
Formulation and Evaluation of the Physicochemical Properties of Extemporaneously Compounded Omeprazole Suspensions Under Controlled Temporal and Environmental Conditions
Thatsanand Xayavong*, Noudy Sengxeu, Phonesouphavanh Mayvanh, Sonesay Thammavong, Khamla Phonsaiyalinkham, Sounantha Souvanlasy, Chanthanom Manithip, David Nugroho, Rachadaporn Benchawattananon, and Theera RittirodPublished Date : June 12, 2025
DOI : https://doi.org/10.12982/NLSC.2025.049
Journal Issues : Number 3, July-September 2025
Abstract Omeprazole (OMP) capsules are commonly prescribed for gastroesophageal reflux disease (GERD). However, liquid formulations are often necessary for specific patient populations, including children, the elderly, and those with dysphagia. As commercially available liquid OMP preparations are limited, extemporaneous suspensions (ES) are frequently compounded. This study evaluated the stability of OMP ES (2 mg/mL) under varying temperature conditions, specifically Lao PDR. Suspensions were stored at 4 ± 3°C, room temperature (RT), and 45 ± 5°C, with physical (color, pH, viscosity, sedimentation, redispersibility) and chemical (HPLC) analyses conducted at predetermined intervals (0, 1, 5, 10, 20, 40, and 60 days). Refrigerated samples exhibited no color change and maintained OMP content within 90-110% of the initial concentration for 60 days. In contrast, color changes were observed at RT and 45 ± 5°C. At RT, OMP content remained within acceptable limits for only 10 days. These results demonstrate that OMP ES is stable for 60 days when refrigerated (4 ± 3°C) and for 10 days at RT. Storage at 45 ± 5°C is not recommended.
Keywords: Formulation, Omeprazole, Extemporaneous suspension, Physical-chemical stability, Temperature
Funding: The authors are grateful for the research funding provided by the ministry of education and sport, science and technology development fund, Laos.
Citation: Xayavong, T., Sengxeu, N., Mayvanh, P., Thammavong, S., Phonsaiyalinkham, K., Souvanlasy, S., Manithip, C., Nugroho, D., Benchawattananon, R., and Rittirod, T. 2025. Formulation and evaluation of the physicochemical properties of extemporaneously compounded omeprazole suspensions under controlled temporal and environmental conditions. Natural and Life Sciences Communications. 24(3): e2025049.
INTRODUCTION
The process of pharmaceutical formulation involves the integration of active pharmaceutical ingredients (APIs) with excipients to create a medicinal product (Hassan, 2012). Pediatric patients who are unable to swallow solid dosage forms, such as tablets or capsules, are often provided with an extemporaneous oral liquid prepared by pharmacists (Kommanaboyina and Rhodes, 1999). In markets, the availability of specific dosage forms for pediatric patients is lower than that for adults. In numerous practice environments, there exists a deficiency of commercially accessible oral liquid dosage forms (Glass and Haywood, 2006). In such instances, individuals who cannot ingest solid dosage forms like tablets or capsules may receive an oral suspension of the medication, which is prepared on-site by a compounding pharmacist (Falconer and Steadman, 2017). There is a notable absence of information regarding the stability, physical properties, chemical properties, microbial aspects, therapeutic potential, and toxicity of extemporaneously prepared drug formulations is lacking (Kommanaboyina and Rhodes, 1999). Stability is the most critical aspect in pharmaceutical formulation. However, certain factors influenced the stability of the drug, including pH and temperature. The stability of a pharmaceutical formulation across various storage conditions is crucial for ensuring the safety, efficacy, and quality of these formulations, as well as for determining the shelf-life of a preparation (Jackson et al., 2010). OMP is a proton pump inhibitor (PPI) that comes in the form of a delayed-release capsule of enteric-coated granules to prevent acid degradation (Oosterhuis and Jonkman, 1989). OMP is a prodrug that is only converted to its active form in the parietal cell, which is the site of action. OMP is used to treat peptic ulcer disease, gastrointestinal reflux, and helicobacter pylori infection (Israel and Hassall, 1998). However, a liquid dosage form of OMP is important for effective treatment of PPI and GERD in debilitated patients and very young children. OMP is marketed in the form of capsules (strength 20 and 40 mg) are the most feasible and accessible product in government healthcare settings. However, they are not suitable for children and patients who cannot swallow capsules. In a previous study, it was found that the OMP content of an ES of 2 mg/ml using the formulation developed by Masih Daneshvari Hospital. Dosage for children in hospital: GERD, ulcer, esophagitis: 1 mg/kg/day 1-2 times daily. Common doses 0.2-3.5 mg/kg/day. May be increased as needed in children 1-6 years due to high metabolic clearance. Critical cases may be given every 6-8 hours to maintain pH > 5 (1.5-2 mg/kg/day) (Takemoto et al., 2003). Data from pharmacy department of Queen Siri kit National Institute of Child Health (QSNICH), Thailand (QSNIC, 2017). Sixty OMP capsules (20 mg) are opened, and their contents are transferred into a mortar for the preparation of the ES. The materials are subsequently triturated into a fine powder and combined with a minimal quantity of vehicle to create a smooth paste. The vehicle is incrementally introduced with continuous agitation, and the suspension is sent to a calibrated container (600 ml). The mortar is washed with an enough solvent and placed into the container. The volume is ultimately calibrated to the designated mark using the car, and the suspension is thoroughly blended. The apparatus created by Masih Daneshvari Hospital has OMP 20 mg in a 1,000 ml Erlenmeyer flask. Utilizing a volumetric flask, 600 ml of an 8.4% sodium bicarbonate solution was incorporated to attain a final concentration. OMP concentration of 2 mg/ml. According to the literature review, OMP is rapidly destroyed at pH values below 7.4, but it remains stable under alkaline conditions pH 9.5 (Boscolo et al., 2020). In addition, reducing OMP ES was prepared by opening sixty capsules of OMP into a mortar and pestle them and then transferring the powder into a 1,000 ml Erlenmeyer flask. The pH was adjusted to 11 with 200 ml sodium hydroxide solution to get a final concentration of 2 mg/ml was above 90% for 28 days under refrigerated and was above 90% for 14 days RT. In addition, the appearance, color and pH remained unchanged. In another study, the concentration of OMP in a 2 mg/ml ES was between 90 and 110% after storage for 60 days at both refrigerated (Sinly et al., 2022). Daytime temperatures in Vientiane capital, Laos. As regards the summer, starting from March raising to 35-37°C between march and May with some daily maximums exceeding 40°C (Lyu et al., 2024). The aim of this study was to develop a suitable vehicle for OMP ES and evaluate its physicochemical properties under different storage conditions such as RT, 4 ± 3°C and 45 ± 5°C.
MATERIAS AND METHODS
Chemical and reagents
Omeprazole standard 99.56% (Lot No. 021099, Vietnam), OMP capsule (20 mg Alliance®, Lot No. COM0903) Exp 20/9/2025, were purchased in Laos Alliance pharmaceutical group co., Ltd, acetonitrile (Lab-scan, Thailand), monobasic sodium phosphate (Univar, Germany), Di-sodium hydrogen phosphate anhydrous (Annular NORMAPUR Product: 102494C, Made in EC), Lactose (Krungthepchemi co., Ltd, Thailand), Sodium hydroxide (Elago Enterprises Pty Ltd, 5 The Cloisters, Cherrybrook, N.S.W 2126, Australia), Xanthan gum (Lot No. 00208126, Austria), SCMC (Krungthepchemi co., Ltd, Thailand), Paraben concentrate (Krungthepchemi co., Ltd, Thailand), Tween 80, Sodium saccharin (Krungthepchemi co., Ltd, Thailand), were used in this study.
Instruments
Electronic balance (Model TA 214i, iTaly), brook field viscometer (Model DV1MLVKJ0, USA), filtration (40/38 Favoirt®, China), hot air oven (Memmert 854 schwabach, Germany), magnetic stirrer (MS7-H550-Pro, china), micropipette (MANUFACTURER/SUPPLIER, Thailand), refrigerated centrifuge (Jouan BR4i), thermometer (EU Article No.620-0865), UV-spectrophotometer (Model CE7200 series, England), high performance liquid chromatography (YL instrument 9300 HPLC system, Korea), Vortex mixer (Model No.SI-T246, USA) were used in this study.
Development of vehicle extemporaneous suspension
The vehicle compositions were chosen according to safety standards for pediatric patients. They ought to be effective at a pH of approximately 11. The components of the formulated vehicles comprise suspending agents, sweetening agents, an anticaking agent, a preservative, and a pH adjusting agent. The ingredients were measured and combined using geometric dilution.
Formulation was prepared by lactose as shown in Table 1. Tween 80 was added as a wetting agent to the mortar and pestle for a smooth paste. Sodium carboxymethylcellulose (SCMC) in amounts of 0.35, 0.45, or 0.5 g is dispersed and mixed with 20 ml of distilled water, then added to the mortar. This mixture is mixed for approximately 10 minutes. Next, 0.5 g of xanthan gum is added, mixed with 20 ml of distilled water (previously dispersed with SCMC) in the mortar, and thoroughly mixed. Then paraben was added and the mixture was mixed until it was homogeneous. Sodium hydroxide buffer (pH 11) was added as buffering agent to control pH. The mortar was filled with distilled water to a final volume of 100 ml and the mixture was transferred from the mortar to 9 x 3 glass bottles and stored in dark place and covered with aluminum foil.
Table 1. Development of extemporaneous suspension.
|
Ingredients |
Amount (g) |
||
|
F1 |
F2 |
F3 |
|
|
Lactose |
0.08 |
0.08 |
0.08 |
|
0.1 % Tween 80 |
4.00 |
4.00 |
4.00 |
|
Sodium carboxymethylcellulose (SCMC) |
0.35 |
0.45 |
0.50 |
|
Xanthan gum |
0.50 |
0.50 |
0.50 |
|
Sodium saccharin |
0.75 |
0.75 |
0.75 |
|
Sodium hydroxide (pH:11) |
10.00 |
10.00 |
10.00 |
|
Paraben concentrate |
2.00 |
2.00 |
2.00 |
|
Purified water qs. (ml) |
100.00 |
100.00 |
100.00 |
Extemporaneous suspension’s physical parameters assessment
The vehicles sampled on days 0, 5, 10, 20, 40 and 60 were reconfigured with water and assessed for physical parameters, including color, sedimentation ratio, re-dispersibility, viscosity, and pH.
Color and sedimentation ratio: The colors were evaluated by the same observer. The suspension remained unaltered. The sedimentation volume (F) was measured at 0, 15, 30 minutes, 1 hour and 24 hours. The experiments were conducted in duplicate.
F = Hu/Ho
Ho represents the beginning height of the suspension, while Hu denotes the height of the sediment at each time interval (Kadiri and Okafor, 2010).
Ease of redispersibility: This is a significant characteristic that indicates the quality of suspensions. The vehicles sampled on days 0, 5, 10, 20, 40 and 60. This was executed in an oversized volumetric bottle. The measurements were conducted as follows. The stored suspensions in a measuring cylinder were inverted by 180°C, and the number of inversions required to achieve a homogeneous suspension was recorded, with inversion deemed to indicate 100% redispersibility (Nyandoro, 2019).
pH measurement: The pH was determined utilizing a pH meter (FiveEasy F20, China).
Viscosity: OMP ES was measured by viscometer in the first and the last day of stability. This viscosity was recorded using rotational viscometer using spindle LV 2 at various speed of 5, 10, 20 and 50 rpm, respectively.
Compounding of OMP ES from capsules
The vehicle exhibiting favorable physicochemical features in liquid form was chosen. The OMP capsules were opened, and their contents were transferred into the vehicle and well mixed. The resultant substance was designated as OMP ES. The concentration of OMP in OMP ES was 2 mg/ml.
Evaluation of OMP ES
OMP ES was assessed in liquid formulations. The physicochemical parameters of OMP ES, such as color, sedimentation ratio, re-dispersibility, and pH, were assessed before to and following a 60 days storage period at room temperature, utilizing the aforementioned methodologies. The liquid form of OMP ES is stored at 4 ± 3°C, room temperature, and 45 ± 5°C for a duration of 60 days. The physical and chemical characteristics of the reassembled OMP ES, including color and pH, were assessed on days 0, 5, 10, 20, 40 and 60, utilizing the aforementioned procedures. The residual proportion of OMP was quantified utilizing HPLC.
Method of analysis by UV–VIS spectrophotometer
Omeprazole standard 0, 5 mg/ml of USP omeprazole in diluent. The calibration of concentration was 50, 100, 150 and 200 μg/ml. The resulting five concentrations were measured spectrophotometrically at range 200 - 400 nm using a UV–VIS spectrophotometer.
High-Performance Liquid Chromatography
HPLC was used in this study to determine the content of OMP. OMP was detected at the wavelength 302 nm (Nataraj et al., 2012). Mobile phase of acetonitrile, phosphate buffer – Dissolve 0.725g monobasic sodium phosphate and 4.472 g of anhydrous disodium hydrogen phosphate. An injection volume of 20 μl was utilized with a C18 column (250 x 4.6mm, 5μ). The mobile phase consisted of a phosphate buffer (pH 7.4) and acetonitrile in a 60:40 v/v ratio. The flow rate was consistently held at 1.0 ml/min.
A standard curve was prepared by serially diluting the stock solution with mobile phase. Solution was made from OMP STD 0, 5 mg/ml of USP omeprazole in diluent. The calibration of concentration was 0, 50, 100, 150 and 200 μg/ml.
Validation of analytical method for determination of the percentage remaining of OMP
The technique validation was conducted in accordance with ICH criteria (Q2(R1)) (2005).
Calibration curve
The OMP STD was weighed to a quantity of 0.5 mg and diluted in distilled water to prepare a stock solution with a concentration of 0.5 mg/ml. The stock solution was filtered using a cellulose acetate membrane with a pore size of 0.45 μm (GE Healthcare UK Limited, USA). The filtrate was further diluted to seven concentrations within the range of 50, 100 and 150 μg/ml. The OMP solutions were assessed for absorbance at a wavelength of 302 nm utilizing a UV-spectrophotometer (Model CE7200 series, England). The curve for calibration was constructed correlating absorbance with concentration. The straight-line regression equation and correlation coefficient (r) were computed utilizing the least-squares method. The linearity was assessed based on the r value and the residual plot. An r value of ≥ 0.99 signifies a strong correlation coefficient. The residual plot, which predicts the discrepancies between observed and expected values, was conducted to verify linearity (Pitaksuteepong et al., 2024).
Limit of detection (LOD)
The limit of detection (LOD) denotes the minimal concentration of an organic micropollutant OMP in a sample that can be accurately identified. The calculation was performed using the subsequent equation (ICH, 2005):
LOD = 3.3(Y/S)
Where Y represents the standard deviation of the y-intercept, while S is the slope of the calibration curve.
Limit of quantitation (LOQ)
The limit of quantitation (LOQ) is the minimum concentration of an organic micropollutant OMP in a sample that can be measured with precision. The calculation was performed using the following equation (ICH, 2005):
LOQ = 10(Y/S)
Where Y denotes the standard deviation of the y-intercept, whereas S signifies the slope of the calibration curve.
Precision
Precision testing was conducted to verify that the analytical approach produces consistent results. The accuracy of the analytical method was assessed at three distinct levels of target concentrations. Intra-day precision was assessed by analyzing three duplicates of each concentration on the same day, whereas inter-day precision was evaluated across three consecutive days. The outcomes were articulated as relative standard deviation [RSD (%) = (Standard deviation/Mean) x 100%] (Kayesh et al., 2013).
Accuracy
Accuracy testing was conducted to verify that the analytical method could precisely measure OMP in a sample utilizing the spiking method. A specified amount of OMP capsule contents was included into the suspension vehicle (blank). The solution was dissolved in distilled water and subsequently filtered. The solutions were diluted to achieve concentrations of 50, 100 and 150 μg/ml. The results were presented as percentage recovery [% recovery = (Recovery concentration / Injected) x 100] of the administered medication (Kayesh et al., 2013).
Determination of the percentage remaining of OMP
The residual percentage of OMP in reconstituted OMP ES was measured on days 0, 1, 5, 10, 20, 40 and 60, with alterations. The reconstituted OMP ES was diluted with distilled water and the solution was subsequently filtered. The filtrate was examined for OMP utilizing HPLC.
Statistical analysis
All samples are shown as mean ± standard deviation. Suspensions containing OMP within the permissible range of 90 to 110% were deemed stable.
RESULTS
Lactose monohydrate can be used as a model for preparing suspension due to its insoluble in water, small particle size, availability, and lower cost compared to OMP. Combine 0.08 g of lactose monohydrate with distilled water to a total volume of 100 ml, then distribute the solution into three cylinders. One cylinder equals 20 ml; the results for lactose are expressed as mean ± SD = 3 ± 1.4142 (n = 3). Crystalline lactose monohydrate are recognized solid forms of lactose monohydrate that exhibit limited solubility in water. Will rapidly descend and aggregate (Rowe et al., 2009). Formulation of an appropriate medicinal suspension. The formulation of a suspension dosage form entails a highly intricate process. The selection of appropriate excipients, such as surfactants and viscosity-enhancing compounds (Ali et al., 2010).
The formulations were developed based on research conducted at Masih Daneshvari Hospital. The study employed xanthan gum and sodium carboxymethyl cellulose (SCMC) as suspending agents, selected for their stability at pH 9.5 (Baniasadi et al., 2012). Sodium saccharin was used as a sweetener and paraben was served as a preservative, Tween 80 served as a wetting agent, and sodium hydroxide was utilized to adjust pH.
Evaluation of 3 formulations
The characteristics (appearance, re-dispersibility, viscosity, pH, sedimentation volume, and color) of the three suspensions were assessed using quality control testing tools.
Appearance and re-dispersibility
All formulations (F1 to F3) manifested as white, viscous solutions. They exhibited same look and behavior regarding re-dispersibility.
Stability of ES
The RT stability test protocol was utilized to determine the stability of the ES. A 100 mL ES was made using the standard recipe presented in Table 2, and the suspension's properties (appearance, redispersibility, viscosity, pH, sedimentation volume, and color) were assessed. The prepared ES was contained within the same glass cylinder and lid used for the glass. The products were stored at RT and at 45 ± 5 °C. Product samples were evaluated after 60 days of storage for description, color, and sedimentation ratio, as presented in Table 2. F1, F2, and F3, maintained at RT for 0, 5, 10, 20, 40, and 60 days, initially produced an ES that was off-white, transitioning to pale yellow after 20 days of storage at 45 ± 5 °C (Figure 2). The sedimentation rate of F3 was the slowest, followed by F2 and F1 (Table 2). Nevertheless, F1 and F2 exhibited greater ease of redispersion (Table 3). The pH of F1 was effectively regulated at approximately pH 11 before and after the stability testing (Table 3). The pH value of the vehicle fluctuated and decreased in relation to temperature and storage duration.
Table 2 Color and sedimentation ratio of the ES during storage stability testing on days 0, 5, 10, 20, 40, and 60 at RT and 45 ± 5 °C relative humidity.
|
Day |
Test condition |
Formulation |
Color |
Sedimentation ratio |
||||
|
0 min |
15 min |
30 min |
1hr |
24 hr |
||||
|
0 |
- |
F1 |
Off-white |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
0.98 ± 0.02 |
0.58 ± 0.06 |
|
F2 |
Off-white |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
0.86 ± 0.02 |
||
|
F3 |
Off-white |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
0.97 ± 0.01 |
||
|
5 |
RT |
F1 |
Off-white |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
0.92 ± 0.05 |
0.67 ± 0.01 |
|
F2 |
Off-white |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
0.86 ± 0.02 |
||
|
F3 |
Off-white |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
0.98 ± 0.01 |
||
|
45 ± 5 °C |
F1 |
Off-white |
1.00 ± 0.00 |
1.00 ± 0.00 |
0.98 ± 0.01 |
1.00 ± 0.00 |
0.58 ± 0.01 |
|
|
F2 |
Off-white |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
0.93 ± 0.03 |
0.76 ± 0.02 |
||
|
F3 |
Off-white |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
0.98 ± 0.01 |
0.86 ± 0.01 |
||
|
10 |
RT |
F1 |
Off-white |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
0.99 ± 0.01 |
0.70 ± 0.01 |
|
F2 |
Off-white |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
0.77 ± 0.01 |
||
|
F3 |
Off-white |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
0.98 ± 0.01 |
||
|
45 ± 5 °C |
F1 |
Off-white |
1.00 ± 0.00 |
1.00 ± 0.00 |
0.99 ± 0.01 |
0.91 ± 0.01 |
0.57 ± 0.01 |
|
|
F2 |
Off-white |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
0.93 ± 0.03 |
0.60 ± 0.01 |
||
|
F3 |
Off-white |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
0.98 ± 0.01 |
0.75 ± 0.02 |
||
|
20 |
RT |
F1 |
Off-white |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
0.91 ± 0.01 |
0.70 ± 0.02 |
|
F2 |
Off-white |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
0.93 ± 0.01 |
0.76 ± 0.03 |
||
|
F3 |
Off-white |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
0.98 ± 0.01 |
||
|
45 ± 5 °C |
F1 |
Pale yellow |
1.00 ± 0.00 |
1.00 ± 0.00 |
0.98 ± 0.01 |
0.91 ± 0.01 |
0.60 ± 0.02 |
|
|
F2 |
Pale yellow |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
0.93 ± 0.03 |
0.65 ± 0.03 |
||
|
F3 |
Pale yellow |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
0.98 ± 0.01 |
0.75 ± 0.02 |
||
|
40 |
RT |
F1 |
Off-white |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
0.91 ± 0.01 |
0.83 ± 0.01 |
|
F2 |
Off-white |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
0.96 ± 0.03 |
0.77 ± 0.01 |
||
|
F3 |
Off-white |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
0.92 ± 0.01 |
||
|
45 ± 5 °C |
F1 |
Pale yellow |
1.00 ± 0.00 |
1.00 ± 0.00 |
0.98 ± 0.02 |
0.91 ± 0.01 |
0.35 ± 0.01 |
|
|
F2 |
Pale yellow |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
0.93 ± 0.03 |
0.51 ± 0.01 |
||
|
F3 |
Pale yellow |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
0.98 ± 0.01 |
0.74 ± 0.03 |
||
|
60 |
RT |
F1 |
Off-white |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
0.91 ± 0.01 |
0.82 ± 0.01 |
|
F2 |
Off-white |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
0.95 ± 0.03 |
0.78 ± 0.01 |
||
|
F3 |
Off-white |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
0.85 ± 0.01 |
||
|
45 ± 5 °C |
F1 |
Pale yellow |
1.00 ± 0.00 |
1.00 ± 0.00 |
0.88 ± 0.02 |
0.88 ± 0.01 |
0.33 ± 0.01 |
|
|
F2 |
Pale yellow |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
0.89 ± 0.03 |
0.50 ± 0.01 |
||
|
F3 |
Pale yellow |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
0.89 ± 0.01 |
0.72 ± 0.03 |
||
Table 3. Re-dispersibility and pH of the ES during storage stability testing on days 0, 5, 10, 20, 40 and 60 at RT and 45 ± 5 °C relative humidity.
|
Day |
Test condition |
Formulation |
Re-dispersibility |
pH |
|
0 |
- |
F1 |
6 |
11.03 ± 0.01 |
|
F2 |
6 |
11.02 ± 0.05 |
||
|
F3 |
7 |
11.04 ± 0.03 |
||
|
5
|
RT |
F1 |
6 |
10.37 ± 0.05 |
|
F2 |
6 |
10.77 ± 0.05 |
||
|
F3 |
7 |
10.77 ± 0.08 |
||
|
45 ± 5 °C |
F1 |
6 |
9.97 ± 0.02 |
|
|
F2 |
6 |
9.96 ± 0,01 |
||
|
F3 |
6 |
9.71 ± 0.04 |
||
|
10 |
RT |
F1 |
7 |
10.76 ± 0.01 |
|
F2 |
7 |
10.56 ± 0.01 |
||
|
F3 |
7 |
10.63 ± 0.07 |
||
|
45 ± 5 °C |
F1 |
6 |
9.95 ± 0.01 |
|
|
F2 |
7 |
9.95 ± 0.02 |
||
|
F3 |
7 |
9.75 ± 0.02 |
||
|
20 |
RT |
F1 |
7 |
10.16 ± 0.02 |
|
F2 |
8 |
10.35 ± 0.01 |
||
|
F3 |
8 |
10.35 ± 0.01 |
||
|
45 ± 5 °C |
F1 |
6 |
9.94 ± 0.01 |
|
|
F2 |
7 |
9.91 ± 0.05 |
||
|
F3 |
7 |
9.72 ± 0.01 |
||
|
40 |
RT |
F1 |
6 |
10.06 ± 0.02 |
|
F2 |
8 |
10.25 ± 0.01 |
||
|
F3 |
8 |
10.25 ± 0.01 |
||
|
45 ± 5 °C |
F1 |
6 |
9.64 ± 0.02 |
|
|
F2 |
7 |
9.86 ± 0.07 |
||
|
F3 |
7 |
9.62 ± 0.01 |
||
|
60 |
RT |
F1 |
6 |
10.09 ± 0.02 |
|
F2 |
7 |
10.24 ± 0.01 |
||
|
F3 |
8 |
10.23 ± 0.01 |
||
|
45 ± 5 °C |
F1 |
6 |
9.67 ± 0.02 |
|
|
F2 |
7 |
9.85 ± 0.07 |
||
|
F3 |
7 |
9.65 ± 0.01 |
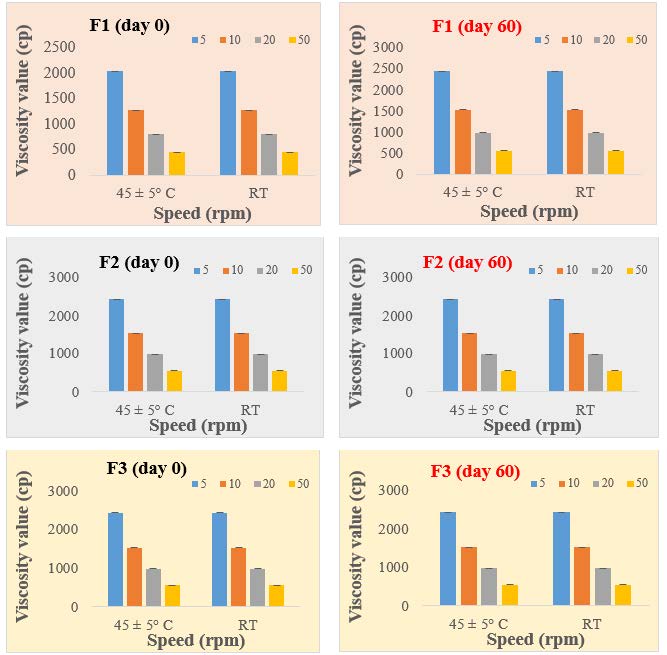
Figure 1. Viscosity values of substances (F1, F2, F3) on Day 0 and Day 60, under two different conditions.
The viscosities day 0 of the 3 formulations ranged from F1= 2030 cP, F2= 2208 cP, F3= 2430 cP, and after 60 days F1= 1949 cP, F2= 2127 cP, F3= 2349 cP. (Figure 1) within the evaluation of suspension specification limits of Viscosity range for HCMC < 5250 cP; Xanthan gum < 8000 cP (Integrated basic drug formulation Sciences) for suspension. However according to strokes law, sedimentation velocity is inversely proportional to the viscosity of the dispersion medium. As viscosity increases, there is decrease in the rate of settling giving rise to good dispersion of particles and enhanced physical stability. Based on this, formulation 1 may be better than the other formulations in terms of physical stability.
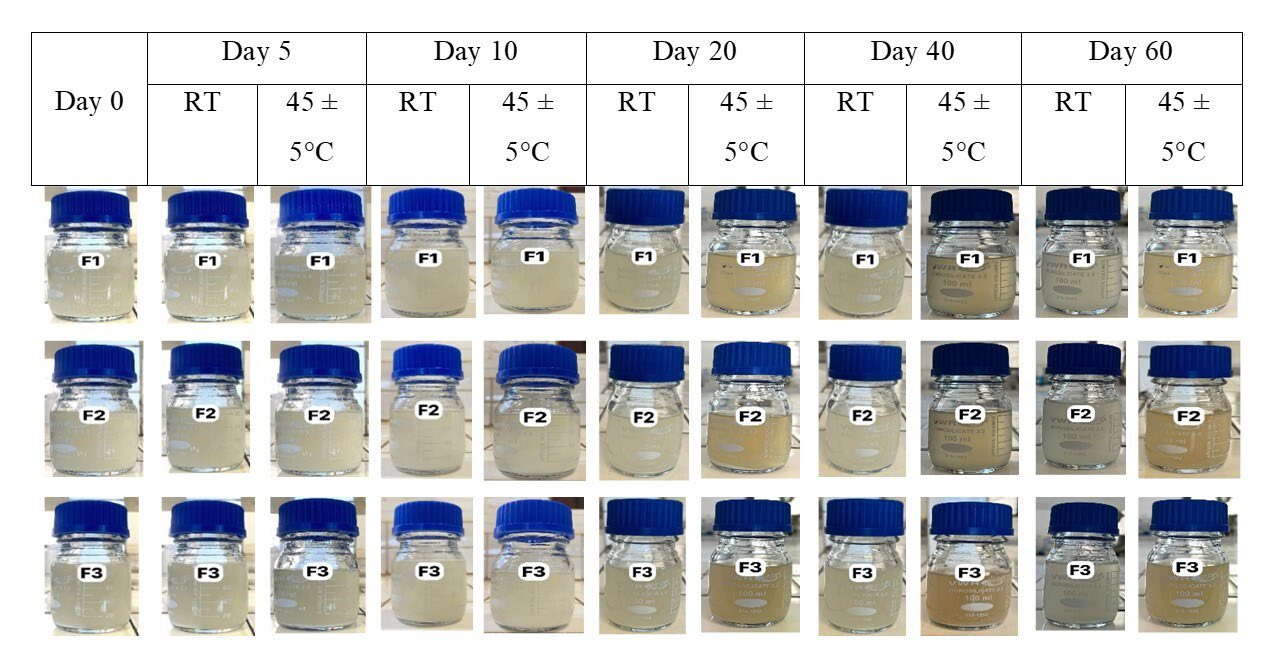
Figure 2. Appearance of the ES during storage stability testing on days 0, 5, 10, 20, 40 and 60 at RT and 45 ± 5°C relative humidity.
The criteria (appearance, re-dispersibility, pH, sedimentation volume, and color) of the three suspensions were assessed using quality control testing tools.
Sedimentation volume and color: an optimal F value near 1 and Color: not change color (original color).
Re-dispersibility: The result was recorded as not easily redisperse, moderately easily redisperse and very easily redisperse (Kadiri and Okafor, 2010).
pH: Rage 7.8 - 11 (Low et al., 2022).
Of the three formulations, F1 had the most favorable physicochemical attributes. Consequently, it was chosen to serve as the medium in further research. The vehicle was combined with the contents of OMP capsules to produce OMP ES at a concentration of 2 mg/ml of OMP. Stored at RT for ten days. The sediment accumulated at the base of the bottle could be readily redisposed through agitation. The pH of the OMP ES exhibits slight variation (Table 4).
Table 4. Physicochemical properties of OMP ES before and after storing at RT for 10 days.
|
Day |
Sedimentation ratio |
Re- dispersibility |
pH |
||||
|
0 min |
15 min |
30 min |
1hr |
24 hr |
|||
|
0 |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
0.98 ± 0.02 |
0.58 ± 0.06 |
6 |
11.00 ± 0.02 |
|
10 |
1.00 ± 0.00 |
1.00 ± 0.00 |
1.00 ± 0.00 |
0.99 ± 0.01 |
0.70 ± 0.01 |
7 |
10.78 ± 0.01 |
Appearance
The criterion for evaluating the suspension's appearance was the preservation of its original white color. The OMP ES samples exhibited a stable hue at 4 ± 3 °C cover a two-month period (Figure 3, Column A). In contrast, the color stability of the OMP ES samples stored at ambient temperature declined, showing a notable change from white/pink to brown/black in just 5 days (Figure 3, Column B). Similarly, the OMP ES samples stored at 45 ± 5 °C had inadequate color stability, changing from white/pink to brown within a single day (Figure 3, Column C).

Figure 3. Color alteration in OMP ES during storage at 4 ± 3 °C (A), RT (B), and 45 ± 5 °C (C). First row: Day 0; second row: Day 1; third row: Day 5; fourth row: Day 10; fifth row: Day 20; sixth row: Day 40; seventh row: Day 60.
Viscosity
Viscosity was assessed on day 0 and again after 60 days. Figure 4A demonstrates that viscosity values decreased with increasing speed for all suspensions on day zero. After a 60 days storage period, viscosity significantly increased at all speeds for the suspensions stored at 4 ± 3 °C (Figure 4B). The suspensions had an initial viscosity, and as the speed increased, the viscosity was found to decrease, as shown in Figure 4. Xanthan gum, which is the main viscosity-enhancing agent in the suspension, is extremely stable (Mudoi et al., 2013). Viscosity decreases with increasing shear rates, flow characteristics are said to be pseudo plastic. This type of flow is encountered with large swollen or dissolved particles. Normally, pseudo plastic flow is exhibited by polymer dispersions like: sodium carboxyl methyl cellulose in water. (Kouchak, 2018).
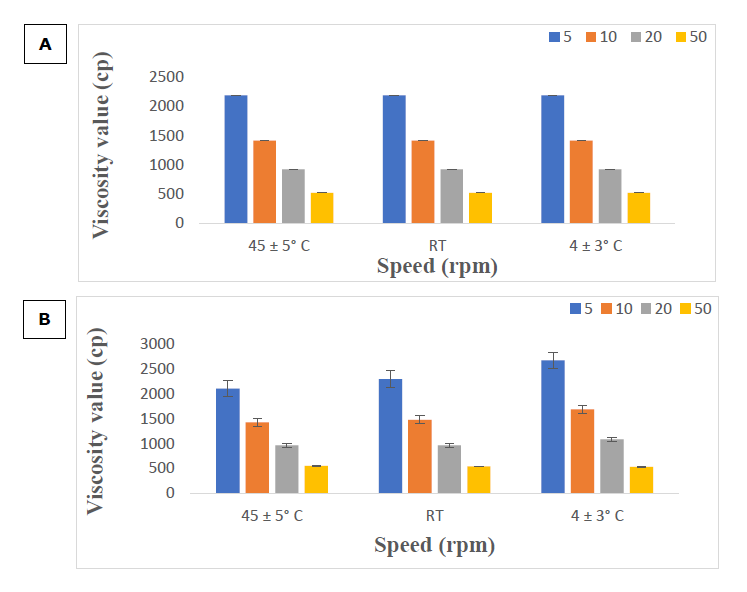
Figure 4. Viscosity value in day 0 under 3 conditions (A) and in day 60 under 3 conditions (B).
pH measurement
OMP ES (2 mg/ml) samples were stored in 100 ml glass bottles, there after placed in plastic bottles coated with aluminum foil, and maintained at cold temperature (4 ± 3 °C), ambient temperature, and 45 ± 5 °C for a period of 60 days. The criterion for stability was the maintenance of the white hue. OMP ES samples preserved at 4 ± 3 °C demonstrated no color change over a 60 days duration (Figure 3, column A). The pH levels were evaluated, resulting in values ranging from 10.08 to 11 under cold conditions. The pH values fluctuated at room temperature (9.46-11) and at 45 ± 5 °C (8.505-11). OMP ES samples stored at 45 ± 5 °C displayed instability, showing unfavorable color changes after 1, 2, and 60 days (Figure 3, columns B and C). OMP ES samples preserved at a refrigerated temperature (4 ± 3 °C) demonstrated no notable fluctuation in pH during the experiment (range 10.08-11, Figure 5, blue line). Similarly, there was no significant change in pH for OMP ES samples stored at RT (range 9.46-11, Figure 5, green line). The sample held at 45 ± 5 °C demonstrated a reduction in pH from 10 to 8.505 after 60 days (Figure 5, red line), indicating instability at this temperature. The suspension lacked a buffering agent, making it vulnerable to pH variations. Hydrolysis, a significant process in drug degradation, can be affected by pH levels (Charde et al., 2013). The pH level affects bacterial growth and the efficacy of preservatives. Thus, pH is a critical factor influencing suspension stability (Stroppel et al., 2023). Samples with varying pH values were assessed throughout time to determine the effect of pH on stability. The suspension maintained a viscous white to brown coloration and was readily re-dispersed (Figure 5). After two months of storage, the appearance and re-dispersibility remained stable, suggesting that the suspension pH likely does not affect these attributes. The labels "column A, B, and C" in Figure 3 appear to be correct. The labels "green, blue, and red" in Figure 5 are accurate, corresponding to the storage conditions: refrigerated, ambient temperature, and 45 ± 5 °C, respectively.
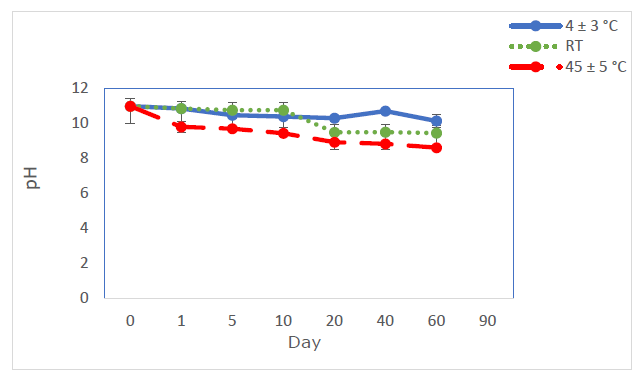
Figure 5. Impact of pH on OMP ES following storage at 4 ± 3 °C (blue line), ambient temperature (green line), and 45 ± 5 °C (red line) (n=3).
Determination of the percentage remaining of omeprazole
The ultraviolet spectra of OMP had a peak absorption wavelength of 302 nm. Thus, a wavelength of 302 nm was used for HPLC analysis to provide maximum sensitivity for the study. Figure 6 depicts the standard curve for OMP. The linear regression of standard curves is represented as X = Y - 0.261/2.0279. The correlation coefficient (R²) was 0.9995 (n=3). The peak regions of the samples were measured, and the concentrations were obtained from the standard. The limit of detection (LOD) and limit of quantification (LOQ) for the analytical method were 2.19 and 5.46 μg/ml, respectively. Table 5 displays intra-day and inter-day precision. The %RSD fell within the approval standards, signifying that the analytical procedure produces reproducible results.
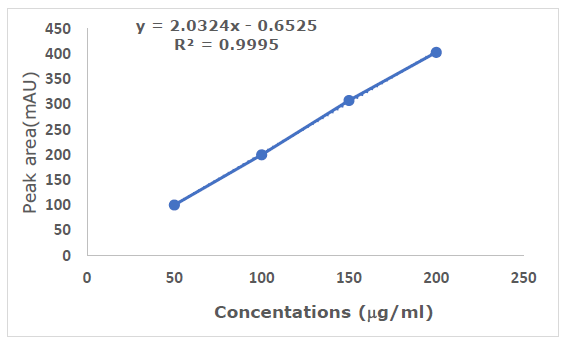
Figure 6. Calibration plot of OMP (n=3).
Table 5 Intra-day and inter-day precision for the analysis method of OMP using HPLC (n=3).
|
Concentration (μg/ml) |
Intra-day Precision |
Inter-day Precision |
||
|
%Recovery |
%RSD |
%Recovery |
%RSD |
|
|
50 |
94.97 - 104.90 |
2.00 - 2.64 |
100.14 |
2.68 |
|
100 |
99.29 - 100.89 |
1.57 - 2.28 |
99.58 |
2.14 |
|
150 |
96.38 - 98.34 |
0.93 - 9.36 |
97.77 |
2.33 |
The recovery percentage fell within the allowed range of 98.0% to 102.0% (Dande et al., 2021), demonstrating that the analytical approach could accurately quantify OMP in OMP ES. Furthermore, these results demonstrated the specificity and selectivity of the analytical approach. OMP ES in liquid form was reconstituted with water and stored at 4 ± 3 °C, as well as at room temperature and 45 °C with 75% relative humidity. The remaining proportion of OMP was between 90% and 110% (Rivai et al., 2018) (Figure 10), indicating stability in the reconstituted OMP ES under three distinct circumstances.
Figures 7, 8, and 9 display the HPLC chromatograms of OMP STD, OMP ES, and the vehicle utilized in the formulation, respectively.
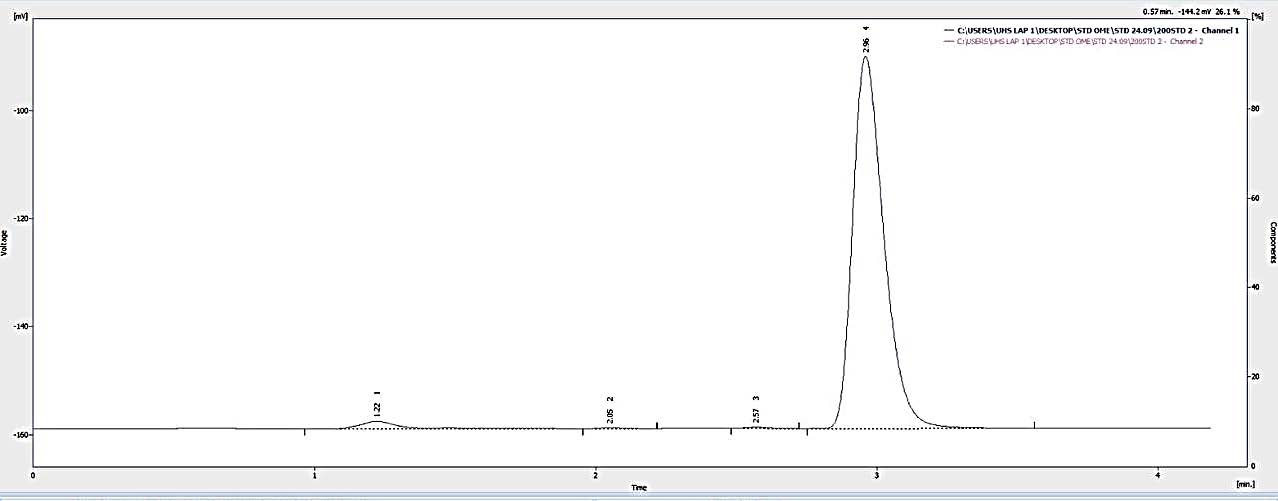
Figure 7. Chromatogram of the omeprazole standard.
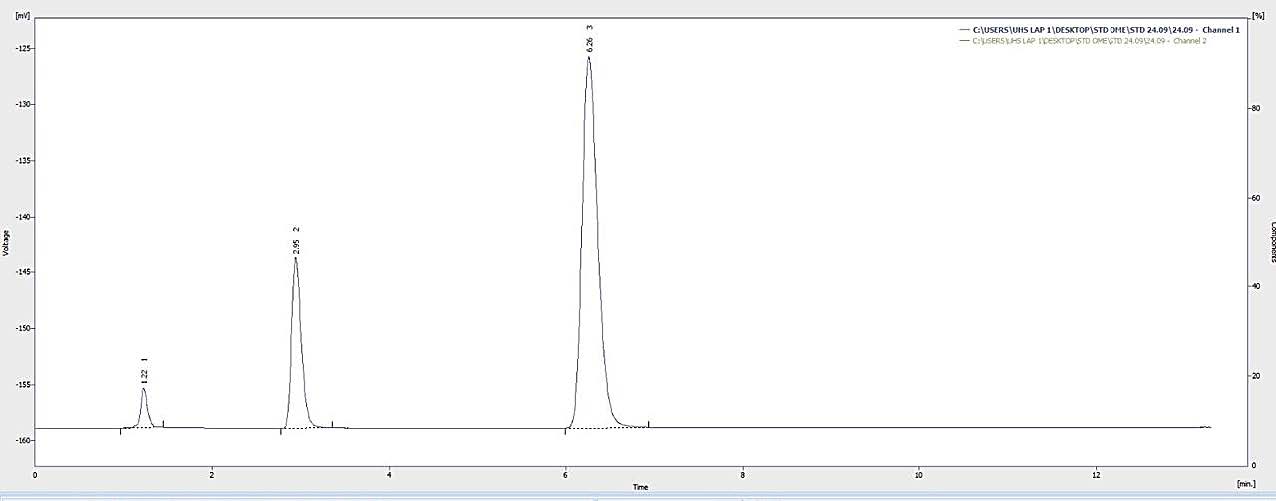
Figure 8. Chromatogram of the omeprazole sample.
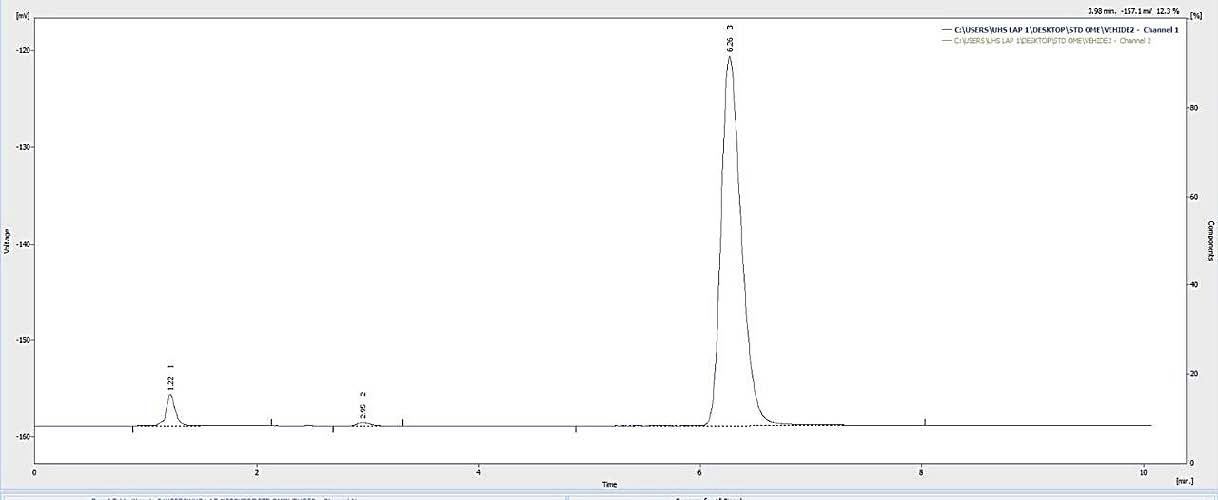
Figure 9. Chromatogram of the vehicle.
For liquid form, OMP ES is stored at 4 ± 3 °C, RT, and 45 ± 5 °C. The remaining percentage of OMP was between 90% and 110% (Figure 10), indicating stability under all three storage conditions.
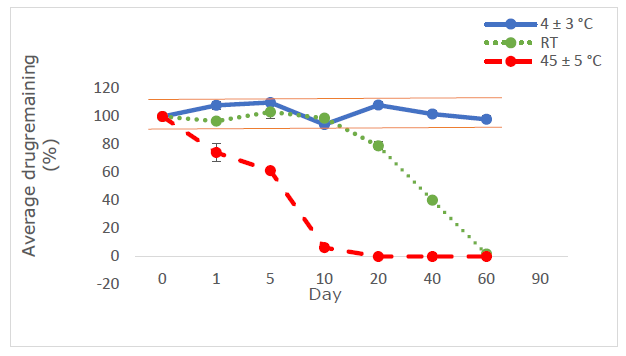
Figure 10. Proportion of medication retained in OMP ES following storage at 4 ± 3 °C (blue line), ambient temperature (green line), and 45 ± 5 °C (red line) (n=3).
DISCUSSION
The purpose of this study was the development of a suspension dosage form, which follows a complex trajectory. The selection of the proper excipients (Kulshreshtha, et al 2009). Lactose can serve as a suspension model due to its insolubility in water, small particle size, availability, and lower cost compared to OMP. The creation of suspensions is designed using a factorial approach for all three formulations. In formulations, SCMC serves as a flocculating agent; its combination yields excellent suspending properties due to the network structures generated by SCMC in water. SCMC is a cellulose ether derivative. Generated by the reaction between sodium mono chloro acetate and alkali cellulose. It is sodium carboxymethyl cellulose. Soluble in water at both elevated and ambient temperatures. Utilize as a stabilizing agent. Viscosity additives and binding agents in food, pharmaceuticals, etc. SCMC remains stable between the pH ranges of 5.0 to 10.0. If the pH is below 5.0, both viscosity and stability will diminish. Exceptional thermal resistance of 160°C (Desplanques et al., 2012). Xanthan gum serves as a suspending agent, with the advantage of maintaining consistent viscosity across a broad spectrum of pH (pH 1-13), temperature, and ionic strength. Xanthan gum is a high molecular weight polymer characterized by a rigid helical structure. It creates intricate molecular aggregates via hydrogen bonding and polymer entanglement (Singhvi et al., 2019), adjusting a pH 11 sodium hydroxide solution, with the products being maintained at room temperature and 45 ± 5 °C.
The ES were subjected to stability testing under RT and 45 ± 5 °C for 60 days. The design of this stability protocol is based on the following reasons: according to U.S. Pharmacopeia (USP-NF, 2017), RT is the correct storage condition for drugs stored in a pharmacy department.
Product samples were examined for color, redispersibility, viscosity, pH, and sedimentation volume after 0, 5, 10, 20, 40 and 60 days of storage. According to Stoke's law, the sedimentation ratio is inversely related to the viscosity of the dispersion medium. As viscosity increases, the settling rate decreases, resulting in improved particle dispersion and greater physical stability. Consequently, formulations 1 and 2 may exhibit superior physical stability compared to the other formulations. The preliminary results indicate that formulation 1 may exhibit superior characteristics regarding redispersibility, viscosity, pH, and sedimentation volume; hence, formulation 1 was selected for adoption. Formulation 1 was chosen for investigation in the stability test of the OMP suspension. Nonetheless, the vehicle exhibited a modest reduction with the rising SCMC concentration, while demonstrating variability. Consequently, the formulation (F1) including 0.1% Tween 80, SCMC, xanthan gum, sodium saccharin, sodium hydroxide, and paraben concentration at 4 % v/v, 0.35 % w/v, 0.5 % w/v, 0.75 % w/v, 0.1 % w/v, and 2 % w/v of the reconstituted solution was chosen for subsequent investigations.
The vehicle underwent stability testing at RT and 45°C, with 75% relative humidity, for a duration of 60 days. 25°C is the appropriate storage temperature for pharmaceuticals maintained in a laboratory at the Faculty of Pharmacy. Furthermore, storage at 45°C was evaluated to confirm the stability of the created vehicle when maintained at ambient temperature in patients' residences, particularly during the summer months. The relative humidity of 75% ± 5% pertains to the typical humidity of Laos's climatic zone IVb, as outlined in the ASEAN Guideline on Stability Study of Drug Products (2010). At the outset of this study, the concentration of all investigated OMP ES was 100%. The residual content of OMP ranged from 90 to 110% after being stored at refrigerated temperatures for up to 60 days. Under room temperature circumstances, the OMP content maintained over 90% for 10 days in glass bottles, but fell below 90% after 1 day at 45 ± 5 °C. This investigation confirmed that the color of OMP ES remained unchanged for 60 days but altered after 60 days at 4 ± 3 °C. The drug's breakdown was swift, leading to distinct peaks on HPLC. The absence of the OMP peak suggests that the OMP preparation lacked stability at elevated temperatures (Figure 9). Previously elucidated the impact of enteric-coated polymers suspended in water on the stability of OMP. The enteric-coated polymer structure comprises phthalate moieties that may induce hydrolysis and breakdown of OMP. In aqueous solutions, the concentration of free acid in certain polymers might impair the efficacy of proton pump inhibitors (PPIs) (Riedel and Leopold, 2005). The chemical stability of OMP in extemporaneous solutions for 28 days at 4 ± 3 °C in this investigation aligns with the findings of a prior work (Baniasadi et al., 2012). This study offers valuable insights into the stability lifetime of OMP drugs, enabling pharmacists to develop extemporaneous solutions and store them securely for future use. The results indicate that refrigeration provides the optimal storage condition for OMP ES among the three temperatures, maintaining the stability of the OMP suspension's physical and chemical characteristics for 60 days. A prior study shown that an OMP suspension exhibited chemical stability under refrigerated conditions. The findings of this study will assist pharmacists in preparing the extemporaneous suspension of OMP. The stability of OMP suspension must be assessed in other vehicles, as the criteria for drug-vehicle selection varies among hospitals. Consequently, pharmacists must examine literature regarding the vehicle that enhances the stability of each drug type to identify an appropriate vehicle.
The objective of this study was to develop a suspension formulation by selecting appropriate excipients. Lactose can serve as a model for suspension since it is insoluble in water, has small particle size, good availability, and low cost compared to OMP (Dominici et al., 2022). The ES formulation was initially developed using suspending agents, sweeteners, anticaking agents, preservatives, and pH adjusters, with the optimal pH for stabilizing OMP found to be 11 (Geng et al., 2019).
The suspending agents used include a mixture of SCMC and xanthan gum. SCMC acts as a flocculating agent to stabilize the suspension and increase viscosity, maintaining stability in the pH range of 5.0 to 10.0. Below pH 5.0, the viscosity and stability decrease. High heat resistance 160°C. Xanthan gum, a high molecular weight polymer with a strong helical structure, forms complex molecular aggregates through hydrogen bonding and polymer entanglement. The advantage of xanthan gum as a suspending agent is its ability to maintain consistent viscosity across a wide pH range (pH 1-13) (Singhvi et al., 2019). Additionally, it has pseudoplastic properties, with high viscosity in static conditions, which decreases when shaken.
The formulation was tested for stability at RT and 45 ± 5 °C for 28 days. The stability protocol was designed based on the following reasons: according to the U.S. Pharmacopeia (USP-NF, 2017), RT is the recommended storage condition for drugs kept in pharmacy departments. Additionally, storage at 45 ± 5 °C was assessed to ensure the formulation’s stability when kept at RT in patient homes, particularly during the summer. The relative humidity of 75% ± 5% corresponds to the typical humidity in climate zone IVb of Lao PDR, according to the ASEAN Guidelines for Stability Studies of Drug Products (2010).
The formulation was also tested for stability at RT and 45 ± 5 °C for 28 days. RT was maintained at the Faculty of Pharmacy, Lao PDR, as it is the appropriate storage condition for drugs. Moreover, storage at 45 ± 5 °C was evaluated to confirm the stability of the drug when stored at RT in patients' homes, especially during the summer months. The relative humidity of 75% ± 5% matches the typical humidity of climate zone IVb of Lao PDR, according to ASEAN guidelines (2010), which sets a short-term expiration date of 28 days for extemporaneously compounded oral products in hospitals to minimize the risk of deterioration and ensure that the product can be prepared in advance (Falconer and Steadman, 2017).
The results indicated that the product samples were checked for color, re-dispersibility, viscosity, pH, and sediment volume after storage at 0, 5, 10, 20, and 28 days. According to Stokes’ law, the rate of sedimentation is inversely related to the viscosity of the dispersion medium. As viscosity increases, the sedimentation rate decreases, resulting in better particle dispersion and improved physical stability. Therefore, F1 and F2 showed superior physical stability compared to other formulations. Preliminary results suggested that F1 might have better re-dispersibility, viscosity, pH, and sediment volume. Hence, F1 was chosen for further stability testing of the OMP suspension. However, the formulation showed a slight decrease in stability as the concentration of SCMC increased, with variability observed. Therefore, F1, which consists of 0.1% Tween 80, SCMC, xanthan gum, sodium saccharin, sodium hydroxide, and preservatives at concentrations of 4% v/v, 0.35% w/v, 0.5% w/v, 0.75% w/v, 0.1% w/v, and 2% w/v, respectively, was selected for further studies. Stability testing showed that the suspension could be prepared in advance stability testing showed that the suspension could be prepared in advance. It can be stored at RT and 45°C.
The suspension was weighed into bottles, and the contents of OMP capsules were added to achieve a concentration of 2 mg/mL of OMP ES in glass bottles. At the start of this study, the OMP ES concentration was monitored at 100%. After storing at refrigeration (4 ± 3 °C), it was observed that the color of OMP ES did not change for 60 days. The pH levels were evaluated, resulting in values ranging from 10.08 to 11 under cold conditions, and the concentration remained between 90% and 110% (Low et al., 2022). When stored at room temperature, the suspension remained stable for 10 days. The pH values fluctuated at room temperature (9.46-11) and at 45 ± 5 °C (pH 8.50 - 11). However, significant degradation of OMP ES occurred quickly after just one day at 45°C, indicating that the formulation is unstable at high temperatures. The degradation of the drug and hydrolysis may depend on the pH, as changes in pH affect microbial growth and the efficacy of preservatives. Thus, pH is a crucial factor to consider for the stability of the product (Sarisuta et al., 1998; Charde et al., 2013).
LIMITATION
pH 11 is not recommended for use with NaOH because NaOH is a highly corrosive substance. Ensuring that a medication retains its potency, purity, and safety throughout its shelf life is paramount for patient well-being. This pursuit often necessitates the meticulous optimization of various formulation parameters, with pH playing a critical role. While our current study has yielded valuable insights into the stability of our target pharmaceutical, it is crucial to acknowledge its inherent limitations, which pave the way for future research and refinement.
CONCLUSION
OMP ES can be stored at 4 ± 3°C for a maximum of 60 days and at RT for up to 10 days, maintaining a drug content above 90 percent. The assay content complies with the permitted specifications. However, the same formulation demonstrated instability when stored at 45 ± 5°C. The OMP ES, stored at 45 ± 5°C, had diminished stability, with significant color changes and drug content dropping below 90% within one day of storage.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the ministry of education and sport, science and technology development fund, Laos for providing financial support and university of health sciences, faculty of pharmacy, Laos or use of their laboratory facilities.
AUTHOR CONTRIBUTIONS
Thatsanand Xayavong, Noudy Sengxeu, Phonesouphavanh Mayvanh, and Sonesay Thammavong assisted in conducting the experiments, performed the statistical analysis and data visualization and wrote the manuscript. David Nugroho, Rachadaporn Benchawattananon, Theera Rittirod, Khamla Phonsaiyalinkham, Sounantha Souvanlasy, and Chanthanom Manithip designed and conducted all of the experiments and wrote the manuscript. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Ali, Y., Kimura, A., Coffey, M.J., and Tyle, P. 2010. Pharmaceutical development of suspension dosage form. Pharmaceutical suspensions: From formulation development to manufacturing. 103: 126.
ASEAN Guideline on Stability Study of Drug Product 2010. Revision 25 the ACCSQ-PPWG, ASEAN, Thailand. https://asean.org/wp-content/uploads/2018/01/ 25PPWG-ANNEX-7 iv-Final-ASEAN-Guideline-on-Stability-Study-Drug-Product-R2.pdf. Accessed March 2023.
Baniasadi, S., Kobarfard, F., and Fahimi, F. 2012. Extemporaneous preparation and stability assessment of omeprazole suspension in a teaching hospital. International Journal of Pharmacy Teaching and Practices. 3: 418-421.
Boscolo, O., Perra, F., Salvo, L., Buontempo, F., and Lucangioli, S. 2020. Formulation and stability study of omeprazole oral liquid suspension for pediatric patients. Hospital Pharmacy. 55(5): 314-322.
Charde, M.S., Kumar, J., Welankiwar, A.S., and Chakole, R.D. 2013. Review: Development of forced degradation studies of drugs. International Journal of Advances in Pharmaceutics. 2(3): 34-39.
Dande, A., Reddy, G.R., and Saithya, G.S. 2021. Stability indicating method development and validation for assay of omeprazole sodium for injection by a rapid RP-HPLC. Asian Journal of Pharmaceutical Research and Development. 9(2): 18-26.
Desplanques, S., Renou, F., Grisel, M., and Malhiac, C. 2012. Impact of chemical composition of xanthan and acacia gums on the emulsification and stability of oil-in-water emulsions. Food Hydrocolloids. 27(2): 401-410.
Dominici, S., Marescotti, F., Sanmartin, C., Macaluso, M., Taglieri, I., Venturi, F., Zinnai, A., and Facioni, M.S. 2022. Lactose: Characteristics, food and drug-related applications, and its possible substitutions in meeting the needs of people with lactose intolerance. Foods. 11(10): 1486.
Falconer, J.R., and Steadman, K.J. 2017. Extemporaneously compounded medicines. Australian Prescriber. 40(1): 5.
Geng, L., Han, L., Huang, L., Wu, Z., Wu, Z., and Qi, X. 2019. High anti acid omeprazole lightweight capsule for gastro-enteric system acid related disorders treatment. Journal of Clinical Gastroenterology and Treatment. 2(5): 068.
Glass, B.D., and Haywood, A. 2006. Stability considerations in liquid dosage forms extemporaneously prepared from commercially available products. Journal of Pharmacy and Pharmaceutical Sciences. 9(3): 398-426.
Hassan, B.R. 2012. Overview on pharmaceutical formulation and drug design. Pharmaceutical Analysis and Acta. 3(10): 1000e140.
International Conference on Harmonization (ICH). 2005. Validation of analytical procedures: Text and methodology Q2(R1). Geneva: International Federation of Pharmaceutical Manufacturers and Associations.
Israel, D.M., and Hassall, E. 1998. Omeprazole and other proton pump inhibitors: pharmacology, efficacy, and safety, with special reference to use in children. Journal of Pediatric Gastroenterology and Nutrition. 27(5): 568-579.
Jackson, M., Lowey, A., and NHS Pharmaceutical Quality Assurance Committee. 2010. Handbook of extemporaneous preparation: A guide to pharmaceutical compounding. Dosage forms. International Current Pharmaceutical Journal. 1(11): 366-369.
Kadiri, J. O., and Okafor, I. S. 2010. Evaluation of the suspending property of Grewia gum in metronidazole suspension. Nigerian Journal of Pharmaceutical Research. 8(1): 247-254.
Kayesh, R., Sultan, M.Z., Rahman, A., Uddin, M.G., Aktar, F., and Rashid, M.A. 2013. Development and validation of a RP-HPLC method for the quantification of omeprazole in pharmaceutical dosage form. Journal of Scientific Research. 5(2): 335-342.
Kommanaboyina, B., and Rhodes, C.T. 1999. Trends in stability testing, with emphasis on stability during distribution and storage. Drug Development and Industrial Pharmacy. 25(7): 857-868.
Kulshreshtha, A.K., Singh, O.N., amd Wall, G.M. 2009. Pharmaceutical suspensions: from formulation development to manufacturing. Springer Science and Business Media.
Low, M., Singh, S., Venkataya, B., Pearson, J., Ibrahim, M., Jarouche, M., Khoo, C., Rowe, J., and Li, C.G. 2022. Stability of omeprazole in a commercial calcium Carbonate based oral suspension at 2, 5 and 10 mg/mL stored under refrigeration (4°C) for 70 days. Journal of Pharmacy Practice and Research. 52: 34-41.
Lyu, Y., Wang, J., Zhi, X., Wang, X., Zhang, H., Wen, Y., Park, E., and Dung, D.T. 2024. The characterization, mechanism, predictability, and impacts of the unprecedented 2023 Southeast Asia heatwave. Nature Partner Journals Climate and Atmospheric Science. 7(1): 246.
Nataraj, K.S., Duza, M.B., Pragallapati, K., and Kumar, D.K. 2012. Development and validation of RP-HPLC method for the estimation of omeprazole in bulk and capsule dosage forms. International Current Pharmaceutical Journal. 1(11): 366-369.
Oosterhuis, B., and Jonkman, J.H. 1989. Omeprazole: Pharmacology, pharmacokinetics and interactions. Digestion: The Official Journal of the Mediterranean Society of Gastrointestinal Surgery. 44(Suppl 1): 9-17.
Pitaksuteepong, T., Kesornmalee, K., and Phanaphai, N. 2024. Development and stability study of oseltamivir reconstitutable dry suspension. Natural and Life Sciences Communications. 23(1): E2024010.
Queen Sirikit National Institute of Child Health. 2017. Queen Sirikit National Institute of Child Health: Info graphic.
Riedel, A., and Leopold, C.S. 2005. Degradation of omeprazole induced by enteric polymer solutions and aqueous dispersions: HPLC investigations. Drug Development and Industrial Pharmacy. 31(2): 151-160.
Rivai, H., Hasanah, R., and Azizah, Z. 2018. Development and validation of omeprazole analysis methods in capsules with absorbance methods and areas under curves methods with UV Vis spectrophotometry. International Journal of Pharmaceutical Sciences and Medicine. 3(3): 21-32.
Rowe, R.C., Sheskey, P., and Quinn, M. 2009. Handbook of pharmaceutical excipients. Libros Digitales-Pharmaceutical Press. London.
Sarisuta, N., Tourtip, T., and Chuarcharoern, S. 1998. Chemical stability and mechanism of degradation of omeprazole in solution. The Thai Journal of Pharmaceutical Sciences. 22(2): 81-88.
Singhvi, G., Hans, N., Shiva, N., and Dubey, S.K. 2019. Xanthan gum in drug delivery applications. p. 121-144. In Md Saquib Hasnain and Amit Kumar Nayak (eds) Natural polysaccharides in drug delivery and biomedical applications. Academic Press, London.
Sinly, C., Saito, H., Anorach, R., Uramaru, N., Kaku, T., Benchawattananon, R., and Rittirod, T. 2022. Stability consideration in extemporaneous omeprazole suspension. Asia-Pacific Journal of Science and Technology. 28(1): APST-28.
Stroppel, L., Schultz-Fademrecht, T., Cebulla, M., Blech, M., Marhöfer, R. J., Selzer, P. M., and Garidel, P. 2023. Antimicrobial preservatives for protein and peptide formulations: An overview. Pharmaceutics. 15(2): 563.
Takemoto, C.K., Hodding, J.H., and Kraus, D.M. 2003. Pediatric dosage handbook. 13th ed., p. 1418. Lexi-Comp, Inc. American Pharmacists Association.
United States Pharmacopeia and National Formulary. 2017. 〈659〉 Packaging and Storage Requirements. Rockville, MD: United States Pharmacopeia Convention.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Thatsanand Xayavong1, *, Noudy Sengxeu1, Phonesouphavanh Mayvanh1, Sonesay Thammavong1, Khamla Phonsaiyalinkham1, Sounantha Souvanlasy1, Chanthanom Manithip2, David Nugroho3, Rachadaporn Benchawattananon3, and Theera Rittirod4
1 Faculty of Pharmacy, University of Health Sciences, Vientiane Capital, Lao People’s Democratic Republic.
2 Lao Red Cross, Vientiane Capital, Lao People’s Democratic Republic.
3 Department of Integrated Science, Faculty of Sciences, Khon Kaen University, Khon Kaen 40002, Thailand.
4 Faculty of Pharmacy, Nakhon Ratchasima College, Nakhon Ratchasima 30000, Thailand.
Corresponding author: Thatsanand Xayavong, E-mail: thatsanandxayavong@gmail.com
ORCID ID:
Thatsanand Xayavong: https://orcid.org/0009-0007-6862-0854
Chanthanom Manithip: https://orcid.org/0000-0001-66-4147
David Nugroho: https://orcid.org/0000-0002-5628-9283
Theera Ritthirod: https://orcid.org/0009-0002-7374-6201
Total Article Views
Editor: Nisit Kittipongpatana,
Chiang Mai University, Thailand
Article history:
Received: December 17, 2024;
Revised: April 20, 2025;
Accepted: May 20, 2025;
Online First: June 12, 2025

