
Fermented Java Plum (Syzygium cumini (L.) Skeels) Juice Using Lactiplantibacillus plantarum: Effect of Fermentation on Its Antioxidant Capacity, α-Glucosidase Inhibition, and Sensory Properties
Anna Rina B. Evangelista-Albacea, Lloyd Earl L. Flandez, Katherine Ann T. Castillo-Israel, and Rona Camille M. Lizardo-Agustin*Published Date : May 15, 2025
DOI : https://doi.org/10.12982/NLSC.2025.045
Journal Issues : Number 3, July-September 2025
Abstract Fruit juices are recently being explored to create functional beverages that promote health and well-being. This study explored the development of fermented Java Plum (Syzygium cumini (L.) Skeels) juice using L. plantarum as a functional beverage with high antioxidants. Java plum juice inoculated with L. plantarum was fermented for 96 hours and its physicochemical, phenolic content, antioxidant activity, α-glucosidase inhibition, and phenolic profile were investigated against an unfermented Java plum juice. Results showed that fermented and unfermented samples were not significantly different in terms of their phenolic and flavonoid content. In contrast, the hydrogen peroxide (H2O2) radical scavenging activity of the juice samples were 1.7 times higher while the 2,2’-azino-biz-(3-ethylbenzothiazoline-6-sulfonic) acid) (ABTS) radical scavenging activity of the juice samples were 4.7 times higher compared to the radical scavenging activity of 0.1% BHT. Also, the fermented juice exhibited a significant one-fold increase in α-glucosidase inhibition activity than its unfermented counterpart at α = 0.05. HPLC analysis revealed that gallic acid is the predominant phenolic acid in the juice followed by catechin and rutin hydrate. In addition, sensory evaluation using the 9-point hedonic scale showed that the Java plum fermented juice is generally acceptable. With these, fermentation can be a viable process in the development of a Java plum juice with its antioxidant capacity conserved while improving its α-glucosidase inhibition activity and sensory attributes.
Keywords: Java plum, Lactic acid fermentation, Lactiplantibacillus plantarum, Antioxidant activity, α-glucosidase inhibition
Funding: This research was funded by the University of the Philippines Emerging Interdisciplinary Research (EIDR) Program (OVPAA-EIDR-C09-15).
Citation: Evangelista-Albacea, A.R.B., Flandez, L.E.L., Castillo-Israel, K.A.T., and Lizardo-Agustin, R.C.M. 2025. Fermented java plum (Syzygium cumini (L.) Skeels) juice using Lactiplantibacillus plantarum: Effect of fermentation on its antioxidant capacity, α-glucosidase inhibition, and sensory properties. Natural and Life Sciences Communications. 24(3): e2025045.
INTRODUCTION
The consumption of plant-based food has been increasing recently due to shifting interests towards healthier diets and sustainable food production. While animal-based food such as dairy-based beverages (i.e. milk, yogurt) offer an excellent source of vitamins and minerals, these products are also associated with high levels of cholesterol and saturated fat which are linked to higher risks for cardiovascular ailments and chronic diseases brought by increased levels of low-density lipoprotein (LDL) (Sharma et al., 2024). Other long-standing challenges with dairy-based beverages are lactose and casein intolerance and allergy to milk proteins (Pérez-Rodríguez et al., 2023). With these concerns in mind, consumers are becoming interested in adding non-dairy-based beverages, particularly those with functional properties, to their diets (Sharma and Mishra, 2013).
Recently, fruit juices have been explored as suitable matrices for the development of functional food. Consumption of fruit juices is linked to body detoxification and has a significant impact on individuals with hypercholesterolemia (Wallace et al., 2020). As a product by itself, fruit juices exert positive health effects due to their antioxidant and anti-aging properties (Ozcelik et al., 2021). The benefits mentioned lead researchers to use fruit juices as substrates for fermentation to diversify the choice for plant-based beverages.
Java plum (Syzygium cumini (L.) Skeels) is a valuable plant that is associated with various health benefits. The tree is widely distributed in Asian countries including Thailand and the Philippines wherein its fruit, seed, leaves, and bark are used in treating cough, dysentery, and inflammation (Sabino et al., 2018; Kishore, 2019). Anthocyanins contribute to the peel’s deep purple color while the combination of sugars, organic acids, and phenolics results in its sweet, sour, and astringent flavor (Ayyanar and Subash-Babu, 2012). Gallic acid is the predominant organic acid while maltose is the predominant sugar which does not offer a high level of sweetness to the fruit. The exotic astringent flavor is brought by its tannins (Sabino et al., 2018). Java plum fruit exerts antihyperlipidemic activity and is traditionally used in the treatment of pharyngitis and splenic diseases (Chagas et al., 2015). Extracts of the fruit also have antidiabetic effects and antiproliferative activity on cancer cells (Aqil et al., 2012; Saurabh et al., 2019). Even while having these properties, the fruit in freeze-dried form does not exhibit oral toxicity as tested in ICR mice therefore having great potential to be used in the development of food products (Estacio et al., 2020).
Fermentation is a viable processing method for the enhancement of nutritional value, organoleptic quality, and shelf-life of food (Torino et al., 2013). Fermentative microorganisms utilize enzymes to soften plant tissue, thus releasing nutrients and bioactive compounds in the food matrix. Softened plant tissues also result in improved digestibility and reduced cooking times, thereby aiding in the retention of nutrients in the fermented food. Biotransformation reactions also result in the formation of unique flavors, aromas, and textures. In terms of shelf-life, fermentative microorganisms suppress the growth of pathogenic microorganisms through the decrease in pH of the food matrix during fermentation and the production of bacteriocins (Xiang et al., 2019). Lactiplantibacillus plantarum is one of the most common microorganisms in fermentation and has provided many benefits. Particularly in fruit juices, L. plantarum has been applied to rabbiteye blueberries (Vaccinium ashei) which increased its superoxide dismutase-like activity, DPPH and alkyl radical scavenging activity, and antiproliferative activity against human cervical carcinoma HeLa cells through induction of apoptosis (Ryu et al., 2019). On the other hand, fermentation of myrtle berries (Myrtus communis L.) using L. plantarum resulted in 5-10 times increase of total phenols, flavonoids, and anthocyanins compared to non-fermented samples and in a 30% increase of DPPH radical scavenging activity (Curiel et al., 2015).
This study explored the development of a fermented beverage through the application of L. plantarum to Java plum juice. The impact of fermentation on the physicochemical attributes, phenolic and flavonoid content, antioxidant activity, α-glucosidase inhibition properties, phenolic profile, and sensory properties of the Java plum juice was also investigated. Correlation among these properties was also evaluated which could aid in the development of a new plant-based fermented beverage.
MATERIAL AND METHODS
Materials
Fresh and fully ripe Java plum fruits were harvested from Urdaneta, Pangasinan, and Calauan, Laguna, Philippines. The fruits were washed and sanitized with dilute sodium hypochlorite solution (200 ppm). After air drying, the fruits were packed in polyethylene bags and were stored in the freezer at -20oC until use in the experiment. Slants of Lactiplantibacillus plantarum BIOTECH 1223 (L. plantarum) were obtained from the Philippine National Collection of Microorganisms at the UPLB National Institute of Molecular Biology and Biotechnology.
Inoculum preparation
The L. plantarum inoculum was prepared by the enrichment of cells in MRS broth (TM Media, India). About ten (10) loopfuls of the microorganism were aseptically transferred in the sterile broth (100 ml) which was incubated at 30-32 °C for approximately 15 hours. Through centrifugation at 3,900 rpm for 10 minutes, the cells were washed twice with 0.85% sodium chloride solution and were then resuspended in sterile distilled water. The optical density of the inoculum was taken at 600 nm and was adjusted to 2.75 absorbance (Abs) to attain a 1010 CFU ml-1 inoculum population.
Fruit juice preparation
Frozen Java plum fruits were deseeded, and the peel and pulp were homogenized to make a puree. The fruit juice was prepared by combining Java plum puree and distilled water (around 1:1) which was then heated to 55-65 °C to aid the extraction of juice from the pulp. Afterward, the pulp was removed by straining through a cheesecloth and the total soluble solids of the juice was adjusted to around 12-13 °Brix by the addition of sugar. Next, the juices were pasteurized at 85-95 °C for 30 minutes and were cooled immediately to room temperature using a water bath. The pasteurized Java plum juice was used for fermentation.
Java plum juice fermentation
Approximately 1010 CFU ml-1 of L. plantarum BIOTECH 1223 (Philippine National Collection of Microorganisms) was inoculated into 100 grams of the pasteurized Java plum fruit juice at the rate of 2.69% weight of the juice. Based on an initial study by the authors regarding fermentation temperature and time, the juice was fermented for 96 hours at 30-32 °C without shaking. A control sample of the pasteurized juice which did not undergo fermentation was kept at 4°C until analysis. Samples for analysis were drawn every 24 hours and juice samples at the 96th hour of fermentation were kept to compare with the control sample.
Analysis of the physicochemical changes in the juice
Changes in the total soluble solids, pH, and titratable acidity of the fermented samples were measured every 24 hours until the end of the fermentation. In addition, changes in the viable cell count of L. plantarum were measured using the spread plate method in MRS agar (TM Media, India).
Analysis of changes in the phenolic content of the juice
Phenolic extract preparation
Phenolics from unfermented and fermented juice samples were extracted using the method by Sartagoda et al. (2021) with modifications. A solvent composed of methanol (Duksan Pure Chemicals Co., Ltd, South Korea), distilled water, and glacial acetic acid (RCI Labscan, Thailand) with a 50:50:1 ratio was prepared. Juice samples were added with the solvent in a 1:2 juice:solvent ratio and were placed in a shaker for 5 minutes. The samples were then filtered through coarse filter paper and kept in amber bottles. The methanolic extracts were kept at 4 °C until use for analysis.
Determination of total phenolic content and total flavonoid content
The total phenolic content (TPC) was measured using the Folin-Ciocalteu colorimetric method by Swain and Hillis (1959) in microplate format. The juice extracts (10 µL) were added with 90 µL triple distilled water and were mixed with 10 µL Folin-Ciocalteu reagent (Loba Chemie Pvt. Ltd., India). The samples were incubated at room temperature for 5 minutes and 100 µL of 7% Na2CO3 was added. Then, samples were diluted to a final volume of 250 uL using triple distilled water and were incubated at room temperature in dark conditions for 2 hours. The absorbance of the samples was measured at 630 nm using a microplate reader (Biobase ELISA microplate reader BK-EL10D, China). A standard curve of gallic acid (Sigma-Aldrich, Germany) was prepared (3 – 100 µg gallic acid/ml) and the results were expressed as mg gallic acid equivalents (GAE) per 100 ml of juice. In addition, the total flavonoid content (TFC) of the samples were analyzed using the method of Reginio et al. (2020) with modifications. The juice extracts (33 µL) were mixed with 133 µL of triple distilled water and 10 µL of 5% NaNO2 solution. The samples were incubated for 5 minutes. Afterwards, 10 µL of 10% AlCl3 ∙ 6H2O was added and was allowed to stand for 6 minutes. Then, 67 µL of 1 M NaOH (Duksan Pure Chemicals Co., Ltd, South Korea) was added and the samples were diluted to a final volume of 330 µL using triple distilled water. The absorbance of the mixture was immediately read at 492 nm. A standard curve of quercetin (Sigma-Aldrich, Germany) was prepared (4 – 250 ug quercetin/ml), and the total flavonoid content of the samples was expressed as mg quercetin equivalents (QE) per 100 ml of juice.
Analysis of antioxidant activity
The hydrogen peroxide scavenging activity, ABTS radical scavenging activity, and ferric reducing power activity (FRAP) of the juice samples were evaluated. The antioxidant activity of the juice samples was measured against the standard food antioxidants, butylated hydroxytoluene (BHT) (0.1%) and butylated hydroxyanisole (BHA) (0.1%) (Sigma-Aldrich, Germany). The antioxidant activity using the three assays was computed using the following formula:
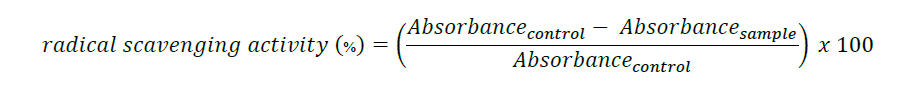
Hydrogen peroxide scavenging activity
The hydrogen peroxide scavenging activity was analyzed using the method of Lizardo et al. (2020) with minor modifications. One hundred microliters (100 µL) of the samples were added with 20 µL H2O2 and were incubated at 37°C for 5 minutes. Afterwards, 30 µL of 1.25 mmol/L ABTS (Sigma-Aldrich, Germany) and 30 µL of peroxidase (1 unit/ml) were added. The mixture was incubated at 37°C for 5 minutes and the absorbance was read at 405 nm. The hydrogen peroxide scavenging activity (%) was calculated using the previously mentioned formula.
ABTS radical scavenging activity
The ABTS radical scavenging activity of the samples was analyzed using the method of González-Palma et al. (2016) with modifications. Samples (5 µL) were added with 195 µL ABTS radical solution and were allowed to incubate at room temperature for 6 minutes. The absorbance was read at 630 nm. The ABTS radical scavenging activity (%) was calculated using the previously mentioned formula.
DPPH radical scavenging activity
The antioxidant activity of the juice samples was determined using the methods described by Lizardo et al. (2020) with modifications. Forty microliters (40 µL) of methanolic extracts and 50% methanol (control) were reacted with 200 uL of DPPH reagent (Loba Chemie Pvt. Ltd., India). The microplate was shaken for 10 seconds and was incubated in the dark for 30 minutes at room temperature. After incubation, the absorbance was read at 492 nm. The DPPH radical scavenging activity (%) was calculated using the previously mentioned formula.
Ferric reducing power activity (FRAP assay)
The reducing power of the samples was determined using the method by Vijayalakshmi and Ruckmani (2016) and Lizardo et al. (2020) with modifications. Samples (50 µL) and 50% methanol (blank) were added with 50 uL of 0.2M phosphate buffer (pH 6.6) and 50 µL of 1% potassium ferricyanide solution. The mixture was shaken and was incubated at 50°C for 20 minutes. After incubation, 50 µL of 10% trichloroacetic acid was added to the mixture. Next, 50 µL of triple distilled water and 10 µL of 0.1% ferric chloride were added. Afterward, the absorbance values of the solutions were read at 620nm against the blank. A higher absorbance value indicates a greater amount of antioxidants in the sample.
α-Glucosidase inhibition acitivity
The α-glucosidase inhibition activity of the samples was determined by the method of Elya et al. (2012) with modifications. Juice samples diluted with distilled water (50 µL) was incubated with 50 µL α-glucosidase (0.1 unit/ml) (Sigma-Aldrich, Germany). Afterward, 50 µL of the p-nitrophenyl-β-glucopyranoside (pNPG) (Sigma-Aldrich, Germany) substrate solution was added and was allowed to stand at 37°C for 20 minutes. Then, 100 µL of 0.1 M sodium carbonate (Na2CO3) was added. A blank control (triple distilled water) was also prepared, and the absorbance of the solutions was read at 405 nm. Calculation of inhibition activity used the same equation used for the antioxidant activity assays.
Phenolic profiling using HPLC
The phenolic compounds of the samples were determined using High-Performance Liquid Chromatography (Shimadzu Inc., Tokyo, Japan) equipped with an LC-20 AD pump with DGU-20A5R degasser, Sil-20 AHT UFLC autosampler, and SPD-M20A diode array detector. Separation of the bioactive compounds was carried out using Inertsil ODS-3 (250 mm x 4.5 mm x 5 µm) reverse phase column protected with an Inertsil ODS-3 (4.0 mm x 10 mm x 5 µm) guard column (Tokyo, Japan). The chromatographic analysis was maintained at 30°C using a CTO-10ASVP column oven (Japan). The mobile phase was composed of solvent A (2 acetonitrile: 98 H2O acidified with acetic acid) and solvent B (98 acetonitrile: 2 H2O acidified with acetic acid). The solvent gradient was as follows: 10–30% B over 6 minutes, maintained until 8 minutes, increased to 50% B over the next 12 minutes, held isocratically until 17 minutes, then elevated up to 90% at 25 minutes, and finally decreased to 10% by 35 minutes, holding steady until 40 minutes. A flow rate of 0.8 ml/minute was used and 20 µL of sample was injected. The identification and quantification of phenolic compounds in the fermented fruit juice extracts were performed by comparing their retention times and peak areas with those of phenolic standards (Sigma-Aldrich, Singapore), measured at their specific absorption maxima: 275 nm for gallic acid, catechin, epicatechin, and syringic acid; 254 nm for rutin hydrate, caffeic acid, ellagic acid, myricetin, and quercetin; 305 nm for p-coumaric acid and resveratrol; and 325 nm for trans-ferulic acid.
Sensory evaluation
The fermented juice sample was subjected to consumer acceptability using a 9-point hedonic scale. An ethical clearance was granted by the San Juan de Dios Educational Foundation Incorporated (IRB Reference No: SJIRB-2023-0003/E-OTR) prior to the sensory evaluation proper. Fifty (50) consumer-type panelists from all genders, age 18-59 years, were pre-screened for product usage, health status, and willingness to participate. The panelists were given 20 ml samples of the pasteurized juice which was assessed for its flavor (overall), flavor (intensity), taste (overall), aftertaste, overall appearance, and overall acceptability.
Experimental design and statistical analysis
The analyses were done in completely randomized design (CRD) and were performed with three independent replicates. The results of the analyses were reported as mean ± standard deviation. Two sample t-test was used to determine differences between the unfermented and fermented samples while Analysis of variance (ANOVA) was used to determine differences of the juice samples’ characteristics with those of the standard antioxidants. In addition, Kendall’s tau correlation was performed to determine the relationship of the phenolic and flavonoid contents with the biological activities. Statistical analyses were performed using Minitab 18 Statistical Software (State College, PA, 2017), XLSTAT 2023.1.6 (Addinsoft, 2023), and jamovi version 2.4.4 (jamovi, 2023).
RESULTS
Physicochemical changes in the juice during fermentation
Java plum fruits and its conversion to unfermented and fermented juice are shown in Figure 1a-b. On the other hand, changes in the physicochemical characteristics of the juice are shown in Figures 2a and 2b. In Figure 2a, the pH of the juice increased very minimally during fermentation from pH 4.33 ± 0.06 to pH 4.43 ± 0.06 after 96 hours of fermentation. On the other hand, the titratable acidity of the juice has a decreasing and increasing pattern after every 24 hours of fermentation. The decrease in titratable acidity is corroborated by the increase in the pH of the juice. In contrast, Figure 2b shows a complete growth curve for L. plantarum and the total soluble solids during fermentation. The growth curve shows a lag, log, stationary, and death phase of the L. plantarum while fermenting the substrates from the juice. There is a significant increase in the bacterial population after 72 hours of fermentation and further fermentation to 96 hours showed a population decline. In contrast, the total soluble solids of the juice were found to decrease from 12 oBrix to 11 oBrix after 48 hours of fermentation.
Figure 1. Conversion of Java plum into a fermented beverage. Java plum fruit (a); unfermented juice (left) and fermented juice (right) (b).
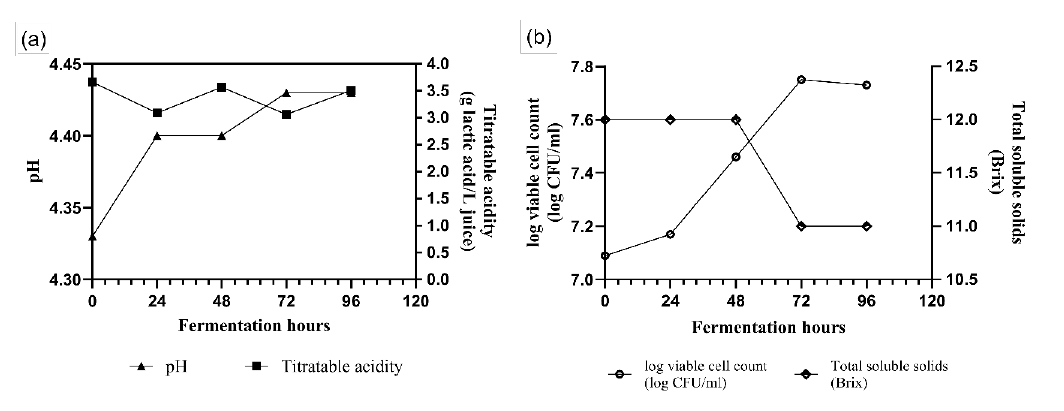
Figure 2. Changes in pH and titratable acidity every 24 hours of fermentation (a); Changes in viable cell count and total soluble solids (TSS) every 24 hours (b).
Changes in the TPC, TFC, antioxidant activity, and α-glucosidase inhibition of the juice
The total phenolic and flavonoid content of the unfermented and fermented juices was determined. In Figure 3a, the total phenolic content of the juice had an insignificant decrease from 99.00 ± 6.17 mg GAE per 100 ml juice to 96.73 ± 4.96 mg GAE per 100 ml juice after fermentation. In contrast, the total flavonoid content of the juice increased from 14.14 ± 0.98 mg QE per 100 ml juice to 16.41 ± 3.38 mg QE per 100 ml juice after fermentation, but the change is similarly insignificant.
The antioxidant activity and inhibition of the α-glucosidase enzyme were measured for the unfermented and fermented juice samples. Figure 3b shows the inhibition activities of the juice samples in comparison with standard inhibitors (0.1% BHT and BHA) against H2O2, ABTS, and DPPH radical species. It was observed that both the unfermented and fermented juice samples have a significantly higher H2O2 radical scavenging activity than 0.1% BHT but they have a significantly lower H2O2 radical scavenging activity than 0.1% BHA. In terms of ABTS radical scavenging activity, both the unfermented and fermented juice samples have a higher activity than 0.1% BHT and the activity of the unfermented juice is comparable to 0.1% BHA. The fermented juice was found to have a significantly lower ABTS radical scavenging activity than 0.1% BHA. For DPPH radical scavenging activity, the unfermented sample had comparable activity with 0.1% BHT. In contrast, the fermented sample had a significantly lower DPPH radical scavenging activity than the other samples.
The ferric-reducing antioxidant power (FRAP) activity of the samples in comparison with the standard inhibitors was also tested. Figure 3c shows that the juice samples are not significantly different in their FRAP activity, but both have significantly lower FRAP activity than 0.1% BHA.
Lastly, as seen in Figure 3d, the α-glucosidase inhibition activity of the juice had a minute increase after fermentation, but the increase is significantly different from that of the unfermented juice at α = 0.05.
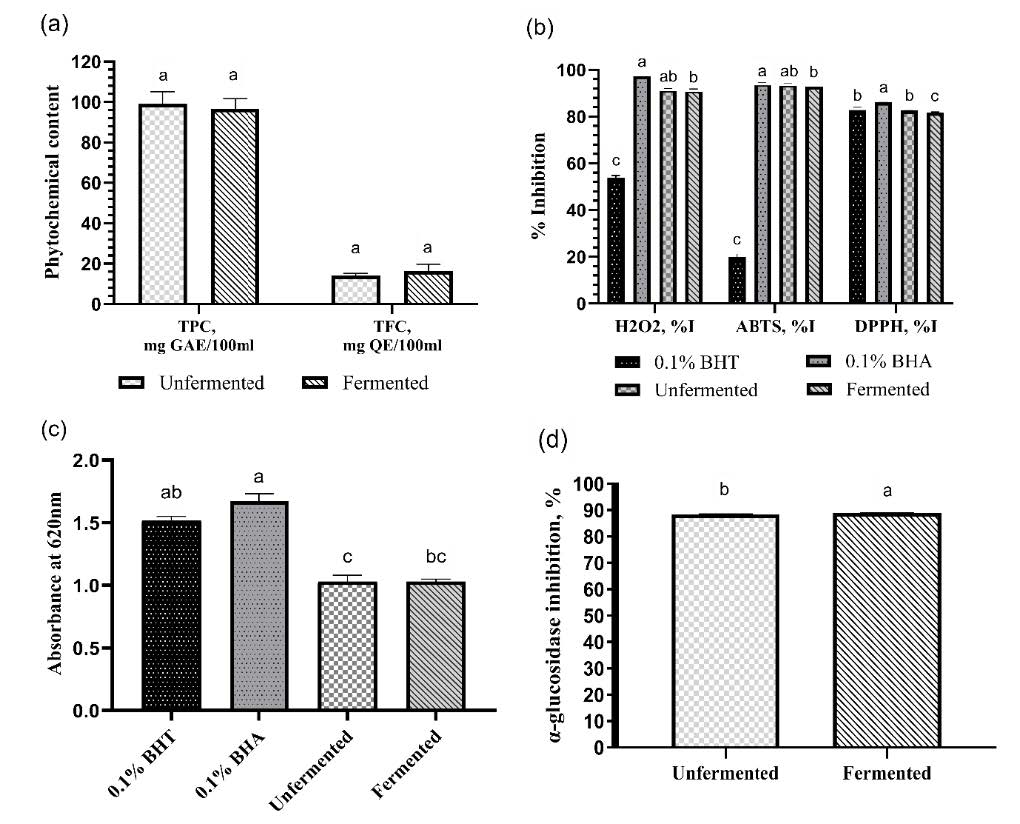
Figure 3. Phenolic content, antioxidant activities, and α-glucosidase inhibition of fermented and unfermented juice. Total phenolic and total flavonoid content (a); % free radical inhibition (b); FRAP activity (c); α-glucosidase inhibition (d); % I - % inhibition. Means with the same letter are not significantly different at α=0.05.
Changes in the phenolic profile in the juice
The phenolic profile of the unfermented and fermented juice samples was analyzed through HPLC. Table 1 shows that gallic acid is the predominant phenolic acid followed by catechin and ellagic acid. In addition, ellagic acid, rutin hydrate, trans-ferulic acid, and syringic acid had significantly (α=0.05) lower concentrations after fermentation while the other phenolic compounds had increased or decreased concentrations but were not significantly different from those of the unfermented sample.
Table 1. Phenolic profile of the unfermented and fermented Java plum juice.
|
Phenolic compound |
RT (min) |
Unfermented juice |
Fermented juice |
|
Gallic acid (GA) |
5.84 |
15.62 ± 2.21 a |
15.52 ± 0.21 a |
|
Catechin (C) |
13.78 |
2.78 ± 0.73 a |
1.67 ± 0.84 a |
|
Ellagic acid (EA) |
14.01 |
2.39 ± 0.35 a |
1.46 ± 0.36 b |
|
Rutin hydrate (RH) |
14.48 |
1.05 ± 0.00 a |
0.51 ± 0.09 b |
|
Myricetin (M) |
14.63 |
0.36 ± 0.01 a |
0.34 ± 0.01 a |
|
Epicatechin (EC) |
14.92 |
0.19 ± 0.05 a |
0.23 ± 0.02 a |
|
Quercetin (Q) |
15.47 |
0.19 ± 0.00 a |
0.19 ± 0.00 a |
|
trans-Ferulic acid (t-FA) |
16.58 |
0.15 ± 0.00 a |
0.14 ± 0.00 b |
|
Syringic acid (SA) |
17.07 |
0.12 ± 0.00 a |
0.11 ± 0.00 b |
|
Resveratrol (R) |
18.05 |
0.10 ± 0.00 a |
0.10 ± 0.00 a |
|
p-Coumaric acid (p-CA) |
18.87 |
0.07 ± 0.01 a |
0.06 ± 0.00 a |
|
Caffeic acid (CA) |
19.35 |
0.06 ± 0.01 a |
0.07 ± 0.00 a |
Note: Means with the same letter are not significantly different at α=0.05
Consumer acceptability of fermented juice
The fermented juice was subjected to a consumer acceptability test using the 9-point hedonic scale. Table 2 shows that the fermented juice had a “ Like very much” rating for overall appearance. On the other hand, the flavor (overall), flavor (intensity), taste (overall), and overall acceptability of the fermented juice had a rating of “Like moderately – Like very much”. The panelists have also commented that the fermented juice had a subtle alcoholic taste with balanced sweetness and sourness. Also, they found the juice refreshing with a pleasing body and little aftertaste.
Table 2. Consumer acceptability responses.
|
Sensory attribute |
Rating |
Description |
|
Flavor (overall) |
7.74 |
Like moderately - Like very much |
|
Flavor (intensity) |
7.38 |
Like moderately - Like very much |
|
Taste (overall) |
7.42 |
Like moderately - Like very much |
|
Aftertaste |
6.82 |
Like slightly - Like moderately |
|
Overall appearance |
8.02 |
Like very much |
|
Overall acceptability |
7.74 |
Like moderately - Like very much |
Note: Hedonic scale: 9 - like extremely; 8 - very much; 7 - like moderately; 6 - like slightly; 5 - neither like nor dislike; 4 - dislike slightly; 3 - dislike moderately; 2 - dislike very much; 1 - dislike extremely
Correlation of the parameters
The correlation of all the measured attributes was determined by conducting the Kendall-tau correlation method. Figure 4 shows the correlation table wherein numbers in bold represent significant (P< 0.05) correlations between the parameters. Values approaching 1 indicate a positive relation wherein a high value of parameter A is associated with a high value of parameter B. Values near 0 indicate no correlation while values nearing -1 indicate a negative correlation, meaning that a high value for parameter A is associated with a low value for parameter B.
The highlights of Figure 4 include a positive correlation between total soluble solids and DPPH and ABTS radical scavenging activity, rutin hydrate, syringic acid, ellagic acid, p-coumaric acid, and trans-ferulic acid. DPPH radical scavenging activity is also positively correlated with rutin hydrate. In contrast, the total soluble solids are negatively correlated with log viable cell count (VCC), α-glucosidase inhibition activity and caffeic acid. Also, log viable cell count is negatively correlated with DPPH radical scavenging activity and rutin hydrate.
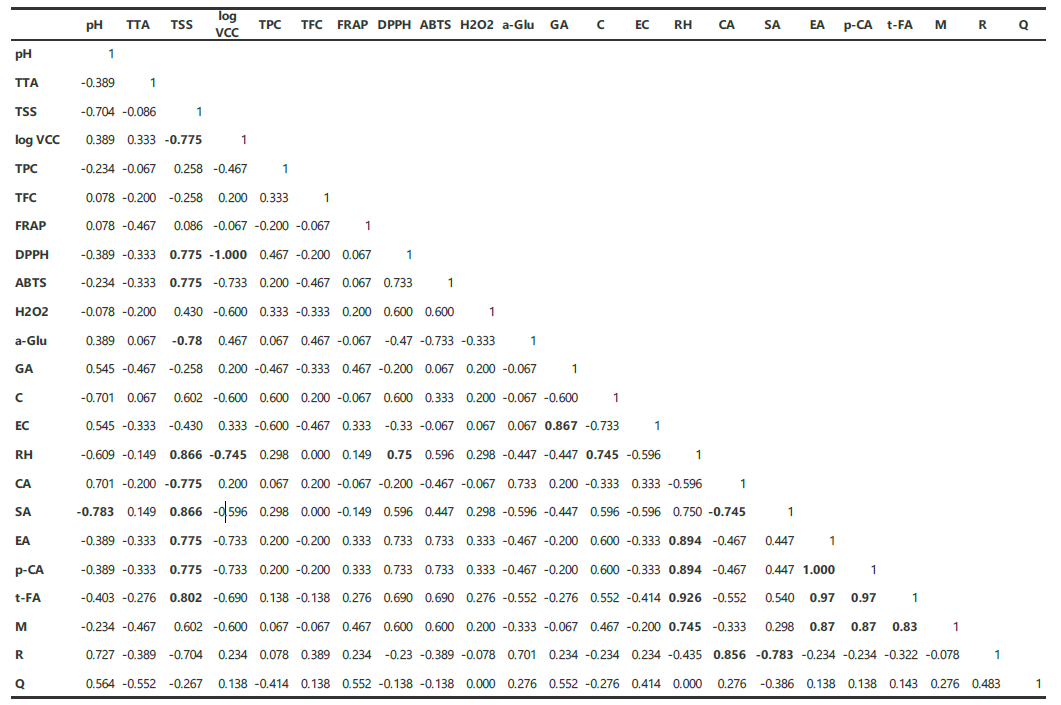
Figure 4. Kendall-tau correlation matrix of the physicochemical characteristics, phenolic and flavonoid contents, antioxidant activity, and phenolic profile of the unfermented and fermented Java plum juice. TTA, total titratable acid; TSS, total soluble solids; log VCC, log viable cell count; TPC, total phenolic content; TFC, total flavonoid content; FRAP, ferric reducing antioxidant power; DPPH, DPPH scavenging activity; ABTS, ABTS scavenging activity; H2O2, H2O2 scavenging activity; a-Glu, α-glucosidase inhibition activity.
DISCUSSION
The development of a functional fermented beverage was explored using Java plum and L. plantarum. Java plum juice as a substrate may have been a stressful medium for L. plantarum as it is acidic and possesses a wide array of phenolic compounds and organic acids which are antagonistic to microorganisms (Di Cagno et al., 2013). The skin and pulp of Java plum fruit contain up to 74 individual phenolic compounds and ethanolic extracts of the edible portion have antagonistic effects against S. aureus, P. aeruginosa, S. epidermidis, and some Candida species (Tavares et al., 2016). Similarly, L. plantarum as the fermenting microorganism was subjected to these antimicrobial effects which may have resulted in a long lag phase of 24 hours. In addition, the L. plantarum cells may have been adjusting to the new medium since there was a change of pH from the pre-culture MRS broth (pH 5.7) to the juice (pH 4.3) which affected its ability to grow spontaneously. The low pH of the juice can result in an extended lag phase and a decrease in the maximum growth rate (Yáñez et al., 2008). With the effect of phenolics and low pH, population growth was only observed after 24 hours with a faster rate after 48 hours which indicates that the bacteria have adjusted to the new environment. The bacterial population had significantly grown from an initial population of 7.09 log CFU ml-1 to a population of 7.73 log CFU ml-1 after 96 hours indicating that the juice was able to support the growth of L. plantarum despite being a harsh medium. The increase in L. plantarum population was marked by a decrease in total soluble solids, indicating that it was used as a substrate for microbial growth.
In this study, the pH of the juice was increasing even if fermentation was occurring which is contrary to the expectation that pH will decrease due to fermentation. However, the pH of the juice might have increased because L. plantarum can metabolize organic acids as carbon sources for fermentation. Accordingly, some L. plantarum strains can metabolize gallic acid, ferulic acid, p-coumaric acid, and caffeic acid (Rodríguez et al., 2009; Muñoz et al., 2017). The mentioned phenolic acids are present in the Java plum juice as corroborated in the HPLC analysis shown in Table 1. The ability of L. plantarum to metabolize these phenolic acids might have caused the increase in pH of the fermented samples in comparison to the unfermented juice. It is expected that after metabolizing these organic acids, lactic acid will be produced during fermentation, thus resulting in a decrease in pH of the juice. However, the amount of lactic acid produced during the fermentation of the Java plum juice might not have been high enough to impart a decrease in pH. This result agrees with the results of Mousavi et al. (2010) wherein fermentation of pomegranate juice with probiotic bacteria, including L. plantarum, resulted in an increase in pH and lower titratable acidity after fermentation.
In relation to its phenolic compounds, a decrease in the total phenolic content after fermentation for 96 hours is similarly expected because of the previously mentioned ability of L. plantarum to metabolize phenolic compounds. On the other hand, the increase in total flavonoid content may be attributed to the breakdown of polyphenols into simpler flavonol compounds by the action of enzymes produced by L. plantarum (Landete et al., 2014). While the TPC and TFC of the final fermented product are not significantly different from the unfermented sample, fermentation still occurred as a fermented aroma and taste were detected by sensory panelists in the fermented sample.
Regarding its antioxidant activity and α-glucosidase inhibition activity, the fermented and unfermented juice exhibited higher than 80% inhibition activity, indicating potency against free radicals and the α-glucosidase enzyme. Free radicals are associated with detrimental oxidative stress to tissues and membranes, resulting in diseases such as cancer, cardiovascular diseases, and rheumatoid arthritis, among many others. On the other hand, α-glucosidase is an enzyme present in the epithelium of the small intestine that catalyzes the cleavage of oligosaccharides into smaller monosaccharides that are more absorbable by the body (Kumar et al., 2011). The action of α-glucosidase increases the postprandial blood glucose levels after a carbohydrate meal which could be detrimental to diabetic patients. In terms of the antioxidant activities, the results indicate that there is no significant difference between the fermented and unfermented samples. This is expected since the values of TPC and TFC where these antioxidant activities are correlated to are also not significantly different between the samples. However, even if this was the case, it is good to note that the antioxidant activities of the fermented and unfermented samples are at par which means that even if the juice samples are fermented, the antioxidant activities of the juice are conserved. In contrast, the observed improvement in the α-glucosidase inhibition of Java plum juice after fermentation agrees with the results of Lizardo et al. (2020) wherein the α-glucosidase activity of cherry silverberry (Elaeagnus multiflora Thunb.) fermented using L. plantarum is significantly higher than that of the unfermented juice. The same results were obtained by Barrón-Álvares et al. (2022) in the fermentation of Cucurbita ficifolia juice using L. plantarum.
As tested through in-vitro methods, the inhibition of the free radicals and α-glucosidase enzyme is possible through the application of fermented Java plum juice which may impart preventive effects from diseases. The fermented juice inhibits 88% of the α-glucosidase enzyme which indicates that its activity of cleaving complex carbohydrates is lesser. With this, it is worth noting that the fermented Java Plum juice may be a supplement to the diet of individuals who are looking into managing their blood glucose levels.
Comparison of the phenolic profile of unfermented and fermented Java plum juice was conducted through HPLC. The decrease in the concentration of some phenolic compounds in Java plum juice suggests that these may have been used as carbon sources by L. plantarum during fermentation. These results agree with the study of Rodríguez et al. (2008) wherein L. plantarum was able to metabolize gallic acid and hydroxycinnamic acids which include p-coumaric acid and ferulic acid. Similarly, Lizardo et al. (2020) reported that the concentration of gallic acid, catechin, and rutin decreased after the fermentation of cherry silverberry fruits. The differences in the phenolic acids metabolized can be attributed to the strain of L. plantarum used, the type of substrate and its components which influence the enzymes produced, and the metabolic pathways that happen during fermentation (Rodríguez et al., 2009).
Aside from evaluating the physicochemical, phenolic and flavonoid content, and phenolic profile of the fermented juice, it was also subjected to consumer acceptability to determine its palatability to consumers. Fermentation imparted a positive impact on the sensory characteristics of the juice as shown by the results. L. plantarum possesses enzymes including tannase, general decarboxylase, and p-coumaric acid decarboxylase which catalyze the production of phenolic compounds that impart positive effects to the sensory characteristics of food (Plessas, 2021). The fruit itself is astringent but after its conversion into a fermented juice, the panelists did not detect any astringency from the sample which may have been due to the action of tannase from L. plantarum during fermentation. Since the Java plum fermented juice is generally acceptable to the panelists, there is potential for this product to be further developed.
Correlation of the attributes was done through Kendall-tau correlation method. The results are expected as higher levels of total soluble solids, which include phenolic acids, would exert more DPPH radical inhibition. The negative correlation of total soluble solids with log VCC is also expected since the increase in bacterial population is a result of the assimilation of total soluble solids. Similar to the study of Lizardo et al. (2020), it can be seen in the HPLC results that the antioxidant activities and enzyme inhibition are not influenced by specific phenolic compounds or the total phenolic content alone. The synergy of these bioactive compounds and the processing techniques applied influence the health benefits of the product.
CONCLUSION
A functional fermented beverage using L. plantarum on Java plum juice was developed. The lactic acid fermentation with L. plantarum resulted in the improvement of its α-glucosidase inhibition activity and sensory characteristics having a “like moderately to like very much” overall acceptability rating. Also, the physicochemical properties, phenolic and flavonoid contents, and antioxidant activities of the juice were conserved even after fermentation. Fermented Java plum juice can be an addition to the wide array of plant-based beverages that promote health.
ACKNOWLEDGEMENTS
The authors thank the Institute of Food Science and Technology, College of Agriculture and Food Science, UP Los Baños for providing the necessary equipment and materials to complete this study.
AUTHOR CONTRIBUTIONS
Anna Rina B. Evangelista-Albacea contributed to the study through conceptualization, methodology, data curation, investigation, analysis, validation, and writing the original manuscript draft. Lloyd Earl L. Flandez assisted in the methodology, investigation, analysis, and review of the manuscript. Rona Camille M. Lizardo-Agustin and Katherine Anne T. Castillo-Israel contributed through conceptualization, methodology, supervision, provision of resources, and review of the manuscript. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Aqil, F., Gupta, A., Munagala, R., Jeyabalan, J., Kausar, H., Sharma, R.J., Singh, I.P., and Gupta, R.C. 2012. Antioxidant and antiproliferative activities of anthocyanin/ellagitannin-enriched extracts from Syzygium cumini L. (Jamun, the Indian blackberry). Nutrition and Cancer. 64: 428–438.
Ayyanar, M. and Subash-Babu, P. 2012. Syzygium cumini (L.) Skeels: A review of its phytochemical constituents and traditional uses. Asian Pacific Journal of Tropical Biomedicine. 2: 240–246.
Chagas, V.T., França, L.M., Malik, S., and Paes, A.M.d.A. 2015. Syzygium cumini (L.) Skeels: A prominent source of bioactive molecules against cardiometabolic diseases. Frontiers in Pharmacology. 6: 259.
Curiel, J.A., Pinto, D., Marzani, B., Filannino, P., Farris, G.A., Gobbetti, M., and Rizzello, C.G. 2015. Lactic acid fermentation as a tool to enhance the antioxidant properties of Myrtus communis berries. Microbial Cell Factories. 14: 67.
Di Cagno, R., Coda, R., De Angelis, M., and Gobbetti, M. 2013. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiology. 33: 1–10.
Elya, B., Basah, K., Mun’im, A., Yuliastuti, W., Bangun, A., and Septiana, E.K. 2012. Screening of α-glucosidase inhibitory activity from some plants of Apocynaceae, Clusiaceae, Euphorbiaceae, and Rubiaceae. Journal of Biomedicine and Biotechnology. 2012: 281078.
Estacio, M.A, Atienza, L., Gapasin, R., Maniwang, J.R., Aranzado, J.R., Mercado, C.J., Dela Cruz, M.A., Fernandez, N.D., Sunico, D.J., Israel, K.A., Bainto, L., and Ilagan, J. 2020. Acute oral toxicity test of selected Philippine indigenous berries as potential food supplements. Current Developments in Nutrition. 4 (Supplement 2):684.
González-Palma, I., Escalona-Buendía, H.B., Ponce-Alquicira, E, Téllez-Téllez, M., Gupta, V.K., Díaz-Godínez, G., and Soriano-Santos, J. 2016. Evaluation of the antioxidant activity of aqueous and methanol extracts of Pleurotus ostreatus in different growth stages. Frontiers in Microbiology. 7: 1099.
Kishore, K. 2019. Phenological growth stages and heat unit requirement of Indian blackberry (Syzygium cumini L., Skeels). Scientia Horticulturae. 249: 455–460.
Kumar, S., Narwal, S., Kumar, V., and Prakash, O. 2011. Alpha-glucosidase inhibitors from plants: A natural approach to treat diabetes. Pharmacognosy Reviews. 5: 19-29.
Landete, J.M., Curiel, J.A., Rodríguez, H., de las Rivas, B., and Muñoz, R. 2014. Aryl glycosidases from Lactobacillus plantarum increase antioxidant activity of phenolic compounds. Journal of Functional Foods. 7: 322–329.
Lizardo, R.C.M., Cho, H-D., Won, Y-S., and Seo, K-I. 2020. Fermentation with mono‐ and mixed cultures of Lactobacillus plantarum and L. casei enhances the phytochemical content and biological activities of cherry silverberry (Elaeagnus multiflora Thunb.) fruit. Journal of the Science of Food and Agriculture. 100: 3687–3696.
Mousavi, Z.E., Mousavi, S.M., Razavi, S.H., Emam-Djomeh, Z., and Kiani, H. 2010. Fermentation of pomegranate juice by probiotic lactic acid bacteria. World Journal of Microbiology and Biotechnology. 27: 123–128.
Muñoz, R., de las Rivas, B., López de Felipe, F., Reverón, I., Santamaría, L., Esteban-Torres, M., Curiel, J.A., Rodríguez, H., and Landete, J.M. 2017. Biotransformation of phenolics by Lactobacillus plantarum in fermented foods. p. 63–83. In: J. Frias, C. Martinez-Villaluenga, E. Peñas [eds]. Fermented Foods in Health and Disease Prevention. Academic Press, London.
Ozcelik, F., Akan, E., and Kinik, O. 2021. Use of Cornelian cherry, hawthorn, red plum, roseship and pomegranate juices in the production of water kefir beverages. Food Bioscience. 42: 101219.
Pérez-Rodríguez, M.L., Serrano-Carretero, A., García-Herrera, P., Cámara-Hurtado, M., and Sánchez-Mata, M.C. 2023. Plant-based beverages as milk alternatives? Nutritional and functional approach through food labelling. Food Research International. 173: 113244.
Plessas, S. 2021. Advancements in the use of fermented fruit juices by lactic acid bacteria as functional foods: Prospects and challenges of Lactiplantibacillus (Lpb.) plantarum subsp. plantarum application. Fermentation. 8: 6.
Reginio, F.C., Qin, W., Ketnawa, S., and Ogawa, Y. 2020. Bio-properties of Saba banana (Musa ‘saba’, ABB Group): Influence of maturity and changes during simulated in vitro gastrointestinal digestion. Scientific Reports. 10: 6701.
Rodríguez, H., Curiel, J.A., Landete, J.M., de las Rivas, B., de Felipe, F.L., Gómez-Cordovés, C., Mancheño, J.M., and Muñoz, R. 2009. Food phenolics and lactic acid bacteria. International Journal of Food Microbiology. 132: 79–90.
Rodríguez, H., Landete, J.M., de las Rivas, B., and Muñoz, R. 2008. Metabolism of food phenolic acids by Lactobacillus plantarum CECT 748T. Food Chemistry. 107: 1393–1398.
Ryu, J.Y., Kang, H.R., and Cho, S.K. 2019. Changes over the fermentation period in phenolic compounds and antioxidant and anticancer activities of blueberries fermented by Lactobacillus plantarum. Journal of Food Science. 84: 2347–2356.
Sabino, L.B.d.S., de Brito, E.S., and Júnior, I.J.d.S. 2018. Jambolan – Syzigium jambolanum. p. 251-256. In S. Rodrigues, E.d.O. Silva, and E.S. de Brito [eds]. exotic fruits reference guide. Academic Press, London.
Sartagoda, K.J., Ilano, M.C., Flandez, L.E., Castillo-Israel, K.A. 2021. Evaluation of the antioxidant activity of Bignay (Antidesma bunius (Linn.) Spreng var. Kalabaw) flesh and seeds as affected by maturity and processing method. Chiang Mai University Journal of Natural Sciences. 20: e2021042.
Saurabh, V., Barman, K., and Singh, A.K. 2019. Synergistic effect of salicylic acid and chitosan on postharvest life and quality attributes of jamun (Syzygium cumini Skeels) fruit. Acta Physiologiae Plantarum. 41: 89.
Sharma, N., Yeasmen, N., Dubé, L., and Orsat, V. 2024. A review on current scenario and key challenges of plant-based functional beverages. Food Bioscience. 60: 104320.
Sharma, V. and Mishra, H.N. 2013. Fermentation of vegetable juice mixture by probiotic lactic acid bacteria. Nutrafoods. 12: 17–22.
Swain, T., and Hillis, W.E. 1959. The phenolic constituents of Prunus domestica. I.—the quantitative analysis of phenolic constituents. Journal of the Science of Food and Agriculture. 10: 63–68.
Tavares, I.M. de C., Lago-Vanzela, E.S., Rebello, L.P.G., Ramos, A.M., Gómez-Alonso, S., García-Romero, E., Da-Silva, R., and Hermosín-Gutiérrez, I. 2016. Comprehensive study of the phenolic composition of the edible parts of jambolan fruit (Syzygium cumini (L.) Skeels). Food Research International. 82: 1-13.
Torino, M.I., Limón, R.I., Martínez-Villaluenga, C., Mäkinen, S., Pihlanto, A., Vidal-Valverde, C., and Frias, J. 2013. Antioxidant and antihypertensive properties of liquid and solid state fermented lentils. Food Chemistry. 136: 1030–1037.
Vijayalakshmi, M. and Ruckmani, K. 2016. Ferric reducing anti-oxidant power assay in plant extract. Bangladesh Journal of Pharmacology. 11: 570-572.
Wallace, T.C., Bailey, R.L., Blumberg, J.B., Burton-Freeman, B., Chen, C-y.O., Crowe-White, K.M., Drewnowski, A., Hooshmand, S., Johnson, E., Lewis, R., Murray, R., Shapses, S.A., and Wang, D.D. 2019. Fruits, vegetables, and health: A comprehensive narrative, umbrella review of the science and recommendations for enhanced public policy to improve intake. Critical Reviews in Food Science and Nutrition. 60: 2174-2211.
Xiang, H., Sun-Waterhouse, D., Waterhouse, G.I.N., Cui, C., and Ruan, Z. 2019. Fermentation-enabled wellness foods: A fresh perspective. Food Science and Human Wellness. 8: 203–243.
Yáñez, R., Marques, S., Gírio, F.M., and Roseiro, J.C. 2008. The effect of acid stress on lactate production and growth kinetics in Lactobacillus rhamnosus cultures. Process Biochemistry. 43: 356–361.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Anna Rina B. Evangelista-Albacea1, Lloyd Earl L. Flandez2, Katherine Ann T. Castillo-Israel1, 2, and Rona Camille M. Lizardo-Agustin1, 2, *
1 Institute of Food Science and Technology, University of the Philippines Los Baños, College, Laguna, 4031, Philippines.
2 Natural Products Development Program, Office of the Vice Chancellor for Research and Extension, University of the Philippines Los Baños, College, Laguna, 4031, Philippines.
Corresponding author: Rona Camille M. Lizardo-Agustin, E-mail: rmlizardo@up.edu.ph
Total Article Views
Editor: Sirasit Srinuanpan,
Chiang Mai University, Thailand
Article history:
Received: January 13, 2025;
Revised: April 23, 2025;
Accepted: April 28, 2025;
Online First: May 15, 2025

