
Protective Effect of Curcuma domestica and Curcuma xanthorrhiza Extracts toward Kidney, Liver, and Pancreatic Organ Dysfunction in Streptozotocin-Induced Diabetes Mellitus Mice
Yora Nindita*, Astika Widy Utomo, Nani Maharani, Endang Mahati, Ilham Fernando Kristiandi, Irfan Kesumayadi, Eny Dyah Kurniawati, Mochamad Ali Sobirin, and Noor Wijayahadi
Published Date : April 3, 2023
DOI : https://doi.org/10.12982/NLSC.2023.029
Journal Issues : Number 2, April-June 2023
ABSTRACT The complications of Diabetes mellitus (DM) in Indonesia still need attention. Curcuma are often used as complementary medicine for antidiabetic. There is no study has been found comparing the effect of Curcuma domestica extracts (CDE) and Curcuma xanthorrhiza extracts (CXE) on the progression of the kidney, liver, and pancreas dysfunction in the mice with streptozotocin (STZ)-induced DM. This study was an experimental study to compare the effect of CDE and CXE on the progression of multi-organ dysfunction such as kidney, liver, and pancreas. The extract was produced by a maceration process with composition 100 gr simplicial and 300 ml ethanol 70%. The mice were induced with 180 mg/kgBW STZ intraperitoneally. The CDE and CXE were given 100 mg/kgBW orally for 21 days. A blood glucose was measured by a glucose meter on days 0, 1, and 21. The histopathological examination of the kidney, liver and pancreas were done on 21 days after the treatment. Data were analyzed using One Way ANOVA and Kruskal-Wallis tests (P <0.05). The results revealed that blood glucose levels showed no significant reduction of CDE and CXE after 21 days of the treatment. Histopathological examination showed a significant protection in kidney inflammation, kidney fibrosis, and pancreatic inflammation (P =0.001, P =0.013, P =0.027 respectively) in CDE and CXE groups. No significant differences were examined in liver inflammation, liver fibrosis, and pancreatic inflammation (P =0.184, P =0.498, P =0.193 respectively). However, there was a significant improvement in liver inflammation by CXE treatment (P =0.041). CXE has a greater potential for hepatoprotective effect compared to CDE.
Keywords: Curcuma extract, Organ dysfunction, Inflammation, Diabetes mellitus, Mice
Funding: The authors are grateful for the research funding provided by the Faculty of Medicine Diponegoro University based on Diponegoro University Number 1173/UN7.5.4.2.1/PP/2020.
Citation: Nindita, Y., Utomo, A.W., Maharani, N., Mahati, E., Kristiandi, I.F., Kesumayadi, I., Kurniawati, E.D., Sobirin, M.A., and Wijayahadi, N. 2023. Protective effect of Curcuma domestica and Curcuma xanthorrhiza extracts toward kidney, liver, and pancreatic organ dysfunction in streptozotocin-induced diabetes mellitus mice. Nat. Life Sci. Commun. 22(2): e2023029.
INTRODUCTION
The lifestyle of modern society is increasingly sedentary leading to epidemiological transition of infectious diseases to degenerative diseases, such as diabetes, hypertension, hyperlipidemia, coronary heart disease, and others. Diabetes mellitus (DM) is a chronic metabolic disease characterized by increased blood sugar levels due to abnormalities in insulin secretion and insulin activity. There are four types of DM, namely type 1 DM, type 2 DM, gestational DM, and other types of DM (American Diabetes Association 2009). Although various prevention efforts have been carried out, type 2 DM is the most prevalent. Approximately 425 million people suffered from DM in 2017, and the number is expected to an increase to 629 million by 2045 (Ogurtsova et al. 2017). In Indonesia, it is estimated that people with diabetes reach 10 million people (6.9%) and occupy the top 7 (seven) countries with the highest DM sufferers in the world (Ogurtsova et al. 2017).
There are a lot of implications from DM, which are an increase in health costs and a decrease in quality of life. Excess free radical production in the hyperglycemic state of DM damages enzymes, cellular machinery, and increases insulin resistance, leading to DM complications (Asmat et al., 2016). Complications of DM are divided into microvascular (diabetic retinopathy, diabetic nephropathy) and macrovascular (coronary heart disease, stroke, diabetic cardiomyopathy). Cardiovascular complications are the main cause of morbidity and mortality in DM patients (Chawla et al. 2016; Fowler 2008). DM is also associated with the liver disorders, such as non-alcoholic fatty liver disease (NAFLD), and causes abnormally elevated the liver function enzymes. NAFLD and hyperglycemic states can damage hepatocytes and also contribute to increased morbidity and mortality among diabetic patients (Saponaro et al. 2015).
Indonesians frequently use traditional herbal medicine for DM. Various types of herbal plants are used by the community as complementary therapies for DM treatment, including temulawak (Curcuma xanthorrhiza) and turmeric (Curcuma domestica) (Kristiana and Suharmiati 2006; Setiawan et al. 2011). Given that DM medications are also found using herbs such as metformin, which is found through extracts of Galega officinalis, the use of herbal remedies in Indonesian society also needs to be evaluated to help identify the new potential for antidiabetic drugs or complementary medicines.(Farnaz Shokri, 2019; Shen, 2014). Thus, in our article, we evaluate and compare the effect of Curcuma domestica extracts (CDE) and Curcuma xanthorrhiza extracts (CXE) on the progression of multi-organ dysfunction such as kidney, liver, and pancreas.
MATERIALS AND METHODS
Materials
This study was an experimental study. C. domestica and C. xanthorrhiza were obtained as simplicial package from Center of Research and Development of Medicinal Plants and Traditional Medicines, Ministry of Health of the Republic of Indonesia. The simplicial were then macerated using ethanol 70%. Male Swiss mice was used for in vivo study. Streptozotocin (STZ) (Nacalai Tesque Inc, Kyoto, Japan) was used for diabetic induction in the mice. The extract was given orally using a CMC vehicle. A glucose meter (EasyTouch GCU, Bioptic Technology, Inc. Taiwan) was used for a blood glucose level.
A maceration process
The maceration process was done using ethanol 70%. The composition was 300 ml of ethanol 70% and 100 gr of each simplicia’s rhizome of C. domestica and C. xanthorrhiza. After 3 days, the extract was filtered using filter paper and evaporated using a rotary vacuum evaporator.
In vivo study
A total of 20 male Swiss mice weighing 25-35 grams at 3 months of age were acclimated for seven days in housing laboratory temperature (20–26 °C) with ad lib food and water supplay. The mice were measured for their random blood glucose levels. The mice with normal blood glucose level (<150 ml/dL) were divided into four groups randomly; (i) negative control (control group) (ii) positive control (STZ group), in which the mice was inducted 180 mg/kgBW STZ intraperitoneally (iii) CDE group, in which the mice was inducted 180 mg/kg STZ intraperitoneally and given 100 mg/kg CDE orally (iv) CXE group, in which the mice was inducted 180 mg/kgBW STZ intraperitoneally and given 100 mg/kgBW CXE orally. After day 1 of STZ induction, the mice were measured for random and fasting blood glucose levels before given the treatment to confirm the diabetic condition. After 21 days of the treatment, the mice were measured for random and the fasting blood glucose level before being terminated. Histopathological examination of kidney, liver, and pancreas was done with haematoxylin-eosin (HE) staining for inflammation scoring and Trichrome Masson and PAS for fibrosis scoring.
Blood glucose level
The blood glucose level was measured several times by a glucose meter (EasyTouch GCU, Bioptic Technology, Inc. Taiwan) through tail clipping method (King et al., 2020). The measurement was carried out on days 0 (before STZ induction), day 1 (after 1-day STZ induction and before the treatment), day 21 (after 21 days of the treatment). Additionally, mice's fasting blood glucose levels were assessed both at the beginning (day 1) and the end of the treatment (day 21). The sample was collected after the mice had fasted for four hours.
Histopathological assessment
Histopathological assessment was carried out in 5 fields of view using 400x magnification for inflammation and fibrosis score in every organ (kidney, liver, and pancreas). The following indicator was used for inflammation scoring; (i) normal (no lymphocyte in parenchyma) (ii) mild (lymphocyte infiltration under 25% area in parenchyma) (ii) mild (lymphocyte infiltration 25-50% area in parenchyma) (iv) severe (lymphocyte infiltration more than 50% area in parenchyma). For fibrosis scoring, the following indicator was used; (i) normal (no fibrosis in parenchyma) (ii) mild (fibrosis infiltration under 25% area in parenchyma) (ii) mild (l fibrosis infiltration 25-50% area in parenchyma) (iv) severe (fibrosis infiltration more than 50% area in parenchyma) (Ibrahim et al., 2018).
Data Analysis
Data were analyzed using One Way Analysis of Variance (ANOVA) with post-hoc LSD, Kruskal-Wallis tests with post-hoc The Mann-Whitney test, Paired sample T-Test and Wilcoxon test using IBM SPSS Statistic for Windows, version 25 (IBM Corp., Armonk, N.Y., USA), with p values <0.05 considered significant.
Ethics Statement
The present study had been approved by the Ethical Committee for Health Research Universitas Diponegoro No.123/EC/KEPK/FK-UNDIP/X/2019.
RESULTS
Sample Characteristic
During the study period, 20 mice from all groups were physically active and healthy. All mice remained complete after the end of the treatment. However, there was a change in the mice weight during the study period as shown in the figure below.
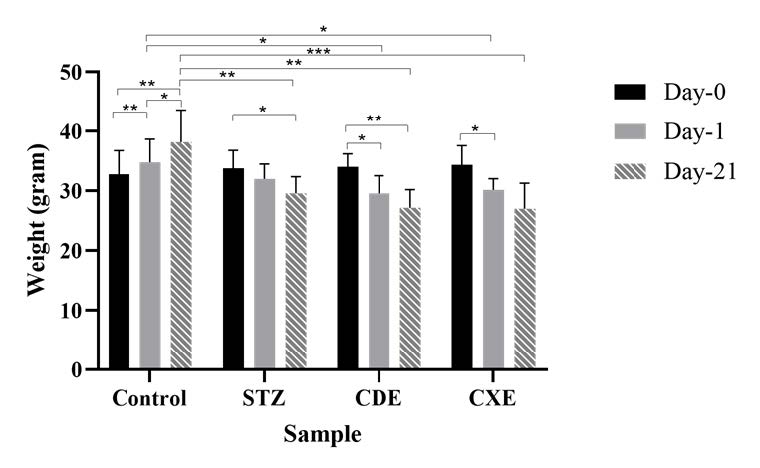
Figure 1. Bar chart of the mice weight on day-0, day-1, and day 21 of STZ induction. Data are presented as mean±SD, *P <0.05, **P <0.01, ***P <0.001
Before STZ induction, the mice weight was within the range of 32-34 grams (P =0.874). One day after STZ induction, the mice’ weight was within the range 29-34 grams (P =0.05). The significant difference was given by the control group from treatment group of CDE and CXE (P =0.012 and P =0.023 respectively). After 21 days of STZ induction, the mice weight was within the range 27-39 grams (P =0.001). The significant difference was given by STZ and the control (P =0.004), the control and treatment group of CDE and CXE (P =0.001 and P <0.001 respectively). Among the group, the control group showed a significant weight gain (P <0.05). Meanwhile, the treatment group and STZ group showed weight loss. A significant weight loss was found in STZ group of day-0 to day-21 (P =0.012), CDE group of day-0 to day-3 (P =0.022) and day-0 to day-21 (P =0.08), and CXE group of day-0 to day-3 (0.028).
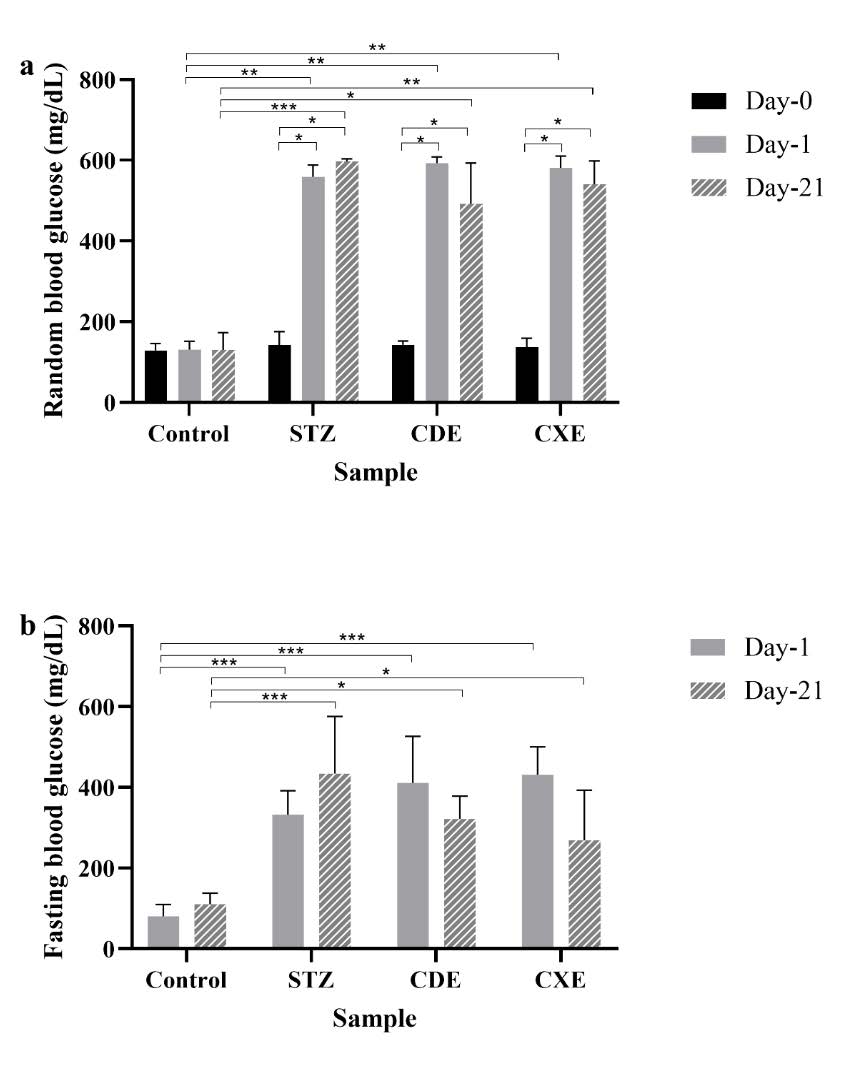
Figure 2. Bar chart with random blood glucose level (a) and fasting blood glucose level (b) on day-0, day-1, and day 21 of STZ induction. Data are presented as mean±SD, *P <0.05, **P <0.01, ***P <0.001.
Random blood glucose level before STZ induction was within the range 129-137 mg/dL and showed no significant difference (P =0.724). One day after STZ induction, the random blood glucose level of STZ and the treatment group increased (the range 558-593 mg/dL), while the control group remain constant with random blood glucose level of 131 ± 20.83 mg/dL and it showed a significant difference (P =0.004). The significant difference on day 1 was given by the control group with STZ and the treatment group of CDE and CXE (P =0.009, P =0.007 and P =0.008 respectively). Meanwhile, the fasting blood glucose level on one day after STZ induction was within the range of 332-431 mg/dL and it showed a significant difference (P <0.001). The control group had the smallest the fasting blood glucose level of 80 ± 29.35 mg/dL on day 1. The significant difference was given by the control group with STZ and the treatment group of CDE and CXE (P <0.001) on day 1.
After 21 days of STZ induction, the random blood glucose level of STZ and the treatment group was within the range of 492-596 mg/dL, while the control group had the smallest random blood glucose level of 130.4 ± 42.55 mg/dL and it showed a significant difference (P =0.002). STZ group had the highest random blood glucose level of 596.8 ± 7.14 mg/dl. The significant difference was given by STZ and the control (P <0.001), the control and the treatment group of CDE and CXE (P =0.048 and P =0.016 respectively). Meanwhile, the fasting blood glucose level of day-21 after STZ induction was within the range 269-434 mg/dl and the control group had the smallest the fasting blood glucose level of 110.8 ± 27.47 mg/dL (P =0.005). The significant difference was given by the control group with STZ and the treatment group of CDE and CXE (P <0.001, P =0.022, and P =0.048, respectively).
Pre and post-analysis with random blood glucose level showed that there was a significant increasing with random blood glucose level on day-0 to day-1 and day-21 in STZ and the treatment group of CDE and CXE (P <0.05). Meanwhile, the fasting blood glucose level showed no significant difference even though the fasting blood glucose level was increasing in the STZ group day-1 to day-21 and decreasing of the fasting blood glucose level on CDE and CXE group day-1 to day-21 (P =0.068).
The effect of CDE and xanthorrhizaCXE in the Kidney.
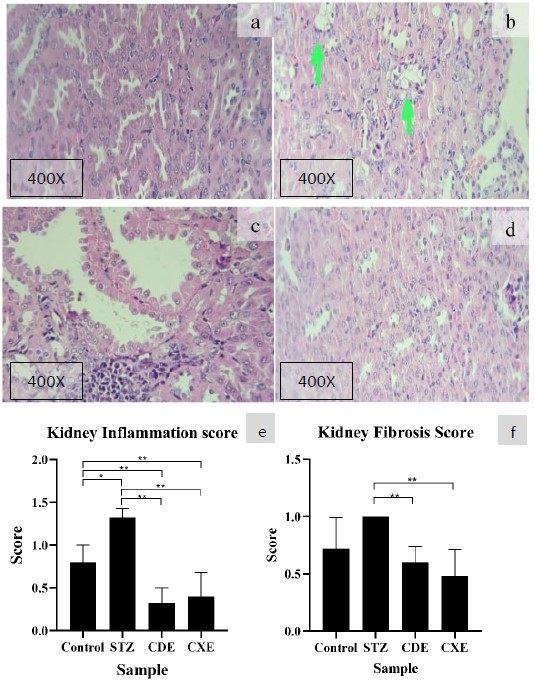
Figure 3. Histopathological lesion found in kidney with HE routine staining with magnification of 400x. The control group shows mild interstitial inflammation (a) the STZ group is the widest spread lesion with enlargement of the tubular lumen, the tubular cast, interstitial inflammation, and fat degeneration (b) the CDE group also shows similar damage with STZ group but with less wide-spread lesion (c) and CXE group also shows similar damage with STZ group but to less wide-spread lesion (d). Bar chart of (e) kidney inflammation score (f) kidney fibrosis score. Data are presented as mean±SD, *P <0.05, **P <0.01, ***P <0.001
The highest kidney inflammation score was STZ group and the lowest kidney inflammation score was CDE with a mean score 1.32 ± 0.11 and 0.32 ± 0.18, respectively. There was a significant difference among the group of kidney inflammation score (P =0.001). The significant difference was found in STZ with the control group (P =0.008) and STZ from treatment group of CDE and CXE (P =0.006 and P =0.007, respectively). The control group also showed a significant differencedifference between the treatment group of CDE and CXE (P =0.007 and P =0.034, respectively). No significant difference between CDE and CXE (P =0.381).
The highest kidney fibrosis score was STZ group and the lowest kidney fibrosis score is CXE with a mean score of 1.00 ± 0.00 and 0.48 ± 0.23, respectively. There was a significant difference among the group of kidney fibrosis score (P =0.013). The STZ group showed a significant differencedifference between the treatment group of CDE and CXE (P =0.005 and P =0.005, respectively). Post-hoc analysis with The Mann-Whitney U test showed that there was no significant difference between STZ with the control group (P =0.053). No significant difference between CDE and CXE (P =0.324).
The effect of CDE and CXE in the Liver
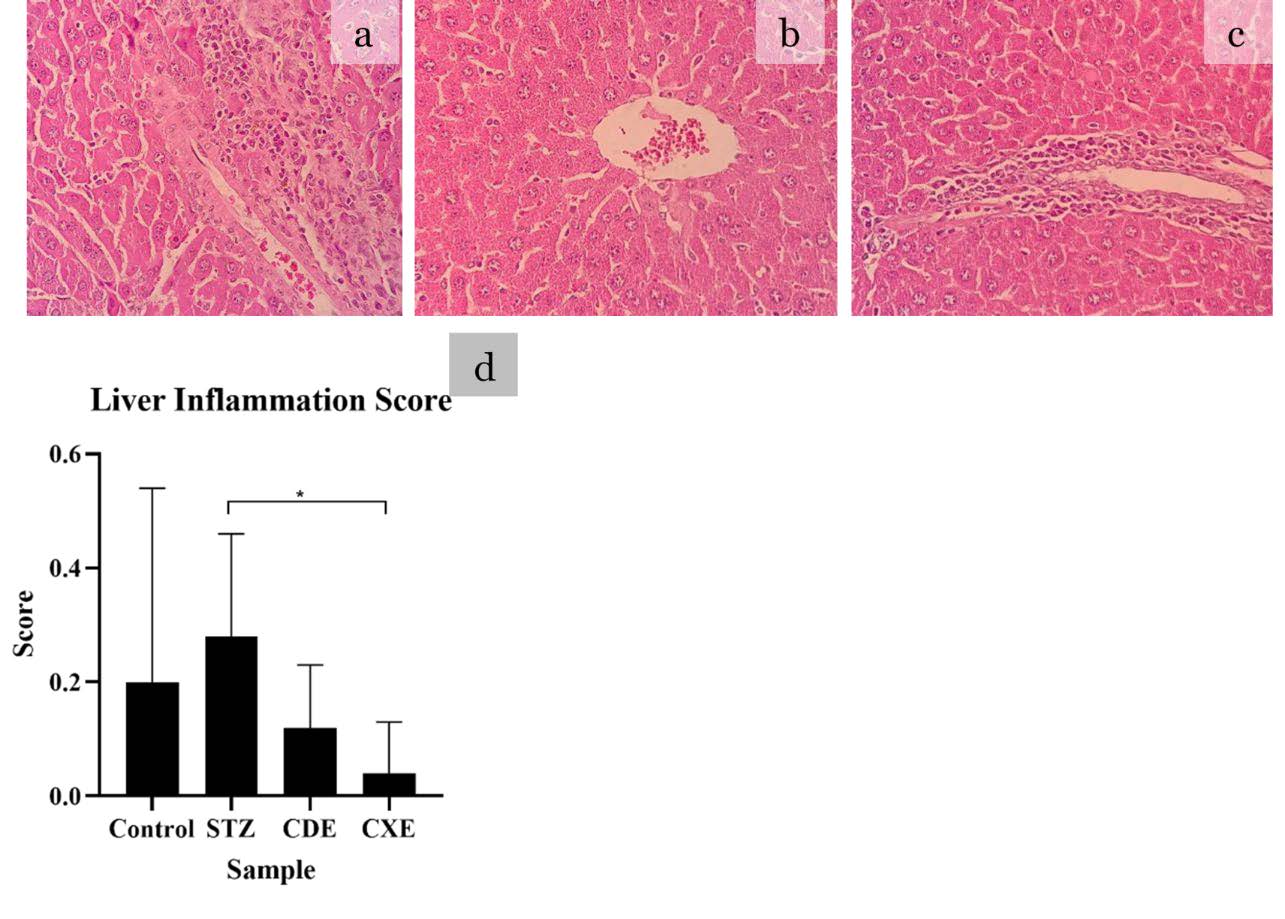
Figure 4. Histopathological lesion found in the liver with HE routine staining with magnification of 400x. All groups showed liver inflammation score below 0. Normal histopathological finding or score 0 (a), mild lymphocyte infiltration score 1 (b), moderate lymphocyte infiltration or score 2 (c) A mean score and SD with statistical analysis is depicted in (d) *P <0.05, **P <0.01, ***P <0.001
The highest liver inflammation score was STZ group and the lowest liver inflammation score was CXE with a mean score 0.28 ± 0.32 and 0.04 ± 0.09, respectively. There was no significant difference among the group (P =0.184). No significant difference between STZ with the control group (P =0.324). The significant difference was only found in STZ with CXE (P =0.041) while no significant difference was found between CDE and CXE (P =0.221).
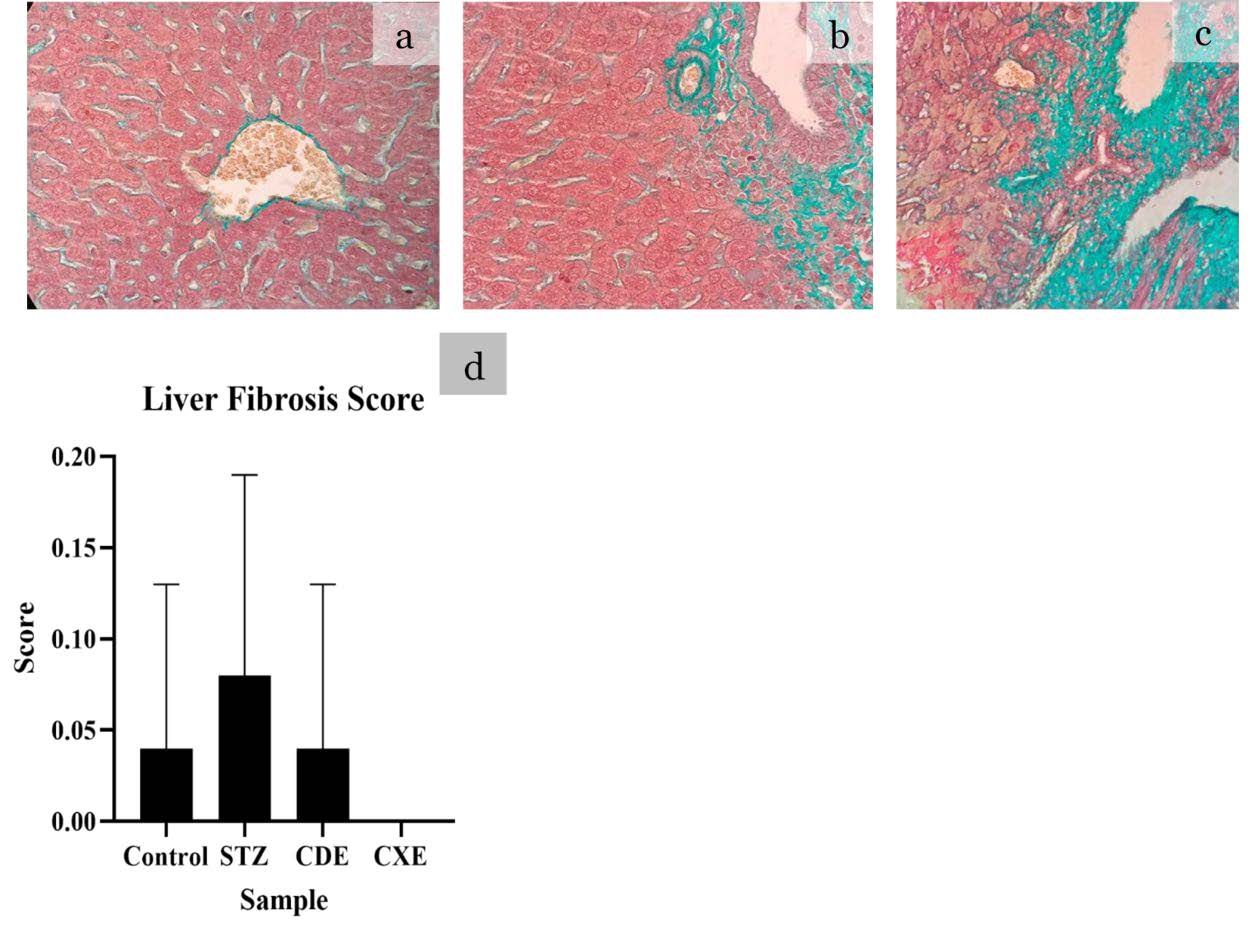
Figure 5. Histopathological lesion found in the liver with TM routine staining with magnification of 400x. All groups showed the mean liver fibrosis score below 0. Moreover, all mice in the CXE group showed normal histopathological level. Normal histopathological finding or score 0 (a) and mild fibrosis or score 1 (b) and moderate fibrosis or score 2 (c). A mean score and SD with statistical analysis is depicted in (d) *P <0.05, **P <0.01, ***P <0.001
With CDE, a smaller fibrosis score was found compared to STZ group. Meanwhile, in the CXE, there was no liver fibrosis although it is not significant (P =0.498).
The effect of CDE and CXE in the Pancreatic
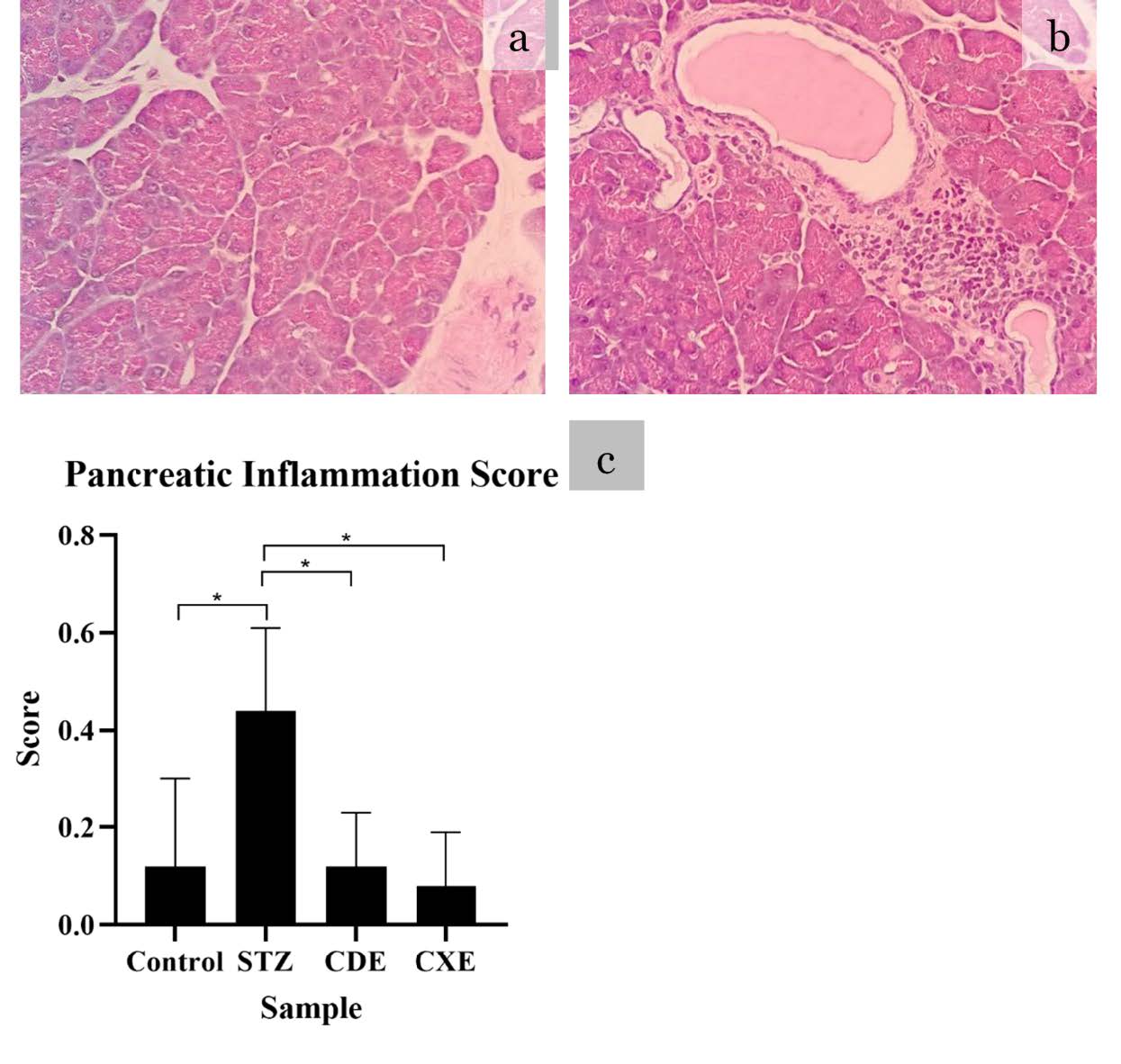
Figure 6. Histopathological lesion found in pancreatic with HE routine staining with magnification of 400x. All groups showed pancreatic inflammation score below 0. Normal histopathological finding or score 0 (a) and mild lymphocyte infiltration score 1 (b). A mean score and SD with statistical analysis is depicted in (c) *P <0.05, **P <0.01, ***P <0.001
The highest pancreatic inflammation score was STZ group and the lowest pancreatic inflammation score was CXE with a mean score 0.44 ± 0.17 and 0.08 ± 0.11, respectively. The Kruskal-Wallis test showed a significant difference among the group (P =0.027). Post-hoc analysis with The Mann-Whitney U test showed that there was a significant difference between STZ with the control group (P =0.031) and STZ from treatment group of CDE and CXE (P =0.017 and P =0.013, respectively). The control group showed no significant difference between the treatment group of CDE and CXE (P =0.817 and P =0.811, respectively). No significant difference between CDE and CXE (P =0.549).
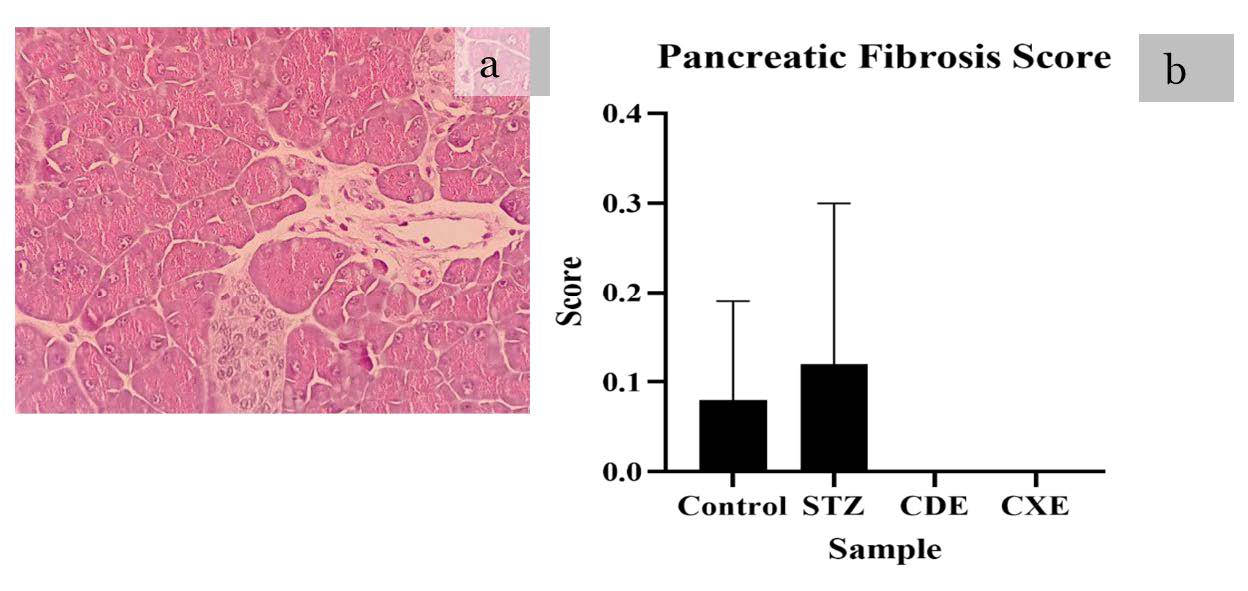
Figure 7. Histopathological lesion found in pancreatic to HE routine staining with magnification of 400x. Control and STZ showed pancreatic fibrosis score below 0. Moreover, the treatment group showed normal finding. Normal histopathological finding or score 0 (a), no histopathological finding with score>1. A mean score and SD with statistical analysis is depicted in (b) *P <0.05, **P <0.01, ***P <0.001
The highest pancreatic fibrosis score was STZ group (a mean score 0.12 ± 0.18 ) and the lowest pancreatic fibrosis score was the treatment group pf CXE and CDE with a mean score 0.00 ± 0.00 in both group. The Kruskal-Wallis test showed no significant difference among the group (P =0.193). The post-hoc analysis with The Mann-Whitney U test showed that there was no significant difference significant difference among the groups (P >0.05).
DISCUSSION
In our study, we have done characterize the identical sample for all treatments. The mice’s weight was within the range 32-34 grams in All groupsthe groups and random blood glucose was within the range 129-137 mg/dL. Non-obese diabetic the mice was considered normal when their random blood glucose level were below 150 mg/dL (Klueh et al. 2006). After STZ induction, 3 weeks treatment led to weight loss. This study is similar with Graham et.al study that found weight loss in the mice after STZ induction ranging from 3.5-3.7 grams (Graham et al. 2011).
There is not standardized hyperglycemia level for the mice or rats because different institutes and investigators use different, nonstandardized, the fasting and nonfasting methods (Furman 2015). The STZ induction led to increase in random blood glucose level ranging from 558-593 mg/dL. Graham et.al. study also found after STZ induction the random blood glucose level was higher than 500 mg/dL (Graham et al. 2011). Our study also found the fasting blood glucose level one day after STZ injection was ranging from 332-431 mg/dL. A similar founding was also observed in another study that found 12 day fasting in day and night time were 398.2 ± 25.4 and 358.7 ± 96.9 mg/dL, respectively (Sun et al. 2016).
C. xanthorrhiza and C. domestica are herbal medicine often used widely in Indonesia. These herbal plants have curcumin and xanthorrhiza substances that have potential effect to reduced blood glucose level. However, our study found no significant difference significant difference after 3 weeks of the treatment with CXE and CDE. In contrast, a study from Essa et al. showed a significant reduction in blood glucose level after 4 weeks of the treatment in rats receiving 100 mg/kg daily dose of CDE (Essa et al. 2019). Hence, no significant difference observed in this study presumably due to the duration of the treatment was only 3 weeks. Moreover, different method used to extract the C. xanthorrhiza and C. domestica can lead to different concentration of active compounds and its antioxidant level.
On histopathological examination, both CXE and CDE showed a significant protection compared to the STZ group in kidney and pancreatic inflammation, and kidney fibrosis. It should be noted that the liver inflammation score gave a significant difference only in the CXE group compared to STZ group, but no significant difference was found between CDE and CXE in liver inflammation score.
CDE contains curcumin, and its hydroxyl and methoxy the groups are responsible for its antioxidant and an anti-inflammatory agent (Rahman et al. 2006). Curcumin downregulate proinflammatory interleukins (IL-1,-2,-6,-8,-12) and cytokines (tumor necrosis factor-alpha (TNF- α), monocyte chemoattractant protein-1 (MCP-1)) through JAK/STAT pathway. An increase TNF- α is responsible for decreased insulin sensitivity (Asghari et al. 2012). The anti-inflammatory effect of curcumin is carried out by downregulating the activity of cyclooxygenase-2 (COX-2), lipoxygenase, xanthine oxidase, and inducible nitric oxide synthase (iNOS) enzymes (Maradana et al. 2013). Curcumin stimulates the expression of Nrf2 and HO-1 and it protects against free radicals.
CXE contains xanthorrhizol. Xanthorrhizol is one of the active compounds which only found in C. xanthorrhiza. Beside xanthorrhizol, CXE also to contain curcumin. Ethanolic extract of CXE was found contain 27% of xanthorrhizol, 7.10% curcumin, and strong antioxidant activity (Kesumayadi et al. 2021). Xanthorrhizol has antihyperglycemic and anti-inflammatory properties (Oon et al. 2015). Kim et al. found that xanthorrhizol and CXE reduced the level of insulin, glucose, free fatty acid, triglyceride in serum, reduced the size of epididymal fat pad and adipocyte, decreased the production of TNF- α, IL-6, IL-1β and CRP in adipose tissue, liver, and muscle. For that reason, xanthorrhizol may prevent fatty liver disease and chronic inflammation (Kim et al. 2014).
No significant difference was found in the liver and pancreatic fibrosis. This might be due to fibrosis is the end process of chronic inflammation and the process takes longer time than the study period (Wynn 2008). However, kidney fibrosis was found in our study. This indicated that the kidney has undergone severe recurring inflammation during the study period.
The pivotal role in diabetic complication are reactive oxygen species (ROS) and oxidative stress, leading to cellular death (Volpe et al., 2018). Previous studies had documented that herbal remedies with antioxidant activity could prevent DM complications, such as Galega officinalis extract and carvacrol could prevent oxidative damage in testicular (Farnaz Shokri, 2019; Shoorei et al., 2020), In our study, the antioxidant properties of CDE and CXE could prevent damage to the kidney and pancreatic. Thus, in the current knowledge, these herbal remedies might be useful as a complementary medicine in diabetic patient. Moreover, these remedies are widely consumed in Indonesia community as a crude extract known Jamu and no toxicity was reported.
There were several limitations of this study. First, there was no quantitative or qualitative measurement of curcumin and xanthorrhizol form the extract in our study. These properties had been widely documented in previous study and its level is abundant. Second, the histopathological scoring was done by modifying only from the percentage of total area and its ordinal data to classify the severity is dynamic, no international standardized scoring was used on histopathological examination. Third, we do not examine and characterize the effective dose and toxic dose of CDE and CXE. Thus, further study examines the dose of CDE and CXE are required. The cross-sectional in Indonesian community or clinical setting study about the side effect of CXE and CDE as the crude extract is also suggested to find its toxicity in human.
CONCLUSIONS
Our study found that CDE and CXE can significantly protect kidney and pancreas and CXE has a greater potential for hepatoprotective effect compared to CDE. A longer study period is recommended to investigate fibrosis damage in the pancreas and the liver.
ACKNOWLEDGEMENTS
The authors are grateful for Faculty of Pharmacy, Chiang Mai University for all facilities. We also thanks Mr.Thomas McManamon for English proof and corrections.
AUTHOR CONTRIBUTIONS
Astika Widy Utomo, Nani Maharani, Endang Mahati, and Eny Dyah Kurniawati assisted in conducting the experiments, performed the statistical analysis and data visualization and wrote the manuscript. Yora Nindita, Mochamad Ali Shobirin, Noor Wijayahadi, Ilham Fernando Kristiandi, and Irfan Kesumayadi designed and conducted all of the experiments and wrote the manuscript. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
American Diabetes Association. 2009. Diagnosis and classification of diabetes mellitus. Diabetes Care. 32: S62–S67.
Asghari, G., Sheikholeslami, S., Mirmiran, P., Chary, A., Hedayati, M., Shafiee, A., and Azizi, F. 2012. Effect of pomegranate seed oil on serum TNF-α level in dyslipidemic patients. International Journal of Food Sciences and Nutrition. 63: 368–371.
Asmat, U., Abad, K., and Ismail, K. 2016. Diabetes mellitus and oxidative stress—A concise review. Saudi Pharmaceutical Journal. 24: 547-553.
Chawla, A., Chawla, R., and Jaggi, S. 2016. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian Journal of Endocrinology and Metabolism. 20: 546–553.
Essa, R., El Sadek, A.M., Baset, M.E., Rawash, M.A., Sami, D.G., Badawy, M.T., Mansour, M.E., Attia, H., Saadeldin, M.K., and Abdellatif, A. 2019. Effects of turmeric (Curcuma longa) extract in streptozocin-induced diabetic model. Journal of Food Biochemistry. 43: 1–10.
Farnaz Shokri, M.S., Hamid Reza Niazkar, Ayda Roudi Rasht Abadi, Hossein Kalarestaghi, and Maliheh Ahin. 2019. Investigation the spermatogenesis and testis structure in diabetic rats after treatment with Galega officinalis extract. Crescent Journal of Medical and Biological Sciences. 6: 31-36.
Fowler, M.J. 2008. Microvascular and macrovascular complications of diabetes. Clinical Diabetes. 26: 77-82.
Furman, B.L. 2015. Streptozotocin-induced diabetic models in mice and rats. Current Protocols in Pharmacology. 70: 5.47.1-5.47.20.
Graham, M.L., Janecek, J.L., Kittredge, J.A., Hering, B.J., and Schuurman, H.J. 2011. The streptozotocin-induced diabetic nude mouse model: Differences between animals from different sources. Comparative Medicine. 61: 356–360.
Ibrahim, K.E., Al-mutary, M.G., Bakhiet, A.O., and Khan, H.A. 2018. Histopathology of the liver, kidney, and spleen of mice exposed to gold nanoparticles. Molecules. 23: 1–14.
Kesumayadi, I., Almas, A.I., Rambe, I.N.H., and Hapsari, R. 2021. Effect of curcuma xanthorrhiza gel on methicillin-resistant staphylococcus aureus-infected second-degree burn wound in rats. Natural Product Sciences. 27: 1–9.
Kim, M.B., Kim, C., Song, Y., and Hwang, J.K. 2014. Antihyperglycemic and anti-inflammatory effects of standardized Curcuma xanthorrhiza Roxb. extract and its active compound xanthorrhizol in high-fat diet-induced obese mice. Evidence-Based Complementary and Alternative Medicine. 2014: 1–10.
King, A.J.F., Gatward, L.F.D., and Kennard, M.R. 2020. Practical Considerations when Using Mouse Models of Diabetes. In A.J.F. King (Ed.), Animal Models of Diabetes. Springer Nature.
Klueh, U., Liu, Z., Cho, B., Ouyang, T., Fieldman, B., Henning, T.P., Kaur, M., and Kreutzer, D. 2006. Continuous glucose monitoring in normal mice and mice with prediabetes and diabetes. Diabetes Technology & Therapeutics. 8: 402–412.
Kristiana, L. and Suharmiati, S. 2006. Analisis rasionalisasi kandungan ramuan diabetes mellitus di laboratorium penelitian dan pengembangan pelayanan pengobatan obat tradisional (LP4OT). Buletin Penelitian Sistem Kesehatan. 9: 107–112.
Maradana, M.R., Thomas, R., and O’Sullivan, B.J. 2013. Targeted delivery of curcumin for treating type 2 diabetes. Molecular Nutrition & Food Research. 57: 1550–1556.
Ogurtsova, K., da Rocha Fernandes, J.D., Huang, Y., Linnenkamp, U., Guariguata, L., Cho, N.H., Cavan, D., Shaw, J.E., and Makaroff, L.E. 2017. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Research and Clinical Practice. 128: 40–50.
Oon, S.F., Nallappan, M., Tee, T.T., Shohaimi, S., Kassim, N.K., Sa’ariwijaya, M.S.F., and Cheah, Y.H. 2015. Xanthorrhizol: A review of its pharmacological activities and anticancer properties. Cancer Cell International. 15: 1–15.
Rahman, I., Biswas, S.K., and Kirkham, P.A. 2006. Regulation of inflammation and redox signaling by dietary polyphenols. Biochemical Pharmacology. 72: 1439–1452.
Saponaro, C., Gaggini, M., and Gastaldelli, A. 2015. Nonalcoholic fatty liver disease and type 2 diabetes: Common pathophysiologic mechanisms. Current Diabetes Reports. 15: 1–13.
Setiawan, A.S., Yulinah, E., Adnyana, I.K., Permana, H., and Sudjana, P. 2011. Efek antidiabetes kombinasi ekstrak bawang putih (Allium sativum Linn.) dan rimpang kunyit (Curcumma domestica Val.) dengan pembanding glibenklamid pada penderita diabetes melitus tipe 2. Majalah Kedokteran Bandung. 43: 26–34.
Shen, M. 2014. A second look at the ancient drug: New insights into metformin. Annals of Translational Medicine. 2: 51.
Shoorei, H., Khaki, A., Shokoohi, M., Khaki, A.A., Alihemmati, A., Moghimian, M., and Abtahi-Eivary, S.H. 2020. Evaluation of carvacrol on pituitary and sexual hormones and their receptors in the testicle of male diabetic rats. Human and Experimental Toxicology. 39: 1019-1030.
Sun, C., Li, X., Liu, L., Canet, M.J., Guan, Y., Fan, Y., and Zhou, Y. 2016. Effect of fasting time on measuring mouse blood glucose level. International Journal of Clinical and Experimental Medicine. 9: 4186–4189.
Volpe, C.M.O., Villar-Delfino, P.H., Dos Anjos, P.M.F., and Nogueira-Machado, J.A. 2018. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death and Disease. 9: 119.
Wynn T. 2008. Cellular and molecular mechanisms of fibrosi. Journal of Pathology. 214: 199–210.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand.
Yora Nindita1, *, Astika Widy Utomo1, Nani Maharani1, Endang Mahati1, Ilham Fernando Kristiandi2, Irfan Kesumayadi2, Eny Dyah Kurniawati3, Mochamad Ali Sobirin4, and Noor Wijayahadi1
1Department of Pharmacology and Therapy, Faculty of Medicine, Universitas Diponegoro, Semarang, 50275 Indonesia.
2Medical Doctor Profession Education, Faculty of Medicine, Universitas Diponegoro, Semarang 50275 Indonesia.
3Department of Anatomical Pathology, Rumah Sakit Umum Daerah Kartini, Jepara, 59421 Indonesia.
4Department of Cardiology and Vascular Medicine, Faculty of Medicine, Universitas Diponegoro, Semarang, 50275 Indonesia.
Corresponding author: Yora Nindita, E-mail: nindita.yora@fk.undip.ac.id
Total Article Views
Editor: Wipawadee Yooin; Veerasak Punyapornwithaya
Chiang Mai University, Thailand
Article history:
Received: April 28, 2022;
Revised: February 10, 2023;
Accepted: February 24, 2023;
Published online: March 13, 2023

