
Effect of Chitosan Oligosaccharide in Denture Adhesive on the Inhibition of Candida albicans and Adhesive Strength to Heat-cured Polymethylmethacrylate
Varat Kanchanomai, Pisaisit Chaijareenont, Phenphichar Wanachantararak, and Patcharawan Silthampitag*Published Date : August 14, 2025
DOI : https://doi.org/10.12982/NLSC.2025.067
Journal Issues : Number 4, October-December 2025
Abstract The objective of this study is to investigate the antifungal effect of various proportions of Chitosan Oligosaccharide (COS) combined in denture adhesive against Candida albicans and examine the adhesion properties of denture adhesive with the best COS proportion, in accordance with ISO 10873:2021. The specimens were coated with denture adhesive combined with COS at concentrations of 0.5, 1, and 1.5-fold of the minimal fungicidal concentration (MFC), and with Nystatin as the positive control. Then immersed in Candida albicans suspension. The number of fungal colonies was recorded after 24 h of immersion. The most effective formulation was further evaluated for its adhesion properties using a Universal Testing Machine, with varying immersion times of 0, 1, 10, 30, 60, and 180 min. Data were analyzed using Two-way ANOVA (P <0.05). The incorporation of COS significantly reduced the colony count compared to the control group. Nystatin exhibited the most pronounced antifungal effect. However, no significant difference was observed between the 1.5 MFC and Nystatin (P <0.05). For adhesion strength, both immersion time and the presence of COS demonstrated significant interactions. In the COS-combined adhesive group, no significant differences were observed across the different immersion times. However, the adhesive strength of all groups met the acceptable standards denture adhesive. In conclusion, combining denture adhesive with 1.5 MFC (180 mg/ml) sterile COS offers a promising option to enhance antimicrobial activity without compromising adhesion strength.
Keywords: Adhesion, Candida albicans, Chitosan, Denture adhesive, Polymethylmethacrylate
Funding: This research was supported by the Faculty of Dentistry, Chiang Mai University. Their financial support made this study possible, and I am deeply grateful for their contribution to this research.
Citation: Kanchanomai, V., Chaijareenont, P., Wanachantararak, P., and Silthampitag, P. 2025. Effect of chitosan oligosaccharide in denture adhesive on the inhibition of Candida albicans and adhesive strength to heat-cured polymethylmethacrylate. Natural and Life Sciences Communications. 24(4): e2025067.
INTRODUCTION
Nowadays, the population worldwide is shifting towards an aging society. The older populations tend to have tooth loss resulting in edentulism condition. Although advances in dental treatment can reduce the level of edentulism, prosthetic treatment is still essential in the older population.
Denture stomatitis is the most common disease in patients wearing dentures with a reported prevalence ranging from 20% to 67% (Perić et al., 2024). It is the opportunistic infection of Candida albicans in oral mucosa caused by various factors, especially ill-fitting denture (Budtz-Jorgensen and Bertram, 1970; Sartawi et al., 2021). Denture stomatitis has received increasing attention in recent years, likely due to their rising global prevalence (Pereira-Cenci et al., 2008). Several ways have been proposed to prevent and treat denture stomatitis. One of them is refreshing the traumatized mucosa underlying ill-fitting denture with an antifungal agent mixed with denture liner or adhesive (Akpan and Morgan, 2002; Sartawi et al., 2021).
Denture adhesive is a material to improve the retention and stability of the denture. Although various antifungal agents are combined with denture adhesive, none of them meet the ideal requirement of antifungal agents. Some antifungal agents have adverse effects such as unpleasant taste, mucosal irritation, diarrhea, nausea, and vomiting (Martínez-Beneyto et al., 2010; Lyu et al., 2016; Rai et al., 2022). Due to the addition of an antifungal agent, it can alter the adhesion strength of denture adhesives. Thus, denture adhesive is continually being developed to improve its function. A novel material with desired properties can better meet the expectations of modern dentistry.
Previous studies have explored the application of chitosan derivatives for the prevention and treatment of denture stomatitis (Namangkalakul et al., 2020). Chitosan Oligosaccharide (COS), a derivative of chitosan polymer is primarily found in crab and shrimp shells. Due to its abundance, it can be commercially sourced at a relatively low cost while supporting the utilization of waste from the seafood processing industry. COS is prepared by partial hydrolysis and deacetylation of chitin or chitosan, resulting in valuable properties such as biocompatibility, biodegradability, non-toxicity, muco-adhesion, and great antimicrobial effect against several bacterial and fungal pathogens (Fakhri et al., 2020).
To our knowledge, limited research has explored the effect of COS combined with denture adhesive. Therefore, the objective of this study is to investigate the antifungal effect of various proportions of Chitosan Oligosaccharide combine with denture adhesive against Candida albicans and examine the adhesion properties of denture adhesive with the best Chitosan Oligosaccharide proportion. The null hypothesis of this study is that different proportions of Chitosan Oligosaccharide combined with denture adhesive demonstrate no different levels of antifungal activity against Candida albicans and the adhesive properties were not different compared to denture adhesive alone.
MATERIALS AND METHODS
Materials
All materials show in Table 1.
Table 1. Lists of materials utilized in this experiment.
|
Material |
Trade name |
Manufacturer |
Lot no. |
|
Heat-cured PMMA |
VERTEXTM Rapid Simplified |
Vertex-Dental B.V., UTRECHT, Netherlands |
WT244P03 |
|
MMA Monomer |
VERTEXTM Rapid Simplified |
Vertex-Dental B.V., UTRECHT, Netherlands |
WT271L12 |
|
Chitosan Oligosaccharide |
Marine Bio Resources: Chitosan Oligosaccharide (COSF) |
Marine Bio Resources Co., Ltd, Samutsakorn, Thailand |
22257/3-1 |
|
Sabouraud dextrose broth |
MERCKTM SABOURAUD 2% dextrose broth |
Merck KGaA, Darmstadt, Germany |
VM922339016 |
|
Sabouraud dextrose agar |
MERCKTM SABOURAUD 4% dextrose agar |
Merck KGaA, Darmstadt, Germany |
VM1053738323 |
|
Denture adhesive |
Polident® |
GlaxoSmithKline, Bangkok Thailand |
JV5W |
Minimal fungicidal concentration (MFC) test
A standard laboratory strain of Candida albicans ATCC 10231 (Department of Medical Science, Ministry of Public Health, Thailand) was inoculated into 2% Sabouraud Dextrose Broth (SDB) and incubated at 37°C for 24 h. The optical density (OD) of the culture at 530 nm was then measured, and the suspension was adjusted to a McFarland No. 0.5 standard (106 CFU/ml). The resulting suspension was combined in an Eppendorf tube, containing 850 µl of sterile distilled water, 50 µl of Candida albicans suspension, 10 mg of denture adhesive (1% wt/vol), and 100 µl of sterile Chitosan Oligosaccharide which varying concentration from 0-200 mg/ml. The mixture was homogenized and incubated at 37°C for 24 h.
For experimental controls, a negative control group consisted of the same mixture without chitosan oligosaccharide (0 mg/ml) to verify the normal growth of Candida albicans in the presence of denture adhesive alone. A positive control group included a Nystatin in place of the chitosan oligosaccharide to confirm the effectiveness of fungal inhibition under standard conditions.
The MFC was determined using the streak plate technique according to the yellowish-brown color and viscosity of the solution. A 1 µl inoculating loop was used to streak each suspension onto Sabouraud Dextrose Agar (SDA), and the plates were then incubated at 37°C for 24 h.
This procedure was performed with modifications based on the method described by Buanpech et al. (2024) and Puangphimolkij et al. (2024).
Specimen preparation
Disks of heat-cured polymethylmethacrylate (PMMA) (Vertex Rapid Simplified, Vertex-Dental B.V., Utrecht, Netherlands) were prepared by the laboratory following the manufacturer's instructions. The disks were trimmed to dimensions of 6 × 8 × 2 mm and immersed in distilled water for 48 h to remove residual monomers. The dimension and surface roughness were standardized using a digital vernier caliper (Mitutoyo, Japan) and a profilometer (Mitutoyo Surftest SJ-310, Kanagawa, Japan). Finally, the specimens were sterilized by ethylene oxide gas (Namangkalakul et al., 2020).
Sample holder and pressure sensitive knob preparation
The sample holder was fabricated with a hole of 22 ± 1 mm diameter and a depth of 0.5 ± 0.1 mm. The pressure-sensitive knob was designed with a circular base, 20 ± 0.5 mm in diameter, in accordance with ISO 10873:2021. Both surfaces of the sample holder and pressure-sensitive knob were abraded with 400-grit waterproof abrasive paper, rinsed with distilled water for 15 s, and allowed to air dry for a minimum of 5 min.
Antifungal activity test
Each specimen disk was randomly coated with 15 mg of denture adhesive (Polident®, GlaxoSmithKline) combined with Nystatin or Chitosan Oligosaccharide with varying concentration, as determined by the MFC. The specimens were placed in a 24-well tissue culture plate and divided into five groups (n = 3 per group) as follows:
Group I: Denture adhesive without COS (negative control)
Group II: Denture adhesive with COS 0.5 MFC
Group III: Denture adhesive with COS 1 MFC
Group IV: Denture adhesive with COS 1.5 MFC
Group V: Denture adhesive with Nystatin (positive control)
A 500 µl of 0.5 McFarland diluted Candida albicans suspension was added to each specimen. The plate was incubated at 37°C for 24 h. After incubation, the specimens were rinsed twice with phosphate-buffered saline (PBS) to remove non-adherent Candida albicans cells. The specimens were then transferred to a 5 ml Eppendorf tube containing 1 ml of SDB and sonicated using an ultrasonic cleaner to collect the adherent Candida albicans cells. The resulting suspension was serially diluted, spread onto SDA plates, and incubated overnight at 37°C. The number of Candida albicans colonies was counted, and the percentage of antifungal activity was calculated in comparison to the negative control group using the following formula:
% Of antifungal activity = (number of colonies in the negative control group – experimental group) x100 / number of colonies in the negative control group
This procedure was performed with modifications based on the method described by Namangkalakul et al. (2024).
Adhesion test
The optimal combination of denture adhesive and COS was selected for further testing of adhesion strength. Regarding the procedure recommended by ISO 10873:2021, the sample holder was filled with denture adhesive and COS, and the surface was flattened. The sample holder was then immersed in distilled water at 37°C for 0, 1, 10, 30, 60, and 180 min. Excess water was removed after each immersion period.
The sample holder was mounted onto a sample stand, and a load of 9.8 ± 0.2 N was applied using a Universal Testing Machine (Model 5565, Instron Co., Canton, MA, USA) with load cell 100 N at a rate of 5 mm/min, using a pressure-sensitive knob. The sample was held in place for 30 s, and then the adhesive strength was measured by pulling at a rate of 5 mm/min. The maximum force applied to the knob was recorded, and the force per unit area was calculated to determine the adhesive strength. Subsequently, the experiment was conducted again using denture adhesive alone as the negative control group (Figure 1).

Figure 1. Sample holder and pressure-sensitive knob attach to the universal testing machine.
Statistical analysis
Statistical analyses were performed using SPSS version 26.0 (SPSS Inc., Chicago, IL, USA) with a confidence level of 95% (P < 0.05). The percentage of remaining fungal cells and the adhesive strength were expressed as means and standard deviations. Data normality was verified using the Shapiro–Wilk test. For fungal cell percentages, One-Way ANOVA was used, while Two-Way ANOVA was applied to analyze the adhesion strength data.
RESULTS
Concentration of Chitosan Oligosaccharides (COS) incorporated into the denture adhesive influenced the growth of Candida albicans colonies. A concentration of 120 mg/ml COS was identified as the lowest effective concentration that inhibited Candida albicans growth, thereby determining the minimal fungicidal concentration (MFC). This MFC was then combined with the adhesive and applied to the specimen to evaluate its antifungal efficacy. (Figure 2).
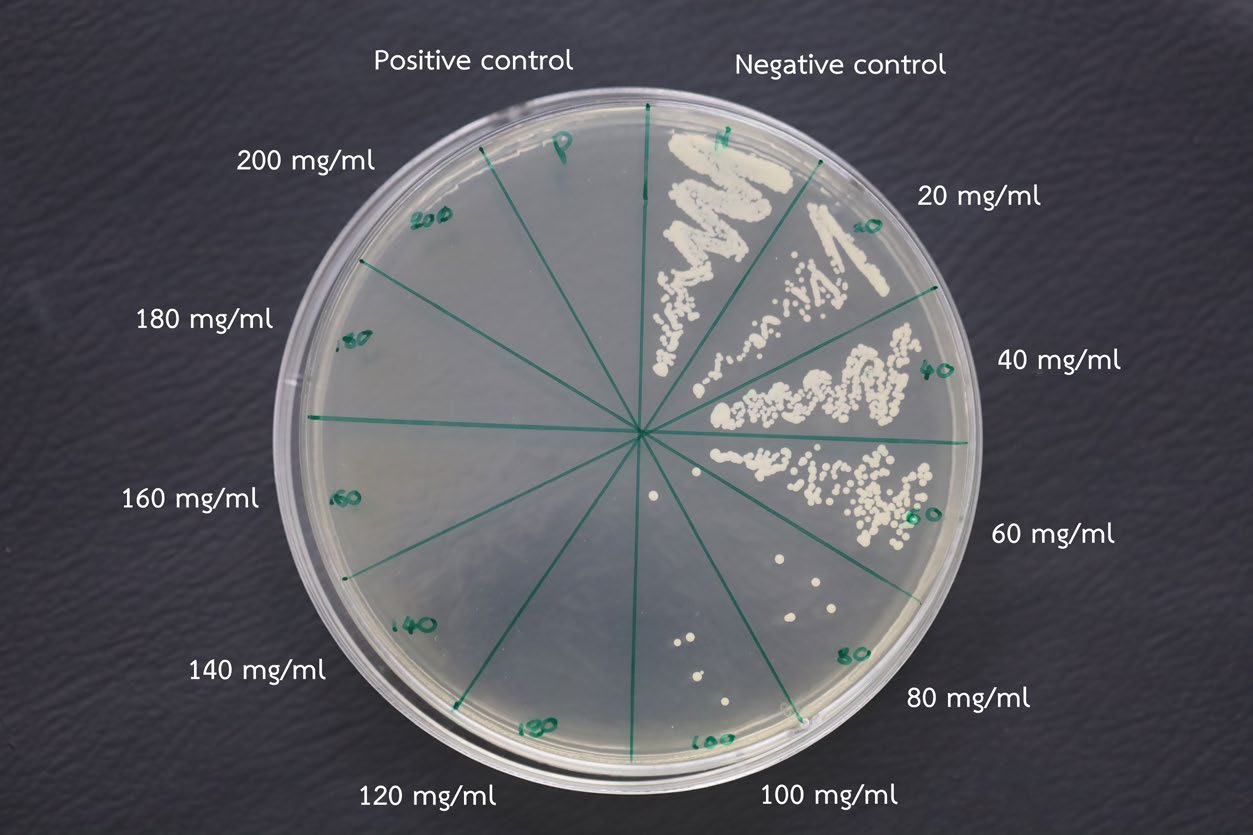
Figure 2. Culture plate showing minimum fungicidal concentration (MFC) test of COS against Candida albicans, with COS concentrations ranging from 20 to 200 mg/ml, alongside a positive control (Nystatin) and a negative control (mixture without COS). Complete inhibition was observed at a COS concentration of 120 mg/ml.
One-way ANOVA revealed that incorporating of COS significantly reduced the colony count relative to the denture adhesive without COS (negative control group). The 0.5 MFC and 1 MFC concentrations exhibited significantly higher colony counts of Candida albicans compared to nystatin. Nystatin demonstrated the most pronounced antifungal effect overall. However, no significant difference was observed between the 1.5 MFC and Nystatin (P < 0.05). Based on these results, 1.5 MFC was selected as the optimal concentration for further evaluation of adhesion strength. (Figure 3).
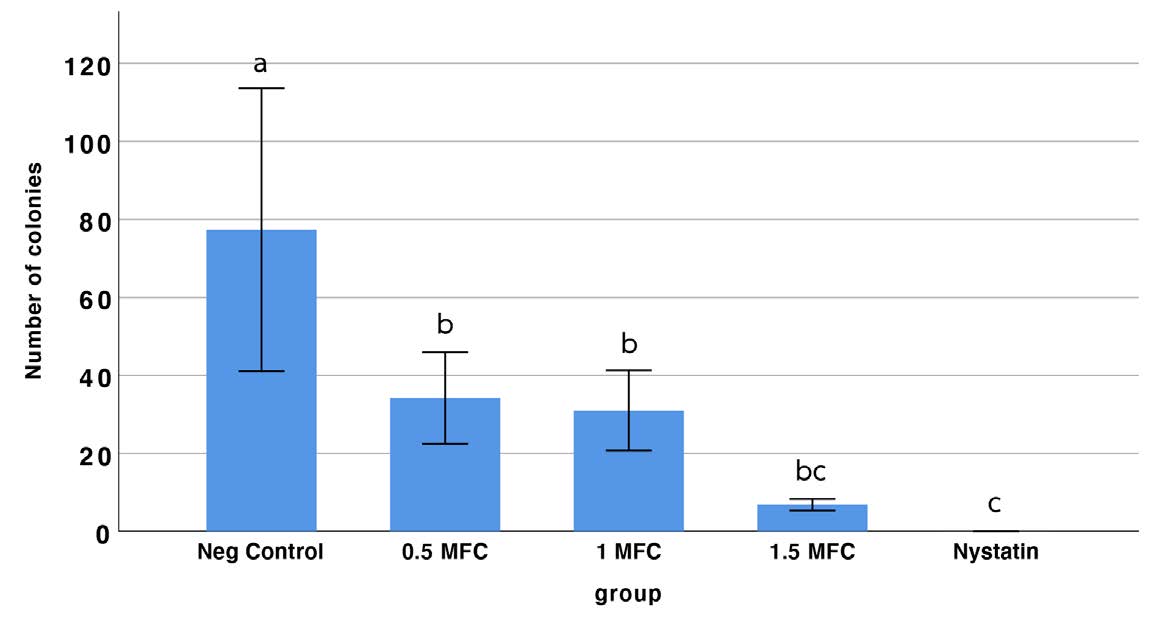
Figure 3. The number of colonies of the various COS concentrations in the denture adhesive compared to the control group (denture adhesive without COS). Bars with the same letter are not significantly different; different letters indicate statistically significant differences among groups (one-way ANOVA, Tukey’s post hoc test, P < 0.05).
Two-way ANOVA revealed significant differences between groups (P < 0.05). Both the immersion time and the presence of chitosan in the adhesive exhibited substantial interactions with the mean adhesion strength. In the adhesive without COS group (control adhesive group), adhesive strength increased after 1 min of immersion, then gradually decreased over 30 min. After that, it stabilized without significant changes. No significant differences were observed in the COS-combined adhesive group across different immersion times. At the initial time, no significant difference in adhesive strength was observed between the two groups. However, the mean adhesive strength of the COS-modified adhesive group was significantly lower than that of the standard adhesive group. Nonetheless, the adhesive strength of all groups met the acceptable standards set by ISO 10873:2021 (Figure 4).
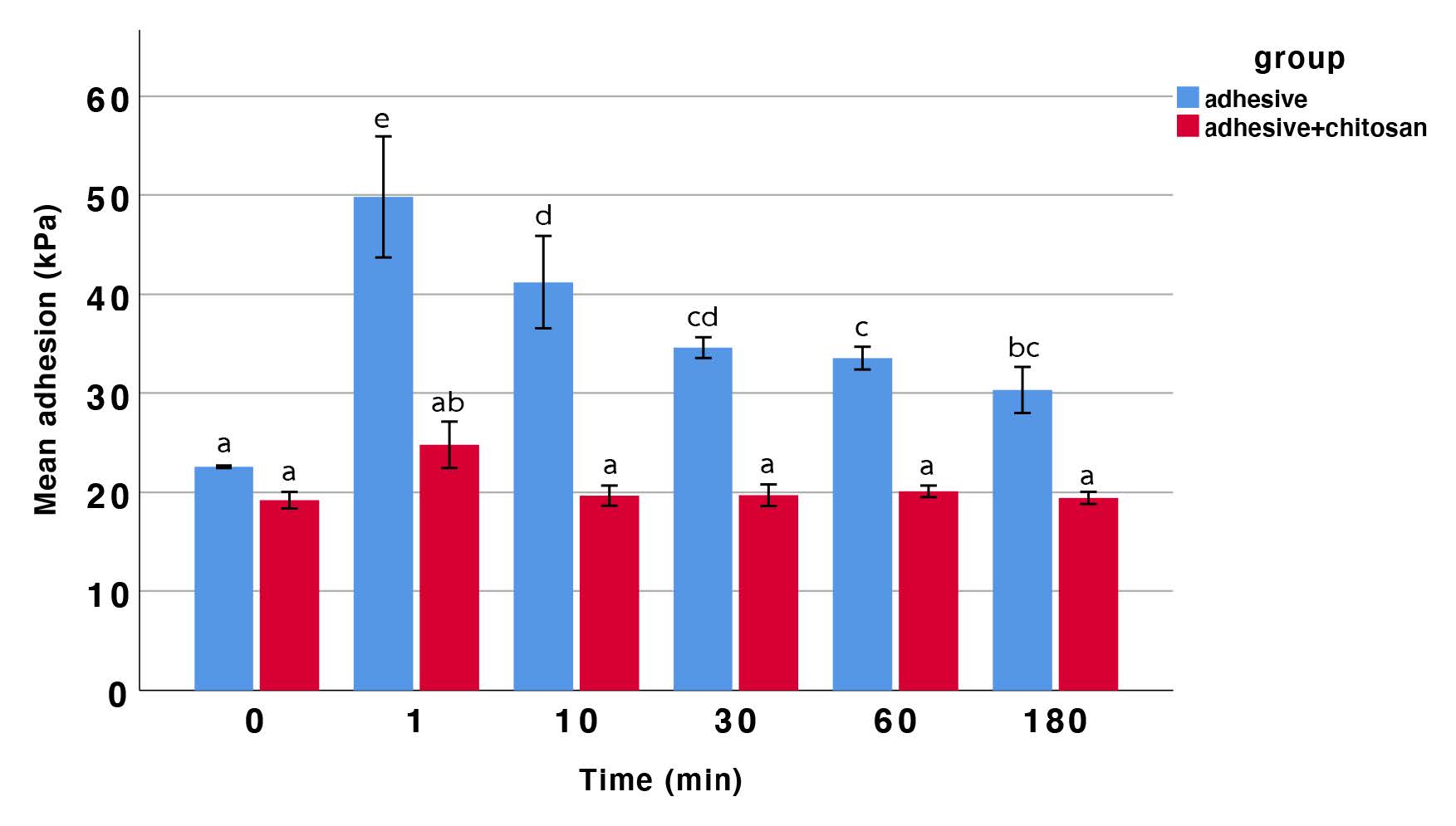
Figure 4. The mean adhesive strength of the control and COS-combined groups over various immersion times. Bars with the same letter are not significantly different; different letters indicate statistically significant differences among groups (two-way ANOVA, Tukey’s post hoc test, P < 0.05).
DISCUSSION
Regarding the results of this study, the null hypothesis that different proportions of Chitosan Oligosaccharide combined in denture adhesive demonstrate no different levels of antifungal activity against Candida albicans, and the null hypothesis that the adhesive properties of Chitosan Oligosaccharide combined in denture adhesive were not different compared to denture adhesive alone, which were rejected.
Results revealed a progressive reduction in the Candida albicans colony count upon incorporating COS into the denture adhesive. These findings are consistent with previous researches that demonstrated promising antifungal activity of chitosan incorporation into materials intended for dental applications (Namangkalakul et al., 2020; Buanpech et al., 2024; Jariyanurut et al., 2024; Puangphimolkij et al., 2024).
Several studies have investigated the antimicrobial effects of COS, demonstrating its significant activity against a wide range of bacterial and fungal pathogens. Low molecular weight and a higher degree of deacetylation in COS correlate with enhanced antimicrobial activity (Atai et al., 2017). However, its antimicrobial potency remains lower compared to that of chitosan. The antimicrobial mechanism of COS is similar to chitin and chitosan. COS interacts with receptors on the microbial cell wall, displacing potassium ions (K+) from the cell membrane, which induces K+ efflux and stimulates extracellular acidification. This disruption leads to changes in the membrane potential and increased calcium ion (Ca2+) uptake, ultimately impairing the microbial cell's vital functions and leading to cell death (Naveed et al., 2019). In contrast, Nystatin binds to the cell membrane and forms polyene-ergosterol complex, causing structural damage and increased membrane permeability, leading to cell death (Coutinho et al., 2004; Groeschke et al., 2006; Lyu et al., 2016). Therefore, ergosterol is uniquely found in the fungal cell membrane, which confers a high degree of specificity to Nystatin in targeting fungal cells. This specificity distinguishes Nystatin from COS, making it more effective and selective in combating fungal infections (Sousa et al., 2023).
Denture adhesives improve the retention, stability, and comfort of removable dentures, enhancing functional performance and minimizing issues such as slippage or discomfort during speech or eating. The exposure to water of the adhesive initiates the adhesion mechanism, which causes the material to swell (Shimazu et al., 2014). The hydration of hydrophobic and hydrophilic components creates a complex morphology that influences key material properties, including adhesive strength, cohesive strength, and viscosity (Fallahi et al., 2018). This process strengthens both adhesive and cohesive characteristics while increasing the viscosity of the medium, thereby reducing the spaces between the denture base and the basal seat (Shay, 1991).
Adhesive force is generated by the interaction of hydrophobic content with a non-polar group of PMMA, while cohesive force and enhanced adhesive qualities are achieved through the cross-linking of long-acting polymers (Polychronakis et al., 2021; Veerapol and Palanuwech, 2023).
A previous study on the physical factors of saliva affecting denture retention demonstrated that viscosity plays a significant role, with higher viscosity correlating to greater retentive force. Therefore, the adhesion strength of denture adhesives to acrylic resins is also expected to be significantly influenced by the viscosity. Adhesives with higher viscosity provide greater adhesion strength when applied at a constant thickness. In contrast, materials with lower viscosity have a greater tendency to flow out of the denture shortly after application, diminishing the adhesive effect and reducing the stability and retention of the denture. A higher content of water-soluble polymers produces materials with increased viscosity, while the higher average molecular weight and larger particle size of these polymers contribute to the elevated viscosity (Kano et al., 2012).
The major adhesive component, primarily alkyl vinyl ether-maleic anhydride (AVE-MA) salts, is presented in all types of adhesives (5-60% by weight). It is mucoadhesive, hydrophilic, and expands when wet. Additionally, the formulation includes a water-insoluble component (20-70% by weight), viscosity index improvers that control overall viscosity (1-20% by weight), water-insoluble plasticizing agents to soften the product (1-10% by weight), gellant agents, and additives for taste and scent, which may also possess medicinal or sensory properties (Veerapol and Palanuwech, 2023). Therefore, the optimal proportion of hydrophobic and hydrophilic components is crucial for achieving the highest adhesive strength. However, the incorporation of COS, which exhibits higher water solubility and lower viscosity compared to chitin or chitosan, may disrupt the adhesive composition, and interfere with the cross-linking process (Muanprasat and Chatsudthipong, 2017). This leads to lower adhesive strength than commercial denture adhesives with the adhesive strength remaining constant over immersion time.
Nevertheless, the adhesive strength of the denture adhesive containing COS remains above the 5 kPa threshold specified by ISO 10873 throughout the immersion period (ISO10873, 2021). However, it is essential to note that this test was conducted under laboratory conditions. Therefore, the results may not accurately reflect the adhesion strength in the oral environment, where factors such as saliva composition and the characteristics of oral mucosa could influence the adhesive performance. Further investigations are necessary to evaluate the effects on different Candida albicans strains and to assess the adhesive strength under oral environment conditions, such as the presence of saliva or the influence of oral mucosa.
CONCLUSION
Based on the findings in this study, it can conclude that denture adhesive combined with sterile COS at a concentration of 1.5 MFC (180 mg/ml) could be used as an optional of a denture adhesive to enhance antimicrobial activity whilst maintain optimum adhesion strength.
ACKNOWLEDGMENTS
I would like to express my sincere gratitude to the Faculty of Dentistry, Chiang Mai University, and the Dental Research Center at the Faculty of Dentistry, Chiang Mai University, for their invaluable support and statistical advice throughout this study. Their guidance and expertise have greatly contributed to the success of this research.
AUTHOR CONTRIBUTIONS
Varat Kanchanomai was responsible for designing and performing the experiments, conducting the statistical analysis and data visualization, and writing the manuscript. Pisaisit Chaijareenont assisted in conducting the mechanical experiments and performed the statistical analysis. Phenphichar Wanachantararak designed and conducted the antifungal experiments and performed the statistical analysis. Patcharawan Silthampitag assisted in conducting all experiments and performed the statistical analysis. All authors have reviewed and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Akpan, A. and Morgan, R. 2002. Oral candidiasis. Postgraduate Medical Journal. 78(922): 455-459.
Atai, Z., Atai, M., Amini, J., and Salehi, N. 2017. In vivo study of antifungal effects of low-molecular-weight chitosan against Candida albicans. Journal of Oral Science. 59(3): 425-430.
Buanpech, P., Chaijareenont, P., Wanachantararak, P., and Silthampitag, P. 2024. Antifungal effect and durability of chitosan oligosaccharide coating on heat-cured polymethylmethacrylate surface. Natural and Life Sciences Communications. 23(1): e2024004.
Budtz-Jorgensen, E. and Bertram, U. 1970. Denture stomatitis. I. The etiology in relation to trauma and infection. Acta Odontologica Scandinavica. 28(1): 71-92.
Coutinho, A., Silva, L., Fedorov, A., and Prieto, M. 2004. Cholesterol and ergosterol influence nystatin surface aggregation: Relation to pore formation. Biophysical Journal. 87(5): 3264-3276.
Fakhri, E., Eslami, H., Maroufi, P., Pakdel, F., Taghizadeh, S., Ganbarov, K., Yousefi, M., Tanomand, A., Yousefi, B., Mahmoudi, S. et al. 2020. Chitosan biomaterials application in dentistry. International Journal of Biological Macromolecules. 162: 956-974.
Fallahi, A., Khadivi, N., Roohpour, N., Middleton, A.M., Kazemzadeh-Narbat, M., Annabi, N., Khademhosseini, A., and Tamayol, A. 2018. Characterization, mechanistic analysis and improving the properties of denture adhesives. Dental Materials. 34(1): 120-131.
Groeschke, J., Solassol, I., Bressolle, F., and Pinguet, F. 2006. Stability of amphotericin B and nystatin in antifungal mouthrinses containing sodium hydrogen carbonate. Journal of Pharmaceutical and Biomedical Analysis. 42(3): 362-366.
ISO10873. 2021. Dentistry — Denture adhesives. In. Geneva: International Organization for Standardization.
Jariyanurut, N., Banthitkhunanon, P., Sukapattee, M., and Wanachantararak, P. 2024. The effect of chlorhexidine-chitosan formulations on Candida albicans biofilm inhibition and the physical properties of polymethyl methacrylate resin. Journal of International Dental and Medical Research. 17(4): 1408-1416.
Kano, H., Kurogi, T., Shimizu, T., Nishimura, M., and Murata, H. 2012. Viscosity and adhesion strength of cream-type denture adhesives and mouth moisturizers. Dental Materials. 31(6): 960-968.
Lyu, X., Zhao, C., Yan, Z.M., and Hua, H. 2016. Efficacy of nystatin for the treatment of oral candidiasis: A systematic review and meta-analysis. Drug Design, Development and Therapy, 10, 1161-1171.
Martínez-Beneyto, Y., López-Jornet, P., Velandrino-Nicolás, A., and Jornet-García, V. 2010. Use of antifungal agents for oral candidiasis: Results of a national survey. International Journal of Dental Hygiene. 8(1): 47-52.
Muanprasat, C. and Chatsudthipong, V. 2017. Chitosan oligosaccharide: Biological activities and potential therapeutic applications. Pharmacology & Therapeutics, 170, 80-97.
Namangkalakul, W., Benjavongkulchai, S., Pochana, T., Promchai, A., Satitviboon, W., Howattanapanich, S., Phuprasong, R., Ungvijanpunya, N., Supakanjanakanti, D., Chaitrakoonthong, T., et al. 2020. Activity of chitosan antifungal denture adhesive against common Candida species and Candida albicans adherence on denture base acrylic resin. Journal of Prosthetic Dentistry. 123(1): e181-e187.
Naveed, M., Phil, L., Sohail, M., Hasnat, M., Baig, M., Ihsan, A.U., Shumzaid, M., Kakar, M.U., Mehmood Khan, T., Akabar, M. et al. 2019. Chitosan oligosaccharide (COS): An overview. International Journal of Biological Macromolecules. 129: 827-843.
Pereira-Cenci, T., Del Bel Cury, A.A., Crielaard, W., and Ten Cate, J.M. 2008. Development of Candida-associated denture stomatitis: New insights. Journal of Applied Oral Science. 16(2): 86-94.
Perić, M., Miličić, B., Kuzmanović Pfićer, J., Živković, R., and Arsić Arsenijević, V. 2024. A systematic review of denture stomatitis: Predisposing factors, clinical features, etiology, and global Candida spp. distribution. Journal of Fungi. 10(5): 328.
Polychronakis, N., Sykaras, N., Polyzois, G., and Lagouvardos, P. 2021. Removal of denture adhesives from PMMA and Polyamide denture base materials. Journal of Applied Oral Science. 29: e20200448.
Puangphimolkij, T., Chaijareenont, P., Wanachantararak, P., and Silthampitag, P. 2024. Effect of chitosan oligosaccharide solution against Candida albicans and color stability of heat-cured polymethylmethacrylate. Journal of International Dental and Medical Research. 17(1): 115-121.
Rai, A., Misra, S.R., Panda, S., Sokolowski, G., Mishra, L., Das, R., and Lapinska, B. 2022. Nystatin effectiveness in oral candidiasis treatment: A systematic review & meta-analysis of clinical trials. Life. 12(11): 1677.
Sartawi, S.Y., Abu-Hammad, S., A Salim, N., and Al-Omoush, S. 2021. Denture stomatitis revisited: A summary of systematic reviews in the past decade and two case reports of papillary hyperplasia of unusual locations. International Journal of Dentistry. 2021: 7338143.
Shay, K. 1991. Denture adhesives. Choosing the right powders and pastes. Journal of the American Dental Association. 122(1): 70-76.
Shimazu, K., Karibe, H., and Ogata, K. 2014. Effect of artificial saliva contamination on adhesion of dental restorative materials. Dental Materials. 33(4): 545-550.
Sousa, F., Nascimento, C., Ferreira, D., Reis, S., and Costa, P. 2023. Reviving the interest in the versatile drug nystatin: A multitude of strategies to increase its potential as an effective and safe antifungal agent. Advanced Drug Delivery Reviews. 199: 114969.
Veerapol, P. and Palanuwech, M. 2023. The effect of eight types of denture adhesives on retention of milled denture base acrylic resin. Srinakharinwirot University Dental Journal. 16(1): 134-147.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Varat Kanchanomai1, Pisaisit Chaijareenont1, Phenphichar Wanachantararak2, and Patcharawan Silthampitag1, *
1 Department of Prosthodontics, Faculty of Dentistry, Chiang Mai University, Chiang Mai 50200, Thailand.
2 Dental Research Center, Faculty of Dentistry, Chiang Mai University, Chiang Mai 50200, Thailand.
Corresponding author: Patcharawan Silthampitag, E-mail: patcharawan.sil@cmu.ac.th
Total Article Views
Editor: Anak Iamaroon,
Chiang Mai University, Thailand
Article history:
Received: May 6, 2025;
Revised: July 22, 2025;
Accepted: August 4, 2025;
Online First: August 14, 2025

