
Xanthan Gum and Propylene Glycol Effects on Physicochemical and Antioxidant Activities of Honey-based Syrup
Aulia Rahma Ardiningsih, Wimala Hardyawati, Mias Isningroom, Cici Darsih, Anggita Sari Praharasti, Muhammad Novrizal Abdi Sahid, and Marlyn Dian Laksitorini*Published Date : May 15, 2025
DOI : https://doi.org/10.12982/NLSC.2025.043
Journal Issues : Number 3, July-September 2025
Abstract Antioxidant supplements from natural ingredients can be an alternative to counteract the effects of free radicals. None of the products use a combination of Chrysanthemum indicum and Centella asiatica extracts formulated as antioxidant supplements, particularly in syrup form. To date, sugar has been commonly used as a syrup base. However, honey can also be used as a base for syrups as a sugar substitute, but it has antioxidant activity. This study evaluated the antioxidant activity of honey-based herbal syrup with varying concentrations of Xanthan Gum and Propylene Glycol. This study aims is to asses two Indonesian honeys' antioxidant capacity against Manuka honey as standard. Ceiba honey was selected as the base for herbal syrup due to its superior antioxidant activity. Subsequently, the antioxidant activity of the three active compounds used in the formula was assessed using DPPH, yielding a result of 272.82 ± 5.167 µg/ml, which falls into the weak category. The antioxidant activity of the honey-based syrup remained unaffected by the addition of propylene glycol and xanthan gum. The honey-based syrup was also subjected to a physical analysis, where pourability, density, and viscosity decreased as the composition of xanthan gum decreased compared to propylene glycol. Meanwhile, higher xanthan gum content increased water activity and a darker appearance. The simplex lattice design (SLD) method identified an optimal formulation containing 0.099% xanthan gum and 10.401% propylene glycol. Freeze-thaw stability testing confirmed the honey-based syrup maintained stable physical properties.
Keywords: Antioxidant supplements, Honey-based syrup, Propylene glycol, Xanthan gum, Stability test
Citation: Ardiningsih, A.R., Hardyawati, W., Isningroom, M., Darsih, C., Praharasti, A.S., Sahid, M.N.A., and Laksitorini, M.D. 2025. Xanthan gum and propylene glycol effects on physicochemical and antioxidant activities of honey-based syrup. Natural and Life Sciences Communications. 24(3): e2025043.
INTRODUCTION
Using herbal medicines for various health purposes has attracted significant public interest. In developing countries, herbal medicine is an important healthcare source for large populations (Kumar et al., 2024). One of the most common formulations for herbal medicine is syrup with sugar as a base. Plant-based syrups can be made with honey, but typically, syrups are formulated using sugar. Diabetes and obesity have become major concerns, one of which is caused by excessive sugar consumption (Palma-Morales et al., 2023). Numerous companies have developed honey-based products. The negative effects mentioned earlier have not been observed in honey consumption (Bahrami et al., 2009). Tropical countries have a wide variety of honey, primarily composed of different sugars, water, and protein. Additionally, it contains phenolic compounds, which have potential as a source of antioxidants (Baloš et al., 2020). The antioxidant activity of Indonesian honey needs to be determined to assess its potential when mixed in herbal syrup formulations.
Consumption of synthesized antioxidant supplements is restricted as they may cause unwanted side effects, so it is better to consume natural antioxidants (Rusman et al., 2020). Chrysanthemum indicum flowers, Centella asiatica herb, and honey are natural ingredients with antioxidant activity that can be used as health supplements (Afjani et al., 2024; Devi et al., 2024; Rahmasari et al., 2024; Syaifabila et al., 2024). Studies showed that C. asiatica herb and C. indicum flower extract exert neuroprotective activities that benefit the brain (Ar Rochmah et al., 2019). C. indicum contains flavonoids, alkaloids, lactones, and sesquiterpenes (Youssef et al., 2020). The antioxidant activity of C. asiatica herbs is obtained from phenolic compounds in the form of triterpenoids (Singh et al., 2014). C. indicum and C.asiatica extracts are used as raw materials for supplements, cosmetics, and herbal drinks.
The syrup is one of the alternative supplement forms that can be formulated using C. indicum and C. asiatica extracts. The syrup's characteristics are affected by the thickeners and solvents used. Xanthan gum is a viscosity enhancer (Adjeng et al., 2023). Xanthan gum can also be easily dispersed in water, and its viscosity remains constant over a wide range of pH levels (Pitaksuteepong et al., 2024). Efforts have been done to improve the solubility of extract in liquid formulation, such as increasing the amount of the solvent, heating the formulation, and introducing a hydrophilic group. The water solubility of natural products is still an incredibly challenging issue, so it is necessary to add a solvent that can increase the solubility of the extract. One of them is propylene glycol, which belongs to the hydrophilic group (Lajoie et al., 2022). The addition of propylene glycol can improve the water solubility of the extract without heating the formula, which can cause degradation in honey compounds (Suan Chua et al., 2014). Optimization of the viscosity enhancer and co-solvent is required to enhance the syrup's sensory quality by improving its pourability and stability and providing a comfortable sensation during use.
Most honey-based medicinal formulations are primarily intended for topical wound care. There are only a few products aimed at alternative routes of administration, such as syrups (Hossain et al., 2021). C. indicum and C. asiatica extracts are rarely combined in one formula, although both have antioxidant properties that could be used as an antioxidant supplement. Therefore, this study aims to evaluate and formulate a honey-based herbal syrup with a combination of C. indicum flower and C. asiatica herb extracts that can be used as an antioxidant supplement. Additionally, this study evaluated the antioxidant activity of two Indonesian honeys. The impact of xanthan gum and propylene glycol addition on the antioxidant activity and physicochemical properties of the honey-based syrup was also investigated in this study.
MATERIAL AND METHODS
Materials
Premium ceiba honey nectar and premium Rambutan honey nectar obtained from PT. Natura Alamindo, Manuka honey was purchased from Comvita Ltd, methanol p.a. was purchased from Merck Ltd., C. indicum L. flower and C. asiatica herb 70% ethanol were purchased from PT. Progo Mulyo, xanthan gum was obtained from Neimenggu Fufeng Biotechnologies Co. Ltd., propylene glycol was purchased from Alfa Kimia, and sodium benzoate was obtained from PT. Gunacipta Multirasa, citric acid, was purchased from PT. Pondasi Inti Sejahtera, sodium citrate obtained from Sentra Kimia Labsains, lemon flavor, and coloring were purchased from Mohler Indonesia, 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) obtained from PT. Smart Lab Indonesia, vitamin C was obtained from Sigma-Aldrich, and Komix Herbal Syrup was purchased from PT. Bintang Toedjooe.
Plant determination
Plant determination was carried out at the Pharmacognosy-Phytochemical Laboratory, Department of Pharmaceutical Biology, Universitas Gadjah Mada, Indonesia. The certificate of determination C. indicum is stated in letter number 38.27.11/UN1/FFA.2/BF/PT/2023 and C. asiatica in letter number 40.27.11/UN1/FFA.2/BF/PT/2023.
Preparation of C. indicum and C. asiatica extracts
The dried C. indicum flower and C. asiatica herbs were extracted by maceration method using 70% (v/v) ethanol in a ratio of 1:7 where the amount of ethanol used is sevenfold of the weight of the dried herbal plant. The maceration were performed at room temperature for 24 hours. Subsequently, re-maceration was performed with the same solvent in a ratio of 1:3 where the amount of ethanol used is three times the weight from the result of first maceration. The extract was concentrated by filtering the mixture through a cloth and evaporating the solvent at 40°C using a water bath. The heating process is stopped when the extract reaches a paste-like consistency. The concentrated ethanolic extract was then stored in a refrigerator at 4°C until use. The percentage of yields (w/w) equation is available at Figure S1.
DPPH radical scavenging activity for the C. asiatica, C. indicum, and honey
The antioxidant activity of honey and extract was measured using the DPPH radical capture test (1,1-diphenyl-2-picryl-hydrazyl). The honey solution was prepared by weighing 3 g of premium nectar Ceiba honey and premium nectar Rambutan honey and then dissolving them in 10 mL of methanol pro analysis. Then, the sample honey solution was diluted 10x. Manuka honey was used as a positive control for honey and was diluted 100 times. Vitamin C was used as a positive control. A hundred milligrams of vitamin C were weighed and dissolved in 100 ml of ethanol p.a. The extract solution was made by weighing 500 mg of C. indicum flower and C. asiatica herbs extracts and dissolving them in 10 mL of methanol pro analysis. C. indicum extract solution was diluted 10x, while C. asiatica extract was diluted 100x. The extract and honey solution were combined by weighing an amount equivalent to 3 g of honey sample and then dissolving them in 10 mL of methanol pro analysis and homogenized using ultrasonic. One milliliter of each sample/standard solution was mixed with 1 mL DPPH-ethanol solution, then methanol (p.a) was added up to 5 mL. After that, the tubes were kept in complete darkness for 30 min. The absorbance of the samples was determined at 517 nm. The percent rate of the DPPH free radicals equation is available in Figure S2 (Supplemental Figure 2).
Total phenolic content (TPC) of honey-based syrup
The TPC of honey-based syrup was determined using the Folin-Ciocalteu method with slight modification, according to Eggleston et al (2021). The syrup (1g) was dissolved in 9 mL of distilled water. The sample (10 µL) was then added 50 µL of Folin- Ciocalteu reagent and incubated at room temperature for 8 min. Around 150 µL of Na2CO3 20% was placed in the vial, and 790 µL of distilled water was added. Furthermore, the mixture was incubated in a dark room for 2 h. The absorbance of the sample was then measured using a microplate reader at 765 nm. Gallic acid was used as standard.
Evaluation of the syrup's antioxidant activity
The antioxidant activity of syrups was measured according to Cici et al. (Darsih et al., 2021) with slight modification. The syrups (1 g) were dissolved in 9 mL of 70% methanol. Approximately 20 µL of extract was added with 80 µL of DPPH solution (1.01 mM). The mixture was incubated at dark room temperature for 30 min. The microplate reader (Multiskan™, Thermo Scientific) was used to measure the absorbance of the mixture at 517 nm. The inhibition percentage of the sample was calculated using:

All measurements were conducted in triplicate.
The formulation of honey-based syrup
The honey-based syrup was prepared using a different formula of xanthan gum and propylene glycol (Tabel 1). A typical daily dose of dried leaves C. asiatica ranges from 300 mg to 680 mg, taken three times daily (Gohil et al., 2010). The currently available product containing Centella asiatica extract is called Paramorina Upbrainina syrup and is administered at a dosage of approximately 1 gram daily. The dried C. indicum can be administered at 2–8 grams divided into three daily administrations (Newall et al., 1996). The daily dose is approximately 900 mg of C. asiatica and 2-8 grams of C. indicum. The syrup is administered at a dosage of 10 mL per dose, taken three times daily. In this study, 3 grams of C. asiatica were combined with 10 grams of C.indicum in a syrup volume of 100 mL, aiming to provide a synergistic antioxidant effect from both extracts. The extract was dissolved with propylene glycol, while xanthan gum was dissolved separately in distilled water. The honey was added to the dissolved extract mixture, followed by the xanthan gum solution and citric acid. In a separate container, sodium benzoate was dissolved in distilled water and added to the syrup mixture. The pH of the syrup was adjusted by adding sodium citrate. After adding the aquadest up to 100 mL, the mixture was stirred until it became homogeneous.
Table 1. Formulation of honey-based syrup containing C. asiatica and C. indicum extract.
|
Ingredients |
Formula |
|||||||
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
|
|
C. asiatica extract (g) |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
|
C. indicum extract (g) |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
|
Honey (g) |
70 |
70 |
70 |
70 |
70 |
70 |
70 |
70 |
|
Propylene glycol (g) |
10.41 |
10.3 |
10.45 |
10.34 |
10.38 |
10.375 |
10.45 |
10.3 |
|
Xanthan Gum (g) |
0.088 |
0.2 |
0.05 |
0.16 |
0.13 |
0.13 |
0.05 |
0.2 |
|
Sodium benzoate (g) |
0.02 |
0.02 |
0.02 |
0.02 |
0.02 |
0.02 |
0.02 |
0.02 |
|
Citric acid (g) |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Sodium citrate (g) |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
|
Lemon flavor and coloring |
qs |
qs |
qs |
qs |
qs |
qs |
qs |
qs |
|
Aquadest (ad) (mL) |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
Note: qs (quantum sufficiat) = add in sufficient quantity until the syrup achieves a brownish-yellow color
Evaluation of honey-based syrup physical properties
An organoleptic test is a test that is based on a sensing process. Organoleptic testing is performed by taking samples of herbal syrup and then observing its physical characteristics, including color, odor, and taste, which can affect consumer acceptance (Kane et al., 2016). The pH of the syrup was determined using the digital pH meter (Hanna pH meter). After immersing the pH meter into the sample, record the pH reading on the meter once a stable value is reached. The pourability test determined how easily the syrup could be dispensed from the primary packaging. Twenty-five milliliters of syrup were poured from a 45° slope. The time required for the syrup to be completely poured from the container was recorded.
One parameter to evaluate the syrup's reproducibility is the specific gravity. The syrup's specific gravity (g/mL) was determined using a pycnometer. The equation is available at Supplemental Figure S3. The syrup's viscosity was measured with an Ostwald viscosimeter with upper and lower capillary limit markers. The liquid was drawn up to the upper mark and then flowed down. The stopwatch was started when the liquid passed the upper mark and stopped when it passed the lower mark on the capillary tube. The equation is available at Figure S4.
The rheometer was also used to determine the viscosity of honey-based syrup following the method with slight modification (Mohamat et al., 2023). The parallel plate with a diameter of 50 mm was used to measurement of viscosity. The gap between the plates was set to 0.1 mm. The analysis was conducted at 25°C with range shear rate set from 0.1 to 100 s-1. The viscosity of samples was expressed as A graph of viscosity (Pa.s) versus shear rate (1/s) and a graph of shear stress (Pa) versus shear rate (1/s).
Water activity is the parameter that can be used to predict product stability and food safety in terms of the probability of microbial growth probability. The lower the water activity, the less likelihood the microbes will grow in the syrup. Ideally, the water activity is less than 0.6. The water activity of honey-based syrup was determined using an Aqualab water activity meter (Aqualab 4TE, Decagon Devices, Inc., WA, USA) at 25°C. The colorimetric analysis of the honey-based syrups was conducted following the method with slight modification by (Mohamat et al., 2023). Chromameter (CR 20 Konica Minolta, Japan) was used to analyze the samples.
Evaluation of honey-based syrup stability
Freeze-thaw stability was used to determine whether a formula would remain stable under various conditions. In this study, the freeze-thaw method was slightly modified by allowing the syrup to stand at room temperature for 2 x 24 hours after completing each cycle and testing the physical properties of the syrup. For the freeze-thaw stability assay, the samples underwent three cycles of freezing at -10°C for 24 hours, thawing at room temperature for 24 hours, and then being transferred to 45°C for 24 hours, followed by another 24 hours at room temperature (Bernal-Chávez et al., 2023). This process was replicated two times. After each cycle, the physical characteristics of the herbal syrup were evaluated to determine its stability. In this study the characteristics test included pH, pourability, density, and viscosity.
Statistical analysis
Antioxidant activity was determined using the DPPH radical scavenging method, and the absorbance profile was used to calculate the percent inhibition and IC50. The antioxidant activity in terms of IC50 value was then analyzed using IBM SPSS Statistics with one-way ANOVA (P<0.05), followed by the post hoc Tukey test. The optimum formula for honey-based syrup was determined based on the physical properties of the herbal syrup formulation, including pourability, viscosity, and density. The analysis of experimental value and predicted value were performed using one sample t-test. Freeze-thaw stability test results were also analyzed by one-way ANOVA. The water activity and color of honey-based syrup data were analyzed using SPSS 16. The data was tested by one-way ANOVA followed by post hoc Duncan's test. Differences at P<0.05 were considered to be significant. The analysis results were presented as triplicates' mean ± SD (standard deviation).
RESULTS
Extraction and characterization of C. indicum flower and C. asiatica herbs
The crude extract of C. indicum L. flowers had a percentage yield of 23.23%. These results have met the requirements for chrysanthemum flower extract yields based on the Farmakope Herbal Indonesia 3rd Edition, which is 22.7%, while the weight of the C. asiatica extract had a yield percentage of 31.09%. This result also met the requirements stated in Farmakope Herbal Indonesia 3rd Edition for the yield of thick extract of C. asiatica herb not less than 7.3%.
Determination of antioxidant activity
The current research provides information on the antioxidant status of two varieties of Indonesian honey and a combination of C. indicum flower and C. asiatica herbs extracts with Indonesian honey. The research conducted by Yap et al. (2023) indicates a decrease in the antioxidant activity of herbal ingredients when combined with other herbal ingredients, which can minimize their impact on pharmacological activity. Consequently, the additional aim of this study is to determine the antioxidant activity of Ceiba and Rambutan premium nectar honey from Indonesia. The study also evaluates the antioxidant activity of the active ingredient mixtures used in the formulation of the honey-based herbal syrup.
The crude extract of C. indicum L. flowers had a percentage yield of 23.23%. These results have met the requirements for chrysanthemum flower extract yields based on the Farmakope Herbal Indonesia 3rd Edition, which is 22.7%. In comparison, the yield of the C. asiatica extract is 31.09%, which met the requirements stated in Farmakope Herbal Indonesia 3rd Edition for the yield of thick extract of C. asiatica herb not less than 7.3%. Further, the antioxidant activity of the extract and its combination were tested using a DPPH radical assay were used.
Table 2. Antioxidant activity of Indonesian Honey, C. indicum, and C. asiatica extracts.
|
Substances |
IC50 (µg/mL) |
Categories |
|
Vitamin C |
2.01 ± 0.01 |
Very strong |
|
Manuka honey |
788 ± 8.78 |
Weak |
|
Ceiba premium nectar honey |
16,910 ± 131.07 |
Weak |
|
Rambutan premium nectar honey |
19,082 ± 229.34 |
Weak |
|
C. asiatica extract |
38.44 ± 0.44 |
Strong |
|
C. indicum extract |
67.11 ± 0.69 |
Strong |
|
Ceiba honey+C.asiaica+C.indicum |
272.82 ± 5.167 |
Weak |
Table 2 showed that the IC50 value of vitamin C as a positive control was 2.01 ± 0.01 µg/ml. According to Surjanto et al., (2019) the strength category of antioxidant activity is categorized as follows: very strong <50 µg/ml, strong 50-100 µg/ml, moderate 101-150 µg/ml, and weak >150 µg/ml. The IC50 of antioxidant activity of C. indicum extract was 67.11 ± 0.69 µg/ml, C. asiatica was 38.44 ± 0.44 µg/ml, which is categorized as a strong and very strong antioxidant. The IC50 of antioxidant activity of Manuka honey, Ceiba nectar honey, and Rambutan nectar honey were identified as weak with IC50 of 788 ± 8.78 µg/ml; 16,910 ± 131.07 µg/ml and 19082 ± 229.34 µg/ml; respectively. Compared to Manuka honey, Ceiba and Rambutan nectar honey shows weaker antioxidant activity. Manuka honey is often considered the gold standard for evaluating the antioxidant activity of other types of honey due to its high phenolic compound content (Becerril‐sánchez et al., 2021). Previous research has shown that tropical honey (from Malaysia) has weak antioxidant activity ranging from 109,760 – 182,560 µg/ml (Sakika et al., 2022). Also, Honey from Magelang, Indonesia, shows weak antioxidant activity (Purwatiningsih et al., 2023). In addition, Omani honey from various regions in the Arabian Peninsula has antioxidant values of 7,800 – 48,600 mg/ml (Al-Farsi et al., 2018). This study found that Manuka honey and both Indonesian honey samples were classified as weak, likely due to the complexity of their composition. However, all honey samples exhibited antioxidant activity, which suggests they are suitable as active ingredients for syrup. Ceiba nectar honey was selected for further syrup formulation based on its lower IC50 value compared to Rambutan nectar honey.
The IC50 of the combination of active compounds was 272.82 ± 5.167 µg/ml, included in the weak category. This finding is in line with research by Yap et al. (2023), which shows the possibility of decreasing the effect of the antioxidant activity caused by the combination of herbs in the formulation. The herbal syrup formula in this study is primarily composed of honey, which serves as the base and accounts for 70% of the mixture. Previous tests have shown that premium Ceiba nectar honey has relatively weak IC50 values for antioxidant activity, which may cause the honey-based syrup to have weak antioxidant activity.
TPC and antioxidant activity of syrup-based honey
Table 3. TPC and scavenging activity of syrup-based honey.
|
Formulation of syrup |
TPC (mg GAE/g sample) |
% inhibition at 1 g/9 mL |
|
F1 |
5.54 ± 0.01 |
81.85 ± 0.01 |
|
F2 |
5.71 ± 0.00 |
81.28 ± 0.20 |
|
F3 |
5.18 ± 0.01 |
80.74 ± 0.10 |
|
F4 |
4.81 ± 0.01 |
81.83 ± 0.29 |
|
F5 |
5.37 ± 0.02 |
78.24 ± 0.10 |
|
F6 |
5.36 ± 0.04 |
81.89 ± 0.83 |
|
F7 |
4.83 ± 0.01 |
81.10 ± 0.08 |
|
F8 |
5.62 ± 0.01 |
81.37 ± 0.78 |
Table 3 revealed that using xantham gum and propylene glycol on syrup-based honey did not affect the antioxidant activity of the products. The observed % inhibition at 1 g/9 mL corresponds to a sample concentration of 1 g dissolved in 9 mL of solvent. The result was also reported that the scavenging activity of syrup-based honey was not positively correlated with phenolic compounds. The antioxidant activity was suggested as related to the C. asitica extract and honey added in syrup. The antioxidant activity of syrup is possible due to the correlation between metabolites such as phenolic, flavonoids, and terpenoids contained in Honey and C. asiatica extracts. Previous research reported that C. asiatica contains flavonoids, tannins, saponins, alkaloids, terpenoids, phenols, and glycosides (Gray et al., 2018). Those metabolites were reported to exhibit antioxidant activity. Honey was reported to contain polyphenol, vitamin, mineral, protein, organic acids, and sugar and exhibited antioxidants (Ahmed et al., 2018).
Formulation of honey-based syrup
In this study, herbal syrup was formulated with a honey base, chosen for its superior health benefits compared to artificial sweeteners commonly used in syrup formulations. Propylene glycol was used as the solvent to increase extract solubility in the formulation (Kuswahyuning et al., 2020). The optimal proportions of xanthan gum and propylene glycol were determined by using the SLD and the Design Expert software. Formulations that meet the criteria for good physical properties can be optimized using the Simplex Lattice Design (SLD) method (Fatimi et al., 2023). Eight experimental formulas were conducted, each using different concentrations of xanthan gum and propylene glycol. The physical properties of the prepared samples were evaluated through organoleptic, pourability, density, viscosity test, rheology, water activity, and color analysis. The organoleptic testing results showed that the honey-based syrup was homogeneous and had a yellow-brown color. It had a distinct aroma of Honey and C. indicum flower extract (Figure 1).

Figure 1. Appearances of honey-based syrup with various concentrations of xanthan gum and propylene glycol according to Table 1.
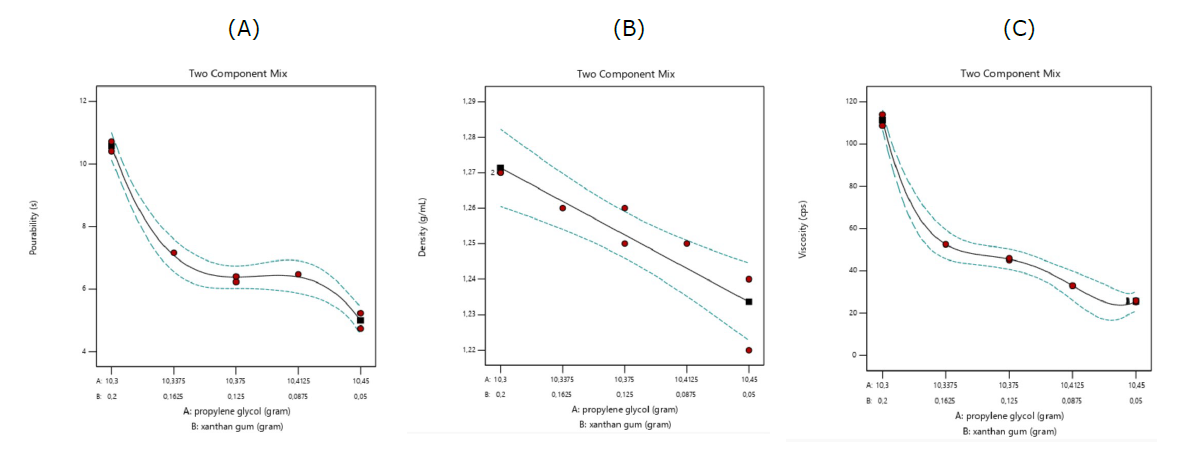
Figure 2. Profile of physical properties of honey-based syrup on expert design. Pourability (A); Density (B); Viscosity (C).
The pourability test aimed to measure the time the syrup took to flow out of the bottle. Formula 7 had the lowest pourability, taking only 4.74 seconds, while Formula 8 had the highest pourability, taking 10.72 seconds (Figure 2a). The test results showed that xanthan gum positively affected the duration of syrup pouring time. An increase in xanthan gum leads to pourability with a longer duration due to the higher viscosity of the syrup. The pourability of the syrup was selected based on its minimum duration to ensure ease of consumption for patients.
The average density range in the honey-based herbal syrup test samples for the entire formula was 1.22 – 1.27 g/mL (Figure 2b). The commercial product (Komix Herbal Syrup®) had a density value of 1.25 g/mL. Preparing honey-based herbal syrup does not involve heating, which ensures that the resulting density range remains relatively consistent. Conversely, heating can result in a decrease in density value, particularly when temperature is elevated.
On the viscosity test, the syrup formulation's lowest viscosity was 25.35 cps, while the highest viscosity was observed in formula 8, which was 114 cps (Figure 2c). The commercial product has a viscosity of 30 cps. Xanthan gum is a hydrocolloid composed of a diverse group of long-chain polymers. It has the unique ability to form a thick dispersion when mixed with water due to its numerous hydroxyl groups (-OH), which enhance its water-binding capacity, increasing the syrup's viscosity (Saha and Bhattacharya, 2010). Previous studies have shown that xanthan gum has a more significant impact on increasing syrup viscosity than glycerin and sweeteners. In this study, xanthan gum was more dominant in increasing the viscosity content than propylene glycol.
Rheology test
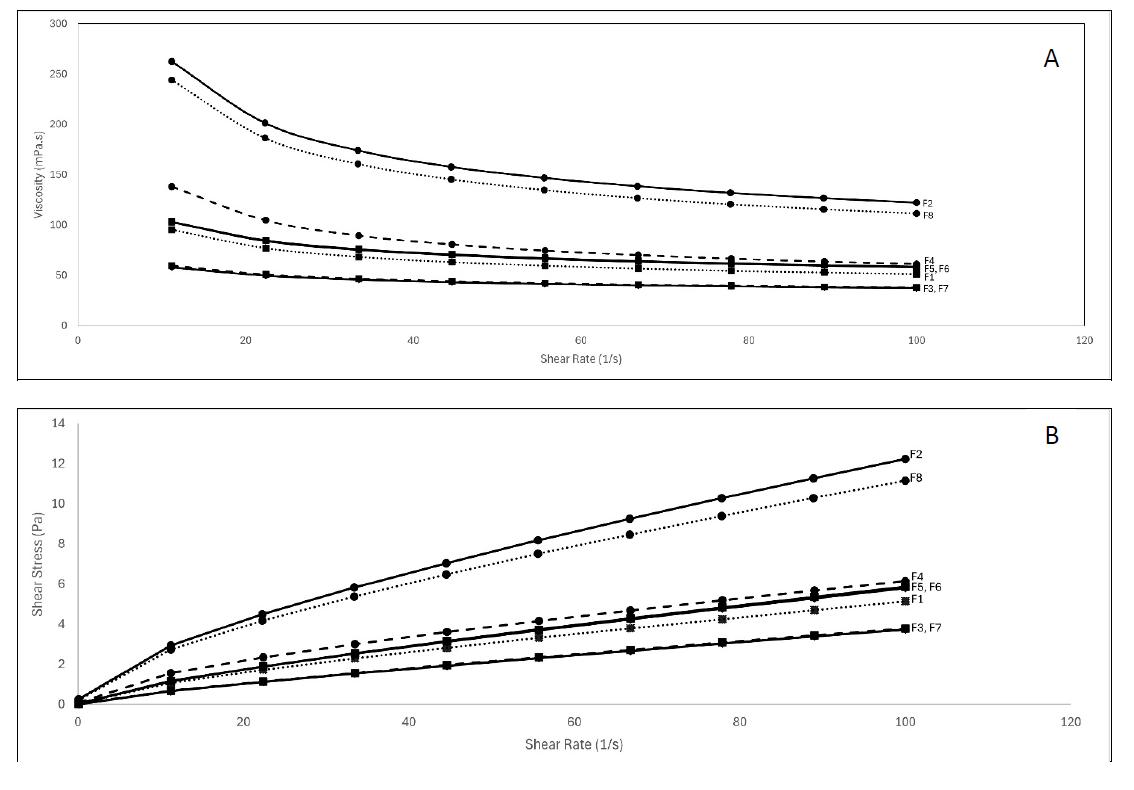
Figure 3. Rheology profile of honey-based syrup. Viscosity vs Shear rate (A). Shear stress vs Shear rate (B).
Figure 3a shows the effect of the propylene glycol and xanthan gum combination on the viscosity value of the syrup mixture at a constant temperature of 25°C. The test was done at the shear rate of 10-100 s-1, resulting in the viscosity value of 37 mPa.s to 262 mPa.s in all samples. Formulas F3 and F7 had the lowest, and F2 had the highest values. The viscosity value is generally relatively constant above the shear rate of 20-s with a slight decrease trend. The F2 and F8 formula stabilizes above the shear rate of 40-s, with a greater decrease than the others. These might be caused by the more significant amount of xanthan gum (0.2 g) on the two formulas compared to the other formulas, which contributes to the greater viscosity value. It is also shown in the result of other formulas, which contributes a higher value of viscosity for greater xanthan gum amounts in the mixture, as we could see that F4 had lower viscosity due to the lower concentration of xanthan gum which was 0.1625 g, continued by F5, F6 at the amount of 0.125 g, and then followed by F1 which had 0.0875 g in it. The lowest amount of xanthan gum was 0.05 g for F3 and F7.
Figure 3b presents the correlation between shear stress and shear rate of honey-based syrup. The graph indicates that the shear stress increased linearly following the shear rate, indicating that the honey-based syrup had Newtonian flow behavior characteristics. Shear stress values were in the 0-12 Pa range in the same order as Figure 3a above. It denoted that shear stress has a positive correlation with viscosity. Xanthan gum is widely used as a thickener due to its high viscosity value. The viscosity for greater xanthan gum concentration (0.5 wt%) in the shear rate of 0-1,000 s-1 was the highest, followed by lower concentration (0.25 wt%), and the lowest concentration (0.1 wt%) has the lowest viscosity value of all. It has a positive correlation between xanthan gum concentration and the resulting viscosity in line with this research (Higiro et al., 2006) also found that through the Huggins plot (specific viscosity/concentration vs concentration) for xanthan gum addition in the locust bean gum (30-120 dl/g) was in higher rates rather than without xanthan gum addition (11-20 dl/g). Carmona et al., (2017) also revealed that the higher the xanthan gum concentration in the formula, the higher its viscosity. They used 0.15-0.40 % m/m in concentration, resulting in 27.4-1,638 Pa.s viscosity value, respectively. The viscosity value of this research was less than the study conducted by (Mohamat et al., 2023), which resulted in a viscosity value of 0.2 – 0.6 Pa.S (200-600 cP), whereas in this research, the values were varied between 0.05-0.275 Pa.S (50-275 cP).
Water activity and Color Analysis
Table 4. Water activity and color analysis of honey-based syrup
|
Sample |
Water activity(aw) |
Colour |
||
|
L* |
a* |
b* |
||
|
Formula 1 |
0.8900 ± 0.00a |
20.7 ± 0.1c |
2.0 ± 0.1c |
2.6 ± 0.8b |
|
Formula 2 |
0.8689 ± 0.00e |
19.8 ± 0.1e |
1.6 ± 0.0d |
1.1 ± 0.8e |
|
Formula 3 |
0.8890 ± 0.00ab |
20.5 ± 0.1d |
1.1 ± 0.1f |
1.0 ± 0.8e |
|
Formula 4 |
0.8895 ± 0.00a,b |
20.6 ± 0.3c,d |
1.4 ± 0.1e |
1.4 ± 0.8d |
|
Formula 5 |
0.8793 ± 0.00c |
20.7 ± 0.1c |
1.7 ± 0.0d |
1.7 ± 0.9c |
|
Formula 6 |
0.8806 ± 0.00c |
21.2 ± 0.0b |
2.3 ± 0.2b |
2.5 ± 1.0b |
|
Formula 7 |
0.8874 ± 0.00b |
20.5 ± 0.1d |
1.0 ± 0.1f |
0.9 ± 1.2f |
|
Formula 8 |
0.8769 ± 0.00d |
21.7 ± 0.0a |
2.9 ± 0.1a |
3.1 ± 0.0a |
Note: The data expressed as mean ± S.D. (n = 3); the different letters within the rows indicate statistically significant differences determined using ANOVA (P < 0.05). The color test was expressed as L*, which is the brightness level; a*, which corresponds to the level of redness; and b*, which corresponds to the level of yellowness. The letters a–f in tables analyzing water activity and color parameters of honey-based syrups typically denote statistical significance groupings based on post-hoc tests. It indicates whether differences between samples or treatments are statistically significant (P <0.05).
Table 4 presented the range of water activity in the honey-based syrup with values between 0.8689 to 0.8900. Water activity manages microbial growth and is also responsible for chemical activities on food related to product quality, stability, and safety. The study reported that adding propylene glycol and xanthan gum to the formula of honey-based syrup changed the water activity at different ratios. The addition of more xanthan gum and less propylene glycol causes the water activity of honey-based to increase. The research also showed that the ratio between xanthan gum and propylene glycol affects the product's color. The study revealed that the honey-based syrup have an L* that ranges between 19.8 and 21.7; a*, which varies between 1.0 and 2.9; and b*, which ranges between 0.9 and 3.1. High propylene glycol and xanthan gum concentrations significantly differed for honey-based syrup's L*, a*, and b* at values (P > 0.05). Using propylene glycol in syrup produced higher L* values, indicating a brighter appearance. Decreasing redness and yellowness of syrup was suggested by a lower value of a*.
Verification of optimum formula
The physical properties of the eight experimental formulations were analyzed using Design Expert software version 13 to validate the optimal formula for honey-based herbal syrup containing C. indicum flower extract and C. asiatica extract. The selection of the optimum formula was based solely on three physical properties: pourability, density, and viscosity. The software calculated a desirability value of 0.872, indicating a highly favorable outcome. According to the software analysis, the ideal formulation is composed of 0.099% xanthan gum and 10.401% propylene glycol. Table 5 shows the predicted values for each response provided by the software. The data obtained is as expected and not significantly different from the prediction value.
Table 5. Comparison of commercial product and Simplex Lattice Design (SLD) Predicted Value with Optimum Formula Test Results.
|
Response |
Commercial value |
Prediction Value |
Experimental Value |
Sig. (2-tailed) |
|
Pourability |
6 |
6.43 |
6.46 |
0.14 |
|
Density |
1.25 |
1.25 |
1.25 |
0.27 |
|
Viscosity |
30.36 |
38.15 |
38.00 |
0.59 |
Stability test of optimum formula
The stability test was applied to the optimum formula of honey-based herbal syrup. Freeze-thaw stability testing is a crucial part of the stability testing process. It determines whether a formula will remain stable under various conditions. The test is conducted by subjecting the formula to multiple freeze-thaw cycles and observing any changes. Physical properties such as pH, pourability, density, and viscosity were tested to determine the herbal syrup's stability and stability (Rahma, 2024). In a single cycle, the honey-based syrup is expected to pass through freezing temperature at -10°C for 24 hours, thawing at room temperature for 24 hours, and then being transferred to 45°C for 24 hours, followed by another 24 hours at room temperature.
In this study, the freeze-thaw method was slightly modified by allowing the syrup to stand at room temperature for 2 x 24 hours after completing each cycle and testing the physical properties of the syrup. In the preliminary test, the syrup preparation was tested for three consecutive days under normal conditions, namely storage at room temperature, and changes in its physical properties were observed. The data on the physical characteristics of the herbal syrup were found to have no significant difference on the first day compared to the second and third days
(Figure 4a). Therefore, modifications to the freeze-thaw cycle can be applied in the stability test of honey-based herbal syrup.
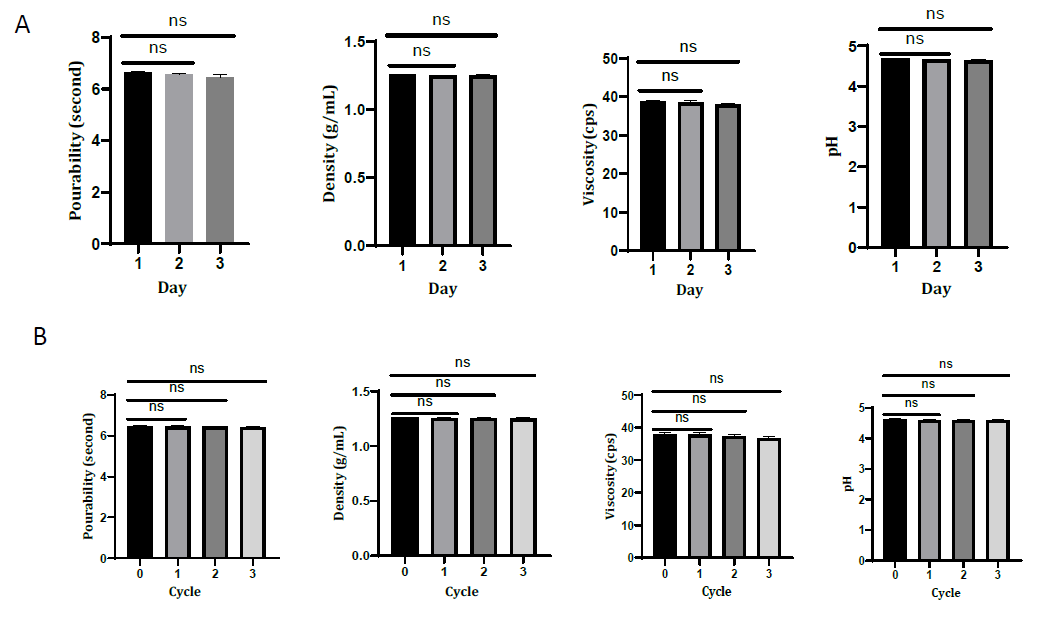
Figure 4. Comparison of pourability, density, viscosity, and pH of honey-based syrup. Result of preliminary test day 2 and day 3 compared to day 1 (A). Result of stability test cycles 1, 2, and 3 compared to cycle 0 (B). The analysis of antioxidant activity was performed using One Way ANOVA followed by Post Hoc Test. Each bar represents mean ± SD, ns = not significant (P-value >0.05).
The freeze-thaw stability test results indicated no significant differences in pourability, density, viscosity, and pH after the syrup passed the test (Figure 4b). This method assesses the impact of extreme temperatures on syrup preparations during storage. The viscosity of a formulation is inversely proportional to temperature. High temperatures can reduce the viscosity of syrup formulation. The decrease in pour time is consistent with the reduction of syrup viscosity. The pH of the syrup also decreased but remained within the acceptable range of 4-7. Therefore, honey-based syrup is stable in its physical properties even when exposed to extreme temperature changes.
As a consequence of the freeze-thaw process, in such circumstances, there is a tendency for alterations to occur in the viscosity of the formula. Previous research mentioned that adding xanthan gum and propylene glycol can help maintain syrup formulas' stability. Xanthan gum is a hydrocolloid with numerous hydrophilic groups. Hydrogen bonds with the hydroxyl groups of the hydrocolloid. This molecular interaction resulted in a stronger interaction between the water molecules and the solid surface. This property helps stabilize the syrup formulation (Zheng et al., 2017). The absence of residue in the freeze-thaw tested syrup may be due to propylene glycol as an extraction solvent.
The stability test for the honey-based syrup produced in this study requires adding a microbial test during the syrup's stability test at extreme temperatures. Microbial contamination can cause changes in the syrup's physiochemical characteristics, including fermentation, turbidity, and changes in color and odor. The microbial content test in the syrup freeze-thaw stability test can improve the stability of the syrup in terms of microbiological properties.
DISCUSSION
This study measured the antioxidant activity of each active substance and the combination of active substances used in herbal syrup formulations. According to Junsi and Siripongvutikorn (2022), C. asiatica has a high value of antioxidant activity and can provide more health benefits. C. indicum flower and C. asiatica herb extracts exhibit strong antioxidant activity, with values of 67.11 µg/mL and 38.44 µg/mL, respectively. However, the herbal syrup in this study was formulated with honey, which has a weak antioxidant activity of 16,910 mg/mL. As a result, the final antioxidant activity of the mixture of the three ingredients falls into the weak category, which measured 272.82 µg/mL.
The novelty of this research is related to the innovation of combining C. indicum flower and C. asiatica extracts in a syrup preparation using thickening agents in the form of xanthan gum and co-solvent propylene glycol. Propylene glycol is added to increase the solubility of the C. indicum and C. asiatica herb extracts to produce a homogeneous syrup preparation. In addition, previous studies have shown that propylene glycol has the same ability as ethanol to dissolve active substances. Thus, it can be an alcohol-free formulation (Laksitorini and Purnomo, 2023). In this study, reducing the concentration of xanthan gum while increasing the amount of propylene glycol results in decreased pourability, density, and viscosity. This is due to the fact that increasing the amount of xanthan gum can greatly enhances the syrup's solubility. Xanthan gum can produce higher viscosity values than other hydrocolloid polysaccharides such as CMC, guar gum, and alginate, even at low concentrations. The optimal formula was created using 0.099% xanthan gum and 10.401% propylene glycol. The syrup preparation in this study uses honey as a base, which is a natural sweetener. Honey constitutes a healthier alternative to conventional high-sugar syrups and offers numerous benefits due to its antioxidant, antibacterial, anti-inflammatory, and wound-healing properties (Nikhat and Fazil, 2022). According to the research to be conducted, it is necessary to optimize the taste of honey-based syrup extracts of C. indicum flowers and C. asiatica herbs due to the bitter aftertaste of the herbal syrup. The stability of the syrup should be evaluated using a freeze-thaw test that includes a microbiological analysis to determine the level of microbial contamination present during storage.
CONCLUSION
In conclusion, Ceiba premium nectar honey is used as a base for herbal syrup due to its higher antioxidant activity than Rambutan premium nectar honey. The optimal formula of honey-based syrup containing C. indicum flower and C. asiatica herb extracts was 0,099% of xanthan gum and 10,401 % of propylene glycol. The utilization of xanthan gum and propylene glycol in honey-based syrup did not influence the antioxidant activity of the products, which produces weak antioxidants. Pourability, density, and viscosity decreased as the composition of xanthan gum decreased compared to propylene glycol, and these results align with predictions and experimental observations. Honey-based syrup in the optimum formula produces good physical stability and can be employed as a potential sugar-free antioxidant supplement that can be consumed within a certain time without the risks associated with excessive sugar consumption.
ACKNOWLEDGEMENTS
The authors would like to thank Mr. Heri Kurniawan from PT Natural Amanindo Utama for providing the authentic ceiba and rambutan nectars used in this study and in Advanced Characterization Laboratories Yogyakarta, National Research and Innovation Agency for providing facilities to conduct the colour test and water activity of the syrup.
AUTHOR CONTRIBUTIONS
Aulia Rahma Ardiningsih assisted in performing most of the experiments, statistical analysis and data visualization, and wrote the manuscript. Wimala Hardyawati performed the honey antioxidant activity test, and Mias Isningrom performed the syrup physical properties test. Cici Darsih performed the syrup's color test and water activity, reviewing and editing the manuscript. Anggita Sari Praharasti conducted the rheological measurement test and evaluation. Muhammad Novrizal Abdi Sahid as a supervisor and input in thin layer chromatography test and antioxidant activity. Marlyn Dian Laksitorini carried out research direction, research funding experimental design, and manuscript finalization. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
REFERENCES
Adjeng, A.N.T., Murrukmihadi, M., Hertiani, T., and Nugroho, A.K. 2023. Optimation of sorbitol, glycerine, and xanthan gum combination in mucolitic syrup of Hibiscus rosa-sinensis leaves extract using mixture design (d-optimal). Rasayan Journal of Chemistry. 16(1): 509–518.
Afjani, Q., Baiti, N., Novrizal, M., Sahid, A., Darsih, C., and Laksitorini, M.D. 2024. The development of plant-based jelly candy for C. indicum L. Flower extract and evaluation of the antioxidant activity. Indonesian Journal of Pharmacy. 35(2): 292–304.
Ahmed, S., Sulaiman, S.A., Baig, A.A., Ibrahim, M., Liaqat, S., Fatima, S., Jabeen, S., Shamim, N., and Othman, N.H. 2018. Review article honey as a potential natural antioxidant medicine : An insight into its molecular mechanisms of action. Oxidative Medicine and Cellular Longevity. 2018: 8367846.
Al-Farsi, M., Al-Amri, A., Al-Hadhrami, A., and Al-Belushi, S. 2018. Color, flavonoids, phenolics and antioxidants of Omani honey. Heliyon. 4(10): e00874.
Ar Rochmah, M., Harini, I.M., Septyaningtrias, D.E., Sari, D.C.R., and Susilowati, R. 2019. C. asiatica prevents increase of hippocampal tumor necrosis factor- α independently of its effect on brain-derived neurotrophic factor in rat model of chronic stress. BioMed Research International. 2019(1): 2649281.
Bahrami, M., Ataie-Jafari, A., Hosseini, S., Foruzanfar, M.H., Rahmani, M., and Pajouhi, M. 2009. Effects of natural honey consumption in diabetic patients: An 8-week randomized clinical trial. International Journal of Food Sciences and Nutrition. 60(7): 618–626.
Baloš, M.M.Ž., Popov, N.S., Radulović, J.Z.P., Stojanov, I.M., and Jakšić, S.M. 2020. Sugar profile of different floral origin honeys from Serbia. Journal of Apicultural Research. 59(4): 398–405.
Becerril‐sánchez, A.L., Quintero‐salazar, B., Dublán‐garcía, O., and Escalona‐buendía, H.B. 2021. Phenolic compounds in honey and their relationship with antioxidant activity, botanical origin, and color. Antioxidants. 10(11): 1700.
Carmona, J.A., Calero, N., Ramírez, P., and Muñoz, J. 2017. Rheology and structural recovery kinetics of an advanced performance xanthan gum with industrial application. Applied Rheology. 2017(1): 2555.
Darsih, C., Indrianingsih, A.W., Poeloengasih, C.D., Prasetyo, D.J., and Indirayati, N. 2021. In vitro antioxidant activity of macroalgae Sargassum duplicatum and Palmaria palmata extracts collected from Sepanjang Beach, Gunungkidul, Yogyakarta. IOP Conference Series: Materials Science and Engineering. 1011(1): 12052.
Debnath, T., Jin, H.L., Hasnat, M.A., Kim, Y., Samad, N.B., Park, P.J., and Lim, B.O. 2013. Antioxidant potential and oxidative dna damage preventive activity of C. indicum extracts. Journal of Food Biochemistry. 37(4): 440–448.
Devi, D.N., Darsih, C., Yuniarti, N., Ardiningtyas, B., and Laksitorini, M.D. 2024. Formulation and antioxidant activity of Gotu Kola jelly candy with plant-based polymers as a gelling agent. Majalah Obat Tradisional. 29(3): 266–275.
Eggleston, G., Boue, S., Bett-Garber, K., Verret, C., Triplett, A., and Bechtel, P. 2021. Phenolic contents, antioxidant potential and associated colour in sweet sorghum syrups compared to other commercial syrup sweeteners. Journal of the Science of Food and Agriculture. 101(2): 613–623.
Fatimi, H.A., Zulkarnain, A.K., and Laksitorini, M.D. 2023. Optimization of 3,4-Dimethoxychalcone and rutin containing gel with simplex lattice design and in vitro-in vivo test as a sunscreen. Pharmaceutical Sciences and Research. 10(2): 89–97.
Gohil, K.J., Patel, J.A., and Gajjar, A.K. 2010. Pharmacological review on Centella asiatica: A potential herbal cure-all. Indian Journal of Pharmaceutical Sciences, 72(5): 546.
Gray, N.E., Alcazar Magana, A., Lak, P., Wright, K.M., Quinn, J., Stevens, J.F., Maier, C.S., and Soumyanath, A. 2018. C. asiatica: Phytochemistry and mechanisms of neuroprotection and cognitive enhancement. Phytochemistry Reviews. 17(1): 161–194.
Higiro, J., Herald, T.J., and Alavi, S. 2006. Rheological study of xanthan and locust bean gum interaction in dilute solution. Food Research International. 39(2): 165–175.
Hossain, M.L., Lim, L.Y., Hammer, K., Hettiarachchi, D., and Locher, C. 2021. Honey-based medicinal formulations: A critical review. Applied Sciences (Switzerland). 11(11): 5159.
Junsi, M., and Siripongvutikorn, S. 2022. Development of herbal juice from C. asiatica: Antioxidant property, nutritional value and shelf life of product. Food Science and Technology (Brazil). 42(1): e93722.
Kane, S.N., Mishra, A., and Dutta, A.K. 2016. Preface: International conference on recent trends in physics. Journal of Physics Conference Series. 755(1): 3–10.
Kikuzaki, H., Hisamoto, M., Hirose, K., Akiyama, K., and Taniguchi, H. 2002. Antioxidant properties of ferulic acid and its related compounds. Journal of Agricultural and Food Chemistry. 50(7): 2161–2168.
Kumar, S., Verma, M., Hajam, Y.A., and Kumar, R. 2024. Honey infused with herbs: A boon to cure pathological diseases. Heliyon. 10(1): e23302.
Kuswahyuning, R., Nanda, T., Sulaiman, S., Galuh, W., Ananingrum, R., and Reno, A. 2020. The influence of propylene glycol/water and ethanol/water binary solvents on the in vitro permeation of Garcinia mangostana L. pericarp extract across shed snakeskin. Indonesian Journal of Pharmacy. 31(3): 187–192.
Lajoie, L., Fabiano-Tixier, A.S., and Chemat, F. 2022. Water as green solvent: Methods of solubilisation and extraction of natural products—past, present and future solutions. Pharmaceuticals. 15(12): 1507.
Laksitorini, M., and Purnomo, H. 2023. Application of hildebrand solubility parameter to identify ethanol-free co-solvent for pediatric formulation. Indonesian Journal of Pharmacy. 34(2): 218–226.
Liu, Y., Shi, S., Wang, Y., Feng, Y., and Luo, C. 2017. Calculation of heavy oil viscosity based on an Arrhenius model. Petroleum Science and Technology. 35(12): 1196–1201.
Mohamat, Noor, N.R.A.M., Yusof, Y.A., Sabri, S., and Zawawi, N. 2023. Differentiation of high-fructose corn syrup adulterated kelulut honey using physicochemical, rheological, and antibacterial parameters. Foods. 12(8): 1670.
Molyneux, P. 2004. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Journal of Science and Technology, 26(2): 211–219.
Newall, C.A., Anderson, L.A., and Phillipson, J.D. 1996. Herbal medicines: A guide for healthcare professionals. Pharmaceutical Press, Michigan.
Nikhat, S., and Fazil, M. 2022. History, phytochemistry, experimental pharmacology and clinical uses of Honey: A comprehensive review with special reference to Unani medicine. Journal of Ethnopharmacology. 282(1): 114614.
Olayemi, O.J., John-Africa, L.B., Chikwendu, C.B., and Isimi, C.Y. 2020. Preliminary evaluation of the physicochemical and antiplasmodial properties of syrup formulations containing the aqueous root extract of Nauclea latifolia (Rubiaceae). Saudi Journal of Medical and Pharmaceutical Sciences. 6(8): 541–547.
Palma-Morales, M., Huertas, J.R., and Rodríguez-Pérez, C. 2023. A comprehensive review of the effect of honey on human health. Nutrients. 15(1): 15133056.
Pitaksuteepong, T., Kesornmalee, K., and Phanaphai, N. 2024. Development and stability study of oseltamivir reconstitutable dry suspension from capsules. Natural and Life Sciences Communications. 23(1): e2024010.
Rahma, A. 2024. Formulation of honey-based syrup combination of Chrysanthemum (C. indicum L.) and Centella (C. asiatica (L.) Urban) extract: Xanthan gum and propylene glycol optimization. Thesis, Gadjah Mada University.
Rahmasari, F.S., Sahid, M.N.A., Siswanti, D.U., Darsih, C., Utami, I.D., Alam, L.P.M., and Laksitorini, M.D. 2024. The development of antioxidant nutraceuticals containing C. indicum L. gummy candy. Majalah Obat Tradisional. 29(1): 37–45.
Rusman, A., Nugroho, A.E., and Pramono, S. 2020. The effect of combination of active fraction Andrographis paniculata (Burm.f) Ness and C. asiatica (i) Urban on the alpha glucocidase inhibitor and antioxidant activities. Indonesian Journal of Pharmacy. 31(1): 56–60.
Saha, D., and Bhattacharya, S. 2010. Hydrocolloids as thickening and gelling agents in food: A critical review. Journal of Food Science and Technology. 47(6): 587–597.
Sakika, K.A., Saiman, M.Z., Zamakshshari, N.H., Ahmed, I.A., Nasharuddin, M.N.A., and Hashim, N.M. 2022. Analysis of antioxidant properties and volatile compounds of honeys from different botanical and geographical origins. Sains Malaysiana. 51(4): 1111–1121.
Singh, S., Singh, D.R., Banu, V.S., and Avinash, N. 2014. Functional constituents (micronutrients and phytochemicals) and antioxidant activity of C. asiatica (L.) Urban leaves. Industrial Crops and Products. 61(1): 115–119.
Suan Chua, L., Ardawati Adnan, N., and roji Sarmidi, M. 2014. Effect of thermal treatment on the biochemical composition of tropical honey samples. International Food Research Journal. 21(2): 773–778.
Syaifabila, I., Yuniarti, N., Darsih, C., and Laksitorini, M.D. 2024. Development of gotu kola (C. asiatica (L.) Urban) gummy candy and its evaluation on antioxidant activity. Journal of Research in Pharmacy. 28(4): 1047–1056.
Yap, V.L., Tan, L.F., Rajagopal, M., Wiart, C., Selvaraja, M., Leong, M.Y., and Tan, P.L. 2023. Evaluation of phytochemicals and antioxidant potential of a new polyherbal formulation TC-16: Additive, synergistic or antagonistic? BMC Complementary Medicine and Therapies. 23(1): 93.
Youssef, F.S., Eid, S.Y., Alshammari, E., Ashour, M.L., Wink, M., and El-Readi, M.Z. 2020. C. indicum and Chrysanthemum morifolium: Chemical composition of their essential oils and their potential use as natural preservatives with antimicrobial and antioxidant activities. Foods. 9(1): 1460.
Zheng, W., Sun, C., and Bai, B. 2017. Molecular dynamics study on the effect of surface hydroxyl groups on three-phase wettability in oil-water-graphite systems. Polymers. 9(8): 370.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Supplementary material
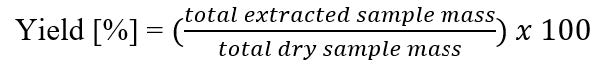
Figure S1. Equation of the percentage of yields (w/w) (Debnath et al., 2013).
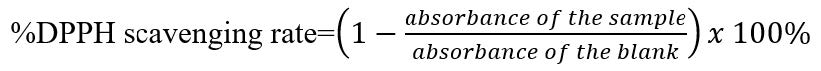
Figure S2. Equation of %DPPH scavenging rate (Kikuzaki et al., 2002)
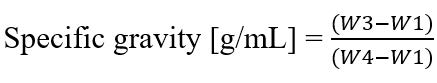
Figure S3. Equation of Specific gravity where the weight of the empty pycnometer is W1, the pycnometer filled with water is W2, the weight of the syrup in the pycnometer is W3, and the weight of the pycnometer filled with the same volume of water is W4 (Olayemi et al., 2020).
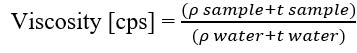
Figure S4. Calculation of Viscosity where the ρ sample is specific gravity sample, ρ water is specific gravity of water, t sample is sample flow time, and t water is water flow time (Liu et al., 2017).
Aulia Rahma Ardiningsih1, Wimala Hardyawati1, Mias Isningroom1, Cici Darsih2, Anggita Sari Praharasti2, Muhammad Novrizal Abdi Sahid3, and Marlyn Dian Laksitorini4, 5, *
1 Undergraduate Program, Faculty of Pharmacy, Universitas Gadjah Mada, Yogyakarta, 55281, Indonesia.
2 Research Center for Food Technology and Processing, National Research and Innovation Agency, Yogyakarta, 55861, Indonesia.
3 Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Universitas Gadjah Mada, Yogyakarta, 55281, Indonesia.
4 Department of Pharmaceutics, Faculty of Pharmacy, Universitas Gadjah Mada, Yogyakarta, 55281, Indonesia.
5 Halal Center (Institute of Halal and Industry System), Universitas Gadjah Mada, Yogyakarta, 55281, Indonesia.
Corresponding author: Marlyn Dian Laksitorini, E-mail: marlyn_fa@ugm.ac.id
Total Article Views
Editor: Sirasit Srinuanpan,
Chiang Mai University, Thailand
Article history:
Received: January 10, 2025;
Revised: April 15, 2025;
Accepted: April 24, 2025;
Online First: May 15, 2025

