
Green Synthesis of Silver Nanoparticles Using Curcuma longa: Evaluation of Antimicrobial Activity, In Vivo Imaging-Based Toxicity, and In Vitro Biocompatibility
Gopinath Kasi, Balamurugan Venkatachalam, Sarinthip Thanakkasaranee, Nattan Stalin, Ramar Dharmaraj, Kittisak Jantanasakulwong, and Pornchai Rachtanapun*Published Date : August 8, 2025
DOI : https://doi.org/10.12982/NLSC.2025.060
Journal Issues : Number 4, October-December 2025
Abstract This study aimed to compare the bioactive profiles and biological activities of methanolic extracts from Curcuma aromatica (CA) and Curcuma longa (CL) rhizomes. Fourier-transform infrared spectroscopy (FTIR) and gas chromatography-mass spectrometry (GC-MS) analyses revealed the presence of bioactive functional groups and identified 19 and 14 phytocompounds in the CA and CL extracts, respectively. The CL extract exhibited higher flavonoid, alkaloid, and phenol contents, along with superior antioxidant activity in 2,2-diphenyl-1-picrylhydrazyl (DPPH) and ferric reducing antioxidant power (FRAP) assays, while CA showed greater metal-chelating activity. The CL extract showed strong antimicrobial activity against Streptococcus pneumoniae and Klebsiella pneumoniae (1.46 cm inhibition zone), while the CA extract exhibited no antimicrobial efficacy. Silver nanoparticles (Ag NPs) synthesized using CL extract displayed an ultraviolet-visible (UV-Vis) absorption peak at 410 nm, spherical morphology, and a face-centered cubic structure with an average crystallite size of 9.32 nm. These Ag NPs exhibited dose-dependent antimicrobial activity against Bacillus megaterium, S. pneumoniae, and K. pneumoniae at a concentration of 100 µg/mL. In vivo toxicity studies of Ag NPs revealed 26.33–76.33% mortality in Artemia salina nauplii at 10 mg/mL after 24 h, while an in vitro cell viability assay in RAW 264.7 cells at 1 µg/mL confirmed cytocompatibility with a stimulatory hormesis effect. These findings suggest that CL extract acts as an antioxidant and antimicrobial agent. The CL extract-mediated synthesis of Ag NPs can be used in antimicrobial coatings, wound dressings, and topical ointments to prevent and treat infections.
Keywords: Antimicrobial activity; Cytocompatibility; Green synthesis; In vivo toxicity; Rhizome extract; Silver nanoparticles
Funding: This research was supported by funding from the NSRF through the Program Management Unit for Human Resources & Institutional Development, Research and Innovation [Grant numbers: B13F660056, B13F670056]. The authors also acknowledge the financial support provided by the 'Reinventing University Program' at Chiang Mai University, Thailand. Additional support was received from the Center of Excellence in Agro Bio-Circular-Green Industry (Agro BCG), Faculty of Agro-Industry, Chiang Mai University. The present study was partially supported by the Thailand Research Fund (TRF) Research Team Promotion Grant, RTA, Senior Research Scholar (N42A671052). This work was further supported by the National Research Council of Thailand (NRCT) through the 'Hub of Talents in Bioplastics for Use in Medical Applications' initiative (Grant No. N34E670071).
Citation: Kasi, G., Venkatachalam, B., Thanakkasaranee, S., Stalin, N., Dharmaraj, R., Jantanasakulwong, K., and Rachtanapun, P. 2025. Green synthesis of silver nanoparticles using Curcuma longa: Evaluation of antimicrobial activity, in vivo imaging-based toxicity, and in vitro biocompatibility. Natural and Life Sciences Communications. 24(4): e2025060.
INTRODUCTION
Nanoparticles (NPs) possess unique nanoscale properties that make them highly valuable for diverse applications in medicine, environmental remediation, food packaging, and industrial processes (Chittasupho et al., 2023; Aminzai et al., 2024; Jaimun et al., 2024; Saravanan et al., 2025). In the medical field, transition metal NPs such as silver (Ag), gold (Au), platinum (Pt), and palladium (Pd) are widely used because of their high adsorption capacity and broad range of applications (Kannan and Maduraiveeran, 2023). Among them, Ag NPs have garnered significant attention due to their cost-effectiveness, availability, and multifunctional properties (Abbas et al., 2024). The Ag NPs are widely used in catalysis, sensors, energy storage, and environmental remediation (Abbas et al., 2024; Khoirotin et al., 2025). Their broad-spectrum antimicrobial activity is particularly remarkable, as they effectively target bacteria and fungi through mechanisms such as reactive oxygen species (ROS) generation, membrane disruption, and enzyme inhibition (Barua and Buragohain, 2024; Pradabsang et al., 2025). These antimicrobial properties are crucial for addressing global challenges, such as antibiotic resistance and microbial contamination (Mahanty et al., 2024). The 2024 World Health Organization, Bacterial Priority Pathogen List (BPPL) highlights the urgency of this issue by classifying 24 antibiotic-resistant bacteria (across 15 families) into three priority tiers: critical, high, and medium. Critical-priority pathogens include carbapenem-resistant Enterobacterales and rifampicin-resistant Mycobacterium tuberculosis. High-priority pathogens encompass fluoroquinolone-resistant Shigella spp. and methicillin-resistant Staphylococcus aureus (MRSA), while medium-priority threats include macrolide-resistant Streptococcus pneumoniae. Therefore, the development of broad-acting antimicrobial agents, such as silver-based nanomaterials, is crucial for combating these threats (Krishnaprasad and Kumar, 2024; Riesenberger et al., 2024).
However, the synthesis method of Ag NPs critically influences their size, morphology, stability, and biological activity (De Barros et al., 2018). Various techniques, including co-precipitation (Dasaradhudu and Srinivasan, 2020), hydrothermal (Tooklang et al., 2024), sol-gel (De Souza et al., 2024), and green synthesis (Balamurugan et al., 2024; Elumalai and Irfan, 2024), have been developed to optimize these properties. Among these, green synthesis approaches are increasingly favored for their eco-friendliness and synergistic enhancement of antimicrobial activity (Kandav and Sharma, 2024). Despite their potential, the toxicity of Ag NPs to normal cells remains a major concern, limiting their application in the biomedical (Ren et al., 2024) and food packaging fields (Sharma et al., 2023; Lieu et al., 2024).
The genus Curcuma is a perennial herbaceous plant of the Zingiberaceae family, with over 120 species identified in tropical regions such as Southeast Asia, India, and Africa (Devi and Sanatombi, 2024). Species such as Curcuma longa (CL) and Curcuma aromatica (CA) are economically valuable due to their medicinal and aromatic properties (Albaqami et al., 2022). CL rhizomes, rich in bioactive compounds such as curcumin, are widely used in traditional medicine and exhibit antioxidant, antibacterial, anti-inflammatory, and antiviral activities (Fuloria et al., 2022; Jyotirmayee and Mahalik, 2022). Similarly, CA is prized for its therapeutic applications in treating ailments such as asthma, skin disorders and rheumatism (Umar et al., 2020; Wuttikul and Sainakham, 2022). These bioactivities are attributed to secondary metabolites like phenolics, flavonoids, and terpenoids, which act as free radical scavengers, disrupt microbial biofilms, and enhance antimicrobial potential (Umar et al., 2020; Fuloria et al., 2022; Wuttikul and Sainakham, 2022).
In recent years, the green synthesis of NPs using plant extracts has emerged as a sustainable alternative to chemical methods (Umar et al., 2020). Plant-based synthesis eliminates toxic reagents, conserves energy, and enables large-scale production (Umar et al., 2020; Vanlalveni et al., 2021). The bioactive compounds in plant extracts not only act as reducing and stabilizing agents but also enhance the antimicrobial activity of the synthesized NPs through synergistic effects (Vanlalveni et al., 2021). However, comparative research on the phytochemical properties and antimicrobial efficacy of different Curcuma species for the synthesis of green NPs is limited. Additionally, the cytocompatibility and toxicity of these NPs remain underexplored, particularly in the context of biomedical applications.
While various methods exist for Ag NP synthesis, the approach using microbial sources such as Streptomyces sp. and Micromonospora sp. has been widely studied. Adiguzel et al. (2018) reported the synthesis of Ag NPs using the cell lysates of Streptomyces sp. AOA21, isolated from soil. The synthesized Ag NPs exhibited antimicrobial activity, with minimum inhibitory concentration (MIC) values of 8 µg/mL for Bacillus cereus, 16 µg/mL for Klebsiella pneumoniae and Escherichia coli, and 32 µg/mL for S. aureus. Similarly, Mazmancı et al. (2023) demonstrated the synthesis of Ag NPs using the culture supernatant of Micromonospora sp. SH121. These Ag NPs exhibited strong antimicrobial activity against a range of pathogens, including Gram-positive and Gram-negative bacteria, as well as fungi. Notably, the Ag NPs also exhibited partial inhibition of biofilm formation in clinical pathogens such as Acinetobacter baumannii, E. coli, K. pneumoniae, and Pseudomonas aeruginosa.
However, microbial-based nanoparticle synthesis is often a time-consuming, multi-step process that requires specialized bacterial culture media, energy, and strict adherence to laboratory safety protocols. In contrast plant-based synthesis an attractive alternative, as it is more cost-effective, eco-friendly, and scalable, with the added benefit of utilizing natural reducing agents and stabilizing compounds found in plant extracts. Previous studies have explored Ag NP synthesis using Curcuma rhizome extracts. Tawre et al. (2022) synthesized Ag NPs using CA aqueous extract, resulting in spherical particles (13 ± 5 nm) with a zeta potential of ─23.8 mV. These Ag NPs demonstrated strong antibacterial activity against multidrug-resistant pathogens, including P. aeruginosa and S. aureus. In addition, Jain et al. (2023) reported the synthesis of Ag NPs using aqueous extracts of CL, Curcuma caesia, and CA, with CL-based Ag NPs exhibiting the most significant anticancer activity, reducing the viability of HT-29 colon cancer cells by 12.6% at 80 µg/mL. In terms of originality, the current work focused on isolating phytocomponents from a methanol extract and using the solid sample for Ag NP synthesis with an aqueous solution. The synthesized Ag NPs were analyzed through three-dimensional testing, including antimicrobial effects, environmental toxicity, and biocompatibility.
The current study investigates and compares the phytochemical profiles and antioxidant capacities of methanolic extracts from CL and CA rhizomes. The Ag NPs were synthesized using the CL extract and thoroughly characterized for their structural, optical, and morphological properties. Their antimicrobial activity was evaluated against human pathogens, while toxicity and biocompatibility were assessed using Artemia salina and RAW 264.7 macrophage cells. These findings offer valuable insights into the safe and practical application of green-synthesized Ag NPs in biomedical and environmental fields.
MATERIALS AND METHODS
Materials
Silver nitrate was procured from Sigma-Aldrich Chemicals (USA). Ethylenediaminetetraacetic acid (EDTA), 2,2-Diphenyl-1-picrylhydrazyl (DPPH), dimethyl sulfoxide (DMSO), Dulbecco's Modified Eagle's Medium (DMEM), microbial media, 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), nalidixic acid and other required chemicals were purchased from Hi-Media (India). Sisco Research Laboratories Pvt. Ltd. (India) supplied the ethanol and methanol for this research. Double-distilled water (DD H2O) and deionized water (DI H2O) were also used for the experiments and required conditions.
Rhizome collection and extraction
C. longa and C. aromatica rhizome materials were collected from Gudalur, Nilgiri District, Tamil Nadu, India, in August 2023. The collected material was cleaned and dried at room temperature (RT). Methanolic extracts of dried rhizome samples (50 g) were obtained via maceration (200 mL, 24 h), followed by solvent evaporation using a rotary vacuum evaporator (45°C), and stored at 4°C for subsequent phytochemical analysis.
Microbial strains
Three Gram-positive bacterial strains (B. megaterium, S. aureus, and S. pneumoniae), two Gram-negative bacterial strains (K. pneumoniae and Shigella flexneri), and one fungal strain (Candida albicans) were used as model microorganisms in this study.
Characterization
The methanol extracts of CL and CA samples, along with Ag NPs, were analyzed using various techniques. The ultraviolet-visible (UV-Vis) spectrophotometer (Shimadzu - UV 1,800) was employed to measure absorbance between 200 to 800 nm. Fourier-Transform Infrared Spectroscopy (FTIR) (Bruker Alpha II) was used to identify functional groups from 4,000 to 500 cm−1. Methanolic extracts of CL and CA were analyzed to determine their bioactive phytochemical components using gas chromatography-mass spectrometry (GC-MS) on a PerkinElmer Turbo Mass Spectrophotometer (USA). The obtained mass spectra were compared with data from the National Institute of Standards and Technology (NIST) 2008 library database and corroborated with the published literature (Nonglang et al., 2022). X-ray diffraction (XRD) analysis was carried out at 25°C using an XPERT-PRO diffractometer (PANalytical, Netherlands) with Ni-filtered Cu Kα radiation (λ = 1.5406 Å), over a 2θ range of 25–80°, at 40 kV and 30 mA, with a step size of 0.05°. The zeta potential of the Ag NPs was measured using a Zetasizer Nano ZSP at 25°C. The surface morphology was examined using scanning electron microscopy (SEM) with Vega3 software, using gold sputtering for conductivity and an accelerating voltage of 15 kV. Energy-Dispersive X-ray Spectroscopy (EDX) analysis was used to identify the elemental composition. High-Resolution Transmission Electron Microscopy (HR-TEM) at 200 kV was used to analyze the particle size, shape, and selected area electron diffraction (SAED) patterns.
Determinations of phytochemical assay
Total phenolics
The total phenol concentration in the CL and CA methanolic extracts was assessed using the Makkar (2003) method. Rhizome extracts (50-600 µL) were combined with 500 µL of 1N Folin-Ciocalteu reagent and a blank. After 5 min, 2.5 mL of 20% sodium carbonate was added, and the mixture was incubated in the dark at RT for 40 min, resulting in a blue color indicating phenolics. Samples were analyzed with UV-Vis absorbance at 725 nm and reported as gallic acid equivalents (GAE). The experiment was conducted in triplicate.
Total alkaloids
The total alkaloid content of CL and CA methanolic extracts was quantified using the 1,10-phenanthroline method (Singh et al., 2004). The extracts were mixed with FeCl3 in HCl and 1,10-phenanthroline in ethanol, incubated at 70 ± 20°C for 30 min, and the absorbance of the resulting red complex was measured at 510 nm. Alkaloid levels were assessed using a colchicine standard curve and calculated as mg per gram based on dry weight.
Total flavonoids
The total flavonoid content in the methanolic extracts of CL and CA was assessed using the Zhishen et al. (1999) method. Each test tube received 50-600 µL of extract and 2 mL of DD H2O, with 2.5 mL DD H2O as a blank. Subsequently, 150 µL of 5% NaNO2 was added and the mixture was kept at RT for 6 min. Then, 150 µL of 10% AlCl3 was added and incubated for another 6 min at RT. Lastly, 2 mL of 4% NaOH was added. Then, the mixture was diluted with DD H2O to 5 mL. The samples were vortexed and left at RT for 15 min. The resulting pink color was measured at 510 nm. The average of each triplicate sample was calculated, and the amount of flavonoids was calculated as in rutin equivalents.
Assessment of antioxidant activity
DPPH assay
The free radical scavenging capacity of methanolic extracts from CL and CA was assessed by Braca et al. (2001) method. Various concentrations (50-600 µL) of extracts were added to 3 mL of 0.004% DPPH in methanol. After 30 min of incubation, the absorbance was measured at 517 nm, and formula (1) was used to calculate the percentage inhibition:
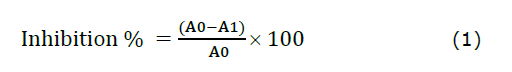
where A0 represents the control absorbance and A1 represents the extract or standard absorbance.
Ferric reducing antioxidant power (FRAP) assessment
The method of Benzie and Strain was used for FRAP analysis, with small modifications (Benzie and Strain, 1996). To make a solution, acetate buffer, TPTZ, and FeCl3·6H2O in a 10:1:1 ratio, and then warmed to 37°C before use. Various concentrations (50-600 μL) of the CL and CA extracts were stored in the dark for 30 min after adding 2,850 µL of FRAP solution. The absorbance of the complex was measured at 593 nm. The obtained results were calculated in µg Trolox equivalents per gram of extract.
Metal chelating assessment
The chelating capacity for ferrous ions (Fe2+) in methanolic extracts from CL and CA was measured by the Dinis et al. (1994) method. Various extract concentrations (50-600 µL) were mixed with 1.6 mL DI H2O and 0.05 mL of 2 mM FeCl2 in each test tube. After 0.5 min of incubation, 0.1 mL of 5 mM ferrozine was added, forming stable, water-soluble magenta complexes with divalent iron. The absorbance of the Fe2+-ferrozine complex was assessed at 562 nm after 10 min at RT and calculated as mg EDTA equivalents per gram of extract.
Antimicrobial activity of methanol extracts of CL and CA
The methanolic extracts of CL and CA were evaluated for their antimicrobial effectiveness against B. megaterium, S. aureus, S. pneumoniae, K. pneumonia, S. flexneri, and C. albicans using the well-diffusion method. The bacterial strains were grown for 24 h in the sterile nutrient broth, while fungal strains were cultured for 48 h. After pouring 20 mL of nutrient agar medium into sterile Petri plates, it was allowed to cool to RT. Subsequently, the bacterial and fungal strains were individually spread on the agar surface. Wells were prepared using a sterilized cork-borer. The solid rhizome extract was dissolved in 100 μL of DMSO and loaded into the wells using a sterile pipette at concentrations of 25, 50, and 100 μg. A disc containing 30 µg of nalidixic acid was used as a positive control. The plates were incubated at 37°C for 24 and 48 h. After incubation, the zones of inhibition were measured and recorded. Each experiment was conducted in triplicate.
Synthesis of Ag NPs using CL methanol extract
The methanolic extract of CL was selected for Ag NP synthesis based on its bioactivity. The solid extract (0.1 g) was dissolved in 1 mL of DD H2O, sonicated for 30 min, and added to 100 mL of 1 mM AgNO3. The mixture was stirred at RT for 30 min. The mixture was then heated at 80°C for 30 min, and the color changed from transparent to brown, indicating Ag NP formation.
Antimicrobial activity of the synthesized Ag NPs using CL methanol extract
The antimicrobial activity of synthesized Ag NPs was tested against B. megaterium, S. aureus, S. pneumoniae, K. pneumoniae, S. flexneri, and C. albicans using the well-diffusion method. Bacteria and fungi were incubated for 24 h and 48 h, respectively. Nutrient agar was poured into Petri dishes, cooled, and inoculated with the strains. Wells were made, and 100 µL of Ag NPs (25, 50, 75, and 100 μg/mL) were added to the wells. A dose of 100 µg/mL was chosen based on the study by Balamurugan et al. (2024). The plates were incubated at 37°C for 24 h (bacteria) and 48 h (fungi), and the inhibition zones were measured. All experiments were performed in triplicate.
Biointerface studies
In vivo imaging-based toxicity assay of Ag NPs using A. salina nauplii
The in vivo toxicity assessment of Ag NPs was performed following the protocol established by Balamurugan et al. (2024). This study investigated the effects of different concentrations of Ag NPs on the growth and survival of A. salina nauplii over a 24 h intermittent flow-through experiment. The experiment commenced with newly hatched nauplii; less than a day old, the state of A. salina was placed in 24-well plates. Each well was filled with 2 mL of seawater for the negative control, while for the positive control, a methanolic extract of CL 0.1% mixture of seawater was used. Ag NPs at concentrations of 2.5, 5.0, 7.5, and 10 mg /mL were prepared by dilution with seawater to reach a total volume of 2 mL in each well (Balamurugan et al., 2024). Subsequently, A. salina nauplii (n = 10) were added to each well for experimental treatment. Each concentration was tested in triplicates in a 24-well plate. After a 12 and 24 h period in the dark, the experimental setup was examined and the number of surviving nauplii was counted. Photographic images were captured using Olympus CX21i LED binoculars loaded with a Magcam DC5 MIPS 5.1 MP 1/2.5 CMOS sensor camera. The mortality rate was calculated using formula (2) to compare the control group with the Ag NP-treated group:
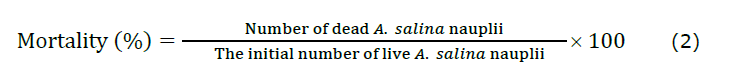
In vitro cell viability assay of Ag NPs
RAW 264.7 macrophages were cultured in DMEM supplemented with 10% FBS at 37°C in 5% CO2. RAW 264.7 macrophages were grown in DMEM with 10% FBS at 37°C under 5% CO2 conditions. At a density of 5 × 104 cells/mL, the cells were placed into a 96-well plate and incubated for 24 h to reach near confluency. The cells were then treated with Ag NP concentrations ranging from 0.1 to 50 μg/mL for another 24 h. The absorbance of the treated sample was measured at 590 nm.
Statistical analysis
Data were replicated and analyzed using IBM SPSS Statistics version 26 (IBM Corp., Armonk, NY, USA) to calculate means and standard deviations. Paired t-tests were conducted to compare the datasets, with statistical significance set at P ≤ 0.05.
RESULTS
UV-Vis spectroscopy and FTIR assessment
The UV-Vis spectra of methanolic extracts from CL and CA rhizome powders are presented in Figure 1A. The CL extract showed two prominent absorption peaks at λmax= 289 and 428 nm, while the CA extract exhibited peaks at λmax = 304 and 377 nm. Absorbance in the 200–350 nm region was observed in both samples, as well as a higher absorbance in the 350–500 nm range for the CL extract. The FTIR spectra of the methanolic extracts of CL and CA are shown in Figure 1B. For the CL extract, characteristic vibration bands were observed at 3,482, 2,925, and 2,862 cm-1, which correspond to N–H stretching (primary amines) and asymmetric and symmetric stretching of –CH2 groups. Additional peaks at 1,697 and 1,449 cm-1 are attributed to the C=C stretching and CH2 bending vibrations, respectively. The bands at 1,172, 1,067, and 534 cm⁻¹ represent C–O stretching, CH3 bending, and C–Cl stretching, respectively. The CA extract spectrum shows a broad O–H stretching vibration at 3,383 cm-1, along with C–H stretching at 3,165 cm-1. Asymmetric and symmetric –CH2 group vibrations were identified at 2,924 and 2,863 cm-1, respectively. The fingerprint region displayed bands at 1,708, 1,461, 1,031, and 534 cm⁻¹, corresponding to C=C stretching, CH₂ bending, and C–O and C–Cl stretching vibrations, respectively.
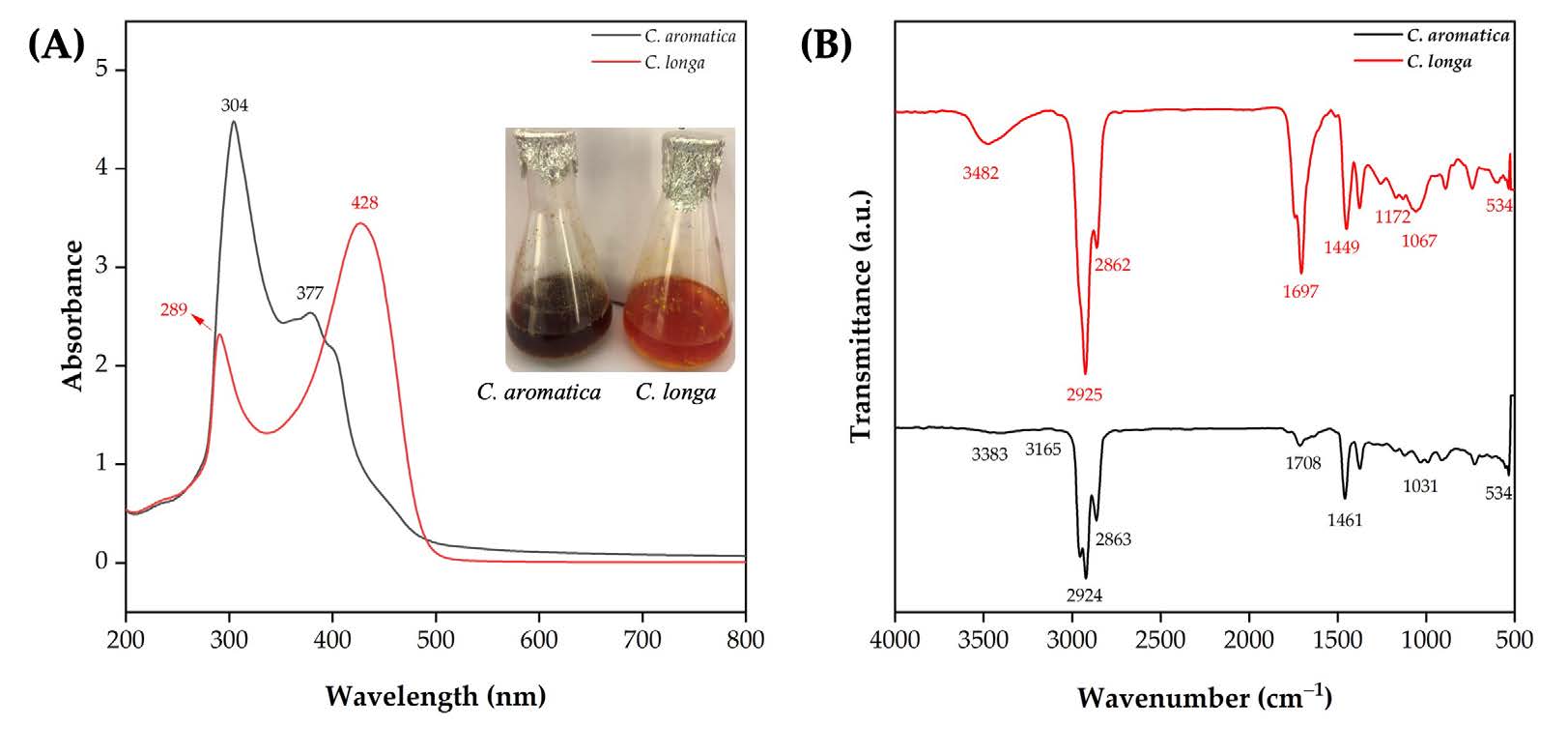
Figure 1. C. longa and C. aromatica rhizome methanol extracts: UV-Vis analysis (A) and FTIR analysis (B).
GC-MS analysis
GC-MS analysis of the methanolic extracts from CL and CA rhizomes identified various phytocompounds (Figure 2). In the CL extract, 14 phytochemicals were detected, with gamma-sitosterol showing the highest peak area percentage. In contrast, 19 compounds were found in the CA extract, with curcumenol being the major component based on peak area percentage. The identification was performed by comparing the retention time, molecular weight, and molecular formula. The compounds detected in the CL and CA extracts are listed in Tables 1 and 2, respectively.

Figure 2. GC-MS chromatogram: C. longa (A) and C. aromatica (B) rhizome methanol extract.
Table 1. GC-MS analysis of the methanol extract of C. longa rhizome.
|
S. No. |
R. Time (min) |
Area% |
Name of Compounds |
Molecular Weight (amu) |
Molecular Formula |
|
1 |
3.136 |
5.60 |
Trimethylsilyl ethaneperoxoate |
148.23 |
C5H12O3Si |
|
2 |
4.149 |
10.50 |
2,2-Dimethoxybutane |
118.17 |
C6H14O2 |
|
3 |
17.676 |
8.64 |
Turmerone |
218.33 |
C15H22O |
|
4 |
17.740 |
4.16 |
Tumerone |
218.33 |
C15H22O |
|
5 |
18.130 |
4.91 |
Curlone |
218.33 |
C15H22O |
|
6 |
20.755 |
7.00 |
n-Hexadecanoicacid |
256.42 |
C16H32O |
|
7 |
25.325 |
1.77 |
1,3,5-Trisilacyclohexane |
126.33 |
C3H6Si3 |
|
8 |
25.461 |
4.28 |
PalmiticAcid, |
256.42 |
C16H32O |
|
9 |
25.667 |
3.55 |
Hexadecanoicacid |
256.42 |
C16H32O |
|
10 |
25.814 |
5.85 |
Bis(2-ethylhexyl)phthalate |
390.55 |
C24H38O4 |
|
11 |
26.210 |
28.47 |
Gamma-Sitosterol |
432.70 |
C29H52O2 |
|
12 |
26.312 |
9.11 |
Gamma-Sitosterol |
432.70 |
C29H52O2 |
|
13 |
26.968 |
2.74 |
1,3,5-Trisilacyclohexane |
126.33 |
C3H6Si3 |
|
14 |
27.625 |
1.60 |
1,3-Benzenedicarboxylicacid |
166.14 |
C8H6O4 |
Note: Retention time (R. Time, min): Time taken for each compound to elute from the GC column.; Area%: Relative abundance of the compound (percentage of the total peak area).; Identified primary compound names: Gamma-sitosterol, palmitic acid, and turmerone.; Molecular weight (amu): Mass of the compound in atomic mass units.; Molecular formula: Chemical formula of the compound.
Table 2. GC-MS analysis of the methanol extract of C. aromatica rhizome.
|
S. No. |
R. Time (min) |
Area% |
Name of Compounds |
Molecular Weight (amu) |
Molecular Formula |
|
1 |
3.782 |
1.62 |
Glycerin |
92.09 |
C3H8O3 |
|
2 |
16.988 |
5.26 |
Epicurzerenone |
230.30 |
C15H18O2 |
|
3 |
18.484 |
36.92 |
Curcumenol |
234.33 |
C15H22O2 |
|
4 |
18.595 |
2.77 |
Cyclodeca[b]furan-2(3H)-one,3a,4,5,8,9,11a-hexahyd |
306.40 |
C17H22O5 |
|
5 |
18.685 |
3.31 |
12.Alpha.-D-5.Alpha.-Androstan-11-One |
274.40 |
C19H30O |
|
6 |
19.024 |
2.90 |
13(16),14-Labdien-8-Ol |
290.48 |
C20H34O |
|
7 |
19.079 |
4.07 |
Decahydro-11-naphthol |
154.25 |
C10H18O |
|
8 |
19.200 |
4.53 |
4H-Inden-4-one,1,2,3,5,6,7-hexahydro-1,1,2,3,3-pent |
206.32 |
C14H22O |
|
9 |
19.591 |
2.91 |
Curcumenone |
234.33 |
C15H22O2 |
|
10 |
19.949 |
1.56 |
2h-1-Benzopyran-3(5h)-One,6,7,8,8a-Tetra |
152.19 |
C9H12O2 |
|
11 |
20.766 |
4.90 |
n-Hexadecanoicacid |
256.42 |
C16H32O |
|
12 |
21.023 |
6.22 |
Nootkaton-11,12-epoxide |
234.33 |
C15H22O2 |
|
13 |
21.303 |
3.64 |
Zederone |
246.30 |
C15H18O3 |
|
14 |
21.400 |
5.06 |
2-Methyl-4-(2,6,6-Trimethyl-1-Cyclohexe |
206.32 |
C14H22O |
|
15 |
22.108 |
1.83 |
ButanoicAcid |
88.11 |
C4H8O2 |
|
16 |
22.159 |
2.80 |
1-(2-Allyl-3,6-Dihydroxyphenyl) Ethanone |
192.21 |
C11H12O3 |
|
17 |
22.414 |
2.30 |
2,4,7,14-Tetramethyl-4-vinyl-tricyclo[5.4.3.0(1,8)]tetradecan-6-ol |
290.50 |
C20H34O |
|
18 |
22.512 |
2.96 |
(3R,3aR,5S,6R,7aR)-3,6,7,7-Tetramethyloctahydro-3a |
222.37 |
C15H26O |
|
19 |
25.661 |
1.26 |
Hexadecanoicacid |
256.42 |
C16H32O |
Note: Retention time (R. Time, min): Time taken for each compound to elute from the GC column.; Area%: Relative abundance of the compound (percentage of total peak area).; Identified primary compound names: Curcumenol, curcumenone, and n-Hexadecanoic acid.; Molecular weight (amu): Mass of the compound in atomic mass units.; Molecular formula: Chemical formula of the compound.
Phytochemicals analysis
The quantification of secondary metabolites in the methanolic extracts of CL and CA is shown in Figure 3A. The CL extract exhibited higher levels of total phenols (2.69 mg/g), alkaloids (17.22 mg/g), and flavonoids (17.61 mg/g) than the CA extract, which recorded 2.43 mg/g, 15.67 mg/g, and 9.94 mg/g, respectively.
Antioxidant activity
Figures 3B and 3C show that methanolic extracts of CL and CA exhibited dose-dependent antioxidant activity in both DPPH and FRAP assays. The CL extract demonstrated consistently higher DPPH radical scavenging activity, achieving up to 90% inhibition at 600 µg/mL, while CA reached only 70% inhibition at the same concentration. In the FRAP assay, CL showed superior ferric-reducing antioxidant power beyond 300 µg/mL compared to CA. However, Figure 3D illustrates that CA had a greater iron chelation capacity, yielding 90 µg EDTA equivalents at 600 µg/mL, compared to CL at 70 µg.
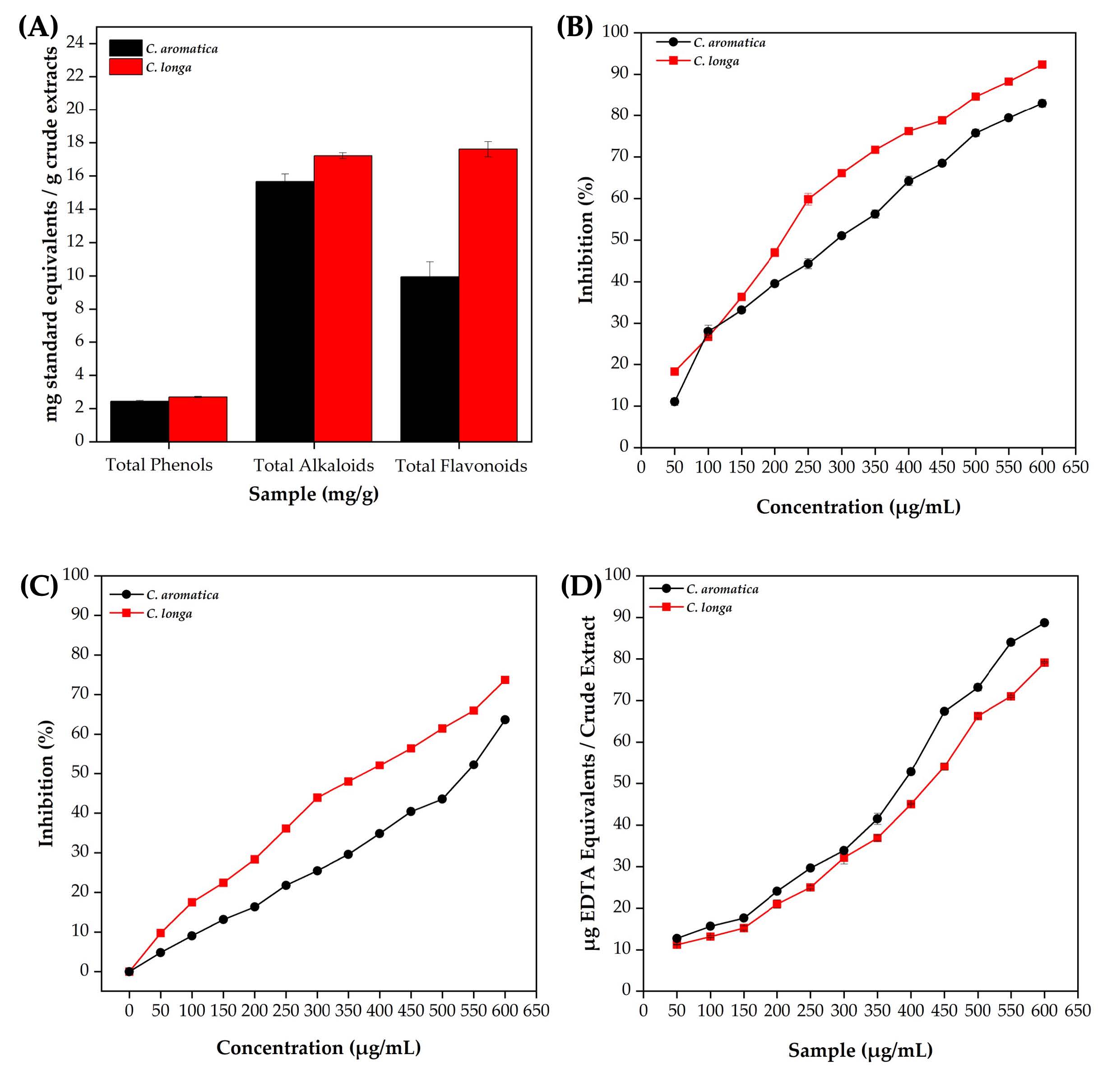
Figure 3. C. longa and C. aromatica rhizome methanol extracts: quantification of total phenols, alkaloids, and flavonoids (A), DPPH (B), FRAP (C), and metal-chelating activity (D).
Antimicrobial activity of CL and CA methanol extracts
Different concentrations (25, 50, and 100 μg) of methanolic rhizome extracts from CL and CA were evaluated against six human pathogenic microorganisms. As illustrated in Figure 4A, CL extract exhibited a dose-dependent increase in antimicrobial activity, while CA showed no zone of inhibition (ZOI) for any of the tested microorganisms. Despite CA containing 19 GC-MS-identified compounds, its extract showed no antimicrobial activity. Conversely, the CL extract, which contained 14 identified compounds, demonstrated significant antimicrobial effects. At 100 μg, CL exhibited the most pronounced antimicrobial activity. Notably, S. pneumoniae and K. pneumoniae showed the largest inhibition zones of 1.46 ± 0.03 cm (Figure 4B). Candida albicans followed with 1.26 ± 0.03 cm, S. aureus and S. dysenteriae with 1.16 ± 0.03 cm, S. flexneri with 1.13 ± 0.03 cm, and B. megaterium with 0.96 ± 0.03 cm.
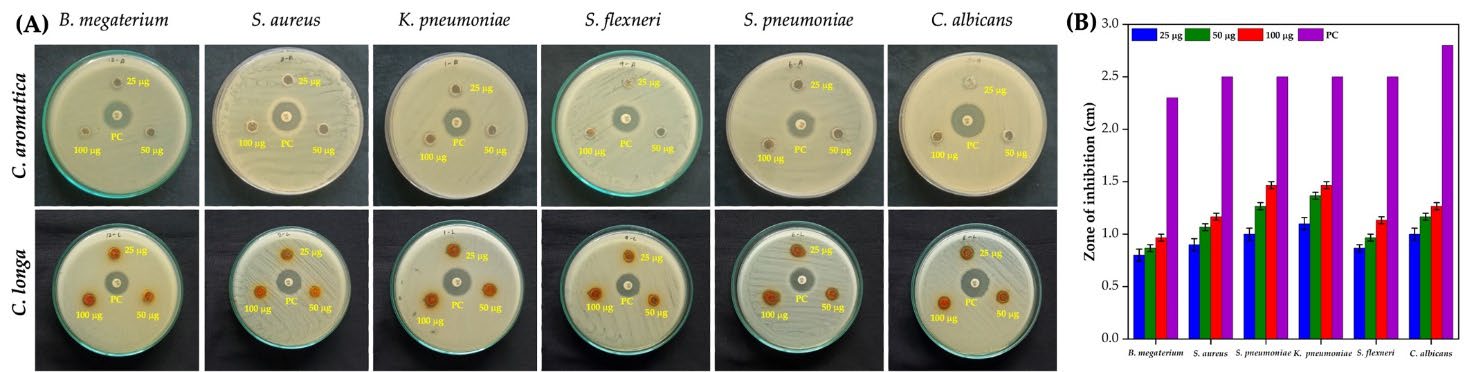
Figure 4. Antimicrobial activity of C. longa and C. aromatica rhizome methanol extracts against various microbial pathogens (A), ZOI at different concentrations of C. longa rhizome methanol extract (B).
Physicochemical properties of synthesized Ag NPs using CL methanol extract
UV-Vis spectroscopy revealed an absorption peak at 410 nm for the synthesized Ag NPs (Figure 5A). The FTIR spectrum (Figure 5B) of the CL methanol extract-synthesized Ag NPs displayed vibration bands at 3,342, 2,970, 2,887, 1,652, 1,375, 1,119, 949, and 520 cm⁻¹. New bands appeared at 1,463, 1,305, 815, and 620 cm⁻¹, confirming NPs formation. XRD analysis (Figure 5C) showed diffraction peaks at 37.09°, 44.59°, 64.67°, and 77.54°, corresponding to the (111), (200), (220), and (311) planes of face-centered cubic (FCC) of Ag, with an average crystallite size of 9.32 nm. Zeta potential analysis showed a stability of −38.6 mV (Figure 5D). SEM imaging (Figure 5E) revealed a predominantly spherical morphology. EDX spectra confirmed elemental silver (Ag) with minor carbon (C) and oxygen (O) peaks (Figure 5F). HR-TEM (Figure 5G) further confirmed spherical Ag NPs with SAED patterns showing high crystallinity. The d-spacing values for the (1 1 1), (2 0 0), (2 2 0), and (3 1 1) planes. The measured particle size ranged from 3.50 to 32.52 nm, with a mean size of 8.81 nm (Figure 5H).
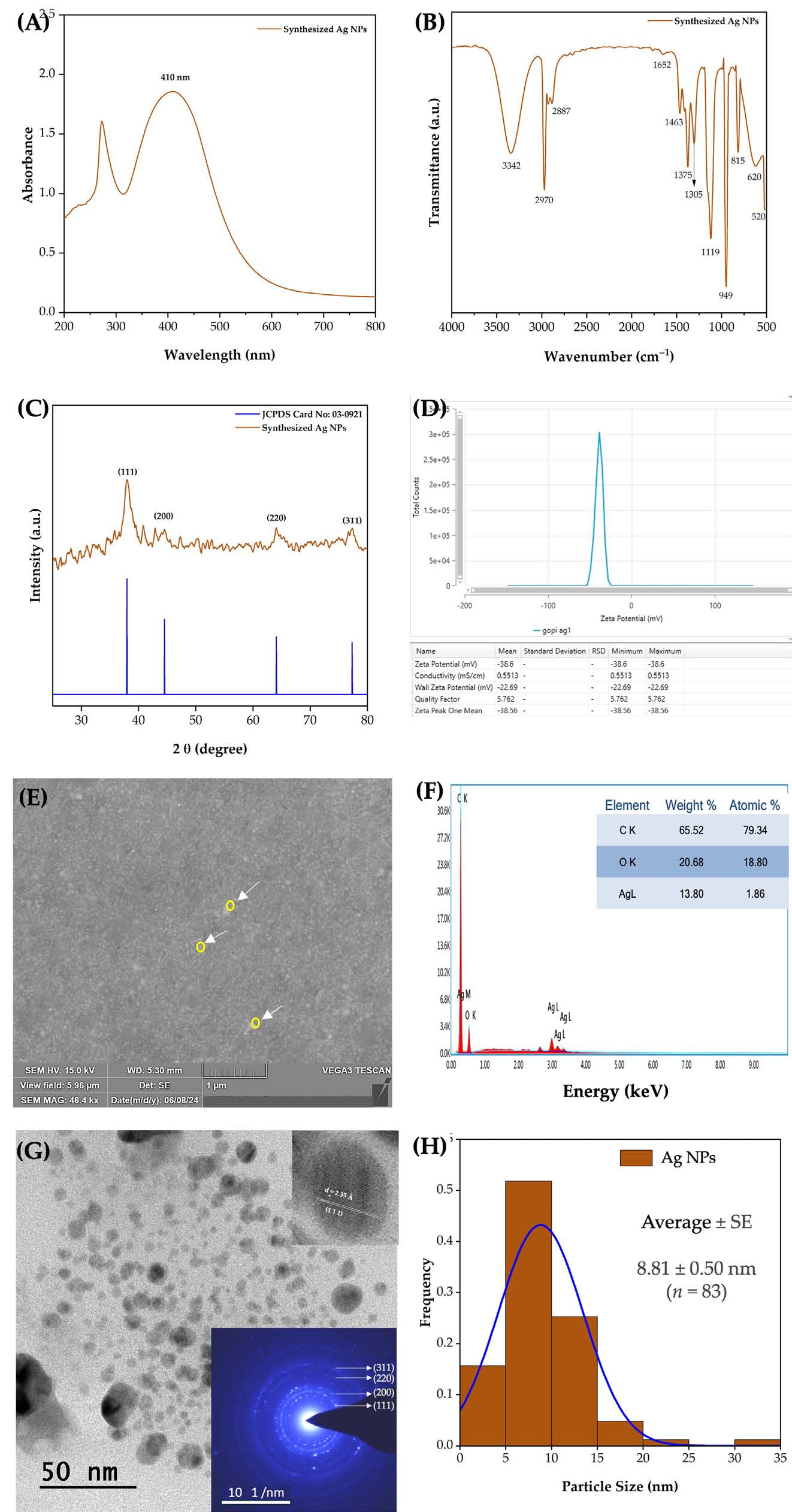
Figure 5. Methanol extract of C. longa rhizome synthesized Ag NPs: UV-Vis spectrum (A), FTIR, (C) XRD pattern (B), Zeta potential (D), SEM image (E), EDX spectrum (F), HR-TEM image with an inset showing SAED analysis (G), and particle size distribution (H).
Antimicrobial activity of Ag NPs using CL methanol extract
The antimicrobial activity of the synthesized Ag NPs was tested against six microorganisms, as shown in Figure 6A–B. The B. megaterium, S. aureus, and S. pneumoniae exhibited ZOI compared to Gram-negative bacteria, highlighting the higher effectiveness of Ag NPs against Gram-positive microorganisms. At the highest concentration (100 μg/mL), the ZOIs for B. megaterium, S. aureus, and S. pneumoniae were 1.2, 1.0, and 1.2 cm, respectively. In contrast, K. pneumoniae and S. flexneri displayed smaller ZOI, with K. pneumoniae increasing from 0.5 cm at 25 μg/mL to 1.2 cm at 100 μg/mL, while S. flexneri ranged from 0.2 cm to 0.7 cm. C. albicans demonstrated moderate susceptibility, with ZOI increasing from 0.4 cm at 25 μg/mL to 1.0 cm at 100 μg/mL. Overall, the results indicated a dose-dependent increase in ZOI values, with higher concentrations (75–100 μg/mL) showing significant inhibition against both Gram-positive and Gram-negative bacteria, as well as C. albicans. It has superior efficacy against Gram-positive strains.
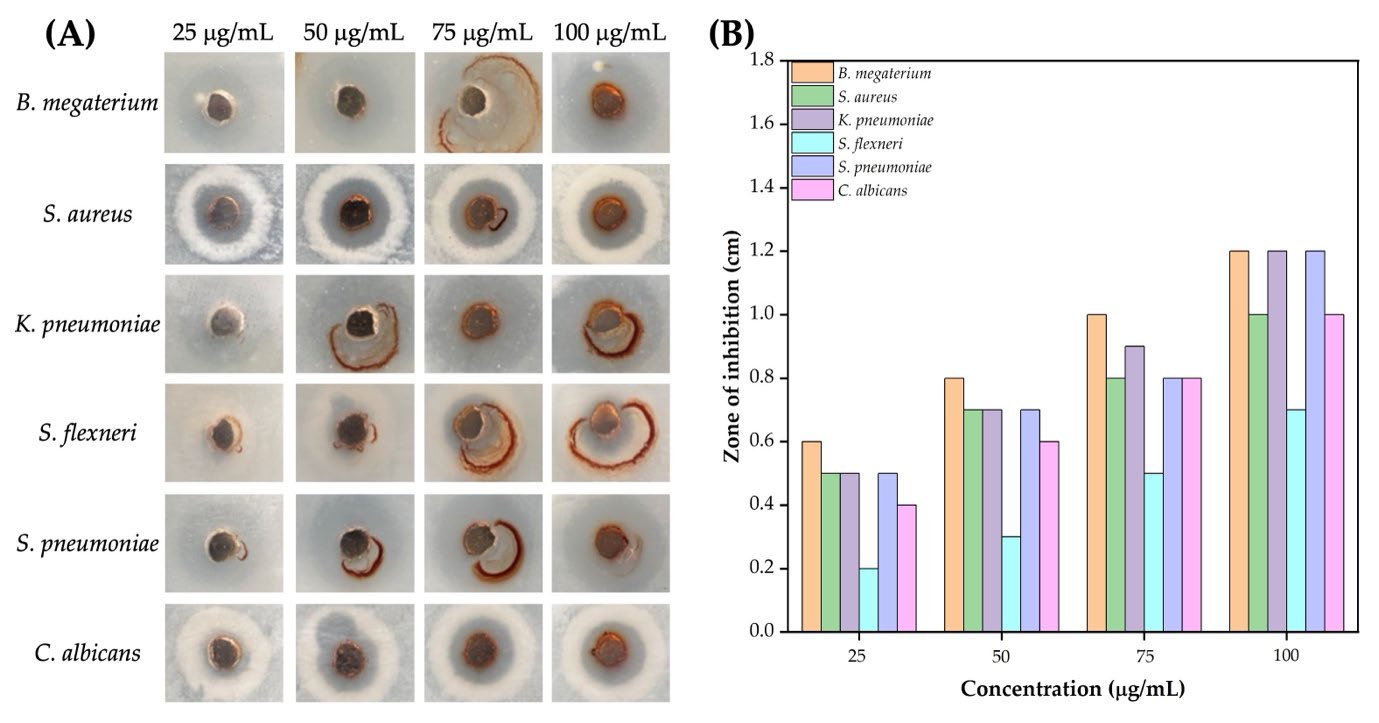
Figure 6. Methanol extract of C. longa rhizome synthesized Ag NPs: Representative images showing the antimicrobial activity against various pathogens at different concentrations (25–100 µg/mL) (A), and ZOI measurements for different microbial strains at varying Ag NP concentrations (B).
Biointerface assessment
In vivo toxicity of Ag NPs
The toxicity of synthesized Ag NPs was assessed in A. salina nauplii over 12- and 24-h exposure periods, using concentrations ranging from 2.5 to 10.0 mg/mL (Figure 7A). At 12 h, Ag NP treatment caused signs of fatigue and reduced swimming speed in nauplii. The mortality rate increased with Ag NP concentration, reaching 13.33%, 16.66%, 26.66%, and 33.33% at 2.5, 5.0, 7.5, and 10.0 mg/mL, respectively (Figure 7B). After 24 h, mortality rates further increased to 26.66%, 43.33%, 56.66%, and 76.66% at the same concentrations, demonstrating a dose- and time-dependent toxic effect. In the negative control group, nauplii exhibited no visible abnormalities, indicating normal growth and survival in seawater. The positive control group, treated with the CL methanol extract alone, showed mild physiological effects without significant structural damage or mortality, suggesting minimal toxicity. At lower concentrations (2.5 mg/mL), slight changes such as minimal visible damage and lethargy were observed. At higher concentrations (5.0 and 7.5 mg/mL), gut discoloration and structural disruption (highlighted by arrows) became evident, indicating gut damage and possible accumulation of Ag NPs. Severe abnormalities, including pronounced gut damage and reduced motility, were observed at 10 mg/mL, reflecting high toxicity.
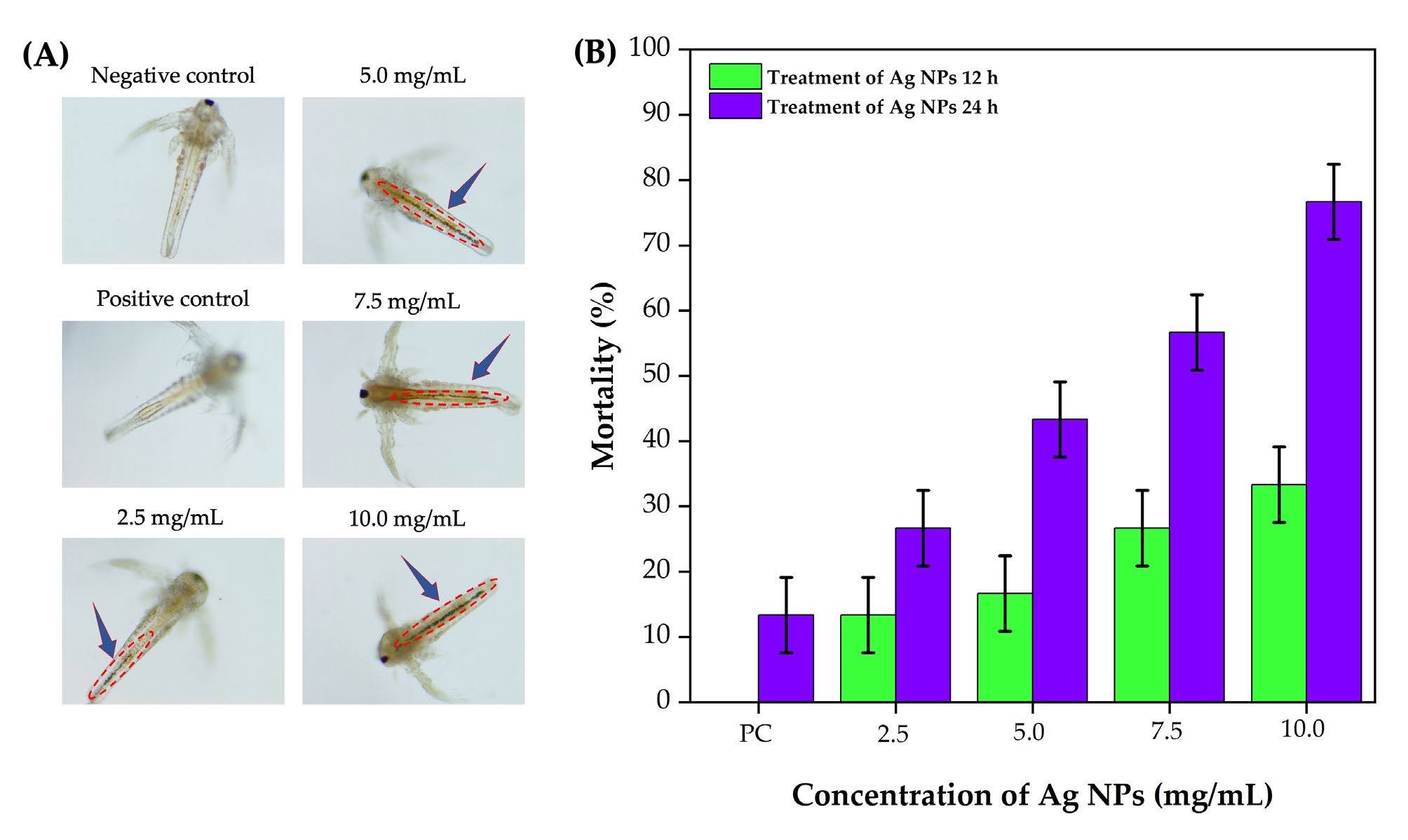
Figure 7. 40X micrographs: Effects of various concentrations of Ag NPs synthesized using C. longa rhizome methanol extract on A. salina nauplii (A). Mortality rate of
A. salina nauplii at 12 and 24 h of exposure to different concentrations of Ag NPs (B).
In vitro cell viability of Ag NPs
The biocompatibility of the synthesized Ag NPs was assessed using RAW 264.7 macrophage cells across a concentration range (0.1–50 µg/mL) (Figure 8A). At 0.1 and 0.5 µg/mL, the Ag NPs treatment enhanced cell viability to over 100%, indicating cell proliferation beyond the control group baseline (Figure 8B). At 1 µg/mL, viability remained slightly above 100%, suggesting continued stimulation of cell growth.
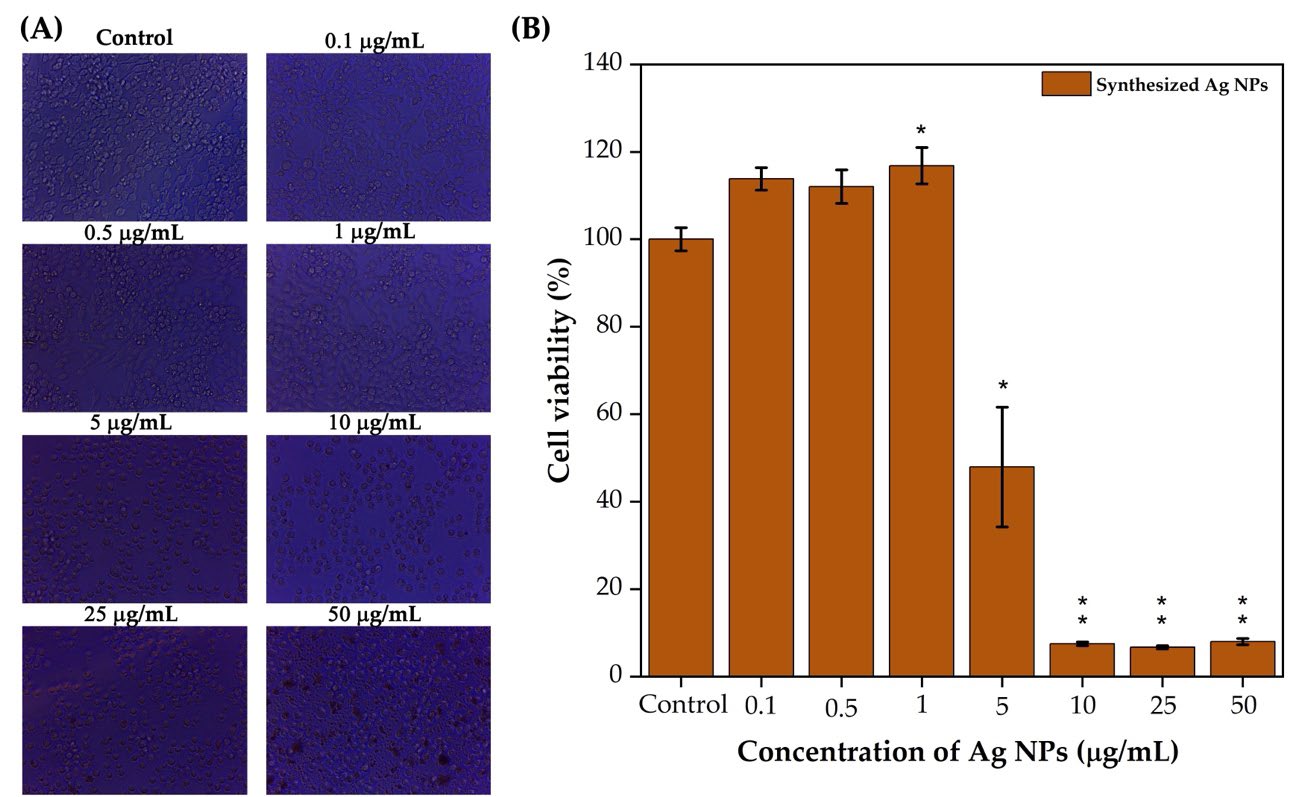
Figure 8. C. longa rhizome methanol extract synthesized Ag NPs tested against RAW 264.7 macrophage cells photographic images of 24 h incubation (micrographs showing 40X magnification) (A). Cell viability against Ag NPs at different concentrations (B). Statistical analysis was performed using an independent samples t-test. Compared with the control, *P < .05, **P < .01, as indicated.
However, a sharp decline in cell viability was observed at 5 µg/mL, where viability dropped to approximately 50%. At higher concentrations (10, 25, and 50 µg/mL), cell viability decreased significantly to below 20%, indicating strong cytotoxic effects and near-complete cell death. These findings confirmed a dose-dependent response, with low concentrations promoting proliferation and high concentrations causing cytotoxicity.
DISCUSSION
UV-Vis and FTIR analysis of the methanolic extract of CL and CA
The UV absorption peak in the 200–350 nm range corresponds to n–π* electronic transitions, which are indicative of aromatic compounds and chromophores, such as hydroxyl and carbonyl groups (Jannah et al., 2018). Furthermore, the absorbance intensity in the 350–500 nm range is attributed to π–π* electronic transitions, primarily associated with curcuminoids, which are the major secondary metabolites (Kim et al., 2013). The higher absorbance intensity associated with π–π electronic transitions in the CL extract suggests a greater concentration of curcuminoids compared to the CA extract. These results confirm the high curcuminoid content in CL, as evidenced by the strong methanol extract absorbance.
FTIR was used to identify functional groups in different organic and inorganic compounds (Othman, 2022). The spectral profile of CL confirmed the presence of curcuminoid structures, with characteristic peaks aligning well with those of earlier studies (Rohman, 2015; Pakkirisamy et al., 2017; Wulandari, 2018). Dewi et al. (2020) reported that the FTIR analysis of CL ethanol extract showed peaks at 3,296, 1,639, 1,512, and 1,282 cm⁻¹, which are attributed to the curcuminoid structure. These peaks were also present in the methanol extract of CL, confirming the presence of curcuminoid derivatives. Other peaks were also observed, verifying the presence of flavonoids, terpenoids, amino acids, phenolic acids, and sterols (Segneanu et al., 2022).
The peaks observed in the CA extract were consistent with the presence of alcohols, phenols, aromatic rings, and nitrogen-containing compounds, indicating a diverse phytochemical composition (Kundu and Nithiyanantham, 2013). Aung and Thu (2024) reported peaks at 3,450, 1,660, 1,460, 1,012, and 723 cm⁻¹ in the FTIR spectrum of the ethanol extract of CA rhizome, which is associated with alcohol and phenol groups, –C=C– stretching, aromatic ring vibrations, C–N bending, and C–H bending. This indicates the presence of flavonoid phenolic compounds, alkaloids, and terpenoids in the extract. These functional groups are well-supported by the methanol extract of CA. The differences observed in the transmittance peaks and specific functional groups in the methanolic extracts of CL and CA indicate distinct phytochemical profiles that significantly influence their biological activities. FTIR analysis showed that the peak intensity of CL was higher than that of CA.
GC-MS analysis of the methanolic extract of CL and CA
The GC-MS results highlighted that both CL and CA rhizomes contain a diverse array of bioactive phytochemicals, particularly essential oils rich in terpenoids. The predominance of gamma-sitosterol in CL and curcumenol in CA suggests potential therapeutic and antimicrobial relevance, as these compounds are known for their biological activities. The essential oil composition of Curcuma species primarily includes monoterpene and sesquiterpene hydrocarbons, which are known for their antibacterial properties (Uribe et al., 1985). Previous reports have identified similar compounds, such as trans-nerolidol, bisabolol, and curcuphenol, in fresh and dried CL rhizomes (Singh et al., 2010), reinforcing the consistency of chemical profiles across different preparations. Additionally, the presence of curcuminoids, such as curcumin, demethoxycurcumin, and bisdemethoxycurcumin, supports the known pharmacological benefits of Curcuma species, including antimicrobial and anticancer properties (Anand et al., 2008).
Phytochemical analysis of the methanolic extract of CL and CA
The elevated phenol, alkaloid, and flavonoid content in the CL extract likely contributes to its superior antioxidant and antibacterial properties. Phenolic compounds and flavonoids are well-known for their free radical-scavenging and antimicrobial effects. This finding is consistent with the work of Burapan et al. (2020), who reported that CL had the highest total phenolic content among several Curcuma species, including C. parviflora and C. latifolia. Additionally, members of the Zingiberaceae family are rich in aromatic compounds, such as terpenes, alcohols, ketones, and phytoestrogens (Tayyem et al., 2006). In particular, CL rhizomes are known to contain more than 235 bioactive compounds, largely consisting of polyphenols and terpenoids (Li et al., 2011; Yuandani et al., 2021), which further supports their strong biofunctional potential.
Antioxidant activity of the methanolic extract of CL and CA
As shown in Figure 3, these results confirm that both CL and CA extracts possess antioxidant properties, but they act through different mechanisms. The higher DPPH and FRAP values of CL suggest stronger radical scavenging and reducing power, consistent with its higher phenolic and flavonoid content. In contrast, CA demonstrated a better metal-chelating ability, indicating a stronger affinity for binding iron ions. The findings surpass several earlier reports: Kirmani et al. (2024) reported an IC50 of 0.7 mg/mL for CL; Nguepi et al. (2024) found SC50 values of 138.75 ± 5.73 µg/mL (DPPH) and 102.23 ± 3.49 µg/mL (FRAP); and Swain and Rautray (2021) observed 86.76% DPPH activity at 500 µg/mL. Additionally, Cousins et al. (2007) noted that drying in vitro-grown CL rhizomes reduced DPPH scavenging but enhanced iron chelation in certain clones. These comparisons confirm the superior antioxidant efficacy of our CL extract and the unique chelation strength of CA, supporting their application in therapeutic or preservative formulations related to oxidative stress.
Further supporting our findings, the antioxidant activity of Astragalus flavesces aqueous leaf extract exhibited DPPH and FRAP activities at 14.41 µg/mL and 3.53 µmol Trolox Equivalents (TE)/mg, respectively (Sahin Yaglioglu et al., 2022). Similarly, the aqueous extract of Echinacea purpurea aerial parts exhibited DPPH and FRAP activities at 21.9 µg/mL and 1.71 mmol TE/mg, respectively (Gecer et al., 2022). These additional comparisons highlight the significant antioxidant potential of our CL extract and the diverse antioxidant profiles of other plant extracts, further supporting their application in therapeutic strategies aimed at combating oxidative stress.
Evaluation of antimicrobial activity of CL and CA methanol extracts
The superior antimicrobial activity of the CL extract, despite having fewer compounds than CA, may be attributed to the high concentration of bioactive molecules, such as flavonoids and phenolics. Kim et al. (2005) reported a phytochemical analysis of CL and ethyl acetate extracts, which contained higher levels of phenolics and flavonoids, along with a medium content of glycosides and steroids. In contrast, the methanol extract exhibited a higher glycoside and steroid content and a moderate phenolic and flavonoid content. Clinical isolates of MRSA, including 13 MRSA strains and one standard methicillin-sensitive S. aureus (MSSA) strain, were exposed to various concentrations of CL (25, 50, and 100 μg/mL). The ethyl acetate extract exhibited the highest antibacterial efficacy, followed by methanol and water extracts. The antibacterial activity of CL is attributed to its high flavonoid content (Gupta et al., 2015). Niamsa and Sittiwet (2009) reported that the aqueous extract of CL rhizome revealed higher ZOI values at a concentration of 500 g/L against E. coli ATCC 25922 (1.82 cm), S. epidermidis ATCC 12228 (1.74 cm), S. aureus ATCC 25923 (1.55 cm), and K. pneumoniae ATCC 10031 (1.39 cm). Curcumin disrupts bacterial membranes, increases permeability, and inhibits DNA and protein synthesis as well as cell signaling, causing electrolyte leakage and cell death (Zheng et al., 2020). Kasta (2020) reported antimicrobial inhibitory zones of 1.58 cm, 1.56 cm, and 1.52 cm for the ethanolic extract of CL rhizome against E. coli, S. aureus, and C. albicans, respectively, at a concentration of 500 mg/mL. Similarly, Hosea et al. (2018) reported the antibacterial activity of the ethanol extract of CL rhizome at 200 mg/mL, with inhibitory zones of 2.53 cm against E. coli and 2.60 cm against S. aureus. In contrast, the water extract exhibited inhibitory zones of 2.13 cm for both bacterial species. The inhibitory zones reported by Kasta (2020) and Hosea et al. (2018) for ethanol and water extracts highlight the significant influence of the solvent type on antimicrobial efficacy. In comparison to previous studies, the current study showed that the methanol extract of CL exhibited superior activity against S. pneumoniae and K. pneumoniae at a concentration of 100 µg, surpassing the ZOI values reported by Niamsa and Sittiwet (2009) for aqueous CL extracts. This suggests that methanolic CL extracts are efficient and cost-effective antimicrobial agents compared to the more concentrated extracts used in earlier studies.
Evaluation of physicochemical properties of Ag NPs
The observed UV-Vis peak at 410 nm is consistent with the surface plasmon resonance of Ag NPs and is slightly lower than that in previous reports using aqueous CL extracts (Sharma et al., 2020). Sharma et al. (2020) synthesized Ag NPs using the aqueous extract of CL rhizomes from Shimla, Mandi, and Bilaspur, resulting in absorption peaks at 421, 419, and 422 nm, respectively. These differences in absorption peaks are related to the phytochemical composition, geographical factors, and environmental conditions, which influence the size, shape, and aggregation of the NPs. Methanol is a polar organic solvent that effectively dissolves a wide range of bioactive compounds, including polar and moderately nonpolar phytochemicals. The methanol extract of CA contains secondary metabolites of flavonoids, phenolics, and terpenoids, which are more effective than the aqueous extracts. These metabolites are crucial for reducing Ag⁺ to Ag⁰ and stabilizing NPs. Consequently, the methanol extracts promoted the formation of smaller, well-dispersed NPs with enhanced surface plasmon resonance effects, resulting in sharper absorption peaks. The FTIR spectrum of Ag NPs synthesized using the CL methanol extract exhibited new bands at 1,463, 1,375, 1,305, 815, and 620 cm⁻¹, in contrast to the CL methanol extract. These bands are likely attributed to CH2 bending modes, C-H bending vibrations, C-N stretching, C-H out-of-plane bending, and N-H groups in proteins. This confirms the production of Ag NPs by reducing Ag²⁺ to Ag⁰. FTIR confirmed the involvement of functional groups such as amines, phenolics, and proteins in both reduction and stabilization processes, consistent with earlier studies (Segneanu et al., 2022). The emergence of new bands in Ag NPs compared to the CL extract indicates surface modifications post-synthesis. Synthesized Ag NPs showed an FCC structure well matched with the JCPDS Card No. 03-0921. Shameli et al. (2012) reported the successful biosynthesis of Ag NPs using CL rhizome powder water extract, where a 24 h stirring time resulted in an average crystallite size of <10 nm with an FCC structure. The −38.6 mV zeta potential indicates a strong colloidal stability. Segneanu et al. (2022) reported that a 1:1 methanol and chloroform extract of CL and its residues were dissolved in methanol and used as a reducing agent to synthesize Ag NPs. It showed a zeta potential of ─22.34 mV. The obtained results demonstrated improved performance. SEM analysis validated the uniform spherical morphology. However, Chintala et al. (2023) reported the synthesis of Ag NPs using CL water extract with a 100 PPM AgNO₃ solution, producing NPs with round, irregular, and amorphous shapes, ranging in size from 65 to 75 nm. EDX analysis confirmed successful reduction of Ag⁺ to Ag⁰. Venkatadri et al. (2020) found that the Ag NPs synthesized from a mixed water extract of Zingiber officinale and CL exhibited a spherical shape, with sizes varying between 20 and 51 nm. In addition, Genc et al. (2020) reported that the synthesis of Ag NPs using Olea europaea leaf extract-derived pure oleuropein was confirmed by SEM analysis, which revealed spherical-shaped particles with an average size of 71 nm. The current study confirmed the successful synthesis of Ag NPs using a CL methanolic extract. Finally, this method provides a one-step, efficient synthesis process that requires only 60 min of stirring at RT. This demonstrates improved stability, reduced energy consumption, and facilitates its application in large-scale commercial production.
Evaluation of antimicrobial activity of Ag NPs
The antibacterial results show that the CL extract with gamma-sitosterol adjoined Ag NPs boosts antimicrobial activity through a synergistic effect. Nurkhaliza et al. (2024) demonstrated that Ag NPs synthesized using hot turmeric powder extract exhibited antibacterial activity against Bacillus subtilis (0.95 cm), S. aureus (1.00 cm), and P. aeruginosa (0.95 cm) at 50 μL concentration. However, no activity was observed against E. coli, likely due to its resistance to Ag NPs and the limited number of bioactive compounds present in the hot turmeric powder extract. Sharma et al. (2020) reported that CL rhizome water extracts from Shimla, Mandi, and Bilaspur synthesized Ag NPs with ZOI values of 1.43 cm, 1.36 cm, and 1.36 cm against S. aureus and 1.06 cm, 1.03 cm, and 1.00 cm against E. coli at 150 µg/mL. Furthermore, Karan et al. (2024a) synthesized Ag NPs using Sambucus nigra leaf extract and reported enhanced antibacterial activity against Salmonella enteritidis, E. coli, S. aureus, and Listeria monocytogenes. The Ag NPs, with a particle size of 27 nm and a zeta potential value of ─51.3 mV, exhibited superior activity, especially against L. monocytogenes (MIC: 16 μg/mL), compared to the leaf extract alone. Additionally, the same research group investigated the synthesis of Ag NPs using Sambucus ebulus leaf extract, showing a notable improvement in inhibition zones (1.2 cm) against L. monocytogenes and Salmonella enteritidis, with slight enhancement for E. coli (0.6 cm) and S. aureus (0.1 cm) compared to the plant extract (Karan et al., 2024b).
The current study demonstrated that Ag NPs synthesized using CL methanol extract exhibited superior antimicrobial activity, with ZOIs of up to 1.2 cm against B. megaterium, S. aureus, and S. pneumoniae at 100 μg/mL, surpassing the ZOIs reported by Sharma et al. (2020) and Nurkhaliza et al. (2024) for Ag NPs synthesized with CL extracts. These findings highlight the enhanced efficacy of CL-synthesized Ag NPs, which can be attributed to the synergistic effects of gamma-sitosterol and other bioactive compounds in the methanol extract. Microbial activity is influenced by factors such as the structure of the bacterial cell wall, size, morphology, dosage of nanoparticle treatment, and synergistic effects (Amini and Shahroodian, 2024). Ag NPs exert their antibacterial action through multiple mechanisms. Initially, Ag NPs bind to bacterial cell membranes through electrostatic interactions between the negatively charged bacterial surface and positively charged Ag⁺ ions released from the NPs (Balalakshmi et al., 2020; Abdelwahab et al., 2024). The interaction can be represented by equation (3-6) (Mammari et al., 2022).
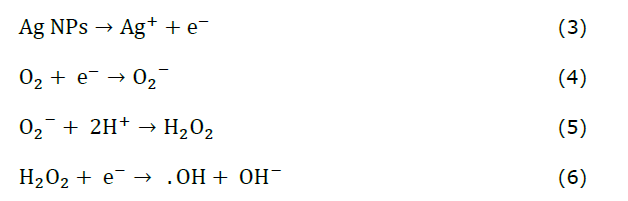
Oxygen molecules (O2) in the environment gain an electron (e⁻), forming superoxide anions (O2⁻). These anions react with protons (H⁺) to produce hydrogen peroxide (H2O2), which penetrates bacterial cells, and generates more reactive species. Additionally, H2O2 reacts with an electron (e⁻) to form hydroxyl radicals (·OH) and hydroxide ions (OH⁻) (Mammari et al., 2022). These highly reactive radicals damage essential cellular components like lipids, proteins, and DNA (Gopinath et al., 2016). Meanwhile, Ag⁺ ions bind to thiol (-SH) groups in enzymes and proteins, inactivating them and blocking essential metabolic pathways. This disruption prevents the bacteria from maintaining cellular functions, ultimately causing cell death (Figure 9) (Gopinath et al., 2016; Balalakshmi et al., 2020).
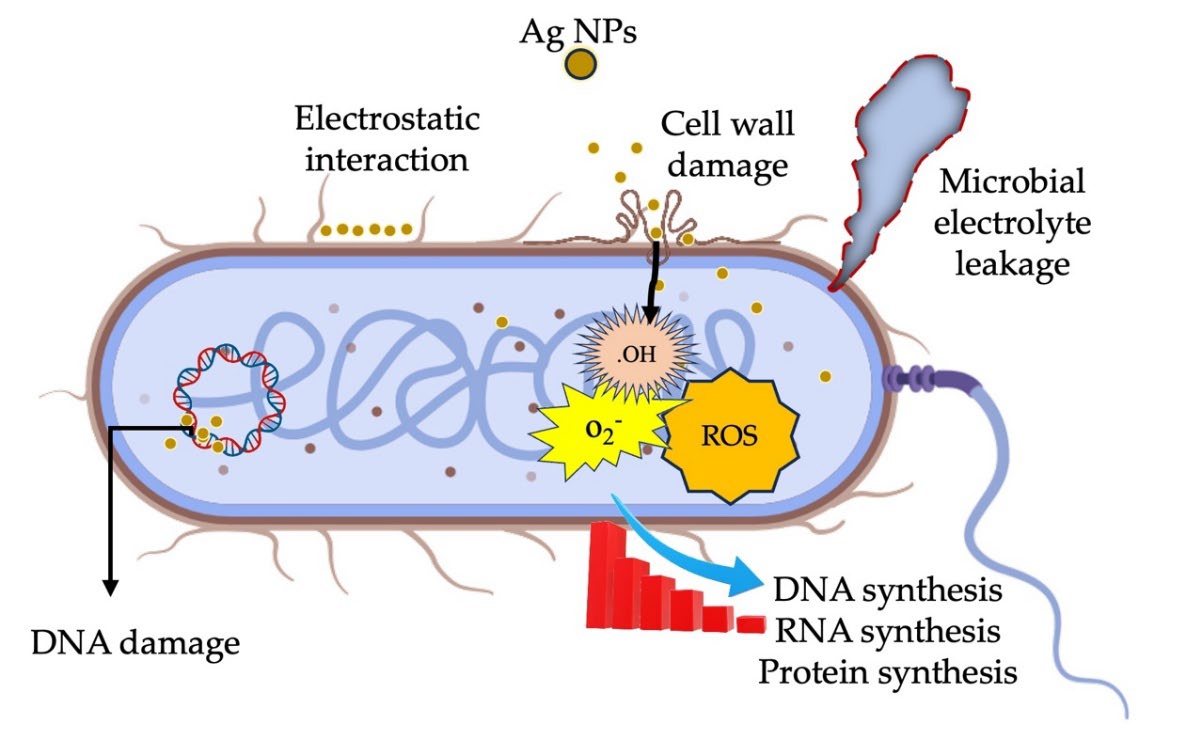
Figure 9. Ag NPs synthesized using C. longa rhizome methanol extract: A schematic depiction of the microbial inhibition mechanism.
Biointerface effect of Ag NPs
Impact of the in vivo safety profile of Ag NPs
The observed mortality and structural damage can be attributed to sustained exposure to ROS and released Ag⁺ ions, which overwhelm the antioxidant defenses of A. salina nauplii. Positively charged NPs bind to negatively charged gut membranes, disrupt respiration, deactivate enzymes, generate peroxides, and cause cell death, with toxicity increasing with Ag NP concentration (Figure 10). Recently, Balamurugan et al. (2024) reported 69% mortality in A. salina at 15 mg/mL Ag NPs after 24 h, with dose-dependent and synergistic effects disrupting gut function and digestion, leading to mortality. The fact that the CL methanol extract alone did not produce significant toxicity reinforces that the observed effects are directly attributable to Ag NPs rather than the plant extract. These results underscore the need to evaluate NPs formulations carefully for biosafety, particularly when considering biomedical or environmental applications.
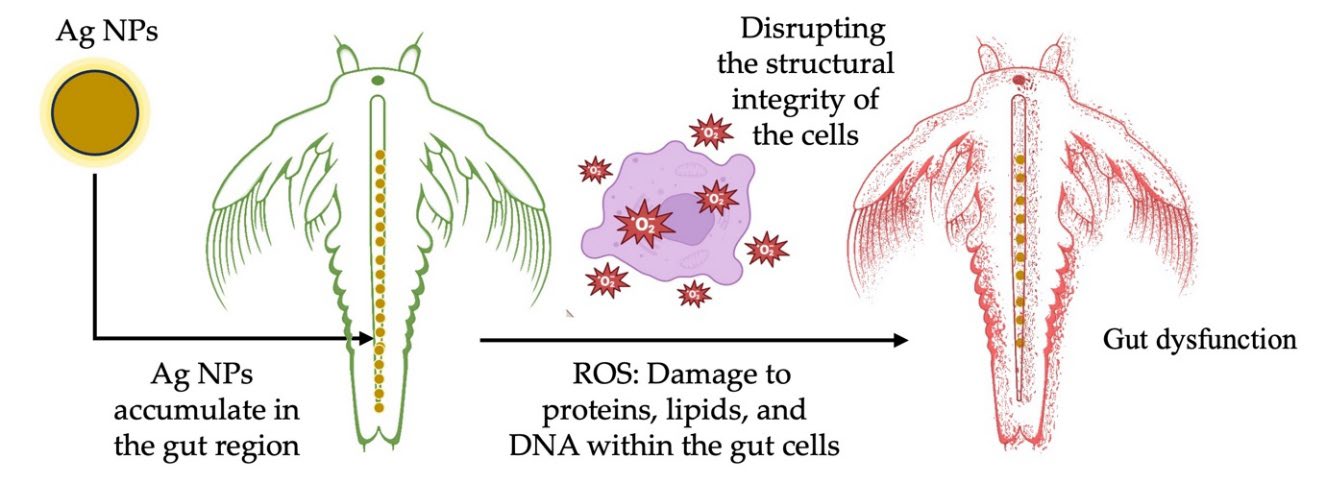
Figure 10. Ag NPs synthesized using C. longa rhizome methanol extract: Toxicity mechanism in A. salina nauplii.
Impact of in vitro cell viability of Ag NPs
Synthesized NPs have frequently been studied using human skin epithelial cells and RAW 264.7 macrophages (Kasi et al., 2024). Assessing the effects of NPs on complex biological systems is vital and encompasses the immune response, inflammatory reactions, cytotoxicity, and other pertinent factors (Gopinath et al., 2023; Rachtanapun et al., 2023; Kasi et al., 2024). Ensuring the safety of NPs is crucial for their wide-ranging applications in biomedical and nanotechnology research (Rachtanapun et al., 2023; Devi et al., 2024). The in vitro results demonstrated that Ag NPs synthesized using CL methanol extract exhibited a biphasic biological response. The increased viability at low concentrations (1 µg/mL) suggests a hormetic effect or macrophage activation, which has been previously reported in nanoparticle-cell interaction studies (Jiao et al., 2014; Trela-Makowej et al., 2024). However, concentrations exceeding 1 µg/mL cause cytotoxic effects. This highlights the importance of optimizing dosage to ensure the safe and effective biomedical application of Ag NPs. The dissolution of Ag NPs and the release of Ag⁺ ions initiate a cascade of chemical reactions, resulting in ROS generation. These ROS, together with Ag⁺, disrupt cellular integrity and function, leading to the observed cytotoxic effects. The interplay of these chemical processes explains the dose-dependent biological response of RAW 264.7 macrophages to Ag NPs. In another study, Mohammadi et al. (2021) reported the synthesis of Ag NPs (10 nm) using ginger rhizome water extract. At concentrations ranging from 0.16 to 1.25 ppm, murine macrophage-derived RAW 264.7 cell viability decreased from 80% to 65% as the dose of Ag NPs was increased. Ongtanasup et al. (2024) found that Ag NPs synthesized from Z. officinale water extract showed over 80% biocompatibility at 100 μg/mL concentration in the L929 cell line. In contrast, Ag NPs synthesized using a 50:50 mixture of ethyl acetate and ethanol exhibited slightly higher cytotoxicity. The biocompatibility of Ag NPs depended on pH, with Ag NPs at pH 5 safe at 68.38 μg/mL and pH 7 and 6 safe at 39.32 μg/mL and 17.52 μg/mL. At 50 μg/mL, Ag NPs caused no significant cell changes, indicating a safety level of biocompatibility. Generally, cell viability above 90% indicates non-cytotoxicity, 60–90% signifies slight cytotoxicity, 30–59% represents moderate cytotoxicity, and below 30% indicates severe cytotoxicity. The synthesized Ag NPs at 1 μg/mL are biocompatible, with cytotoxicity influenced by quantum confinement, size, shape, charge, and stability (Pareek et al., 2018).
Based on the comparative analysis presented in Table 3. The current study demonstrates superior performance by integrating comprehensive assessments of antimicrobial efficacy, in vivo toxicity, and in vitro biocompatibility. Unlike previous reports that focused primarily on antibacterial or anticancer activity, our study uniquely combined a broad-spectrum microbial panel (including both Gram-positive and Gram-negative bacteria and fungi), A. salina toxicity, and cytocompatibility in RAW 264.7 macrophages. The synthesized Ag NPs exhibited effective inhibition zones and maintained at 117% cell viability, highlighting their potential as safe and multifunctional antimicrobial NPs.
Table 3. Comparative analysis of antimicrobial activity and toxicity of C. longa indicated with Ag NPs.
|
Raw materials |
Properties |
Microbial and biological test targets |
Method of antimicrobial and toxicity |
Performance |
Reference |
|
C. longa rhizome powder |
Reaction time: 24 h
TEM: 5–35 nm
Shape: Decahedral, ellipsoidal, oblong spheres, and triangular. |
E. coli O157:H7 and Listeria monocytogenes |
Colony Forming Unit (CFU) |
4 log CFU/mL reduction at 24 h |
(Alsammarraie et al., 2018) |
|
C. longa rhizome powder |
Reaction time: Vigorous stirring at 60°C for 15 min
Shape: spherical |
E. coli ATCC 25922 S. aureus ATCC 29213 and S. pneumoniae |
Disk diffusion |
15 µL showed ZOI at 1.2, 1.1 and 0.9 cm |
(Al-Saif et al., 2018) |
|
C. longa tuber powder |
Reaction time: 37°C for 24 h
TEM: 32 nm
Shape: Spherical |
S. aureus and
|
Antibiofilm |
0.7 µg/mL showed a 90% decrease biofilm formation |
(Kamble and Shinde, 2018) |
|
C. longa rhizome powder |
– |
P. aeruginosa and S. aureus |
Antibiofilm |
The IC50 values for 0.19 and 0.16 mg/mL |
(Suwal et al., 2025) |
|
C. longa and Zingiber officinale rhizome (1:1) |
TEM: 20–51 nm
Shape: Spherical |
HT-29 cells |
Anti-cancer activity |
IC50 at 150.8 µg/mL |
(Venkatadri |
|
C. longa rhizomes |
Reaction time: Incubation at 30 min
|
a) B. subtilis S. aureus S. faecalis K. pneumoniae P. aeruginosa E. coli and C. albicans b) MCF 7 cells |
a) Well diffusion
b) In Vitro cytotoxicity |
a) 300 µL of Ag NPs showed ZOI at 1.5, 1.9, 2.9, 2.0, 2.4, 1.9 and 1.8 cm
b) IC50 value at 1.84 µg/mL |
(Ganesan |
|
C. longa rhizome |
Reaction time: Stirring at 37°C for 30 min and heating at 80°C for 30 min
TEM: 3.50–32.52 nm
Shape: Spherical |
a) B. megaterium S. aureus K. pneumoniae S. flexneri S. pneumoniae and C. albicans b) A. salina nauplii
c) RAW 24.7 macrophages |
a) Well diffusion
b) In Vivo toxicity
c) In Vitro cytotoxicity |
a) 100 µg/mL of Ag NPs showed ZOI at 1.2, 1.0, 1.2, 0.7, 1.2 and 1.0 cm
b) Mortality rate of 76.33% at 10 mg/mL c) 1 μg/mL showed cytocompatibility |
Our work |
Note: Antimicrobial activity: Disk/well diffusion assays.; Bacterial reduction (log CFU/mL): The reduction in the number of colony-forming units per milliliter, expressed on a logarithmic scale.; Cytotoxicity (IC₅₀, µg/mL): The concentration of a compound that reduces cell viability by 50%, expressed in micrograms per milliliter (µg/mL).;Transmission Electron Microscopy (TEM): A technique used to visualize the morphology and size of nanoparticles at high resolution.; Toxicity: Cell viability assays; Zones of inhibition (ZOI): Measurement of the area around an antimicrobial agent where bacterial growth is inhibited, expressed in centimeter (cm).
CONCLUSIONS
This study successfully compared the bioactive profiles and biological activities of CL and CA rhizomes, focusing on their phytochemical composition, antioxidant, and antimicrobial potential. The methanolic extract of CL demonstrated superior antioxidant activity and antimicrobial properties, attributed to its high content of gamma-sitosterol, palmitic acid, and turmerone. Compared to CL, CA exhibited a stronger metal-chelating capacity, highlighting the distinct biological activities of the two species. The Ag NPs synthesized by the CL extract exhibited an average size of 8.81 nm and demonstrated strong antimicrobial activity, particularly against Gram-positive bacteria, with ZOI values up to 1.46 cm. The NPs exhibited high stability, with a zeta potential of −38.6 mV, supporting their potential in nanomedicine. In vivo toxicity assessments revealed dose-dependent mortality in A. salina nauplii, with a mortality rate of 76.33% at 10 mg/mL, suggesting a synergistic effect. Biocompatibility tests on RAW 264.7 macrophage cells showed enhanced viability at low concentrations (1 µg/mL) owing to the quantum size of the Ag NPs, whereas cytotoxicity was observed at higher doses. These results suggest that CL-derived Ag NPs hold promise as nanomedicines for pharmaceutical and biological applications.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the Faculty of Agro-Industry, Chiang Mai University, for providing instrumental support. Special thanks are also extended to the Botany Departments of Alagappa University and Periyar University, as well as the Department of Life Science at Gachon University, for their valuable facilities and assistance in conducting the biological activity studies.
AUTHOR CONTRIBUTIONS
Gopinath Kasi: Conceptualization, Data curation, Software, Validation, Writing - original draft; Balamurugan Venkatachalam: Data curation, Formal Analysis, Validation, Visualization, Writing - review & editing; Sarinthip Thanakkasaranee: Data curation, Formal Analysis, Software, Supervision, Validation, Visualization, Writing - review & editing. Nattan Stalin: Data curation, Formal Analysis, Validation, Writing - review & editing. Ramar Dharmaraj: Data curation, Formal Analysis, Validation, Writing - review & editing. Kittisak Jantanasakulwong: Formal Analysis, Writing - review & editing. Pornchai Rachtanapun: Conceptualization, Formal analysis, Funding acquisition, Investigation, Supervision, Validation, Visualization, Writing - review & editing.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Abbas, R., Luo, J., Qi, X., Naz, A., Khan, I.A., Liu, H., Yu, S., and Wei, J. 2024. Silver nanoparticles: Synthesis, structure, properties and applications. Nanomaterials. 14(17): 1425.
Abdelwahab, M.A., Nabil, A., El-Hosainy, H., Tahway, R., and Taha, M.S. 2024. Green synthesis of silver nanoparticles using curcumin: A comparative study of antimicrobial and antibiofilm effects on Acinetobacter baumannii against chemical conventional methods. Results in Chemistry. 7: 101274.
Adiguzel, A.O., Adiguzel, S.K., Mazmanci, B., Tunçer, M., and Mazmanci, M.A. 2018. Silver nanoparticle biosynthesis from newly isolated Streptomyces genus from soil. Materials Research Express. 5(4): 045402.
Al-Saif, S.S. A.-L., Awad, M.A., and Siddiqui, M.I. 2018. Formation, characterization and pathogen activities of green synthesis of Curcuma silver nanoparticles. Journal of Computational and Theoretical Nanoscience. 15(4): 1300-1306.
Albaqami, J.J., Hamdi, H., Narayanankutty, A., Visakh, N.U., Sasidharan, A., Kuttithodi, A.M., Famurewa, A.C., and Pathrose, B. 2022. Chemical composition and biological activities of the leaf essential oils of Curcuma longa, Curcuma aromatica and Curcuma angustifolia. Antibiotics. 11(11): 1547.
Alsammarraie, F.K., Wang, W., Zhou, P., Mustapha, A., and Lin, M. 2018. Green synthesis of silver nanoparticles using turmeric extracts and investigation of their antibacterial activities. Colloids and Surfaces B: Biointerfaces. 171: 398-405.
Amini, S.M. and Shahroodian, S. 2024. Antibacterial activity of silver and gold nanoparticles that have been synthesized by curcumin. Inorganic and Nano-Metal Chemistry. 55(5): 520-526.
Aminzai, M.T., Yildirim, M., and Yabalak, E. 2024. Metallic nanoparticles unveiled: Synthesis, characterization, and their environmental, medicinal, and agricultural applications. Talanta. 280: 126790.
Anand, P., Thomas, S.G., Kunnumakkara, A.B., Sundaram, C., Harikumar, K.B., Sung, B., Tharakan, S.T., Misra, K., Priyadarsini, I.K., and Rajasekharan, K.N. 2008. Biological activities of curcumin and its analogues (congeners) made by man and mother nature. Biochemical Pharmacology. 76(11): 1590-1611.
Aung, M.M.M. and Thu, Y.Y. 2024. Extraction, isolation and identification of active constituents from Curcuma aromatica Salisb (Taw-Sanwin). 4th Myanmar Korea Conference on Research Journal. 4: 155-165.
Balalakshmi, C., Alharbi, N.S., Kadaikunnan, S., Khaled, J.M., Alanzi, K.F., Gopinath, K., Arumugam, A., and Govindarajan, M. 2020. Development of chitosan/agar-silver nanoparticles-coated paper for antibacterial application. Green Processing and Synthesis. 9(1): 751-759.
Balamurugan, V., Ragavendran, C., and Arulbalachandran, D. 2024. Eco-friendly green synthesis of AgNPs from Elaeocarpus serratus fruit extract: Potential to antibacterial, antioxidant, cytotoxic effects of colon cancerous cells (HT-29) and its toxicity assessments of marine microcrustacean Artemia nauplii. Molecular Biology Reports. 51(1): 418.
Barua, N. and Buragohain, A.K. 2024. Therapeutic potential of silver nanoparticles (AgNPs) as an antimycobacterial agent: A comprehensive review. Antibiotics. 13(11): 1106.
Benzie, I.F.F. and Strain, J.J. 1996. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Analytical Biochemistry. 239(1): 70-76.
Braca, A., De Tommasi, N., Di Bari, L., Pizza, C., Politi, M., and Morelli, I. 2001. Antioxidant principles from Bauhinia tarapotensis. Journal of Natural Products. 64(7): 892-895.
Burapan, S., Kim, M., Paisooksantivatana, Y., Eser, B.E., and Han, J. 2020. Thai Curcuma species: Antioxidant and bioactive compounds. Foods. 9(9): 1219.
Chintala, S., Laishram, R., Mondal, P., Pal, K., Kantamraju, P., Ghosh, S., Karmakar, K., Chakdar, H., Mukhopadhyay, R., and Sen, R. 2023. Turmeric (Curcuma longa L.) rhizome extract mediated silver nanoformulation exhibits enhanced antifungal property against Rhizoctonia solani and boosts innate immunity of rice. Industrial Crops and Products. 206: 117616.
Chittasupho, C., Samee, W., Tadtong, S., Jittachai, W., Managit, C., and Athikomkulchai, S. 2023. Cytotoxicity, apoptosis induction, oxidative stress, and cell cycle arrest of Clerodendrum chinense flower extract nanoparticles in HeLa cells. Natural and Life Sciences Communications. 22(4): e2023057.
Cousins, M., Adelberg, J., Chen, F., and Rieck, J. 2007. Antioxidant capacity of fresh and dried rhizomes from four clones of turmeric (Curcuma longa L.) grown in vitro. Industrial Crops and Products. 25(2): 129-135.
Dasaradhudu, Y. and Srinivasan, M.A. 2020. Synthesis and characterization of silver nano particles using co-precipitation method. Materials Today: Proceedings. 33: 720-723.
De Barros, C.H.N., Cruz, G.C.F., Mayrink, W., and Tasic, L. 2018. Bio-based synthesis of silver nanoparticles from orange waste: Effects of distinct biomolecule coatings on size, morphology, and antimicrobial activity. Nanotechnology, Science and Applications. 11: 1-14.
De Souza, C.C., Ramos, G.Q., Katak, R.d.M., Muniz, V.A., Roque, R.A., Ferreira, N.S., Matos, R.S., Xing, Y., and Filho, H.D. 2024. Eco-friendly synthesis of silver nanoparticles via Cassava starch: Structural analysis and biocidal applications against Aedes aegypti and pathogenic bacteria. Journal of Sol-Gel Science and Technology. 113: 518-533.
Devi, L., Kushwaha, P., Ansari, T.M., Kumar, A., and Rao, A. 2024. Recent trends in biologically synthesized metal nanoparticles and their biomedical applications: A review. Biological Trace Element Research. 202(7): 3383-3399.
Devi, S.T. and Sanatombi, K. 2024. Bioactive compounds and biological activities of Curcuma species. In: H.N. Murthy, K.Y. Paek and S.Y. Park (Eds.). Bioactive Compounds in the Storage Organs of Plants. Reference Series in Phytochemistry. Cham (Switzerland): Springer. p. 337–393.
Dewi, S., Saepudin, E., and Rahayu, D. 2020. Reduction of curcuminoid extract from turmeric (Curcuma longa) rhizomes and its antibacterial activities. IOP Conference Series: Materials Science and Engineering. 902: 012072.
Dinis, T.C.P., Madeira, V.M.C., and Almeida, L.M. 1994. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Archives of Biochemistry and Biophysics. 315(1): 161-169.
Elumalai, A. and Irfan, N. 2024. Fabrication of silver nanoparticles using Acalypha paniculata extract, AI-based interaction analysis and its activity explication. Natural and Life Sciences Communications. 23(3): e2024035.
Fuloria, S., Mehta, J., Chandel, A., Sekar, M., Rani, N.N.I.M., Begum, M.Y., Subramaniyan, V., Chidambaram, K., Thangavelu, L., and Nordin, R. 2022. A comprehensive review on the therapeutic potential of Curcuma longa Linn. in relation to its major active constituent curcumin. Frontiers in Pharmacology. 13: 820806.
Ganesan, S., Mehalingam, P., and Selvam, G.S. 2019. Green synthesis of silver nanoparticles from de-oiled rhizomes of Curcuma longa L. and its biomedical potential. In: M. Rajan, K. Anand and A. Chuturgoon (Eds.). Proceedings of the International Conference on Nanomedicine (ICON-2019). ICON 2019. Springer Proceedings in Materials. Cham (Switzerland): Springer. p. 94–106.
Gecer, E.N., Erenler, R., Temiz, C., Genc, N., and Yildiz, I. 2022. Green synthesis of silver nanoparticles from Echinacea purpurea (L.) Moench with antioxidant profile. Particulate Science and Technology. 40(1): 50-57.
Genc, N., Yildiz, I., Chaoui, R., Erenler, R., Temiz, C., and Elmastas, M. 2020. Biosynthesis, characterization and antioxidant activity of oleuropein-mediated silver nanoparticles. Inorganic and Nano-Metal Chemistry. 51(3): 411-419.
Gopinath, K., Gnanasekar, S., Al-Ghanim, K.A., Nicoletti, M., Govindarajan, M., Arumugam, A., Balalakshmi, C., and Thanakkasaranee, S. 2023. Fabrication of neodymium (Nd), cadmium (Cd) and Nd:Cd doped hybrid copper oxide nanocomposites: Evaluation of their antibacterial activity and cytotoxicity against human L132 cell line. Ceramics International. 49(18): 29933-29947.
Gopinath, K., Kumaraguru, S., Bhakyaraj, K., Mohan, S., Venkatesh, K.S., Esakkirajan, M., Kaleeswarran, P., Alharbi, N.S., Kadaikunnan, S., and Govindarajan, M. 2016. Green synthesis of silver, gold and silver/gold bimetallic nanoparticles using the Gloriosa superba leaf extract and their antibacterial and antibiofilm activities. Microbial Pathogenesis. 101: 1-11.
Gupta, A., Mahajan, S., and Sharma, R. 2015. Evaluation of antimicrobial activity of Curcuma longa rhizome extract against Staphylococcus aureus. Biotechnology Reports. 6: 51-55.
Hosea, Z.Y., Kator, L., and Rhoda, E.H. 2018. Phytochemical properties and antimicrobial activities of aqueous extract of Curcuma longa (turmeric) rhizome extract. Asian Journal of Research in Crop Science. 2(1): 43142.
Jaimun, R., Kanha, N., and Sangsuwan, J. 2024. Indicator films containing Dendrobium orchid flower extract and TiO2 nanoparticles for monitoring fish fillet spoilage. Natural and Life Sciences Communications. 23(3): e2024036.
Jain, A., Jain, P., Soni, P., Tiwari, A., and Tiwari, S.P. 2023. Design and characterization of silver nanoparticles of different species of Curcuma in the treatment of cancer using human colon cancer cell line (HT-29). Journal of Gastrointestinal Cancer. 54(1): 90-95.
Jannah, R., Rafi, M., Heryanto, R., Kautsar, A., and Septaningsih, D. 2018. UV-Vis spectroscopy and chemometrics as a tool for identification and discrimination of four Curcuma species. International Food Research Journal. 25(2): 643-648.
Jiao, Z.-H., Li, M., Feng, Y.-X., Shi, J.-C., Zhang, J., and Shao, B. 2014. Hormesis effects of silver nanoparticles at non-cytotoxic doses to human hepatoma cells. PLoS One. 9(7): e102564.
Jyotirmayee, B. and Mahalik, G. 2022. A review on selected pharmacological activities of Curcuma longa L. International Journal of Food Properties, 25(1): 1377-1398.
Kamble, S.P. and Shinde, K.D. 2018. Anti-biofilm activity against Gram-positive bacteria by biologically synthesized silver nanoparticles using Curcuma longa. Pharmaceutical Nanotechnology. 6(3): 165-170.
Kandav, G. and Sharma, T. 2024. Green synthesis: An eco friendly approach for metallic nanoparticles synthesis. Particulate Science and Technology. 42(5): 874-894.
Kannan, P. and Maduraiveeran, G. 2023. Metal oxides nanomaterials and nanocomposite-based electrochemical sensors for healthcare applications. Biosensors. 13(5): 542.
Karan, T., Erenler, R., Gonulalan, Z., and Kolemen, U. 2024a. Biogenic synthesis of silver nanoparticles using Sambucus nigra leaves: Elucidation, antimicrobial, antioxidant activities and quantification of phenolics. Chemical Papers. 78(1): 473-481.
Karan, T., Gonulalan, Z., Erenler, R., Kolemen, U., and Eminagaoglu, O. 2024b. Green synthesis of silver nanoparticles using Sambucus ebulus leaves extract: Characterization, quantitative analysis of bioactive molecules, antioxidant and antibacterial activities. Journal of Molecular Structure. 1296: 136836.
Kasi, G., Thanakkasaranee, S., Stalin, N., Arumugam, A., Jantanasakulwong, K., Panyathip, R., Sukunta, J., Tanadchangsaeng, N., Worajittiphon, P., and Rachtanapun, P. 2024. Enhancement of antimicrobial properties and cytocompatibility through silver and magnesium doping strategies on copper oxide nanocomposites. Journal of Alloys and Compounds. 1007: 176481.
Kasta, G. 2020. Antimicrobial activity of ethanol extract of rhizome turmeric (Curcuma longa L.) for growth of Escherichia coli, Staphylococcus aureus and Candida albicans. Asian Journal of Pharmaceutical Research and Development. 8(3): 5-8.
Khoirotin, Faaizatunnisa, N., and Munasir. 2023. Green synthesis of Fe3O4 nanoparticles using green betel leaf extract for methylene blue adsorption. Natural and Life Sciences Communications. 22(3): e2023042.
Kim, H.-J., Kim, D.-J., Karthick, S., Hemalatha, K., Raj, C.J., Ok, S., and Choe, Y. 2013. Curcumin dye extracted from Curcuma longa L. used as sensitizers for efficient dye-sensitized solar cells. International Journal of Electrochemical Science. 8(6): 8320-8328.
Kim, K.-J., Yu, H.-H., Cha, J.-D., Seo, S.-J., Choi, N.-Y., and You, Y.-O. 2005. Antibacterial activity of Curcuma longa L. against methicillin-resistant Staphylococcus aureus. Phytotherapy Research. 19(7): 599-604.
Kirmani, F., Saddiqe, Z., Saleem, S., Ali, F., and Haq, F.U. 2024. Phytochemical investigation and antibacterial activity of Curcuma longa against multi-drug resistant bacteria. South African Journal of Botany. 164: 137-145.
Krishnaprasad, V.H. and Kumar, S. 2024. Antimicrobial resistance: An ultimate challenge for 21st century scientists, healthcare professionals, and policymakers to save future generations. Journal of Medicinal Chemistry. 67(18): 15927–15930.
Kundu, S. and Nithiyanantham, U. 2013. In situ formation of curcumin stabilized shape-selective Ag nanostructures in aqueous solution and their pronounced SERS activity. RSC Advances. 3(47): 25278-25290.
Li, S., Yuan, W., Deng, G., Wang, P., Yang, P., and Aggarwal, B. 2011. Chemical composition and product quality control of turmeric (Curcuma longa L.). Pharmaceutical Crops. 2: 28–54.
Lieu, D.M., Dang, T.K.T., and Nguyen, T.H. 2024. Green synthesized silver nanoparticles, a sustainable approach for fruit and vegetable preservation: An overview. Food Chemistry: X. 23: 101664.
Mahanty, R., Saha, I., Halder, J., Rajwar, T. K., Dash, P., Das, C., Pradhan, D., Sarangi, M. K., Dubey, D., and Rai, V.K. 2024. Biosurfactant-based silver nanoparticles: Recent advances and their applications in antimicrobial drug resistance. Journal of Drug Delivery Science and Technology. 92: 105382.
Makkar, H.P. 2003. Quantification of tannins in tree and shrub foliage: A laboratory manual. Kluwer Academic Publishers, Dordrecht, The Netherlands. Springer Science & Business Media
Mammari, N., Lamouroux, E., Boudier, A., and Duval, R.E. 2022. Current knowledge on the oxidative-stress-mediated antimicrobial properties of metal-based nanoparticles. Microorganisms. 10(2): 437.
Mazmancı, B., Könen Adıgüzel, S., Sadak, Y.S., Yetkin, D., Ay, H., and Adıgüzel, A.O. 2023. Antimicrobial, antibiofilm, and anticancer potential of silver nanoparticles synthesized using pigment-producing Micromonospora sp. SH121. Preparative Biochemistry & Biotechnology. 53(5): 475-487.
Mohammadi, M., Zaki, L., KarimiPourSaryazdi, A., Tavakoli, P., Tavajjohi, A., Poursalehi, R., Delavari, H., and Ghaffarifar, F. 2021. Efficacy of green synthesized silver nanoparticles via ginger rhizome extract against Leishmania major in vitro. PloS One. 16(8): e0255571.
Nguepi, M.S.D., Itoe, U.S., Itoe, F A., Ntieche, D.W., Dize, D., Kemzeu, R., Noubissi, P.A., Kouemou, N., Fokou, P.V.T., and Tincho, M.B. 2024. Persea americana, Curcuma longa and Allium sativum extracts exhibit cercaricidal, anti-inflammatory and anti-oxidant activities. South African Journal of Botany. 169: 268-275.
Niamsa, N. and Sittiwet, C. 2009. Antimicrobial activity of Curcuma longa aqueous extract. Journal of Pharmacology and Toxicology. 4(4): 173-177.
Nonglang, F.P., Khale, A., Wankhar, W., and Bhan, S. 2022. Pharmacognostic evaluation of Eranthemum indicum extracts for its in-vitro antioxidant activity, acute toxicology, and investigation of potent bioactive phytocompounds using HPTLC and GCMS. Beni-Suef University Journal of Basic and Applied Sciences. 11(1): 129.
Nurkhaliza, F., Risana, M.Z., Pubasari, A., Priatmoko, S., Prastya, M.E., and Andreani, A.S. 2024. Comparative study of well diffusion and disc diffusion method to investigate the antibacterial properties of silver nanoparticles synthesized from Curcuma longa extracts. E3S Web of Conferences.503: 09003.
Ongtanasup, T., Kamdenlek, P., Manaspon, C., and Eawsakul, K. 2024. Green-synthesized silver nanoparticles from Zingiber officinale extract: Antioxidant potential, biocompatibility, anti-LOX properties, and in silico analysis. BMC Complementary Medicine and Therapies. 24(1): 84.
Othman, N. 2022. IR spectroscopy in qualitative and quantitative analysis. In: M. El-Azazy, K. Al-Saad and A.S. El-Shafie (Eds.). Infrared Spectroscopy: Perspectives and Applications. London (UK): IntechOpen. p. 57–72.
Pakkirisamy, M., Kalakandan, S.K., and Ravichandran, K. 2017. Phytochemical screening, GC-MS, FT-IR analysis of methanolic extract of Curcuma caesia Roxb (Black Turmeric). Pharmacognosy Journal. 9(6): 952-956.
Pareek, V., Gupta, R., and Panwar, J. 2018. Do physico-chemical properties of silver nanoparticles decide their interaction with biological media and bactericidal action? A review. Materials Science and Engineering: C. 90: 739-749.
Pradabsang, C., Sukrong, S., and Warisnoicharoen, W. 2025. Green synthesis of antimicrobial silver nanoparticles using edible plants. Natural and Life Sciences Communications. 24(3): e2025054.
Rachtanapun, P., Rachtanapun, C., Jantrawut, P., Thanakkasaranee, S., Kasi, G., Tantala, J., Panraksa, P., and Chaiwarit, T. 2023. Carboxymethyl chitosan-based materials in packaging, food, pharmaceutical, and cosmetics. In: R. Jayakumar (Eds.). Multifaceted Carboxymethyl Chitosan Derivatives: Properties and Biomedical Applications. Advances in Polymer Science, vol 292. Cham (Switzerland): Springer Nature. p. 139–203.
Ren, Y., Zhang, Y., and Li, X. 2024. Application of AgNPs in biomedicine: An overview and current trends. Nanotechnology Reviews. 13(1): 20240030.
Riesenberger, B., Rodriguez, M., Marques, L., Cervantes, R., Gomes, B., Dias, M., Pena, P., Ribeiro, E., and Viegas, C. 2024. Filling the knowledge gap regarding microbial occupational exposure assessment in waste water treatment plants: A scoping review. Microorganisms. 12(6): 1144.
Rohman, A., Sudjadi., Devi., Ramadhani, D., and Nugroho, A. 2015. Analysis of curcumin in Curcuma longa and Curcuma xanthorriza using FTIR spectroscopy and chemometrics. Research Journal of Medicinal Plant. 9: 179-186.
Sahin Yaglioglu, A., Erenler, R., Gecer, E.N., and Genc, N. 2022. Biosynthesis of silver nanoparticles using Astragalus flavesces leaf: Identification, antioxidant activity, and catalytic degradation of methylene blue. Journal of Inorganic and Organometallic Polymers and Materials. 32(10): 3700-3707.
Saravanan, M., Mahalakshmi, B., Afeeza, KLG., Subriya, S., Sridevi, M., and Dilipan, E. 2025. Formulation and characterization of sodium alginate-based nanoparticle doped with seagrass (Syringodium isoetifolium) extract: In vitro anti-inflammatory, antioxidant and anti-diabetic activities. Natural and Life Sciences Communications. 24(2): e2025035.
Segneanu, A.-E., Vlase, G., Lukinich-Gruia, A. T., Herea, D.-D., and Grozescu, I. 2022. Untargeted metabolomic approach of Curcuma longa to neurodegenerative phytocarrier system based on silver nanoparticles. Antioxidants. 11(11): 2261.
Shameli, K., Ahmad, M.B., Zamanian, A., Sangpour, P., Shabanzadeh, P., Abdollahi, Y., and Zargar, M. 2012. Green biosynthesis of silver nanoparticles using Curcuma longa tuber powder. International Journal of Nanomedicine. 2012 (7): 5603-5610.
Sharma, M., Monika, Thakur, P., Saini, R.V., Kumar, R., and Torino, E. 2020. Unveiling antimicrobial and anticancerous behavior of AuNPs and AgNPs moderated by rhizome extracts of Curcuma longa from diverse altitudes of Himalaya. Scientific Reports. 10: 10934.
Sharma, S., Sharma, N., and Kaushal, N. 2023. Utilization of novel bacteriocin synthesized silver nanoparticles (AgNPs) for their application in antimicrobial packaging for preservation of tomato fruit. Frontiers in Sustainable Food Systems. 7: 1072738.
Singh, D.K., Srivastava, B., and Sahu, A. 2004. Spectrophotometric determination of Rauwolfia alkaloids: Estimation of reserpine in pharmaceuticals. Analytical Sciences. 20(3): 571-573.
Singh, G., Kapoor, I., Singh, P., de Heluani, C.S., de Lampasona, M.P., and Catalan, C.A. 2010. Comparative study of chemical composition and antioxidant activity of fresh and dry rhizomes of turmeric (Curcuma longa Linn.). Food and Chemical Toxicology. 48(4): 1026-1031.
Suwal, N., Bashyal, S., Paudyal, P., Khanal, D.P., Suwal, N., Dhaubanjar, S., Thapa, R., and Paudel, K.R. 2025. Comparative assessment of antibiofilm and antioxidant activities between Curcuma longa silver nano particles and ethanolic extract of Curcuma longa. Next Research. 2(3): 100447.
Swain, S. and Rautray, T.R. 2021. Estimation of trace elements, antioxidants, and antibacterial agents of regularly consumed Indian medicinal plants. Biological Trace Element Research. 199(3): 1185-1193.
Tawre, M.S., Shiledar, A., Satpute, S.K., Ahire, K., Ghosh, S., and Pardesi, K. 2022. Synergistic and antibiofilm potential of Curcuma aromatica derived silver nanoparticles in combination with antibiotics against multidrug-resistant pathogens. Frontiers in Chemistry. 10: 1029056.
Tayyem, R. F., Heath, D.D., Al-Delaimy, W.K., and Rock, C.L. 2006. Curcumin content of turmeric and curry powders. Nutrition and Cancer. 55(2): 126-131.
Tooklang, P., Audtarat, S., Chaisen, K., Thepsiri, J., Chingsungnoen, A., Jittabut, P., and Dasri, T. 2024. Functionalization of silver nanoparticles coating cotton fabrics through hydrothermal synthesis for improved antimicrobial properties. Nano Express. 5(2): 025009.
Trela-Makowej, A., Orzechowska, A., and Szymańska, R. 2024. Less is more: The hormetic effect of titanium dioxide nanoparticles on plants. Science of the Total Environment. 910: 168669.
Umar, N.M., Parumasivam, T., Aminu, N., and Toh, S.-M. 2020. Phytochemical and pharmacological properties of Curcuma aromatica Salisb (wild turmeric). Journal of Applied Pharmaceutical Science. 10(10): 180-194.
Uribe, S., Ramirez, J., and Peña, A. 1985. Effects of beta-pinene on yeast membrane functions. Journal of Bacteriology. 161(3): 1195-1200.
Vanlalveni, C., Lallianrawna, S., Biswas, A., Selvaraj, M., Changmai, B., and Rokhum, S.L. 2021. Green synthesis of silver nanoparticles using plant extracts and their antimicrobial activities: A review of recent literature. RSC Advances. 11(5): 2804-2837.
Venkatadri, B., Shanparvish, E., Rameshkumar, M., Arasu, M.V., Al-Dhabi, N.A., Ponnusamy, V.K., and Agastian, P. 2020. Green synthesis of silver nanoparticles using aqueous rhizome extract of Zingiber officinale and Curcuma longa: In-vitro anti-cancer potential on human colon carcinoma HT-29 cells. Saudi Journal of Biological Sciences. 27(11): 2980-2986.
Wulandari, R., Sudjadi, Martono, S., and Rohman, A. 2018. Liquid chromatography and fourier transform infrared spectroscopy for quantitative analysis of individual and total curcuminoid in Curcuma longa extract. Journal of Applied Pharmaceutical Science. 8 (9): 107-113.
Wuttikul, K. and Sainakham, M. 2022. In vitro bioactivities and preparation of nanoemulsion from coconut oil loaded Curcuma aromatica extracts for cosmeceutical delivery systems. Saudi Journal of Biological Sciences. 29(12): 103435.
Yuandani, Jantan, I., Rohani, A.S., and Sumantri, I.B. 2021. Immunomodulatory effects and mechanisms of Curcuma species and their bioactive compounds: A review. Frontiers in Pharmacology. 12: 643119.
Zheng, D., Huang, C., Huang, H., Zhao, Y., Khan, M.R.U., Zhao, H., and Huang, L. 2020. Antibacterial mechanism of curcumin: A review. Chemistry and Biodiversity. 17(8): e2000171.
Zhishen, J., Mengcheng, T., and Jianming, W. 1999. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chemistry. 64(4): 555-559.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Gopinath Kasi1, Balamurugan Venkatachalam2, Sarinthip Thanakkasaranee1, 3, 4, Nattan Stalin5, Ramar Dharmaraj6, Kittisak Jantanasakulwong1, 3, 4, and Pornchai Rachtanapun1 ,3, 4, *
1 Division of Packaging Technology, School of Agro-Industry, Faculty of Agro-Industry, Chiang Mai University, Chiang Mai 50100, Thailand.
2 Department of Botany, Periyar University, Salem, Tamil Nadu, 636 011, India.
3 Center of Excellence in Agro Bio-Circular-Green Industry, Chiang Mai University, Chiang Mai 50100, Thailand.
4 Center of Excellence in Materials Science and Technology, Chiang Mai University, Chiang Mai 50200, Thailand.
5 Department of Life Science, Gachon University, Seongnam 13120, Republic of Korea.
6 Department of Botany, Alagappa University, Karaikudi, Tamil Nadu 630003, India.
Corresponding author: Pornchai Rachtanapun, E-mail: pornchai.r@cmu.ac.th
ORCID: Pornchai Rachtanapun: https://orcid.org/0000-0001-8157-4433
Total Article Views
Editor: Sirasit Srinuanpan,
Chiang Mai University, Thailand
Article history:
Received: June 10, 2025;
Revised: July 11, 2025;
Accepted: July 15, 2025;
Online First: August 8, 2025

