
Decreased Activity of the Hypothalamic-Pituitary-Adrenal Axis after Acute Aerobic Exercise in Obese Women
Sugiharto*, Desiana Merawati, Adi Pranoto, and Hendra SusantoPublished Date : July 10, 2023
DOI : https://doi.org/10.12982/NLSC.2023.037
Journal Issues : Number 3, July-September 2023
Abstract The increase of cortisol hormone and blood pressure indicates the active Hypothalamic-Pituitary-Adrenal axis (HPA-axis) and Sympathetic-Adreno-Medullary-axis (SAM-axis). Someone with obesity tends to have a more active HPA-axis and SAM-axis. Exercise is proclaimed as an effective model to reduce the HPA-axis and SAM-axis activities. This study aims to describe the HPA-axis and SAM-axis activities on the moderate-intensity aerobic. The participants of this study were 20 obese female teenagers aged 20 to 24 years old with sufficient physical fitness. They were divided into three groups of CTL (n=7, control group), IAE (n=7, interval time aerobic exercise) and CAE (n=7, continuously aerobic exercise). IAE was carried out with moderate-intensity running followed by active recovery through low-intensity running with five repetitions. It was carried out for 35 minutes. The CAE was carried out with moderate-intensity continuously running for 30 minutes. Both IAE and CAE were completed using treadmills. The HPA-axis was estimated using cortisol hormone indicator, while the cortisol hormone was measured using ELISA Kit. The SAM-axis was examined using the heart response. The data were analyzed using a One-Way ANOVA test with SPSS version 21. The results suggest significant different cortisol hormone secretion between before and after the moderate-intensity interval time and continuously aerobic exercise (P ≤ 0.01). Different blood pressure and heartbeat were also observed before and after the aerobic (P ≤ 0.01). Moderate-intensity interval time and continuously aerobic exercise reduces cortisol hormone secretion, blood pressure, and heartbeat, thus, it lessens the HPA-axis and SAM-axis activities.
Keywords: HPA-axis, SAM-axis, Aerobic exercise, Obesity
Funding: There is no financial funding in this study, funding comes from personal finance.
Citation: Sugiharto, Merawati, D., Pranoto, A., and Susanto, H. 2023. Decreased activity of the hypothalamic-pituitary-adrenal axis after acute aerobic exercise in obese women. Natural and Life Sciences Communications. 22(2): e2023037.
INTRODUCTION
Obesity has been recorded to occur not only in adults and the elderly but also in children and adolescents (Wang and Lobstein, 2006; Pranoto et al., 2023), positioning obesity as a complex public health issue (Reljic et al., 2021). Obesity increases the risk of comorbidities, such as cardiovascular (Wu et al., 2020), and also psychological problems (Tayagi et al., 2021). A previous study has identified that in general, obese people are susceptible to experiencing stress and depression caused by discontentment toward their body shape (Carraça et al., 2021). Stress and depression are psychological problems that provoke metabolism disorders, complications (Xiao et al., 2020), increasing blood pressure, and circulating glucose levels (Marques et al., 2021). Obese women are highly sensitive to their body shape (Heraclides et al., 2012) and are reported to have higher stress levels than women with normal weight and men (Besral and Widiantini 2015). A higher cortisol hormone has been observed in obese women than women with normal weight (Xiao et al., 2020). A high level of cortisol hormone indicates the active HPA-axis (Xiao et al., 2020). A similar pattern has been observed on SAM-axis, resulting in an increase in heart rate and blood pressure (Arvidson et al., 2020). A high concentration of cortisol hormone causes Hyperinsulinemia and growth factor that may induce maladaptive accumulation of visceral adipose tissue, dyslipidemia, and increasing blood pressure (Köchli et al., 2021). Cortisol hormone is also an essential factor that modulates eating behavior, its high reactivity signifies higher food intake than people with low cortisol hormone reactivity (Marques et al., 2021). Increasing cortisol hormone is an indicator of active HPA-axis (Wen et al., 2021). Therefore, efforts to decrease HPA-axis are urgently required to inhibit and prohibit the increase of obesity, reducing the risk factor of obesity. However, a systemic and physiological approach that is safe, continuous, and can be carried out long term remains unrecognized.
Psychological and physiological disorders may disrupt homeostasis, increasing the HPA-axis activities (Wen et al., 2021). HPA-axis is a neuroendocrine axis carrying an essential role in the stress response regulation through accelerating the cortisol hormone secretion (Lucassen et al., 2012). Besides, an increase of the HPA-axis is correlated with a decrease in insulin and an increase in various harmful physical and mental health risks (Köchli et al., 2021). The previous study explains that active HPA-axis increase the risk of non-communicable disease, lower immunity, increase susceptibility toward infection, and affect the severity of infectious diseases (Glaser and Kiecolt-Glaser, 2005). Active HPA-axis prompts other medical issues (Heraclides et al., 2012), such as the increase in gluconeogenesis (Foss and Dyrstad, 2011), increase risk of diabetes Mellitus type 2 (Heraclides et al, 2012), disrupt gonadotropin-releasing hormone (GnRH), and obstruct the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) (Berga, 2019). Regular aerobic exercise can induce beneficial physiological adaptation toward the HPA-axis activity and may be used as the fundamental act to regulate obesity (Messerli-Bürgy et al., 2019).
Aerobic exercise carries physiological effects that maintain the energy balance and reduce negative physiological issues (Carraça et al., 2021; Pranoto et al., 2023). Previous studies have identified the effects of exercises on our health, somatic, cardiovascular, and maintains our mental health (Arvidson et al., 2020). Exercise also decreases stress and depression, while increasing lipid metabolism (Caplin et al., 2021; Susanto et al., 2023). Besides, it enhances physical health, prohibits diseases, and prolongs life (Marques et al., 2021). A study indicates that exercise with proper intensity lowers HPA-axis activities (Wen et al., 2021), decreasing the cortisol hormone secretion (Skurvydas et al., 2017; Martínez-Díaz and Carrasco, 2021). However, studies on the effects of exercise intensity and type in reducing the HPA-axis remain inconclusive. Aerobic exercise is presumed to decrease HPA-axis and cortisol hormone secretion (Arvidson et al., 2020), but another study indicates that moderate-intensity endurance exercise is more effective in decreasing the cortisol hormone and HPA-axis activities than weight exercise (Davitt et al. 2017). A different study states that the ergo cycle 60% VO2max decreases cortisol hormone on obese subjects (Kong et al, 2016), but in high-intensity, it enhances the HPA-axis activity (Martínez-Díaz and Carrasco, 2021). In contrast, for someone with a sedentary lifestyle, high-intensity interval training increases maximal aerobic capacity and reduces the cortisol hormone (Syamsudin et al., 2021; Irandoust and Taheri 2018). Distinctive results from those studies signify inconsistent exercise responses from the obese subjects (Carraça et al., 2021). The different body composition and level of physical fitness between obese and non-obese individuals are expected to be the causative factor (Gar et al., 2020). However, exercise is a physiological response stimulus on HPA-axis and SAM-axis (Bizzarri et al., 2020), in secreting cortisol hormone and blood pressure (Köchli et al., 2021). Therefore, this study investigated the changes in HPA-axis activity on obese women after aerobic exercise, using cortisol hormone. The findings were expected to be a new approach in reducing obesity, related to lowering stress and enhancing our health.
MATERIALS AND METHODS
Experimental design
This study involved 21 obese female adolescents, with body mass index (BMI) of 26–33 kg/m2, aged 20-24 years old, percentage of body fat (PBF) ≥ 30%, normal fasting blood glucose, normal Hb, relatively good volume oxygen maximal, and healthy according to the doctor check-up. The research method and stages had been explained to and understood by the research participants before they declared their willingness to participate. The research stages had been approved by Health Research Ethics Committee FK-UB (No.26/EC/KEPK-S1/02/2020).
Physical exercise protocol
Twenty-one female adolescents that had matched the criteria were divided into groups of CTL (n = 7, control group), IAE (n = 7, interval time aerobic exercise), and CAE (n = 7, continuously aerobic exercise). The interval time and continuously aerobic exercise used the medium intensity of 60% – 70% HRmax. IAE group exercised for 35 minutes by running at the determined intensity, followed by active recovery with low intensity running. CAE groups carried out non-stop continuously running with moderate-intensity for 30 minutes. The exercise was carried out at Sports Health Service Center, Malang City Health Office, starting at 6 a.m. The participants’ heartbeat and blood pressure were continuously supervised through the treadmill monitor.
Anthropometrics, body composition, and physical condition
This study involved obese female adolescents, selected based on the criteria of anthropometrics, body composition, and physical fitness. The anthropometrics used indicators of body weight, body height, and BMI. Meanwhile, the body composition measurements used indicators of PBF, fat mass (FM), free fat mass (FFM), and muscle mass (MM). The body fitness estimation used an indicator of volume oxygen maximal, measured using the Astrand method and ergo cycle.
Blood sampling for HPA-axis activity analysis
Analysis of HPA-axis activity was carried out using the hormone cortisol indicator, measured before and after the aerobic exercise. The blood sampling for cortisol hormone examination was carried out from the cubital vein. A 3 ml of blood was taken and centrifuged. The obtained serum was stored at -80°C. For the FBG and Hb examination, the blood was taken from capillaries using a fingerstick. ELISA kit (Cat.No E-EL-0157), the method was used to measure the cortisol hormone. Meanwhile, the blood pressure, as the SAM-axis activity parameter, was measured using a digital sphygmomanometer OMRON HEM-7156 Deluxe.
Statistical analysis
The data were analyzed using a One-way ANOVA test, followed by Tukey’s HSD post hoc test, with a significance value of 5%. Before the One-way ANOVA test, the prerequisite test of normality and homogeneity tests were carried out using Shapiro-Wilk, and Levene test. If the obtained data were normal and homogeneous, Uji Sign Test Wilcoxon, Kruskal-Wallis Test was carried out.
RESULTS
Results of the analysis on the research participants’ characteristics in each group are presented in Table 1.
Table 1. Characteristics of research participants.
|
Parameters |
Group |
P-value |
||
|
CTL (n=7) |
IAE (n=7) |
CAE (n=7) |
||
|
Age (years) |
21.86 ± 1.35 |
22.00 ± 1.41 |
21.86 ± 1.35 |
0.97 |
|
Weight (kg) |
76.61 ± 7.15 |
76.01 ± 5.34 |
69.97 ± 6.28 |
0.12 |
|
Height (m) |
1.60 ± 0.06 |
1.61 ± 0.05 |
1.55 ± 0.04 |
0.09 |
|
BMI (kg/m2) |
29.83 ± 1.46 |
29.56 ± 1.06 |
29.14 ± 1.34 |
0.61 |
|
PBF (%) |
45.50 ± 2.89 |
43.53 ± 4.01 |
45.01 ± 3.54 |
0.56 |
|
FM (kg) |
34.84 ± 4.16 |
33.27 ± 4.57 |
32.83 ± 7.27 |
0.77 |
|
FFM (kg) |
41.66 ± 4.34 |
42.93 ± 3.09 |
39.49 ± 3.32 |
0.22 |
|
MM (kg) |
39.19 ± 3.97 |
40.33 ± 2.83 |
37.19 ± 3.05 |
0.22 |
|
SBP (mmHg) |
115.14 ± 4.56 |
113.86 ± 4.18 |
114.57 ± 3.65 |
0.84 |
|
DBP (mmHg) |
76.14 ± 4.34 |
75.29 ± 4.35 |
74.86 ± 3.29 |
0.83 |
|
RHR (bpm) |
78.71 ± 7.89 |
80.86 ± 8.05 |
79.57 ± 4.61 |
0.84 |
|
VO2max (mL/kg/min) |
27.55 ± 2.12 |
26.64 ± 1.15 |
28.61 ± 3.32 |
0.32 |
|
FBG (mg/dL) |
95.43 ± 3.10 |
94.14 ± 3.39 |
93.43 ± 2.99 |
0.50 |
|
Hb (g/dL) |
14.61 ± 0.91 |
14.81 ± 1.14 |
14.94 ± 1.09 |
0.84 |
Note: Description: BMI: Body mass index; PBF: Percentage of body fat; FM: Fat mass; FFM: Free fat mass; MM: Muscle mass; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; RHR: Resting heart rate; VO2max: Maximum oxygen volume; Hb: Hemoglobin; FBG: Fasting blood glucose; CTL: Control group; IAE: interval time aerobic exercise; CAE: continuously aerobic exercise, P-Value was obtained from one-way ANOVA test. Data are presented with mean ± SD.
There were no significant different participants’ characteristics from each group (Tabel 1) (P ≥ 0.05). The effects of exercise on HPA-axis activity with cortisol hormone indicators from each group are illustrated in Figure 1.
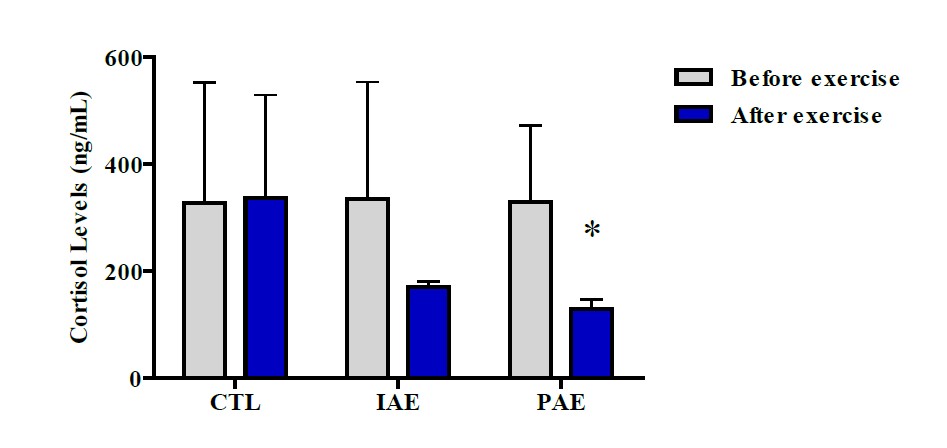
Figure 1. Level of cortisol hormone before and after aerobic exercise. Data were presented with mean ± SD. P -Value was obtained from Wilcoxon Sign Test by comparing the cortisol hormone before and after the exercise. (*) Significant vs. Pretest (P ≤ 0.05).
The results of the Wilcoxon Sign Test on the CTL group showed no significant difference in cortisol hormone level (327.36 ± 225.61 vs. 336.64 ± 192.47 ng/mL, (P ≥ 0.05)). Similarly, no significant difference in cortisol hormone level was also observed in the IAE groups between before and 10 minutes after the exercise (P ≥ 0.05). However, in the CAE group, there was a significantly different cortisol hormone, before and 10 minutes after the exercise (P ≤ 0.05). In the CAE group, the participants’ cortisol hormone level after the exercise is lower than before the exercise, indicating that aerobic exercise decreases cortisol hormone. Before the exercise, the level of cortisol hormone in the IAE group was (334.99 ± 218.06 ng/mL), while after the exercise it was (170.34 ± 10.15 ng/mL), while for the CAE group the cortisol level before and after exercise were (328.65±143.09 ng/mL) and (129.98 ± 16.53 ng/mL), respectively (Figure 1).
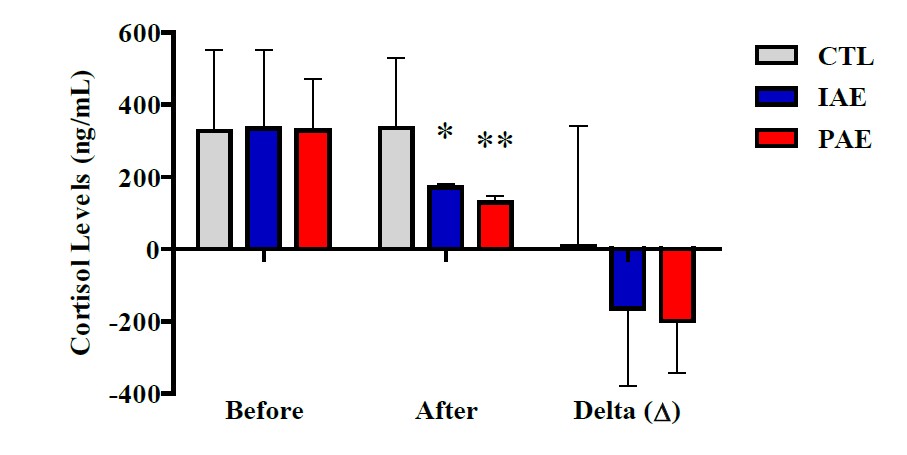
Figure 2. Cortisol level based on the blood sampling time from each group.
Data are presented with mean ± SD. P-Value was obtained from Kruskal-Wallis Test. (*) Significant vs. CTL (P ≤ 0.01). (**) Significant vs. IAE (P ≤ 0.01).
Based on the Kruskal-Wallis Test results (Figure 2), no different cortisol hormone level was observed on the three groups before exercise and delta (Δ) (P ≥ 0.05), but after exercise, there was a significant difference (P ≤ 0.001). Similarly, the results of the Mann Whitney Test also showed significantly different cortisol hormone levels after exercise between IAE with CTL groups (P = 0.003), CAE with CTL (P = 0.002), and CAE with IAE (P = 0.002) groups.
The effects of aerobic exercise on the SAM-axis, investigated using heart rate indicators and blood pressure, on each group, before and after the exercise are illustrated in Figure 3.
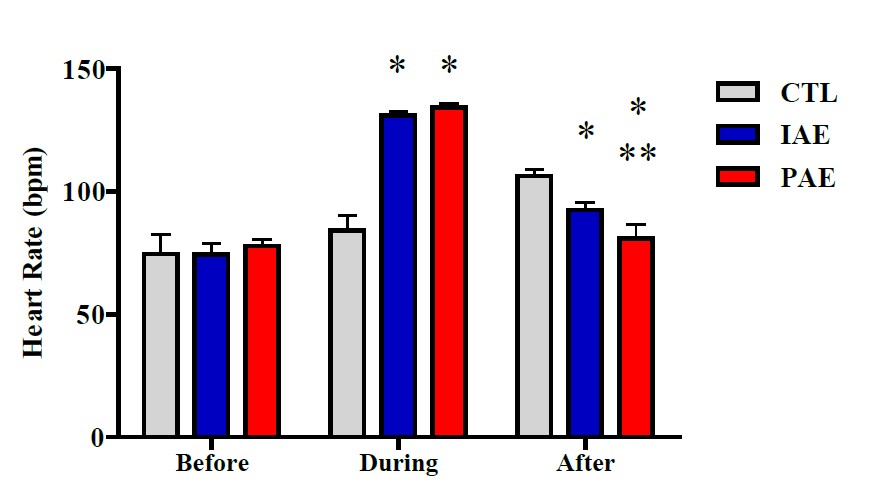
Figure 3. Average heart rate before, during, and after the exercise in all group. Data is presented with mean ± SD. P -Value was obtained from a one-way ANOVA test by comparing participants’ heart rate before exercise vs. during exercise vs. after exercise. (*) Significant vs. CTL (P ≤ 0.001). (**) Significant vs. IAE (P ≤ 0.001).
One-way ANOVA test results showed no significant different heartbeat in all groups, before the exercise (P ≥ 0.05), but a significantly different heartbeat was observed after the exercise (P ≤ 0.001) (Figure 3). The data were further analyzed using Tukey's HSD post-hoc test. The results signified that during the exercise, there was a significant heartbeat difference between CTL and IAE (P ≤ 0.001), as well as CTL and CAE (P ≤ 0.001), while between IAE and CAE the difference was not significant (P ≥ 0.05). A significantly different heartbeat was also observed 10 minutes after the exercise, between IAE with CTL (P ≥ 0.05), CAE with CTL (P ≤ 0.001), and CAE with IAE (P ≤ 0.001).
Effects of aerobic exercise on systole and diastole blood pressure before, during, and after the exercise on the three groups are presented in Figures 4 and 5.
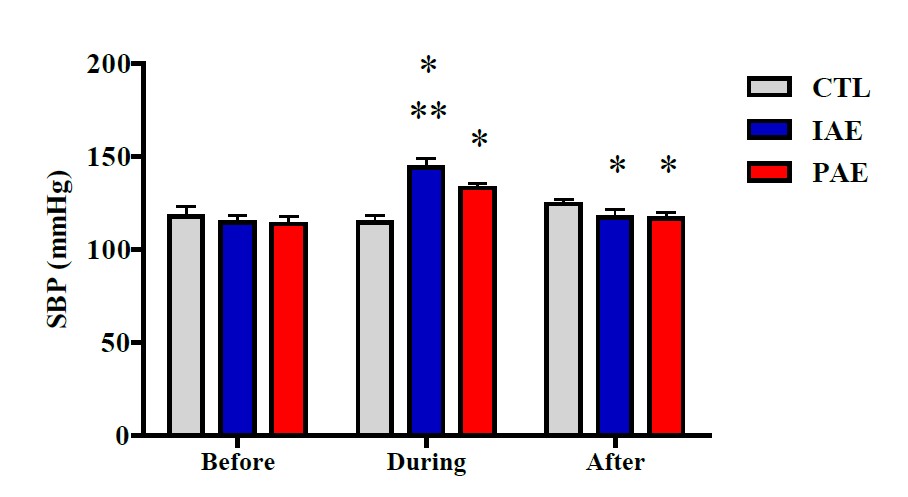
Figure 4. Average systolic blood pressure before, during, and after the exercise in all group. Data is presented with mean ± Standard Deviation (SD). P-Value was obtained from One-way ANOVA test by comparing the systolic blood pressure before exercise vs. during exercise vs. after exercise. (*) Signifikan vs. CTL. (**) Signifikan vs. CAE.

Figure 5. Average diastolic blood pressure pretest, during, and posttest in all group. Data is presented with mean ± Standard Deviation (SD). P-Value was obtained from a One-way ANOVA test by comparing the diastolic blood pressure pre-exercise vs. during-exercise vs. post-exercise. (*) Significant vs. IAE (P ≤ 0.001). (**) Significant vs. CAE (P ≤ 0.001).
The results of the One-way ANOVA test showed no significant difference in systole blood pressure in all groups, before the exercise (P ≥ 0.05), but a significant difference was observed during and after the exercise (P ≤ 0.001). Further analysis using Tukey's HSD posthoc test showed significant different systolic blood pressure during the exercise, between CTL with IAE (P ≤ 0.001), CTL with CAE (P ≤ 0.001), and CAE with IAE (P ≤ 0.001). Significant different systolic blood pressure was also observed after the exercise, between IAE with CTL (P ≤ 0.01), and CAE with CTL (P ≤ 0.01), but the difference between CAE and IAE (P ≥ 0.05) was not significant (Figure 4). According to the One-way ANOVA test results, there was no significant diastole blood pressure difference, before the exercise (P ≥ 0.05). However, a significant difference was observed during the exercise (P ≤ 0.001). Further analysis using Tukey's HSD posthoc test showed significantly different systole blood pressure between CTL with IAE, (P ≤ 0.001) and CTL with CAE (P ≤ 0.001), while the difference was not significant between CAE with IAE (P ≥ 0.05). After the exercise, the diastolic blood pressure between CAE with CTL (P ≤ 0.05) was significantly different. In contrast, nonsignificant different diastolic blood pressure after the exercise was observed between CTL with IAE, IAE with CAE (P ≥ 0.05) (Figure 5).
DISCUSSION
The active HPA-axis potentially results in psychological and physiological disorders which may disrupt homeostasis (Wen et al., 2021). Increase of cortisol hormone secretion is the indicator of active HPA-axis (Köchli et al., 2021) and stress (Martínez-Díaz and Carrasco, 2021). Consequently, it positions HPA-axis monitoring as an urgent matter in reducing the stress level on people with obesity and risk factors of obesity’s comorbidities. The findings showed different cortisol hormone levels, before and after the exercise, with higher cortisol levels was observed before exercise than after the exercise (Figure 2). It signifies that obese people have higher cortisol levels than non-obese individuals (normal body weight). The previous study on people with normal body weight shows that the cortisol level in women and men are 136.66 ng/mL and 119.63 ng/mL, respectively (Espelund et al., 2005). Besides, obese people also present higher heart rates and blood pressure (Figures 3-5). The high level of cortisol hormone, heart rate, and blood pressure indicates the active HPA-axis and Sam-axis. This finding is identical with a previous study that identifies the inclination of stress on young women who experienced obesity (Ricotti et al., 2020), with a more active HPA-axis, compared to the normal body weight group (Gar et al., 2020; Xiao et al., 2020).
The active HPA-axis on the obesity group does not only play an essential role in the development of hypertension and metabolism disorders in the future (Köchli et al., 2021), but it also contributes to increase risks of obesity and other metabolic diseases (Bose et al., 2009). With the active HPA-axis, increasing cortisol hormone carries an essential role in leptin and ghrelin signaling that affects the energy balance and is a dominant factor in modulating eating behavior. The obese individual with high cortisol hormone levels tends to have high food intake than people with lower cortisol levels (Marques et al., 2021). It is suspected to lower the level of taste threshold sensitivity which affects appetite (Fehm-Wolfsdorf et al. 1989). Therefore, HPA-axis activity regulation is urgent to reduce cortisol hormone, obesity rate, and the risk factors related to obesity. The findings suggest that moderate-intensity aerobic exercise lowers the cortisol hormone secretion, heartbeat, and blood pressure (Figure 2-5). This result is linear with another study identifying that exercises neutralize and suppress the acceleration of stress hormone (Stults-Kolehmainen and Sinha, 2014), and decreases HPA-axis activity (Martínez-Díaz and Carrasco, 2021).
HPA-axis is one of the neuro-endocrine axes with an important role in the stress response regulation that modulates cortisol hormone secretion in humans (Rutters et al., 2012). The finding also showed decreasing HPA-axis activity, represented by lower cortisol hormone secretion after exercise with 60% VO2max intensity (Budde et al., 2015). The stress reactivity also decreased, following the reduced cortisol hormone, heartbeat, and heart rate variability on the groups practicing aerobic exercise, compared with the control group (Arvidson et al., 2020). A study on Wistar rats running on a treadmill with 14-16 m/min speed while listening to music show a decrease in cortisol hormone (Sugiharto et al., 2019). Moderate-intensity exercise also reduces HPA-axis reactivity toward stressors on adults (Wen et al., 2021).
A decrease in cortisol hormone secretion indicates the changes in HPA-axis reactivation, enhancing the feeling of joy, content, and pleasure (Arvidson et al., 2020). Lower HPA-axis activity is one of the factors that enhance metabolic health (Al-Samerria and Radovick et al., 2021). The results of this present study signify that exercise can mediate the decrease of HPA-axis activity. Exercise is a physiological stimulus proclaimed to change the HPA-axis and Sam-axis activities (Bizzarri et al., 2021). The alteration of HPA-axis activity, marked by the decrease of cortisol hormone secretion, carries extensive effects on metabolism improvement (Hoekstra et al., 2010). Lower cortisol hormone secretion increases the susceptibility of leptin and insulin, stimulating the raise of NPY/AgRP neurons in ARC (Xiao et al., 2020) and enhancing lipid metabolism (Wirtz et al., 2009). Our further analysis identified different derivations of cortisol hormone between the participant joining interval time and continuously aerobic exercise. The heart rate and blood pressure of participants with continuously aerobic exercise are lower than participants with interval time aerobic (Figures 2-5). The continuously aerobic is presumed to be easier and more uncomplicated for the obese groups than the interval time aerobic. Since the interval time aerobic was followed by a recess (recovery), the exercise was disjointed, which may make the exercise harder and become more uncomfortable. Consequently, it brings not only psychological but also physiological effects that impact body responses toward the exercises demand (Sharon-David and Tenenbaum, 2017). Once the body is capable of completing the exercise demand, exercise can be a beneficial media to cope with the exercise intensity (Köchli et al., 2021), so that the exercise can be enjoyable and establish a greater mood (Kim & Jee et al., 2020), and reduce stress, decreasing the HPA-axis activity (Martínez-Díaz and Carrasco, 2021). In contrast, the misery and uncomfortable feeling during the exercise affect the decreasing level of cortisol hormone secretion, heart rate, and blood pressure. Therefore, we conclude that these two types of exercises can be used as the approach to shape balance energy and stress, related to obesity.
The continuously aerobic exercise can be adopted as a method and strategy to transform the obesity group’s lifestyle, to regulate the energy balance, physiological health, psychological health, and metabolic health. However, this study also has a number of limitations. First, our participants were only limited to females, we did not involve male participants. Second, we only used acute exercises, so we could not measure the level of adaptation on the exercise effects. Nevertheless, the present findings provide fundamental and essential information to prevent the increase of the obesity rate and the risks factors related to obesity. Besides, the findings also confirm that exercise, physiologically, carries extensive physiological functions, primarily in preventing non-communicable disease. Continuously aerobic exercise is recommended to be an approach to reduce HPA-axis and SAM-axis. Besides, we recommend future studies to use molecular-based physiological concepts relevant to exercise and stress in regulating energy in obesity groups as it is essential to find solvency for the obesity problem and to habituate modern society to exercise.
CONCLUSION
The research findings suggest that the acute version of both types of exercises, the continuously and interval time aerobic, decreases the HPA-axis and SAM-axis in the female obesity group. However, the continuously aerobic was observed to decrease HPA-axis and SAM-axis better than the interval time aerobic. Therefore, we recommend the female obese group to use continuously aerobic.
ACKNOWLEDGEMENTS
The authors thank the Faculty of Sports Science, Universitas Negeri Malang for providing research support facilities.
AUTHOR INFORMATION
Sugiharto assisted in conducting the experiments, and wrote the manuscript. Desiana Merawati, Hendra Susanto designed and conducted all of the experiments and wrote the manuscript. Adi Pranoto performed the statistical analysis and data visualization, and wrote the manuscript. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Al-Samerria, S. and Radovick, S. 2021. The role of insulin-like growth factor-1 (IGF-1) in the control of neuroendocrine regulation of growth. Cells. 10(10): 2664.
Arvidson, E., Dahlman, A. S., Börjesson, M., Gullstrand, L., and Jonsdottir, I. H. 2020. The effects of exercise exercise on hypothalamic-pituitary-adrenal axis reactivity and autonomic response to acute stress-a randomized controlled study. Trials. 21(1): 888.
Berga SL. 2019. Chapter 18: Stress-induced anovulation. In stress: Physiology, Biochemistry, and pathology 1st edition. United States of America: Academic Press. 213-226.
Besral B. and Widiantini W. 2015. Determinan stres pada pegawai kementerian kesehatan republik Indonesia. Kesmas: National Public Health Journal. 9(3): 222-228.
Bizzarri, C., Colabianchi, D., Giannone, G. A., Di Luigi, L., and Cappa, M. 2021. Exercise-induced GH secretion is related to puberty. Journal of Endocrinological Investigation. 44(6): 1283–1289.
Bose, M., Oliván, B., and Laferrère, B. 2009. Stress and obesity: The role of the hypothalamic-pituitary-adrenal axis in metabolic disease. Current Opinion in Endocrinology, Diabetes, and Obesity. 16(5): 340–346.
Budde H, Machado S, Ribeiro P, and Wegner M. 2015. The cortisol response to exercise in young adults. Frontiers in Behavioral Neuroscience. 9: 13.
Carraça, E. V., Encantado, J., Battista, F., Beaulieu, K., Blundell, J. E., Busetto, L., van Baak, M., Dicker, D., Ermolao, A. et al. 2021. Effect of exercise exercise on psychological outcomes in adults with overweight or obesity: A systematic review and meta-analysis. Obesity Reviews. 22 (Suppl 4): e13261.
Caplin, A., Chen, F. S., Beauchamp, M. R., and Puterman, E. 2021. The effects of exercise intensity on the cortisol response to a subsequent acute psychosocial stressor. Psychoneuroendocrinology. 131: 105336.
Davitt, P. M., Henderson, G. C., Walker, A. J., and Arent, S. M. 2017. Postprandial hormone response after endurance or resistance exercise in obese women. Comparative Exercise Physiology. 13(4): 227-235.
Espelund, U., Hansen, T. K., Højlund, K., Beck-Nielsen, H., Clausen, J. T., Hansen, B. S., Orskov, H., Jørgensen, J. O. L., and Frystyk, J. 2005. Fasting unmasks a strong inverse association between ghrelin and cortisol in serum: studies in obese and normal-weight subjects. The Journal of Clinical Endocrinology & Metabolism. 90(2): 741-746.
Fehm-Wolfsdorf G, Scheible E, Zenz H, Born J, Fehm HL. Taste thresholds in man are differentially influenced by hydrocortisone and dexamethasone. Psychoneuroendocrinology. 1989. 14(6): 433-440.
Foss, B. and Dyrstad, S. M. 2011. Stress in obesity: Cause or consequence?. Medical Hypotheses. 77(1): 7-10.
Gar, C., Rottenkolber, M., Haenelt, M., Potzel, A. L., Kern-Matschilles, S., Then, C., Seissler, J., BidlingmIAEr, M., and Lechner, A. 2020. Altered metabolic and hormonal responses to moderate exercise in overweight/obesity. Metabolism: Clinical and Experimental. 107: 154219.
Glaser, R. and Kiecolt-Glaser, J. K. 2005. Stress-induced immune dysfunction: implications for health. Nature Reviews Immunology. 5(3): 243-251.
Heraclides, A. M., Chandola, T., Witte, D.R., and Brunner, E. J. 2012. Work stress, obesity and the risk of type 2 diabetes: Gender‐specific bidirectional effect in the Whitehall II study. Obesity. 20(2): 428-433.
Hoekstra, M., Korporaal, S. J., Li, Z., Zhao, Y., Van Eck, M., and Van Berkel, T. J. 2010. Plasma lipoproteins are required for both basal and stress-induced adrenal glucocorticoid synthesis and protection against endotoxemia in mice. American journal of physiology. Endocrinology and Metabolism. 299(6): E1038–E1043.
Irandoust, K. and Taheri, M. 2018. Effect of a high intensity interval exercise (HIIT) on serotonin and cortisol levels in obese women with sleep disorders. Women’s Health Bulletin. 6(1): e83303.
Kim, J. and Jee, Y. 2020. EMS-effect of exercises with music on fatness and biomarkers of obese elderly women. Medicina. 56(4): 158.
Köchli, S., Botha-Le Roux, S., Uys, A. S., and Kruger, R. 2021. Cardiorespiratory fitness, blood pressure and ethnicity are related to salivary cortisol responses after an exercise test in children: The exAMIN youth SA study. International Journal of Environmental Research and Public Health. 18(15): 7898.
Kong, Z., Sun, S., Liu, M., and Shi, Q. 2016. Short-term high-intensity interval exercise on body composition and blood glucose in overweight and obese young women. Journal of Diabetes Research. 2016: 4073618.
Lucassen, E. A. and Cizza, G. 2012. The hypothalamic-pituitary-adrenal axis, obesity, and chronic stress exposure: Sleep and the HPA axis in obesity. Current Obesity reports. 1(4): 208–215.
Marques, C. G., Dos Santos Quaresma, M., Nakamoto, F. P., Magalhães, A., Lucin, G. A., and Thomatieli-Santos, R. V. 2021. Does modern lifestyle favor neuroimmunometabolic changes? a path to obesity. Frontiers in Nutrition. 8: 705545.
Martínez-Díaz, I. C., and Carrasco, L. 2021. Neurophysiological stress response and mood changes induced by high-intensity interval training: A pilot study. International Journal of Environmental Research and Public Health. 18(14): 7320.
Messerli-Bürgy, N., Horsch, A., Schindler, C., Boichat, A., Kriemler, S., Munsch, S., Crottet, B., Marquez-Vidal, P. M., Borghini, A., and Puder, J. J. 2019. Influence of acute physical activity on stress reactivity in obese and normal weight children: A randomized controlled trial. Obesity Facts. 12(1): 115–130.
Pranoto, A., Cahyono, M. B. A., Yakobus, R., Izzatunnisa, N., Ramadhan, R. N., Rejeki, P. S., Miftahussurur, M., Effendi, W. I., Wungu, C. D. K., and Yamaoka, Y. 2023. Long-term resistance–endurance combined training reduces pro-inflammatory cytokines in young adult females with obesity. Sports. 11(3): 54.
Pranoto, A., Rejeki, P.S., Miftahussurur, M., Setiawan, H. K., Yosika, G. F., Munir, M., Maesaroh, S., Purwoto, S.P., Waritsu, C., and Yamaoka Y. 2023. Single 30 min treadmill exercise session suppresses the production of pro-inflammatory cytokines and oxidative stress in obese female adolescents. Journal of Basic and Clinical Physiology and Pharmacology. 34(2): 235-242.
Reljic, D., Frenk, F., Herrmann, H. J., Neurath, M. F., and Zopf, Y. 2021. Effects of very low volume high intensity versus moderate-intensity interval exercise in obese metabolic syndrome patients: A randomized controlled study. Scientific Reports. 11(1): 2836.
Ricotti, R., Solito, A., Mariotti Zani, E., Caputo, M., Genoni, G., Barone-Adesi, F., Mancioppi, V., Agosti, E., Aimaretti, G., Bellone, S., and Prodam, F. 2020. The relationship between cortisol and IGF-I influences metabolic alteration in pediatric overweight and obesity. European Journal of Endocrinology. 182(3): 255–264.
Rutters, F., La Fleur, S., Lemmens, S., Born, J., Martens, M., and Adam, T. 2012. The hypothalamic-pituitary-adrenal axis, obesity, and chronic stress exposure: Foods and HPA axis. Current Obesity Reports. 1: 199–207.
Sharon-David, H. and Tenenbaum, G. 2017. The Effectiveness of exercise interventions on coping with stress: Research synthesis. Studies in Sport Humanities. 22: 19-29.
Skurvydas, A., Verbickas, V., Eimantas, N., Baranauskiene, N., Cernych, M., Skrodeniene, E., Daniuseviciute, L., and Brazaitis, M. 2017. Psychological and physiological biomarkers of neuromuscular fatigue after two bouts of sprint interval exercise. Frontiers in Psychology. 8: 2282.
Stults-Kolehmainen, M. A. and Sinha, R. 2014. The effects of stress on physical activity and exercise. Sports Medicine. 44(1): 81–121.
Sugiharto, Merawati, D., and Susanto, H. 2019. The physiological effects of campursari (crossover music genre) allegro in sports on circulating cortisol and testosterone levels. International Conference on Biology and Applied Science (ICOBAS) AIP Conference Proceedings. 2120: 070005.
Susanto, H., Sugiharto, Taufiq, A., Pranoto, A., and Dwi Trijoyo Purnomo, J. 2023. Dynamic alteration of plasma levels of betatrophin in younger female onset obesity post acute moderate-intensity exercise training. Saudi Journal of Biological Sciences. 30(2): 103546.
Syamsudin, F., Wungu, C. D. K., Qurnianingsih, E., and Herawati, L. 2021. High-intensity interval training for improving maximum aerobic capacity in women with sedentary lifestyle: a systematic review and meta-analysis. Journal of Physical Education and Sport. 21(4): 1788-1797.
Tayagi, N.K., Solanky, A., Jamali, S.N., Azharuddin, M., Ali, K., & Ahmad, I. (2021). Aerobic exercise, in combination with listening music, changes post-exercise cardiac autonomic function in collegiate overweight and obese individuals. Asian Journal of Sports Medicine 11(1): e97122.
Wang, Y. and Lobstein, T. 2006. Worldwide trends in childhood overweight and obesity. International Journal of Pediatric Obesity. 1(1): 11–25.
Wen, C., Chou, C. P., Belcher, B. R., Weigensberg, M. J., Black, D. S., and Spruijt-Metz, D. 2021. The acute relationship between affective states and stress biomarkers in ethnic minority youths. International Journal of Environmental Research and Public Health. 18(23): 12670.
Wirtz, P. H., Ehlert, U., Bärtschi, C., Redwine, L. S., and von Känel, R. 2009. Changes in plasma lipids with psychosocial stress are related to hypertension status and the norepinephrine stress response. Metabolism: Clinical and Experimental. 58(1): 30–37.
Wu, J., Zhao, F., Zhang, Y., Xue, J., Kuang, J., Jin, Z., Zhang, T., Jiang, C., Wang, D., and Liang, S. 2020. Effect of one-year growth hormone therapy on cardiometabolic risk factors in boys with obesity. BioMed Research International. 2020: 2308124.
Xiao, Y., Liu, D., Cline, M. A., and Gilbert, E. R. 2020. Chronic stress, epigenetics, and adipose tissue metabolism in the obese state. Nutrition & Metabolism. 17: 88.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand.
Sugiharto1,*, Desiana Merawati1, Adi Pranoto2, and Hendra Susanto3
1 Department of Sport Science, Faculty of Sport Science, Universitas Negeri Malang, Malang, Indonesia.
2 Doctoral Program of Medical Science, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia.
3 Department of Biology, Faculty of Mathematics and Natural Sciences, Universitas Negeri Malang, Malang, Indonesia.
Corresponding author: Sugiharto, E-mail: sugiharto@um.ac.id
Total Article Views
Editor: Veerasak Punyapornwithaya,
Chiang Mai University, Thailand
Article history:
Received: February 25, 2022;
Revised: March 26, 2023;
Accepted: March 29, 2023;
Published online: April 19, 2023

