
The Effect of Optimum Photoperiod from Blue LED Light on Growth of Chlorella Vulgaris in Photobioreactor Tank
Narong Kamolrat*, Settakorn Kamuang, Thiamthep Khamket, Pichasit Sangmek, and Suphasit SitthaphanitPublished Date : July 10, 2023
DOI : https://doi.org/10.12982/NLSC.2023.038
Journal Issues : Number 3, July-September 2023
Abstract Study for investigated the optimum photoperiod from blue LED light for the growth of Chlorella vulgaris in culture tank. Photoperiods from blue LED Light (Light/Dark) were set up at 24:0, 16:8 and 12:12 h. The initial number of C. vulgaris cells was 1.6 x 105 ± 0.12 cells mL-1. Culture medium was using commercially available fertilizers. After 17 days of cultivation, the results showed that the 24:0 h photoperiod had better growth of algae than 16:8 and 12:12 h, with maximum number of cells of 1.64 x 107 ± 0.23 cells mL-1 specific growth rate (SGR) was 0.17 ± 0.05, -0.06 ± 0.11 and -0.19 ± 0.1% day-1 with statistically significant differences (P < 0.05) in day 5 of cultivation. The stationary phase continued up to day 3 of cultivation, between the treatment. The optimum temperature for growth ranged from 35.5–38.5°C. The results showed that blue LED lighting at 24:0 h is the optimum photoperiod for cultivation of C. vulgaris.
Keywords: Photobioreactor tank, Photoperiod, Blue LED light, Chlorella vulgaris, Growth rate
Funding: The authors would like to thank the Division of Research Management and Academic Services, Kasetsart University, ChaloemPhrakiat Sakon Nakhon Province Campus for the research funding.
Citation: Kamolrat, N., Kamuang, S., Khamket, T., Sangmek, P., Sitthaphanit, S. 2023. The effect of optimum photoperiod from blue LED light on growth of chlorella vulgaris in photobioreactor tank. Natural and Life Sciences Communications. 22(3): e2023038.
INTRODUCTION
The ornamental fish business in Thailand that generates income into the country with a value of at least 13.78 million $/ year (Raja et al., 2019), as a result, small-scale farmers were increasing every year. To cultivation ornamental fish for high survival rates, it was necessary to use good quality live feed and which comes from a clean and sterile culture system. Moina sp. was the most important live feed for ornamental fish (Petmanee, 1999). To obtain a quality Moina sp., it was necessary to use Chlorella sp. The significance of Chlorella sp. lies in their versatile applications in various fields. Chlorella sp. are used as a source of nutritional supplements for human consumption, as well as for the production of biofuel (Khoeyi et al., 2012; Severes et al., 2017). Moreover, Chlorella sp. is an essential feed source for zooplankton, aquatic animals, and larvae. C. vulgaris culture in Thailand can be produced form open system (outdoors) in the cement pond (Thewaratmaneekun et al., 2006) and close system inside the laboratory. The cost of culture area and energy from each system was still a limitation for small-scale farmers. Therefore, the development of equipment for algae cultivation solves the problem of such limitations. The design of equipment in a small tank culture unit with a control system must be studies to find suitable factors for growth of C. vulgaris was nutrients, light, and temperature. The use of LED lighting for a microalgae-culture is adapted from plant factory. The LED bulbs can be controlled to suit the growth of plants. With a lower energy consumption rate than fluorescent lamps. This result was higher energy efficiency and longer service life (Lertrat, 2012; Pradu, 2015) From this system, certain colors of light will be selected from light-emitting diodes. The wavelength range required for plant and plankton growth was selected: blue-light with wavelength 400 - 500 nm, red-light at wavelength 600-700 nm (Helena et al., 2016), and distant red-light wavelength 700-800 nm. (Watjanatepin and Boonmee, 2017). It is known that chlorophyll a and b have the best photon absorption at wavelength 400-480 nm (blue) and 630-680 nm (red) (Gacomelli, 1998). Developed as a microalgae tank that receives light factor from LED bulbs. From the studies have shown that LED light have influences of C. vulgaris growth compared to Fluorescent (Solomon et al., 2011), it was found that the light from blue LED lamp was able to make the algae growth of Chlorella sp. (Metsoviti et al., 2020) and blue light have a higher potential to stimulate the growth of C. vulgaris than other colored LED lights (Kamolrat et al., 2019). Furthermore, our findings indicate that blue LED light with a photoperiod of 12:12 in an indoor setting was the most effective condition for stimulating nutrient-rich biomass production of C. ellipsoidea, as evidenced by significant differences (P < 0.05) in cell density, cell dry weight, protein content, lipid content, and cell size compared to white, green, and red LED lights (Baidya et al., 2021). Therefore, blue LED light was selected by studying the duration of the blue LED light that can maximize C. vulgaris growth rate and the effect of energy from LED bulbs as temperature in cultured tank. From a study on microalgae, it was discovered that the growth rate of microalgae is influenced by the duration of photoperiod, with intervals between 12:12 and 16:8 hours depending on the species (Baidya et al., 2021; Gunawan et al., 2018; Hollis et al., 2019; Khoeyi et al., 2012). Increasing the frequency of light exposure can significantly enhance productivity and photosynthetic efficiency in microalgae (Krzemińska et al., 2014). The objective of this study was to identify the optimal photoperiod required to achieve the maximum growth rate of C. vulgaris that was cultured in a photobioreactor tank to develop efficient aquaculture equipment that can be used by ornamental fish farmers to produce natural feed within their own households, C. vulgaris was cultured in a photobioreactor tank.
MATERIALS AND METHODS
Set up of experimental tank
The prefabricated culture tank was set up in a 20-liter container, with equipped 30 blue LED lights core for illumination (Module LED model 5630, 1.5w 150 lumen, China), by an automatic controller and the water vortex system with water pump (Figure. 1). The value of light energy was measured as unit of the photosynthetic photon flux density (PPFD) controlled at 363.60 ± 4.13 µmol m-2 s-1

Figure 1. The bioreactor tank in size 20-liter having a core with LED light and automatic controller (a) Cultivation condition of C. vulgaris under blue LED lights sources (b).
Preparation of the algal sample
The algal samples of C. vulgaris TISTR 8261 were sourced from the Department of Fisheries and cultured within the culture room at the fishery farms at Nong Han Chaloem Phrakiat Park. The cell number was expanded to the desired amount. The average initial cell number of the algae used in the experiment was 1.6×105 ± 0.12 cells mL-1.
Preparation of the culture medium
The preparation of the algal culture medium was modified from the formula of the Department of Fisheries (Thewaratmaneekun et al. 2006). The culture medium consists of 0.5 g L-1 of urea, 0.2 g L-1 of Phosphorus pentoxide, 0.2 g L-1 of Potassium oxide, glucose as the source of carbon at 0.5 g L-1 and 0.4 g L-1 of lime. The medium was then mixed by using distilled water as a solvent into 15 liters.
Optimum photoperiod test
Using the automatic light switch system, photoperiod was set up at different light to dark (light: dark) periods of 24:0, 16:8 and 12:12 h. The experiment was done in completely randomized design (CRD) with three replications.
Data collection
Algal samples were collected daily for counting the algal cell throughout the culture period with hemocytometer (BOECO 1/10 mm, Germany) (Absher, 1973).

Analysis of specific growth rates (Phatarpekar et al., 2000)
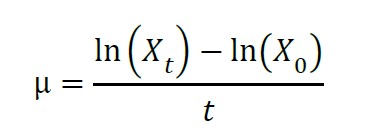
μ = Specific Growth Rate (SGR)
Xt = cell count at time
X0 = initial cell count at time
t = time (day)
The temperature of the water was monitored daily at 6 am and 1 pm. All treatments were culture of 17 days period.
Statistical analysis of the data
The data on growth rate was analyzed using the Analysis of Variance (ANOVA) and the comparison was done with the Scheffe’ test at 95 percent confidence level using SPSS V.20 statistics program.
RESULTS
Growth performance of C. vulgaris
Under blue LED light condition with light-to-dark period of 24:0, 16:8, and 12:12 h, the algae grew into the exponential phase in day 2 of cultivation and the growth rates were increased until day 3 (Figure. 2). The cell growth varied along the photoperiods with the cell number of 1.40 × 107 ± 1.39, 1.05 × 107 ± 0.63 and 0.65 × 107 ± 1.13 cells mL-1, for 24:0, 16:8, and 12:12 h, respectively. At 24:0 h light period, the growth rate was significantly higher than the other photoperiods (P < 0.05). The cell growth at 24:0 h light period has increased continually until day 5, and reached death phase on day 6, whereas at period of 16:8 and 12:12 h, the algae cells have decreased and reached death phase on day 5. The number of algae cells at day 5 for the photoperiods were 1.64 × 107 ± 0.23, 0.95 × 107 ± 0.85 and 0.52 × 107 ± 0.77 cells mL-1, respectively.

Figure 2. The density of C. vulgaris cell culture at different light/dark cycle (24:0 h, 16:8 h and 12:12 h of photoperiod for 17 days).
Table 1 showed SGR during the exponential phase of the algal cells. It was found that all the photoperiods reached the exponential phase from day 1 was 332.66 ± 2.16, 278.69 ± 4.01 and 206.59 ± 5.68 % day-1 with statistically significant differences (P < 0.05) between the treatment. Meanwhile stationary phase was reached on day 3 was 24.21 ± 7.37, 20.08 ± 18.89 and 20.17 ± 1.86 % day-1 not statistically significant difference between the treatment. It was evident that, the light periods at 24:0 h had significant effect on the algal growth rate in the exponential phase. However, there was a decrease in growth rate on day 4 in all the photoperiods, show that the death phase began in all the photoperiods.
Table 1. SGR of the C. vulgaris grown from Lag phase to Death phase culture period of experiment.
|
Day |
|
SGR (% day-1) |
|
|
24:0 |
16:8 |
12:12 |
|
|
1 |
3.33 ± 0.02a |
2.79 ± 0.04b |
2.07 ± 0.06c |
|
2 |
0.9 ± 0.03a |
1.19 ± 0.16ab |
1.42 ± 0.17b |
|
3 |
0.24 ± 0.07 |
0.20 ± 0.19 |
0.20 ± 0.02 |
|
4 |
-0.01 ± 0.04 |
-0.03 ± 0.17 |
-0.02 ± 0.16 |
|
5 |
0.17 ± 0.05a |
-0.06 ± 0.11ab |
-0.19 ± 0.10b |
|
6 |
-0.45 ± 0.12a |
-0.21 ± 0.06ab |
-0.01 ± 0.20b |
|
7 |
-0.17 ± 0.08 |
0.00 ± 0.16 |
0.04 ± 0.22 |
|
8 |
0.03 ± 0.12 |
-0.17 ± 0.17 |
-0.14 ± 0.20 |
|
9 |
-0.06 ± 0.10 |
-0.09 ± 0.13 |
-0.11 ± 0.16 |
|
10 |
0.03 ± 0.03a |
0.01 ± 0.04a |
-0.16 ± 0.02b |
|
11 |
-0.09 ± 0.06 |
-0.43 ± 0.21 |
-0.25 ± 0.09 |
|
12 |
-0.26 ± 0.16 |
-0.31 ± 0.24 |
-0.42 ± 0.14 |
|
13 |
-0.51 ± 0.15 |
-0.37 ± 0.33 |
-0.75 ± 0.19 |
|
14 |
-0.67 ± 0.10a |
-1.60 ± 0.19b |
-1.66 ± 0.18b |
|
15 |
-1.55 ± 0.15 |
-1.85 ± 0.15 |
-1.97 ± 0.31 |
|
16 |
-1.73 ± 0.08a |
-1.11 ± 0.15b |
-01.40 ± 0.30ab |
Note: Mean ± se in rows with the different alphabets were statistically different at the significant level of 0.05 when compared by Scheffe’ test.
Effects of temperature inside the culture tank
In this study, temperature of the water was measured in the morning before the lighting system was switched on at 6 am. The various photoperiod of 24:0, 16:8 and 12:12 h recorded a temperature value of 35.5, 33.1 and 32.6°C, respectively at 24:0 h was statistically significant differences (P < 0.05) with the other experiment. After switching off the light, the temperature inside the tank changed according to the period the light was switched off (Table 2.). The temperature at 1 pm had an average value of 37.0, 36.3 and 35.7°C a higher than 6 am for the photoperiod of 24:0, 16:8 and 12:12 h, respectively. The temperature inside the culture tank varied according to the daily weather condition, however, the suitable temperature for C. vulgaris cultivation must not exceed 39°C (Hosakul, 1972; Singh and Singh, 2015), but throughout the culture period of 17 days, it was found that temperature was not higher than 39°C
Table 2. water temperature in culture tank.
|
Temp. (°C) |
Light/dark cycle of photoperiod |
|||||
|
24:0 h |
16:8 h |
12:12 h |
||||
|
6 am |
1 pm |
6 am |
1 pm |
6 am |
1 pm |
|
|
Max |
37.0 |
38.5 |
34.3 |
38.4 |
33.7 |
38.0 |
|
Min |
34.2 |
35.7 |
31.7 |
34.7 |
31.6 |
34.1 |
|
Average |
35.5a |
37.0a |
33.1 b |
36.3ab |
32.6 b |
35.7b |
DISCUSSION
The highest value of growth rate of the algae was at 24:0 h of photoperiod. This period was significantly different compared with the other photoperiods (exponential phase). Sufficient growth factors such as nutrients and light were required. It was found that on Day 2 of the experiment 24:0 16:8 and 12:12 h have different growth rates. Light/dark at 24: 0 h had slower stationery and death phases, with the highest number of cells (1.63 x 107 cells mL-1). Therefore, the different growth comes from the period of exposure. The exposure time for the algae was 16:8 h or more (Krzemińska et al., 2014; Gunawan et al., 2018). Therefore, the blue LED light used in this study can stimulate photosynthetic process of the chlorophyll a and b in the algae cell, thereby allowing the algae to grow fast (Kamolrat et al., 2019), fat accumulation in Chlorella sp. biomass (Das et al., 2011; Shu et al., 2012; Teo et al., 2014). The PPFD of blue LED can stimulate biomass and fat content in C. vulgaris, which must be greater than 200 µmol m-2 s-1 energy level (Atta et al., 2013; Blair et al., 2014; Singh and Singh, 2015). In this experiment, The PPFD energy value of the LED lamp was 363.60 µmol m-2 s-1 showed that higher light intensity affects algal growth, with PPFD working with wavelength. This will stimulate the activity of Chlorophyll a and b to extract nitrogen in the form of NH4 and CO2 to be used in cell formation (Yan and Zheng, 2014). In normal conditions, the natural exposure to algae was 12:12 h when exposed to 16: 8 h algae can grow continuously and when the exposure was increased to 24: 0 h, the algae could still grow. The dark period should be limited to about 20% of the cycle time to maintain a biomass yield on light energy as high as under continuous illumination of this PFFD. The efficiency was found to decrease when longer darker periods, 50% of the cycle time, was applied the light: dark cycle ratio also influences the productivity as well as the byproducts (Janssen et al., 2001). Consist of photosystem II function and content responded primarily to instantaneous growth light intensities during the photoperiod (Li et al., 2017). While diel carbon fixation and Ribulose‐1, 5‐bisphosphate Carboxylase Oxygenase (RUBISCO) content responded more to photoperiod duration than to instantaneous light intensity. Changing photoperiods caused species-specific changes in the responses of photochemical yield (e- /photon) to growth light intensity (Li et al., 2017). It was demonstrated that the algal factor in this experiment had an effect on similarly accelerated growth, such as light from blue LED PPFD, which C. vulgaris can accept to 2,000 µmol m-2 s-1, at reaching the saturation point of light even if there was sufficient nutrient, C. vulgaris will not grow. (Hollis et al., 2019). In terms of increasing the open light time as a result, plankton has a longer period of photosynthesis. Cell division will increase (Vasumathi et al., 2012). The growth rate was due to the exposure time. However, when considering the SGR, it was found that the lag phase was rapid and stationary phase was short. It was found that when C. vulgaris received the appropriate nutrients and light intensity, photosynthesis was continued processed and algae grew quickly. The short lag phase was due to the not having to adapt much in the beginning plankton due to the use of conventional or similar nutrition from sources that were expanded in the tank. The environment inside the culture tanks was not much different from the source that was taken, so C. vulgaris can adapt quickly. To study the effect of temperature from LED lamps on heat accumulation in the experimental tank. This was because the LED lamp will provide PPFD and heat energy.
Usually, the temperature of the LED lamp is lower than the fluorescent lamp. But when the light is continuous at 24:0 h, it can build up the heat inside the experimental tank. Therefore, it is necessary to measure the water temperature in the tank that it was at the appropriate level. In general, each species of algae required suitable temperature for growth and temperature with different growth inhibitory effects, for example, Chlorella sp. was grown at 36°C and was inhibited at 42°C (Hosakul, 1972; Singh and Singh, 2015). It was found that the heat energy from the LED lamp at 24:0 h had influence on the temperature inside the culture tank. It was able to maintain a constant temperature inside the tank at a level of 35.5-38.5°C (Table 2), higher than 16: 8 and 12:12 hours, and had no effect on the growth rate. When the temperature inside the tank was constant, C. vulgaris does not need to adapt much, allowing it to grow normally. Although the temperature inside the tank was still influenced by the outside temperature, at 24:0 h it was better to maintain the temperature inside the tank.
From the aforementioned, the composition of the tank was an important part of this study. In general, algae lighting has been found to provide light from outside the container. A container for raising algae, such as a flash or a plastic bag, causing algae to absorb light through the surface of the container with the illumination angle from either side or around the vessel (Gunawan et al., 2018; Satthong et al., 2019; Shu et al., 2012; Yan and Zheng, 2014). From this study, light in the middle of the tank. The light axis and the cylindrical body make the light spread better. Coupled with the color of the experimental tank, which is an opaque white plastic, it does not absorb light but increases the reflectivity of light. The water circulation system affects the diffusion of reflected light in many directions and helps in the circulation of nutrients and C. vulgaris cells from settling. Therefore, C. vulgaris cells have a greater chance of receiving direct light. The culture medium for raising algae BBM, BG-11 (El-fayoumy et al., 2020) and N8 culture medium were generally preferred, but in this study modified culture media using commercially available fertilizers were used. It has been shown that tank culture system can increase the yield of C. vulgaris. Based on specific growth rates reported by Singh and Singh (2015) under irradiances from 30 µmol m-2 s-1 to 550 µmol m-2 s-1 under light: dark cycle. Investigator observed the Chlorella sp. required minimum irradiance to sustain net growth. Maximum SGR increased from 0.12 % day-1 at 10°C, to 0.66 % day-1 at 30°C. On the day stationary phase, the SGR value of 24 h was 0.17 % day-1. The SGR value was in the reported range. Therefore, the results of the study show that this invented device along with the modified formulation has the ability to produce Chlorella sp. However, the temperature should be kept at a constant level. The research was carried out between September and October in Sakon Nakhon Province, during the transitional period from the rainy season to the winter season. Therefore, this experimental tank can cultivate C. vulgaris in low light and cool conditions such as rainy, winter season or a area with limited light form outside which makes it difficult to cultivate algae. Part of the cost of this experiment includes the operational expenses of the experimental tank, which amounts to 41.36 $ for 24 hours of electricity usage, with a daily consumption of 1.14 $. Moreover, the medium cost for 15 liters is 0.034 $. When cultivation is taken into consideration, a culture tank is the optimal choice for small-scale farmers who aim to grow C. vulgaris in a confined space with limited natural light or when the weather is not suitable for algae cultivation thus enabling year-round cultivation. In conclusion, the findings of the study demonstrate that the variations in the techniques employed for algae cultivation were significant. Previous investigations on the culture of C. vulgaris commonly employed external light sources to illuminate the algae cultivation in transparent containers. However, in this experiment, opaque white containers and internal light sources were used to limit the distribution of light outside the containers, resulting in improved lighting for C. vulgaris. This, in turn, LED to continuous growth of the algae when light and medium were provided at sufficient levels. The findings of this study can be used to optimize the cultivation of C. vulgaris by selecting the appropriate blue LED photoperiod for this photobioreactor.
Despite the promising results, there were limitations to this experiment. The PPFD light energy from LED lamps used in the experiment was limited, which could have an impact on the growth of C. vulgaris. Additionally, the cultivation system used was a single crop, and the tank size was relatively small, which could limit production capacity. Moreover, the culture medium used was turbid, which reduced the distribution of light inside the tank. The presence of a 24:0 h culture C. vulgaris can impact the degradation of LED bulbs, thus prompting the need to either select a bulb type with an extended lifespan and an enhanced lighting control system. These limitations can be addressed through further research and development. For instance, could be achieved by increasing the tank size and improving the efficiency of the device, such as change LED lamp power. Furthermore, the effect of different culture medium compositions on the production of C. vulgaris could also be investigated to further improve the efficiency of the cultivation process. This research aims to develop a prototype device that is compact and suitable for limited farming spaces. In the industry, this device can be utilized to expand the equipment set and increase production capacity.
CONCLUSION
Under The applied culture tank conditions, blue LED lighting at 24:0 h, C. vulgaris algae were able to grow at maximum. The temperature inside the tank is at a level that C. vulgaris can grow efficiently. However, the temperature should be kept at a constant level. Therefore, this experimental device can cultivate C. vulgaris in low light and cool conditions such as rainy season or winter which makes it difficult to cultivate. However, the culture tank system needs to be improved the culture tank system such as quality of light, water circulation, air ventilation system and Chlorella sp. culture medium.
ACKNOWLEDGEMENTS
The authors would like to thank the Division of Research Management and Academic Services, Kasetsart University, ChaloemPhrakiat Sakon Nakhon Province Campus for the research funding.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Absher, M. 1973. Hemocytometer counting. p.395-397. In Tissue culture. Academic Press, Oklahoma.
Atta, M., Idris, A., Bukhari, A., and Wahidin, S. 2013. Intensity of blue LED light: A potential stimulus for biomass and lipid content in freshwater micro algae Chlorella vulgaris. Bioresource Technology. 148: 373–378.
Baidya, A., Akter, T., Islam, M. R., Shah, A. K. M. A., Hossain, M. A., Salam, M. A., and Paul, S. I. 2021. Effect of different wavelengths of LED light on the growth, chlorophyll, β-carotene content and proximate composition of Chlorella ellipsoidea. Heliyon, 7(12): 1-8.
Blair, M.F., Kokabian, B., and Gude, V. G. 2014. Light and growth medium effect on Chlorella vulgaris biomass production. Journal of environmental chemical engineering. 2(1): 665-674.
Das, P., Lei, W., Aziz, S.S., and Obbard, J.P. 2011. Enhanced algae growth in both phototrophic and mixotrophic culture under blue light. Bioresource Technology. 102(4): 3883–3887.
El-fayoumy, E.A., Shanab, S.M.M., and Shalaby, E.A. 2020. Metabolomics and Biological activities of Chlorella vulgaris grown under modified growth medium (BG11) composition. Chiang Mai University Journal Natural Sciences. 19(1): 91-123.
Gacomelli, G.A. 1998. Greenhouse glazing and solar radiation transmission workshop. Center for Controlled Environment Agriculture, Rutgers University, Cook College, New Jersey.
Gunawan, T. J., Ikhwan, Y., Restuhadi, F., and Pato, U. 2018. Effect of light intensity and photoperiod on growth of Chlorella pyrenoidosa and CO2 Biofixation. In E3S Web of Conferences (Vol. 31, p. 03003). EDP Sciences.
Helena, S., Zainuri, M., and Suprijanto, J. 2016. Microalgae Dunaliella salina (Teodoresco, 1905) growth using the LED light (light limiting dioda) and different media. Aquatic Procedia. 7: 226-230.
Hollis, L., Ivanov, A.G., and Hüner, N. P. A. 2019. Chlorella vulgaris integrates photoperiod and chloroplast redox signals in response to growth at high light. Planta. 249:1189–1205.
Hosakul, K. 1972. The selection and growth characteristics of some local microalgae tolerating high temperature. Master of Science in Microbiology, Faculty of Science and Arts, Kasetsart University. Bangkok.
Janssen, M., Slenders, P., Tramper, J., Mur L.R., and Wijffels R.H. 2001. Photosynthetic efficiency of Dunaliella tertiolecta under short light/dark cycles. Enzyme and Microbial Technology. 29(4): 298–305.
Kamolrat, N., Phattaralephon, J., and Sitthaphanit, S. 2019. Effects of LED light on growth performance of Chlorella vulgaris. Khon Kaen Agriculture Journal. 47(3): 559–566.
Khoeyi, Z.A., Seyfabadi, J., and Ramezanpour, Z. 2012. Effect of light intensity and photoperiod on biomass and fatty acid composition of the microalgae, Chlorella vulgaris. Aquaculture International. 20: 41–49.
Krzemińska, I., Pawlik-Skowrońska, B., Trzcińska, M., and Tys, J. 2014. Influence of photoperiods on the growth rate and biomass productivity of green microalgae. Bioprocess and Biosystems Engineering. 37: 735–741.
Lertrat, K. 2012. Plant production technology of the 21st century. Khon Kaen Agriculture Journal. 40 Supply. 4: 1–8.
Li, G., Talmy, D., and Campbell, D.A. 2017. Diatom growth responses to photoperiod and light are predictable from diel reductant generation. Journal of Phycology. 53(1): 95-107.
Metsoviti, M.N., Papapolymerou, G., Karapanagiotidis, I.T., and Katsoulas, N. 2020. Effect of intensity and quality on growth rate and composition of Chlorella vulgaris. Plants. 9(1): 31.
Petmanee, T. 1999. Plankton culture guide. Institute of Aquaculture Male Implantation. Songkhla, Thailand.
Phatarpekar, P.V., Sreepada, R.A., Pednekar, C., and Achuthankutty, C.T. 2000. A comparative study on growth performance and biochemical composition of mixed culture of Isochrysis galbana and Chaetoceros calcitrans with monocultures. Aquaculture. 181(1-2): 141-155.
Pradu, T. 2015. Efficiency of LED lamps to increase lighting and reduce energy use in the Library and Educational Media Center. Library and Educational Media Center Walailak University. 2(3): 50–56.
Raja, K. P. A., Padmavathy, S., and Sampathkumar, J. S. 2019. Present and future market trends of Indian ornamental fish sector. International Journal of Fisheries and Aquatic Studies. 7(2): 06-15.
Satthong, S., Saego, K., Kittrungloadjanaporn, P., Nuttavut, N., Amornsamankul, S., and Triampo, W. 2019. Modeling the effects of light sources on the growth of algae. Advances in Difference Equations. 170: 1–6.
Severes, A., Hegdeb, S., D'Souzaa, L., and Hegdec, S. 2017. Use of light emitting diodes (LEDs) for enhanced lipid production in micro-algae based biofuels. Journal of Photochemistry and Photobiology, B: Biology. 170: 235–240.
Shu, C.H., Tsai, C.C., Liao, W.H., Chen, K.Y., and Huang, H.C. 2012. Effects of light quality on the accumulation of oil in a mixed culture of Chlorella sp. and Saccharomyces cerevisiae. Journal of Chemical Technology & Biotechnology. 87: 601–607.
Singh, S.P., and Singh, P. 2015. Effect of temperature and light on the growth of algae species: A review. Renewable and Sustainable Energy Reviews. 50: 431–444.
Solomon, E.P., Berg, L.R., and Martin, D.W. 2011. Biology. Nineth. Edition. Brooks/Cole, Cengage Learning, California. Civil and Environmental Engineering Department. Mississippi State University. Mississippi State. USA. 2: 665–674.
Teo, C.L., Atta, M., Bukhari, A., Taisir, M., Yusuf, A.M., and Idris, A. 2014. Enhancing growth and lipid production of marine microalgae for biodiesel production via the use of different LED wavelengths. Bioresource Technology. 162: 38–44.
Thewaratmaneekun, P., Sejkit, S., and Watcharakonyothin, T. 2006. Moina culture. A publication for Fisheries Learning Center, Department of Fisheries, Ministry of Agriculture and Cooperatives, Bangkok.
Vasumathi, K.K., Premalatha, M., and Subramanian, P. 2012. Parameters influencing the design of photobioreactor for the growth of microalgae. Renewable and Sustainable Energy Reviews. 16(7): 5443-5450.
Watjanatepin, N., and Boonmee, C. 2017. Which color of light from the light emitting diodes is optimal for plant cultivation. Journal of Sciences and Technology. 25(1): 158–176.
Yan, C., and Zheng, Z. 2014. Performance of mixed LED light wavelengths on biogas upgrade and biogas fluid removal by microalga Chlorella sp. Applied Energy. 113: 1008–1014.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Narong Kamolrat1, *, Settakorn Kamuang2, Thiamthep Khamket2, Pichasit Sangmek1, and Suphasit Sitthaphanit1
1 Department of Agriculture and Resources, Faculty of Natural Resources and Agro-Industry, Kasetsart University Chalermphrakiat Sakon Nakhon Province Campus, Sakon Nakhon 47000, Thailand.
2 Department of Electric Engineering and Computer, Faculty of Science and Engineering, Kasetsart University Chalermphrakiat Sakon Nakhon Province Campus, Sakon Nakhon 47000, Thailand.
Corresponding author: Narong Kamolrat, E-mail: narong.ka@ku.th
Total Article Views
Editor: Sirasit Srinuanpan,
Chiang Mai University, Thailand
Article history:
Received: February 7, 2022;
Revised: March 27, 2023;
Accepted: March 30, 2023;
Published online: April 21, 2023

