability, and was free from microbial contamination. This formulation exhibits antioxidant activity, promotes fibroblast proliferation and migration, and facilitates wound repair. This finding suggests that a live LGG-loaded emulsion with optimized pH may apply for topical applications targeting skin wound healing.
Keywords: Emulsion, Formulation, Lacticaseibacillus rhamnosus GG, Stability, Topical, Wound healing
Citation: Pantub, K., Dumkliang, E., Tanasupawat, S., and Thamacharoensuk, T. 2025. Evaluation of emulsion stability and skin healing efficacy of topical formulations containing viable Lacticaseibacillus rhamnosus GG. Natural and Life Sciences Communications. 24(4): e2025066.
INTRODUCTION
Skin microbial imbalance is intricately linked to the development of various skin-associated disorders, including atopic dermatitis, rosacea, acne vulgaris, and psoriasis (Hsu et al., 2020; Pessemier et al., 2021; Moniaga et al., 2022). While many studies have focused on oral probiotic administration (Krutmann, 2012; Wieërs et al., 2020; Sinha et al., 2021; Mahmud et al., 2022), topical treatment also presents an interesting therapeutic strategy. Despite a limited number of trials, clinical studies have begun to reveal the effectiveness of topical applications. For example, probiotics like Lactobacillus bulgaricus, L. acidophilus, or L. plantarum have been shown to improve acne treatment by reducing skin colonization by Cutibacterium acnes (Muizzuddin et al., 2012; Bowe and Rath, 2019). Lacticaseibacillus rhamnosus GG (LGG), previously known as Lactobacillus rhamnosus GG until its genus was reclassified (Zheng et al., 2020), is well-known probiotic strains most studied by scientific report (Yang et al., 2025). This strain combined with prebiotics in topical formulations provides an effective treatment for sunburn (Kaur and Rath, 2019). By adding live LGG, the formulation can help maintain or restore the optimal acidic pH (typically ranging from 4 to 6), which is essential for the skin microbiome. This balance is vital for preventing harmful bacteria, enhancing keratinocyte differentiation processes, leading to improved skin barrier function and overall skin health (Grice and Segre, 2011; Lukic et al., 2021; Steele, 2022). However, the survival of probiotics is challenged by several factors, such as the environment during processing and storage. To address these challenges, microencapsulation techniques have been widely studied and shown to effectively protect LGG from environmental stresses, including acidity, oxidative stress, oxygen, and temperature (Mahmoud et al., 2020). For this reason, the encapsulation technique is applied to protect probiotics from damage caused by environmental factors, using the same process as in a previous study, which involved whey protein isolate and glutinous rice starch to preserve bacterial viability and develop emulsions for skincare applications (Thamacharoensuk et al., 2020).
Numerous cosmetic products claim to contain probiotics, while they only include extracts or lysates. Such products should be more appropriately classified as postbiotic formulations. Postbiotics have been commonly used in skincare as non-living microorganisms, including bacterial lysates, ferments, and filtrates (Almeida et al., 2023; Dou et al., 2023). The formulation can be prepared in both hydrophilic and lipophilic forms, such as toners, mists, serums, gels, lotions, creams, and masks. From a research and development perspective, it is possible to specify storage conditions and shorten the period after opening, as appropriate. Proper packaging is also crucial; it should be able to protect LGG from environmental factors. Emulsions are an effective form of skincare that can be prepared using an additional cold process to ensure that the probiotics are not sterilized by heat. The combination of water and oil makes it ideal for holistic skincare. The emulsion represents dispersions consisting of two liquid phases that are incapable of mixing but can be combined through mechanical shear and surfactant (Goodarzi and Zendehboudi, 2019). Within the pharmaceutical and cosmetic industries, there is a growing interest in emulsions as carriers of bioactive compounds. However, the production of such systems presents challenges due to their proneness to destabilization during storage. Emulsion instability can be attributed to various physical and chemical mechanisms, including oxidation, creaming, coalescence, flocculation, phase inversion, and others (Ravera et al., 2012). The incorporation of antioxidants can help protect ingredients from deterioration caused by the oxidation process (Sessa et al., 2013; Sharmeen et al., 2021). Therefore, tocopheryl acetate and ethyl panthenol are used in these emulsions to enhance and preserve stability. In this study, we aim to evaluate the feasibility of using live LGG in cosmetics, monitor the growth of potentially dangerous bacterial contamination, and reassess the possibility of biological activity in the formula with the best physical characteristics. In parallel, encapsulated LGG bacteria were also investigated to examine their compatibility with the formulations. Stability tests were performed under ambient temperature to explore their practical application in daily life. The findings of this study provide important preliminary information for probiotic cosmetics. They may serve as a prototype for further development, highlighting the importance of maintaining bacterial viability and stability, and also enabling a clearer direction for studying dermatological effects.
MATERIAL AND METHODS
Materials
Lacticaseibacillus rhamnosus GG (LMG 18243) was obtained from the Belgian Co-ordinated Collections of Microorganisms Bacterial Collection (BCCM) in Ghent, Belgium. This strain was isolated from Human faeces. Culture media, including de Man Rogosa Sharpe (MRS), Sabouraud Dextrose Agar (SDA), and Tryptic Soy Agar (TSA), were purchased from Sigma (Bangkok, Thailand). Sodium Polyacrylate (and) polyisobutene, cyclomethicone, tocopheryl acetate (vitamin E), and ethyl panthenol (vitamin B5) were purchased from Chanjao Longevity Co., Ltd. Bangkok, Thailand. The normal human skin fibroblasts (43rd-44th passage) provided by Manose Health and Beauty Research Center Co., Ltd Chiang Mai, Thailand.
Preparation of encapsulated LGG and LGG cells
LGG cells were cultivated in 500 ml of MRS broth at 37°C for 48 hours and harvested by centrifugation at 3,000 rpm for 6 min. They were washed three times in phosphate saline buffer (pH 7.4). Cell density was determined by standardizing with a 0.5 McFarland standard and the surface-spread plate technique on MRS agar in duplicate to obtain a final concentration of ~108 CFU/ml. The resulting bacterial concentration was either used for encapsulation or used directly for live cell. Encapsulated LGG was prepared by the spray-drying technique following Thamacharoensuk et al. (2020) conditions. The wall materials were a mixture of whey protein isolated and glutinous rice flour in a 4:1 ratio and autoclaved at 121°C for 15 mins. Whey protein was dispersed into autoclaved DI water at 25°C. Then followed by glutinous rice flour, stirred for 10 mins, and adjusted to pH 7.5 with 1M NaOH before LGG dispersion. The mixture was calculated to be 20% w/w of wall material concentration. The condition of the spray-dried technique was set at 140°C for the inlet temperature and 60°C for the outlet temperature. And the viability of LGG after encapsulation was performed to ensure that the cells remained viable after the encapsulation process.
To enumerate the encapsulated LGG, microparticles were disintegrated using a cordless pellet pestle in phosphate buffer (pH 7.4) and cells viability were counted using the surface-spread plate technique. This method had been previously shown not to affect the LGG cells viability (Thamacharoensuk et al., 2020). The results were expressed as CFU/ml.
To determine the percentage of yield of encapsulated LGG from production, the total solids before spray drying and the recovery after spray drying were applied. The total solid was a weighted combination of wall material and core material. The proportion of the two factors was calculated following the equation in the previous study (Boonsom and Dumkliang, 2020) as follows:
% process yield = (total recovery / total solids sprayed) × 100
Preparation and evaluation of LGG topical formulation
The composition ingredients used in the topical formulation are listed in Table 1.
Table 1. The weight percentage compositions of various ingredients in LGG-loaded emulsion.
|
Phases |
Ingredients |
Content %w/w |
||||||||
|
Control |
pH-adjusted |
Preservative added |
||||||||
|
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
F7 |
F8 |
F9 |
||
|
A |
Sodium Polyacrylate (and) Polyisobutene |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
|
Cyclomethicone |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
|
|
Tocopheryl Acetate |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
|
|
B |
Ethyl Panthenol |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
|
Water |
q.s. |
q.s. |
q.s. |
q.s. |
q.s. |
q.s. |
q.s. |
q.s. |
q.s. |
|
|
C |
Encapsulated LGG |
- |
1 |
- |
- |
1 |
- |
- |
1 |
- |
|
LGG cells |
- |
- |
1 |
- |
- |
1 |
- |
- |
1 |
|
|
D |
Lactic acid |
- |
- |
- |
pH4 |
pH4 |
pH4 |
- |
- |
- |
|
E |
Phenoxy- ethanol |
- |
- |
- |
- |
- |
- |
1 |
1 |
1 |
Topical formulations were separated into three groups: control base emulsions (F1-F3), pH-adjusted formulations (F4-F6), and preservatives added (F7-F9). According to our screening, the lipid phase was preheated above 65-70 °C and then mixed into the aqueous phase at the same temperature. The pre-emulsion was homogenized until it cooled down below 40-45 °C. Lactic acid was added as a pH adjuster to compare with phenoxyethanol added in another formulation to examine the suitable condition for LGG loading. The encapsulated LGG powder (1% w/w) was dispersed into the pre-emulsion (F2, F5, F8). The mixture was simultaneously stirred to achieve LGG dispersion. LGG cells were calculated to be equivalent to the concentration of LGG powder (CFU/g) and added to the remaining formulations (F3, F6, and F9). In addition, emulsions obtained from the method were characterized by visualization, pH measurement, and viscosity evaluation immediately after preparation. Stability testing was conducted under two conditions: storage at ambient temperature with light exposure for 8 weeks to simulate normal usage conditions, and accelerated heating-cooling cycles between 40°C and 4°C for 6 cycles, with monitoring of physical characteristics and bacterial viability.
Viability of LGG
The viability of LGG in the topical formulation was immediately evaluated after preparation and at post storage conditions. Briefly, samples were performed by 10-fold serial dilution and spread-plated on MRS agar at 37°C for 24-48 hours (Pertami et al., 2024). Total viable counts of plates were determined and expressed as CFU/ml. The experiment was performed in duplicate.
Microbial contamination test
Contamination monitoring is essential for the development of probiotic cosmetics to ensure that the formulations are not harmful. Microbial contamination tests were performed using the surface-spread plate technique (Detmer et al., 2010; Sanders, 2012). The LGG formulation (0.1 gram) was dispersed in 1 ml of sterile distilled water using a vortex mixer. Then, 0.1 ml of the suspension was spread onto agar using a spreader glass, while sterile distilled water served as a control. According to the Ministry of Public Health in Thailand, cosmetics must be free of aerobic bacteria, yeast, and mold (total aerobic plate count), including four types of pathogens: Pseudomonas aeruginosa, Staphylococcus aureus, Candida albicans, and Clostridium spp. (only herbal cosmetics) (FDA Thai, 2016). The samples were then incubated at specific conditions as shown in Table 2, and the number of colonies was counted to calculate the CFU/g of the sample.
Table 2. The conditions of microbial contamination test.
|
Microorganisms |
Nutrient mediums |
Testing conditions |
|
Aerobic bacteria |
Nutrient agar |
Incubate at 37°C, 24-48 h |
|
Aerobic mold |
Sabouraud Dextrose Agar |
Incubate at 28-30 °C, 3-5 days |
|
Clostridium spp. |
Egg Yolk Agar |
Incubate under anaerobic condition using Anaerocult® A at 37°C, 24-48 h |
|
S. aureus |
Mannitol Salt Agar |
Incubate at 37°C, 24-48 hrs |
|
P. aeruginosa |
Centrimide Agar |
Incubate under anaerobic condition using Anaerocult® A at 37°C, 24-48 h |
|
C. albicans |
HiCromeTM Candida Differential Agar |
Incubate at 28-30 °C, 3-5 days |
Antioxidant activity
As a result of stability studies, the best characteristic formula was tested for antioxidant activity. Free radical scavenging activity was performed according to the previous method using l,l-diphenyl-2-picrylhydrazyl (DPPH) (Manosroi et al., 2010; Maneechai et al., 2023). DPPH solution was added to different tested samples in methanol (0.01, 0.1, 1, 10, and 100 mg/ml) in each well of a 96-well microtiter plate. The reactions were conducted for 15 min in the dark at ambient temperature. The absorbance was measured at 517 nm against methanol without DPPH as a blank. Ascorbic acid was used as the standard antioxidant. The experiment was performed in four replications. The concentration of sample required to scavenge 50% of DPPH (SC50) was determined. The DPPH radical-scavenging activity was calculated according to the equation in the previous study (Yakaew et al., 2020) as follows:
% DPPH radical scavenging activity = 1 - [Asample/Ablank] × 100
where Asample and Ablank are the absorbances of the sample and blank.
Proliferation of normal human skin fibroblast activity and viability tests
The percentage of cell growth from SRB assay
The best-characteristic formula was repeatedly prepared and tested for cell proliferation activity. The human skin fibroblasts (HSF) were cultured under standard conditions using completed Dulbecco's modified Eagle medium (DMEM) supplemented with 10% FBS and 1% penicillin/streptomycin as a cell culture medium. The LGG formulation was weighed and solubilized in a sterile medium. The formulation was filtered with a 0.2-micron membrane. Ascorbic acid was utilized as the comparative group, while HSF in completed DMEM without formulation served as the control group. HSF cells were plated at a density of 1×105 cells/well and then left for cell attachment on the plate overnight in 5% CO2 at 37°C. Cells were exposed to the mixture of completed DMEM with the LGG formula in various concentrations for 24 h. Six serial concentrations ranged from 0.0001-10 mg/ml. The adherent cells were dyed after incubation with a sulforhodamine B (SRB) dye solution following the SRB assay (Papazisis et al., 1997; Manosroi et al., 2010; Singpanna et al., 2023). The bound dye was solubilized and the absorbance was measured at 540 nm by a microplate reader. The assays were done in three independent and separate experiments. The absorbances were recorded and used to calculate the percentage of cell growth (G) according to the equation in the previous study (Kansom et al., 2019) as follows:
% G = (Asample - Ablank / Acontrol - Ablank) x 100
Where Asample are the absorbances of six serial solutions of LGG formulation or ascorbic acid
Acontrol are the absorbances of the HSF in completed DMEM
Ablank are the absorbances of the solution of LGG formulation in a sterile medium
The percentage of cell viability from SRB assay
As in the above experiment, the LGG formulation candidate was selected. The procedure was also repeated in the viability assay. Ascorbic acid was utilized as the comparative group, while LGG formulation served as the sample. Comparatives and samples are independent and separate experiments. Weighed and solubilized in a sterile medium, and then filtered with the 0.2-micron membrane. Six serial solutions were prepared and diluted to 0.0001-10 mg/ml with a sterile medium. For screening, an SRB assay was applied to investigate toxicity against HSF following previous calculation method (Vichai and Kirtikara, 2006). Percentage viability was calculated using the following equation and compared the ascorbic acid with the LGG formulation.
%Cell viability = Viable cells / Total cells (dead and viable) x 100
The highest concentration that showed no toxicity to cell lines was selected for wound healing activity.
Wound healing activity
The activity of the formulation on proliferation and migration of fibroblasts was evaluated using the scratch assay (Liang et al., 2007; Marasri et al., 2024; Taresh et al., 2025). Briefly, fibroblasts were cultivated until a monolayer of cells was formed. An artificial linear wound was created into the monolayers and individually examined to ensure a uniform gap size. Then, cells were exposed to the LGG formula at a selected concentration (1 mg/ml). Ascorbic acid at the same concentration was utilized as the comparative group. Images were obtained from the same fields after scratching (t0) and after 8, 24, and 48 hours using a microscope. The wound area was analyzed using ImageJ. Untreated scratched cells represented the control.
Statistical analysis
Statistical analysis was conducted using Minitab 17. All experimental results are presented as mean ± SD. The characterization data (n=3) and LGG viability of emulsions (n=2) were statistically analyzed to compare values after preparation and after storage. A paired t-test was used to evaluate differences within the same formulation across time points. Cell growth and cell viability from SRB assay results involved comparing multiple concentrations within the same treatment using one-way analysis of variance (ANOVA). Data are presented with n=3 for cell growth and n=4 for cell viability assays. Significance was accepted at P < 0.05 for all experiment designs.
RESULTS
Physical properties of LGG-loaded emulsions
The preliminary evaluation after preparation is shown in Table 3. All formulations (F1-F9) initially exhibited a homogeneous texture, opaque white color, and no phase separation, indicating acceptable emulsion formation. Slight variations in viscosity were observed, likely due to differences in key components used (Table 1), such as LGG cells, encapsulated LGG, and preservatives. To investigate the stability and impact of LGG form, formulations were divided into three groups: control base emulsions (F1-F3), pH-adjusted emulsions (F4-F6, adjusted to pH 4 using lactic acid), and preservative-added emulsions (F7-F9, containing 1% phenoxyethanol).
Table 3. Stability studies of emulsions.
|
Formulations |
Conditions |
||||||
|
After preparation |
Ambient temp. |
Heating-cooling cycle |
|||||
|
pH |
Viscosity (cps) |
pH |
Viscosity (cps) |
pH |
Viscosity (cps) |
||
|
F1 |
6.31 ± 0.35 |
25,771 ± 667 |
6.49 ± 0.28 |
26,633 ± 47 |
6.99 ± 0.03 |
20,600 ± 57** |
|
|
F2 |
6.19 ± 0.07 |
29,093 ± 278 |
unstable |
unstable |
|||
|
F3 |
4.45 ± 0.25 |
31,907 ± 82 |
contaminated |
unstable |
|||
|
F4 |
4.06 ± 0.07 |
37,600 ± 623 |
4.05 ± 0.18 |
28,293 ± 310** |
4.58 ± 0.07** |
37,367 ± 694** |
|
|
F5 |
3.85 ± 0.12 |
34,300 ± 82 |
contaminated |
4.77 ± 0.11* |
35,133 ± 47* |
||
|
F6 |
4.04 ± 0.07 |
35,507 ± 471 |
3.90 ± 0.02 |
35,667 ± 47 |
4.10 ± 0.14 |
35,197 ± 285 |
|
|
F7 |
6.22 ± 0.11 |
25,100 ± 216 |
5.43 ± 0.23 |
24,767 ± 330** |
5.92 ± 0.13 |
25,093 ± 335 |
|
|
F8 |
5.71 ± 0.32 |
29,587 ± 66 |
unstable |
unstable |
|||
|
F9 |
4.72 ± 0.71 |
33,833 ± 47 |
5.46 ± 0.07 |
33,667 ± 125 |
unstable |
||
Note: * indicates a statistically significant difference within formulations between after-preparation and storage conditions.,
** indicates a statistically significant difference between storage conditions., P-value < 0.05, n=3 (mean ± SD).
After preparation, the pH-adjusted emulsions presented lower pH values, ranging from 3.85 to 4.06. Among these, F6, containing LGG cells, maintained both pH and viscosity after preparation. Encapsulated LGG in F5 slightly increased the viscosity (34,300 ± 82 cps), while free LGG cells in F4 caused a higher viscosity change, possibly due to interactions between bacterial cells and the emulsion matrix. After ambient storage, the stability outcomes varied depending on both LGG type and formulation additives. Phase separation occurred in F2 and F8, both containing encapsulated LGG, indicating possible destabilization effects of the capsule material (e.g., swelling or release of contents). Microbial contamination was found in F3, which contained LGG cells, and F5, with pH adjusted, which contained encapsulated LGG. Notably, both formulations without phenoxyethanol still showed contamination, indicating that pH adjustment alone is not sufficient to control microbial growth when preservatives are absent. Formulation 6 and 9, containing LGG cells but with either acid or preservative adjustment, remained physically and microbiologically stable, showing only minimal pH and viscosity changes (no significant change at a P-value less than 0.05). These results suggest that encapsulated LGG may negatively impact emulsion stability under ambient conditions, especially in the absence of preservatives.
Accelerated stability in heating-cooling 6 cycles showed that cracking or instability occurred in four formulations. F2 and F3 (control base emulsions with encapsulated and free LGG, respectively) were unstable. F8 and F9 (preserved emulsions) also showed cracking, even with phenoxyethanol, indicating that thermal stress could overcome the preservative protection, particularly in the presence of LGG or capsule materials. In contrast, F1 (no LGG), F4 (pH-adjusted, no LGG), and F7 (preserved, no LGG) maintained their physical integrity, indicating that the presence of LGG, especially in the capsule form, may reduce durability under thermal stress.
From the results of physical characteristics under storage conditions, F6 (pH-adjusted emulsion with LGG cells) demonstrated the best balance of microbial stability, physical stability, and LGG survivability under both ambient and accelerated conditions. It showed no contamination, maintained pH and viscosity, and did not crack during stress testing. These results highlight that pH adjustment, when combined with unencapsulated LGG cells, may provide a more stable and favorable formulation, particularly in the absence of preservatives. Thus, F6 was selected for further activity evaluation due to its superior performance in maintaining emulsion integrity and LGG viability.
Viability of LGG
The encapsulated LGG from the spray drying technique gives 78.02% process yield and the survival of microorganisms is 96.52%, which are consistent with the previous studies that showed process yield and survival rate of encapsulated LGG over 74.44% and 97.83% (Thamacharoensuk et al., 2020).
According to the encapsulation studies, LGG viability under storage conditions showed that formulations F2, F5, and F8 maintained higher survival rates at ambient temperature. In these formulations, the number of viable LGG increased from 5.72 ± 0.2 to 8.68 ± 0.1 log CFU/mL compared to immediately after preparation. The highest survival rate was 136.89 ± 2.45%, highlighting the effectiveness of the encapsulation in preserving LGG. Under accelerated heating–cooling cycles, encapsulated LGG exhibited only a slight reduction in viability, from 6.40 ± 0.2 to 6.16 ± 0.2 log CFU/mL.
In contrast, LGG-loaded emulsions without encapsulation (F3, F6, and F9) demonstrated a decline in viable LGG counts from 5.72 ± 0.2 to 3.79 ± 0.1 log CFU/mL under both ambient and accelerated heating–cooling conditions. As expected, non-encapsulated LGG showed a greater loss in viability compared to encapsulated counterparts. This is likely due to the absence of protective matrices, such as whey protein isolate and glutinous rice starch, which in the encapsulated formulations serve as both protective barriers and nutritional sources for LGG cells.
The lowest survival rate was observed in formulation F6 across both storage conditions; however, the reduction was not statistically significant (P > 0.05), with viability remaining above 73.65 ± 4.54% (data not shown).
Microbial contamination test
The result from the total aerobic plate (bacteria and mold) count and four types of pathogens in the LGG-loaded emulsion is shown in Figure 1. The selected formula was free from unwanted microorganisms, thus complying with Thai legal requirements. Therefore, the developed emulsion can be safely utilized for eye cream, sensitive skin, and even children under 3 years old (FDA Thai, 2016).
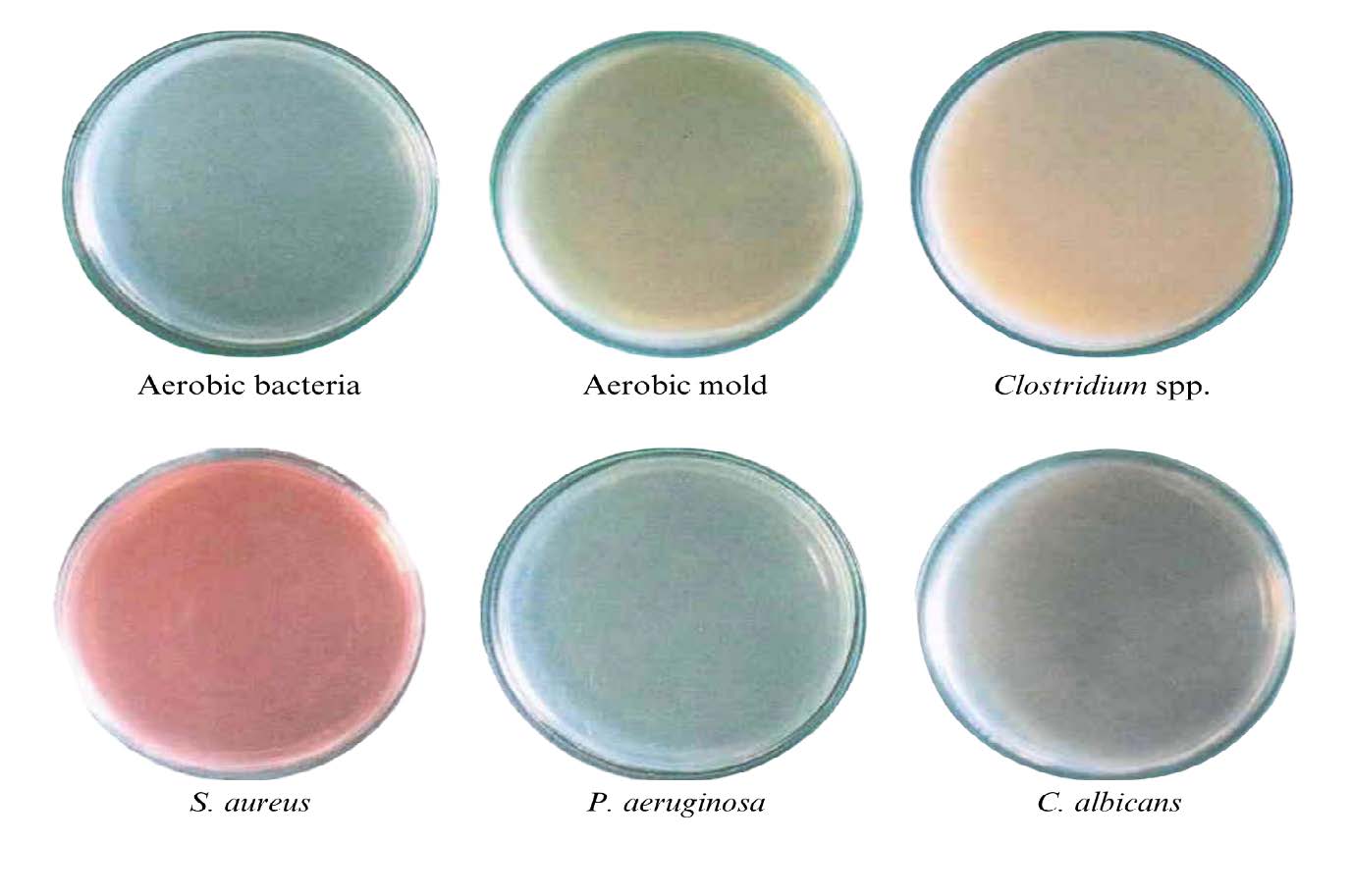
Figure 1. Microbial contamination test of LGG-loaded emulsion.
Antioxidant activity
To evaluate the antioxidant potential of the LGG-loaded emulsion, we investigated the in vitro radical scavenging activity by utilizing the DPPH radical scavenging assays. The SC50 value of the LGG-loaded emulsion, corresponding to the sample concentration required to scavenge radicals by 50%, was determined to be 2.18 ± 0.14 mg/ml. Ascorbic acid, a potent agent with antioxidative properties, that served as a positive control, was determined to be 0.03 ± 0.01 mg/ml (data not shown). Low SC50 values indicate strong antioxidant activity. In comparison to ascorbic acid, The LGG-infused emulsion was less efficient in antioxidant activity against the DPPH radical than ascorbic acid by more than 70%.
Proliferation of normal human skin fibroblast activity and viability tests
The percentage of cell growth from SRB assay
The percentage of cell growth from the experiment with the SRB assay is shown in Table 4. It was found that ascorbic acid had the highest cell stimulative effect at 0.1 mg/ml, which was 44.05 ± 1.70%. Compared to the LGG-loaded emulsion, 0.1 mg/ml showed a higher stimulative effect, which gave 45.41 ± 3.41%. Furthermore, the LGG-loaded emulsion exhibited the highest activity at a concentration of 1 mg/ml (49.23 ± 6.39%). This means that when the emulsion containing LGG was observed specifically at the 1 mg/ml and indicates the magnitude of this effect, likely in comparison to a control. Whereas the effect of ascorbic acid was decreased (25.29 ± 2.44%), this could suggest that ascorbic acid is either unstable, less effective at more than 1 mg/ml, or possibly cytotoxic at that level, resulting in lower observed biological activity.
Table 4. The percentage of cell growth from SRB assay after treatment.
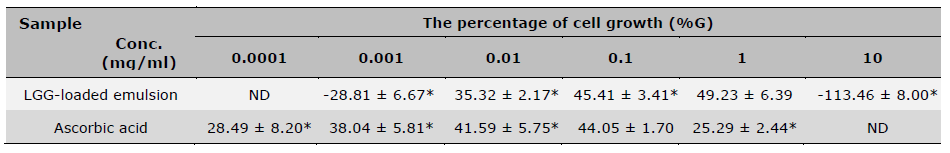
Note: * indicates a statistically significant difference among concentrations within the same group., P-value < 0.05, n=3 (mean ± SD), ND means not tested.
Table 5. The percentage of cell viability from SRB assay after treatment.
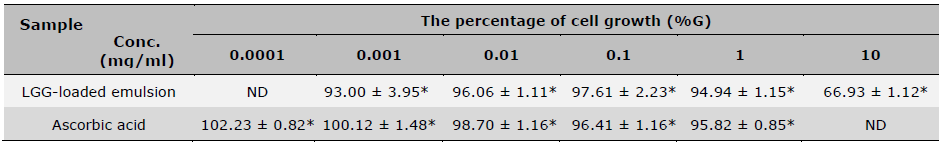
Note: *indicates a statistically significant difference among concentrations within the same group., n=4 (mean ± SD), ND means not tested.
The percentage of cell viability from SRB assay
As shown in Table 5, cell viability treated with the LGG-loaded emulsion is consistently more than 90% from 0.001-1 mg/ml concentrations, which was non-toxic to human dermal fibroblast cells. At 10 mg/ml, the viability decreases to 66.93 ± 1.12%, which provides evidence indicating dose-dependent cytotoxicity. In contrast, ascorbic acid maintained high viability more than 95% at all tested concentrations, with a slight proliferative effect at 0.0001 mg/ml (102.23 ± 0.82%). These results suggest that both samples are non-cytotoxic at lower concentrations, though LGG-loaded emulsion becomes cytotoxic at high doses.
Wound healing activity
Based on the SRB assay, both the LGG-loaded emulsion and ascorbic acid at a concentration of 1 mg/ml were further evaluated for their wound healing activity. Figure 2 presents the results of the scratch assay, which was conducted to investigate the effects on cell proliferation and/or migration. Black arrows indicate wound edges, and scale marking shown in each panel (units in µm). The LGG-loaded emulsion exhibited an initial migration at 24 h and reached maximum stimulatory effects at 48 h. The results indicated that the LGG-loaded emulsion promoted wound closure more rapidly than the control (untreated cells) and stimulated a similar response in the comparative group. While ascorbic acid is well known for promoting collagen synthesis and antioxidant (Pullar et al., 2017; Bechara et al., 2022; Boo, 2022). The LGG-loaded emulsion was more effective than ascorbic acid, leading to more complete wound closure at the final time point. This suggests that LGG-derived biocomponents may stimulate cell migration and tissue regeneration, possibly through mechanisms different from their antioxidant effects.
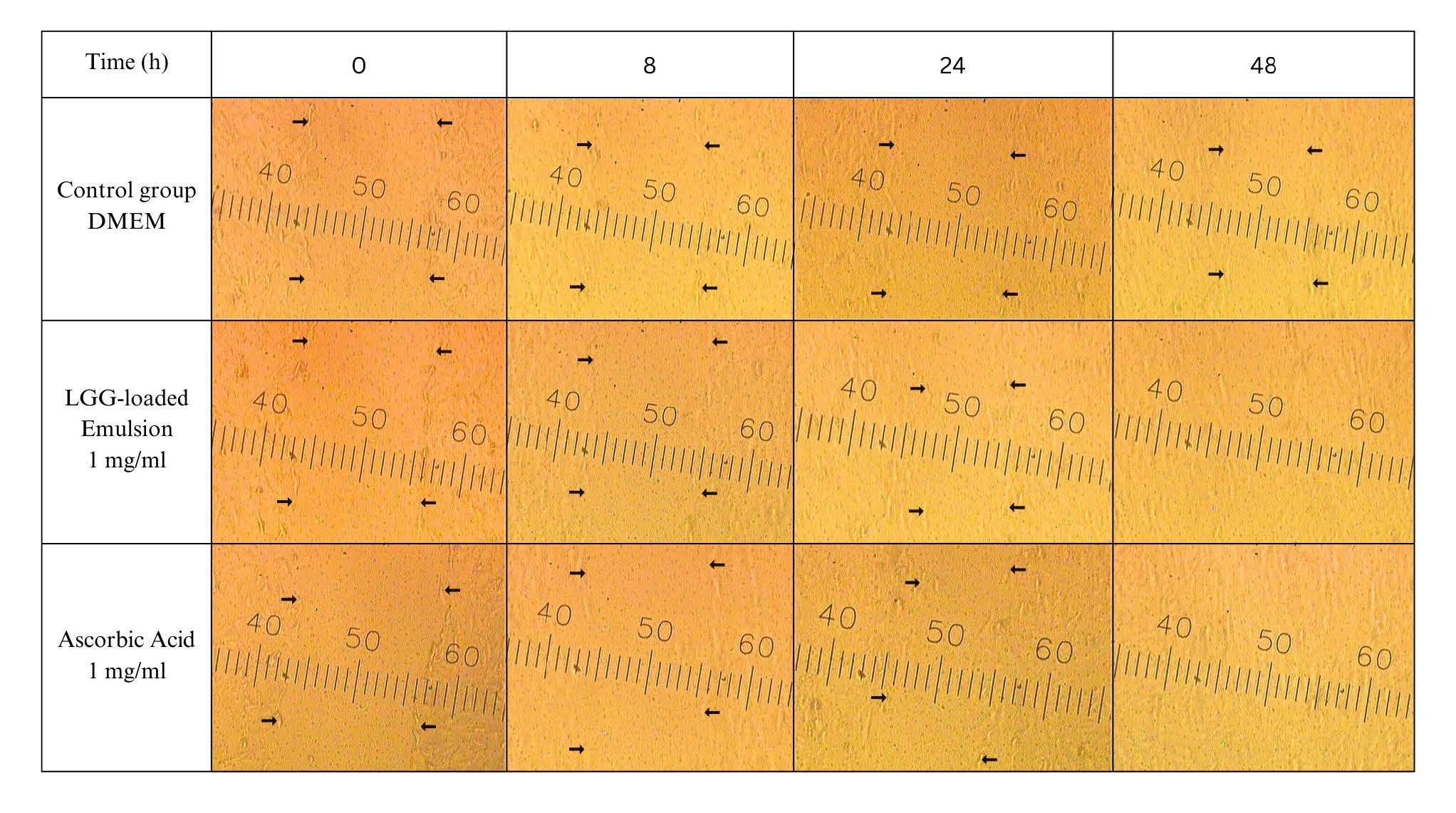
Figure 2. Effect of LGG-loaded emulsion on the proliferative and/or migratory activities of fibroblasts in the scratch assay.
DISCUSSION
The first consideration is the results of the viability tests; the encapsulation process is effective in protecting LGG. Similarly, the results from developing the encapsulated LGG-loaded emulsion (F2, F5, and F8) showed an increase in the survival rate. The whey protein used in encapsulated LGG plays a protective role against environmental factors (Pimentel-González et al., 2009; Solghi et al., 2020). It has been reported as providing probiotics with enhanced resilience in high-stress environments (Picot and Lacroix, 2004). Previous studies have illustrated the protective effect exerted by the encapsulation of probiotic microorganisms when they encounter unfavorable circumstances, and they have shown that the microorganisms displayed considerably greater viability in comparison to non-encapsulated cells (Lian et al., 2003; Picot and Lacroix, 2004). However, physical tests indicated that encapsulated LGG affected the stability of the developed emulsion. Moreover, the wall materials, such as whey protein and the formula’s water content, may provide nutrition for microorganisms and contribute to contamination or cracking in some formulations. The presence of numerous LGG cells may have increased the osmotic pressure of the emulsion. A difference in osmotic pressure could have facilitated the transportation of water from droplets lacking cells (smaller droplets) to droplets containing cells (larger droplets) and may have accelerated the process of coalescence, thereby leading to the instability of formulations (VanderGheynst et al., 2013). Nonetheless, these factors did not cause contamination by unwanted microorganisms in the formulation. The selected emulsion formula (F6) exhibits good physical properties and an acceptable survival rate. In this research, an emulsion favorable for LGG does not require encapsulation, only an appropriate pH level.
A notable finding from the study was the enhancement of cell proliferation in human fibroblasts. At 0.1 mg/ml, the LGG-loaded emulsion demonstrated comparable efficacy to ascorbic acid. Jung et al. (2019) demonstrated that the lysates of L. rhamnosus could improve the skin barrier function using a reconstructed human epidermis model. Similar studies showed Lactobacillus and lactic acid bacteria could balance the pH of the skin (Reisch, 2017) and positively promote skin fibroblasts (Zhang et al., 2023). Previous studies have shown that proteins produced from LGG strains, such as Msp1 and Msp2, can directly aid wound healing by promoting cellular processes involved in tissue repair and regeneration. Secreted substances can enhance the host’s immune response, create an unfavorable environment for pathogens, help regulate skin inflammation and contribute to a balanced skin environment (Steele, 2022; Badaluta et al., 2024). The key point is that postbiotics cannot actively modulate and balance the skin’s microbial ecosystem through direct competition and colonization. Moreover, the results of the wound healing tests further validate the effectiveness of LGG in an emulsion dosage form. Although this prominence may be attributed to tocopheryl acetate, it is important to note that its concentration had been reduced by approximately 105-fold from the initial formulation. When considering the overall components, it can be concluded that the results are very similar to those of a pure ascorbic acid solution. Furthermore, the modest antioxidant activity test results help explain the hypothesis that tocopheryl acetate may not be the primary component for promoting wound healing. Mohammedsaeed et al. (2015) using lysates of the acid-lactic bacteria L. rhamnosus GG and L. reuteri, observed accelerated gap closure using human keratinocytes cultured in vitro. In a recent study, Moreira et al. (2021) showed that oral treatment with L. rhamnosus LPR was able to accelerate wound closure, improve blood supply, and attenuate scar formation. These findings underscore the wound healing potential of LGG strains and support the use of live LGG-loaded emulsions as a promising biofunctional alternative or complementary agent to conventional treatments like vitamin C. However, it is important to note that this study was conducted using in vitro models, which presents a limitation in fully extrapolating the results to real-world applications. Nonetheless, the outcomes provide a valuable foundation for the further development of LGG-based formulations aimed at meeting the criteria for cosmetic or dermatological products. Future studies should include in vivo evaluations and long-term shelf-life stability assessments to validate efficacy and commercial viability.
CONCLUSION
This study demonstrates the successful development of a pH-controlled emulsion using lactic acid, which enables the incorporation of live LGG into a stable cosmetic formulation. The emulsion maintained favorable physical characteristics under ambient storage and thermal stress conditions without loss of LGG viability, supporting its potential for long-term topical use. Importantly, the optimized emulsion not only exhibited antioxidant activity but also enhanced fibroblast proliferation and migration, two key biological processes involved in wound healing and tissue repair. These findings suggest that the live LGG may provide skin benefits beyond microbiome enhancement, particularly in the context of restorative skin care. The proposed mechanism of action may be due to the production of bio-metabolites derived from LGG, including organic acids (lactic and acetic), hydrogen peroxide, and bacteriocins, which can contribute to skin barrier function and maintenance of the acid mantle. The formulation is free from microbial contamination, enhancing its safety. However, further studies are needed to optimize additional formulation parameters such as water activity, moisture content, and release kinetics, as well as to assess in vivo efficacy and long-term skin compatibility. Overall, this research presents a novel probiotic-based emulsion platform with potential for advanced skincare applications in both dermacosmetics and skin therapies, paving the way for future research into topical systems that integrate the microbiome.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the Faculty of Pharmaceutical Sciences, Chulalongkorn University, for providing the Lacticaseibacillus rhamnosus GG bacterial strain that was crucial for this research.
AUTHOR CONTRIBUTIONS
Ketrawee Pantub assisted in the experimental design of the formulation, designed efficacy tests and stability studies, performed the statistical significance calculations, and wrote the manuscript. Tanatip Thamacharoensuk assisted in designing the preparation of the Lacticaseibacillus rhamnosus GG strain, verified bacterial viability, tested for bacterial contamination, and wrote the manuscript. Ekachai Dumkliang was primarily responsible for the encapsulation process. Somboon Tanasupawat sourced and prepared the Lacticaseibacillus rhamnosus GG bacterial strain for use. All researchers have read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Almeida, C.V.D., Antiga, E., and Lulli, M. 2023. Oral and topical probiotics and postbiotics in skincare and dermatological therapy: A concise review. Microorganisms. 11(6): 1420.
Badaluta, V.A., Curutiu, C., Ditu, L.M., Holban, A.M., and Lazar, V. 2024. Probiotics in wound healing. International Journal of Molecular Sciences. 25(11): 5723.
Bechara, N., Flood, V.M., and Gunton, J.E. 2022. A systematic review on the role of vitamin C in tissue healing. Antioxidants. 11(8): 1605.
Boo, Y.C. 2022. Ascorbic acid (vitamin C) as a cosmeceutical to increase dermal collagen for skin antiaging purposes: Emerging combination therapies. Antioxidants. 11(9): 1663.
Boonsom, T. and Dumkliang, E. 2020. Response surface optimization on microencapsulation of lemongrass essential oil using spray drying. Key Engineering Materials. 859: 271-276.
Bowe, W.P. and Logan, A.C. 2011. Acne vulgaris, probiotics and the gut-brain-skin axis-back to the future?. Gut Pathogens. 3: 1.
Detmer, A., Jorgensen, C., and Nylen, D. 2010. A guidance document on microbiological control of cosmetic products. Kobenhavn: Danish Ministry of the Environment Agency, Denmark. pp. 1-43.
Dou, J., Feng, N., Guo, F., Chen, Z., Liang, J., Wang, T., Guo, X., and Xu, Z. 2023. Applications of probiotic constituents in cosmetics. Molecules. 28(19): 6765.
Food and Drug Administration Thailand. 2016. Specifications of cosmetics prohibited from manufacturing, importing, or selling [Internet]. Nonthaburi (TH): Ministry of Public Health; [cited 2024 May 3]. Available from: https://cosmetic.fda.moph.go.th/interesting-law/category/cosmetic-laws
Goodarzi, F. and Zendehboudi, S. 2019. A comprehensive review on emulsions and emulsion stability in chemical and energy industries. The Canadian Journal of Chemical Engineering. 97(1): 281-309.
Grice, E.A. and Segre, J.A. 2011. The skin microbiome. Nature Reviews Microbiology. 9(4): 244–253.
Hsu, D.K., Fung, M.A., and Chen, H.L. 2020. The role of skin and gut microbiota in the pathogenesis of psoriasis, an inflammatory skin disease. Medicine in Microecology. 4: 100016.
Jung, Y.O., Jeong, H., Cho, Y., Lee, E.O., Jang, H.W., Kim, J., and Lim, K.M. 2019. Lysates of a probiotic, Lactobacillus rhamnosus, can improve skin barrier function in a reconstructed human epidermis model. International Journal of Molecular Sciences. 20(17): 4289.
Kansom, T., Dumkliang, E., Patrojanasophon, P., Sajomsang, W., Saeeng, R., Zhu, W.M., and Opanasopit, P. 2019. Folate-functionalized amphiphilic chitosan polymeric micelles containing andrographolide analogue (3A. 1) for colorectal cancer. Key Engineering Materials. 819: 15-20.
Kaur, K. and Rath, G. 2019. Formulation and evaluation of UV protective synbiotic skin care topical formulation. Journal of Cosmetic and Laser Therapy. 21(6): 332-342.
Krutmann, J. 2012. Pre-and probiotics for human skin. Clinics in Plastic Surgery. 39(1): 59-64.
Liang, C.C., Park, A.Y., and Guan, J.L. 2007. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nature Protocols. 2(2): 329-333.
Lian, W.C., Hsiao, H.C., and Chou, C.C. 2003. Viability of microencapsulated bifidobacteria in simulated gastric juice and bile solution. International Journal of Food Microbiology. 86(3): 293-301.
Lukic, M., Pantelic, I., and Savic, S.D. 2021. Towards optimal pH of the skin and topical formulations: From the current state of the art to tailored products. Cosmetics. 8(3): 69.
Mahmoud, M., Abdallah, N.A., El-Shafei, K., Tawfik, N.F., and El-Sayed, H.S. 2020. Survivability of alginate-microencapsulated Lactobacillus plantarum during storage, simulated food processing and gastrointestinal conditions. Heliyon. 6(3): e03541.
Mahmud, M.R., Akter, S., Tamanna, S.K., Mazumder, L., Esti, I.Z., Banerjee, S., and Pirttilä, A.M. 2022. Impact of gut microbiome on skin health: Gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes. 14(1): 2096995.
Maneechai, P., Leelapornpisid, P., and Poomanee, W. 2023. Multifunctional biological activities and cytotoxic evaluation of Bouea macrophylla for cosmetic applications. Natural and Life Sciences Communications. 22(2): e2023030.
Manosroi, A., Jantrawut, P., Akihisa, T., Manosroi, W., and Manosroi, J. 2010. In vitro anti-aging activities of Terminalia chebula gall extract. Pharmaceutical Biology. 48(4): 469-481.
Marasri, P., Sookkhee, S., Wanachantararak, P., and Owittayakul, D. 2024. Anti-inflammatory activity and wound healing ability of coconut oil mouthwash on gingival fibroblast cell in vitro. Natural and Life Sciences Communications. 23(4): e2024059.
Mohammedsaeed, W., Cruickshank, S., McBain, A.J., and O’Neill, C.A. 2015. Lactobacillus rhamnosus GG lysate increases re-epithelialization of keratinocyte scratch assays by promoting migration. Scientific Reports. 5(1): 16147.
Moniaga, C.S., Tominaga, M., and Takamori, K. 2022. An altered skin and Gut microbiota are involved in the modulation of itch in atopic dermatitis. Cells. 11(23): 3930.
Moreira, C.F., Cassini-Vieira, P., Canesso, M.C.C., Felipetto, M., Ranfley, H., Teixeira, M.M., and Barcelos, L.S. 2021. Lactobacillus rhamnosus CGMCC 1.3724 (LPR) improves skin wound healing and reduces scar formation in mice. Probiotics and Antimicrobial Proteins. 13: 709-719.
Muizzuddin, N., Maher, W., Sullivan, M., Schnittger, S., and Mammone, T. 2012. Physiological effect of a probiotic on skin. Journal of Cosmetic Science. 63(6): 385-395.
Papazisis, K.T., Geromichalos, G.D., Dimitriadis, K.A., and Korsaris, A.H. 1997. Optimization of the sulforhodamine B colorimetric assay. Journal of Immunological Methods. 208: 151–158.
Pertami, S.B., Yunitasari, E., Budiono, B., Yulifah, R., Astuti, N.P., Herawati, T., Arifah, S.N., and Atho’illah, M.F. 2024. Bioactive compounds and antioxidant activity in Greek-style yoghurt infused with shield aralia leaves. Natural and Life Sciences Communications. 23(3): e2024045.
Pessemier, B.D., Grine, L., Debaere, M., Maes, A., Paetzold, B., and Callewaert, C. 2021. Gut–skin axis: Current knowledge of the interrelationship between microbial dysbiosis and skin conditions. Microorganisms 9(2): 353.
Picot, A. and Lacroix, C. 2004. Encapsulation of bifidobacteria in whey protein-based microcapsules and survival in simulated gastrointestinal conditions and in yoghurt. International Dairy Journal. 14(6): 505-515.
Pimentel-González, D.J., Campos-Montiel, R.G., Lobato-Calleros, C., Pedroza-Islas, R., and Vernon-Carter, E.J. 2009. Encapsulation of Lactobacillus rhamnosus in double emulsions formulated with sweet whey as emulsifier and survival in simulated gastrointestinal conditions. Food Research International. 42(2): 292-297.
Pullar, J.M., Carr, A.C., and Vissers, M. 2017. The roles of vitamin C in skin health. Nutrients 9(8): 866.
Ravera, F., Dziza, K., Santini, E., Cristofolini, L., and Liggieri, L. 2012. Emulsification and emulsion stability: The role of the interfacial properties. Advances in Colloid and Interface Science. 288: 102344.
Reisch, M. 2017. The microbiome comes to cosmetics. Chemical & Engineering News. 95(19): 30-34.
Sanders, E.R. 2012. Aseptic laboratory techniques: Plating methods. Journal of Visualized Experiments. 63: e3064.
Sessa, M., Casazza, A.A., Perego, P., Tsao, R., Ferrari, G., and Donsì, F. 2013. Exploitation of polyphenolic extracts from grape marc as natural antioxidants by encapsulation in lipid-based nanodelivery systems. Food and Bioprocess Technology. 6: 2609-2620.
Sharmeen, J.B., Mahomoodally, F.M., Zengin, G., and Maggi, F. 2021. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules. 26(3): 666.
Singpanna, K., Pornpitchanarong, C., Patrojanasophon, P., Rojanarata, T., Ngawhirunpat, T., Pamornpathomkul, B., and Opanasopit, P. 2023. Gold nanoparticles for enhanced skin permeation of a protein drug. Natural and Life Sciences Communications. 22(4): e2023065.
Sinha, S., Lin, G., and Ferenczi, K. 2021. The skin microbiome and the gut-skin axis. Clinics in Dermatology. 39(5): 829-839.
Solghi, S., Emam-Djomeh, Z., Fathi, M., and Farahani, F. 2020. The encapsulation of curcumin by whey protein: Assessment of the stability and bioactivity. Journal of Food Process Engineering. 43(6): e13403.
Steele, C. 2022. Lactobacillus rhamnosus GG: A review of clinical use and efficacy. Nutritional Medicine Journal. 1 (3): 70-116.
Taresh, R.K., Sahib, A.A., Nawar, F.A., Hassen, T.F., Hamoodah, Z.J., and Madhi, R. 2025. Synthesis and characterization of inulin polymer and study its role on wound healing. Natural and Life Sciences Communications. 24(1): e2025008.
Thamacharoensuk, T., Boonsom, T., Tanasupawat, S., and Dumkliang, E. 2020. Optimization of microencapsulated Lactobacillus rhamnosus GG from whey protein and glutinous rice starch by spray drying. Key Engineering Materials. 859: 265-270.
VanderGheynst, J.S., Guo, H.Y., Cheng, Y.S., and Scher, H. 2013. Microorganism viability influences internal phase droplet size changes during storage in water-in-oil emulsions. Bioprocess and Biosystems Engineering. 36: 1427-1434.
Vichai, V. and Kirtikara, K. 2006. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nature Protocols. 1: 1112-1116.
Wieërs, G., Belkhir, L., Enaud, R., Leclercq, S., Philippart de Foy, J.M., Dequenne, I., and Cani, P.D. 2020. How probiotics affect the microbiota. Frontiers in cellular and Infection Microbiology. 9: 454.
Yakaew, S., Phimnuan, P., Tiensomjitr, K., Nakyai, W., Nuengchamnong, N., Ross, G., and Viyoch, J. 2020. Hom–Kularb–Dang rice bran extract for the prevention of UVB-damage against human skin fibroblast. Chiang Mai University Journal of Natural Sciences. 19(1): 34-50.
Yang, Y., Sui, J., Liao, W., Wang, S., Pan, D., Sun, G., and Xia, H. 2025. Clinical evidence on the health benefits and safety of probiotic Lacticaseibacillus rhamnosus: A systematic review. Probiotics and Antimicrobial Proteins. https://doi.org/10.1007/s12602-025-10646-8.
Zhang, X., Xu, J., Ma, M., Zhao, Y., Song, Y., Zheng, B., and Meng, L. 2023. Heat-killed Lactobacillus rhamnosus ATCC 7469 improved UVB-induced photoaging via antiwrinkle and antimelanogenesis impacts. Photochemistry and Photobiology. 99(5): 1318-1331.
Zheng, J., Wittouck, S., Salvetti, E., Franz, C., Harris, H.M.B., and Mattarelli, P. 2020. A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. International Journal of Systematic and Evolutionary Microbiology. 70: 2782–2858.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Ketrawee Pantub1, Ekachai Dumkliang2, Somboon Tanasupawat3, and Tanatip Thamacharoensuk4, *
1 Department of Cosmetic Science and Technology, Faculty of Pharmaceutical Sciences, Burapha University, Chonburi 20131, Thailand.
2 Drug Delivery System Excellence Center (DDSEC), Department of Pharmaceutical Technology, Faculty of Pharmaceutical Sciences, Prince of Songkla University, Songkhla 90110, Thailand.
3 Department of Biochemistry and Microbiology, Faculty of Pharmaceutical Sciences, Chulalongkorn University, Bangkok 10330, Thailand.
4 Department of Pharmaceutical Technology, Faculty of Pharmacy, Eastern Asia University, Pathum Thani 12110, Thailand.
Corresponding author: Tanatip Thamacharoensuk, E-mail: tanatip@eau.ac.th
ORCID: Tanatip Thamacharoensuk: https://orcid.org/0009-0004-3063-1863
Total Article Views
Editor: Sirasit Srinuanpan,
Chiang Mai University, Thailand
Article history:
Received: November 12, 2024;
Revised: July 31, 2025;
Accepted: August 1, 2025;
Online First: August 15, 2025

