
Development of Mucoadhesive Chitosan-Iodoacetamide Buccal Tablets for Local Delivery of Dextromethorphan
Yin Yin Myat, Mont Kumpugdee Vollrath, Nitjawan Sahatspan, Tanasait Ngawhirunpat, Chaiyakarn Pornpitchanarong*, and Prasopchai Patrojanasophon*Published Date : July 10, 2023
DOI : https://doi.org/10.12982/NLSC.2023.041
Journal Issues : Number 3, July-September 2023
Abstract This study aimed to synthesize a chitosan (CHI) derivative, chitosan-iodoacetamide (CHI-IA), with enhanced mucoadhesive properties and develop buccal mucoadhesive tablets of CHI-IA for local delivery of dextromethorphan (DXT). Mucoadhesive CHI-IA was synthesized by a chemical reaction between CHI and iodoacetic acid (IA) in different weight ratios. The synthesized CHI-IA was characterized by Fourier-transformed infrared spectroscopy (FT-IR) and nuclear magnetic resonance spectroscopy (NMR) to confirm the chemical structure and assure successful synthesis. DXT was selected as the model drug to be incorporated into the matrix tablet to develop mucoadhesive buccal cough relief tablets. The mucoadhesive strength of the CHI-IA tablets was determined by a texture analyzer. Moreover, the drug content and the release characteristics of DXT from the mucoadhesive tablets were evaluated. The FT-IR and NMR spectra of CHI-IA indicated the conjugation of IA on the structure of CHI. The CHI-IA tablets exhibited a 4-time increase in mucoadhesion force compared with that of unmodified CHI. Moreover, a gradual release of DXT from the tablets was observed, and the release kinetics best fit with the Higuchi model (R2 = 0.9912). Therefore, the CHI-IA could be applied as the mucoadhesive carrier system for drug delivery.
Keywords: Chitosan, Iodoacetic acid, Mucoadhesive, Buccal tablets, Dextromethorphan
Funding: The research was supported by the Research and Creative Fund, Faculty of Pharmacy, Silpakorn University.
Citation: Myat, Y.Y., Vollrath, M.K., Sahatspan, N., Ngawhirunpat, T., Pornpitchanarong, C., and Patrojanasophon, P. 2023. Development of mucoadhesive chitosan-iodoacetamide buccal tablets for local delivery of dextromethorphan. Natural and Life Sciences Communications. 22(3): e2023041.
INTRODUCTION
Mucoadhesive drug delivery is receiving great interest in the development of drug delivery system (Andersen et al., 2015). The term mucoadhesion is the adhesion of a mucoadhesive material, or drug delivery system, to the surface of the mucous membrane through physical or chemical interactions. The phenomena offer the prolonged residence time of drug at the site of action or absorption, reduces the drug degradation, extends the therapeutic effect of drugs by controlling the release pattern, augmenting the bioavailability of the therapeutic drug by preventing the first-pass metabolism, and improved patient satisfaction (Ways et al., 2018).
Mucoadhesive materials can be either natural polymer, semi-synthetic polymer, or synthetic polymers. They are classified into two generations according to their chemical structure and the interaction formed with the mucin glycoprotein of the mucous membrane. The first generation mucoadhesive materials refers to those that weakly and non-specifically bind with the mucous membrane (Russo et al., 2016; Chatterjee et al., 2017). These polymers would form physical entanglement, ionic interaction, hydrogen bond, or van der Waal force with the mucus. Examples of the polymer in this generation include, cationic, anionic, and non-ionic polymers, e.g., chitosan, polyacrylic acid, and methyl cellulose, respectively. The second generation mucoadhesive polymers are the polymers that can specifically form a strong covalent interaction with the component of the mucous membrane, most commonly the mucin glycoprotein. These polymers are usually functionalized with specific moieties to allow covalent bond formation with the mucin (Surendranath et al., 2022). Thiolated, acrylated, catechol-functionalized, and maleimide-grafted materials are examples of the second generation mucoadhesive polymers which have been proven to increase mucoadhesive capability from the first generation mucoadhesive materials (Bernkop-Schnurch, 2005; Tonglairoum et al., 2016; Shitrit and Bianco-Peled, 2017; Pornpitchanarong et al., 2020). Moreover, recent research has reported the capability of methacrylate, phenylboronic acid, and aldehyde-functionalized polymer with improved mucoadhesion properties (Brannigan and Khutoryanskiy, 2019; Brotherton et al., 2022). However, the mucoadhesive materials with a potent adhesion that allow specific binding are still limited and further research and development of such polymer is required. Recently, the bioconjugation researches are gaining more attention. Several literatures have mentioned the use of selective conjugation with iodine-bounded compounds to attach proteins which contain cysteine that has reactive thiol side chain. Besides, the knowledge can be used and applied with mucoadhesive drug delivery (Chen and Gao, 2022).
Chitosan (CHI), a cationic polysaccharide co-polymer of N-acetyl-glucosamine and glucosamine, is commonly used in the mucoadhesive dosage forms, including mucoadhesive tablets for oral, buccal, and vaginal delivery (Sogias et al., 2012; Abruzzo et al., 2015; Cazorla-Luna et al., 2019). It can be cultivated from chitin by the deacetylation process (Bagan et al., 2012; Li et al., 2022). CHI possesses various marvelous advantage which are mucoadhesive property, biodegradability, biocompatibility, antimicrobial property, and low toxicity (Khangtragool et al., 2009; Andersen et al., 2015). The mucoadhesion capacity of CHI is due to the existence of hydroxyl (-OH) and amine (-NH2) groups in the backbone structure (Bagan et al., 2012). These groups can bind to the mucous membrane using hydrogen bonds or the negative charge of sialic acid of mucin glycoproteins through electrostatic interaction (Ways et al., 2018). So, the reason for the good mucoadhesion capacity of CHI might be owing to the chemical bond and electrostatic interactions between the positive charge of CHI and the negative charge of mucin (Andersen et al., 2015). Iodoacetic (IA), an alkylating agent, can react with cysteine residues in the proteins resulting in a strong adhesion force with the mucin. In this study, a derivative of CHI polymer substituted with an iodoacetyl functional group (chitosan-iodoacetamide; CHI-IA) was synthesized using a convenient synthesis route to improve the mucoadhesive properties of CHI. The synthesized CHI-IA was then used to prepare mucoadhesive tablets for drug delivery, where dextromethorphan (DXT) was used as a model drug. DXT comparable commercial dosage form is a lozenge which required frequent administration due to the solubility. The buccal mucoadhesive tablet would help the retain a drug in the oral cavity, prolong its action, and reduce the dosing frequency. To the best of our knowledge, a precise comparative determination of CHI-IA mucoadhesive property to the parent CHI has not been reported.
MATERIALS AND METHODS
Materials
Chitosan (CHI, Number-averaged MW 11,000 g/ mol) was acquired from OliZac Technologies Co., Ltd. (Bangkok, Thailand). Iodoacetic acid (IA), N-Ethyl-N′-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), and DXT HBr (meet USP testing specifications) were procured from Sigma-Aldrich (St. Louis, MO, USA). All other reagents, and solvents were used without additional purification.
Synthesis of chitosan-iodoacetamide
The synthesis of CHI-IA was performed by a carbodiimide coupling reaction using a two-step procedure following the synthesis scheme shown in Figure 1 (Shen et al., 2020). Firstly, various weight ratios of IA to CHI (1:1, 2:1, 3:1), 0.36 g of NHS (3.13 mmol), and 0.6 g of EDC (3.13 mmol) were dissolved into 2 mL of DMF in a small vial and stirred with slight warming until the color of the mixture changed into a dark brown color. The reaction was continued by stirring for 24 h in an ambient temperature to get a stable ester intermediate. After that, 0.58 g of CHI was dissolved in 7 mL of 1 M hydrochloric acid in a round bottom flask and mixed for 5 min before 18 mL of deionized water was added. The mixture was mixed using a magnetic stirrer. Then, the formerly prepared mixture of IA, EDC, and NHS was slowly added to the CHI solution with constant stirring. Afterward, the pH was modified to 5.0 with 1 M NaOH. The physical appearance of the reaction mixture transformed from dark brown into a yellowish clear solution after the pH adjustment. The reaction was continued for 24 h at an ambient atmosphere with continuous stirring to complete the reaction. The reactant solution was purified by dialysis in deionized water for 3 days with water replacement every 6 h followed by lyophilization.
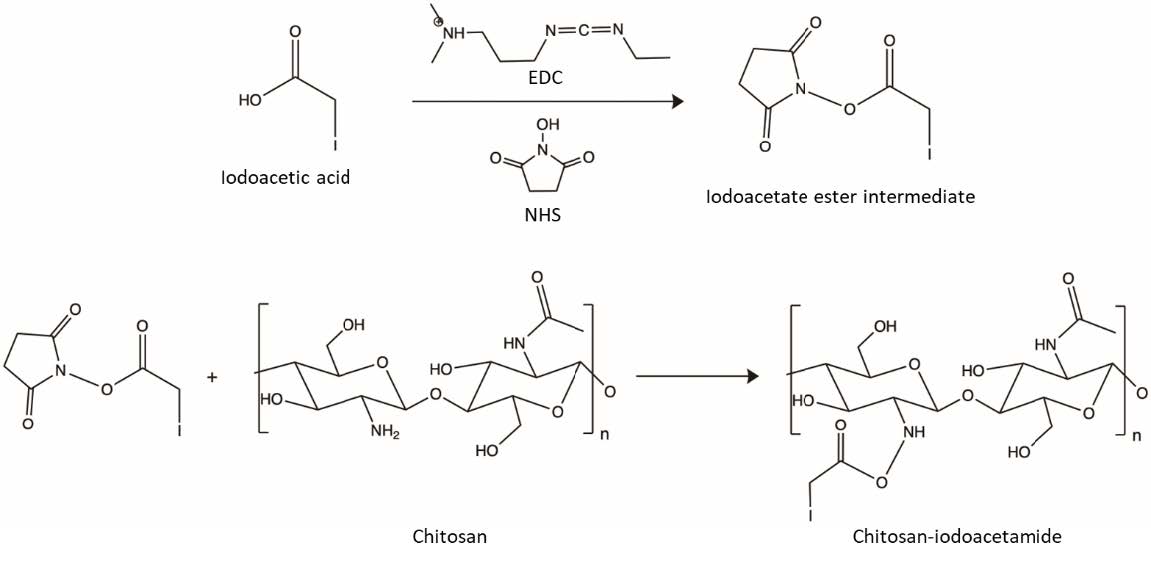
Figure 1. Synthesis scheme of CHI-IA using a two-step procedure.
Characterizations of chitosan-iodoacetamide
1H- Nuclear magnetic resonance spectroscopy
The chemical structure of CHI-IA, CHI, and IA was characterized using 1H-Nuclear magnetic resonance spectroscopy (1H-NMR). The spectrometer was 300 MHz Bruker AVANCE III HD (Billerica, MA, USA) performed at 298 K. The samples were dissolved in deuterated acetic acid (2 %v/v). The spectra were collected and reported as chemical shift (δ) in part per million (ppm).
Fourier-transformed infrared spectrophotometer
The FT-IR analyses for CHI, IA, and CHI-IA were performed using an attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectrophotometer (Nicolet iS5, Thermo Fisher Scientific, MA, USA) equipped with a single bounce diamond. The analysis was recorded as an average of 32 scans with 16 cm-1 resolution at the wavenumber ranging from 500 to 4,000 cm-1.
Determination of iodine content
To determine the degree of functionalization of CHI-IA, the amount of iodine in the structure was analyzed using inductive coupled plasma-mass spectrometer (ICP-MS) (Model-Agilent Technologies, 1100 series, Santa Clara, CA, USA) with Mira-Mist (PEEK) nebulizer at 0.1 rpm. Samples of 10 mg was digested in concentrated HNO3 at 180°C for 15 min in a closed-system microwave digestion instrument. After cooling, the solutions were diluted in ultrapure water before the analysis (Todorov and Gray, 2016).
Preparation of mucoadhesive tablets
For the determination of mucoadhesive property and the drug release study, mucoadhesive tablets of CHI-IA with DXT and CHI with DXT were prepared by direct compression. The polymer was thoroughly blended with DXT. Then, the mixture of CHI or CHI-IA and DXT was compressed into a tablet using a single punch tableting machine N 29352 (Specac Ltd. Science & Innovation Centre, UK) with the compression pressure of 2000 G and hold for 1 min. Tablets of blank CHI-IA and unmodified CHI were also prepared using the same procedure to be used as a control.
Ex vivo mucoadhesion study
The mucoadhesive performance of the CHI and its derivative on the buccal mucosa was evaluated using a texture analyzer (TA.XT Plus, Stable Micro Systems, Hamilton, USA) determining the force used to separate the mucoadhesive tablets from the mucosa. The compressed polymer tablets (CHI-IA and CHI) were fixed to the upper probe of the texture analyzer. The buccal mucosa was excised evenly and washed with artificial saliva before being placed onto the tissue holder of the texture analyzer. The surface of the buccal mucosa was dispersed with 500 µL of the artificial saliva. The probe was lowered down to press on the tissue surface, then hold for 5 sec. The force (N) required to separate the mucoadhesive tablets from the buccal mucosa and the work of adhesion obtained from the area under the detachment curve were determined (Nafee et al., 2004).
Drug content and in vitro drug release study
The DXT content in the DXT/CHI-IA tablet was quantified. The drug in the tablet was extracted by dissolving the tablet in 15 mL deionized water and shaken at 100 rpm for 24 h. The drug solution was further diluted to fit the standard curve and quantified using UV spectrometer at 278 nm (VICTOR NivoTM, Perkin Elmer, MA, USA).
The release characteristics of DXT from DXT/CHI-IA tablets were investigated compared with the commercially available DXT lozenges. The release experiment was carried out in 50 mL artificial saliva (pH 6.8) contained in a glass bottle. The test was performed at 37˚C in a shaking incubator shaken at 75 rpm. At a predetermined timepoints (0, 5, 10, 15, 30 min, and 1, 2, 4 h), 5 mL of the release medium was withdrawn the same amount of fresh medium was added. Then, the sample solution was analyzed by a UV spectrometer at 278 nm.
Statistical Analysis
All tests were performed in triplicate and the data were expressed as mean ± SD. The statistical difference was analyzed using a One-way analysis of variance at 95% confidence interval.
RESULTS
Characterizations of chitosan-iodoacetamide
The CHI derivative (CHI-IA) was synthesized by reacting the amino-functional groups of CHI at its C-2 position with the carboxylic acid group of IA. This is accomplished by utilizing a carbodiimide-mediated crosslinking reaction. The initiation of the carboxylic acid of IA was completed by the coupling of EDAC by the carbodiimide group. The resultant short-lived O-acylisourea active ester was converted into N-hydroxysuccinimidyl ester by the addition of NHS which is a more reactive and stable complex. The reactive ester complex conjugated with the amine group of CHI and became an amide functional group under pH 5 (Shen et al., 2020).
The structure of CHI-IA was confirmed by FTIR and 1H-NMR analysis. The FTIR spectrum of CHI is presented in Figure 2(a). A broad band between 3,000–3,500 cm-1 represented the characteristic peak of the O–H stretching. The band at 1,588 cm-1 was attributed to the N–H bending of the amine group in the glucosamine backbone. Besides, the N–H stretching of amines is displayed in the region of 3,286–3,361 cm-1. The weak absorption peak at 1,646 cm-1 corresponded to the C=O of secondary amide. The peaks at 2,874, 1,417, 1,323, and 1,248 cm-1 were due to the CH2 contained in the polysaccharide structure of CHI (Sahatsapan et al., 2018). In the spectrum of CHI-IA, the single band at 3324 cm-1 corresponds to N–H stretching which was overlapping the O–H stretching. A strong peak at 1630 cm-1 was the C=O stretching of the new amide group. The peak shown at 1417 cm-1 and 1373 cm-1 were probably belonged to CH2 and CH3 bending and 2874 cm-1 were the CH stretching of the conjugated IA. Also, the peaks at 1,237 cm-1 matched the C–O stretching of IA.
The NMR spectrum of the CHI-IA was shown in Figure 2(b). Firstly, the characteristic peak of CH2I of IA appeared at 3.5 ppm in the spectrum of IA (Shen et al., 2020). In the spectrum of CHI, a characteristic peak of CH3 of N-acetyl glucosamine was seen at 2.04 ppm. Moreover, the peaks between 3.4 to 3.8 would be the H-3, H-4, H-5, and H-6 of CHI (Abdelgawad et al., 2017a). The spectrum of CHI-IA was similar to those in CHI. Significantly, the H-2 signal of the substituted glucosamine group in the derivative appeared at 3.1 which was slightly shifted from the unsubstituted H-2 of CHI at 3.0 ppm.
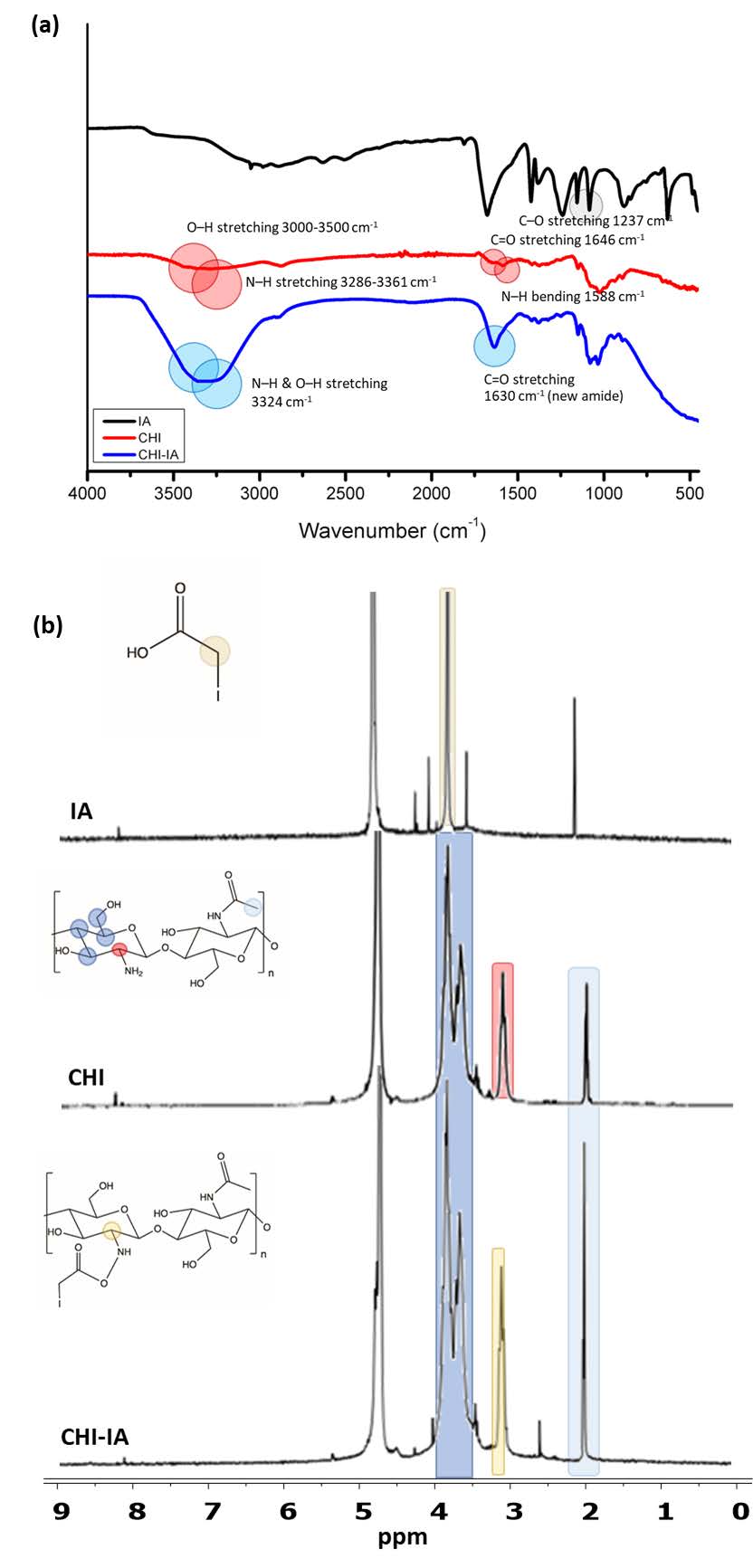
Figure 2. FTIR spectra (a) and 1H-NMR spectra of IA, CHI, and CHI-IA (b).
The results from FTIR and 1H-NMR confirmed the formation of amide bonds. Nevertheless, the observation of the grafted iodine could not be detected in neither FTIR nor the 1H-NMR spectra. By that, the content of iodine in CHI-IA was quantified by ICP-MS to establish the degree of substitution of IA on CHI. The results obtained by the ICP-MS are expressed in Table 1. According to the results, there were no considerable differences in iodine content in each ratio of CHI:IA. However, at the CHI:IA of 1:1, the highest percentage of synthesis yield (20%) was obtained compared to other ratios. Therefore, this ratio was selected for further investigations.
Table 1. The degree of substitution of different CHI:IA ratios compared with the % yield.
|
CHI:IA |
% Yield |
Degree of substitution |
|
1:1 |
20.00 |
10.90 ± 0.64 |
|
1:2 |
12.10 |
11.00 ± 0.86 |
|
1:3 |
6.90 |
9.00 ± 0.45 |
Ex vivo mucoadhesion study
The mucoadhesive property of CHI-IA was performed using a texture analyzer to evaluate the adhesion force of the CHI derivative compared to the unmodified CHI. The adhesion of the polymer and its synthesized derivative was examined indirectly where the maximum detachment force was recorded to determine the strength of adhesion. As shown in Figure 3, the detachment force of CHI-IA was four times higher than the unmodified CHI under an identical measurement environment and parameters. The work of adhesion which was calculated from the area under the detachment curve for CHI was 0.73 ± 0.11 N.mm and CHI-IA was 1.34 ± 0.07 N.mm (P <0.05). According to the maximum detachment force and the work of adhesion, CHI-IA showed to have greater mucoadhesive capability compared to CHI.
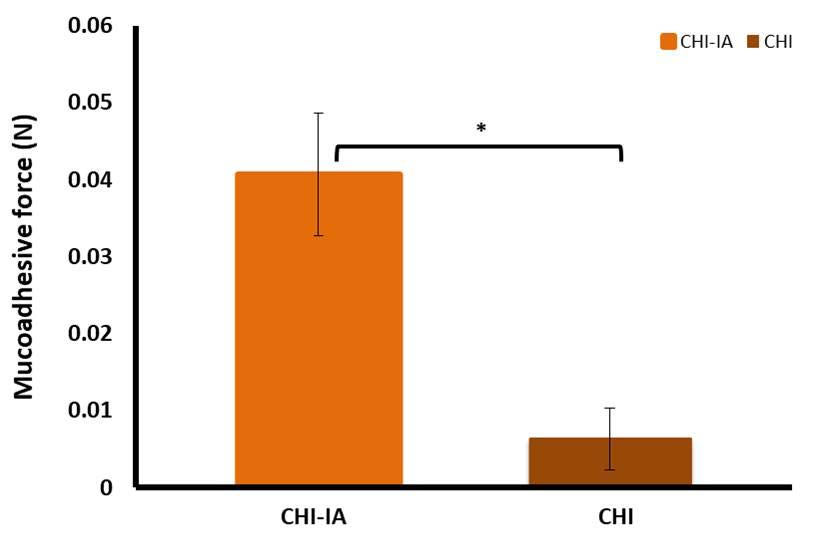
Figure 3. Ex vivo mucoadhesion force of CHI-IA in the porcine buccal mucosa compared with CHI.
Drug content and in vitro drug release study
DXT was added to the polymer blend prior to the compression to contain 5 mg per tablet. The drug content after the tablet preparation was analyzed and reported as %labeled amount. It was observed that 101.59 ± 1.21% of DXT was found. The drug release profile of DXT from CHI-IA tablets was determined in artificial saliva pH 6.8 and compared with commercially available DXT lozenges. The findings are displayed in Figure 4. The commercial DXT lozenges exhibited immediate release and reached a hundred percent of DXT within 30 min. The DXT from CHI-IA tablets showed a more gradual release and the release was completed in 4 h. In addition, no initial burst release of the drug was observed.
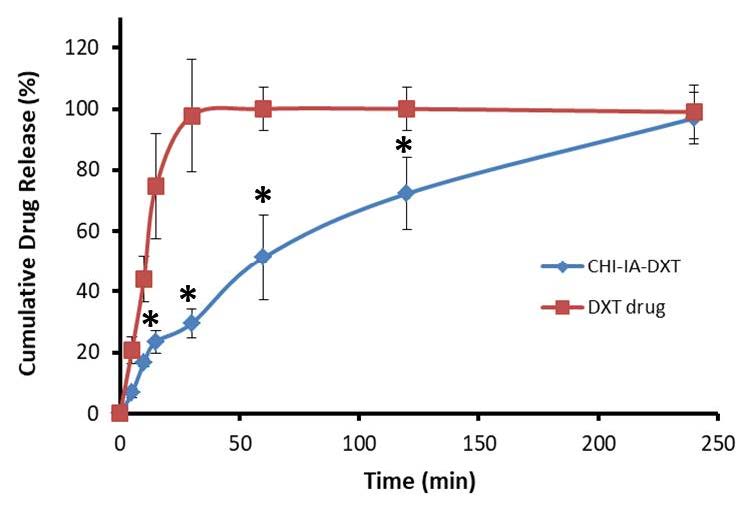
Figure 4. In vitro release profile of DXT from CHI-IA tablets compared with commercial DXT lozenges.
The release profile of DXT was fitted to kinetic models of zero-order, first-order and Higuchi models to investigate the mechanism responsible for the release of DXT from the polymer tablet, and the results are presented in Figure 5. According to the regression correlation coefficient, the release of DXT from CHI-IA polymer best fit with the Higuchi model with an R2 of 0.9912, while the zero-order model showed an R2 of 0.9064 and 0.9795 for first order kinetic model.
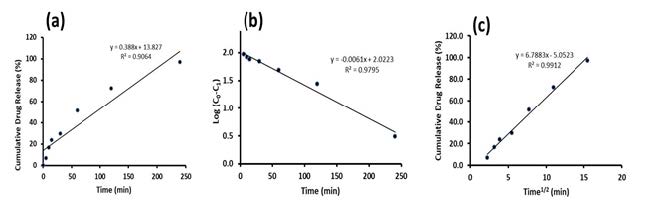
Figure 5. Kinetic models of DXT release from CHI-IA tablets zero-order model (a) first-order model (b) Higuchi model (c).
DISCUSSION
The synthesis of CHI-IA was successful through the two-step reaction. The structure of the synthesized CHI derivative was confirmed by spectroscopic analyses. The substitution of iodoacetic acid on the CHI backbone was confirmed and quantified using ICP-MS where the weight ratio between CHI and IA of 1:1 was adequate to achieve the highest degree of substitution. This could be because the amount of IA acid added was excess in terms of molar ratio to the molar quantity of amine functional group in the CHI structure introduced to the reaction. Moreover, the degree of substitution was similar to. Abdelgawad et al, (2017) that found 12-17% substitution; however, CHI used in our study has a lower MW and noted that the method to determine iodine content was not identical (Abdelgawad et al., 2017b). The safety profile of the synthesized polymer, though not established in this work, could be expected to be desirable. CHI has long been used in conventional drug formulations and massively acquired in the development of novel system for its advantages and biocompatibility property. Also, the grafted iodoacetamide acid group used in the bioconjugation mechanism is known and studied to the attachment with peptides and proteins in biomedical uses. Thus, The CHI-IA synthesized could be beneficial in a drug delivery system (Shen et al., 2020; Williams et al., 2008).
The CHI-IA with 11% degree of iodine moiety substituted in this study showed significantly greater mucoadhesive capability compared to the unmodified CHI upon tested on the adhesion force. This may be due to the strong interaction between CHI-IA with mucin in the mucous membrane since it can form a covalent bond with cysteine groups presented on the mucin glycoprotein (Aitken and Learmonth, 2002). In the bioconjugation studies, α-haloacetate and α-haloacetamide moieties can bind with a nucleophilic functional group in proteins, most reactively between iodine and the sulfhydryl group of cysteine. The bond formed was the irreversible covalent bond of thioether linkage resulting in much improved mucoadhesion capability compared to CHI which could loosely bind with the mucous membrane via ionic interaction and H-bond, that was also present in the CHI-IA interaction (Agarwal and Aggarwal, 2015; Hermanson, 2013; Kumar et al., 2022).
The drug content found in the mucoadhesive tablet was within the acceptable range for residing in the United States Pharmacopeia 2022 specification on DXT tablet that should contain 90-110% of the drug. The release of DXT from the commercial lozenge was rapid due it is commonly manufactured with high water solubility component e.g., sugar, sorbitol, etc. Whereas, the release of DXT from the CHI-IA compressed tablet was gradual and completed at 4 h because the drug was hydrophilic that it rapidly dissolves in the aqueous environment. The polymer matrix of the tablet helped to retard its release to a desirable time period. The release profile best fitted the Higuchi model which was precisely in concordance with the system developed that was a homogeneous dispersion of the drug in a swellable polymer matrix. The Higuchi release model confirmed that the release of DXT from polymer tablets was by diffusion through the polymer matrix (Paczkowska et al., 2020). The term diffusion mechanism is related to the transport of drugs from the polymer matrix into the dissolution medium which is depending on the concentration gradient (Karthikeyan et al., 2020; Pornpitchanarong et al., 2022). Besides, the result was rational and could precisely explain the drug delivery system developed.
CONCLUSION
CHI-IA, a mucoadhesive polymer, was successfully synthesized. The mucoadhesive capacity of the CHI-IA polymer tablet was superior compared to unmodified CHI in which covalent bond between iodoacetamide and sulfhydryl group of cysteine in the mucin glycoprotein was supposedly responsible for the improvement. The developed polymer could be applicable as a mucoadhesive agent to carry the DXT in form of a buccal tablet. Moreover, the polymer tablets could retard the release of DXT for 4 h. The release kinetics fit well with the Higuchi model signifying that the release was due to diffusion of the drug from the insoluble polymer matrix. Above all, the developed CHI derivative could be accounted as a member of the covalently bonded mucoadhesive material and would be beneficial in further developments of transmucosal drug delivery.
ACKNOWLEDGMENTS
The author would like to acknowledge the faculty of Pharmacy, Silpakorn University for the facility and instrument supports.
AUTHOR CONTRIBUTIONS
Yin Yin Myat dedicated in conducting the experiments and writing the first draft of the manuscript. Mont Kumpugdee Vollrath and Tanasait Ngawhirunpat guided the direction of the research. Nitjawan Sahatsapan facilitate and supervise the experiment. Chaiyakarn Pornpitchanarong supervise the experiment and project, and revised the manuscript. Prasopchai Patrojanasophon, initiated the project, acquiring the funding, administrating, and supervise the project.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
REFERENCES
Abdelgawad, A.M., El-Naggar M.E., Hudson S.M., and Rojas O.J. 2017a. Fabrication and characterization of bactericidal thiol-chitosan and chitosan iodoacetamide nanofibres. International Journal of Biological Macromolecules. 94(Pt A): 96-105.
Abdelgawad, A.M., El-Naggar M.E., Hudson S.M., and Rojas O.J. 2017b. Fabrication and characterization of bactericidal thiol-chitosan and chitosan iodoacetamide nanofibres. International Journal of Biological Macromolecules. 94: 96-105.
Abruzzo, A., Cerchiara T., Bigucci F., Gallucci M.C., and Luppi B. 2015. Mucoadhesive buccal tablets based on chitosan/gelatin microparticles for delivery of propranolol hydrochloride. Journal of Pharmaceutical Sciences. 104(12): 4365-4372.
Agarwal, S. and Aggarwal S. 2015. Mucoadhesive polymeric platform for drug delivery; a comprehensive review. Current Drug Delivery. 12(2): 139-156.
Aitken, A., Learmonth M. 2002. Carboxymethylation of cysteine using iodoacetamide/ iodoacetic acid. In: Walker JM, editor. The protein protocols handbook. Totowa, NJ: Humana Press. p. 455-456.
Andersen, T., Bleher S., Eide Flaten G., Tho I., Mattsson S., and Skalko-Basnet N. 2015. Chitosan in mucoadhesive drug delivery: Focus on local vaginal therapy. Marine drugs. 13(1): 222-236.
Bagan, J., Paderni C., Termine N., Campisi G., Lo Russo L., Compilato D., and Di Fede O. 2012. Mucoadhesive polymers for oral transmucosal drug delivery: A review. Current Pharmaceutical Design. 18(34): 5497-5514.
Bernkop-Schnurch, A. 2005. Thiomers: A new generation of mucoadhesive polymers. Advanced Drug Delivery Reviews. 57(11): 1569-1582.
Brannigan, R.P. and Khutoryanskiy V.V. 2019. Progress and current trends in the synthesis of novel polymers with enhanced mucoadhesive properties. Macromolecular Bioscience. 19(10): 1900194.
Brotherton, E.E., Neal T.J., Kaldybekov D.B., Smallridge M.J., Khutoryanskiy Vitaliy V., and Armes S.P. 2022. Aldehyde-functional thermoresponsive diblock copolymer worm gels exhibit strong mucoadhesion. Chemical Science. 13(23): 6888-6898.
Cazorla-Luna, R., Notario-Perez F., Martin-Illana A., Ruiz-Caro R., Tamayo A.,, Rubio J., and Veiga M.D. 2019. Chitosan-based mucoadhesive vaginal tablets for controlled release of the anti-hiv drug tenofovir. Pharmaceutics. 11(1).
Chatterjee, B., Amalina N., Sengupta P., and Mandal U. 2017. Mucoadhesive polymers and their mode of action: A recent update. Journal of Applied Pharmaceutical Science. 7: 195-203.
Chen, F.J. and Gao J. 2022. Fast cysteine bioconjugation chemistry. Chemistry. 28(66): e202201843.
Hermanson, G.T. 2013. Chapter 2 - functional targets for bioconjugation. In: Hermanson GT, editor. Bioconjugate techniques (third edition). Boston: Academic Press. p. 127-228.
Karthikeyan, M., Deepa M.K., Bassim E., Rahna C., and Raj K. 2020. Investigation of kinetic drug release characteristics and in vitro evaluation of sustained-release matrix tablets of a selective cox-2 inhibitor for rheumatic diseases. Journal of Pharmaceutical Innovation. 16: 1-7.
Khangtragool, A., Ausayakhun S., Leesawat P., and Molloy R. 2009. Evaluation of the use of chitosan in ocular drug delivery of vancomycin. Chiang Mai University Journal of Natural Sciences. 8(1): 1-9.
Kumar, R., Islam T., and Nurunnabi M. 2022. Mucoadhesive carriers for oral drug delivery. Journal of Controlled Release. 351: 504-559.
Li, M., Kamdenlek P., Kuntanawat P., Eawsakul K., Porntaveetus T., Osathanon T., and Manaspon C. 2022. In vitro preparation and evaluation of chitosan/pluronic f-127 hydrogel as a local delivery of crude extract of phycocyanin for treating gingivitis. Chiang Mai University Journal of Natural Sciences. 21(4):1-13.
Nafee, N.A., Ismail F.A., Boraie N.A., Mortada L.M. 2004. Mucoadhesive delivery systems. I. Evaluation of mucoadhesive polymers for buccal tablet formulation. Drug Development and Industrial Pharmacy. 30(9): 985-993.
Paczkowska, M., Chanaj-Kaczmarek J., Romaniuk-Drapala A., Rubis B., Szymanowska D., Kobus-Cisowska J., Szymanska E., Winnicka K., and Cielecka-Piontek J. 2020. Mucoadhesive chitosan delivery system with chelidonii herba lyophilized extract as a promising strategy for vaginitis treatment. Journal of Clinical Medicine. 9(4): 1208.
Pornpitchanarong, C., Rojanarata T., Opanasopit P., Ngawhirunpat T., Bradley M., and Patrojanasophon P. 2022. Maleimide-functionalized carboxymethyl cellulose: A novel mucoadhesive polymer for transmucosal drug delivery. Carbohydrate Polymers. 288: 119368.
Pornpitchanarong, C., Rojanarata T., Opanasopit P., Ngawhirunpat T., and Patrojanasophon P. 2020. Catechol-modified chitosan/hyaluronic acid nanoparticles as a new avenue for local delivery of doxorubicin to oral cancer cells. Colloids and Surfaces B: Biointerfaces. 196: 111279.
Russo, E., Selmin F., Baldassari S., Gennari C.G.M., Caviglioli G., Cilurzo F., Minghetti P., and Parodi B. 2016. A focus on mucoadhesive polymers and their application in buccal dosage forms. Journal of Drug Delivery Science and Technology. 32:113-125.
Sahatsapan, N., Rojanarata T., Ngawhirunpat T., Opanasopit P., and Tonglairoum P. 2018. 6-maleimidohexanoic acid-grafted chitosan: A new generation mucoadhesive polymer. Carbohydrate Polymers. 202: 258-264.
Shen, J., Nada A.A., Abou-Zeid N.Y., and Hudson S.M. 2020. Synthesis of chitosan iodoacetamides via carbodiimide coupling reaction: Effect of degree of substitution on the hemostatic properties. Carbohydrate Polymers. 229: 115522.
Shitrit, Y. and Bianco-Peled H. 2017. Acrylated chitosan for mucoadhesive drug delivery systems. International Journal of Pharmaceutics. 517(1-2): 247-255.
Sogias, I.A., Williams A.C., and Khutoryanskiy V.V. 2012. Chitosan-based mucoadhesive tablets for oral delivery of ibuprofen. International Journal of Pharmaceutics. 436(1-2): 602-610.
Surendranath, M., M.R R., and Parameswaran R. 2022. Recent advances in functionally modified polymers for mucoadhesive drug delivery. Journal of Materials Chemistry B. 10(31): 5913-5924.
Todorov, T.I. and Gray P.J. 2016. Analysis of iodine in food samples by inductively coupled plasma-mass spectrometry. Food Additives and Contaminants - Part A Chemistry, Analysis, Control, Exposure and Risk Assessment. 33(2): 282-290.
Tonglairoum, P., Brannigan R.P., Opanasopit P., and Khutoryanskiy V.V. 2016. Maleimide-bearing nanogels as novel mucoadhesive materials for drug delivery. Journal of Materials Chemistry B. 4(40): 6581-6587.
Ways, T.M.M., Lau W.M., and Khutoryanskiy V.V. 2018. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers (Basel). 10(3): 267.
Williams, D.K., Meadows C.W., Bori I.D., Hawkridge A.M., Comins D.L., and Muddiman D.C. 2008. Synthesis, characterization, and application of iodoacetamide derivatives utilized for the aliphat strategy. Journal of the American Chemical Society. 130(7): 2122-2123.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Yin Yin Myat1, Mont Kumpugdee Vollrath2, Nitjawan Sahatspan1, 3, Tanasait Ngawhirunpat1, Chaiyakarn Pornpitchanarong1, *, and Prasopchai Patrojanasophon1,*
1 Pharmaceutical Development of Green Innovations Group (PDGIG), Department of Industrial Pharmacy, Silpakorn University, Nakhon Pathom, 73000, Thailand
2 Faculty of Pharmaceutical and Chemical Engineering, Berliner Hochschule für Technik, 13353 Berlin, Germany
3 Department of Materials Science and Engineering, School of Molecular Science and Engineering, Vidyasirimedhi Institute of Science and Technology (VISTEC), Rayong, 21210, Thailand.
Corresponding author: Chaiyakarn Pornpitchanarong E-mail: pornpitchanaron_c@su.ac.th,
Prasopchai Patrojanasophon E-mail: patrojanasophon_p@su.ac.th
Total Article Views
Editor: Nisit Kittipongpatana,
Chiang Mai University, Thailand
Article history:
Received: February 8, 2023;
Revised: March 24, 2023;
Accepted: April 12, 2023;
Published online: April 24, 2023

