
Effects of Magnolia Bark Extract and Hydrated Silica Concentrations on the Performance of Compressed Toothpaste Tablets
Saowalak Phonsee, Tasana Pitaksuteepong, Rungarun Kriangkrai, and Worawut Kriangkrai*Published Date : July 31, 2025
DOI : https://doi.org/10.12982/NLSC.2025.059
Journal Issues : Number 4, October-December 2025
Abstract This study investigated the formulation and performance characteristics of compressed toothpaste tablets incorporating magnolia bark extract and varying concentrations of hydrated silica. The tablets were prepared using a direct compression method. The effects of hydrated silica content on key performance parameters, including physicochemical properties, cleaning efficacy, antimicrobial activity, and stability, were evaluated. Increasing concentrations of hydrated silica in the powder formulation reduced its flowability, as evidenced by decreases in the compressibility index, Hausner's ratio, and angle of repose. Upon compression into tablets, higher silica content resulted in reduced tablet hardness without significantly affecting friability or foaming properties. All formulations complied with pharmacopoeial standards for weight variation, hardness, and friability. The tablet containing 15% w/w hydrated silica demonstrated a cleaning efficacy of 50.31%, closely comparable to conventional paste-based toothpaste (54.99%). The optimized formulation exhibited a Relative Dentin Abrasivity (RDA) of 84.15 and a Relative Enamel Abrasivity (REA) of 8.23, indicating a medium abrasive level suitable for daily use. Accelerated stability testing showed no changes in the tablet's physical properties; however, a slight reduction in flavor intensity was observed over time. Antimicrobial assessments revealed that tablets containing magnolia bark extract produced inhibition zones against Streptococcus mutans comparable to those achieved with chlorhexidine. These findings offer valuable insights into the development of environmentally sustainable oral care products that maintain therapeutic efficacy while incorporating natural antimicrobial agents.
Keywords: Toothpaste tablets, Magnolia bark extract, Cleaning efficacy, Hydrated silica, Antimicrobial activity
Citation: Phonsee, S., Pitaksuteepong, T., Kriangkrai, R., and Kriangkrai, W. 2025. Effects of magnolia bark extract and hydrated silica concentrations on the performance of compressed toothpaste tablets. Natural and Life Sciences Communications. 24(4): e2025059.
INTRODUCTION
The escalating environmental concerns associated with plastic waste have prompted the development of sustainable alternatives in various consumer products. Traditional toothpaste tubes contribute significantly to plastic pollution and are challenging to recycle due to their multi-material composition (Malea et al., 2020). This has led to increased interest in eco-friendly oral care solutions such as toothpaste in chewable tablet form or “toothpaste tablets”. This offers benefits like reduced packaging waste, ease of transport, and accurate dosing (Nithish kumar et al., 2023). Moreover, the toothpaste tablets gained popularity in cosmetic development due to its various advantages, such as ease of use, low manufacturing costs, being preservative-free, relatively stable, shipping, and storage (Padmanabh et al., 2022).
Plant-derived bioactives have gained significant attention for toothpaste. Ogboji et al. (2018) conducted a physicochemical evaluation to assess the antimicrobial properties of a green toothpaste formulated using natural ingredients, including turmeric, aloe vera, guava, mint, neem, and lemon. The herbal extract exhibited good antimicrobial properties. Magnolia bark extract, derived from Magnolia officinalis, is rich in phenolic compounds such as magnolol and honokiol. These compounds exhibit strong antimicrobial, antioxidant, and anti-inflammatory properties (Ogata et al., 1997; Chang et al., 1998). Notably, these substances demonstrated inhibitory effects on Streptococcus mutans, the primary bacterium responsible for dental caries, by disrupting biofilm formation and bacterial adhesion (Sakaue et al., 2016). Furthermore, magnolia compounds have shown potential for therapeutic applications in dentistry with relatively low cytotoxicity (Lee et al., 2011). In addition to active botanical ingredients, excipients such as abrasives play a critical role in toothpaste formulation. Hydrated silica is a widely used mild abrasive agent that contributes to mechanical plaque removal and tooth polishing without damaging enamel (Wiilknitz, 1997). The balance of abrasiveness and tablet integrity is crucial for developing effective compressed toothpaste tablets.
This study investigates the antimicrobial efficacy of magnolia bark extract and the influence of varying concentrations of hydrated silica on the performance of compressed toothpaste tablets. The research evaluates key formulation parameters, including powder flowability, tablet thickness, hardness, friability, foaming ability, cleaning ability, abrasivity, and antimicrobial activity, to determine the optimal balance between natural functionality and formulation stability. The outcomes offer valuable insights for the development of effective, eco-friendly, and consumer-appealing oral care products within the field of cosmetic science.
Especially, this work provides a useful model for product development and offers valuable information to guide the cosmetics industry in the production of toothpaste tablets.
MATERIAL AND METHODS
Materials
Table 1 presents seven toothpaste tablet formulations (F0-F6) designed to evaluate the effects of magnolia bark extract and hydrated silica. Magnolia bark extract (LEMA-14A, Forecus Co., Ltd, Thailand) was incorporated at a concentration of 2% w/w in F1-F6 for antimicrobial properties. F0 serves as the negative control, containing 0% magnolia bark extract. To study the effect of varying concentrations of hydrated silica (Zeodent 113, Evonik, Germany), F1-F6 were designed. The hydrated silica was used as an abrasive agent to aid in the removal of dental plaque and surface stains. Dicalcium phosphate dihydrate (Merck, Germany), calcium carbonate (Merck, Germany), and sodium bicarbonate (Solvay, Belgium) were functioned as abrasives to enhance cleaning efficacy. Xylitol (Roquette, France) was used as tablet diluent. It also improved the taste and texture of toothpaste formulations (Agiba and Eldin, 2019) and provided dental health benefits such as reducing the risk of dental caries (Nayak et al., 2014). Sorbitol powder (Roquette, France) was used as a sweetener and provides a pleasant mouthfeel. Poloxamer 407 (BASF, Germany) and sodium coco sulfate (BASF, Germany) were used as cleansing agents. Sodium fluoride (Sigma-Aldrich, USA) was added as an anticaries agent. All other chemicals were pharmaceutical or food grade as indicated, ensuring the safety and quality required for oral care applications.
Table 1. The composition of toothpaste tablets.
|
Ingredients (% w/w) |
Formulations |
||||||
|
F0 |
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
|
|
Xylitol |
51.96 |
51.46 |
50.46 |
48.46 |
46.46 |
43.46 |
38.46 |
|
Sorbitol powder |
15.00 |
15.00 |
15.00 |
15.00 |
15.00 |
15.00 |
15.00 |
|
Poloxamer 407 |
3.00 |
3.00 |
3.00 |
3.00 |
3.00 |
3.00 |
3.00 |
|
Sodium coco sulfate |
1.50 |
1.50 |
1.50 |
1.50 |
1.50 |
1.50 |
1.50 |
|
Hydrated silica |
2.00 |
2.00 |
3.00 |
5.00 |
7.00 |
10.00 |
15.00 |
|
Sodium fluoride |
0.22 |
0.22 |
0.22 |
0.22 |
0.22 |
0.22 |
0.22 |
|
PVP K-30 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
|
Calcium carbonate |
6.00 |
6.00 |
6.00 |
6.00 |
6.00 |
6.00 |
6.00 |
|
CI 16035 |
0.02 |
0.02 |
0.02 |
0.02 |
0.02 |
0.02 |
0.02 |
|
Magnolia bark extract |
0.00 |
0.50 |
0.50 |
0.50 |
0.50 |
0.50 |
0.50 |
|
Peppermint flavor |
1.50 |
1.50 |
1.50 |
1.50 |
1.50 |
1.50 |
1.50 |
|
Menthol crystals |
0.50 |
0.50 |
0.50 |
0.50 |
0.50 |
0.50 |
0.50 |
|
Methyl diisopropyl Propionamide |
0.30 |
0.30 |
0.30 |
0.30 |
0.30 |
0.30 |
0.30 |
|
Dicalcium phosphate dihydrate |
10.00 |
10.00 |
10.00 |
10.00 |
10.00 |
10.00 |
10.00 |
|
Sodium bicarbonate |
6.00 |
6.00 |
6.00 |
6.00 |
6.00 |
6.00 |
6.00 |
|
Magnesium stearate |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
|
Total |
100.00 |
100.00 |
100.00 |
100.00 |
100.00 |
100.00 |
100.00 |
Preparation of toothpaste tablets
The toothpaste table was firstly formulated in powder blend. The composition of toothpaste tablets is shown in Table 1. The first stage was base sweeteners followed by adding a mixture of xylitol and sorbitol powder combined with abrasives ingredients (hydrated silica, dicalcium phosphate dihydrate). The colorant was pre-blended with calcium carbonate using geometric dilution until a uniform mixture was achieved and then incorporated into the formulation. The liquid phase containing magnolia bark extract, flavoring agents, menthol crystals, and methyl diisopropyl was mixed and stirred until a clear solution was obtained, then added into the powder mixture using appropriate mixing equipment. The liquid was gradually absorbed by the powder components, resulting in a uniform and cohesive blend. Functional agents (poloxamer 407, sodium coco sulfate, sodium fluoride, PVP K-30, and sodium bicarbonate, were added. All ingredients were passed through a 60-mesh sieve to ensure uniform particle size distribution. The powder mixture was mixed for 5 min. Magnesium stearate was further added at the final stage of mixing and blended for 1 min. The powder mixture was compressed into tablets (diameter: 10 mm; biconvex; average tablet weight: 500 mg) using a single-punch tablet press (TDP-1.5, Zhejiang Capsulcn Machinery Co., Ltd., China). The target thickness of the tablets was set at 5.5 mm.
Powder characterization
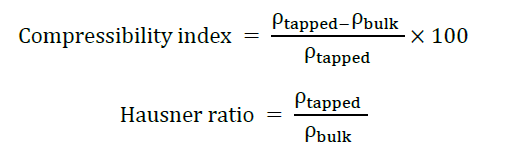
The powder blend was visually inspected under standardized lighting to evaluate color uniformity and texture. pH was measured by dispersing 10 g of powder blend in 10 mL of deionized water and stirring to form a suspension, followed by measurement using a calibrated pH meter (acceptable pH range for oral care product: pH 6–8. Moisture content was determined using a moisture analyzer (HE53, Mettler Toledo, Switzerland) with loss on drying method at 105°C until constant weight. All tests were conducted in triplicate. The angle of repose was measured by the fixed cone method with a funnel mounted at a fixed height (10 cm) above a flat horizontal surface. The funnel had a standard orifice diameter of 10 mm, allowing the powder to flow freely and form a conical pile. The height and diameter of the resulting cone were measured, and the angle of repose was calculated. Bulk and tapped densities were obtained by measuring the volume of the powder before and after tapping, respectively, using a digital tapped density apparatus (TDA-2, Campbell Electronics, India). The Compressibility Index and Hausner’s Ratio were calculated using the following formulas:
Physicochemical characterization of the toothpaste tablets
Organoleptic properties, including appearance, color, and odor, were evaluated visually to assess aesthetic quality. Weight variation was determined by individually weighing 20 randomly selected tablets, calculating the average weight, and computing the percentage deviation of each tablet using the following equation.
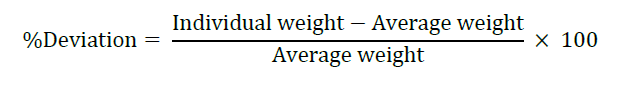
Tablet thickness was measured in 10 tablets using a digital vernier caliper, and the mean ± standard deviation was reported. Tablet hardness was assessed using an analog push-pull gauge (NK-500, Wenzhou Weidu Electronics Co., Ltd., China) on 10 tablets. Friability was evaluated using a Roche friabilator (FTA-20N, Campbell, USA) by subjecting 12 pre-weighed tablets to 100 rotations at 25 rpm for 4 min, followed by reweighing to calculate percentage weight loss.
Foam test
The foam test was performed following Annisa et al. (2023), where 10 mL of a 1% w/v slurry of the toothpaste tablet was added to 50 mL of distilled water in a graduated cylinder (250 mL, Pyrex®, Germany). After vigorous shaking for 30 seconds, the foam height was measured immediately and after 5 min to assess foam stability.
Cleaning ability
The cleaning ability of the toothpaste tablets was evaluated using a method adapted from Ogboji et al. (2018), with stained eggshells used as enamel analogs as shown in Figure 1. Firstly, hard-boiled eggs were stained by immersion in a prepared solution consisting of 200 mL of boiling water, 15 mL of vinegar, and 1 mL of red food dye. The eggs were soaked in the solution for 5 min to allow uniform surface staining of the eggshell, then divided into two halves. The test included 6 toothpaste tablet formulations (F1–F6), 2 commercial toothpaste tablet brands (Brand A and Brand B), and a conventional toothpaste (Brand C) for comparison. To study cleaning ability, toothpaste tablets were crushed into powder. The powder (0.35 g) was mixed with 1.5 mL of distilled water to form a slurry. Similarly, 0.35 g of commercial toothpaste was used. Stained eggs were brushed by an Oral-B® Pro-Health Precision Clean electric toothbrush. One side of the egg was brushed with toothpaste slurry and another side was brushed with a distilled water. The color removal was assessed using a colorimetric spectrophotometer (Mini scan EZ 4500S, HunterLab, USA), and calculated using the following equation:
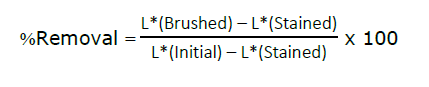
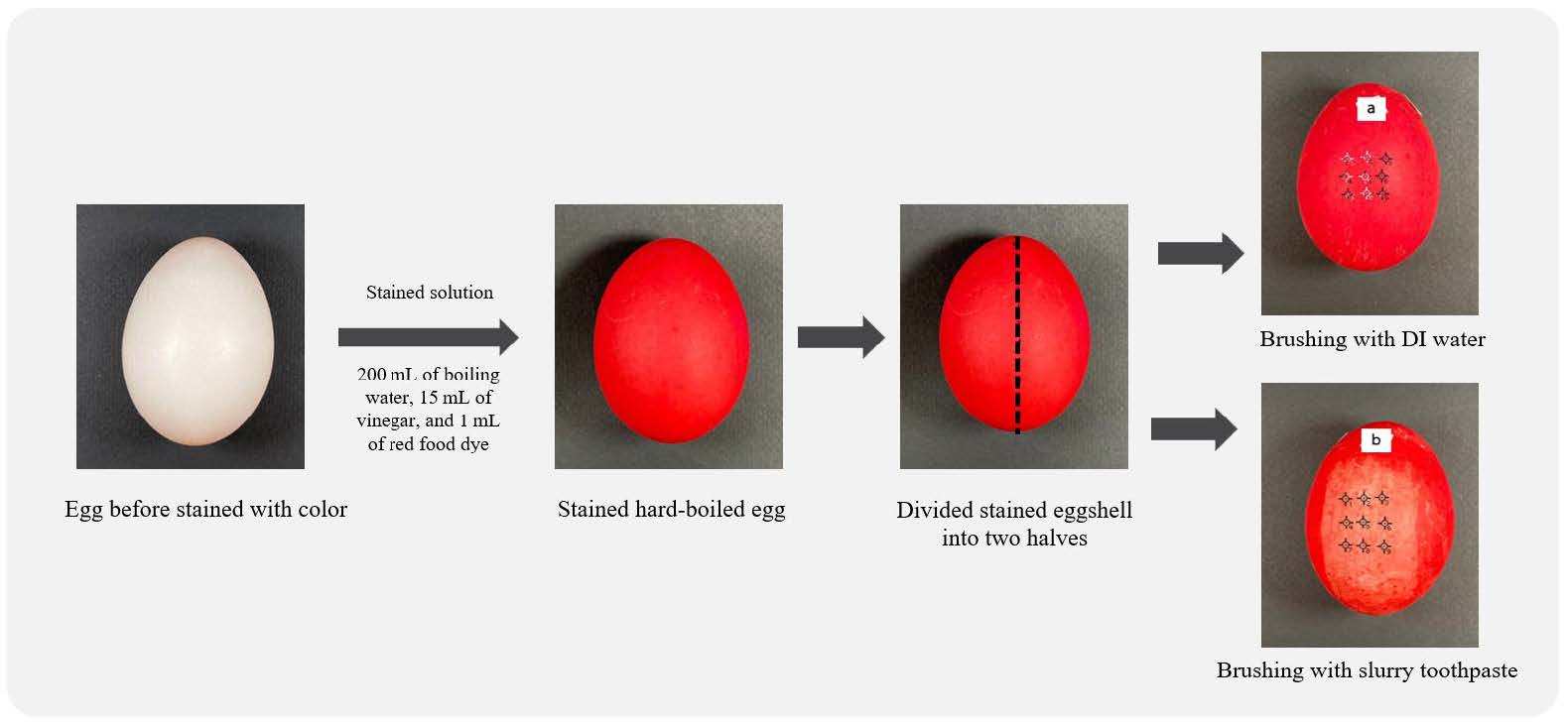
Figure 1. Stained eggshell preparing for cleaning ability test.
Abrasivity test
The abrasivity of the toothpaste tablets was evaluated according to ISO 11609:2007 guidelines, with testing conducted by the Oral Biology Research Center (OBRC) to ensure compliance with international standards. A brushing simulator (V-8 cross brushing machine, SABRI Dental Enterprise, Inc., Villa park, IL, USA) and human enamel specimens were used for the test. Tablets were ground and mixed with distilled water to create a slurry, simulating real-use conditions. Brushing was performed under standardized parameters (200 g load, 60 strokes/min, 1,500 cycles). For each test, 25 g of toothpaste or toothpaste tablet (crushed into powder) was mixed with 40 mL of distilled water to form a slurry. After preparation, 3 mL of this slurry was used for the brushing simulation.
Enamel abrasion was assessed by measuring the weight loss of the specimens before and after brushing. Abrasivity was expressed as Relative Enamel Abrasivity (REA) and Relative Dentin Abrasivity (RDA), calculated using standard formulas. RDA values were interpreted according to ISO 11609 classifications.
Antimicrobial test
The antimicrobial efficacy of the toothpaste tablets was evaluated using the agar well diffusion method against Streptococcus mutans ATCC 25175, which was obtained from the Cosmetics and Natural Products Research Center, Faculty of Pharmaceutical Sciences, Naresuan University. Wells on Mueller-Hinton agar were filled with toothpaste slurry, and a commercial toothpaste served as a control to compare the antimicrobial performance of the formulated toothpaste tablets. The tested samples included: toothpaste tablets containing 0.5% magnolia bark extract, toothpaste tablets with 0.15% chlorhexidine (CHX), a commercial toothpaste, a placebo toothpaste tablet (without active ingredients), a 0.5% magnolia bark extract solution, and a 0.15% CHX solution.
The toothpaste dilutions were prepared by dispersing 10 mg of each sample in 500 μL of double-distilled water. The antimicrobial activity was evaluated using the agar well diffusion method. Agar plates were inoculated with Streptococcus mutans, and wells (6 mm in diameter) were created using a sterile cork borer. Each well was filled with the prepared dilution, and the plates were incubated at 37°C for 24 h. Zones of inhibition were measured to assess antibacterial efficacy. After 24 h of incubation at 37°C, inhibition zones were measured to assess antimicrobial activity. All plates were made in triplicates.
Stability test
The stability of the toothpaste tablets was evaluated following the ASEAN Guideline on Stability Study of Drug Product (R1). Tablets were stored in glass containers and kept under accelerated conditions at 40°C ± 2°C/75% RH ± 5% for 1, 3, and 6 months. At each time point, samples were evaluated for visual defects, hardness, friability, and weight variation. Visual changes were scored on a 0–5 scale (0 = no change; 5 = extremely changed).
RESULTS
Effects of magnolia bark extract and hydrated silica concentrations on the physicochemical properties of powder blends
All prepared powder blends formulations (F0–F6) resulted in fine, light pink powders that passed through a 60-mesh sieve, indicating suitable particle size distribution for tablet compression (Figure 2). Moisture content ranged from 3.98% to 4.34% across formulations. The incorporation of magnolia bark extract did not significantly alter the physicochemical properties of the powder blends (Table 2).

Figure 2. Final powder blended (light pink fine powder).
Increasing the concentration of hydrated silica from 2% (F1) to 15% (F6) exhibited the decreasing in bulk and tapped densities. Correspondingly, the compressibility index and Hausner’s ratio increased, alongside the angle of repose, indicating reduced flowability and higher inter-particle friction with higher silica content (F3-F6). Moisture content remained relatively stable across all formulations (3.98% - 4.34%). The pH slightly increased with higher amounts of hydrated silica.
Table 2. Physicochemical properties of powder blends.
|
Moisture content (%) |
Tapped density (g/ml) |
Bulk density (g/ml) |
Compressibility index (%) |
Hausner’s ratio |
Angle of repose (θ) |
pH (1% w/v) |
|
|
F0 |
4.02 ± 0.20 |
0.84 ± 0.01 |
0.73 ± 0.01 |
13.50 ± 0.16 |
1.16 ± 0.01 |
30.20 ± 0.60 |
6.88 ± 0.02 |
|
F1 |
3.98 ± 0.18 |
0.86 ± 0.01 |
0.75 ± 0.01 |
13.04 ± 0.14 |
1.15 ± 0.00 |
29.90 ± 0.66 |
6.92 ± 0.01 |
|
F2 |
4.02 ± 0.21 |
0.92 ± 0.01 |
0.75 ± 0.01 |
18.63 ± 0.20 |
1.23 ± 0.00 |
38.87 ± 0.40 |
6.88 ± 0.01 |
|
F3 |
4.21 ± 0.40 |
0.92 ± 0.01 |
0.71 ± 0.01 |
23.53 ± 0.92 |
1.31 ± 0.02 |
43.83 ± 0.38 |
6.90 ± 0.00 |
|
F4 |
4.16 ± 0.34 |
0.92 ± 0.01 |
0.64 ± 0.01 |
30.84 ± 1.57 |
1.45 ± 0.03 |
41.68 ± 1.68 |
7.29 ± 0.01 |
|
F5 |
4.22 ± 0.11 |
0.85 ± 0.00 |
0.57 ± 0.00 |
33.17 ± 0.55 |
1.50 ± 0.01 |
45.24 ± 0.41 |
7.11 ± 0.00 |
|
F6 |
4.34 ± 0.55 |
0.72 ± 0.01 |
0.44 ± 0.00 |
38.74 ± 0.90 |
1.63 ± 0.02 |
41.95 ± 0.32 |
7.26 ± 0.01 |
Effects of magnolia bark extract and hydrated silica concentrations on the physicochemical properties of toothpaste tablets
Compressed toothpaste tablets were produced with a target weight of 500 mg, thickness around 5 mm, and diameter of 10 mm. Weight variation across all formulations was within the acceptable ±5% limit, ranging from 497.51 mg to 502.30 mg. Slight weight variations were observed with increasing hydrated silica (F1-F6). Tablet hardness decreased as hydrated silica concentration increased from 2% to 15%. Friability values ranged from 0.21% (F1) to 0.42% (F4), increasing slightly at higher hydrated silica concentrations.
Table 3. Physicochemical properties of toothpaste tablets.
|
Physicochemical properties (mean ± SD) |
||||
|
Weight variation (mg) |
Thickness (mm) |
Hardness (kg/cm2) |
Friability (%) |
|
|
F0 |
502.30 ± 6.50 |
5.60 ± 0.05 |
5.75 ± 0.48 |
0.22 ± 0.02 |
|
F1 |
500.90 ± 6.65 |
5.57 ± 0.04 |
5.62 ± 0.53 |
0.21 ± 0.03 |
|
F2 |
501.56 ± 3.31 |
5.59 ± 0.08 |
5.28 ± 0.49 |
0.35 ± 0.04 |
|
F3 |
498.62 ± 4.22 |
5.61 ± 0.03 |
5.11 ± 0.45 |
0.32 ± 0.06 |
|
F4 |
499.59 ± 2.48 |
5.56 ± 0.09 |
5.20 ± 0.35 |
0.42 ± 0.08 |
|
F5 |
498.53 ± 6.52 |
5.57 ± 0.04 |
4.95 ± 0.42 |
0.41 ± 0.05 |
|
F6 |
497.51 ± 8.14 |
5.62 ± 0.06 |
4.90 ± 0.55 |
0.38 ± 0.11 |
Effects of magnolia bark extract and hydrated silica concentrations on dental care properties of the toothpaste tablets
Foam test
Foam height was measured to assess the foaming capacity of the toothpaste tablets. The formulations tested included F1–F6, commercial toothpaste tablet brands (A and B), and a conventional toothpaste (Brand C). The results are summarized in Table 4. F1 showed the highest foam height among the formulated tablets (7.22 ± 0.35 cm). Foam height slightly decreased with increasing hydrated silica concentration. The formulated tablets showed foaming properties superior to the commercial tablets and comparable to conventional toothpaste. Conventional toothpaste (Brand C) produced the highest foam height (10.20 ± 0.52 cm).
Table 4. Foam height of toothpaste tablets formulations.
|
Formulations |
Foam height (cm) |
|
F0 |
7.20 ± 0.15 |
|
F1 |
7.22 ± 0.35 |
|
F2 |
7.02 ± 0.15 |
|
F3 |
6.98 ± 0.05 |
|
F4 |
7.15 ± 0.12 |
|
F5 |
6.52 ± 0.05 |
|
F6 |
6.82 ± 0.30 |
|
Commercial toothpaste tablet brand A |
4.33 ± 0.51 |
|
Commercial toothpaste tablet brand B |
1.20 ± 0.30 |
|
Conventional toothpaste brand C |
10.20 ± 0.52 |
Cleaning ability
The cleaning efficiency of the toothpaste tablet formulations was evaluated by measuring the percentage of stain removal. The results for all formulations, including commercial products, are presented in Table 5. Incorporating 0.5% magnolia bark extract (F1) did not significantly differ from the control (F0) in cleaning ability. Formulation F6 (15% hydrated silica) demonstrated the highest stain removal (56.31 ± 0.62%), which was comparable to conventional toothpaste Brand C (57.27 ± 0.66%). Formulations F5 and F6 showed cleaning efficacy similar to the conventional toothpaste.
Table 5. Cleaning test of toothpaste samples.
|
Formulations |
%Removal (Mean ± SD) |
|
DI Water |
16.61 ± 1.01 |
|
F0 |
35.47 ± 1.13 |
|
F1 |
36.64 ± 0.81 |
|
F2 |
45.52 ± 0.78 |
|
F3 |
45.86 ± 0.65 |
|
F4 |
44.66 ± 0.57 |
|
F5 |
55.67 ± 0.08 |
|
F6 |
56.31 ± 0.62 |
|
Commercial toothpaste tablet brand A |
33.71 ± 1.20 |
|
Commercial toothpaste tablet brand B |
20.55 ± 0.61 |
Abrasivity test
Relative Dentin Abrasivity (RDA) and Relative Enamel Abrasivity (REA) were determined for formulations F1 (lowest silica) and F6 (highest silica), a commercial tablet (Brand A), and conventional toothpaste (Brand C). F6 exhibited the highest abrasivity among the formulated tablets (RDA 84.15 ± 2.01, REA 8.23 ± 0.82). F1 showed moderate abrasivity (RDA 51.05 ± 1.64, REA 2.04 ± 0.15). Commercial tablet Brand A had the lowest abrasivity (RDA 24.02 ± 1.06, REA 1.03 ± 0.54). Conventional toothpaste Brand C showed the highest overall abrasivity (RDA 123.08 ± 4.05, REA 13.03 ± 2.00) (Table 6).
Table 6. Abrasivity values of toothpaste samples.
|
Formulation |
Type of toothpaste samples |
RDA Mean ± Sem |
REA Mean ± Sem |
|
F1 |
Tablet |
51.05 ± 1.64 |
2.04 ± 0.15 |
|
F6 |
Tablet |
84.15 ± 2.01 |
8.23 ± 0.82 |
|
Commercial toothpaste tablet brand A |
Tablet |
24.02 ± 1.06 |
1.03 ± 0.54 |
|
Conventional toothpaste brand C |
Paste |
123.08 ± 4.05 |
13.03 ± 2.00 |
Antimicrobial activity of toothpaste tablets containing magnolia bark extract
Antimicrobial activity was assessed using the agar well diffusion method against Streptococcus mutans. Solutions of 0.50% magnolia bark extract and 0.15% CHX showed the largest inhibition zones (28.45 ± 0.39 mm and 30.27 ± 0.27 mm, respectively). Toothpaste tablets containing 0.50% magnolia bark extract exhibited an inhibition zone of 10.71 ± 0.47 mm, while tablets with 0.15% CHX showed an inhibition zone of 14.52 ± 2.41 mm. Placebo tablets and standard toothpaste demonstrated lower activity (8.85 ± 1.04 mm and 9.66 ± 1.15 mm, respectively), and DI water showed no inhibition (Table 7)
Table 7. Antimicrobial activity zone of inhibition.
|
Sample |
Avg. diameter of inhibition zone (mm) |
|
0.50% w/w magnolia toothpaste tablets |
10.71 + 0.47 |
|
0.15% w/w CHX toothpaste tablets |
14.52 ± |
|
Placebo toothpaste tablets |
8.85 ± |
|
Standard toothpaste |
9.66 ± |
|
0.50% magnolia bark extract |
28.45 ± |
|
0.15% CHX |
30.27 ± |
|
DI water |
0.00 |
Stability test of toothpaste tablets containing magnolia bark extract
Stability testing was conducted on formulation F6 under ambient and accelerated conditions (40°C, 75% RH) for 6 months. Physical properties including appearance, color, taste, weight variation, thickness, hardness, and friability were evaluated. All results are summarized in Table 8. Under ambient conditions, the tablets showed good stability. Under accelerated conditions, minimal changes were observed; color and taste intensity decreased over time, correlating with a slight weight reduction. Tablet hardness decreased from an initial 5.51 ± 0.60 kg/cm² to 4.22 ± 0.45 kg/cm² after 6 months at accelerated conditions.
Table 8. The general appearance of formulation F6 after stability studies.
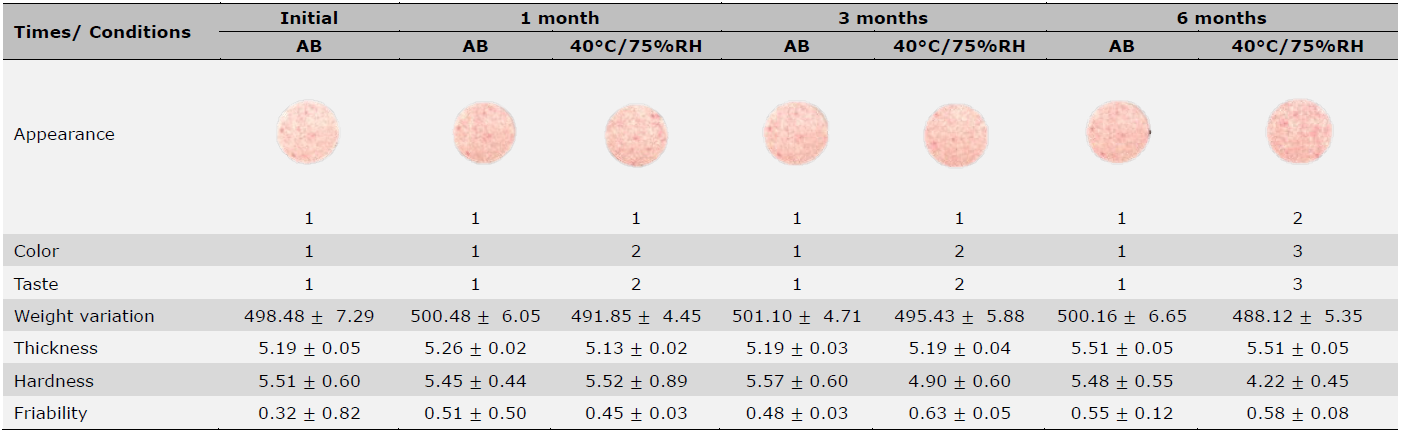
Note: AB = ambient temperature, RH = relative humidity
DISCUSSION
This study focused on developing compressed toothpaste tablets using magnolia bark extract and varying hydrated silica concentrations, evaluating their physicochemical and dental care properties.
The physicochemical analysis of the powder blends indicated that while magnolia bark extract had little impact, increasing hydrated silica concentration negatively affected flowability. The decrease in bulk and tapped densities with higher silica content is consistent with the porous nature of hydrated silica (Alrobaian et al., 2023). The observed lower flowability (increased Compressibility Index, Hausner’s Ratio, and angle of repose) at higher silica levels (F3-F6) suggest that incorporating glidants might be necessary for efficient large-scale production. The slight increase in pH with higher silica content could be attributed to the weakly basic nature of hydrated silica, potentially offering a buffering effect beneficial for maintaining oral pH. This behavior is consistent with findings in oral care formulations, where hydrated silica not only serves as an abrasive agent but also plays a role in modulating the pH of the product to maintain oral health conditions (Vranić et al., 2004).
Regarding the compressed tablets, all formulations met the pharmacopoeial requirements for weight variation. Tablet thickness was set at 5.6 mm to achieve the tablet hardness within the range of 5-6 kg/cm², which is considered appropriate for common chewable tablets (Van Hove et al., 2023). The decrease in tablet hardness with increased hydrated silica concentration suggests that silica interferes with inter-particle bonding. This reduction in hardness is attributed to the influence of hydrated silica on the bonding between ingredients, making the tablets softer and less compressible (Jonat et al., 2005). The concurrent slight increase in friability at higher silica levels could be linked to the inherent abrasiveness of silica potentially increasing surface brittleness (Akitomo et al., 2024), although all formulations remained well within the acceptable limits for tablet integrity.
The dental care properties revealed important formulation effects. While higher silica content slightly reduced foam height, potentially due to interference with surfactant function as suggested by Vranić et al. (2004). The formulated tablets generally outperformed commercial tablets and approached the foaming level of conventional toothpaste, which is significant for consumer acceptance. Conventional toothpaste typically exhibits high foaming properties due to their relatively high content of surfactants and foaming agents. Importantly, cleaning efficacy improved with higher hydrated silica concentrations, with F6 (15% hydrated silica) achieving stain removal comparable to conventional toothpaste. This aligns with the known role of hydrated silica as an abrasive agent enhancing mechanical cleaning (Pilecco et al., 2024).
The abrasivity tests confirmed the influence of hydrated silica concentration, a known abrasive agent (Johannsen et al., 2013). F6, with the highest silica content, showed the highest RDA and REA among the formulated tablets, yet remained within the medium abrasivity range (RDA < 100) considered safe for daily use according to ISO standards. This contrasts with the conventional toothpaste tested, which fell into the higher abrasivity category. The lowest silica formulation (F1) and the commercial tablet exhibited lower abrasivity, suggesting suitability for users needing gentler options. This highlights the possibility of tuning abrasivity by adjusting silica levels to balance cleaning needs with enamel and dentin preservation.
The antimicrobial testing demonstrated the potential of magnolia bark extract as a natural active ingredient. The extract itself showed strong inhibition against Streptococcus mutans, a key pathogen responsible for periodontitis and tooth decay (Pitaksuteepong et al., 2024) comparable to CHX. While the activity was reduced when incorporated into the tablet due to the dilution effects. The magnolia-containing tablets still exhibited significantly better antimicrobial performance than the placebo and standard toothpaste tested. This supports previous findings on the anti-biofilm and bactericidal effects of magnolia compounds against cariogenic bacteria (Komarov et al., 2017). This result suggests its viability as a natural alternative for oral care products.
Stability testing under both ambient and accelerated conditions demonstrated good physical stability for the optimized formulation (F6). However, under accelerated conditions, partial degradation of the flavoring agents was observed, as indicated by a reduction in taste intensity and slight weight loss. This degradation is attributed to the impact of heat and moisture on the flavoring components (González-González et al., 2022). Additionally, the observed decrease in hardness under stress conditions highlights the need for careful consideration of appropriate packaging and storage recommendations.
CONCLUSION
This study successfully formulated toothpaste tablets incorporating magnolia bark extract as a natural antimicrobial agent. The optimized levels of hydrated silica could modify cleaning properties. The findings demonstrated that the tablets achieved medium abrasivity suitable for daily use, with cleaning efficacy comparable to conventional toothpaste. The antimicrobial activity of the toothpaste tablets against Streptococcus mutans was equal to chlorhexidine, highlighting the potential as a natural alternative for oral care. The formulations exhibited excellent physical stability and met pharmacopoeial standards for quality. These results provide valuable insights into the formulation of sustainable oral care products that align with environmental goals while maintaining therapeutic efficacy.
ACKNOWLEDGMENTS
The authors thank the Faculty of Faculty of Pharmaceutical Sciences at Naresuan University, Phitsanulok, Thailand for providing instruments and facilities.
AUTHOR CONTRIBUTIONS
Saowalak Phonsee and Worawut Kriangkrai contributed to conceptualization, methodology, and investigation. Saowalak Phonsee prepared the original draft, performed review and editing. Tasana Pitaksuteepong and Rungarun Kriangkrai were responsible for data visualization. Worawut Kriangkrai also contributed to writing-original draft preparation, writing-review and editing, and provided supervision for the project. All authors have read and agreed to the published version of the manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Agiba, A.M. and Eldin, A.B. 2019. Insights into formulation technologies and novel strategies for the design of orally disintegrating dosage forms: A comprehensive industrial review. International Journal of Pharmacy and Pharmaceutical Sciences. 11(9): 8-20.
Akitomo, T., Kusaka, S., Kametani, M., Mitsuhata, C., Naka, S., Matsumoto-Nakano, M., Nakano, K., and Nomura, R. 2024. High-performance silica-containing professional mechanical tooth-cleaning paste that effectively adsorbs pigments. Pediatric Dental Journal. 34(2): 62-69.
Alrobaian, M., Alalaiwe, A., Almalki, Z.S., and Fayed, M.H. 2023. Application of response surface methodology to improve the tableting properties of poorly compactable and high-drug-loading canagliflozin using nano-sized colloidal silica. Pharmaceutics. 15(11): 2552.
Annisa, M., Harsini, H., and Murti, Y.B. 2023. Evaluation of herbal gel toothpaste formulated using bay leaf essential oil on physicochemical characteristics and extrinsic stain removal. Journal of HerbMed Pharmacology. 12(3): 425-431.
Chang, B., Lee, Y., Ku, Y., Bae, K., and Chung, C. 1998. Antimicrobial activity of magnolol and honokiol against periodontopathic microorganisms. Planta Medica. 64(4): 367-369.
González-González, O., Ramirez, I.O., Ramirez, B.I., O’Connell, P., Ballesteros, M.P., Torrado, J.J., and Serrano, D.R. 2022. Drug stability: ICH versus accelerated predictive stability studies. Pharmaceutics. 14(11): 2324.
Johannsen, G., Georg, T., Annsofi, J., and Liljeborg, A. 2013. The importance of measuring toothpaste abrasivity in both a quantitative and qualitative way. Acta Odontologica Scandinavica. 71(4): 508-517.
Jonat, S., Hasenzahl, S., Gray, A., and Schmidt, P.C. 2005. Influence of compacted hydrophobic and hydrophilic colloidal silicon dioxide on tableting properties of pharmaceutical excipients. Drug Development and Industrial Pharmacy. 31(7): 687-696.
Komarov, G.N., Hope, C.K., Wang, Q., Adejemi, A.A., Smith, P.W., Burnside, G., Inui, T., and Higham, S.M. 2017. Dental plaque regrowth studies to evaluate chewing gum formulations incorporating magnolia bark extract. Journal of Functional Foods. 37: 612-617.
Lee, Y.-J., Lee, Y.M., Lee, C.-K., Jung, J.K., Han, S.B., and Hong, J.T. 2011. Therapeutic applications of compounds in the Magnolia family. Pharmacology & Therapeutics. 130(2): 157-176.
Malea, A., Tzotzis, A., Manavis, A., and Kyratsis, P. 2020. Innovative and sustainable toothpaste packaging design. Journal of Graphic Engineering and Design. 11: 19-29.
Nayak, P.A., Anand, N.U., and Khandelwal, V. 2014. The effect of xylitol on dental caries and oral flora. Clinical, Cosmetic and Investigational Dentistry. 6: 89-94.
Nithish Kumar, A., Sivakumar, G., and Baskaran, A. 2023. An alternative to toothpaste with chewable toothpaste tablets -a review. Paripex - Indian Journal of Research. 12(4): 130-132.
Ogata, M., Hoshi, M., Shimotohno, K., Urano, S., and Endo, T. 1997. Antioxidant activity of Magnolol, honokiol, and related phenolic compounds. Journal of the American Oil Chemists' Society. 74: 557-562.
Ogboji, J., Chindo, I.Y., Jauro, A., Boryo, D.E.A., and Lawal N.M. 2018. Formulation, physicochemical evaluation and antimicrobial activity of green toothpaste on Streptococcus mutans. International Journal of Advanced Chemistry. 6(1): 108-113.
Padmanabh, S.K.D., Makhiya, M., Mulchandani, V., Jhamb, V., Trivedi, M., and Upendrabhai, M.J. 2022. A comparative clinical evaluation of plaque removal efficacy of a chewable toothpaste tablet with conventional toothpaste in children – A randomized clinical trial. Saudi Journal of Oral Sciences. 9(3): 185-189.
Pilecco, R.O., da Rosa, L., Tribst, J., Kleverlaan, C., Özcan, M., and Feilzer, A. 2024. Relative abrasive potential of silica-based conventional and tablet dentifrices on enamel and dentin. PLoS One. 19: 0313637.
Pitaksuteepong, T., Chatthong, A., Meepian, A., and Jobsri, J. 2024. Development of toothpaste containing Morus alba stem extract and its antimicrobial activity against oral pathogens. Natural and Life Sciences Communications. 23(4): e2024060.
Sakaue, Y., Domon, H., Oda, M., Takenaka, S., Kubo, M., Fukuyama, Y., Okiji, T., and Terao, Y. 2016. Anti-biofilm and bactericidal effects of Magnolia bark-derived magnolol and honokiol on Streptococcus mutans. Microbiology and Immunology. 60(1): 10-16.
Van Hove, B., Kanagale, P., Quinten, T., Gaiki, S., Collignon, K., Swar, Y., Shah, J., Verheyen, E., Preda, F.-M., Samanta, A., et al. 2023. Development of a new age-appropriate, chewable tablet of mebendazole 500 mg for preventive chemotherapy of soil-transmitted helminth infections in pre-school and school-age children. European Journal of Pharmaceutics and Biopharmaceutics. 188: 217-226.
Vranić, E., Lacević, A., Mehmedagić, A., and Uzunović, A. 2004. Formulation ingredients for toothpastes and mouthwashes. Bosnian Journal of Basic Medical Sciences. 4(4): 51-58.
Wiilknitz, P. 1997. Cleaning power and abrasivity of European toothpastes. Advances in Dental Research. 11(4): 576-579.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Saowalak Phonsee1, Tasana Pitaksuteepong1, Rungarun Kriangkrai2, and Worawut Kriangkrai1, 3, *
1 Department of Pharmaceutical Technology, Faculty of Pharmaceutical Sciences, Naresuan University, Phitsanulok 65000, Thailand.
2 Department of Oral Biology, Faculty of Dentistry, Naresuan University, Phitsanulok 65000, Thailand.
3 Center of Excellence for Natural Health Product Innovation, Naresuan University, Phitsanulok 65000, Thailand.
Corresponding author: Worawut Kriangkrai, E-mail: Wg.kriangkrai@gmail.com
ORCID: Worawut Kriangkrai: https://orcid.org/0000-0001-9367-4343
Total Article Views
Editor: Anak Iamaroon,
Chiang Mai University, Thailand
Article history:
Received: May 2, 2025;
Revised: June 30, 2025;
Accepted: July 9, 2025;
Online First: July 31, 2025


 2.41
2.41