
Novel Denture Cleanser Formulated From Virgin Coconut Oil and The Anionic Emulsifier Against Candida albicans Biofilms Formed on 96-Wells Plate and Acrylic Resin Surfaces
Wariya Siriyod, Phenphichar Wanachantararak, Thanapat Sastraruji, Pisaisit Chaijareenont, Wantida Chaiyana, Siriwoot Sookkhee, and Darunee Owittayakul*Published Date : July 10, 2023
DOI : https://doi.org/10.12982/NLSC.2023.047
Journal Issues : Number 3, July-September 2023
Abstract The objectives of this study were to investigate the inhibitory effect of coconut oil denture-cleansing (CDC) formula against Candida albicans biofilm formed on 96-wells plate and acrylic surfaces at various immersion times and to determine the effects on physical properties of heat-cured acrylic surfaces. A total of twenty-seven CDC formulas were prepared. All CDC formulas were evaluated the stability and anticandidal activity by freeze-defrost cycles and broth microdilution assays, respectively. The formula 22th (F22) containing 0.3 g of anionic emulsifier at a ratio 40:60 of virgin coconut oil to distilled water showed the highest stability and anticandidal activity. The percent inhibition against C. albicans biofilm formed on 96-well plate and acrylic resin surfaces of the F22 after 8 hours of immersion were 86.87 ± 0.65%, and 91.19 ± 1.81%, respectively. However, the F22 had significantly lower inhibitory activity than 0.12% chlorhexidine gluconate (P <0.05). Furthermore, flexural strength of acrylic resin specimens was determined using a 3-point bending test and surface roughness was measured with a profilometer. The flexural strength and the change in surface roughness of F22 were not significant different compared with chlorhexidine and distilled water after 8 hours immersion for 30 days (P >0.05). In conclusions, F22 which containing 0.3 g of anionic emulsifier at the ratio of coconut oil to distilled water at 40:60 exhibited the potent inhibitory activity to Candida albicans biofilms and had no significant effect on the flexural strength and surface roughness of acrylic resins after immersion for 30 days.
Keywords: Denture cleanser, Coconut oil, Anionic emulsifier, Biofilm, Candida albicans, Acrylic resins
Funding: We are grateful for the research funding provided by the Faculty of Dentistry, Chiang Mai University, Chiang Mai, Thailand.
Citation: Siriyod, W., Wanachantararak, P., Sastraruji, T., Chaijareenont, P., Chaiyana, W., Sookkhee, S., and Owittayakul, D. 2023. Novel denture cleanser formulated from virgin coconut oil and the anionic emulsifier against Candida albicans biofilms formed on 96-wells plate and acrylic resin surfaces. Natural and Life Sciences Communications. 22(3): e2023047.
INTRODUCTION
Denture stomatitis is an inflammatory disease resulting from a polymicrobial biofilm infection at the denture surface–palatal mucosa interface. Its incidence is occurring up to 70% of older patients (Gendreau et al., 2011). Old age should be regarded as a normal inevitable biological phenomenon. During the latter half of the 20th century, the age demographic changed dramatically with a greater proportion of elderly people within the population. This change has had a major impact on the delivery of oral healthcare products (Akar et al., 2008). The increase of the number of elderlies may lead to an increase in the number of people requiring removable dentures which can result in denture stomatitis. Inadequate cleaning of removable dentures promotes the accumulation and adhesion of biofilm, which is one of the main causes of prosthesis failure (Neppelenbroek, 2015). It has been widely reported that denture biofilm acts as a reservoir of microbial community which is similar to that of dental biofilm, except for an increase in Candida spp., that can cause local infections, especially Candida-related denture stomatitis (Gleiznys et al., 2015) Candida albicans is most common microorganism that adheres the inner surface of the denture bases and has been identified as the primary risk factor of denture stomatitis (Ramage et al., 2004). Besides poor denture cleanliness, local and systemic conditions such as xerostomia and diabetes, particularly found in older adults are predominant factors in the pathology of denture stomatitis (Bozdemir et al., 2019). Various methods were reported to eliminate C. albicans such as brushing, ultrasonic, sodium hypochlorite (NaOCl), chlorhexidine gluconate (CHX), natural extracts, and the combination method. Mechanical method by brushing dentures alone is ineffective against microbial biofilms on dentures (Glass et al., 2001). The combination of brushing and chemical immersion in denture cleansers is the recommended method for removing biofilms on dentures (Felton et al., 2011). An ideal denture cleanser should have bactericidal and fungicidal properties, remove biofilms without affecting denture materials’ properties or stains. It should be nontoxic, compatible with denture material, short acting (≤ 8 hours), simple to use, acceptable taste, and cost-effective (Felton et al., 2011). However, there is no ideal chemical agent. Chemical denture cleansers such as NaOCl, CHX and alkaline peroxides are most widely used. NaOCl and CHX were reported for candidal biofilm eradication efficacy. Alkaline peroxides were not effective in eliminating Candida (Ribeiro Rocha et al., 2020) in the 15 minutes period specified by the manufacturer. Nevertheless, this solution showed an adverse effect on denture materials. Sodium hypochlorite is not used as immersion products. Exposure of longer than ten minutes in this solution may cause the damage of denture. This solution can damage the denture base to cobalt chromium alloy surface. Moreover, overnight immersion of NaOCl can affect to the color stability and flexural strength of acrylic resin (Barbosa et al., 2007), can increase the surface roughness (Vieira et al., 2010), exerts the malodor, and has a bad taste (Barnabé et al., 2004). Both 0.12%, and 2.0% CHX-based treatments exhibited the similar ability to remove denture biofilm. Moreover, long term soaking of dentures caused acrylic staining. CHX should not be applied with nystatin because their combination creates a salt, which interferes the efficacy of the drugs (Barkvoll et al., 1989). CHX at 0.12% can affect the microhardness of the denture base by continue exposure, and alter the dimensional stability of self-cure acrylic resin (Arora et al., 2011). Alkaline peroxide solution can decrease the flexural strength of acrylic resins (Shah et al., 2015) and causes lightening of acrylic resin on extended use (Ghalichebaf et al., 1982). Therefore, a new or alternative choice for denture cleanser with anticandidal activity and non-toxic substance should be investigated for example, mouthwash containing propolis, white vinegar, lemongrass extract (Dany et al., 2015). Coconut oil contains a plentiful source of medium-chain fatty acids (MCFAs), especially lauric acid (45-56%) and capric acid (4-10%) (Nevin et al., 2006). These MCFAs can destroy candidal cell membrane (Mukhtar et al., 2020) and has potential anticandidal activity against C. albicans (Yildirim-Bicer et al., 2014). Among several MCFAs, 2.5 mM of lauric acid, 5 mM of capric acid, and 1.25 mM of monocaprin were effective to kill C. albicans within ten minutes of incubation time (Bergsson et al., 2001). Bergsson et al’s study reported that capric acid affected the ultrastructure of C. albicans as demonstrated by TEM. After treatment with 10 mM of capric acid for 30 minutes, the results demonstrated the disorganization of cytoplasm and cell membrane.
Coconut oil-pulling was carried out in the preventive therapy to maintain oral hygiene (Asokan et al., 2009). Our former study reported high patient satisfaction score with coconut oil-pulling because of its advantages such as good taste, pleasing scent, and fewer allergic reactions compared with CHX (Owittayakul et al., 2018). Recently, Intharakaewsri et al. developed anticandidal mouthwash from coconut oil that was added propylene glycol and distilled water to reduce its cost. This innovative mouthwash was formulated with virgin coconut oil: propylene glycol: distilled water at the ratio at 60:30:10 demonstrated the efficacy to reduce C. albicans biofilm as equal to nystatin with the percent of inhibition at 83.75+ 5.75, and 82.36+4.61, respectively (Intarakaewsri et al., 2020). Unfortunately, this formula of coconut oil was not stable. The separation of the coconut oil layer and water layer was observed within a few minutes resulting in the inappropriate denture-cleansing agent. Hence, other emulsifying agent are required to solve this problem and achieve a homogeneous solution. The objectives of the present study were to develop a denture-cleansing formula from virgin coconut oil with an emulsifier and to investigate its efficacy in order to reduce C. albicans biofilm. In addition, the effect on physical properties of heat-cured acrylic surfaces were evaluated. The null hypothesis were that there is no difference of the anticandidal activity between the developed cleansing formula from virgin coconut oil and 0.12% CHX, and the developed cleansing formula does not affect the physical properties of acrylic resin, such as flexural strength and surface roughness, at 30 days of immersion.
MATERIALS AND METHODS
Preparation of coconut oil denture-cleansing (CDC) formula
A total of 27 CDC formulas were prepared by using virgin coconut oil (VCO; CoCo Delight®, GPO, Pathumthani, Thailand) and diluted with distilled water at the ratio of 10-90%v/v. Then, sodium polyacrylate and C13-14 isoparaffin and Laureth-7 (tradename AquagelTM 45; Chem Sources Ltd., Bangkok, Thailand) was separately added at 0.1, 0.2 or 0.3 %w/v. The ingredients were mixed together using a magnetic stirrer.
Emulsion stability test
The stability test was performed within two days at room temperature according to the freeze-defrost cycles (Daher et al., 2014). The formulas which exhibited the homogeneous appearance were selected. Four incubation cycles of each formula at 4°C were completed and then incubated again at 45°C for 24 hours. After the incubation cycle completed, the formulas which demonstrated an unchanged appearance were then selected for further evaluation.
Candida albicans strains
C. albicans ATCC10231 was obtained from the laboratory of Department of Medical Science, Ministry of Public Health, Nonthaburi, Thailand. Sabouraud Dextrose agar (SDA; DifcoTM; Bacton Dickinson, Sparks, MD) was used as the culture medium of C. albicans while Sabouraud dextrose broth (SDB; DifcoTM; Bacton Dickinson, Sparks, MD) was used to determine the minimum inhibitory concentration (MIC) and the minimum fungicidal concentration (MFC). All strains were separately cultured in SDB at 37°C for 24 hours under aerobic condition. At the end of the incubation period, one mL of culture was centrifuged at 2,000 rpm/minute for ten minutes. Cell-free supernatant was then decanted. Candidal cells were resuspended with one mL of Phosphate Buffer Saline (PBS) and then adjusted at the optical density of 600 nm to be equal to McFarland No.1 (106 CFU/mL) (Chanpa et al., 2023).
Determinations of MIC and MFC
The MIC and MFC values of 0.12% CHX, 100% VCO, The selected CDC formula and AquagelTM 45 against C. albicans were determined by using broth microdilution assay with slight modification (Rodríguez-Tudela et al., 2003). Positive and negative controls were 0.12% CHX and 0.1 to 1.0 %w/v of AquagelTM 45 which was dissolved in distilled water. One hundred µL of candidal suspension and 100 µL of sample solutions were mixed into a 96-well plate and incubated at 37°C for 24 hours. The lowest concentration of the tested compounds that can inhibit the visual growths of C. albicans was recorded as MIC. The growth of the tested pathogen in the non-visible wells over the MIC well were subculturing with inoculating loop. A sample from each well was subsequently streaked on the surface of SDA plate. The plates were then incubated at 37°C for 24 hours. The lowest concentration of the tested compounds exhibiting the absence of C. albicans colony on SDA plate was recorded as the MFC.
Colony Enumeration
Colony enumeration was performed to evaluate C. albicans inhibitions of the selected CDC formula (Madeira et al., 2016). C. albicans suspension was adjusted to a final concentration of 1.5×106 CFU/mL. One hundred µL of C. albicans solution and 100 µL of the sample were mixed into 96-wells plate and incubated at 37°C for 24 hours. Then, ten µL of suspension from each well was dropped into 90 µL of SDB and was then serially diluted with PBS solution. The 100 µL of suspended solution was plated in triplicated onto SDA plate. The plates were aerobically incubated for 24 hours at 37°C before counting the colonies. The percentage of reduction was calculated using the following formula;
[(Numbers of colonycontrol - Numbers of colonysample)/Numbers of colonycontrol] × 100%
Biofilm Formation
One hundred µL of each isolate of C. albicans which adjusted the optical density of McFarland No. 1 was dropped into each well of the pellicle-prepared 96-wells plate. One hundred µL of SDB was separately dropped into each well. The tested 96-wells plate was then incubated at 37°C for 90 minutes for initial adherence (Santos et al., 2016). After the end of incubation period, the whole supernatant in each well was gradually decanted before being washed twice with 200 µL of PBS solution. One hundred µL of SDB was immediately dropped into each well and replaced every 24 hours until the incubation was completed after 48 hours. After the end of the incubation period, the whole supernatant in each well was completely drained before being washed twice with 200 µl of PBS solution. The growth of candidal biofilm was measured according to the previous study (Chanpa et al., 2023).
Inhibitory activity against C. albicans biofilms formed on 96-well plate
The MFC value of 0.12% CHX solution against C. albicans in freshly prepared biofilms was determined at 48 hours of the incubation period as described in the previous report (Chanpa et al., 2023). C. albicans biofilms that formed on the surface of 96-well plates were measured after incubated with the selected CDC formulas at 37°C for 20 minutes, 8 hours, and 12 hours. After the incubation period, the treated biofilm of each well was separately removed by a sterilized disposable inoculating loop. The biofilm suspension was serially two-fold diluted in PBS and plated in triplicate onto a SDA plate to enumerate the C. albicans colonies. Inhibitory activity on biofilm was calculated as described above as a percentage of candidal inhibition. The formula which exhibited the strongest activity was selected for further investigation with regard to candidal viability.
Inhibition of candidal viability and biofilm detected by fluorescence microscopy
C. albicans biofilm was prepared on the 96-wells plate which was then incubated with the selected CDC formula for inhibition time. The plate was washed twice with PBS, then removed the solution before stained with FUNTM-1 to determine the candidal viability and appearance of biofilm extracellular matrix. Prior to staining, the plate was transferred to a new well of 96-wells plate and incubated with 2 mL of PBS containing 10 µM FUNTM-1 fluorescent dye (Invitrogen, Thermo Fisher Scientific, Waltham, MA) and 25 µg/mL of concanavalin A (Con A)-Alexa Fluor 488 conjugate (Invitrogen, Thermo Fisher Scientific, Waltham, MA) for 45 minutes at 37°C in dark condition (Montelongo-Jauregui et al., 2019). This investigation was completed in triplicate. After the incubation period, fluorescence microscopy (Olympus EX41 microscope, Olympus Co., Shenzhen, Guangdong, PRC) was performed to observe the candidal viability at 200x magnification. The FUNTM-1 stain passively diffuses into a variety of cell types and initially stains the cytoplasm with a diffusely distributed dye fluorescence. For yeasts and fungi, live cells stain red but dead cells stain bright yellowish green without any red color. Concanavalin A (Con A) is one of the most widely used lectins in cell biology which exhibits green fluorescence. It selectively binds to α-mannopyranosyl and α-glucopyranosyl residues at the glycan of C. albicans.
Anticandidal activity against biofilm formed C. albicans on acrylic resin disc
Heat-cured acrylic resin discs (Hexa Ceram dental laboratory, Chiang Mai, Thailand) with a diameter of 6 × 2 mm. were fabricated, immersed in distilled water at 37°C for 48 hours to reduce the remaining monomers, and then sterilized with ethylene oxide gas (Paranhos et al., 2009).
Biofilm formation was prepared on the sterilized discs of acrylic resin according to the previous study (Chanpa et al., 2023). The anticandidal activity of the selected CDC formula was determined at 37°C for 20 minutes, 8 hours, and 12 hours. Briefly, two hundred µL of the selected CDC formula was dropped into each well which placed the C. albicans biofilm on acrylic discs. After incubation with various cleansing times, each acrylic disc was washed twice with PBS and then transferred into a new centrifuge tube that contained 300 µL of the SDB. Biofilm attached on the acrylic resin was separately sonicated using an ultrasonic vibrator at 1,000 Hz for five minutes to break down attachment (Choonharuangdej et al., 2021). Ten µL of the suspension was diluted into a new centrifuge with 990 µL of SDB twice. The anticandidal activity against C. albicans biofilm on acrylic resin disc was determined by colony enumeration and the percentage of fungal inhibition was calculated.
Effect on flexural strength (Fs) of heat-cured acrylic resin
Fifty-six rectangular heat-cured acrylic resin sheets with a size of 65 × 10 × 3 mm were fabricated, polished, and then immersed in distilled water at 37°C for 48 hours to eliminate residual monomer (Jorge et al., 2006). The polishing process was performed by using 400-grit silicon carbide abrasive paper (TOA Paint Public Com. Ltd., Thailand) under running water. Sequential sandpapering was done using a micromotor, and then specimens were polished on a wet rag wheel with a slurry of pumice. The polished specimens were checked their dimension using a vernier caliper. The fifty-six specimens were divided into four groups (n=14) including the baseline group (initiation value), the control group (immersion with distilled water, the two treated groups (immersion with the selected CDC formula, and 0.12% CHX). For each cycle of sample immersion, the sample was rinsed through distilled water for three minutes, dried, then kept at room temperature for 24 hours (Ghazal et al., 2019). The cycling of each sample was repeated every day for 30 days. The flexural strength was measured according to the three-point bending test (Arora et al., 2011) by using the universal hardness tester (Instron 5566 universal testing machine, Instron®, Norwood, MA) with a crosshead speed at five mm/minute and with the support span width of 50 mm applying the load until specimen fracture. The maximum load exerted at failure was recorded in Newton (N). The flexural strength (Fs) and flexural modulus (Fm) of each sample were calculated from the following equations (ISO, 2013).
Flexural strength (MPa) = 3PL/bd2
Flexural modulus (MPa) = L3m/4bd3
with P, maximum load; L, span length (65 mm); b, acrylic width (10 mm); d, acrylic thickness (3 mm); m, the slope of the modulus line (N/mm).
Effect on surface roughness (Ra) of heat-cured acrylic resin
Thirty specimens with a size of 25 × 14 × 3 mm were fabricated, prepared and polished as described above. A roughness tester (Surftest SJ-310, Mitutoyo Co., Kanagawa, Japan) was used to measure the surface roughness of each rectangular specimen at baseline. The baseline values were not exceeding 0.2 µm to simulate the clinically acceptable level of surface roughness (Neppelenbroek et al., 2005). Thirty acrylic sheets were divided into three groups (n=10): distilled water, the CDC formula, and CHX. Each sample was immersed for 8 hours as described above (Bollen et al., 1997). Sample cycling was repeated every day for a 30 days-period. Surface roughness was determined at days 0, 15 and 30. Each specimen were performed 2.4 mm in length and cutoff value of 0.5 mm/s in the regions corresponding of the marks of the specimen (Madeira et al., 2016). The mean value of surface roughness was calculated from the three lines. The resolution of the record data was 0.01 µm.
Statistical analysis
Normal distribution of data was tested by Shapiro-Wilk test. One-way analysis of variance (One Way-ANOVA) and Tukey’s multiple comparisons were used to compare the means between groups and the paired-sample T-test was computed to compare the means within the same group using IBM software SPSS 17.0 (SPSS Inc., Chicago, Illinois, USA). All tests were performed using a confidence level of 95%.
RESULTS
Stability evaluation of CDC formulas with AquagelTM 45
Details of each CDC formula exhibited with % AquagelTM 45, % VCO and their physical stability at day 1 and day 2 were shown in Table 1. Figure 1 demonstrated that the 27 CDC formulas were obtained according to the variation of % AquagelTM 45 in a range of 0.1 to 0.3% and their stabilities at 48 hours of incubation. Only five CDC formulas, F19 to F23, were presented the homogeneous appearance (H) after 48 hours of incubation at room temperature. The results revealed that the application of 0.3%w/v of AquagelTM 45 was effective to stabilize the mixture of oil and water Details of each CDC formula exhibited with ingredients, and physical stability at day one and day two were shown in Table 1.

Figure 1. Stability evaluation of CDC formulas at 48 hours of incubation.
Table 1. CDC formulas, ingredients and their physical stabilities.
|
Formulas |
Ingredients |
Physical Stabilities |
||
|
%w/v AquagelTM 45 |
% VCO |
Day 1 |
Day 2 |
|
|
F1 |
|
10 |
CS |
CS |
|
F2 |
|
20 |
CS |
CS |
|
F3 |
|
30 |
S |
S |
|
F4 |
|
40 |
CS |
CS |
|
F5 |
0.1 |
50 |
CS |
CS |
|
F6 |
|
60 |
CS |
CS |
|
F7 |
|
70 |
CS/C |
CS |
|
F8 |
|
80 |
S/C |
S/C |
|
F9 |
|
90 |
S/C |
S/C |
|
F10 |
|
10 |
H/C |
H/C |
|
F11 |
|
20 |
H/C |
H/C |
|
F12 |
|
30 |
H/C |
H/C |
|
F13 |
|
40 |
S/C |
S/C |
|
F14 |
0.2 |
50 |
H/C |
H/C |
|
F15 |
|
60 |
H/C |
H/C |
|
F16 |
|
70 |
H/C |
H/C |
|
F17 |
|
80 |
S/C |
S/C |
|
F18 |
|
90 |
H/C |
H/C |
|
F19 |
|
10 |
H |
H |
|
F20 |
|
20 |
H |
H |
|
F21 |
|
30 |
H |
H |
|
F22* |
|
40 |
H |
H |
|
F23* |
0.3 |
50 |
H |
H |
|
F24 |
|
60 |
H/C |
H/C |
|
F25 |
|
70 |
H/C |
H/C |
|
F26 |
|
80 |
H/C |
H/C |
|
F27* |
|
90 |
S/C |
S/C |
Note: CS, Clear separation; S, Separation; H, Homogeneous; C, Colloidal appearance; *, Selected formulas
To confirm the appropriate %VCO for development of the stable CDC formulas, three formulas in different %VCO, F22, F23, and F27, were selected to investigate with the freeze-defrost cycles for one and two days, and with an extreme thermal test for eight days. Figure 2 demonstrates that two CDC formulas (F22 and F23), demonstrated homogeneous emulsion at the end of freeze-defrost cycle and extreme thermal stability test. These CDC formulas were stable and used for further investigations.

Figure 2. Stability evaluation of the selected CDC formulas with 0.3%w/v AquagelTM 45 at one and two days of incubation at room temperature, and after eight days of extreme thermal incubation.
Determination of MIC and MFC values of CHX
The MIC and MFC values of CHX were 0.0075% and 0.015%w/v CHX, therefore fungicidal. In comparison, 0.1% to 1% AquagelTM 45 did not inhibit C. albicans in 96-wells plate or on the SDA plate.
Colony enumeration
Figure 3 shows the significant difference between 0.12% CHX, CDC formulas F22 and F23, compared with 100% virgin coconut oil (VCO) and 0.3% AquagelTM 45 after 24-hours of incubation against C. albicans (P <0.001). The mean values of inhibitory activities were 97.73 ± 1.24%, 94.19 ± 2.24% and 91.18 ± 2.11%, whereas the mean percentage of inhibition of the VCO and 0.3% AquagelTM 45 were only 44.55 ± 3.00% and 2.16 ± 0.50%, respectively. The CDC formulas F22 and F23 differed on the percent ratio between VCO and distilled water such as 40:60, and 50:50 %v/v, respectively.

Figure 3. Percentage of inhibition against C. albicans after 24-hour incubation; *, P < 0.05. The red bars of these data were the mean value of each data group.
Figure 4 demonstrates the mean value of percentage inhibition against C. albicans biofilms-formed on 96-wells plate by CFU enumeration. The results exhibited the highest anticandidal activity ranked in order to 0.12% CHX, CDC formula F22, CDC formula and F23, 100% VCO and 0.3% of AquagelTM 45, respectively. CHX at 0.12% showed the highest inhibition after immersion for 8 and 12 hours whereas its high level of inhibitory activity was shown at 92.49 ± 1.47% after 20 minutes of immersion. For the activity of emulsifier, immersion in 0.3% AquagelTM 45 for 20 minutes, 8 hours and 12 hours did not inhibit the C. albicans biofilms, 1.16 ± 0.78%, 1.44 ± 0.74%, and 1.28 ± 0.38%, respectively. For the activity of VCO, there were the significant differences of inhibitory activity compared with 0.12% CHX at all immersion time (P <0.05). At 20 minutes of immersion, the significant differences (P <0.05) were detected after compared among 0.12% CHX, the CDC formula F22, the CDC formula F23, and 100% VCO with the inhibitions at 92.49 ± 1.47%, 79.21 ± 2.18%, 38.49 ± 0.71%, and 22.72 ± 1.15%, respectively. At 8 and 12 hours of immersion, the significant differences at P <0.001 were also detected after compared among 0.12% CHX, the CDC formula F22, the CDC formula F23, and 100% VCO with the inhibitions at 99.56 ± 1.02% and 99.81 ± 0.38, 86.87 ± 0.65% and 91.94 ± 0.83, 79.02 ± 0.83% and 82.81 ± 1.44, and 52.81 ± 1.16 and 52.09 ± 1.01%, respectively. The inhibitory activity of CDC formula F22 showed significantly higher than that of the CDC formula F23 at all immersion times (P <0.05). As the results, F22 was chosen because of its stability and anticandidal effect. Although, the CDC formula F22 exhibited high value of candidal inhibition within 20 minutes of immersion, eight-hours of immersing time was chosen because of higher percentage inhibition of C. albicans. Moreover, the American College of Prosthodontists suggested to use a short acting (8 hours) substance (Felton et al., 2011).
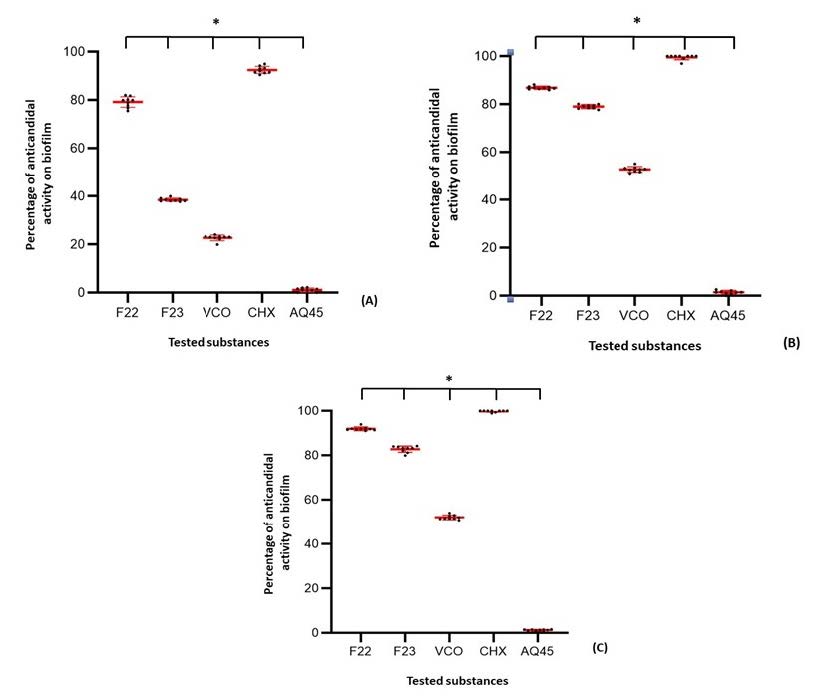
Figure 4. Percentage inhibition of C.albicans biofilms of five different substances at three immersion times (A) 20 min, (B) 8 hour, (C) 12 hour; *, P<0.05. The red bars of these data were the mean value of each data group.
Inhibition of candidal viability and biofilm detected by fluorescence microscopy
Figure 5 reveals that candidal biofilm and its extracellular matrix were decreased after immersed in the selected CDC formula F22 whereas inhibition was demonstrated in the immersion of 0.12% CHX solution both with the FUNTM-1 and Con A stainings. After eight hours of immersion, the treated C. albicans biofilms were stained with those fluorescent dye. The red fluorescence signal of FUNTM-1 appeared to be concentrated in candidal cells which indicated that the C. albicans cells was alive.
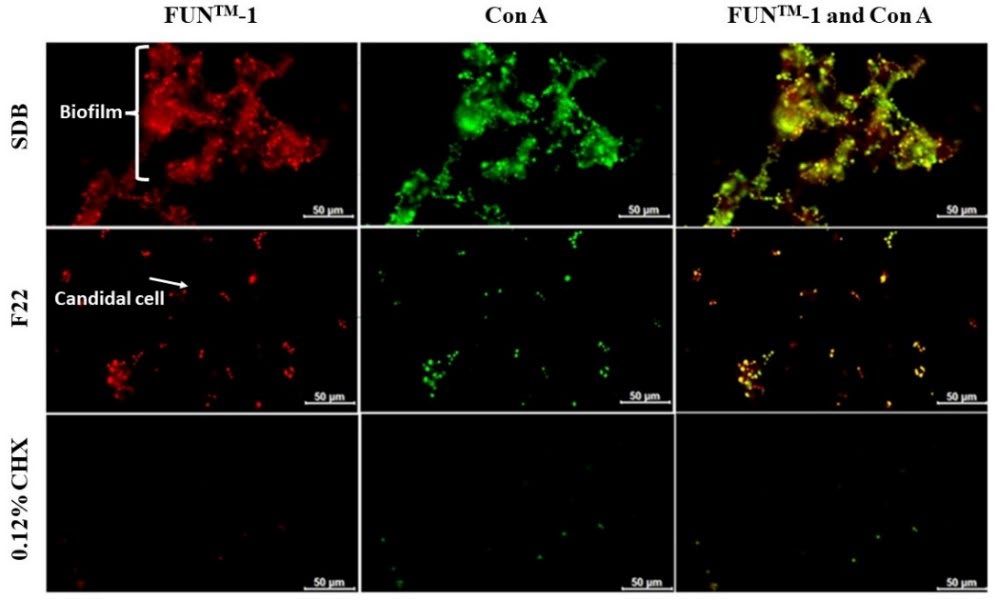
Figure 5. Inhibition of candidal viability and biofilm formation detected by fluorescence microscopy after eight hours of immersion. Biofilm formation of C. albicans in SDB, the CDC formula F22, and 0.12% CHX solution, respectively. CHX was performed as the positive control.
Anti- C. albicans biofilm formed on acrylic resin discs at 8 hours of immersion
Figure 6 demonstrates the mean percentage inhibition of C. albicans biofilm-formed on acrylic resin surfaces. At 8 hours of immersion, 0.12% CHX exhibited the greatest percent inhibition of C. albicans biofilms with 98.59 ± 0.53%, followed by the CDC F22 (91.19 ± 1.81%), 100% VCO (41.54 ± 1.34%), and 0.3% AquagelTM 45 (2.06 ± 0.82%). The CDC F22 presented high values of percentage inhibition of C. albicans biofilm. However, the percentage inhibition of 0.12% CHX group was significantly greater than that of the others (P <0.05).
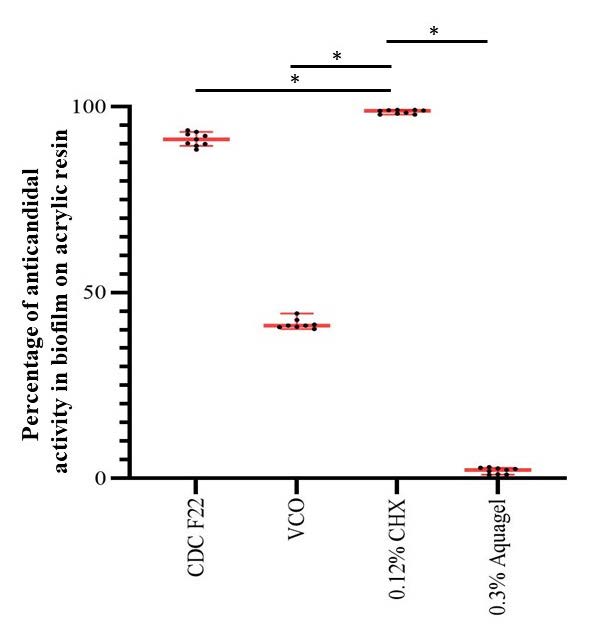
Figure 6. Percentage inhibition against C. albicans biofilms formed on acrylic resin surface by CFU calculating at 8 hours of immersion.
*, Significant differences were shown in comparison with 0.12% CHX (P < 0.05). The red bars of these data were the mean value of each data group.
Investigation on flexural strength of heat-cured acrylic resin after immersion with CDC formula F22
Table 2 demonstrates the mean values of flexural strength and flexural modulus of acrylic resin after 8 hours of immersion at room temperature for 30 days. The results showed the flexural strength and flexural modulus of baseline group had slightly higher than those of other groups. However, the results of the One-way ANOVA test found no significant difference in flexural strength among four groups. The statistical analysis showed no significant difference in flexural strength and flexural modulus after 8 hours of simulated immersion (P=0.328, and 0.931, respectively).
Table 2. Means and standard deviation flexural strength and flexural modulus of all test groups at 30 days immersion period.
|
Groups (n=14) |
Flexural strength (MPa) |
Flexural modulus (GPa) |
|
No treatment |
131.630 ± 22.290 |
2.498 ± 3.490 |
|
Distilled water |
117.900 ± 16.120 |
2.444 ± 1.230 |
|
0.12 % CHX |
120.930 ± 20.250 |
2.470 ± 1.360 |
|
The CDC formula F22 |
122.920 ± 22.090 |
2.477 ± 1.230 |
Note: Data were analyzed by One-way ANOVA test. No significantly different at P >0.05
Investigation of surface roughness of heat-cured acrylic resin after immersion with the CDC formula F22
Table 3 presents the surface roughness and the changes in roughness (∆Ra) of heat-cured acrylic resin after immersion in three different solutions. The results of paired-sample T-test revealed that the roughness values of three groups was not significantly different at day 0, 15, and 30 (P >0.05). Furthermore, analysis of the One-way ANOVA showed no significant difference in ∆Ra between three different solutions (P =0.210).
Table 3. Means and standard deviation of surface roughness (Ra, µ) of three solutions.
|
Groups (n=10) |
Baseline*, ** |
Day 15* |
Day 30** |
∆Ra*** (Day 0 – Day 30) |
|
Distilled water |
0.101 ± 0.046 |
0.108 ± 0.050 |
0.107 ± 0.049 |
-0.002 ± 0.009 |
|
012% CHX |
0.092 ± 0.048 |
0.092 ± 0.0447 |
0.100 ± 0.478 |
0.009 ± 0.019 |
|
The CDC formula F22 |
0.078 ± 0.048 |
0.078 ± 0.0472 |
0.077 ± 0.042 |
0.007 ± 0.011 |
Note: *, **Data were analyzed by paired-sample T-test; *** Data were analyzed by One-way ANOVA test. No significantly different at P <0.05
DISCUSSION
This study focused on developing a stable CDC formula which demonstrated a homogenous solution, possessed the required anticandidal activity with minimal alteration to the physical properties of acrylic resin. The null hypotheses were accepted: there was no significant difference of the anticandidal activity between the developed CDC formula and 0.12% CHX, and the CDC formula does not affect the physical properties of acrylic specimens at 30 days of immersion.
The CDC product was formulated according to three phases of compositions: VCO, distilled water and emulsifier. There are three different types of emulsifying agents based on the behavior in aqueous solutions including anionic, cationic, and nonionic emulsifiers (Levine et al., 1989). Adding emulsifiers facilitates reducing the interfacial tension between water and oil phases, allows the creation of smaller droplets and increases the emulsion status. The emulsifier structure includes a hydrophobic portion that dissolves in the oil phase and a hydrophilic portion that may be either charged or uncharged and dissolves in the aqueous phase (Tadros, 2009). The application of emulsifier to the development of coconut oil related products was reported in very few studies. Most of all, they belong to the nonionic type such as propylene glycol (1, 2-propanediol) (Intarakaewsri et al., 2020), Tween 80 (Polyoxyethylene sorbitan mono-oleate) (Fitriyani et al., 2018), Tween 85 (Polyoxyethylene sorbitan tri-oleate), Span 85 (Sorbitan tri-oleate), Span 80 (Sorbitan mono-oleate), Span 20 (Sorbitan monolaurate) (Gani et al., 2015). However, the previous study of Intharakaewsri et al reported a non-stable formulation which consisted of VCO, distilled water and emulsifier (Intarakaewsri et al., 2020). Therefore, an anionic emulsifier was chosen instead of a nonionic emulsifier in this study. AquagelTM 45 is a universal emulsifier that can emulsify a variety of oils. It is an anionic polyacrylic acid emulsifier that is stable, non-corrosive, environment-friendly, and compatible with polymer substrates. It swells rapidly and creates a gel or cream immediately without neutralization or heat. It shows the ease of preparation, no heat requirement, and no foam production. These characteristics of AquagelTM 45 were advantageous, therefore, we used it in the preparation of CDC formulas in the present study.
In this study, 27 formulations were developed, however, only two CDC formulas (F22 and F23) were stable and exhibited homogeneous appearances. These formulas were composed of VCO, distilled water and AquagelTM 45 in a ratio of 40-50%, 50-60%, and 0.3%, respectively. The results indicated that AquagelTM 45 was an appropriate emulsifier to stabilize the mixture of VCO and distilled water.
C. albicans ATCC 10231 was used because it is a strain derived from the mouth and throat of a human (Fani et al., 2014). The selected CDC formulas in our investigation which were prepared from 40-50% VCO exhibited significantly higher percentage inhibition of C. albicans growth compared to the other formulas that contained different percentages of VCO. The obtained findings indicated the application of emulsifier in a 40-50% ratio increases the anticandidal activity of VCO. The study of Fitriyani and Andina reported that 50% VCO possessed the largest average inhibitory zone than that of other % VCO (Fitriyani et al., 2018). Furthermore, Intarakaewsri et al reported that the mouthwash formulated in the ratio of VCO, propylene glycol and distilled water at 60:30:10 was able to reduce viable cells in the fungal biofilms by 83.75 ± 5.75% that was significantly higher than that in 100% VCO (42.83 ± 7.61%). In addition, there was no statistical difference between this formulation and that of nystatin (82.36 ± 4.61%) (Intarakaewsri et al., 2020). Regarding the anticandidal activity of VCO, Ogbolu et al reported the anticandidal activity of VCO in vitro (Ogbolu et al., 2007). MCFA in coconut oil can inhibit Gram-positive bacteria, Gram-negative bacteria, fungi, protozoa and viruses (Khoramnia et al., 2013). VCO is composed of greater than 99% triglycerides, with free fatty acids making up less 0.2% (Marina et al., 2009). VCO triglycerides can be hydrolyzed with lipase and water to form monoglycerides, diglycerides, glycerol, and free fatty acids. Monoglycerides and free fatty acids have been reported to exhibit the antimicrobial activity (Bhattacharyya et al., 2020). The exact mechanism by which VCO exerts antimicrobial effects is still unknown. It has been suggested that VCO must be metabolized to release its component MCFAs, caprylic acid (C8), capric acid (C10), and lauric acid (C12) to exert its antimicrobial effects (Ogbolu et al., 2007). Of these metabolites, lauric acid may have the most antimicrobial activity. A proposed mechanism for the antibacterial effects of VCO suggests that membrane lipids are solubilized as the VCO fatty acids integrate into the membrane (Shilling et al., 2013).
The physical characteristics of acrylic resin particularly the flexural strength and roughness are the crucial parameters. Currently, there is no published data about the effect of coconut oil-based denture cleansers on the physical properties of acrylic denture materials. The parameters were assessed after 8 hours of immersion with F22 according to the recommendation of by the American College of Prosthodontists (Felton et al., 2011). Moreover, 8 hours of immersion is initiated the overnight denture hygiene care by patients (Degirmenci et al., 2020). Flexural strength of acrylic denture was measured to determine the longevity of prosthesis. Poor flexural strength causes higher incidence of fracture of dentures (Barbosa et al., 2007). Furthermore, immersion in denture cleansers may reduce the flexural strength of acrylic resins (Sato et al., 2005). According to ISO 20795-1 for denture base polymers, the 3-point flexural test is frequently used to measure the flexural strength of denture base resins (ISO, 2013). The results of the present study revealed that mean flexural strength of acrylic sheets after 8 hours of immersion in the CDC formula F22 for 30 days was 122.92 ±22.09 MPa, which had no significantly change in flexural strength compared with baseline. The flexural strength value of the CDC formula F22 group was still above the minimum requirement at 65 MPa which indicated by ISO 20795.1.2013 (ISO, 2013). Flexural modulus is the ratio of stress to strain in flexural deformation. It demonstrates the stiffness or rigidity of a material within the elastic range (Chaijareenont et al., 2012). A lower flexural modulus is favorable in increasing the absorbed energy before fracture of the denture base, but a higher flexural modulus is recognized as clinically advantageous (Ucar et al., 2012). From the results of the present study, the flexural modulus value of acrylic sheets after eight hours of immersion in the CDC formula F22 for 30 days had no significant change compared with non-treatment and distilled water groups. The flexural modulus value of The CDC formula F22 was higher than the indicated minimum (2 GPa) (ISO, 2013). Considering the surface roughness, the increase of roughness usually relates to the colonization of candida cells and biofilm formation (Paranhos Hde et al., 2013). Moreover, excessive surface roughness of acrylic denture base leads to difficulty in removal of biofilm and increases the risk of Candida-associated denture stomatitis (Valentini et al., 2017). The previous study reported that the surface roughness value is greater than 0.2 μm may promote bacterial colonization and plaque formation (Bollen et al., 1997). In this study, mean surface roughness after an eight-hour immersion in the CDC formula F22 for 30 days was 0.077 µm which was acceptable within the range reported by Bollen et al (Bollen et al., 1997). In relation to CHX group, the result of this present study was in agreement with the former study by Schwinding et al that revealed CHX can cause a slight increase in surface roughness, but had no significant on Ra (Schwindling et al., 2014).
According to the American College of Prosthodontists (Felton et al., 2011), the CDC formula F22 had the desirable properties, which including homogenous mixture, fungicidal properties, short action (≤8 hours), ease of use and non-damaging the heat-curing acrylic resin surfaces. In terms of the appropriate immersion period, the results of the present study revealed that the CDC formula F22 had the highest anticandidal activity at 12-hour immersion, which was longer than the recommendation of eight hours. Therefore, denture immersion with the CDC formula F22 for eight hours, and a combination of brushing and chemical immersion may increase the efficiency in eliminating C. albicans on dentures. However, the eight-hour immersion of acrylic resin in the CDC formula F22 for 30 days in experimental conditions did not lead to significant changes in flexural strength and surface roughness, indicating the solution was safe to use. Nevertheless, further clinical trials concerning effect of long-term application of the CDC formula on the strength and roughness should be conducted.
The limitation of the present study is that a single species was used in an in vitro biofilm model. Whereas mixed species and polymicrobial biofilms, which are more resistant than single species biofilm, are found in real life. Moreover, the effects of the solutions on acrylic resin were analyzed without biofilms. Another limitation of our study was that only chemical cleaning method by chemical substances was simulated. As a result of this, the physical values would be less than in vivo study due to several factors such as mastication process and mechanical brushing or tap water could affect the physical properties of acrylic denture bases.
CONCLUSION
After formulation, the CDC formula F22 exhibited a stable emulsion with minimum amount of emulsifier used and demonstrated the highest anticandidal activity. Within the eight hours of immersion, the formula effectively reduced C. albicans biofilms formed on 96-well plates and acrylic resin sheet. Moreover, CDC formula F22 did not alter two physical properties of the acrylic resin sheets. Moreover, the CDC formula F22 had no significant effect on flexural strength and surface roughness of the acrylic resin after 30 days of immersion. In conclusion, the CDC formula F22 is an effective product made from virgin coconut oil that can be applied as an alternative denture cleanser for eradication of biofilm-formed C. albicans, and prevention of denture stomatitis caused by this pathogen.
ACKNOWLEDGMENTS
We wish to thank the volunteer in this study, staffs and technicians at the Comprehensive Dental Clinic, Faculty of Dentistry. This research would have been impossible without the approval of the Faculty of Dentistry Human Experimentation Committee, Faculty of Dentistry, Chiang Mai University (21/2021). This study received approval from the Institutional Biosafety Committee of Chiang Mai University, code number CMUIBC A-056400.
AUTHOR CONTRIBUTIONS
Wariya Siriyod wrote the research proposal, prepared the IBC document, designed and performed the experiments, and wrote the manuscript draft. Darunee Owittayakul provided the conceptualization, consulted on the research proposal, submitted the research funding, wrote the manuscript draft and proofread the research work. Phenphichar Wanachantararak designed the experiments, consulted on the IBC document, provided the resources, consulted on the experiments. Thanapat Sastraruji provided the statistic analysis, and performed the experiments. Pisaisit Chaijareenon provided the equipment and techniques for the physical characteristics. Wantida Chaiyana designed the experiments and provided the chemicals and techniques for formulating emulsions. Siriwoot Sookkhee consulted on the research proposal, performed the experiments, analyzed the statistical data, wrote the manuscript and proofread the research work. All authors have read and approved the final published version of manuscript.
CONFLICT OF INTEREST
No potential conflicts of interest relevant to this article were reported.
REFERENCES
Ajay, R., Suma, K., and Ali, S. A. 2019. Monomer modifications of denture base acrylic resin: A systematic review and meta-analysis. Journal of Pharmacy and Bioallied Sciences. 11(Suppl 2): S112-S125.
Akar, G. C. and Ergül, S. 2008. The oral hygiene and denture status among residential home residents. Clinical Oral Investigations. 12(1): 61-65.
Andes, D., Nett, J., Oschel, P., Albrecht, R., Marchillo, K., and Pitula, A. 2004. Development and characterization of an in vivo central venous catheter candida albicans biofilm model. Infection and Immunity. 72: 6023-6031.
Arora, S., Khindaria, S. K., Garg, S., and Mittal, S. 2011. Comparative evaluation of linear dimensional changes of four commercially available heat cure acrylic resins. Contemporary Clinical Dentistry. 2(3): 182-187.
Asokan, S., Emmadi, P., and Chamundeswari, R. 2009. Effect of oil pulling on plaque induced gingivitis: a randomized, controlled, triple-blind study. The Indian Journal of Dental Research. 20(1): 47-51.
Barbosa, D. B., de Souza, R. F., Pero, A. C., Marra, J., and Compagnoni, M. A. 2007. Flexural strength of acrylic resins polymerized by different cycles. Journal of Applied Oral Science. 15(5): 424-428.
Barkvoll, P. and Attramadal, A. 1989. Effect of nystatin and chlorhexidine digluconate on Candida albicans. Oral Surgery, Oral Medicine Oral Pathology and Oral Radiology. 67(3): 279-281.
Barnabé, W., de Mendonça Neto, T., Pimenta, F. C., Pegoraro, L. F., and Scolaro, J. M. 2004. Efficacy of sodium hypochlorite and coconut soap used as disinfecting agents in the reduction of denture stomatitis, Streptococcus mutans and Candida albicans. Journal of Oral Rehabilitation. 31(5): 453-459.
Bergsson, G., Arnfinnsson, J., Steingrímsson, O., and Thormar, H. 2001. In vitro killing of Candida albicans by fatty acids and monoglycerides. Antimicrobial Agents and Chemotherapy. 45(11): 3209-3212.
Bhattacharyya, A., Sinha, M., Singh, H., Patel, R. S., Ghosh, S., Sardana, K., et al. 2020. Mechanistic insight in to the antifungal effects of a fatty acid derivative against drug-resistant fungal infections. Frontiers in Microbiology. 11: 2116.
Bollen, C. M., Lambrechts, P., and Quirynen, M. 1997. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dental Material. 13(4): 258-269.
Bozdemir, E., Yilmaz, H. H., and Orhan, H. 2019. Oral mucosal lesions and risk factors in elderly dental patients. Journal of Dental Research, Dental Clinics, Dental Prospects. 13(1): 24-30.
Castro, R. D. and Lima, E. O. 2013. Anti-candida activity and chemical composition of Cinnamomum zeylanicum blume essential oil. Brazilian Archives of Biology and Technology. 56(5): 749-755.
Chaijareenont, P., Takahashi, H., Nishiyama, N., and Arksornnukit, M. 2012. Effect of different amounts of 3-methacryloxypropyltrimethoxysilane on the flexural properties and wear resistance of alumina reinforced PMMA. Dental Materials Journal. 31(4): 623-628.
Chanpa, P., Owittayakul, D., Wanachantararak, P., Chaiyana, W., and Sookkhee, S. 2023. Formulation of coconut oil mouthwash with mixed emulsifier and its growth inhibition of Candida albicans biofilms. Chiang Mai University Journal of Natural Science. 22(1): e20233016.
Choonharuangdej, S., Srithavaj, T., and Thummawanit, S. 2021. Fungicidal and inhibitory efficacy of cinnamon and lemongrass essential oils on Candida albicans biofilm established on acrylic resin: An in vitro study. Journal of Prosthetic Dentistry. 125(4): 707.e701-707.e706.
Daher, C., Fontes, I., Rodrigues, R., Damasceno, G., Soares, D., Aragão, C. F. S., et al. 2014. Development of O/W emulsions containing Euterpe oleracea extract and evaluation of photoprotective efficacy. Brazilian Journal of Pharmaceutical Sciences. 50: 639-652.
Dany, S. S., Mohanty, P., Tangade, P., Rajput, P., and Batra, M. 2015. Efficacy of 0.25% Lemongrass oil mouthwash: A Three arm prospective parallel clinical study. Journal of Clinical and Diagnostic Research. 9(10): Zc13-17.
Degirmenci, K., Atala, M. H., and Sabak, C. 2020. Effect of different denture base cleansers on surface roughness of heat polymerised acrylic materials with different curing process. Odovtos International Journal of Dental Sciences. 22(3): 145-153.
Fani, M. M., Kohanteb, J., and Araghizadeh, A. 2014. Inhibitory activity of Myrtus communis oil on some clinically isolated oral pathogens. Medical Principles and Practice. 23(4): 363-368.
Felton, D., Cooper, L., Duqum, I., Minsley, G., Guckes, A., Haug, S., et al. 2011. Evidence-based guidelines for the care and maintenance of complete dentures: A publication of the American College of Prosthodontists. Journal of Prosthodontics. 20 Suppl 1: S1-s12.
Fitriyani, U.,Andina, M. (2018). Virgin coconut oil inhibits Candida albicans growth In vitro. Paper presented at the Proceedings of the 2nd Sylah Kuala International Conference on Medical and Health Science 2018, Banda Aceh, Indonesia.
Gani, S. S. A. and Adisah, S. Z. 2015. Phase behaviour study of swiftlet nest using virgin coconut oil with non-ionic surfactants. Malaysian Journal of Analytical Sciences. 19: 184-193.
Gendreau, L. and Loewy, Z. G. 2011. Epidemiology and etiology of denture stomatitis. Journal of Prosthodontics. 20(4): 251-260.
Ghalichebaf, M., Graser, G. N.,Zander, H. A. 1982. The efficacy of denture-cleansing agents. Journal of Prosthetic Dentistry. 48(5): 515-520.
Ghazal, A. R. A., Idris, G., Hajeer, M. Y., Alawer, K., and Cannon, R. D. 2019. Efficacy of removing Candida albicans from orthodontic acrylic bases: An in vitro study. BMC Oral Health. 19(1): 71.
Glass, R. T., Goodson, L. B., Bullard, J. W., and Conrad, R. S. 2001. Comparison of the effectiveness of several denture sanitizing systems: A clinical study. Compendium of Continuing Education in Dentistry. 22(12): 1093-1096.
Gleiznys, A., Zdanavičienė, E., and Žilinskas, J. 2015. Candida albicans importance to denture wearers. A literature review. Stomatologija. 17(2): 54-66.
Intarakaewsri, T., Owittayakul, D., and Wanachantararak, P. 2020. Development of virgin coconut oil mouthwash against Candida albicans biofilms. Chiang Mai Dental Journal. 41(3): 55-64.
ISO. 2013. ISO 20795-1 DentistryBase polymers -- Part 1: Denture base polymers. Geneva
Jorge, J. H., Giampaolo, E. T., Vergani, C. E., Machado, A. L., Pavarina, A. C., and Carlos, I. Z. 2006. Effect of post-polymerization heat treatments on the cytotoxicity of two denture base acrylic resins. Journal of Applied Oral Science 14(3): 203-207.
Khoramnia, A., Ebrahimpour, A., Ghanbari, R., Ajdari, Z., and Lai, O. M. 2013. Improvement of medium chain fatty acid content and antimicrobial activity of coconut oil via solid-state fermentation using a Malaysian Geotrichum candidum. BioMed Research International. 2013: 954542.
Levine, S., Bowen, B. D., and Partridge, S. J. 1989. Stabilization of emulsions by fine particles I. Partitioning of particles between continuous phase and oil/water interface. Colloids and Surfaces. 38(2): 325-343.
Madeira, P. L., Carvalho, L. T., Paschoal, M. A., de Sousa, E. M., Moffa, E. B., da Silva, M. A., et al. 2016. In vitro effects of lemongrass extract on candida albicans biofilms, human cells viability, and denture surface. Frontiers in Cellular and Infection Microbiology. 6: 71.
Marina, A. M., Che Man, Y. B., Nazimah, S. A. H.,Amin, I. 2009. Chemical properties of virgin coconut oil. Journal of the American Oil Chemists' Society. 86(4): 301-307.
Montelongo-Jauregui, D., Saville Stephen, P., and Lopez-Ribot Jose, L. 2019. Contributions of Candida albicans dimorphism, adhesive interactions, and extracellular matrix to the formation of dual-species biofilms with Streptococcus gordonii. mBio. 10(3): e01179-01119.
Mukhtar, N. I., Abllah, Z., Mohamad, A. N., Shahdan, I. A.,Haron, U. A. 2020 Mechanism of antifungal activity of virgin coconut oil on cell membrane of Candida albicans. Journal of International Dental and Medical Research. 13(3): 903-908.
Neppelenbroek, K. H. 2015. The importance of daily removal of the denture biofilm for oral and systemic diseases prevention. Journal of Applied Oral Sciences. 23(6): 547-548.
Neppelenbroek, K. H., Pavarina, A. C., Vergani, C. E., and Giampaolo, E. T. 2005. Hardness of heat-polymerized acrylic resins after disinfection and long-term water immersion. Journal of Prosthetic Dentistry. 93(2): 171-176.
Nevin, K. G. and Rajamohan, T. 2006. Virgin coconut oil supplemented diet increases the antioxidant status in rats. Food Chemistry. 99(2): 260-266.
Ogbolu, D. O., Oni, A. A., Daini, O. A., and Oloko, A. P. 2007. In vitro antimicrobial properties of coconut oil on Candida species in Ibadan, Nigeria. Journal of Medicinal Food. 10(2): 384-387.
Okonogi, S., Phumat, P., Khongkhunthian, S., Suttiat, K., and Chaijareenont, P. 2021. Denture-soaking solution containing piper betle extract-loaded polymeric micelles; Inhibition of Candida albicans, clinical study, and effects on denture base resin. Antibiotics (Basel). 10(4): 440.
Owittayakul, D., Palee, K., Khongkhunthian, S., Langkapin, W., Wanachantararak, P., and Bhatia, P. 2018. Efficacy of coconut oil and 0.12 % chlorhexidine mouthrinses in reduction of plaque and gingivitis: A two-week randomized clinical trial. The Journal of the Dental Association of Thailand. 68: 360-369.
Paranhos Hde, F., Peracini, A., Pisani, M. X., Oliveira Vde, C., de Souza, R. F., and Silva-Lovato, C. H. 2013. Color stability, surface roughness and flexural strength of an acrylic resin submitted to simulated overnight immersion in denture cleansers. Brazilian Dental Journal. 24(2): 152-156.
Paranhos, H. F., Silva-Lovato, C. H., de Souza, R. F., Cruz, P. C., de Freitas-Pontes, K. M., Watanabe, E., et al. 2009. Effect of three methods for cleaning dentures on biofilms formed in vitro on acrylic resin. Journal of Prosthodontics. 18(5): 427-431.
Paterson, D. L., Mulazimoglu, L., Casellas, J. M., Ko, W. C., Goossens, H., Von Gottberg, A., et al. 2000. Epidemiology of ciprofloxacin resistance and its relationship to extended-spectrum beta-lactamase production in Klebsiella pneumoniae isolates causing bacteremia. Clinical Infectious Diseases. 30(3): 473-478.
Ramage, G., Tomsett, K., Wickes, B. L., López-Ribot, J. L.,Redding, S. W. 2004. Denture stomatitis: a role for Candida biofilms. Oral Surgery Oral Medicine Oral Pathology Oral Radiology and Endodontology. 98(1): 53-59.
Ribeiro Rocha, G. D. S., Neves Duarte, T., de Oliveira Corrêa, G., Nampo, F. K., and de Paula Ramos, S. 2020. Chemical cleaning methods for prostheses colonized by Candida spp.: A systematic review. Journal of Prosthetic Dentistry. 124(6): 653-658.
Rodríguez-Tudela, J. L., Barchiesi, F., Bille, J., Chryssanthou, E., Cuenca-Estrella, M., Denning, D., et al. 2003. Method for the determination of minimum inhibitory concentration (MIC) by broth dilution of fermentative yeasts. Clinical Microbiology and Infection. 9: i-viii.
Santos, J. D., Piva, E., Vilela, S. F., Jorge, A. O., and Junqueira, J. C. 2016. Mixed biofilms formed by C. albicans and non-albicans species: a study of microbial interactions. Brazilian Oral Research. 30: S1806-83242016000100232.
Sato, S., Cavalcante, M. R., Orsi, I. A., Paranhos Hde, F., and Zaniquelli, O. 2005. Assessment of flexural strength and color alteration of heat-polymerized acrylic resins after simulated use of denture cleansers. Brazilian Dental Journal. 16(2): 124-128.
Schwindling, F. S., Rammelsberg, P., and Stober, T. 2014. Effect of chemical disinfection on the surface roughness of hard denture base materials: A systematic literature review. International Journal of Prosthodontics. 27(3): 215-225.
Shah, V. R., Shah, D. N., Chauhan, C. J., Doshi, P. J.,Kumar, A. 2015. Evaluation of flexural strength and color stability of different denture base materials including flexible material after using different denture cleansers. The Journal of the Indian Prosthodontic Society. 15(4), 367-373.
Shilling, M., Matt, L., Rubin, E., Visitacion, M. P., Haller, N. A., Grey, S. F., et al. 2013. Antimicrobial effects of virgin coconut oil and its medium-chain fatty acids on Clostridium difficile. Journal of Medicinal Food. 16(12): 1079-1085.
Tadros, T. F. 2009. Emulsion science and technology: A general introduction. Emulsion Science and Technology. 1(1): 1-55.
Ucar, Y., Akova, T., and Aysan, I. 2012. Mechanical properties of polyamide versus different PMMA denture base materials. Journal of Prosthodontics. 21(3): 173-176.
Valentini, F., Luz, M. S., Boscato, N., and Pereira-Cenci, T. 2017. Surface roughness changes in denture liners in denture stomatitis patients. International Journal of Prosthodontics. 30(6): 561–564.
Vieira, A. P., Senna, P. M., Silva, W. J., and Del Bel Cury, A. A. 2010. Long-term efficacy of denture cleansers in preventing Candida spp. biofilm recolonization on liner surface. Brazilian Oral Research. 24(3): 342-348.
Yildirim-Bicer, A. Z., Peker, I., Akca, G., and Celik, I. 2014. In vitro antifungal evaluation of seven different disinfectants on acrylic resins. BioMed Research International. 2014: 519098.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Wariya Siriyod1, Phenphichar Wanachantararak2, Thanapat Sastraruji2, Pisaisit Chaijareenont3, Wantida Chaiyana4, Siriwoot Sookkhee5, and Darunee Owittayakul1, *
1 Department of Family and Community Dentistry, Faculty of Dentistry, Chiang Mai University, Chiang Mai, Thailand.
2 Dental Research Center, Faculty of Dentistry, Chiang Mai University, Chiang Mai, Thailand.
3 Department of Prosthodontic, Faculty of Dentistry, Chiang Mai University, Chiang Mai, Thailand.
4 Department of Pharmaceutical Science, Faculty of Pharmacy, Chiang Mai University, Chiang Mai, Thailand.
5 Department of Microbiology, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand.
Corresponding author: Darunee Owittayakul E-mail: jeeleeja@gmail.com
Total Article Views
Editor: Anak Iamaroon,
Chiang Mai University, Thailand
Article history:
Received: December 28, 2022;
Revised: Aprill 21, 2023;
Accepted: May 24, 2023;
Published online: June 22, 2023

