
Exploring the Use of Pineapple Peel Extract for Underarm Care: Antioxidant and Anti-ageing Properties for Serum Formulation and Stability Testing
Kittima Saephan, Chutikan Yanukul, Pattana Sripalakit and Aurasorn Saraphanchotiwitthaya*Published Date : July 31, 2025
DOI : https://doi.org/10.12982/NLSC.2025.061
Journal Issues : Number 4, October-December 2025
Abstract Pineapple peel (PP), a significant waste product from the agricultural industry, presents both environmental challenges and potential for value-added applications. This study aimed to upcycle PP into a valuable skincare ingredient, particularly for developing an underarm care product. We prepared the PP extract by maceration and then evaluated its total phenolic content, antioxidant activity, and inhibitory effects on skin-related enzymes. The PP extract had a total phenolic content of 14.76 ± 0.67 mg gallic acid equivalent/g. It demonstrated potent antioxidant activity, capable of scavenging free2,2-diphenyl-1-picrylhydrazyl radicals with a half-maximal inhibitory concentration (IC50) of 340.03 ± 2.80 µg/mL, and it had a Trolox equivalent antioxidant capacity of 0.35 ± 0.04 mM Trolox/g. The extract exhibited moderate anti-tyrosinase activity with an IC50 of 1.03 ± 0.08 mg/mL, as determined by the dopachrome method but showed limited inhibition of elastase and collagenase, as determined by the spectrophotometric method. We developed eight underarm skincare serum formulations to demonstrate practical applications and assessed their properties. Formulation F8 emerged as the optimal choice based on physical, chemical, and microbiological stability tests when stored at 2-8 °C. These findings suggest that PP extract offers promising properties for skin health applications while providing a sustainable solution for agricultural waste management. Future research should explore long-term stability and involve clinical trials to develop a more comprehensive and safer product.
Keywords: Anti-oxidant activity, Aging, Extract, Waste reduction, Stability
Funding: This work was supported by Naresuan University (NU), Phitsanulok, Thailand and the National Science, Research and Innovation Fund (NSRF), Thailand (Grant No. R2567B048).
Citation: Saephan, K., Yanukul, C., Sripalakit, P., and Saraphanchotiwitthaya, A. 2025. Exploring the use of pineapple peel extract for underarm care: Antioxidant and anti-ageing properties for serum formulation and stability testing. Natural and Life Sciences Communications. 24(4): e2025061.
INTRODUCTION
Pineapple (Ananas comosus (L.) Merril) is a tropical fruit with over 100 cultivars, widely cultivated across Southeast Asia and other tropical regions (García-Villegas et al., 2022). The Smooth Cayenne or Batavia cultivar has been the leading variety since the 1880s (Beauman, 2005) and holds particular significance in Thailand, where production in 2023 reached 1.45 million tons. Notably, 70% to 80% of this pineapple is processed into export products such as canned pineapple and juice (MAC, 2023). The Food and Agriculture Organization estimates that pineapple waste products, including peel and core, account for 50–65% of the total fruit weight, which can pose environmental challenges (Hikal et al., 2022). Therefore, reusing or adding value to pineapple waste is a practical approach to tackling the environmental and economic impacts.
Pineapple peel (PP) is a rich source of bioactive compounds, including high amounts of alkaloids, flavonoids, and saponin, with moderate levels of phenolics, tannins, and terpenoids (Hikal et al., 2021, 2022). The diverse composition of PP, containing fiber, vitamins, enzymes, and essential oils, supports its potential applications in the food, pharmaceutical, textile, and cosmetic industries. Previous studies have highlighted the therapeutic potential of PP, including anti-cancer, antibacterial, and antifungal activities. Additionally, it is a source of antioxidant compounds that can be applied in cosmetics (Hikal et al., 2021). Notably, PP extracts are effective against tyrosinase, an enzyme involved in skin pigmentation processes (Musika et al., 2021). While PP extracts have been used in facial masks (Maulidia et al., 2020) and wound-healing products (Rocha et al., 2019), their application in underarm care formulations remains unexplored. We focused on pineapple peel (PP) because it is easily accessible and can be procured in large amounts, which makes it convenient for research purposes.
Underarm skin issues significantly impact self-confidence, particularly for women, due to axillary hyperpigmentation characterized by increased melanocytic activity and inflammatory responses (Castanedo-Cazares et al., 2013). Strategies to minimize dark underarm pigmentation include avoiding irritating products (Zirwas and Moennich, 2008), using brightening ingredients, and considering laser treatments. Skin pigmentation plays a crucial role in aging processes, with melanin overproduction potentially leading to disorders like freckles and melasma. Tyrosinase, a key enzyme in melanin generation, has become a focus for developing melanogenesis inhibitors, with natural compounds offering safer alternatives to synthetic skin-whitening agents (Pillaiyar et al., 2017; Zolghadri et al., 2019). Recent studies highlight phenolic compounds and cinnamic acid derivatives as promising anti-tyrosinase agents, presenting potential alternatives to traditional inhibitors like kojic acid and hydroquinone (Güven et al., 2023).
Skin aging is affected by external factors including ultraviolet (UV) radiation, air pollution, diet, alcohol, and smoking, as well as internal factors such as genetics and physiological changes. Skin aging can lead to aesthetic issues such as wrinkles, freckles, age spots, and reduced firmness, which can result in emotional, mental, and psychosocial challenges (Costa et al., 2022). Oxidative stress is involved in the aging process by contributing to an elevation in reactive oxygen species and a reduction in the levels of both enzymatic and non-enzymatic defenses (Papaccio et al., 2022). The antioxidative defense mechanism has a significant role in helping skin protect against the detrimental impacts of oxidative damage. Various plants and their phytochemicals have been found to have antioxidative properties (Costa et al., 2022; Michalak, 2022; Yeniçeri et al., 2024). Moreover, skin aging and loss of firmness are caused by collagenase, which degrades collagen, and elastase, which breaks down elastin. Interventions targeting these enzymatic pathways have the potential to comprehensively address underarm skincare issues.
In recent years, the cosmetics industry has shifted toward using sustainable and ecofriendly ingredients sourced from agricultural by-products. In the present study, we focused on PP waste, which contains various bioactive compounds that have numerous biological properties. We aimed to explore the cosmetic benefits of PP extract, particularly its antioxidant, anti-tyrosinase, anti-collagenase, and anti-elastase properties. Furthermore, we formulated an underarm skincare serum containing PP extract and evaluated its stability.
MATERIALS AND METHODS
Materials
The pineapple peel (Ananas comosus L. Merril) of the Smooth Cayenne variety was obtained from a local fruit shop in Phitsanulok, Thailand. Folin-Ciocalteu reagent, 6-hydroxy-2,5,7,8-tetramethyl chroman-2-carboxylic acid (Trolox), 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,4,6–tris (2-pyridyl)-s-triazine (TPTZ), N-[3-(2-furyl) acryloyl]-Leu-Gly-Pro-Ala (FALGPA), N-succinyl-Ala-Ala-Ala-p-nitroanilide (SANA), L-Dopa, tyrosinase, porcine pancreatic elastase (PP), collagenase, gallic acid, ascorbic acid, kojic acid, epigallocatechin gallate (EGCG) were purchased from Sigma Chemical Company (St. Louis, MO, USA). Iron (III) chloride hexahydrate, ferrous (II) sulphate, Tris-HCl buffer, and Tricine buffer were purchased from Merck (Darmstadt, Germany). The ingredients used in the formulation such as Carbopol U21, sodium carboxymethyl cellulose, butylene glycol, Microcare PHC, sodium hyaluronate (Double hyaluron liquid, HA 5%), lemon peel bioferment, and encapsulated menthol were purchased from Chemipan Corporation Co., Ltd. (Bangkok, Thailand).
Preparation of PP extract
PP was washed and dried completely in a hot oven (50°C). Then, the dried PP was pulverized into a powder with a blender. Thirty-five grams of dried powder were macerated in 200 mL of a water-ethanol solution (1:1 ratio) for 2 h. Sonication was performed every 30 min for 5 min at each time in a water bath. The mixture was filtered and dried completely using a rotary evaporator. The yield of the extract was determined by calculating the dry weight of the extract per dry plant weight. The extract was stored in a sealed container in the refrigerator until tested. For further study, solutions of various concentrations of extracts were prepared. Dimethyl sulfoxide was used as a solvent; its maximum concentration was 1% (v/v).
Determination of total phenolic content (TPC)
The TPC of the PP extract was assessed using the Folin-Ciocalteu method (Singleton et al., 1999). Twenty microliters of the extract solution (1 mg/mL) was mixed with 100 μL of 10% (v/v) Folin-Ciocalteu reagent in a 96-well plate. Subsequently, 80 μL of 7.5% (w/v) sodium carbonate was added and mixed thoroughly. After incubation for 30 min, the absorbance at 760 nm was measured using a microplate reader (Eon, Bio-Tek, U.S.A). The TPC of the extract was calculated using linear regression based on a standard curve of gallic acid (50-150 µg/mL) and is expressed as milligrams of gallic acid equivalent (GAE) per gram of dry extract.
2,2-Diphenyl-1-picrylhydrazyl (DPPH) scavenging assay
The antioxidant activity of the extract was tested by evaluating its capability to scavenge DPPH radicals, following a published protocol (Blois, 1958) with some modifications. The extract solution (0.05-2 mg/mL) and 0.2 M DPPH were prepared in ethanol. Subsequently, 50 μL of the extract solution and 100 μL of the DPPH solution were added to the wells of a 96-well plate. After shaking, the mixture was incubated in the dark at room temperature for 30 min. The absorbance of the reaction mixture was determined at 517 nm using a 96-well microplate reader (Eon, Bio-Tek, USA). The percentage inhibition of DPPH by the extract was calculated as follows:
% Inhibition = [(Abscontrol − Abssample) / Abscontrol] × 100
where Abscontrol is the absorbance of the control sample and Abssample is the absorbance of the test sample. A dose-response curve was plotted using the percentage of inhibition and concentrations. Linear regression was utilized to determine the half-maximal inhibitory concentration (IC50)—that is, the concentration needed to inhibit 50% of the DPPH radicals. Compared with the standard Trolox (10-50 μg/mL), the Trolox equivalent antioxidant capacity (TEAC) was determined as millimoles of Trolox equivalents (TE) per gram of sample.
Ferric reducing antioxidant power (FRAP) assay
The antioxidant activity of the extract was also determined based on the Ferric-reducing capability of the extract through the colorimetric method as described previously (Benzie and Strain, 1996), with some modifications. Extract solutions were prepared in acetate buffer (0.2 mg/mL). Three hundred micromolar acetate buffer (pH 3.6), 20 mM FeCl3, and 10 mM 2,4,6-tris (2-pyridyl)-s-triazine (TPTZ) in a 10:1:1 (v/v/v) ratio were mixed to produce the FRAP reagent. Thirty microliters of the tested solutions were combined with 270 μL of the FRAP reagent and then incubated in the dark at room temperature for 30 min. The absorbance of the extract was measured at 595 nm using a 96-well microplate reader to determine the reducing ability of ferric iron (Fe3+) to ferrous iron (Fe2+). Iron(II) sulfate (FeSO4; 20-100 μM) was used to generate a calibration curve. The FRAP of the PP extract was calculated using linear regression based on a standard curve of FeSO4 and is expressed in millimoles of ferrous equivalents per gram of extract.
Anti-tyrosinase activity assay
The tyrosinase inhibition property of the extract was assessed using the dopachrome assay (Zhang et al., 2009), with some modifications. The extract was diluted in phosphate buffer (pH 6.8) at 0.05-2 mg/mL. The, 40 µL of this sample was combined with 40 μL of the substrate (0.8 mM L-DOPA) and 80 μL of phosphate buffer. The mixture was incubated in the dark at room temperature for 10 min. Afterwards, 40 μL of tyrosinase in phosphate buffer (125 units/mL) was introduced. The mixture was incubated in the dark at room temperature for 20 min. Kojic acid (20-100 μg/mL) was used as a positive control. The absorbance of the mixture was determined at 492 nm using a 96-well microplate reader. The percentage of tyrosinase activity inhibition was determined using the following equation:
% Inhibition = [(Abscontrol – Abssample) / Abscontrol] × 100
A dose-response curve was plotted using the percentage of inhibition and concentrations. Linear regression was utilized to determine the IC50.
Anti-elastase activity assay
The elastase inhibition property of the extract was evaluated using a spectrophotometric method, similarly to a previous study (Laothaweerungsawat et al., 2020), with some modifications. The extract solution (50-400 μg/mL), porcine pancreatic elastase at 5 μg/mL, and the substrate—N-succinyl-Ala-Ala-Ala-p-nitroanilide (SANA) at 0.18 mg/mL—were prepared by dissolving in 200 mM Tris-HCl buffer (pH 8.0). One hundred sixty microliters of the extract solution was combined with 20 μL of elastase in a 96-well plate and incubated at room temperature for 20 min. Next, 20 μL of SANA was added to each well, and the plate was incubated for 60 min. Epigallocatechin gallate (EGCG; 20-200 μg/mL) was used as a positive control. The absorbance was read at 410 nm with a 96-well microplate reader. The percentage of anti-elastase activity was calculated using the following equation:
% Inhibition = [(Abscontrol − Abssample) / Abscontrol] × 100
A dose-response curve was plotted using the percentage of inhibition and concentrations. Linear regression was utilized to determine the IC50.
Anti-collagenase activity assay
The anti-collagenase activity of the extract was assessed with a spectrophotometric method (Thring et al., 2009), with some modifications. The extract (50-400 μg/mL), collagenase (0.25 mg/mL), and the substrate, namely 0.8 mM N-[3-(2-furyl) acryloyl]-Leu-Gly-Pro-Ala (FALGPA), were prepared in 50 mM Tricine buffer (pH 7.5). In a 96-well plate, 160 μL of the extract was combined with 20 μL of collagenase and incubated at room temperature for 20 min. Subsequently, 20 μL of FALGPA was added to initiate the reaction. EGCG (20-200 μg/mL) was utilized as a positive control. The absorbance of the reaction mixture was measured at 340 nm using a microplate reader. The percentage of anti-collagenase activity was calculated using the following equation:
% Inhibition = [(Abscontrol − Abssample) / Abscontrol] × 100
A dose-response curve was plotted using the percentage of inhibition and concentrations. Linear regression was utilized to determine the IC50.
Formulation of a serum containing PP extract
Eight formulations of serum containing PP extract (F1-F8) were prepared by varying the concentration of the extract as well as the type and concentration of the thickening agent (sodium carboxymethylcellulose [SCMC], carbomer, or Carbopol Ultrez U21) and moisturizing agent (glycerin and butylene glycol). First, the thickening agents were dispersed in distilled water to form a mucilage (part 1). Then, sodium hyaluronate, glycerin, lemon peel ferment, and menthol were dissolved in distilled water and mixed well (part 2). The PP extract was dissolved in butylene glycol (part 3). Parts 1 and 2 were mixed, and then part 3 was added and stirred to achieve a homogeneous mixture. The preservative and fragrance were added to the mixture and mixed well. If necessary, 5% (w/w) citric acid was added slowly to adjust the pH to 5.5. All serum formulations were evaluated for physical quality such as color, precipitation, homogeneity, viscosity, spreadability, and flowability. Additionally, the pH was measured and microbiological stability was evaluated. The best formulation (F8) was chosen for stability testing.
Stability testing
Stability testing was conducted by storing the F8 serum in a glass bottle at 2-8°C, room temperature (27 ± 2°C), or 45°C for 3 months. Additionally, the serum was subjected to an accelerated heating-cooling cycle, specifically six cycles where the temperature alternated between 4°C (for 24 h) and 45°C (for 24 h; Saraphanchotiwitthaya et al., 2023). At the end of the storage period, the physical and chemical properties and microbiological contamination of the stored F8 serum were evaluated and compared with freshly prepared F8 serum.
Physical characterization of the serum
The color, clarity, and precipitation of the serum containing PP extract were determined by observation. The homogeneity was assessed by spreading 0.5 g of the serum on a clean, dry glass slide, covered with a coverslip, and observed under a light microscope. A serum with a uniform distribution and without lumps and aggregates was defined as being homogenous. In addition, the viscosity of the serum was determined with a Brookfield DV-III viscometer (AMETEK, USA) at 20-100 rpm, using cone spindle type CP40. The spreadability of the serum was evaluated using the parallel plate technique (Cirri et al., 2012). The serum (0.1 g) was placed on a glass plate marked with a circular area (1 cm in diameter) and then covered with another glass plate and a 200-g weight. The test sample was allowed to settle for 5 min, after which time the spread diameter (in cm) on the glass was measured. Finally, a preliminary flowability test was conducted by dropping 0.1 g of the sample onto a glass slide inclined at a 45° angle. The time it took for the sample to flow along the 7.5-cm length of the glass slide was recorded. The flow rate was calculated by dividing the distance by the time (cm/s).
Measurement of pH
The serum (2 mL) was stirred gently to achieve uniformity. The pH was measured with a digital pH meter (SevenCompact Duo S213-meter, Mettler-Toledo International Inc., USA) with an electrode (pH Sensor InLab® Solids Pro-ISM, Mettler-Toledo International Inc.) as the indicator. The probe was immersed in the sample to measure the pH three times, and the average was calculated.
Microbial contamination test
Microbiological stability was assessed using the spread plate technique to determine the total viable count. A serum sample (0.1 g) was spread on the nutrient agar surface of a Petri dish, followed by incubation at 37°C for 48 h. Then, the numbers of colonies were counted and are expressed are colony-forming units (CFU) per gram. A contaminated sample had a count of > 1,000 CFU/g (Huang et al., 2024).
Statistical analysis
The experiments were carried out in triplicate (n = 3). One-way analysis of variance was used to determine significant differences (P < 0.05). The results are presented as mean ± standard deviation (SD).
RESULTS
Preparation of PP extract and total phenolic content determination
The PP was macerated in a water-ethanol solution (1:1, v/v) to obtain the PP extract. The yield was 24.03% ± 2.24% relative to the dry material (w/w). The resulting extract was viscous and deep brown in color. The PP extract had a TPC of 14.76 ± 0.56 mg GAE/g extract.
Antioxidant activity assay
Table 1 presents the details regarding the antioxidant activity of the PP extract. It presented an IC50 of 340.03 ± 2.80 µg/mL to scavenge DPPH free radicals. The TEAC was 0.35 ± 0.04 mM TE/g extract. Finally, the FRAP was 9.39 ± 0.28 μmol Fe2+/g extract.
Aging-related enzymes inhibitory activity assay
We used the dopachrome assay to evaluate the anti-tyrosinase activity of the PP extract. It presented an IC50 of 1.03 mg/mL, indicating strong tyrosinase inhibition. Of note, this value is 13 times lower than that of kojic acid, a well-known tyrosinase inhibitor. The PP extract mildly inhibited elastase, with an IC50 of 7.89 ± 0.42 mg/mL, which is weaker than that of EGCG. The PP extract inhibited collagenase, with an IC50 of 9.11 ± 0.42 mg/mL. It was less potent than EGCG. The results of aging-related enzyme inhibitory activity are presented in Table 2.
Table 1. Antioxidant activity of the PP extract.
|
Test sample |
Antioxidant activity |
||
|
DPPH assay |
FRAP assaya
|
||
|
IC50 (μg/mL) |
TEAC (mM Trolox/g extract) |
||
|
PP extract |
340.03 ± 2.80 |
0.35 ± 0.04 |
9.39 ± 0.28 |
|
Ascorbic acid |
10.70 ± 0.13 |
11.05 ± 0.62 |
30.06 ± 2.64 |
|
Trolox |
29.58 ± 0.14 |
- |
38.77 ± 0.71 |
Note: aThe data are expressed as μmol Fe2+/g for the PP extract, and μmol Fe2+/mg for ascorbic acid and Trolox.
Table 2. Aging-related enzyme inhibitory activity of the PP extract.
|
Test sample |
IC50 (mg/mL) |
||
|
Anti-tyrosinase activity |
Anti-elastase activity |
Anti-collagenase activity |
|
|
PP extract |
1.03 ± 0.08 |
7.89 ± 0.42 |
9.11 ± 0.49 |
|
Kojic acid |
0.08 ± 0.01 |
- |
- |
|
Ascorbic acid |
0.26 ± 0.03 |
- |
- |
|
EGCG |
- |
0.12 ± 0.01 |
0.50 ± 0.01 |
Formulation of underarm serum containing PP extract
We produced eight formulations of a cosmetic underarm serum containing PP extract (F1-F8) for skin moisturizing, nourishing, and lightening. F1-F3 and F4-F7 included 2% and 1% (w/w) PP extract, respectively. The proportion of Carbopol 940, Carbomer Ultrez 21, and SCMC as a thickening agent varied among the formulations (0.5% and 1%, w/w). In addition, we added glycerin and butylene glycol so that the serum would have a moisturizing effect. Butylene glycol was present as a solvent for the extract. Finally, we included a mixture of phenoxyethanol and chlorphenesin (Microcare PHC) as preservatives. The final pH of each formulation was adjusted to 5.5.
The formulation containing 1% (w/w) PP extract and 1% (w/w) SCMC exhibited satisfactory viscosity, clarity, and a medium shade of orange color, making it suitable for topical application. We developed this formulation into the F8 serum by adding two skin-nourishing components, lemon peel bioferment and sodium hyaluronate, as well as encapsulated menthol to produce a long-lasting cooling effect and to reduce the sticky sensation. All ingredients could be mixed uniformly without precipitation. Of note, adding the extract solution to the mucilage of the thickeners resulted in decreased viscosity and slightly decreased clarity of the preparations (Table 3). The F8 serum was clear, orange-gold in color, and had a mild pineapple and menthol scent. The pH was 5.49 ± 0.02. It had a low viscosity (30.95 ± 1.78 cP), moderate spreadability (10.17 ± 0.58 cm), and flowed easily (1.22 ± 0.07 cm/s). The serum was homogenous, without lumps and aggregates. F8 was subjected to the stability testing.
Stability testing
The F8 serum was stored at 2-8 °C, room temperature (27 ± 2°C), or 45°C for 3 months, and also subjected to a heating-cooling cycle. Figure 1 shows the appearance of the F8 serum under these various conditions. Under the heating-cooling cycle, the odor and clarity of F8 remained unchanged, and there was no precipitation or microbial growth. However, there was a slight darkening in color, a minor decrease in pH, and a reduction in viscosity. After storage for 3 months, the odor and clarity of the F8 serum remained consistent, with no phase separation or precipitation. The color of the F8 serum stored at 2-8 °C and room temperature remained unchanged, maintaining an orange-gold color. However, when stored at 45°C, the F8 serum darkened to a yellowish-brown color.
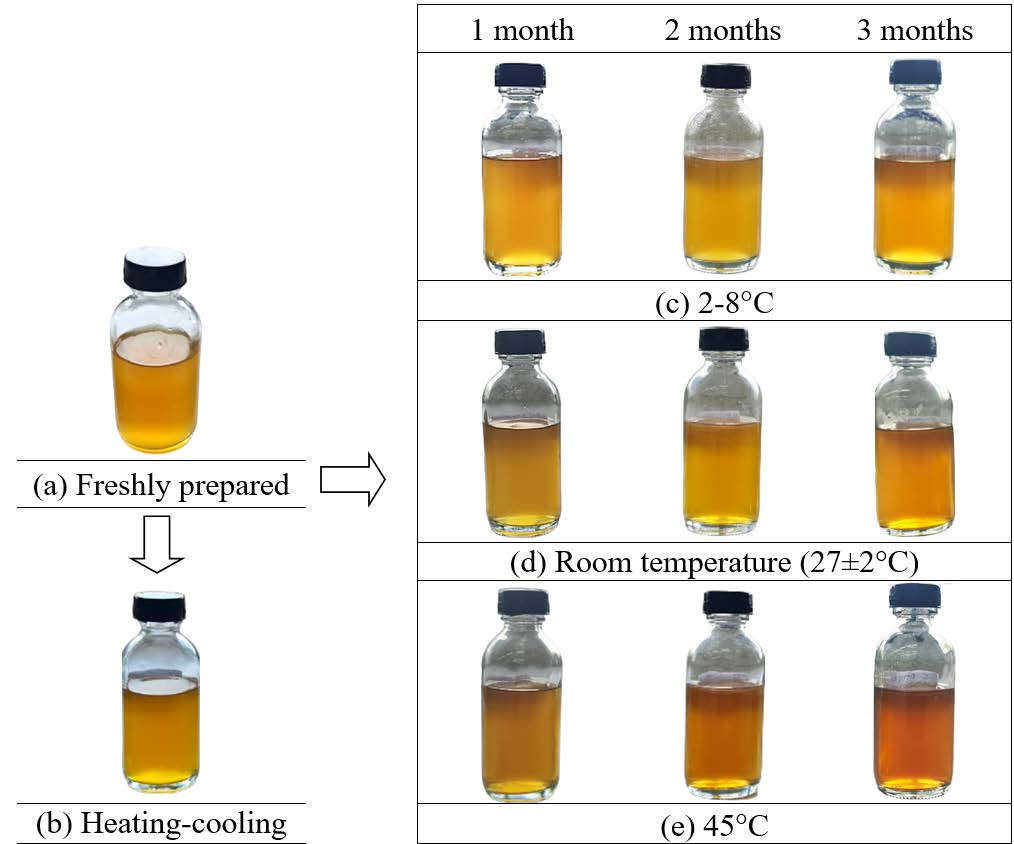
Figure 1. Physical appearance of the F8 serum: freshly prepared (a); subjected to the heating-cooling cycling (b); and stored for 3 months at 2-8 °C (c), room temperature (27 ± 2°C) (d), and 45°C (e).
The viscosity of the F8 serum decreased over time, with the most significant reduction observed at 45°C. It exhibited an initial pH of 5.5. While the pH decreased slightly during storage for 3 months at all temperatures, storage at 2-8 °C led to the most stable pH. There was no microbial contamination. The stability assessment of the F8 serum is shown in Figure 2 and Table 3.
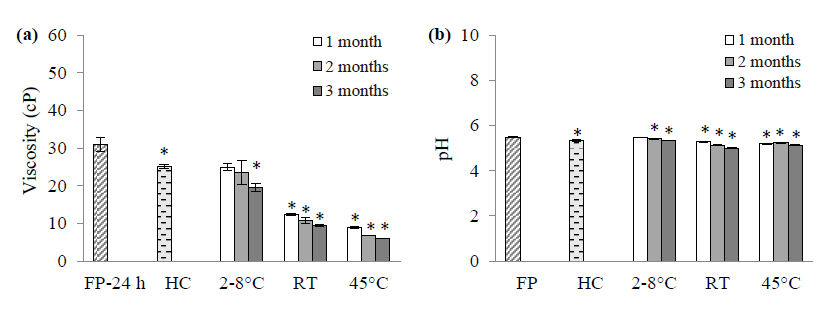
Figure 2. Viscosity (a) and pH (b) of the F8 serum: freshly prepared (FP); subjected to the heating-cooling cycling (HC); and stored for 3 months at 2-8 °C, room temperature (27 ± 2°C) and 45°C. *P < 0.05 compared with FP (n = 3).
Table 3. Stability assessment of the F8 serum.
|
Characteristic |
Freshly prepared formulation |
Heating and cooling cycle |
Formulation stored for 3 months |
||
|
2-8 °C |
Room temperature (27 ± 2°C) |
45°C |
|||
|
Color |
Orange gold |
Dark orange gold |
Orange gold |
Orange gold |
Orange-brown |
|
Odor |
Mild pineapple scent with light menthol |
Mild pineapple scent with light menthol (no change from the freshly prepared formulation) |
|||
|
Precipitation |
No |
No precipitation (no change from the freshly prepared formulation) |
|||
|
Viscosity (cP)a |
30.95 ± 1.78 |
25.07 ± 0.58* |
19.56 ± 1.09* |
9.48 ± 0.33* |
6.00 ± 0.09* |
|
pH |
5.49 ± 0.02 |
5.33 ± 0.04* |
5.33 ± 0.02* |
5.00 ± 0.02* |
5.13 ± 0.01* |
|
Microbial growth |
No |
No microbial growth (no change from the freshly prepared formulation) |
|||
Note: The data are presented as the mean ± standard deviation of triplicate experiments, *P < 0.05 compared with the freshly prepared formulation. aViscosity was measured at 24 h; low < 100, moderate 100-1000, high > 1,000 cP.
DISCUSSION
We prepared a PP extract by maceration in a water-ethanol solution, resulting in a rather high yield. We used sonication to enhance the extraction efficacy and 50% (v/v) ethanol for extraction due to safety concerns related to cosmetic applications. A previous study showed that 50% (v/v) ethanol is an effective solvent for plant extraction compared with other solvents (Maneechai et al., 2023). The ethanol in this mixture facilitates solvent penetration into the plant tissue, thus disrupting the cell wall structure and enhancing the extraction efficiency (Zuorro et al., 2019).
The TPC of our PP extract (14.76 ± 0.56 mg GAE/g extract) is higher than the findings from the previous studies. Lourenço et al. (2021) produced a PP extract using 80% ethanol and obtained a TPC of 11.10 ± 0.01 mg GAE/g dry extract, while de Oliveira et al. (2009) stated a TPC of 9.1 mg GAE/g dry extract from different pineapple waste extracts. However, various factors such as the pineapple variety, plant extraction methods, and solvents can result in variations in the TPC. For example, Li et al. (2014) extracted Bali PP using methanol reflux and achieved a TPC of 31.98 mg GAE/g extract. Furthermore, Polanía et al. (2022) utilized ultrasonication to extract golden PP fermented with Rhizopus oryzae, leading to a much higher TPC of 866.26 mg GAE/g extract.
We investigated several biological activities related to cosmetics applications, including anti-oxidant, anti-tyrosinase, anti-elastase and anti-collagenase activities. The assessment of antioxidant activity involves various assays that measure the ability of a solution to scavenge free radicals, including DPPH, catalase, superoxide dismutase, 2,2'-azinobis(3-ethylbenzothiazoline-6-sulfonic acid), and glutathione peroxidase. We evaluated the ability of our PP extract to reduce the DPPH radical (purple colored). This process involves the donation of a hydrogen atom to the DPPH radical, leading to the production of a reduced form of 1,1-diphenyl-2-picryl hydrazine (yellow colored), which can be quantified using spectrophotometry (Costa et al., 2022). We also evaluate the FRAP, which involves reducing ferric-tripyridyl triazine (Fe3+-TPTZ) to blue-colored ferrous-tripyridyl triazine (Fe2+-TPTZ; Benzie and Strain, 1996). Our PP extract inhibited DPPH free radicals and reduced Fe3+. The DPPH radical scavenging activity of our PP extract shows moderate potency when compared to previous studies. Azizan et al. (2020) produced a PP extract using 100% ethanol and obtained an IC50 of 353.10 ± 21.34 μg/mL. However, other studies have reported variable antioxidant effects of PP. Li et al. (2014) reported an IC50 of 1.13 ± 0.03 mg/mL for a Bali PP methanolic extract. Saraswaty et al. (2017) used varying proportions of ethanol and water to produce PP extracts and found an IC50 of 0.8-1.3 mg/mL for dried extracts and 0.25-0.59 mg/mL for fresh extracts. Additionally, Lourenço et al. (2021) used 80% ethanol to produce a PP extract and demonstrated antioxidant activity of 91.79 ± 1.98 and 174.50 ± 9.98 µmol Trolox/g extract using the DPPH and FRAP assays, respectively. The differences in these findings may be attributed to variations in pineapple sources, varieties, extraction techniques, and assay methods. Furthermore, the potency of the antioxidant activity of the extract may vary depending on the assays used for the measurements.
The production of melanin depends on the enzyme tyrosinase. This enzyme converts L-DOPA into dopachrome—an intermediate in the melanin pathway. We used a dopachrome assay to evaluate anti-tyrosinase activity, using L-DOPA as a substrate. The reddish-brown color of dopachrome, resulting from the oxidation of L-DOPA, can be quantified using a spectrophotometer. Our PP extract exhibited significant tyrosinase inhibitory activity, similar to a previous study (Musika et al., 2021). Their PP extract inhibited tyrosinase by 56.93% ± 5.46% when used at a concentration of 0.5 mg/mL. However, differences in pineapple sources and extraction techniques may impact the observed anti-tyrosinase activity.
Phenolic compounds, particularly polyphenols, are essential antioxidants in cosmetics due to their significant benefits for skin health. These compounds are renowned for their antioxidant, anti-inflammatory, and anti-aging properties, making them invaluable in cosmetic formulations. Vrianty et al. (2019) reported the presence of luteolin in both PP and pineapple core extracts, suggesting its potential contribution to its antioxidant and anti-tyrosinase properties. Additionally, a phenolic-enriched fraction from Arbutus unedo demonstrated antioxidant and anti-tyrosinase activities, further supporting their potential as valuable components in cosmeceutical products (Habachi et al., 2022).
Elastase breaks down elastin, a protein responsible for maintaining skin elasticity and resilience. The prevention of skin firmness loss and a reduction in the appearance of wrinkles can be achieved by inhibiting elastase activity. We evaluated the anti-elastase activity of our PP extract by using an assay employing SANA as a substrate to react with porcine pancreatic elastase. This enzyme can cleave SANA, producing N-Succinyl-(Ala)3 and p-nitroaniline. The amount of yellow-colored p-nitroaniline produced can be measured using a spectrophotometric method and compared with EGCG as a positive control. Our PP extract mildly inhibited elastase, suggesting that it could be used as a co-ingredient in skincare products aimed at maintaining skin elasticity and youthfulness. There has been limited investigation regarding the elastase inhibitory activity of PP extract, with only a report of activity from a pineapple extract (IC50 = 16.79 ± 1.62 μg/mL; Wijaya et al., 2020). However, the study of the anti-elastase activity of various Thai plants revealed a marked variation in their inhibitory activity, ranging from 1.33% to 88.31% (0.5 mg/mL); most of them exhibited either no or weak inhibition of elastase activity (Chaikhong et al., 2022). Although PP extract may not be very potent, requiring a higher concentration to exert a substantial inhibitory effect on elastase, the addition of 1% (w/w) PP extract to the serum is higher than the obtained IC50 for the anti-elastase activity assay (0.79% w/w). Thus, PP extract could contribute to maintain skin elasticity, to delay the appearance of wrinkles, and to promote a youthful appearance when used in a sufficient quantity and consistently.
Collagenase breaks down collagen, an essential component for skin elasticity and firmness. Inhibition of collagenase activity can potentially prevent premature skin aging. We evaluated the anti-collagenase activity of our PP extract by using FALGPA as a collagenase substrate. We measured the resulting product spectrophotometrically and compared it with EGCG as a positive control. The PP extract inhibited collagenase, indicating that it has mild anti-wrinkle activity and might be useful as a supplement with other anti-wrinkle ingredients to produce an optimal effect. There has been limited evaluation of the anti-collagenase activity of PP. The authors of a previous related study showed that freeze-dried pineapple juice powder and triple-coated pineapple granules exhibited high matrix metalloproteinase 2 (MMP-2) inhibitory activity, specifically 50.74% and 48.48%, respectively (Ruksiriwanich et al., 2019). MMP-2 may have a role as an interstitial collagenase in vivo and be sufficient for the degradation of both the basement membrane collagen IV and connective tissue collagens (Aimes et al., 1995). Therefore, the collagenase inhibitory activity of PP might be ascribed to the residual fruit flesh and juice that contaminated the peel samples, in addition to the active components in the PP responsible for this activity.
We prepared a series of serum formulations (F1-F8) that included our PP extract to address skin moisturizing, nourishing, and lightening. The formulations varied in their PP extract content and thickening agent types and concentrations. Among these, we selected the F8 serum—containing 1% (w/w) PP extract, 1% (w/w) SCMC as a thickening agent, and lemon peel bioferment and sodium hyaluronate for skin nourishing and to improve skin whitening and elasticity. We also added encapsulated menthol to produce a long-lasting cooling effect and to reduce the sticky sensation. PP extract of 1%w/w was added to the formulation, which was 29.4 and 9.7 times higher than the IC50 values by anti-DPPH and anti-tyrosinase assays, respectively. These concentrations indicate the potential of the formulation to delay skin aging and to promote skin brightening when applied consistently over time. The F8 serum exhibits desirable characteristics and properties, making it well-suited for underarm application. Its light texture, spreadability, non-sticky consistency, and appropriate viscosity contribute to a comfortable user experience. Additionally, the inclusion of PP extract further enhances its suitability for underarm care, providing potential benefits such as skin brightening and antioxidant protection.
The stability test results indicated that the F8 serum should be stored at 2-8 °C to minimize changes to the color, viscosity, and pH. Notably, the incorporation of the PP extract significantly reduced the viscosity of the F8 serum (measured 24 h after formulation to allow an adequate swelling time) compared with the base serum, suggesting an interaction between the extract and SCMC that disrupted the polymer’s structure. The viscosity of SCMC is influenced by several factors, including the swelling time and pH. SCMC gradually degrades over time, leading to reduced viscosity (Wang et al., 2022), elevated temperatures, as observed with the heating-cooling cycle and storage at 45°C, further decreased SCMC viscosity. While the formulation was designed to have a lightweight, non-sticky feel, the observed viscosity reduction may not substantially impact the user experience. Although there was a slight pH decrease at all storage temperatures over 3 months, the pH remained within the acceptable range for skin health. There was no microbial growth due to the presence of appropriate preservatives. However, additional microbiological testing is necessary to comply with the International Standards of Microbiological Quality in Cosmetics.
CONCLUSION
The valorization and recycling of PP can help reduce waste and add value to this agricultural byproduct. Our findings indicate that PP extract exhibits antioxidant and anti-tyrosinase activity in vitro, and exerts a subtle anti-wrinkle effect. This suggests that PP extract is a promising ingredient for skin care products, including underarm care. The F8 serum, which incorporates PP extract into a lightweight formulation with a non-greasy texture, provides a refreshing and cooling sensation suitable for daily underarm care. To maintain its stability, the product should be stored at 2-8 °C, avoiding direct exposure to heat. This study contributes to environmental sustainability by minimizing waste and supporting self-reliant community-based economies. Future research should explore additional thickeners, conduct long-term stability studies, and initiate human clinical trials to develop a comprehensive and safe product prototype.
ACKNOWLEDGMENTS
The authors thank the Faculty of Pharmaceutical Sciences, Naresuan University for providing instruments.
AUTHOR CONTRIBUTIONS
Kittima Saephan and Chutikan Yanukul contributed to the research design, conducted experiments, and collected primary data. Pattana Sripalakit provided supervision for data analysis. Aurasorn Saraphanchotiwitthaya supervised the project and drafted the manuscript. All authors reviewed and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Aimes, R.T. and Quigley, J.P. 1995. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. Journal of Biological Chemistry. 270(11): 5872-5876.
Azizan, A., Lee, A.X., Abdul Hamid, N.A., Maulidiani, M., Mediani, A., Abdul Ghafar, S.Z., Zolkeflee, N.K.Z., and Abas, F. 2020. Potentially bioactive metabolites from pineapple waste extracts and their antioxidant and α-glucosidase inhibitory activities by 1H NMR. Foods. 9(2): 173.
Beauman F. 2005. The pineapple: King of fruits. London: Chatto & Windus. p. 247.
Benzie, I.F.F. and Strain, J.J. 1996. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Analytical Biochemistry. 239: 70–76.
Blois, M.S. 1958. Antioxidant determinations by the use of a stable free radical. Nature. 181: 1199–1200.
Castanedo-Cazares, J.P., Lárraga-Piñones, G., Ehnis-Pérez, A., Fuentes-Ahumada, C., Oros-Ovalle, C., Smoller, B.R., and Torres-Álvarez, B. 2013. Topical niacinamide 4% and desonide 0.05% for treatment of axillary hyperpigmentation: A randomized, double-blind, placebo-controlled study. Clinical, Cosmetic and Investigational Dermatology. 6: 29-36.
Chaikhong, K., Chumpolphant, S., Rangsinth, P., Sillapachaiyaporn, C., Chuchawankul, S., Tencomnao, T., and Prasansuklab, A. 2022. Antioxidant and anti-skin ageing potential of selected Thai plants: In vitro evaluation and in silico target prediction. Plants (Basel). 12(1): 65.
Cirri, M., Bragagni, M., Mennini, N., and Mura, P. 2012. Development of a new delivery system consisting in “drug–in cyclodextrin–in nanostructured lipid carriers” for ketoprofen topical delivery. European Journal of Pharmaceutics and Biopharmaceutics. 80(1): 46-53.
Costa, E.F., Magalhães, W.V., and Di Stasi, L.C. 2022. Recent advances in herbal-derived products with skin anti-ageing properties and cosmetic applications. Molecules. 27: 7518.
de Oliveira, A.C., Valentim, I.B., Silva, C.A., Bechara, E.J.H., Barros, M.P., Mano, C.M., and Goulart, M.O.F. 2009. Total phenolic content and free radical scavenging activities of methanolic extract powders of tropical fruit peels. Food Chemistry. 115: 469–475.
García-Villegas, A., Rojas-García, A., Villegas-Aguilar, Md.C., Fernández-Moreno, P., Fernández-Ochoa, Á., Cádiz-Gurrea, Mdl.L., Arráez-Román, D., and Segura-Carretero, A. 2022. Cosmeceutical potential of major tropical and subtropical fruit by-products for a sustainable revalorization. Antioxidants. 11(2): 203.
Güven, Z.B., Saracoglu, I., Nagatsu, A., Yilmaz, M.A., Basaran, A.A. 2023. Anti-tyrosinase and antimelanogenic effect of cinnamic acid derivatives from Prunus mahaleb L.: Phenolic composition, isolation, identification and inhibitory activity. Journal of Ethnopharmacology. 310: 116378.
Habachi, E., Rebey, I.B., Dakhlaoui, S., Hammami, M., Sawsen, S., Msaada, K., Merah, O., and Bourgou, S. 2022. Arbutus unedo: Innovative source of antioxidant, anti-inflammatory and anti-tyrosinase phenolics for novel cosmeceuticals. Cosmetics. 9(6): 143.
Hikal, W.M., Mahmoud, A.A., Said-Al Ahl, H.A.H., Bratovcic, A., Tkachenko, K.G., Kačániová, M., and Rodriguez, R.M. 2021. Pineapple (Ananas comosus L. Merr.), waste streams, characterisation and valorisation: An overview. Open Journal of Ecology. 11: 610-634.
Hikal, W.M., Said-Al Ahl, H.A.H., Tkachenko, K.G., Bratovcic, A., Szczepanek, M., and Rodriguez, R.M. 2022. Sustainable and environmentally friendly essential oils extracted from pineapple waste. Biointerface Research in Applied Chemistry. 12: 6833-6844.
Huang, J., Hitchins, A.D., Tran, T.T., and McCarron, J.E. 2024. Bacteriological Analytical Manual Chapter 23: Methods for Cosmetics. [Internet] U.S. Food and Drug Administration. Available from: https://www.fda.gov/food/laboratory-methods-food/bam-chapter-23-methods-cosmetics [Accessed 2024 Nov 2].
Laothaweerungsawat, N., Sirithunyalug, J., and Chaiyana, W. 2020. Chemical compositions and anti-skin-ageing activities of Origanum vulgare L. essential oil from tropical and Mediterranean region. Molecules. 25: 1101.
Li, T., Shen, P., Liu, W., Liu, C., Liang, R., Yan, N., and Chen, J. 2014. Major polyphenolics in pineapple peels and their antioxidant interactions. International Journal of Food Properties. 17: 1805-1817.
Lourenço, S.C., Campos, D.A., Gómez-García, R., Pintado, M., Oliveira, M.C., Santos, D.I., Corrêa-Filho, J.C., Moldão-Martins, M., and Alves, V.D. 2021. Optimization of natural antioxidants extraction from pineapple peel and their stabilization by spray drying. Foods. 10(6): 1255.
Maulidia, M.L., Darsono, F.L., and Wijaya, S. 2020. Effect of pineapple (Ananas comosus) peel viscous extract concentrations in the clay face mask preparation. Asian Journal of Pharmacognosy. 4(1): 31-42.
Michalak, M. 2022. Plant-derived antioxidants: Significance in skin health and the ageing process. International Journal of Molecular Sciences. 23: 585.
Ministry of Agriculture and Cooperatives. 2024. MAC, Thailand, has established a working group to develop a pineapple plant law, supporting stable and sustainable careers [Internet]. Bangkok: Ministry of Agriculture and Cooperatives. Available from: https://www.moac.go.th/news-preview-451491791899 (Accessed 2024 Nov 2).
Musika, S., Pokratok, N., Pliankratoke, J., Khongla, C., Kupradit, C., and Ranok, A., and Mangkalanan, S. 2021. Antioxidant, antityrosinase and antibacterial activities of fruit peel extracts. International Journal of Agricultural Technology. 17(4): 1447-1460.
Papaccio, F., D’Arino, A., Caputo, S., and Bellei, B. 2022. Focus on the contribution of oxidative stress in skin ageing. Antioxidants (Basel). 11: 1121.
Pillaiyar, T., Manickam, M., and Namasivayam, V. 2017. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. Journal of Enzyme Inhibition and Medicinal Chemistry. 32: 403-425.
Polanía, A.M., Londoño, L., Ramírez, C., and Bolívar, G. 2022. Influence of ultrasound application in fermented pineapple peel on total phenolic content and antioxidant activity. Fermentation. 8: 314.
Rocha, D.A., Queiroz, E., Botelho, L.N.S., Fráguas, R.M., Mendes dos Santos, C., Abreu, C.M.P., and de Sousa, R.V. 2019. In vitro enzyme activity and in vivo healing activity of the protein extract from pineapple peel. Acta Scientiarum. Biological Sciences. 41: e41466.
Ruksiriwanich, W., Chaitep, T., Lertwicha, R., and Jantrawut, P. 2019. Disease prevention functional foods from the high potential MMP-2 inhibition activity of pineapple juice granule. International Journal of Pharmacy and Pharmaceutical Sciences. 11(4): 16-22.
Saraphanchotiwitthaya, A., Saibuathong, N., Boonsirirungrueng, T., and Sripalakit, P. 2023. Formulation development and evaluation of chewable gels containing Jatuphalathika extract. Natural and Life Sciences Communications. 22(4): e2023060.
Saraswaty, V., Risdian, C., Primadona, I., Andriyani, R., Andayani, D.G.S., Mozef, T. 2017. Pineapple peel wastes as a potential source of antioxidant compounds. IOP Conference series: Earth and Environmental Science. 60: 012013.
Singleton, V.L., Orthofer, R., and Lamuela-Raventos, R.M. 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymology. 299:152-178.
Thring, T.S., Hili, P., and Naughton, D.P. 2009. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complementary and Alternative Medicine. 9: 27.
Vrianty, D., Laila, Q.R., Widowati, W., Putra, F.S.A., Fibrina, D., Fachrial, E., and Lister, I.N.E. 2019. Comparison of antioxidant and anti-tyrosinase activities of pineapple (Ananas comosus) core extract and luteolin compound. Journal Kedokteran Brawijaya 30(4): 240-246.
Wang, F., Sun, Z., Li, X., Wang, K., Chen, D., and Li, Z. 2022. Study on factors influencing the viscosity of sodium carboxymethyl cellulose used in capsule-mucous sealers. Geofluids. 2022(1): 4198311.
Wijaya, L., Lister, I.N.E., Fachrial, E., and Girsang, E. 2020. Scavenge ABTS and inhibition of elastase enzyme activity from ethanol extract of pineapple (Annas cosmusus (L.) Merr) core. American Scientific Research Journal for Engineering, Technology, and Sciences. 70(1): 106-113.
Yeniçeri, E., Altay, A., Koksal, E., Altın, S., Taslimi, P., Yılmaz, M.A., Cakir, O., Tarhan, A., and Kandemir, A. 2024. Phytochemical profile by LC-MS/MS analysis and evaluation of antioxidant, antidiabetic, anti-Alzheimer, and anticancer activity of Onobrychis argyrea leaf extracts. European Journal of Integrative Medicine. 66: 102337.
Zhang, X., Hu, X., Hou, A., and Wang, H. 2009. Inhibitory effect of 2,4,2',4'-tetrahydroxy-3-(3-methyl-2-butenyl)-chalcone on tyrosinase activity and melanin biosynthesis. Biological and Pharmaceutical Bulletin. 32: 86-90.
Zirwas, M.J. and Moennich, J. 2008. Antiperspirant and deodorant allergy: Diagnosis and management. The Journal of Clinical and Aesthetic Dermatology. 1(3): 38-43.
Zolghadri, S., Bahrami, A., Khan, M.T.H., Munoz-Munoz, J., Garcia-Molina, F., Garcia-Canovas, F., and Saboury A.A. 2019. A comprehensive review on tyrosinase inhibitors. Journal of Enzyme Inhibition and Medicinal Chemistry. 34: 279-309.
Zuorro, A., Iannone, A., and Lavecchia, R. 2019. Water-organic solvent extraction of phenolic antioxidants from brewer’s spent grain. Processes. 7: 126.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Kittima Saephan1, Chutikan Yanukul1, Pattana Sripalakit2, 3 and Aurasorn Saraphanchotiwitthaya1, 2, *
1 Department of Pharmaceutical Technology, Faculty of Pharmaceutical Sciences, Naresuan University, Phitsanulok 65000, Thailand.
2 Pharmaceutical Biotechnology Research Unit, Faculty of Pharmaceutical Sciences, Naresuan University, Phitsanulok 65000, Thailand.
3 Department of Pharmaceutical Chemistry and Pharmacognosy, Faculty of Pharmaceutical Sciences, Naresuan University, Phitsanulok 65000, Thailand.
Corresponding author: Aurasorn Saraphanchotiwitthaya, E-mail: aurasorns@nu.ac.th
ORCID: Aurasorn Saraphanchotiwitthaya: https://orcid.org/0000-0002-7166-7027
Total Article Views
Editor: Nisit Kittipongpatana,
Chiang Mai University, Thailand
Article history:
Received: November 2, 2024;
Revised: January 24, 2025;
Accepted: July 21, 2025;
Online First: July 31, 2025

