
In-vivo Wound Healing Activity of Acalypha paniculata
Elumalai A.*, Shanmugapandiyan P., Vijay Kumar V., Kaviarasan L.Published Date : August 6, 2025
DOI : https://doi.org/10.12982/NLSC.2025.062
Journal Issues : Number 4, October-December 2025
Abstract The wound healing properties of the ethanol extract of dried aerial parts of Acalypha paniculata (APE) were investigated using excision and incision wound models in Wistar strain albino rats. In this experimental study, second-degree burn wounds were created in twenty prescreened rats, which were divided into four groups of five animals each. Group I served as the control and received normal saline, Group II was treated with povidone-iodine as the standard drug, and Groups III and IV were administered topically APE at doses of 250 and 500 µl/wound respectively. For the excision wound model, wound healing was assessed using parameters such as the percentage of wound contraction and epithelialization duration on days 2, 6, 10, and 14, along with histopathological evaluations. In both models, rats treated with APE demonstrated significantly enhanced wound healing activity (P <0.001) compared to the control group. This was evident from the accelerated wound contraction, shorter epithelialization period, increased skin tensile strength, and complete healing within 18 days for excision and seven days for incision method. Histopathological analysis further confirmed these findings, showing well-developed hair follicles, denser collagen fibers, reduced inflammatory cells, mature fibroblasts, and enlarged capillary-sized blood vessels, comparable to the outcomes observed in the standard treatment group. Our study demonstrates that the positive effects of APE on wound healing can be attributed to its ability to reduce wound size.
Keywords: Acalypha paniculata, Ethanolic extract, Wound healing activity, Antioxidant activity
Citation: Elumalai, A., Shanmugapandiyan, P., Vijay Kumar, V., and Kaviarasan, L. 2025. In-vivo wound healing activity of Acalypha paniculata. Natural and Life Sciences Communications. 24(4): e2025062.
INTRODUCTION
Wounds are skin injuries caused by various external or internal factors, including temperature, pH changes, chemicals, friction, trauma, or radiation, leading to a break in the epithelial lining. Wound healing is a complex and dynamic process involving multiple coordinated cellular events to efficiently repair damaged tissue. This process typically progresses through three phases: inflammation, proliferation, and remodelling (Kruzicova et al., 2023; Almuhanna, 2024; Mamun et al., 2024). During these phases, damaged tissue is repaired, lost tissue is replaced, and the epithelium is restored. Key cells involved include keratinocytes, fibroblasts, vascular endothelial cells, and immune cells, which facilitate inflammation, cell migration, and angiogenesis. A primary goal of wound healing is to restore the epithelial barrier, which is crucial for preventing infection and fluid loss. The World Health Organization (WHO) estimates that 80% of the population in developing countries relies on traditional medicine, primarily plant-based drugs, for their primary healthcare needs. India's rich biodiversity offers natural products that promote wound healing by providing safe, effective, and affordable options (Kumar et al., 2007; Palani et al., 2024; Pathak et al., 2024). Traditional plants contain bioactive phytochemicals that accelerate healing, regenerate tissue, and offer antiseptic and anti-inflammatory benefits, improving blood flow at the wound site. However, wound healing can be complicated by bacterial infections, stress, and nutritional deficiencies, increasing the demand for plants with antimicrobial properties. An imbalance in free radical generation and antioxidants can induce oxidative stress, causing tissue damage and delaying wound healing. Reducing excessive free radicals and the inflammatory response during the acute inflammatory stage, along with promoting collagen synthesis, are key strategies for enhancing the wound healing process. Therefore, eliminating free radicals could be crucial in healing chronic wounds (Adhav et al., 2015; Ramamoorthy et al., 2019; Shedoeva et al., 2019; Vasanthakumari et al., 2024).
Acalypha paniculata a commonly used, most popular herb in Ayurvedic medicine, is familiarly known for its anti-inflammatory, anti-rheumatic, hepatoprotective and anti-diabetic properties (Matthew, 1983; Sarvalingam et al., 2017; Elumalai et al., 2024; Elumalai and Irfan, 2024; Elumalai et al., 2024). More recently, it is being used to treat ulcers and bacterial infections. Previous surveys suggest that the plant is a promising wound healing agent that warrants further investigation. To date, no reports are available on the wound healing effects of Acalypha paniculata aerial parts when applied topically. Thus, the present study was conducted to scientifically prove the wound healing activity on experimentally induced excision and incision wound healing models in Wistar albino rats.
MATERIAL AND METHODS
Collection of plant material
Fresh aerial parts of Acalypha paniculata (AP) were collected from the Parvathamalai hill region, Thiruvannamalai District, Tamil Nadu, India (2.4352°N, 78.9684°E). A local herbalist assisted in identifying the plant, and it was authenticated by the Department of Pharmacognosy at the Siddha Central Research Institute (CCRS), Chennai (Reference: H12092201S). AP typically blooms from June to January. The plant material was shade-dried, powdered, and extracted with ethanol (1:5 w/v) at 60°C for 6 hours. The extract was then evaporated, dried, and stored.
Experimental animals
Inbred adult Wistar rats of either sex, weighing approximately 170-180 ± 2.5g, were obtained from the Cape Biolab animal breeding and animal experimentation facility. The animal study protocol was approved by CPSEA (approval number: CBLRC/IAEC/02/02-2024). Rats were housed in cages to acclimatize to standard laboratory conditions (20-22 °C, with a humidity-controlled environment and a 12-hour dark/light cycle) for 7 days, with ad libitum access to food and water.
Wound healing activity by excision wound model in rats
A total of 20 rats were divided into four groups, each containing five rats, with identical wounds. Group 1 was the control group containing untreated rats; Groups 2 and 3 were treated with 250 mg and 500 mg of prepared extracts, respectively; and Group 4 was treated with standard povidone iodine ointment. Following the method conducted by Ghashghaii et al., four groups of five rats each were anesthetized using ketamine hydrochloride (50 mg/kg, i.p., body weight). The back fur of the rats was shaved to create a clear area for the wound. A circular full-thickness excision wound of 1 cm in diameter was made using toothed forceps, a surgical blade, and pointed scissors. After the procedure, the rats were allowed to recover from anesthesia and were housed individually in cages. The animals were closely monitored for any signs of infection. If infection was detected, the affected rats were excluded from the study and replaced. The control group rats were kept under standard conditions. Different doses of APE (250 and 500 µl/wound) were topically applied to the treatment groups twice daily for 7 days, then once daily until the wounds healed. Similarly, povidone iodine cream was applied to the positive control group twice daily for 7 days, then once daily until the wounds healed. Wound contraction was evaluated and measured as a percentage on the 7th, 14th, and 21st days after wound formation (Cherukuri et al., 2023; Ubhrani et al., 2023; Mahadevi et al., 2024). Granulation tissue was collected on the 21st day for the determination of hydroxyproline content. The percentage of wound contraction was calculated using the formula:

Incision wound model
The experimental animals and grouping of rats were conducted as previously mentioned in the excision wound model. The hair on the dorsal part of all the rats was removed using a depilatory cream. After anesthetizing the rats with an intraperitoneal injection of ketamine (50 mg/kg), they were secured to the operation table in a prone position. A paravertebral straight incision, 6 cm in length, was made at least 1.5 cm lateral to the vertebral column using a blade. A full-thickness excision wound was created along the marked area using toothed forceps, a surgical blade, and pointed scissors. After achieving complete haemostasis, the wound was closed with interrupted sutures placed 1 cm apart. The wound area was mopped with cotton swabs soaked in 70% alcohol, and the rats were caged individually to recover from anesthesia. The rats were closely monitored for any signs of infection. If signs of infection appeared, the affected animals were excluded from the study and replaced. The rats were treated daily with APE and the standard from day 0 to the 9th post-wounding day. The stitches were removed on the 8th day, and the breaking strength of the wound was measured on the 10th post-wounding day using the continuous, constant water flow technique described by Lee (1968). The skin-breaking strength was assessed in the evening of the 10th day after the final application of APE and the standard treatment. The excised skin samples were fixed in buffered formalin, embedded in paraffin, and stained using Hematoxylin and Eosin for histological examination (Chakraborty et al., 2024).
Statistical analysis
The results are expressed as mean ± standard error of mean (SEM). The statistical significance was analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s Multiple Comparisons Test employing statistical software, Graph Pad 6, Differences between groups were considered significant at P <0.05 levels.
RESULTS
Excision wound model
The wound healing effect of APE was evaluated by measuring the wound area in rats on days 7, 14, and 21. Rats treated with APE showed a significantly faster reduction in wound area compared to the control group. Daily progression of wound contraction following topical application of APE at concentrations of 250 and 500 mg/kg is presented in Table 1 and Figure 1. The wound contraction activity of APE increased in a dose-dependent manner. APE demonstrated a comparable onset of action in reducing wound size and exhibited significant effects (P < 0.001) across all observed days compared to the standard treatment group. By day 21, no wound area was observed in either the APE-treated groups or the standard drug group. The percentage of wound closure in the excision model was consistent with the observed wound contraction results.
Table 1. Percentage wound contraction in excision wound model in rats.
|
Post wounding days |
Percentage wound contraction (%) |
|
|||
|
Group 1 |
Group 2 (Standard) |
Group 3 (APE 250) |
Group 4 (APE 500) |
||
|
5 |
34 |
33 |
32 |
33 |
|
|
10 |
41 |
75 |
66 |
73 |
|
|
15 |
49 |
89 |
79 |
90 |
|
|
20 |
51 |
99 |
91 |
99 |
|
In the excision wound model, hydroxyproline levels were higher in the treatment groups than in the control group, showing better collagen formation and improved wound healing. The control group had the lowest values, increasing slowly from 12.12 ± 1.57 µg/mg on Day 7 to 16.26 ± 1.41 µg/mg on Day 28. The standard group showed a steady rise, reaching 31.12 ± 31.57 µg/mg by Day 28, though there was high variation. The APE-treated groups showed a dose-related effect. APE at 250 mg/kg increased levels to 21.53 ± 1.21 µg/mg by Day 28, while 500 mg/kg reached 30.14 ± 1.43 µg/mg, close to the standard. These results suggest that APE, especially at 500 mg/kg, helps wound healing by boosting collagen production.
Table 2. Estimation of hydroxyproline content in excision wound model in rats.
|
Group 1 (Control) |
Group 2 (Standard) |
Group 3 (APE 250) |
Group 4 (APE 500) |
|
12.12 ± 1.57 |
19.24 ± 2.34 |
16.31 ± 1.24 |
21.31 ± 1.34 |
|
13.51 ± 2.21 |
23.51 ± 1.48 |
19.35 ± 1.58 |
22.41 ± 1.54 |
|
14.41 ± 1.23 |
26.38 ± 3.91 |
20.14 ± 1.71 |
24.38 ± 2.72 |
|
16.26 ± 1.41 |
31.12 ± 31.57 |
21.53 ± 1.21 |
30.14 ± 1.43 |
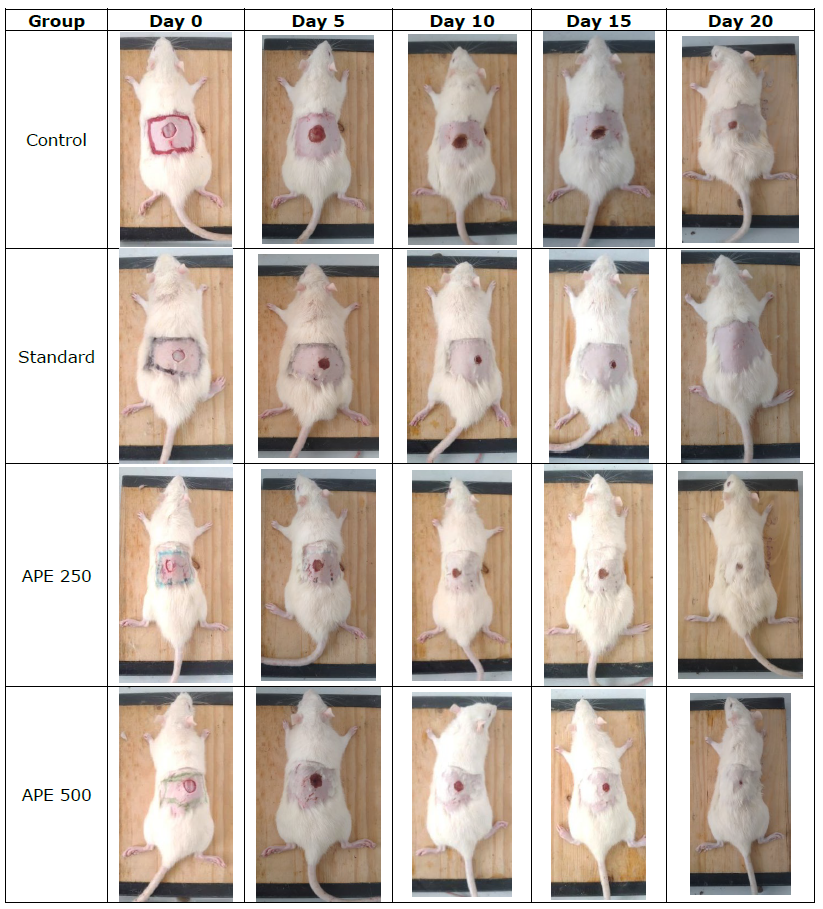
Figure 1. Photograph of excision wound test result.
Incision wound model
In excision wound model, the APE showed a promising result by increasing both the dry granulation tissue weight and granulation tissue breaking strength compared to the control group (P >0.001). The photomicrographs of the granulation tissue obtained from the animal wound are shown in Figure 2.
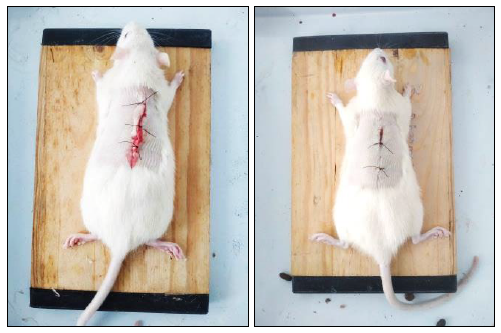
Figure 2. Photograph of incision wound test result.
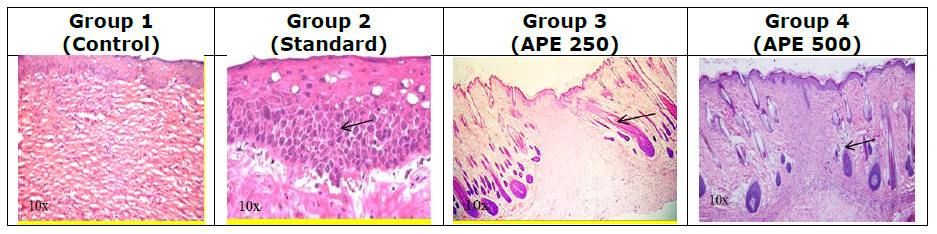
Figure 3. Histopathology of incision wound test.
Histological analysis of wound tissue on Day 21 revealed distinct differences among the experimental groups. The control group showed incomplete epithelialization, loosely arranged collagen fibers, and persistent inflammatory cell infiltration, indicating delayed healing. In contrast, the standard-treated group exhibited complete re-epithelialization with well-organized collagen bundles, abundant fibroblasts, and neovascularization (Figure 3). APE at 250 µl/wound demonstrated moderate healing, with partial collagen organization, reduced inflammation, and visible fibroblast proliferation. Notably, APE at 500 µl/wound resulted in well-formed epithelium, dense and aligned collagen fibers, restored hair follicles, and minimal inflammation, closely resembling normal skin architecture. These findings confirm that APE, particularly at the higher dose, promotes effective wound healing comparable to standard treatment.
DISCUSSION
Wound healing is a complex process that involves several mechanisms, including hemostasis, inflammation, matrix synthesis and deposition, angiogenesis, fibroplasia, epithelialization, contraction, and remodeling. Any disruption in these processes can prolong tissue damage and delay recovery (Subramanian et al., 2023; Athilli et al., 2024; Marasri et al., 2024). Angiogenesis is a crucial physiological and pathological process in wound healing and chronic inflammation. In the early phase of injury, the activation of granular leukocytes migrating to the wound site results in the production of reactive oxygen species (ROS). These ROS play an important role in inflammatory processes as mediators of injury, directly damaging skin structures. The dual function of angiogenesis is to supply oxygen and essential nutrients to the lesion area and to accelerate granulation tissue formation (Insuan et al., 2021; Saputera et al., 2022).
Control of the inflammation phase during the wound healing process in the skin will increase the quality of wound healing. Wound dressings, foams, or gels containing synthetic medications with topical antibiotics, and other healing agents are used to heal wounds. These agents can be extremely costly to the patient due to prolonged treatment. Several medicinal plants have shown significant wound healing potential due to their antioxidant, anti-inflammatory, and collagen-boosting properties. For instance, Curcuma amada and oregano oil-based hydrogels enhanced re-epithelialization in rats (Mahadevi et al., 2024). Dodonaea viscosa flavonoid fractions promoted fibroblast proliferation and tensile strength (Subramanian et al., 2023). Similarly, Centella asiatica and Citrus aurantium extracts accelerated granulation and epithelial regeneration in rodent models (Cherukuri et al., 2023; Ubhrani et al., 2023). This study assessed antimicrobial, antioxidant and wound healing efficacy of Acalypha paniculata ethanolic extract. For the first time, the present results showed that APE possess wound healing efficacy in an excisional and incision wound model in rats, While in anti-bacterial activity, the APE demonstrated antibacterial effects against and common wound contaminants.
A. paniculata grows in the wild in the northern parts of Tiruvannamalai districts including Western Ghats. Over the ages, the local people in Parvathamalai hills have incorporated the use of aerial parts of A. paniculata as oral nutraceuticals in arthritic and diabetic conditions. Phytochemical analysis revealed the presence of different components such as alkaloids, flavonoids, tannins and phenols. Our previous studies have been reported ethanolic extract was shown to have more bioactive constituents and identified different components were carried out by the GCMS analysis (Elumalai and Irfan, 2024).
In this study, it was observed that excision wounds treated with APE improved wound contraction and the period of reepithelialisation compared to control and standard treated excisional wounds with no sign of skin irritation. All parameters tested in the present study indicate the healing process of the wound. The contraction of the wound was measured every 5 days to understand the wound healing progress to determine complete wound healing. The epithelialization time was significantly reduced from 20 days (control) to 16 days for both the 250 and 500 µg extract. Enhanced epithelialization and wound contraction may result from the APE ability to boost collagen synthesis and promote epithelialization by facilitating cell proliferation or increasing epithelial cell viability (Golmohammadii et al., 2021).
Histological investigations on the healing of infected wounds were conducted using H&E stain to monitor epidermal regeneration and the presence of inflammatory cells. APE-treated wounds demonstrated facilitated epithelialization with thick epithelial tissues and collagen deposition in the dermis. Additionally, there was improved granulation, reduced inflammatory infiltration, and accelerated tissue repair processes. The development of new epithelium and increased capillary density indicated angiogenesis, confirming wound healing.
The presence of collagen in APE-treated tissues improved tensile strength. Inflammatory cells in the wound area indicated the initial phase of healing, and their absence 20 days after wounding suggested complete wound healing. The increased epidermal height and capillary density, along with fewer inflammatory cells in treated wounds, indicated enhanced wound healing. Throughout the experiment, the progression of new epithelium covering the defected area was greater in APE-treated rats compared to the standard treatment. The re-epithelialization process was completed by the 20th day in the APE-treated rats.
The results of the incision wound model, which showed increased wound tensile strength on the 10th post-wounding day, support the crude extract's wound healing ability. The breaking strength was calculated as a parameter in the incision wound model to illustrate how well the restored tissue resisted breaking under tension. Rats treated with APE 250, APE 500, and iodine ointments showed an increase in breaking strength. In contrast, the control group had a breaking strength which was twofold smaller than that of the APE-treated group. The increase in tensile strength may be associated with the promotion of collagen synthesis, angiogenesis, and stabilization of fibers, thereby improving the circulation of oxygen and nutrient supply, which are vital for the wound healing cascade. This effect could be due to the enhancement of collagen maturation, which provides strength and integrity to the wound matrix, facilitated by the presence of secondary metabolites in APE, such as alkaloids, flavonoids, tannins and saponins.
CONCLUSION
The results of the current study on wound healing in rats support our findings on the effect of A. paniculata extract on the infected wounds in diabetic rats. Therefore, the results obtained justify the use of the aerial parts of APE for wound healing which upholds the traditional use of the plant.
AUTHOR CONTRIBUTIONS
All authors contributed significantly to the conception, design, execution, and interpretation of the study. Elumalai conducted the experiments, Manoj analyzed the data, and Dr. Shanmugapandiyan drafted the manuscript. All authors reviewed and approved the final version of the manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
STATEMENT FOR DATA SHARING
The data supporting the findings of this study are available upon reasonable request from the corresponding author.
STATEMENT FOR ETHICAL APPROVAL
This study was conducted in accordance with ethical guidelines and was approved by Ethics Committee Approval No.: CBLRC/IAEC/02/02-2024. Informed consent was obtained from all participants where applicable.
REFERENCES
Adhav, R., Mantry, P., and Darwhekar, G.N. 2015. Wound healing medicinal plants of India: A review. International Journal of Pharmacognosy. 2(1): 6-10.
Almuhanna, Y. 2024. Effect of Ducrosia anethifolia methanol extract against methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa biofilms on excision wounds in diabetic mice. Frontiers in Cellular and Infection Microbiology. 14: 1386483.
Athilli, L. and Balasubramaniam, A. 2024. In vitro and in vivo wound healing potential of Commiphora caudata. Indian Journal of Pharmaceutical Education and Research. 58(1s): s262-s273.
Cherukuri, S., Paramanayagam, A., and Prabakaran, R. 2023. Evaluation of cutaneous wound healing activity of Citrus aurantium fruit peel extract-based ointment in albino rats. Research Journal of Pharmacy and Technology. 16(1): 250-254.
Chakraborty, S., Sen, S., Das, A., Ghosh, P., and Mukherjee, K. 2024. Fermented polyherbal formulation ameliorates the severity of acute multiple antibiotic-resistant Pseudomonas aeruginosa-infected burn wounds in a rat burn model. Journal of Pathogens. 13: 3601954.
Elumalai, A., Irfan, N., Haja Nazeer Ahamed, Vijaya Vara Parsad, M., Ismail, Y., Ashok Kumar, P., and Prabhu D. 2024. Impact of green-synthesized silver nanoparticles in Wistar rats: Behavioral, biochemical, and histopathological insights from acute and sub-acute oral exposure. Archives of Razi Institute. 80(3): 769-781.
Elumalai, A. and Irfan, N. 2024. Histological assessment of Acalypha paniculata and its phytoconstituent systemisation. Studies on Ethno-Medicine. 18(2): 24-31.
Elumalai, A. and Irfan, N. 2024. Fabrication of silver nanoparticles using Acalypha paniculata extract, AI-based interaction analysis, and its activity explication. Natural and Life Sciences Communications. 23(3): e2024035.
Golmohammadii, A., Namjou, A., and Heidarian, E. 2021. Evaluation of hydroalcoholic extract of licorice root (Glycyrrhiza glabra L.) on wound healing of gastrotomized male Wistar rats: Histopathological changes. Iranian Journal of Veterinary Surgery. 16(2): 75–83.
Insuan, O., Thongchuai, B., Chaiwongsa, R., Khamchun, S., and Insuan, W. 2021. Antioxidant and anti-inflammatory properties of essential oils from three Eucalyptus species. Chiang Mai University Journal of Natural Sciences. 20(4): e2021091.
Kruzicova, A., Chalupova, M., Kuzminova, G., Parak, T., Klusakova, J., Sopuch, T., and Suchy, P. 2023. Effect of novel carboxymethyl cellulose-based dressings on acute wound healing dynamics. Veterinarni Medicina. 68(10): 403-411.
Kumar, B., Vijayakumar, M., Govindarajan, R., and Pushpangadan, P. 2007. Ethnopharmacological approaches to wound healing: Exploring medicinal plants of India. Journal of Ethnopharmacology. 114(2): 103-113.
Mahadevi, R., Shiva Kumar, M.S., Natarajan, D., Sengottuvelu, S., and Kavitha, R. 2024. Anti-inflammatory and wound healing properties of Curcuma amada and oregano essential oil-derived hydrogel on Wistar rats. Journal of Chemical and Health Research. 14(4): 817-827.
Mamun, A.A., Shao, C., Geng, P., Wang, S., and Xiao, J. 2024. Recent advances in molecular mechanisms of skin wound healing and its treatments. Frontiers in Immunology. 15: 1395479.
Marasri, P., Sookkhee, S., Wanachantararak, P., and Owittayakul, D. 2024. Anti-inflammatory activity and wound healing ability of coconut oil mouthwash on gingival fibroblast cell in vitro. Natural and Life Sciences Communications. 23(4): e2024059.
Matthew, K.M. 1983. The Flora of Tamil Nadu Carnatic (Vol. 3[2]). Diocesan Press, Madras.
Pathak, D. and Mazumder, A. 2024. A critical overview of challenging roles of medicinal plants in improvement of wound healing technology. DARU Journal of Pharmaceutical Sciences. 32: 379-419.
Palani, N., Vijayakumar, P., Monisha, P., Ayyadurai, S., and Rajadesingu, S. 2024. Electrospun nanofibers synthesized from polymers incorporated with bioactive compounds for wound healing. Journal of Nanobiotechnology. 22(1): 211.
Ramamoorthy, R., Muthalagu, M., Andra, S., Kumaravel, R., and Selvaraj, P. 2019. Investigation on antimicrobial, antioxidant, and cytotoxicity properties of triple bark extract formulated using traditional medicinal plants. SN Applied Sciences. 1: 772.
Sarvalingam, A., Vijayakumar, D., Pavithra, C., Sharmila, S., and Rajendran, A. 2017. Inventory and ethnomedicinal plants used by rural people of Eastern Ghats of Tamil Nadu, India. Journal of Ecobiotechnology. 9: 5-12.
Saputera, D., Nirwana, I., Kridanto, M.J., and Surboyo, M.D.C. 2022. The role of ellagic acid on inflammatory protein NFKB1 and HSP70 with STITCH prediction. Chiang Mai University Journal of Natural Sciences. 21(1): e2022003.
Shedoeva, A., Leavesley, D., Upton, Z., and Fan, C. 2019. Wound healing and the use of medicinal plants. Evidence-Based Complementary and Alternative Medicine. 2019: 2684108.
Subramanian, S., Duraipandian, C., Alsayari, A., Senthil Kumar, R., and Venkatesan, M. 2023. Wound healing properties of a newly formulated flavonoid-rich fraction from Dodonaea viscosa Jacq. leaves extract. Frontiers in Pharmacology. 14: 1096905.
Ubhrani, G., Talati, H., Bhatt, P., Sorathia, K., and Suhagia, B. 2023. Preliminary wound healing activity of polyherbal formulation containing Cinnamon zeylanicum, Centella asiatica and Moringa oleifera. Hacettepe University Journal of the Faculty of Pharmacy. 43(3), 204-211.
Vasanthakumari, K., Sharmila, C., Jaya Priya, S., and Vadivel, V. 2024. Wound healing mechanisms of Couroupita guianensis fruit pulp: An ethnomedicine used by traditional healers in India. Natural Product Research. 38(4): 634-638.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Elumalai A.*, Shanmugapandiyan P., Vijay Kumar V., and Kaviarasan L.
School of Pharmacy, Sathyabama Institute of Science and Technology, Jeppiaar Nagar, Rajiv Gandhi Salai, Chennai - 600 119. Tamilnadu, India.
Corresponding author: Elumalai A., E-mail: elumalai.a.pharmacy@sathyabama.ac.in
Total Article Views
Editor: Veerasak Punyapornwithaya,
Chiang Mai University, Thailand
Article history:
Received: February 22, 2025;
Revised: July 9, 2025;
Accepted: July 22, 2025;
Online First: August 6, 2025

