
Effects of Longan Biochar as Filter Materials on Plant Responses and Wastewater Treatment by Typha angustifolia L.
Pakawat Janyasupab, Hans Brix, and Arunothai Jampeetong*Published Date : July 10, 2023
DOI : https://doi.org/10.12982/NLSC.2023.035
Journal Issues : Number 3, July-September 2023
Abstract This study investigated the effects of filter materials and Typha angustifolia L. (cattail) plantation on wastewater treatment efficiency and assessed the growth responses of cattail in the different filter materials. Five treatments of lab-scale experiment were set-up: unplanted and planted columns filled with either gravel or longan biochar, and columns without any filters and plants were used as a control. Without plantation, longan biochar filters removed 97% of biochemical oxygen demand (BOD5), 96% of total suspended solids (TSS), 86% of ammonium (NH4-N), 82% of total Kjeldahl nitrogen (TKN), and 75% of nitrate (NO3-N). Compared with the unplanted gravel-based system, 87% of BOD5 and 89% of TSS were removed but NH4-N, TKN, and NO3-N removal was only 27%, 37%, and 34%, respectively. However, the pollutant removal in gravel-based systems was as high as the removal in longan biochar-based systems when planted with cattail. It removed 84% and 81% of NH4-N and TKN, respectively, and removed 90% of total phosphorus (TP) whereas the TP removals in the other four treatments were only 13-25%. The study indicates that longan biochar is efficient for removing N and for filtering organic matters from wastewater. Hence, longan biochar may have promise for the use as a filter material in constructed wetlands (CWs). The biochar, however, may have adverse effects on the plants. Therefore, the proportion of biochar in the filter material must be optimized to obtain the most effective plant growth and water treatment before the system can be applied in real-life systems.
Keywords: Cattail, Longan biochar, Gravel, Nutrient, Wastewater treatment
Citation: Janyasupab, P., Brix, H., and Jampeetong, A. 2023. Effects of longan biochar as filter materials on plant responses and wastewater treatment by Typha angustifolia L.. Natural and Life Sciences Communications. 22(3): e2023035.
INTRODUCTION
Wastewaters from households, farming, livestock, and various industries are still commonly discharged into natural water bodies without adequate treatment and causing serious consequences for the organisms living in these natural water bodies. Constructed wetlands (CWs) can be used to treat different types of wastewaters and are often popular because they are low-cost, environment-friendly, and have a relatively high treatment efficiency for many pollutants (Kivaisi, 2001; Biswas and Rana, 2014). CW systems improve water quality through many physically, chemically, and biologically processes such as sedimentation, microbial degradation, and nutrients uptake by wetland plants (Kadlec, 2009). Furthermore, wetland plant biomass produced in the systems can be used as a soil amendment or animal feeding after being harvested (Quilliam et al., 2015; Verhofstad et al., 2017).
The bed filter material in the CWs is an important component as it supports the plant growth, it provides a filter for the wastewater passing through it, and it provides the surface area for the attachment of microorganisms. Generally, filter materials in CWs could be divided into natural occurring materials, natural occurring aggregates, processed and modified materials, and waste materials (Ballantine and Tanner, 2010). Gravel, sand, and soil are most commonly used because they are cheap and can be found easily. However, other materials such as materials produced from agricultural wastes and products like activated carbon are also interesting as they can increase treatment efficiency of the CWs (Chong et al., 2009).
Biochar is charcoal produced by pyrolyzing organic material, e.g. agricultural wastes, at relatively high temperatures (more than 300°C) in the absence of oxygen or at very low oxygen concentrations (Lehmann and Joseph, 2012). Biochar retains a high percentage of carbon and minerals, and has been used for improving soil quality and enhancing plant growth. Previous studies reported that biochar pyrolyzed from different plant species had different physical characteristics and chemical components (Mukherjee et al., 2011; Pituya et al., 2017). Some kinds of biochar have been applied for agricultural activities. For example, fig and corncob biochar, added into soil could improve soil quality, resulting in the increasing of biomass and yield of wheat and corn (Ibrahim et al., 2015; Kizito et al., 2019). In terms of environmental clean-up, its high porosity and surface area encouraged the use of biochar as a filter media in CWs for supporting microorganisms and pollutant adsorption (Dalahmeh et al., 2016). Previous studies found that CWs added with biochar derived from corncob, Chinese cork oak, and bamboo were effective to remove chemical oxygen demand (COD) and NH4-N, at 59-77% and 68-96%, respectively (Kizito et al., 2017; Feng et al., 2020; Ajibade et al., 2021). Furthermore, addition of biochar from chipped hemp fibre and coconut shell in CWs reduced over 90% of PO4-P (Bolton et al., 2019; Xing et al., 2021). There are, however, only few studies on the effects of biochar on growth of wetland plants and their performance in biochar-filled CWs. For example, biochar produced from alder (Alnus sp.) was reported to stimulate growth and biomass of cattail (Typha latifolia L.) leading to an increase of total N, NO3-N, and PO4-P removal efficiency (Kasak et al., 2018). Also, it was found that biochar produced from giant reed (Arundo donax L.) can stimulate growth of water celery (Oenanthe javanica (Blume) DC.) and enhance nutrient uptake, resulting in over 80% of NO3-N and total N removal (Li et al., 2019). However, adverse effects of biochar on plant growth have also been documented (Liao et al., 2014). Toxicity of soluble forms of organic compounds in biochar such as alcohols, ketones, phenols, and polycyclic aromatic hydrocarbons (PAHs), which had been decomposed from plant polymers (cellulose, hemicellulose, and lignin) after biomass pyrolysis, could inhibit plant growth (Godlewska et al., 2021). Amount and kind of organic compounds accumulated in each biochar type depends on quantities of lignin, cellulose, and hemicellulose composed in each pyrolyzed plant species (Gale et al., 2016), which may affect the plant growth as well as influence the treatment system efficiency. Thus, biochar selection for the use in CWs is important to gain the most effective on plant growth, plant tolerance, and wastewater treatment efficiency.
Longan (Dimocarpus longan Lour.) is a fast-growing evergreen tree yielding high income for farmers in northern Thailand. However, longan branches have to be cut after harvesting the fruits and hence become agricultural waste. The farmers usually burn the branches outdoor causing dust and smoke leading to air pollution and bring respiratory system disease to people. Converting this waste into biochar is an interesting solution, not only to reduce air pollution from burning but also benefit commercially to the farmers and local people (Nematian et al., 2021). For its application, Suksawat et al. (2017) reported that the longan trees planted with soil mixed with longan biochar grew better than longan trees planted with only soil. Furthermore, longan biochar, which produced from longan wood, generally has higher pore volume and surface area than non-woody biochar (Kizito et al., 2017; Tomczyk et al., 2020). Thus, it is expected to show an efficient suspended solids entrapment and good habitat provision for microorganisms concerning N elimination in CWs. However, the efficiency of longan biochar on wastewater treatment and its effects on wetland plant growth is not yet known.
Typha angustifolia L. (cattail) is an emergent plant in family Typhaceae. It is distributed mostly in tropical and temperate wetlands, including Thailand. Cattail planted in gravel and sand-filled CWs had high growth rates and the systems had high potential to remove BOD5, COD, TKN, and TP (81%, 71-82%, 50-88%, and 65%, respectively) (Kantawanichkul et al., 2009; Pongthornpruek, 2017). Besides, cattail was reported to grow well in CWs filled with porous materials such as oil palm shell and zeolite (Chong et al., 2009). However, growth responses and pollutant removal efficacy of cattail planted in biochar-based systems in tropical regions have not been investigated. Thus, this study aimed to i) investigate growth, leaf pigments and some minerals in tissue of cattail when grown on longan biochar compared with the plant grown on gravel, and ii) assess water treatment efficiency of unplanted and cattail-based systems using longan biochar as a filter compared to gravel-filter systems. In order to observe the effects of filters on pollutants removal efficiency, treating wastewater in systems without filters was tested in comparison. The outcomes of this study would provide benefit information for filter materials and plant species selection to treat wastewater in the large scale CWs.
MATERIALS AND METHODS
Filter media used in the study
Gravel (Ø = 7–10 mm) and longan biochar produced from longan branches were selected as filter materials for this study. Longan biochar was purchased from Warm Heart Foundation, Phrao district, Chiang Mai province, Thailand, where cut longan branches were received from local farmers. In the production process, longan branches had been cut and pyrolyzed in a furnace at 450°C for two hours. Then, biochar particles were crushed by machine to reduce their size (Ø = 3-5 mm) before packaging and transporting to the laboratory. Before the use, the biochar was sieved using the mesh (size of opening 1.5 mm) in order to remove ashes. Physicochemical characteristics of longan biochar (specific surface area, pore volume, cation exchange capacity (CEC), and mineral contents) have been determined and reported in our previous study (Janyasupab and Jampeetong, 2022). To minimize toxic contaminants in biochar which may possibly be harmful to the plants and reduce pollutants removal efficiency, the biochar was soaked with tap water which was renewed every day for two weeks (Artiola and Wardell, 2017).
Plant preparation and experimental design
Rhizomes of cattail were collected from natural wetlands in Chiang Mai province, Thailand. They were cut into 150-300 mm long pieces and placed in trays (width 300 mm × length 450 mm × height 50 mm) containing soil. The rhizomes were watered every day. After approximately four weeks, new shoots produced from rhizomes (400-600 mm height; 35-45 g fresh weight) were selected for the experiment.
Laboratory column experiments were carried out in the greenhouse at the Department of Biology, Faculty of Science, Chiang Mai University. The columns were constructed from polyvinyl chloride (PVC) pipe (Ø = 15 cm × height 55 cm). In the gravel-based columns, gravel (Ø = 10-15 mm) was placed in the column for 50 cm height. For the longan biochar-based columns, gravel was loaded at the bottom to a height of 10 cm in order to promote water flow and then the biochar (Ø = 3-5 mm) was added to a height of 40 cm (totally 50 cm) (Figure 1A). These two kinds of filter column systems were planted with cattail. In order to determine the efficiency of plant and filter material separately, unplanted columns filled with either gravel or longan biochar and a control (column had neither filter nor plant) were constructed. The experiment was arranged in a completely randomized design with five treatments (three replicates per treatment, totally 15 columns): gravel-unplanted, gravel filter-planted, longan biochar-unplanted, longan biochar filter-planted, and no filter-unplanted (control) (Figure 1B).
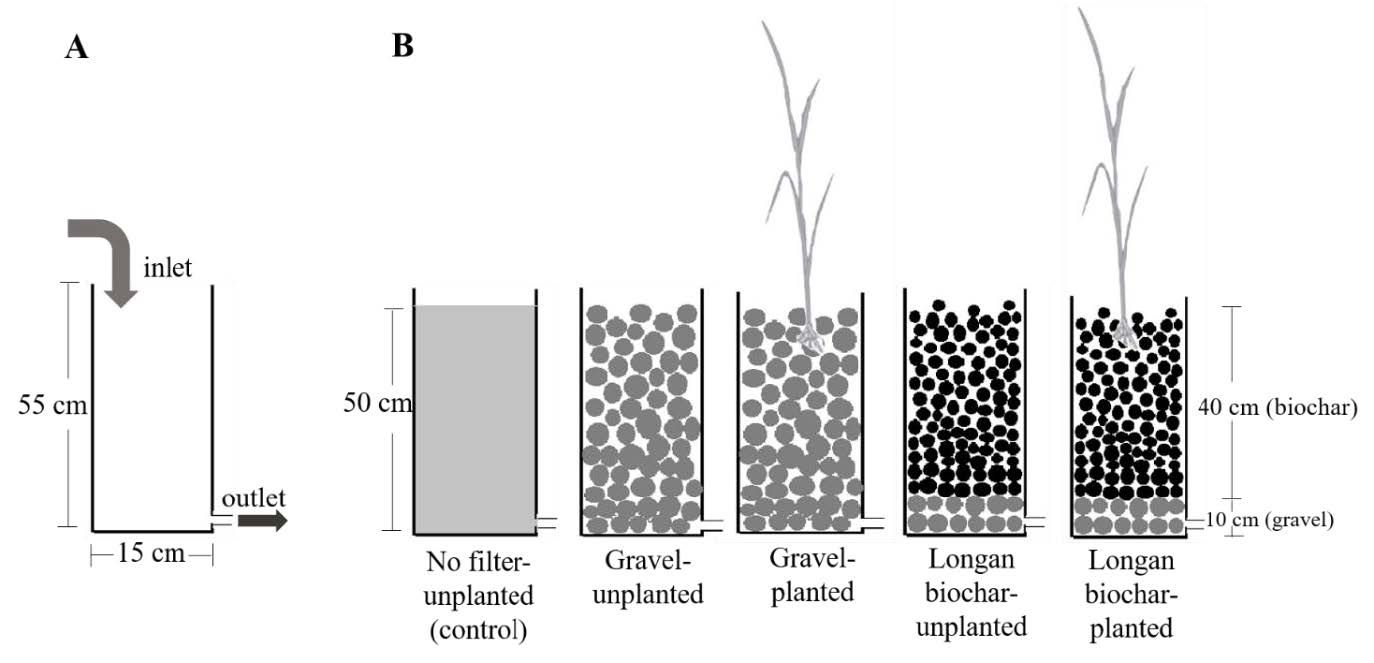
Figure 1. (A) Diagram of column characteristics and water entering and draining direction and (B) the experimental set-up of five systems containing different filter materials with and without growing cattail.
Operation and water analysis
Wastewater used in this study was collected from the Wastewater Treatment Unit, Chiang Mai University. The light condition in the greenhouse was 13 h light/11 h dark and the air temperature was approximately 27.5°C. Wastewater and sludge were filled into each column: four liters in the filtered systems and 9.8 liters in the non-filtered system, and acclimatized by flooding for 30 days to allow plants to grow and biofilm to developed. Then, the wastewater was drained from the columns. The untreated wastewater was sampled (three replicates; characteristics of the wastewater have been shown in table 1) to analyze water quality before being loaded into the columns and being adopted under hydraulic retention time of 3 days (Ajibade et al., 2021). Then, the wastewater was sampled (800 mL per sample) at the outlet point of each column for water analysis before drained and renewed (Figure 1A). The process was repeated four more times to yield five replicates (15 days in total). Totally, 45 days were operated on this experiment.
Table 1. Water quality and pollutant concentrations of wastewater from CMU campus (mean ± SE).
|
Parameter |
Units |
Concentrations (mean ± SE) |
|
DO |
mg L-1 |
0.0 ± 0.0 |
|
BOD5 |
mg L-1 |
76 ± 12 |
|
pH |
- |
7.3 ± 0.3 |
|
EC |
µS cm-1 |
1,410 ± 55 |
|
TDS |
ppm |
710 ± 23 |
|
TSS |
mg L-1 |
90 ± 15 |
|
NO3-N |
mg L-1 |
2.1 ± 0.4 |
|
NH4-N |
mg L-1 |
22.1 ± 2.7 |
|
TKN |
mg L-1 |
25.6 ± 5.7 |
|
TP |
mg L-1 |
4.1 ± 0.9 |
Note: Mean and standard error were calculated based on 15 replicates during 5 renewals (3 replicates for each round).
For water analysis, dissolved oxygen (DO) and biochemical oxygen demand (BOD5) were analyzed using an azide modification method (4500-O C) (APHA-AWWA-WEF, 2017). The pH, electrical conductivity (EC), and total dissolved solid (TDS) were measured by a multi-parameter analyzer (CyberScan PC 300, Eutech Instruments Pte Ltd., Singapore). Total suspended solids (TSS) were analyzed by filtering water samples through GF-C (Whatman® Glass Microfiber Filters; Ø = 47 mm) (2540 D) and weighing (APHA-AWWA-WEF, 2017). Nitrate (NO3-N) was measured using a UV-method (Oscarson et al., 1988). Ammonium (NH4-N) was measured by a modified salicylate method (Quikchem Method no. 10-107-06-3B; Lachat Instruments, Milwaukee, WI, USA). Total Kjeldahl nitrogen (TKN) was determined using the Kjeldahl method (Nelson and Sommers, 1980). For TP analysis, water samples were digested using sulfuric-nitric acids and measured by a stannous chloride method (4500-P D) (APHA-AWWA-WEF, 2017). Then, removal percentages of BOD5, TSS, NO3-N, NH4-N, TKN, and TP were calculated with the equation:

Where Ci and Ce are concentrations of influent and effluent, respectively.
Plant growth and biochemical study
At the end of the experiment, all plants were harvested and cleaned with tap water. Total plant height was recorded, then, all plants were separated into 3 parts (roots, rhizomes, and leaves) and freeze-dried to constant weight. Relative growth rates (RGRs) were calculated with equation:
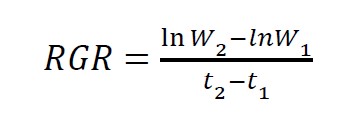
Where W1 and W2 are initial and final dry weight (g), and t1 and t2 are initial and final time (days) (Evans, 1972). Concentrations of chlorophyll a (Chl a), chlorophyll b (Chl b), total chlorophylls (Chl a+b), and carotenoids in the leaves were analyzed from freeze-dried samples according to Lichtenthaler (1987). For plant mineral analysis, the dried plant materials of each plant part were digested in acid solutions at high temperature (220-360°C). Then, TKN was analyzed using the Kjeldahl method (Nelson and Sommers, 1980). P was analyzed by the vanadomolybdate method (Hanson, 1950). K was analyzed using atomic absorption spectroscopy (AAS) (SpectrAA, Varian, Australia) described by Robinson (1960).
FTIR analysis of longan biochar
To identify the functional groups and chemical bonding, longan biochar was analyzed by fourier-transform infrared spectroscopy (FTIR) to detect chemical compounds which may affect the plant growth and the water treatment efficiency. Biochar samples (before and after the experiment) were finely ground and were analyzed using FTIR spectrometer (Thermo Fisher Scientific, USA) by scanning at wavenumber 4,000-400 cm-1 with 4 cm-1 resolution.
Statistical analysis
The software Past326b (Hammer and Harper, 2020) was used for statistical analysis. Effluent DO concentrations and pollutant removal efficiency were analyzed by T-test and two-way analysis of variance (ANOVA). Differences in plant growth, biomass, and biochemical parameters were tested with the T-test. Honestly Significant Differences (HSD) were performed at 5% significance level.
RESULTS
Water quality
The effluent pH varied among treatments (Figure 2A). Generally, effluent pH of the unplanted gravel-based systems and the control were unchanged from the influent. However, pH dropped from 7.3 to 6.2 after treating wastewater through the cattail-gravel based columns. In contrast, pH in planted and unplanted longan biochar-based systems slightly increased from 7.3 to 7.7 and 7.4, respectively. Similarly, EC and TDS of the wastewater treated by cattail-gravel based systems decreased approximately 34%, while EC and TDS in control and unplanted gravel-based systems scarcely variated. However, EC and TDS slightly increased (12-19%) in both planted and unplanted longan biochar-based systems (Figure 2B-2C).
DO concentration was solely influenced by filter material (Table 2). Influent DO was at 0 mg L-1 while the concentrations increased to 2.0-2.3 mg L-1 in both planted and unplanted longan biochar-based systems. Effluent DO in planted and unplanted gravel-based systems were 1.0-1.2 mg L-1 (Table 3; Figure 2D). The DO in the control remained at 0 mg L-1 (Figure 2D).

Figure 2. Average (A) pH, (B) electrical conductivity (EC), (C) total dissolved solid (TDS), and (D) dissolved oxygen (DO) of wastewater flooded in each column for 3 days. The horizontal lines on each bar show mean inlet concentrations from table 1.
The pollutants removal was mostly affected by both filter materials and plant with significant interaction effect (Table 2). Without plantation, longan biochar showed higher efficiency on BOD5, TSS, NH4-N, TKN, and NO3-N removal than gravel (Figure 3A-3E; Table 3). However, plantation of cattail in gravel-based system helped to increase BOD5, NH4-N, TKN, and NO3-N removal to be better than unplanted gravel-based system (Table 4). Especially NH4-N and TKN removal, the efficiency was substantially increased to be as high as planted longan biochar-based system (Table 3). Meanwhile, BOD5, TSS, NH4-N, and TKN removal in control was significantly lower than filtered and planted systems, only 63%, 81%, 2%, and 15%, respectively (Figure 3A-3D). High TP removal was found only in planted gravel-based system (90%). While, the systems used longan biochar as a filter material either planted or unplanted with cattail had low TP removal efficiency (19-26%) and it was not significant different from the control (14%) (Table 3-4; Figure 3F).
Table 2. Results of two-way ANOVA (F-ratio) of DO concentration and BOD5, TSS, NH4-N, TKN, NO3-N, and TP removal efficiency in the systems filled with different filter materials (gravel and longan biochar) with and without plantation of cattail.
|
|
Main effects |
Interactions |
|
|
Filter materials |
Plantation |
Filter materials × Plantation |
|
|
DO concentration (mg L-1) |
45.8*** |
0.0 |
1.2 |
|
BOD5 removal (%) |
73.8*** |
15.3** |
6.3* |
|
TSS removal (%) |
8.2* |
2.6 |
1.6 |
|
NH4-N removal (%) |
79.8*** |
72.8*** |
68.5*** |
|
TKN removal (%) |
41.0*** |
41.2*** |
47.2*** |
|
NO3-N removal (%) |
131.3*** |
13.2** |
7.2* |
|
TP removal (%) |
69.0*** |
72.5*** |
102.0*** |
Note: *P <0.05, ** P <0.01, and *** P < 0.001
Table 3. Results of T-test on DO concentration and BOD5, TSS, NH4-N, TKN, NO3-N, and TP removal efficiency (mean ± SE) of wastewater treated with different filter material.
|
|
|
Filter materials |
F-ratio |
|
|
Gravel |
Longan biochar |
|||
|
DO (mg L-1) |
Unplanted |
1.0±0.0a |
2.3±0.2b |
8.9** |
|
|
Planted |
1.2±0.2a |
2.1±0.1b |
3.4** |
|
BOD5 removal (%) |
Unplanted |
87.1±1.3a |
97.4±0.7b |
0.3** |
|
|
Planted |
93.1±1.0a |
98.7±0.3b |
2.9** |
|
TSS removal (%) |
Unplanted |
89.3±2.2a |
95.7±1.0b |
1.7* |
|
|
Planted |
93.8±1.1 |
96.2±1.5 |
7.2 |
|
NH4-N removal (%) |
Unplanted |
27.6±5.2a |
85.9±2.4b |
2.6*** |
|
|
Planted |
84.5±3.0 |
86.8±2.9 |
1.0 |
|
TKN removal (%) |
Unplanted |
37.6±4.5a |
81.6±2.2b |
1.4*** |
|
|
Planted |
81.6±4.2 |
80.1±2.1 |
2.7 |
|
NO3-N removal (%) |
Unplanted |
34.2±1.8a |
75.1±3.5b |
2.5*** |
|
|
Planted |
52.5±2.4a |
77.9±2.4b |
0.1*** |
|
TP removal (%) |
Unplanted |
18.6±3.0 |
25.6±6.0 |
2.9 |
|
|
Planted |
90.9±0.9b |
19.4±5.1a |
3.4*** |
Note: Different letters superscripts indicate significant differences between treatment, * P < 0.05, ** P < 0.01, and *** P < 0.001
Table 4. Results of T-test on DO concentration and BOD5, TSS, NH4-N, TKN, NO3-N, and TP removal efficiency (mean ± SE) of wastewater treated with and without plantation of cattail.
|
|
Filter materials |
Unplanted |
Planted |
F-ratio |
|
DO (mg L-1) |
Gravel |
1.0±0.0 |
1.2±0.2 |
7.0 |
|
|
Longan biochar |
2.3±0.2 |
2.1±0.1 |
1.8 |
|
BOD5 removal (%) |
Gravel |
87.1±1.3a |
93.1±1.0b |
0.0* |
|
|
Longan biochar |
97.4±0.7 |
98.7±0.3 |
5.1 |
|
TSS removal (%) |
Gravel |
89.3±2.2 |
93.8±1.1 |
3.1 |
|
|
Longan biochar |
95.7±1.0 |
96.2±1.5 |
3.3 |
|
NH4-N removal (%) |
Gravel |
27.6±5.2a |
84.5±3.0b |
1.2*** |
|
|
Longan biochar |
85.9±2.4 |
86.8±2.9 |
0.0 |
|
TKN removal (%) |
Gravel |
37.6±4.5a |
81.6±4.2b |
0.2** |
|
|
Longan biochar |
81.6±2.2 |
80.1±2.1 |
0.0 |
|
NO3-N removal (%) |
Gravel |
34.2±1.8a |
52.5±2.4b |
1.3** |
|
|
Longan biochar |
75.1±3.5 |
77.9±2.4 |
1.2 |
|
TP removal (%) |
Gravel |
18.6±3.0a |
90.9±0.9b |
3.2*** |
|
|
Longan biochar |
25.6±6.0 |
19.4±5.1 |
0.4 |
Note: Different letters superscripts indicate significant differences between treatment, * P < 0.05, ** P < 0.01, and *** P < 0.001
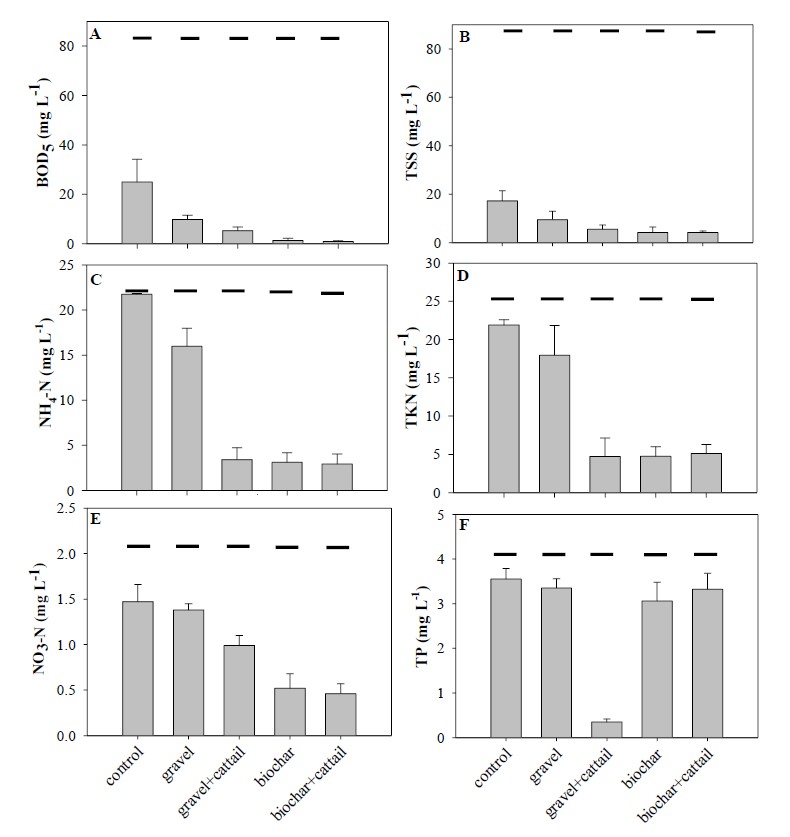
Figure 3. (A) Biochemical oxygen demand (BOD5), (B) total suspended solid (TSS), (C) NH4-N concentrations, (D) total Kjeldahl nitrogen (TKN) concentrations, (E) NO3-N concentrations, and (F) total phosphorus (TP) concentrations of wastewater flooded in each column for 3 days. The horizontal lines on each bar show mean inlet concentrations from table 1.
Plant growth and biochemical analysis
The growth of cattail was significantly different when grown on gravel and longan biochar. Cattail grown on gravel had higher RGRs, plant dry biomass, and plant height than the plant grown on longan biochar. Furthermore, concentrations of Chl a, Chl b, and Chl a+b in leaves were higher in the plants grown on gravel, but carotenoid concentrations were not significantly different across treatments (Table 5). From mineral analysis, filter media did not affect N concentration in the plant tissue (Figure 4A), but K and P concentration in roots and K concentration in leaves of cattail grown on longan biochar were significantly higher than in the plants grown on gravel (Figure 4B-4C).
Table 5. Results of T-test of relative growth rate (RGR), plant biomass, plant height, chlorophylls and carotenoid content of cattail (mean ± SE) grown on gravel and longan biochar-based systems for 45 days.
|
|
Filter materials |
F-ratio |
|
|
Gravel |
Longan biochar |
||
|
RGR (g g-1 d-1) |
0.017 ± 0.001b |
0.009 ± 0.000a |
7.2** |
|
Total biomass (g DM) |
19.3 ± 0.2b |
11.3 ± 0.5a |
4.1*** |
|
Root biomass (g DM) |
0.6 ± 0.1b |
0.3 ± 0.1a |
0.1* |
|
Rhizome biomass (g DM) |
6.4 ± 0.6a |
5.8 ± 0.3a |
3.4 |
|
Leaf biomass (g DM) |
12.3 ± 0.5b |
5.2 ± 0.1a |
11.2** |
|
Height (mm) |
1,573 ± 62b |
1,243 ± 68a |
8.0* |
|
Chlorophyll a (mg g-1 DM) |
2.0 ± 0.2b |
1.0 ± 0.2a |
0.9* |
|
Chlorophyll b (mg g-1 DM) |
1.3 ± 0.2b |
0.5 ± 0.0a |
4.1* |
|
Chlorophyll a+b (mg g-1 DM) |
3.2 ± 0.4b |
1.5 ± 0.2a |
1.8* |
|
Total carotenoids (mg g-1 DM) |
0.06 ± 0.01a |
0.07 ± 0.03a |
2.8 |
Note: Different letters superscripts indicate significant differences between treatment, * P < 0.05, ** P < 0.01, and *** P < 0.001

Figure 4. Concentrations of (A) total nitrogen, (B) phosphorus, and (C) potassium in roots, rhizomes, and leaves of cattail grown on gravel-based system ( ) and longan biochar-based system ( ) for 45 days. Different letters indicate significant differences of mineral content within the same part of plant.
TIR analysis of longan biochar
The peaks before and after the wastewater treatment were not significantly different (Figure 5). Peaks at 440.5 and 440.8 cm-1 and the weak peaks at 2,313.5 cm-1 did not match to any functional groups. The peaks of 520.0, 520.3, 596.6, 598.3, 649.7, and 649.9 cm-1 corresponded to the bonding of carbon with any halogen elements. The peaks at 696.4 and 696.6 cm-1 belong to benzene derivative. The peaks observed at 741.8 and 742.1 cm-1 belong to C-H (bending) while 901.2, 906.2, 960.6, and 966.0 cm-1 belong to C=C (bending). Furthermore, the peaks of 1042.0 cm-1 belong to C-N (stretching) while 1,199.8 and 1,200.7 cm-1 peaks belong to C-O (stretching).

Figure 5. FTIR analysis of longan biochar (A) before (B) after the experiment.
DISCUSSION
It is obvious that both filter materials and plantation with cattail play important roles in pollutant removal. In control systems without filter materials and plants, the organic solids were reduced through sedimentation and microbial degradation consuming oxygen diffused from the atmosphere (Metcalf and Eddy, 2004). Therefore, TSS and BOD5 decreased while diffused air was presumed to be consumed by microorganisms so that DO remained at 0 mg L-1. Addition of filters supplemented lower water flow and provided surface areas for organic solids entrapment (Maiga et al., 2017), therefore, TSS and BOD5 could be removed better compared with the system without filter while oxygen diffused to the system could be partly preserved. However, kinds of filters significantly affect treatment efficiency. Overall, longan biochar was more efficient than gravel on reducing TSS and BOD5 especially in unplanted system, which could be influenced by its dimension that was smaller. The small particle size usually provides more surface to entrap organic solids (Ávila et al., 2014). Similarly, previous studies reported that adding biochar produced from wood as a filter in CWs increased efficiency of BOD5 and TSS removal from wastewater compared with conventional gravel or sand-based CWs (de Rozari et al., 2015; Zhou et al., 2019; Feng et al., 2020). Furthermore, the particle with light weight and high pore volume encouraged more oxygen diffusion and preservation (Saeed and Sun, 2012), so longan biochar could improve more DO concentration than gravel. Zhou et al. (2020) also reported that DO concentrations of wastewater in wood biochar-added CWs were higher than DO of wastewater in CWs filtered with only conventional sand. With plantation, most wetland plants can cope with low O2 conditions by producing aerenchyma in their internal structure that allow air transportation from their shoots to underground stems and roots, for release into the rhizosphere (Shelef et al., 2013). Additional oxygen released from high root biomass of cattail planted on gravel could be consumed by microorganisms to encourage more degradation activity (El-Sherbeny et al., 2013), causing better BOD5 removal compared with unplanted gravel-based system.
Removal of nitrogen and phosphorus also depends on type of filters and plantation with significant interactions between these two factors. Longan biochar showed high efficiency on N removal reliably due to smaller size and higher porous structure than gravel which was expected to provide more surface to support the attachment and growth of nitrifiers and denitrifiers. More abundant of nitrifying and denitrifying bacteria in biochar-based CWs than in gravel-based CWs has been reported in the previous study (Kizito et al., 2017). Furthermore, DO concentration affected nitrifying activity which influenced on NH4-N and TKN removal. NH4-N was nitrified at approximately 0.5 mg L-1 DO, whereas the nitrification rate moderately increased with DO concentrations (How et al., 2018). Higher DO concentration in longan biochar-based system might enhance more nitrifying activity leading to better NH4-N removal compared with unplanted gravel-based system which only 27% of NH4-N was removed. Meanwhile, the control system was under anoxic conditions where nitrifying bacteria might not have been functional so that the effluent NH4-N concentration was maintained nearly as the influent. In addition, CEC of biochar encouraged NH4-N adsorption, concurrent with a release of other alkali metal cations such as K+, Ca2+, and Mg2+ (Dai et al., 2016; Munera-Echeverri et al., 2018; Pan et al., 2021). The CEC of longan biochar used in this study was 13 cmol kg-1 (Janyasupab and Jampeetong, 2022), which was effective to adsorb some NH4-N while slight pH, EC, and TDS increasing in treated wastewater believably resulted from alkali metal ions releasing. Similarly, TKN in longan biochar-based systems was also mainly removed through nitrification and N adsorption causing 80% removal. However, low DO and absence of NH4-N adsorption ability in unplanted gravel-based system led to only 37% of TKN removal while anoxic condition in control treatment where nitrifying activity was inhibited, lacking of TKN removal (only 14%) was found. Denitrification was a main process to remove NO3-N which generally increased with a decrease of DO (Fennel et al., 2005), however, NO3-N was better removed in longan biochar-based systems although DO concentrations were higher than gravel-based and control treatments. A substantial promotion of denitrifying activity could be influenced by carbon from biochar which acted as electron donor to denitrifiers (Li et al. 2018). Higher efficient of NO3-N removal in biochar-added systems than the systems wholly filled with conventional gravel or sand was also found in the previous studies (Gupta et al. 2016; Zhou et al. 2020). On TP reduction, the control system could be mainly influenced by sedimentation of organic P (NIWA, 2020). According to filter materials dependency, longan biochar was expected to entrap more particulate P due to its high pore volume and high surface area. However, accumulated PO4-P in biochar structure can be released and caused P increasing in the systems. So, the P removal in longan biochar-based systems were not different from unplanted gravel-based and control systems. PO4-P releasing were also found in some biochar types which caused the increasing of soluble P concentrations in water or soil after being used (Wang et al., 2015; Jin et al., 2016). On the other hands, a higher root biomass of cattail planted on gravel-based system presented high efficient to take up nutrients causing the best TP removal and improved NH4-N removal to be as high as both planted and unplanted longan biochar-based system. A mass balance calculated based on TKN and TP removal and plant biomass using the procedure determined by Breen (1990) showed that N and P accumulation in the tissues of cattail planted on gravel accounted for 61% and 43% of the removed TKN and TP, respectively. However, plant roots which took up NH4+ balanced the charge in their cells by extruding H+ to the external (Imler et al., 2019). Thus, decreasing of pH in planted gravel-based system was found. Furthermore, cattail not only took up N and P, but also other conductive mineral ions (Zingelwa and Wooldridge, 2009). In this study, 34% reduction of EC and TDS in planted gravel-based system reliably resulted from mineral ions uptake of cattail. Similar result was found in the study of Mudavanhu et al. (2014) which revealed that EC and TDS of wastewater in CWs planted with Typha capensis (Rohrb.) N.E. Br. decreased approximately 20-30% within 3 days compared to the unplanted CW where EC and TDS remained unchanged. However, cattail planted on longan biochar hardly support N and P removal. In longan biochar-based systems, N were mostly eliminated through microbial mechanisms and by NH4-N adsorption onto biochar. So, N supplied for plant was limited. To prevent nutrients deficiency, plant roots can bind on surface or internal pores of biochar to take up nutrients from the particles (Prendergast-Miller et al., 2014). In present study, cattail root binding to longan biochar was observed so that the plant could take up adsorbed NH4-N from the particles. N uptake was indicated by mass balance calculation which revealed that N accumulation in the tissues of cattail planted on longan biochar accounted for 24% of removed TKN. Furthermore, the binding of root to longan biochar may results in the uptake of soluble P and conductive alkali cations such as K+ from the particles which caused higher P concentration in root and K concentration in root and leaf than cattail planted on gravel. The increasing P and K concentrations in the tissue of the plants grown on biochar were also reported in the previous studies (Prendergast-Miller et al., 2014; Jia et al., 2019). However, growth and biomass of the root of cattail planted on longan biochar was significantly lower than the root of cattail planted on gravel so that the role of cattail in longan biochar-based system on P and conductive ions reductions was limited. Therefore, significant differences of TP, EC, and TDS concentrations between planted longan biochar-based system and unplanted longan biochar-based system were scarcely presented.
Vegetation is an important component in CWs. This study found that cattail grew better in gravel-based systems than in longan biochar-based system. This could be influenced by plant growth conditions resulted from different filter material which affected pH and chemical compounds in treatment systems. Brix et al. (2002) reported that T. latifolia showed the highest RGR, high N uptake rates, and high adenine nucleotides production when growing at pH 6.5. However, T. latifolia grown at pH 7 showed significantly lower growth, ATP synthesis, and N uptake. Gonzaga et al. (2018) found that filling excessive coconut husk and orange bagasse biochar into soil led to an increase of pH from 5.8 to 7.2 - 8.4, resulting in growth reduction of maize. Also, chemical compounds from biochar may inhibit plant growth. FTIR analysis revealed that longan biochar used in this study contained organic compounds of benzene derivation, C=C bonding, and C-H bonding which are resemble to the structure of ethylbenzene. These compounds have been reported to be toxic to plant root resulting in biomass reduction and leaf chlorosis (Canadian Council of Ministers of the Environment, 2004; Yan and Zhou, 2011). Therefore, high root biomass of cattail planted on gravel could release more O2 to improve BOD5 removal and could take up more N and P causing better removal than unplanted gravel-based system. Meanwhile, low root biomass of cattail planted on longan biochar, which was affected by pH increasing and chemical compounds, caused very low O2 releasing and low nutrient uptake leading to insignificant difference on pollutant removal from unplanted longan biochar-based system. However, the low growth rate of cattail in longan biochar-based system was inconsistent to the study by Kasak et al. (2018) which found that cattail planted in alnus biochar-added CW grew better than CWs without biochar. The differences may be influenced by longan biochar volume which was fully filled in present study while biochar in the previous study was only 10% added. Although positive effect on plant growth was found but the system could remove only 20-25% of total N and NO3-N (Kasak et al. 2018). To gain the most effective on plant growth and pollutants elimination, the relative amount of longan biochar to fill into the systems should be studied prior to the application.
CONCLUSION
Results from this study showed that the filter materials in CWs and the presence of plants interactively affect pollutants removal in the system. Filters consisting of longan biochar had 1.5-2.5 times higher removals of NO3-N than gravel and 2 times higher effluent DO concentrations at a hydraulic retention time of three days. The porous structure of longan biochar and higher surface area in the treatment system encourage the entrapment of organic matter leading to a higher BOD5 removal percentage. The presence of plants (Typha) also contributed significantly to the treatment performance as up 61% of the nitrogen removed, and up to 43% of the P removed were bound in the biomass of the plant grown on gravel. Therefore, the removal of NH4-N and TKN in gravel-based system were as high as in both planted and unplanted longan biochar-based system and presented the most efficient on TP removal. The growth and treatment performance of the plants were greatly retarded in filters composed on pure longan biochar as compared to plants in gravel filters. This may be caused by toxic effects of the biochar. Hence, to alleviate the toxic effects of biochar on the plant growth, the proportion of longan biochar in CW filters should be studied to discover the most appropriate proportion for wastewater treatment and plant growth.
ACKNOWLEDGEMENTS
This research was supported by the Science Achievement Scholarship of Thailand (SAST) and partially supported by Chiang Mai University. Also, we would like to thank the Faculty of Science, Chiang Mai University for a grant partially supporting this research. Finally, we thank the Wastewater Treatment Unit, Chiang Mai University for kindly giving permission to collect water samples. We thank
Mr. Alvin Yoshinaga for helpful comments on this manuscript and his help to read and correct the English writing.
AUTHOR CONTRIBUTIONS
Pakawat Janyasupab conducted the experiments, performed the statistical analysis, and wrote original draft of the manuscript. Hans Brix commented and edited the manuscript. Arunothai Jampeetong designed the experiment, supervised P. Janyasupab, commented, and edited the manuscript. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no conflict of interests.
REFERENCES
Ajibade, F.O., Wang, H., Guadie, A., Ajibade, T.F., Fang, Y., Sharif, H.M.A., Liu, W., and Wand, A. 2021. Total nitrogen removal in biochar amended non-aerated vertical flow constructed wetlands for secondary wastewater effluent with low C/N ratio: Microbial community structure and dissolved organic carbon release conditions. Bioresource Technology. 322: 124430.
APHA-AWWA-WEF. 2017. Standard Methods for the Examination of Water and Wastewater (23th ed.). American Public Health Association/American Water Works Association/Water Environment Federation, Washington, D.C.
Artiola, J.F., and Wardell, L. 2017. Guide to making and using biochar for gardens in Southern Arizona. Cooperative Extension, Tucson.
Ávila, C., Nivala, J., Olsson, L., Kassa, K., Headley, T., Mueller, R.A., Bayona, J.M., and García, J. 2014. Emerging organic contaminants in vertical subsurface flow constructed wetlands: Influence of media size, loading frequency and use of active aeration. Science of the Total Environment. 494-495: 211-217.
Ballantine, D.J., and Tanner, C.C. 2010. Substrate and filter materials to enhance phosphorus removal in constructed wetlands treating diffuse farm runoff: A review. New Zealand Journal of Agricultural Research. 53(1): 71-95.
Biswas, J.K., and Rana, S. 2014. Treatment wetlands as ecotechnological tools for regenerative reclamation of wastewater: Experiences from working with Kalyani model. Journal of Clean Energy Technologies. 2(1): 23-27.
Bolton, L., Joseph, S., Greenway, M., Donne, S., Munroe, P., and Marjo, C.E. 2019. Phosphorus adsorption onto an enriched biochar substrate in constructed wetlands treating wastewater. Ecological Engineering. 142: 100005.
Breen, P.F. 1990. A mass balance method for assessing the potential of artificial wetlands for wastewater treatment. Water Research. 24(6): 689-697.
Brix, H., Dyhr-Jensen, K., and Lorenzen, B. 2002. Root-zone acidity and nitrogen source affects Typha latifolia L. growth and uptake kinetics of ammonium and nitrate. Journal of Experimental Botany. 53(379): 2442-2450.
Canadian Council of Ministers of the Environment. 2004. Canadian soil quality guidelines for the protection of environmental and human health: Ethylbenzene (2004). In: Canadian environmental quality guidelines, 1999, Canadian Council of Ministers of the Environment, Winnipeg.
Chong, H.L.H., Ahmad, M.N., and Lim, P.E. 2009. Growth of Typha angustifolia and media biofilm formation in constructed wetlands with different media. Borneo Science. 25: 11-21.
Dai, L., Li, H., Tan, F., Zhu, N., He, M., and Hu, G. 2016. Biochar: A potential route for recycling of phosphorus in agricultural residues. GCB Bioenergy. 8(5): 852-858.
Dalahmeh, S.S., Lalander, C., Pell, M., Vinnerås, B., and Jönsson, H. 2016. Quality of greywater treated in biochar filter and risk assessment of gastroenteritis due to household exposure during maintenance and irrigation. Journal of Applied Microbiology. 121: 1427-1443.
de Rozari, P., Greenway, M., and El Hanandeh, A. 2015. An investigation into the effectiveness of sand media amended with biochar to remove BOD5, suspended solids and coliforms using wetland mesocosms. Water Science and Technology. 71(10): 1536-1544.
El-Sherbeny, G.A., Zahran, M.A., and Ghanem, M.I. 2013. Water hyacinth as a bio-agent for wastewater treatment in Egypt. Journal of Environmental Sciences. 42(2): 309-320.
Evans, C.G. 1972. The quantitative analysis of plant growth. Blackwell, Oxford.
Feng, L., Wang, R., Jia, L., and Wu, H. 2020. Can biochar application improve nitrogen removal in constructed wetlands for treating anaerobically-digested swine wastewater? Chemical Engineering Journal. 379: 122273.
Fennel, K., Follows, M., and Falkowski, P.G. 2005. The co-evolution of the nitrogen, carbon and oxygen cycles in the Proterozoic ocean. American Journal of Science. 305(6-8): 526545.
Gale, N.V., Sackett, T.E., and Thomas, S.C. 2016. Thermal treatment and leaching of biochar alleviates plant growth inhibition from mobile organic compounds. PeerJ. 2385: 1-25.
Godlewska, P., Ok, Y.S., and Oleszczuk, P. 2021. THE DARK SIDE OF BLACK GOLD: Ecotoxicological aspects of biochar and biochar-amended soils. Journal of Hazordous Materials. 403: 123833.
Gonzaga, M.I.S., Mackowiak, C., de Almeida, A.Q., and de Carvalho Junior, J.I.T. 2018. Positive and negative effects of biochar from coconut husks, orange bagasse and pine wood chips on maize (Zea mays L.) growth and nutrition. Catena. 162: 414-420.
Gupta, P., Ann, T., and Lee, S. 2016. Use of biochar to enhance constructed wetland performance in wastewater reclamation. Environmental Engineering Research. 21(1): 36-44.
Hammer, O., and Harper, D.A.T. 2020. Paleontological Statistics, version 4.01, reference manual. Natural History Museum, University of Oslo, Oslo.
Hanson, W.C. 1950. The photometric determination of phosphorus in fertilizers using the phosphovanado-molybdate complex. Journal of the Science of Food and Agriculture. 1(6): 172-173.
How, S.W., Lim, S.Y., Lim, P.B., Aris, A.M., Ngoh, G.C., Curtis, T.P., and Chua, A.S.M. 2018. Low-dissolved-oxygen nitrification in tropical sewage: An investigation on potential, performance and functional microbial community. Water Science and Technology. 77(9): 2274-2283.
Ibrahim, O.M., Bakry, A.B., El Kramany, M.F., and Elewa, T.A. 2015. Evaluating the role of bio-char application under two levels of water requirements on wheat production under sandy soil conditions. Global Journal of Advanced Research. 2(2): 411–418.
Imler, C.S., Arzola, C.I., and Nunez, G.H. 2019. Ammonium uptake is the main driver of rhizosphere pH in southern highbush blueberry. HortScience 54(5): 955-959.
Janyasupab, P., and Jampeetong, A. 2022. Performance of porous substrate for domestic wastewater treatment under prolonged hydraulic retention time. Applied Environmental Research. 44(3): 45-58.
Jia, W., Wang, C., Ma, C., Wang, J., Sun, H., and Xing, B. 2019. Mineral elements uptake and physiological response of Amaranthus mangostanus (L.) as affected by biochar. Ecotoxicology and Environmental Safety. 175: 58-65.
Jin, Y., Liang, X., He, M., Liu, Y., Tian, G., and Shi, J. 2016. Manure biochar influence upon soil properties, phosphorus distribution and phosphatase activities: A microcosm incubation study. Chemosphere. 142: 128-135.
Kadlec, R. 2009. Comparison of free water and horizontal subsurface treatment wetlands. Ecological Engineering. 35(2): 159-174.
Kantawanichkul, S., Kladprasert, S., and Brix, H. 2009. Treatment of high-strength wastewater in tropical vertical flow constructed wetlands planted with Typha angustifolia and Cyperus involucratus. Ecological Engineering. 35: 238-247.
Kasak, K., Truu, J., Ostonen, I., Sarjas, J., Oopkaup, K., Paiste, P., Kõiv-Vainik, M., Mander, Ü., and Truu, M. 2018. Biochar enhances plant growth and nutrient removal in horizontal subsurface flow constructed wetlands. Science of the Total Environment. 639: 67-74.
Kivaisi, A.K. 2001. The potential for constructed wetlands for wastewater treatment and reuse in developing countries: A review. Ecological Engineering. 16(4): 545-560.
Kizito, S., Lv, T., Wu, S., Ajmal, Z., Luo, H., and Dong, R. 2017. Treatment of anaerobic digested effluent in biochar-packed vertical flow constructed wetland columns: Role of media and tidal operation. Science of the Total Environment. 592: 197-205.
Kizito, S., Luo, H., Lu, J., Bah, H., Dong, R., and Wu, S. 2019. Role of nutrient-enriched biochar as a soil amendment during maize growth: Exploring practical alternatives to recycle agricultural residuals and to reduce chemical fertilizer demand. Sustainability. 11: 3211.
Lehmann, J., and Joseph, S. 2012. Biochar for environmental management: Science and Technology. Routledge, London.
Li, J., Fan, J., Zhang, J., Hu, Z., and Liang, S. 2018. Preparation and evaluation of wetland plant-based biochar for nitrogen removal enhancement in surface flow constructed wetlands. Environmental Science and Pollution Research. 25: 13929-13737.
Li, J., Fan, J., Liu, D., Hu, Z., and Zhang, J. 2019. Enhanced nitrogen removal in biochar-added surface flow constructed wetlands: Dealing with seasonal variation in the north China. Environmental Science and Pollution Research. 26: 3675–3684.
Liao, S., Pan, B., Li, H., Zhang, D., and Xing, B. 2014. Detecting free radicals in biochars and determining their ability to inhibit the germination and growth of corn, wheat and rice seedlings. Environmental Science and Technology. 48(15): 8581-8587.
Lichtenthaler, H.K. 1987. Chlorophylls and carotenoids: Pigments of photosynthetic membranes. Methods in Enzymology. 148: 350-382.
Maiga, Y., von Sperling, M., and Mihelcic, J.R. 2017. Constructed wetlands. In J.B. Rose, and B. Jiménez-Cisneros (eds), Water and sanitation for the 21st century: health and microbiological aspects of excreta and wastewater management (global water pathogen project). (J.R. Mihelcic and M.E. Verbyla (eds), Part 4: Management of risk from excreta and wastewater - Section: Sanitation system technologies, pathogen reduction in sewered system technologies), Michigan State University, E. Lansing, MI, UNESCO.
Metcalf, L., and Eddy, H.P. 2004. Wastewater engineering, treatment and reuse. McGraw-Hill, New York.
Mudavanhu, N., Ndeketeya, A., and Masaya, N. 2014. An assessment of phytoremediation capacity of Eichhornia crassipes and Typha capensis for the removal of total dissolved solids in plastic recycling industry wastewater. IOSR Journal of Environmental Science Toxicology and Food Technology 8(1): 86-92.
Mukherjee, A., Zimmerman, A.R., and Harris, W. 2011. Surface chemistry variations among a series of laboratory-produced biochars. Geoderma. 163: 247-255.
Munera-Echeverri, J.L., Martinsen, V., Strand, L.T., Zivanovic, V., Cornelissen, G., and Mulder, J. 2018. Cation exchange capacity of biochar: An urgent method modification. Science of the Total Environment. 642: 190-197.
Nelson, D.W., and Sommers, L.E. 1980. Total nitrogen analysis of soil and plant tissue. Journal Association of Official Analytical Chemists. 63(4): 770-778.
Nematian, M., Keske, C., and Ng’ombe, J.N. 2021. A techno-economic analysis of biochar production and the bioeconomy for orchard biomass. Waste Management. 135: 467-477.
NIWA. 2020. Constructed wetlands to reduce contaminant loss from pastoral farms. The National Institute of Water and Atmospheric Research, Auckland.
Oscarson, P., Ingemarsson, B., Ugglas, M., and Larsson, C.M. 1988. Characteristics of NO3- uptake in Lemna and Pisum. Plant and Soil. 111: 203-205.
Pan, X., Gu, Z., Chen, W., and Li, Q. 2021. Preparation of biochar and biochar composites and their application in a Fenton-like process for wastewater decontamination: A review. Science of The Total Environment. 754: 142104.
Pituya, P., Sriburi, T., and Wijitkosum, S. 2017. Properties of biochar prepared from Acacia wood and coconut shell for soil amendment. Engineering Journal. 21(3): 63-76.
Pongthornpruek, S. 2017. Treatment of piggery wastewater by three grass species growing in a constructed wetland. Applied Environmental Research. 39(1): 75-83.
Prendergast-Miller, M.T., Duvall, M., and Sohi, S.P. 2014. Biochar–root interactions are mediated by biochar nutrient content and impacts on soil nutrient availability. European Journal of Soil Science. 65(1): 173-185.
Quilliam, R.S., van Niekerk, M.A., Chadwick, D.R., Cross, P., Hanley, N., Jones, D.L., Vinten, A.J.A., Willby, N., and Oliver, D.M. 2015. Can macrophyte harvesting from eutrophic water close the loop on nutrient loss from agricultural land? Journal of Environmental Management. 152: 210–217.
Robinson, J.W. 1960. Atomic absorption spectroscopy. Analytical Chemistry. 32(8): 17A-29A.
Saeed, T., and Sun, G. 2012. A review on nitrogen and organics removal mechanisms in subsurface flow constructed wetlands: Dependency on environmental parameters, operating conditions and supporting media. Journal of Environmental Management. 112: 429-448.
Shelef, O., Gross, A., and Rachmilevitch, S. 2013. Role of plants in a constructed wetland: Current and new perspectives. Water. 5: 405-419.
Suksawat, C., Ariyadet, C., Sutigoolabud, P., and Sangchyoswat, C. 2017. Towards a zero-waste model in longan farms: Impact of longan biochar and corn mulch on longan plantation soils. Journal of Thai Interdisciplinary Research. 12(2): 1-7.
Tomczyk, A., Sokołowska, Z., and Boguta, P. 2020. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Reviews in Environmental Science and Bio/Technology. 19: 191-215.
Verhofstad, M.J.J.M., Poelen, M.D.M., van Kempen, M.M.L., Bakker, E.S., and Smolders, A.J.P. 2017. Finding the harvesting frequency to maximize nutrient removal in a constructed wetland dominated by submerged aquatic plants. Ecological Engineering. 106: 423-430.
Wang, Y., Lin, Y., Chiu, P., Inhoff, P., and Guo, M. 2015. Phosphorus release behaviors of poultry litter biochar as a soil amendment. Science of The Total Environment. 512-513: 454-463.
Yan, S., and Zhou, Q. 2011. Toxic effects of Hydrilla verticillata exposed to toluene, ethylbenzene and xylene and safety assessment for protecting aquatic macrophytes. Chemosphere. 85: 1088-1094.
Xing, T., Yun, S., Li, B., Wang, K., Chen, J., Jia, B., Ke, T., and An, J. 2021. Coconut-shell-derived bio-based carbon enhanced microbial electrolysis cells for upgrading anaerobic co-digestion of cow manure and aloe peel waste. Bioresource Technology. 338: 125520.
Zhou, X., Wang, R., Liu, H., Wu, S., and Wu, H. 2019. Nitrogen removal responses to biochar addition in intermittent-aerated subsurface flow constructed wetland microcosms: Enhancing role and mechanism. Ecological Engineering. 128: 57-65.
Zhou, L., Wang, J., Xu, D., Li, Y., Yao, B., and Howard, A. 2020. Responses of nitrogen transformation and dissolved oxygen in constructed wetland to biochar and earthworm amendment. Environmental Science and Pollution Research. 27: 29475-20484.
Zingelwa, N.S., and Wooldridge, J. 2009. Uptake and accumulation of mineral elements from winery and distillery effluents by Typha latifolia and Phragmites australis. South African Journal of Enology and Viticulture. 30(1): 43-48.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand. https://cmuj.cmu.ac.th
Pakawat Janyasupab1, Hans Brix2, and Arunothai Jampeetong1, *
1 Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand
2 Department of Biology, Aarhus University, 8000 Aarhus C, Denmark.
Corresponding author: Arunothai Jampeetong, E-mail: arunothai.2519@gmail.com
Total Article Views
Editor: Tonapha Pusadee,
Chiang Mai University, Thailand
Article history:
Received: October 18, 2022;
Revised: March 10, 2023;
Accepted: March 27, 2023;
Published online: April 11, 2023

