
Multifunctional Biological Activities and Cytotoxic Evaluation of Bouea macrophylla for Cosmetic Applications
Pawarisa Maneechai, Pimporn Leelapornpisid, and Worrapan Poomanee*Published Date : April 3, 2023
DOI : https://doi.org/10.12982/NLSC.2023.030
Journal Issues : Number 2, April-June 2023
ABSTRACT
This study aimed to investigate the biological activities, cytotoxicity, phytochemical constituents and stability profile of Bouea macrophylla Griff. peel extracts for cosmeceutical applications. Extraction using maceration or fractionation was optimized using various solvents; ethyl acetate, ethanol, 50%v/v ethanol. The antioxidant activities were determined using DPPH and ABTS radical scavenging, lipid peroxidation inhibition and FRAP assays. Anti-tyrosinase activity was also performed for implying skin depigmentation effects. Total phenolics content, total flavonoids content and total anthocyanin content were also investigated. In addition, ex vivo cytotoxicity and anti-inflammatory effects of the selected extract were studied. Moreover, high performance liquid chromatography (HPLC) technique was used to analyze and quantify phytochemical components of the extract to study the stability profile. The results revealed that the highest percentage yield was shown in hydroethanolic extract (BPHE). Regarding phytochemical contents, BPHE contained 83.91 ± 0.00 mg gallic acid equivalent (GAE)/g extract, 12.98 ± 0.01 mg quercetin equivalent (QE)/g extract. Additionally, BPHE exhibited the strongest antioxidant and anti-tyrosinase properties along with good anti-inflammatory effects. Furthermore, BPHE had no cytotoxicity to human fibroblast cells. The HPLC results showed two major peaks in BPHE, i.e., gallic and ellagic acids. Accordingly, B. macrophylla peel extract could be a promising bioactive ingredient to develop further as anti-aging cosmetic and cosmeceutical products.
Keywords: Bouea macrophylla Griff. peel extract, Antioxidant, Anti-tyrosinase, Anti-inflammatory, Cytotoxicity
Funding: This study is part of a master’s thesis at Chiang Mai University which was funded by the Graduate School and the Faculty of Pharmacy at Chiang Mai University, Thailand.
Citation: Maneechai, P., Leelapornpisid, P., and Poomanee, W. 2023. Multifunctional biological activities and cytotoxic evaluation of Bouea macrophylla for cosmetic applications. Nat. Life Sci. Commun. 22(2): e2023030.
INTRODUCTION
It has been proven that oxidative stress contributes to increased production of reactive oxygen species (ROS) which is chiefly associated to skin aging and elevated tyrosinase enzyme, melanogenesis-related proteins and mRNA levels in melanocytes leading to increased melanogenesis (Chen, Liu, Zhao, and Qiu, 2021). Likewise, skin inflammation triggered by pro-inflammatory mediators which is also induced by ROS during stressful situations plays a crucial role in skin aging and a number of skin problems (Checa and Aran, 2020). As a result, current cosmetic and cosmeceutical products are composed of active agents which potentially ameliorate oxidative stress, inflammation as well as skin hyperpigmentation (Salvioni et al., 2021). It has been ubiquitously reported that several natural products, herbs, or even waste materials from agricultural, food and beverage industries have been extensively applied in cosmetics owing to their great potentials of antioxidation, anti-inflammation and depigmentation. In general, most natural ingredients containing secondary metabolites such as phenolics, flavonoids, tannins, amino acids and vitamins which have shown a multiple of beneficial effects for treating several skin problems (Santos, Corrêa, and Chorilli, 2015). Consequently, a number of scientific research have attempted to explore promising cosmetic ingredients from natural resources.
Marian plum (Bouae macrophylla Griff.) has been considered one of the economic crops of Thailand widely grown in several areas especially in Nakhon Nayok, Phitsanulok and Ang Thong Provinces. Three different cultivars in Thailand are categorized by fruit characteristics and tastes including sour B. macrophylla, sweet B. macrophylla and mayong chid (B. oppositifolia). Among all cultivars of Thai B. macrophylla, the sweet B. macrophylla is regarded as an important local economic fruit that Thai farmers grow for commercial purposes from February to March due to its unique sweet taste. Also, the sweet B. macrophylla fruit is widely consumed owing to its lower selling price compared with B. oppositifolia. Related research has revealed that the ethanolic extracts from leaves of B. macrophylla in different types including sour B. macrophylla, sweet B. macrophylla and B. oppositifolia cultivated in Nakhon Nayok province contain high total phenolic contents (Thummajitsakul & Silprasit, 2017). Furthermore, methanolic extracts of ripe and unripe fruits of B. macrophylla were reported for their high concentrations of alkaloids, flavonoids, saponins, sterols, triterpenes, phenolics, tannins and vitamin C. Additionally, the ethanolic extract of sweet B. macrophylla seed from ripe fruit presents a strong antioxidant property, which was equivalent to Trolox, a water-soluble form of tocopherol (Dechsupa, Kantapan, Tungjai, & Intorasoot) [0]. From these reports, the extracts of the B. macrophylla fruit, leaves and seeds were important sources of natural antioxidants (Sukalingam, 2018). However, no evidence regarding chemical compositions, biological properties, or safety profiles of Thai sweet B. macrophylla peels, considered as a waste product from fruit processing industries, has been revealed until now. Therefore, the peel of sweet B. macrophylla is interesting to investigate regarding as bioactive substances and biological potential for cosmeceutical applications.
As a consequence, the aims of this study were to investigate the biological activities of B. macrophylla peel extract for the first time in terms of antioxidant, anti-tyrosinase and anti-inflammatory properties along with cytotoxicity concerning human skin fibroblast cells implying a safety profile. Moreover, pre-formulation studies, in terms of phytochemical contents and stability of the extract, were evaluated. These findings possibly add economic value to agricultural waste and provide a promising active ingredient for cosmeceutical applications.
MATERIALS AND METHODS
Chemical materials
Solvents used for extraction including ethyl acetate and ethanol were analytical grade. Solvents used for HPLC analysis as acetonitrile, methanol and phosphoric acid (H3PO4) were HPLC grade. The 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2'-azino-bis (3-ethyl benzothiazoline-6-sulphonic acid) (ABTS), linoleic acid, 2,2'-azobis (2-amidonopropane) dihydrochloride (AAPH), ammonium thiocyanate (NH₄SCN), tyrosinase from mushroom, 4-amino-3-hydroxyphenylalanine (L-tyrosine) and 3,4-dihydroxy-L-phenylalanine (L-dopa) were purchased from Fluka (Buchs, Switzerland). Gallic acid (GA), ellagic acid (EA), L-ascorbic acid, kojic acid and Trolox were purchased from Sigma-Aldridge Inc. (Schnelldorf, Germany). Beta-arbutin was purchased from Namsiang Co., Ltd. (Thailand).
Plant materials
The sweet B. macrophylla fruits were harvested from the B. macrophylla plantation in Phitsanulok Province, Thailand. The peels of sweet B. macrophylla were then collected. The plant materials were identified and authenticated the Medicinal plant innovative center, Faculty of Pharmacy, Chiang Mai University with herbarium code of 0023323. The peels were cleaned to remove the any dirt on the surface and cut into small pieces. They were incubated at 50°C for 24 hours in a hot-air oven. The dried plant materials were ground into fine powder using an electric blender before extraction and stored in an airtight container until use.
Plant extraction
The dried sweet B. macrophylla peels were extracted using solvents presenting different polarities by conventional maceration and solvent fractionation techniques relying on extracting potential and polarities for polyphenol extraction. Firstly, the dried plant was macerated in three cycles of 95% v/v ethanol and 50% v/v ethanol for 48 hours. Before use, the sample was mixed with solvents at 1:2 (w/v) ratio, and the mixture was then filtered through Whatman No.1 filter paper. The 95% v/v ethanolic solution was evaporated using a rotary evaporator (Buchi® Rotavapor R-300, Thailand) at 45°C to obtain crude ethanolic extract (BPE), whereas the 50% v/v ethanolic extract solution was evaporated to remove ethanol before being spray-dried with 2% w/v maltodextrin as a carrier (Buchi® Mini Spray dryer B-290, Thailand) at 140°C inlet and 100°C outlet temperatures to produce hydroethanolic extract (BPHE). Secondly, the dried plants were fractionated firstly with ethyl acetate, followed by fractionation with 95% v/v ethanol, respectively. The concentrated ethyl acetate fraction (BPEA) and the ethanolic fraction (BPEE) were then obtained using evaporation. The crude extracts were stored in airtight containers and kept in a refrigerator (SF-C697, Sanyo, Japan) at 4°C for further use.
The percentage yield was expressed as the mass of crude extract obtained per dried weight of B. macrophylla peels and was calculated by Truong et al. (2019) [0] according to the equation below.
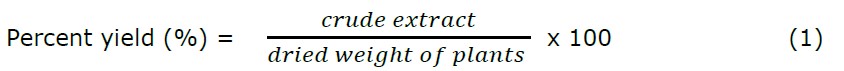
Phytochemical screening testing
Obtained extracts of B. macrophylla peels were evaluated for the presences of alkaloids, flavonoids, phytosterol, saponins, tannins and phenolics. The color changes indicated positive results following standard procedures.
Testing for alkaloids
Each extract was dissolved in 3 ml of ethanol, then 5 ml of 2 N of hydrochloric acid (HCl) was added and the mixture was heated in water bath. The mixture was then filtered and 3 ml of mixture was treated with a few drops of Dragendorff’s reagent. A black precipitate indicated the presence of alkaloids. Absolute ethanol served as a negative control while Ergotamine tartrate served as a positive control (Parbuntari et al., 2018).
Testing for flavonoids
Each extract was dissolved in 5 ml of ethanol. Then, a few drops of conc. HCl and magnesium ribbon were added and a pink color was observed within 3 minutes, indicating the presence of flavonoids. Quercetin served as a positive control while absolute ethanol was a negative control (Singh and Saxena, 2017).
Testing for phytosterols
Each extract was dissolved in chloroform and filtered. Then 1 ml of acetic acid anhydride was added and mixed thoroughly. After that, the reaction mixture was acidified by 2 ml of concentrated sulfuric acid (H2SO4). The presence of purple-red ring at the interface of two layers or blue-green color in the acetic acid anhydride layer indicated the presence of phytosterols (Pradnya and Alka, 2014).
Testing for saponins
Each extract was dissolved with 20 ml of deionized water and boiled in a water bath. After that, the mixture was cooled. The solution was then shaken vigorously in a graduated cylinder for 15 minutes. The presence of stable foam indicated the presence of saponins (Weli et al., 2018).
Testing for tannins and phenolics
The extract was dissolved in 10 ml of 0.9% w/v NaCl solution and heated by sonicator for 15 minutes, followed by filtration. Then, 1% w/v of gelatin salt reagent was added. The white precipitation indicated the presences of tannins. Positive test was also confirmed by addition of a few drops of 1% w/v ferric chloride (FeCl3) solution to the mixture. The presence of phenolics demonstrated a bluish-black color. A positive control was GA, while a negative control was deionized water
Determination of total phenolic content
Quantification of total phenolic content (TPC) was carried out using the Folin-Ciocalteu colorimetric method with modifications (Poomanee et al., 2018). The extract was dissolved in 50% v/v ethanol (0.5 ml). Then, the reagent mixture, consisting of 2 ml Folin-Ciocalteu reagent (Merck®, Germany), 7.5 ml sodium carbonate solution (Na2CO3), and 4 ml distilled water was mixed with the extract solution and left at room temperature. After 30 minutes, the absorbance was measured using a UV-VIS spectrophotometer (Shimadzu, UV-2600i, Japan) at a wavelength of 765 nm. The GA calibration curve, which is a regression of GA concentration (X) and absorbance (Y) was produced. The TPC values of the extracts was calculated based on the regression equation of Y = 0.0057X + 0.0177;
RESULTS
Bouea macrophylla Griff. peel extracts
Percentage yields and characteristics of the obtained extracts are shown in Table 1. The maceration of dried B. macrophylla peels using various solvents exhibited differences in their characteristics of the B. macrophylla crude extracts. The maximum yield was obtained from BPHE, followed by BPEE, BPE, and BPEA, respectively.
Table 1. The percentage yields and characteristics of B. macrophylla peel extracts.
|
Samples |
Types of solvents |
Extraction yield (%w/w) |
Characteristics |
|
BPEA |
Ethyl acetate fraction |
2.81 ± 1.70d |
Dark brown, viscous liquid |
|
BPHE |
Hydroethanolic extract |
45.46 ± 4.20a |
Brown-yellow, dry power |
|
BPEE |
Ethanolic fraction |
28.06 ± 3.50b |
Light brown, viscous liquid |
|
BPE |
Crude ethanolic extract |
20.18 ± 3.09c |
Brown, viscous liquid |
Note: Each value was showed as mean ± standard deviation (SD) of triplicate (n = 3), Superscript letters (a,b,c,d) indicate significant differences (P<0.05) between sample analyzed by One-Way ANOVA with multiple comparison using Tukey test.
Phytochemical screening of the extracts
This study performed the preliminary phytochemical screening of the different extracts from B. macrophylla peels showing the presences of secondary metabolites including phytosterol, saponin, tannin, and phenolic extracts as shown in Table 2. Most chemical components of the B. macrophylla peels were tannins and phenolics owing to high yields of BPE, BPEE, and BPHE, whereas phytosterols were found in only BPEA and BPE. These phytochemical compounds could offer biological activities of the plants.
Table 2. The phytochemical components of B. macrophylla peel extracts.
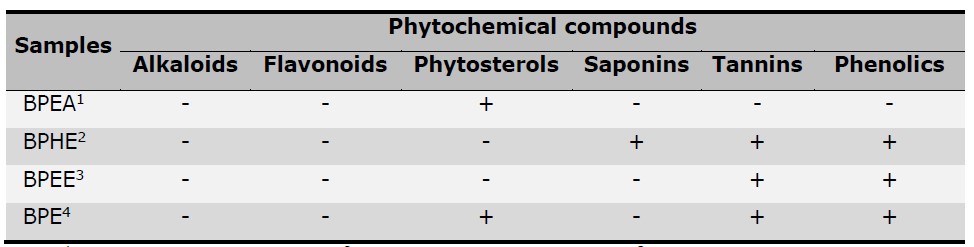
Note: 1BPEA: Ethyl acetate fraction, 2BPHE: Hydroethanolic extract, 3BPEE: Ethanolic fraction,
4BPE: Crude ethanolic extract; Positive (+) and negative (-) signs indicate the presence and absence of phytochemical compounds respectively.
Total phenolic, flavonoid, and anthocyanin contents of the extracts
The result demonstrated the extracts were composed of phenolic and flavonoid constituents in significantly different amounts as shown in Table 3. The highest TPC was found in BPE followed by BPHE, BPEE, and BPEA. In the case of flavonoids, BPEA presented the highest TFC among all extracts. Moreover, the anthocyanin was not detected in the extracts.
Table 3. Total phenolic and total flavonoid contents of B. macrophylla peel extracts.
|
Samples |
Total phenolic content |
Total flavonoid content |
|
BPEA |
76.75 ±0.09d |
31.95 ±0.10a |
|
BPHE |
80.25 ±0.01b |
6.04 ±0.01d |
|
BPEE |
78.50 ±0.09c |
6.63 ± 0.01b |
|
BPE |
87.35 ±0.09a |
6.33 ±0.01c |
Note: * mg GAE/ g extract is mg gallic acid equivalent per gram of extract.
** mg QE/ g extract is mg quercetin equivalent per gram of extract.
Superscript letters (a,b,c,d) indicate significant differences (P<0.05) between sample analyzed by One-Way ANOVA with multiple comparison using Tukey test
Antioxidant activities of B. macrophylla peel extracts
Regarding antioxidant assays in terms of DPPH and ABTS scavenging, linoleic acid peroxidation along with FRAP assays revealed the potential of the B. macrophylla peel extracts to attenuate oxidative stress through comprehensive pathways. Concerning the various extracts, BPE, BPEE and BPHE exerted a notable DPPH scavenging properties, whereas BPEA was not as strong as the other extracts as shown in Table 4. However, these are less potent than Trolox, GA, EA, and L-ascorbic acid (P < 0.05). Each extract also presented antioxidation through the ABTS scavenging assay. Considering the two assays, among all extracts, BPHE revealed the highest free radical scavenging activity because it showed the lowest IC50 on ABTS radicals and a low IC50 on DPPH radicals with no significant difference from BPEE and BPE. Furthermore, according to the linoleic acid peroxidation assay, BPHE extract exhibited the best inhibitory effect on lipid peroxidation indicating a similar manner to the results of ABTS and DPPH assays. In the FRAP assay, the abilities to reduce TPTZ-Fe3+ to TPTZ-Fe2+ of all extracts were shown in Table 4 for which the reducing effect of BPHE was the greatest.
Table 4. Effects of B. macrophylla peel extracts in various solvents on different antioxidant models and anti-tyrosinase activity.
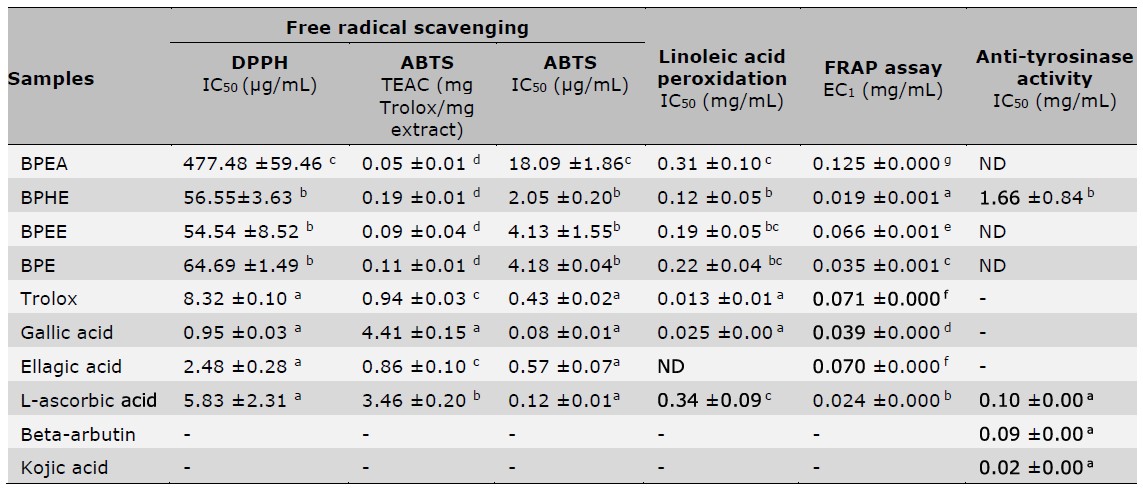
Note:Superscript letters (a,b,c,d,e,g) indicate significant differences (P<0.05) between sample analyzed by One-Way ANOVA with multiple comparison using Tukey test; ND = not detected, EC1 is equivalent concentration, which represented ferric reducing ability equivalent to 1 mM FeSO4.
Tyrosinase inhibition activity of B. macrophylla peel extracts
The B. macrophylla peel extracts were studied for inhibiting tyrosinase enzyme through the substrate of L-tyrosine. Table 4 indicates that only BPHE was capable of inhibiting tyrosinase through the L-tyrosine pathway which exhibited an IC50 value of 1.66 ± 0.84 mg/ml, whereas other extracts revealed no activity. In compared with the reference standard compounds that are commonly used as active components in skin lightening cosmetic products, such as beta-arbutin, kojic acid and L-ascorbic acid, BPHE was regarded to display low tyrosinase inhibitory activity.
Anti-inflammatory activity of the selected extracts
Before assessing anti-inflammatory efficacy, the cytotoxicity of the chosen extracts on the RAW 264.7 cell line was firstly assessed. The result of BPHE showed no toxicity to the cells. After that, the anti-inflammatory effect of BPHE on LPS-stimulated RAW 264.7 macrophages was evaluated in a term of inhibitory effect on NO production. Triamcinolone acetonide is a commonly used as an anti-inflammatory drug. As shown in Figure 1. BPHE could inhibit NO production from LPS-induced macrophages up to 24.76 ± 3.73% at concentrations of 0.5 mg/ml, while triamcinolone acetone can inhibit NO production was 28.56 ± 3.96% at a concentration of 0.5 mg/ml. Furthermore, the extract presented no significant difference from triamcinolone acetonide at all doses (P > 0.05).
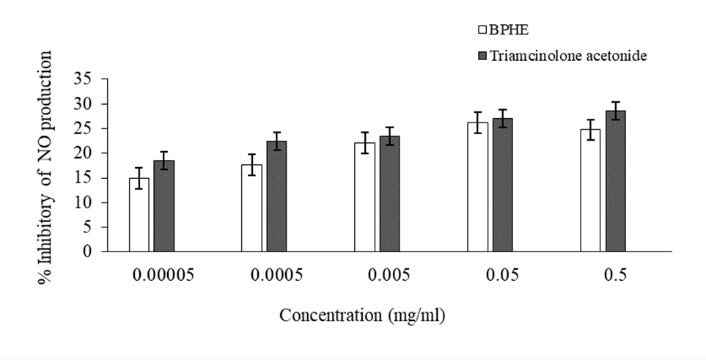
Figure 1. Percent inhibition on nitric oxide (NO) of the hydroethanolic extracts (BPHE) and ellagic acid on LPS-induced RAW 264.7 cell line; (Mean ± SD; n = 4).
In vitro cytotoxicity in human fibroblast cells of the selected extract
Owing to the greatest biological properties of BPHE, this extract was chosen for investigation of cytotoxicity. The results shown in Figure 2, revealed percent cell viability following the treatment with BPHE extract and ellagic acid at different concentrations ranging from 0.0001 to 1 mg/ml. It was shown that the BPHE and ellagic acid were not hazardous to human fibroblast cells at all doses. The percent cell viability of the BPHE-treated cells and ellagic acid-treated cells varied 95.95 - 110.85% and 90.85 - 111.90%, respectively. The positive control of a cytotoxic agent was sodium lauryl sulfate (SLS), which shown toxicity against human fibroblast cells at concentrations of 0.1 and 1 mg/ml with percent cell viability of 46.32 ± 3.44% and 24.06 ± 1.61%, respectively.
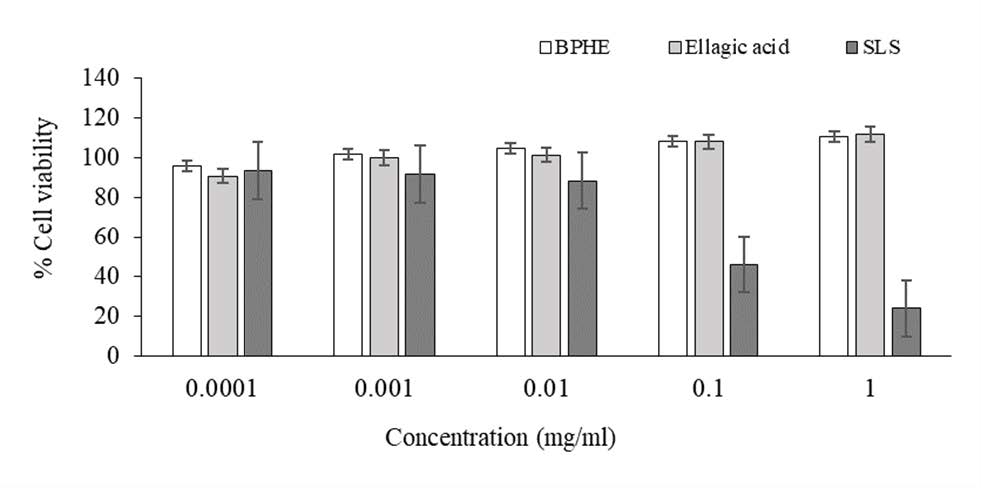
Figure 2. Cytotoxicity of the hydroethanolic extract (BPHE) and ellagic acid on human fibroblast cells comparing with sodium lauryl sulfate (SLS); (Mean ± SD; n = 4).
HPLC fingerprint of the selected extracts
The chemical compositions of the BPHE were determined using the HPLC. The chromatograms as shown in Figure 3, revealed that gallic acid and ellagic acid were identified in BPHE at retention times of 7.07 ± 0.00 minutes and 23.12 ± 0.01 minutes, respectively. It is worth noting that gallic acid and ellagic acid could be used as marker of the extract which might determine the biological property of the extract. The gallic acid and ellagic acid content of BPHE extract contained 5.90 ± 4.24 mg and 2.40 ± 1.41 mg per gram of extract. In addition, the amounts of gallic acid and ellagic acid were used to be indicators for stability testing.
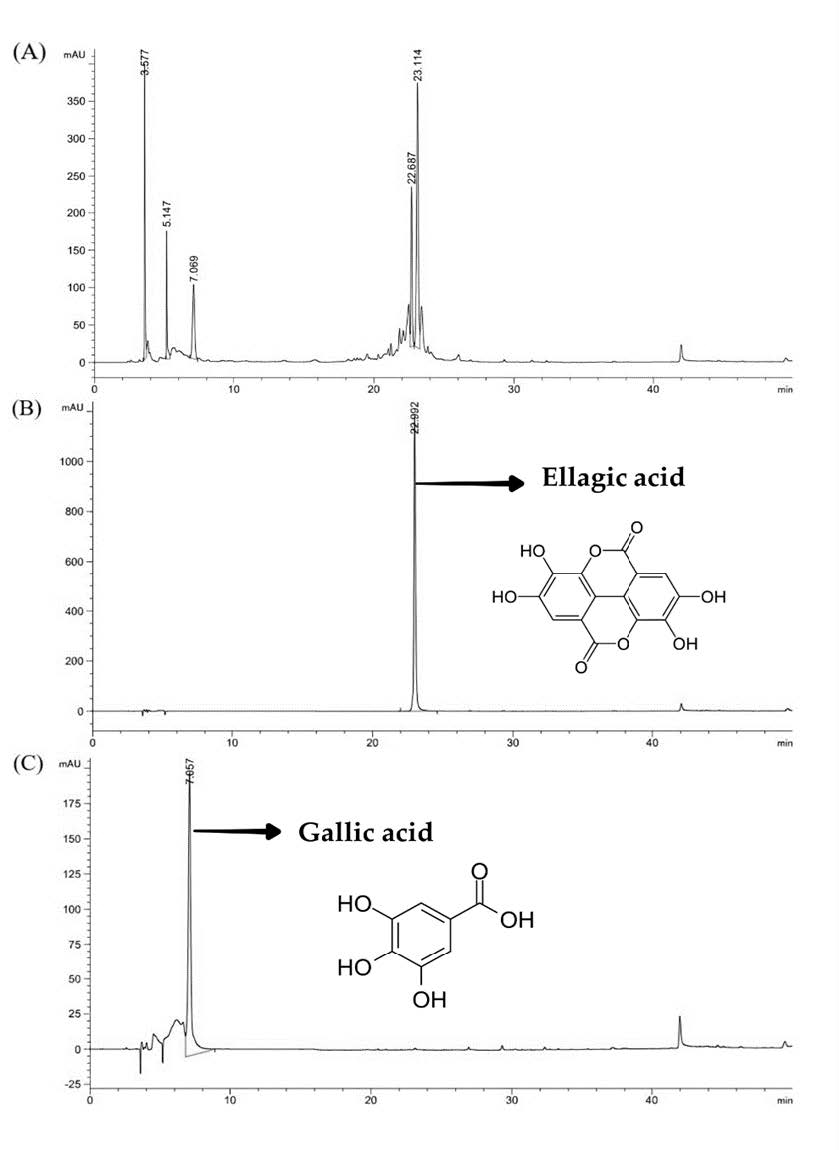
Figure 3. HPLC chromatograms of (A) BPHE, (B) Ellagic acid (40 ppm), and (C) Gallic acid (80 ppm).
Stability test of the selected extracts
The stability profile of BPHE in terms of DPPH scavenging and anti-tyrosinase properties at various conditions was explored. After storage under heating-cooling (HC) for 6 cycles as an accelerated condition and 45°C conditions for 30 days, the physical appearances of the samples changed slightly in which darker yellow in color and no precipitation were presented compared to the initial.
Figure 4 illustrated DPPH scavenging property of BPHE compared to the initial after storage in various conditions. Antioxidant effects of all samples after storage under all including 4°C, high temperature (45°C), HC, room temperature under light (RTL) and without light (RTD) were significant decreased from the initial (P < 0.05). Likewise, the tyrosinase inhibitory activity of the extract was significantly decreased in all conditions. In particular, under 45°C and RTL, the highest reductions of the effect from the initial were observed. Additionally, chemical stability of BPHE containing gallic acid and ellagic acid were shown in terms of percent reduction from the initial as shown in Figure 5. The highest reduction of ellagic acid were found after storage under HC and RTL while the highest reduction of gallic acid were found after storage under 45°C and RTL which were in correspondence with the attenuation of its biological effect.
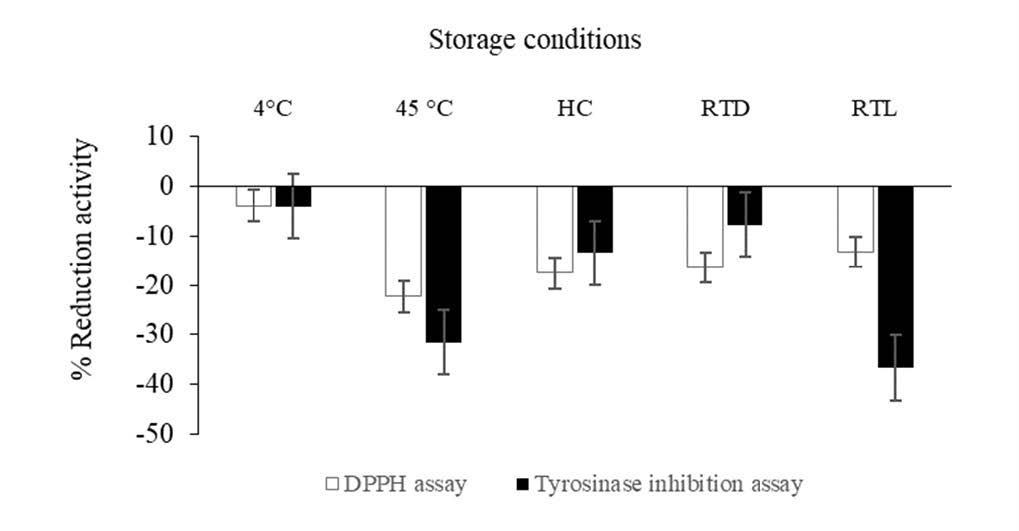
Figure 4. The percentage reduction DPPH antioxidant activity and tyrosinase inhibitory activity of BPHE after stability testing compared to the initial testing.
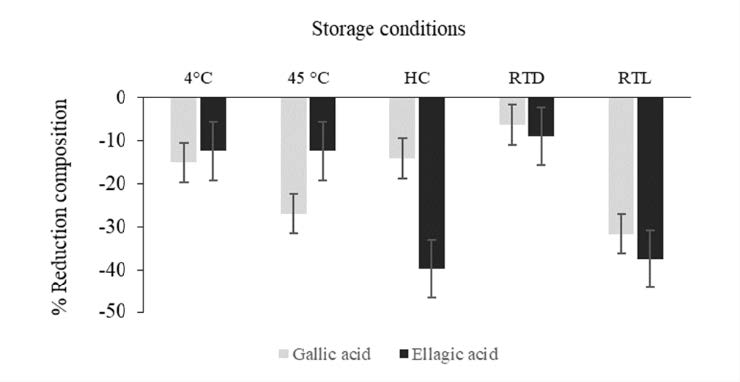
Figure 5. The percentage reduction composition of chemical stability of BPHE from day 0 at various conditions.
DISCUSSION
In the present study, B. macrophylla peel which was a waste product from fruit consumption and processing, was firstly investigated for its biological potentiality in terms of antioxidant, anti-tyrosinase and anti-inflammatory activities in order to be applied as a cosmeceutical active ingredient. Initially, the extracting solvents were optimized. Maceration is one of the conventional extraction methods that is easy and inexpensive because it only requires a basic instrument (Tambun, Alexander, and Ginting, 2012). In addition, this technique was widely utilized to extract thermally unstable compounds (Zhang, Lin, and Ye, 2018). Importantly, solvents used for extraction have a crucial influence on the types and quantities of secondary metabolites from the medicinal plants (Rebey et al., 2012). It was reported that ethyl acetate, water and aqueous combinations of ethanol, methanol and acetone are the most commonly used solvents for polyphenols extraction from plant materials (Ajila et al., 2010). Three extracting solvents presenting different polarities as ethyl acetate, ethanol and 50% v/v ethanol were included in our experiment. Our findings revealed that B. macrophylla peel, which were extracted by 50% v/v ethanol using maceration method gave the maximum percent yield implying that hydroethanolic solvent is the most efficient solvent for extracting B. macrophylla peel rather than the other solvents. This indicates that a great quantity of polar polyphenols resided in the peel. In addition, it was reported that the mixture of water and ethanol potentially augmented the extracting capacity since plant tissue is easily swelled by protic solvent. Subsequently, the structures of plant protein such as cell wall were denatured and loosen by ethanol that enhance the penetration of solvent molecules (Zuorro, Iannone, and Lavecchia, 2019). A previous study also reported that B. macrophylla seeds extracted from 50% v/v ethanol showed the highest yield compared to others from different concentrations of ethanol in water (25%, 75% and 95% v/v), which was in correspondence with our results (Dechsupa et al., 2018). The most found polyphenol compounds in plant extracts are phenolics, flavonoids and tannins which were highly capable of neutralizing free radicals and showed strong antioxidant capacity (Prabh and Vasantha, 2011; Arina and Harisun, 2019; Adam, Razali, Arapoc, Aziz, and Marsiddi, 2021). In previous study, Sukalingam et al. (2018) reported that methanolic extracts of ripe and unripe fruits of B. macrophylla contained the highest concentrations of alkaloids, flavonoids, saponins, sterols, triterpenes, phenolics, tannins and vitamin C, followed by ethanolic, aqueous and hexane extract, respectively. Likewise, our phytochemical screening results demonstrated that phytochemical compositions of B. macrophylla peel extracts were phytosterols, saponins, tannins and phenolics depending on polarity of the solvents and contributing to their promising biological activities. It has been widely proven that most of the tannins and phenolics are extracted effectively by ethanol or polar solvents than by non-polar solvents which was correlated to our results (Nawaz et al., 2020). Meanwhile, phytosterols and flavonoids present in a lipophilic manner can be easily extracted by nonpolar solvents such as ethyl acetate, petroleum ether and hexane (Uddin et al., 2018). Moreover, the presence of phytosterols in crude BPE but not in BPEE indicated that fractionation using ethyl acetate following by ethanol could partially purified the extract. In addition, it is worth noting that our results of total phenolic and flavonoid contents of BPEA were not in correspondence with those of phytochemical screening test which might provide false negative results of flavonoids and phenolics in the BPEA.
Our findings firstly demonstrated that extracts of B. macrophylla peel have strong antioxidant activities owing to the presences of phenolics and flavonoids. Polyphenol compounds are well-known for their antioxidant activity preventing human, animal and plant cells against the harmful effects of free radicals (Ghasemzadeh and Ghasemzadeh, 2011). The antioxidant capacity of polyphenols is due to the hydroxyl (-OH) moiety existing in the chemical structure that have the capacity to inhibit or prevent a target molecule from ROS peroxide, hyperoxide and lipid peroxyl (Pourmorad, Hosseinimehr, and Shahabimajd, 2006; Yamagishi and Matsui, 2011; Wu et al., 2011). In addition, the high amount of polyphenolic compounds was in correspondence with higher antioxidant activity since there were more active compounds to scavenge the available free radicals (Shahidi, Janitha, and Wanasundara, 1992). The extracts of B. macrophylla peel have highest ability to scavenge ABTS free radicals followed by DPPH radicals due to IC50 values reported from ABTS assay was apparently lower than DPPH assay. This finding could be explained that ABTS radical is more sensitive to antioxidant compounds due to faster kinetics reacting with the hydroxyl contained compound (Ilyasov et al., 2020). Furthermore, the experimental systems of ABTS and DPPH assays were different in a term of physical property of the radicals. The ABTS-radical scavenging system was high polarity since aqueous solution serves as a dissolving solvent of the free radicals, whereas DPPH-radical scavenging system was low polarity owing to its lipophilicity in nature (Lee et al., 2015). This finding also implied that the active substances in the extracts might be probable to be hydrophilic substances which was corresponding to the characteristic of BPHE. Furthermore, the oxidation of cellular lipids is known as lipid peroxidation that is the chain reactions of oxidative degradation of lipids. It plays an important role in the degradation of cellular functions leading to various degenerative disease and even skin aging problems (Ayala, Muñoz, and Argüelles, 2014). Our results demonstrated that BPHE had the highest inhibitory capacity on lipid peroxidation. Moreover, BPHE has the greatest ability of ferric reducing antioxidant power. Therefore, BPHE has outstanding antioxidant activities compared to other extracts.
Melanin is the key characteristic that causes dark spots, freckles, melisma and melanomas. Due to melanin production consists of complex oxidation and enzymatic processes, with tyrosinase being one of the most specific enzymes (Ayala et al., 2014; Sun, Guo, Zhang, and Zhuang, 2017). Besides, ROS are also important in melanogenesis and the catalyzed reactions of tyrosinase that produce dopaquinone lead to an increase in melanin synthesis (Zuo et al., 2018). Various skin whitening agents possess anti-melanogenic properties via antioxidant properties and directly inhibitory effects or regulatory action on tyrosinase activity. Hence, tyrosinase inhibitors are promising targets in cosmetics and skin pigmentation treatments. From our results, the tyrosinase inhibitory activity of B. macrophylla extract was also revealed of which only BPHE exerted this effect. Therefore, BPHE had a potential skin-whitening agent through both antioxidant and anti-tyrosinase effects.
Inflammation reaction which leading to extensive skin deterioration is also attributed to oxidative stress. In addition, the cell responding to cytotoxic compounds is usually followed by the production of inflammatory cytokines (Cui et al., 2018). Nitric oxide (NO) is regarded as one of the major inflammatory mediators contributing the prolong inflammatory and immune responses (Sharma et al., 2007). The bioactive compounds can inhibit the activity of inducible nitric oxide synthase (iNOS) and scavenge free radicals (Wiegand and Hipler, 2009). This research also investigated the potential of B. macrophylla peel extract to regulate NO production and secretion from lipopolysaccharide (LPS)-activated RAW 264.7 cells, a mouse macrophage cell line. RAW 264.7 cells are frequently used to investigate the anti-inflammatory effects of pharmaceutical agents (Adebayo, Ondua, Shai, and Lebelo, 2019). The ability of the BPHE to inhibit NO production from LPS-induced RAW 264.7 cells was shown at a concentration of 0.05 mg/ml. These results strongly supported an anti-inflammatory activity of B. macrophylla peel extract. Taken together, BPHE could be a promising active ingredient for cosmeceutical applications.
Cosmetics and personal care products should be concerned about safety and devoid of dangerous compounds that harm to humans and other living things (Hwang et al., 2018). Although natural substance has beneficial effects and many comprehensive mechanisms related to free radicals and oxidative stress, yet it is important to consider the safety assessment. Our study revealed that the cytotoxicity of the extract on human fibroblasts through sulforhodamine B (SRB) assay. It was reported that BPHE and ellagic acid which was a one of the major compounds in BPHE at concentrations of 0.0001-1 mg/ml had no toxicity to human fibroblasts. Therefore, the B. macrophylla peel extracts were safe for human use.
Aside from safety assessment, standardized profiles are the most important step for quality assurance in the natural cosmetics which is simplified by chemical and chromatographic procedures (Seo et al., 2016; Barthe et al., 2021). Phytochemical compositions in the study of Dechsupa et al. (2018) revealed that B. macrophylla seed kernel extracts demonstrated eighteen compounds by using HPLC at a wavelength of 270 nm. Furthermore, gallic acid and ellagic acid were successfully identified. In present study, BPHE was chosen for HPLC fingerprint analysis due to its significant antioxidant, anti-tyrosinase as well as its anti-inflammatory properties. The HPLC chromatogram of BPHE exhibited two identified peaks including gallic acid and ellagic acid. These compounds could be used as a BPHE biomarker for further product development and quality control. However, other phytochemical compounds found in the extract might play a role in its biological potential which were interesting for further investigation.
According to the stability testing, the results suggested that both decline in gallic acid and ellagic acid might have an effect on the decrease in antioxidant property and tyrosinase inhibition of the extract. These results possibly provided the information for further developing of an innovative cosmetic formulation in order to overcome these stability limitations of the extract. Moreover, the storage condition of the extract should be controlled in terms of low temperature and light-protection system.
CONCLUSIONS
This study firstly reported the multifunctional biological activities of B. macrophylla peel extracts in terms of antioxidation, anti-tyrosinase and anti-inflammatory effects along with the phytochemical constituents, safety and stability profiles for cosmetic applications. Our study revealed that hydroethanolic extract of B. macrophylla peel exerted the greatest biological properties with a good safety profile in human fibroblasts. Gallic acid and ellagic acid were identified in this extract. These findings implied that B. macrophylla peel extract has high potential to be a bioactive ingredient for cosmeceutical applications. However, the results of stability test suggested that cosmeceutical applications of B. macrophylla peel extract might require the development of optimized delivery systems to enhance its stability especially under light and high temperature conditions.
ACKNOWLEDGEMENTS
The authors are grateful for Faculty of Pharmacy, Chiang Mai University for all facilities. We also thanks Mr.Thomas McManamon for English proof and corrections.
AUTHOR CONTRIBUTIONS
Pawarisa Maneechai chiefly conducted the experiments, performed the statistical analysis and wrote the manuscript with data visualization by Pimporn Leelapornpisid and Worrapan Poomanee who designed all of the experiments and revised the manuscript. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest in this work.
REFERENCES
Adam, Z., Razali, R., Arapoc, D.J., Aziz, A.H.A, and Marsiddi, N.A. 2021. DPPH radical scavenging and folin-Ciocalteu assays: simple and reliable methods to quantify antioxidant activity and total phenolic content. Nuclear Engineering and Technology. 1–8.
Adebayo, S.A., Ondua, M., Shai, L.J., and Lebelo, S.L. 2019. Inhibition of nitric oxide production and free radical scavenging activities of four South African medicinal plants. Journal of Inflammation Research, 12: 195–203.
Ajila, C.M., Brar, S.K., Verma, M., Tyagi, R.D., Godbout, S., and Velero, J.R. 2010. Extraction and analysis of polyphenols: Recent trends. Critical Reviews in Biotechnology. 31: 1–22.
Arina, M.Z.L. and Harisun, Y. 2019. Effect of extraction temperatures on tannin content and antioxidant activity of Quercus infectoria (Manjakani), Biocatalysis and Agricultural Biotechnology. 19: 1–5.
Ayala, A., Muñoz, M., and Argüelles, S. 2014. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal, Oxidative Medicine and Cellular Longevity. 1–31.
Barthe, M., Bavoux, C., Finot, F., Mouche, I., Petrenci, C., Forreryd, A., et al. 2021. Safety testing of cosmetic products: Overview of established methods and new approach methodologies (NAMS). Cosmetics. 8: 1–18.
Checa, J. and Aran, J. 2020. Reactive oxygen species: drivers of physiological and pathological processes. Journal of Inflammation Research. 13: 1057–1073.
Chen, J., Liu, Y., Zhao, Z., and Qiu, J. 2021. Oxidative stress in the skin: Impact and related protection. International Journal of Cosmetic Science. 43: 495–509.
Cui, H., Duan, F., Jia, S., Cheng, F., and Yuan, K. 2018. Antioxidant and tyrosinase inhibitory activities of seed oils from Torreya Grandis Fort. Ex Lindl. BioMed Research International. 2018: 5314320.
Dechsupa, N., Kantapan, J., Tungjai, M., and Intorasoot, S. 2018. Maprang “Bouea macrophylla Griffith” seeds: Proximate composition, HPLC fingerprint, and antioxidation, anticancer and antimicrobial properties of ethanolic seed extracts. Heliyon. 5: 8405–8440.
Ghasemzadeh A. and Ghasemzadeh, M. 2011. Flavonoids and phenolic acids: Role and biochemical activity in plants and human. Journal of Medicinal Plants Research. 5: 6697–6703.
Hwang, J., Ma, J., Park, J., Jung, H., and Park, Y. 2018. Anti-inflammatory and antioxidant effects of Mok, a polyherbal extract, on lipopolysaccharide‑stimulated RAW 264.7 macrophages. International Journal of Molecular Medicine. 43: 26–36.
Ilyasov, I., Beloborodov, V., Selivanova, I., and Terekhov, R. 2020. ABTS/PP decolorization assay of antioxidant capacity reaction pathways, International Journal of Molecular Sciences. 21: 1–27.
Lee, K.J., Oh, Y.C., Cho, W.K., and Ma, J.Y. 2015. Antioxidant and anti-inflammatory activity determination of one hundred kinds of pure chemical compounds using offline and online screening HPLC assay. Evidence-Based Complementary and Alternative Medicine. 1–13.
Lee J. 2005. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. Journal of AOAC International. 88: 1269–1278.
Nawaz, H., Shad, M., Rehman, N., Andaleeb, H., and Ullah, N. 2020. Effect of solvent polarity on extraction yield and antioxidant properties of phytochemicals from Bean (Phaseolus vulgaris) seeds. Brazilian Journal of Pharmaceutical Sciences. 56: 1–9.
Parbuntari, H., Prestica, Y., Gunawan, R., Nurman, M.N., and Adella, F. 2018. Preliminary phytochemical screening (qualitative analysis) of cacao leaves (Theobroma Cacao L.). EKSAKTA: Berkala Ilmiah Bidang MIPA. 19: 40–45.
Poomanee, W., Chaiyana, W., Mueller, M., Viernstein, H., Khunkitti, W., and Leelapornpisid, P. 2018. In-vitro investigation of anti-acne properties of Mangifera indica L. kernel extract and its mechanism of action against Propionibacterium acnes. Anaerobe. 52: 64–74.
Poomanee, W., Chaiyana, W., Intasai, N., and Leelapornpisid, P. 2015. Biological activities and characterization of the pod extracts from sompoi (Acacia concinna linn) grown in northern Thailand, International Journal of Pharmacy and Pharmaceutical Sciences. 7: 237–241. Retrieved from https://innovareacademics.in/journals/index.php/ijpps/article/view/5463
Pourmorad, F., Hosseinimehr, S.J., and Shahabimajd, V. 2006. Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. African Journal of Biotechnology. 5: 1142–1145.
Pradnya, A. and Alka, C. 2014. Field test for the detection of phytersterols. International Research Journal of Pharmacy. 5: 734–736.
Prabh, M.R. and Vasantha, K. 2011. Antioxidant, cytotoxicity and polyphenolic content of Calotropis procera (Ait.) R. Br. Flowers. Journal of Applied Pharmaceutical Science. 1: 136–140. Retrieved from https://japsonline.com/abstract.php? article_id=190&sts=2
Rebey, I.B., Bourgou, S., Debez, I.B.S., Karoui, I.J., Sellami, I.H., Msaada, K., et al. 2012. Effects of extraction solvents and provenances on phenolic contents and antioxidant activities of cumin (Cuminum cyminum L.) seeds. Food and Bioprocess Technology. 5: 2827–2836.
Salvioni, L., Morelli, L., Ochoa, E., Labra, M., Fiandra, L., Palugan, L., et al. 2021. The emerging role of nanotechnology in skincare. Advances in Colloid and Interface Science. 293: 1–23.
Shahidi, F., Janitha, P.K., and Wanasundara, P.D. 1992. Phenolic antioxidants. Critical Reviews in Food Science and Nutrition. 32: 67–103.
Sharma, J.N., Omran, A.A.I., and Parvathy, S.S. 2007. Role of nitric oxide in inflammatory diseases, Inflammopharmacology. 54: 252–259.
Santos, B., Corrêa, M., and Chorilli, M. 2015. Sustainability, natural and organic cosmetics: Consumer, products, efficacy, toxicological and regulatory considerations. Brazilian Journal of Pharmaceutical Sciences. 51: 17–26.
Seo, J.H., Kim, J.E., Shim, J.H., Yoon, G., Bang, M.A., Bae, C.S., et al. 2016. HPLC analysis, optimization of extraction conditions and biological evaluation of Corylopsis coreana Uyeki Flos. Molecules. 21: 94.
Singh, G. and Saxena, R.K. 2017. Phytochemical analysis of Tinospora cordifolia by using different solvent extract, International Journal of Current Research. 9: 61213–61215.
Sukalingam, K. 2018. Preliminary phytochemical analysis and in vitro antioxidant properties of Malaysian ‘Kundang’ (Bouea macrophylla Griffith). Trends in Phytochemical Research. 2: 261–266.
Sun, L., Guo, Y., Zhang, Y., and Zhuang, Y. 2017. Antioxidant and anti-tyrosinase activities of phenolic extracts from rape bee pollen and inhibitory melanogenesis by cAMP/MITF/TYR pathway in B16 mouse melanoma cells. Frontiers in Pharmacology. 8: 104.
Tambun, R., Alexander, V., and Ginting, Y. 2021. Performance comparison of maceration method, soxhletation method, and microwave-assisted extraction in extracting active compounds from soursop leaves (Annona Muricata): a review. IOP Conference Series: Materials Science and Engineering. 1122: 1–7.
Thummajitsakul, S. and Silprasit, K. 2017. Genetic differentiation and antioxidant activities of Bouea macrophylla Griffith in Nakhon Nayok province. Journal of Applied Biological Chemistry. 60: 41–47.
Truong, D.H., Nguyen, D.H., Ta, N.T.A., Bui, A.V., Do, T.H., and Nguyen, H.C. 2019. Evaluation of the use of different solvents for phytochemical constituents, antioxidants, and in vitro anti-inflammatory activities of Severinia buxifolia. Journal of Food Quality. 8178294
Torres-Rodríguez, M.L., Erika-García, C., Berhow, M., and Mejia, E. 2016. Anti-inflammatory and antioxidant effect of Calea urticifolia lyophilized aqueous extract on lipopolysaccharide-stimulated raw 264.7 macrophages. Journal of Ethnopharmacology. 188: 266–274.
Uddin, M.S., Ferdosh, S., Akanda, M., Ghafoor, K., Rukshana, A.H., Ali E., et al. 2018. Techniques for the extraction of phytosterols and their benefits in human health: A review. Separation Science and Technology. 53: 2206–2223.
Vichai, V. andKritara, K. 2006. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nature Protocols. 1: 1112–1116.
Weli, A.M., Salmi, S., Hoqani, H.A., and Hossain, M.A. 2018. Biological and phytochemical studies of different leaves extracts of Pteropyrum scoparium. Beni-Suef University Journal of Basic and Applied Sciences. 7: 481–486.
Wiegand, C. and Hipler, U.C. 2009. Evaluation of biocompatibility and cytotoxicity using keratinocyte and fibroblast cultures. Skin Pharmacology and Physiology. 22: 74–82.
Wu, Y.Y., Li, W., Xu, Y., Jin, E.H., and Tu, Y.Y. 2011. Evaluation of the antioxidant effects of four main theaflavin derivatives through chemiluminescence and DNA damage analyses. Journal of Zhejiang University-SCIENCE B. 12: 744–751.
Yamagishi, S. and Matsui, T. 2011. Nitric oxide, a Janus-faced therapeutic target for diabetic microangiopathy-friend or foe?. Pharmacological Research. 64: 187–194.
Zhang, Q., Lin, L., and Ye, W. 2018. Techniques for extraction and isolation of natural products: A comprehensive review. Chinese Medicine. 13: 1–26.
Zuo, A., Dong, H., Yu, Y., Shu, Q., Zheng, L., and Yu, X. 2018. The anti-tyrosinase and antioxidant activities of flavonoids dominated by the number and location of phenolic hydroxyl groups, Chinese Medicine, 13, 51–63.
Zuorro, A., Iannone, A., and Lavecchia, R. 2019. Water-organic solvent extraction of phenolic antioxidants from brewer’s spent grain. Processes. 7: 126.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand.
Pawarisa Maneechai1, Pimporn Leelapornpisid1, 2, and Worrapan Poomanee 1, 2, *
1 Department of Pharmaceutical Sciences, Faculty of Pharmacy, Chiang Mai University, Chiang Mai 50200, Thailand.
2 Innovation Center for Holistic Health, Nutraceuticals, and Cosmeceuticals, Faculty of Pharmacy, Chiang Mai University, Chiang Mai 50200, Thailand.
Corresponding author: Worrapan Poomanee, E-mail: worrapan.p@cmu.ac.th
Total Article Views
Editor: Wipawadee Yooin; Veerasak Punyapornwithaya
Chiang Mai University, Thailand
Article history:
Received: November 25, 2022;
Revised: February 7, 2023;
Accepted: February 24, 2023;
Published online: March 7, 2023

