
Nutritional and Phytochemical Content of Freeze-Dried Fruits of Two Philippine Bignay (Antidesma bunius (L.) Spreng) Cultivars
Jonina Marie J. Tengco, Liezl M. Atienza*, Dianne Jane A. Sunico, Ann C. Cayetano, Aimee Sheree A. Barrion, Maria Amelita C. Estacio, and Katherine Ann T. Castillo-IsraelPublished Date : April 3, 2023
DOI : https://doi.org/10.12982/NLSC.2023.028
Journal Issues : Number 2, April-June 2023
ABSTRACT
Bignay (Antidesma bunius (L.) Spreng) is a fruit-bearing tree that is indigenous to the Philippines and in different parts of Asia with bignay-common and bignay-kalabaw as the two most locally abundant cultivars. Its fruits are being consumed as food and have been recently studied for its antioxidant properties. However, several chemical constituents of bignay fruit are still yet to be explored. Therefore, this study aimed to determine the vitamins, minerals, and phytochemicals present in bignay-common and bignay-kalabaw fruit. Using HPLC analysis, bignay-kalabaw was observed to have higher vitamin A and E contents than bignay-common. On the other hand, bignay-common was higher in terms of vitamin C content with 25.996 ± 0.688 mg/100 g freeze-dried fruit compared to bignay-kalabaw. Mineral analysis using ICP-OES revealed that bignay-kalabaw fruit had more calcium, copper, and manganese content than bignay-common. Conversely, bignay-common had significantly higher iron content than bignay-kalabaw. Qualitative screening using methanolic extracts revealed that both cultivars possessed 9 out of the 14 phytochemicals tested, namely tannins, saponins, flavonoids, quinones, terpenoids, phenols, coumarins, steroids, and phlobatannins. The findings obtained in the present study indicate that the fruits of these bignay cultivars contain health-promoting compounds and have potential to be used as food supplements. Further in vitro studies for the two bignay cultivars are recommended.
Keywords: Antidesma, ‘Common’, ‘Kalabaw’, Nutritional, Phytochemical
Funding: This study was funded by the Philippine Council for Health Research (PCHRD) and Science Education Institute (SEI) of the Department of Science and Technology (DOST), Philippines.
Citation: Tengco, J.M.J., Atienza, L.M., Sunico, D.J.A., Cayetano, A.C., Barrion, A.S.A., Estacio, M.A.C., and Castillo-Israel,K.A.T. 2023. Nutritional and phytochemical content of freeze-dried fruits of two Philippine bignay (Antidesma bunius (L.) Spreng) cultivars. Nat. Life Sci. Commun. 22(2): e2023028.
INTRODUCTION
Bignay (Antidesma bunius (L.) Spreng), from the Euphorbiaceae family, is a fruit-bearing tree native to the Philippines and other Asian countries. Its fruits grow in clusters and are colored green then turn to red then black upon ripening (Belina-Aldemita et al., 2013). The Philippines has two commonly grown cultivars namely Bignay-common and Bignay-kalabaw, wherein the ‘kalabaw’ cultivar have larger leaf and fruit sizes compared to the ‘common’ cultivar (Castillo-Israel et al., 2020). Bignay fruits are rich sources of several macro- and micronutrients such as vitamins A and C, and minerals like calcium, phosphorus, and iron (FNRI-DOST, 2007), as well as several phytochemicals (Islam et al., 2018). Some studies in the Philippines have also shown functional properties of bignay fruits and leaves, such as its antioxidant (Sartagoda et al. 2021), antimicrobial (Lizardo et al., 2015), and anti-inflammatory (Muñoz et al., 2021). The ‘common’ and ‘kalabaw’ cultivars have also exhibited considerable lipid-lowering activity (Crieta et al., 2021).
Currently, there is a lack of literature on the chemical composition of bignay-common and bignay-kalabaw fruits found in the Philippines, specifically in terms of vitamin, mineral, and phytochemical contents. Therefore, this study aimed to characterize the two cultivars in relation to their nutritional and functional properties to serve as baseline data for future studies and product development of these fruits.
MATERIALS AND METHODS
Plant collection and sample preparation
Around 200 kgs each of fresh fully ripe bignay-common and bignay-kalabaw fruits were harvested from Los Baños, Laguna, Philippines. Fruits were collected from June to August following the harvest season of bignay. The seeds and flesh were then manually separated, and seeds were discarded. The fruit flesh was homogenized, then freeze-dried at 20°C and 40 mTorr pressure using VirTis Co. (Gardiner, NY) for at least 48 hours. After which, the freeze-dried Bignay fruits were crushed using a mortar and pestle, then filtered using an 80-mesh U.S. standard sieve. The freeze-dried samples were stored in metallized bags and stored at -20°C until use.
Vitamin analysis
Vitamin A. Samples were weighed into a 50-ml volumetric flask, followed by 0.3 g ascorbic acid, 20 ml of ethanol, and 1.0 ml of 50% KOH solution. The resulting solution was swirled then refluxed for 45 minutes in a hot water bath at 90°C. After which, the condenser was washed with 2 ml 60:40 acetonitrile (AcN):glacial acetic acid (HAc). The solution was cooled in running water then filled to 50 ml with AcN. The solution was then filtered using a disposable filter membrane into an 8-ml HPLC vial. A standard solution of vitamin A acetate was also prepared using the same procedure. A 20 µl of the standard solution was then injected in the HPLC unit. The vitamin A contents were determined with the wavelength set at 313 nm.
Vitamin C. Samples were first weighed into a 25-ml volumetric flask, then 15 ml of 2% acetic acid was added. The solution was swirled and sonicated for 15 minutes. After, the solution was cooled and filled to a volume of 25 ml with 2% acetic acid. The solution was filtered using a disposable filter membrane into an 8-ml HPLC vial. A standard solution of vitamin C was also prepared using the same procedure. A volume of 20 µl of the standard solution was injected into the HPLC unit and the vitamin C contents of the samples were determined at 280 nm.
Vitamin E. Samples were weighed into a 25-ml volumetric flask, then, 15 ml of methanol was added before sonicating the mixture for 15 minutes. The solution was cooled and brought to a volume of 25 ml with methanol. After which, the solution was filtered using a disposable filter membrane into an 8-ml HPLC vial. A reference standard was also prepared using vitamin E acetate. Twenty (20) µl of the standard solution was injected into the HPLC unit, then the vitamin E contents were determined at 280 nm.
Mineral Analysis
To determine the mineral content, the bignay fruits were analyzed using Inductively Coupled Plasma – Optical Emission Spectroscpy (ICP-OES). The concentrations of the following minerals were determined: macrominerals (calcium, potassium, magnesium), microminerals (boron, chromium, copper, iron, manganese, zinc), and trace metals (lead and nickel). Samples were first prepared using microwave digestion. Using an analytical balance, 0.2500 grams of the freeze-dried samples were accurately weighed, and transferred into the microwave polytetrafluoroethylene (PTFE) vessels. Concentrated nitric acid and 30% hydrogen peroxide were added inside each vessel at a ratio of 7:3 (v/v). The PTFE vessels were hermetically sealed, then placed inside the digester. The digestion program consisted of a 20-min gradual increase in temperature to 200°C from 0°C, a 20-min isothermal step at 200°C (1800 W) and then a ventilated cooling stage. After cooling to room temperature, all the digests were filtered through a Whatman filter paper (grade 42), then collected in plastic vials. All digests were then subjected to ICP-OES for the quantification of minerals and trace metals (Brand: Teledyne Leeman Labs; Model: Prodigy7).
Table 1. ICP-OES operating conditions.
|
Instrument |
Prodigy high-dispersive ICP |
|
Spectrometer |
High resolution echelle polychromator |
|
RF generator |
40 MHz “free running” |
|
Argon flow |
Coolant: 18 L min-1 |
|
Auxiliary: 0.8 L min-1 |
|
|
Nebulizer: 36 psi |
|
|
Nebulizer |
Pneumatic (glass concentric) |
|
Spray chamber |
Glass cyclonic |
|
Hydride generator |
Leeman Labs. Inc. Part No. 130-1070 |
|
Three channel peristaltic pump (0.9 mL min-1) |
|
|
T connector |
|
|
Reaction coil |
|
|
Output power (1.3 kW) |
|
|
Plasma viewing (Radial) |
|
|
Sample uptake delay |
50 s |
|
Integration time |
40 s |
Qualitative Analysis for Phytochemicals
The bignay fruits were screened for the presence of phytochemicals, specifically tannins, saponins, flavonoids, quinones, terpenoids, phenols, coumarins, steroids, phytosteroids, phlobatannins, alkaloids, glycosides, cardiac glycosides, and anthraquinones. The procedures used were that of Sofowora (1993) and Harborne (1973) as cited by Jayapriya and Shoba (2014). Scores (-, +, ++, +++) were recorded and given based on the intensity of the color (Surmaghi et al., 1992).
Sample preparation. Methanol extracts were prepared using freeze-dried samples of bignay-common and bignay-kalabaw. Two (2) grams of the sample were added to approximately 5 ml of 50:50 w/v methanol: distilled water solution. The resulting solution was placed in an orbital mechanical shaker for 10 minutes before placing in a centrifuge at 3000 rpm for 5 minutes. After which, the supernatant was decanted then filtered with Whatman filter paper (Shimada et al., 1992). The analysis was carried out in triplicates.
Test for tannins. To 1 ml of the berry extract, 2 ml of 5% ferric chloride was added. The formation of a dark blue or greenish black indicates the presence of tannins. Scores were noted as: no color development = negative (-); slightly dark blue or greenish black color = weakly positive (+); distinct dark blue or greenish black color = positive (++); heavy dark blue or greenish black color = strongly positive (+++).
Test for saponins. To 2 ml of the berry extract, 2 ml of distilled water was added before shaking in a test tube lengthwise for 15 minutes. The formation of a 1 cm layer of foam indicates the presence of saponins. Scores were recorded as follows: no foam = (-); foam at least 1 cm = (+); foam more than 1 cm but less than 2 cm high = (++); and foam greater than 2 cm high = (+++).
Test for flavonoids. To 2 ml of the berry extract, 1 ml of 2N sodium hydroxide was added. The formation of yellow color indicates the presence of flavonoids. Scores were noted as: no color development = (-); slight yellow color = (+); distinct yellow color = (++); heavy yellow color = (+++).
Test for alkaloids. To 2 ml of the berry extract, 2 ml of concentrated hydrochloric acid was added, followed by the addition of a few drops of Mayer’s reagent. The presence of green color or white precipitate indicates the presence of alkaloids. Scores were recorded as: no color development = (-); slight green color and opaqueness = (+); distinct turbidity and green color, white precipitate = (++); heavy green color and heavy precipitate produced = (+++).
Test for quinones. To 1 ml of the extract, 1 ml of concentrated sulfuric acid was added. The formation of a red color indicates the presence of quinones. Scores were noted as: no color development = (-); slight reddish color = (+); distinct red color = (++); heavy red color = (+++).
Test for glycosides. To 0.5 ml of the berry extract, 3 ml of chloroform and 10% ammonia solution were added. The formation of a pink color indicates the presence of glycosides. Scores were recorded as: no color development = (-); slight pinkish color = (+); distinct pink color = (++); heavy pink color = (+++).
Test for cardiac glycosides. To 0.5 ml of the extract, 2 ml of glacial acetic acid and a few drops of 5% ferric chloride were added. This was under layered with 1 ml of concentrated sulfuric acid. The formation of a brown ring at the interface indicates the presence of cardiac glycosides. Scores were recorded as: no ring formation = (-); slight, undefined ring formation = (+); distinct brown ring formation = (++); heavy brown ring formations = (+++).
Test for terpenoids. To 0.5 ml of the extract, 2 ml of chloroform was added followed by the careful addition of concentrated sulfuric acid. The appearance of a red brown color at the interface indicates the presence of terpenoids. Scores were recorded as: no color development = (-); slight reddish-brown color = (+); distinct red-brown color = (++); heavy red-brown color = (+++).
Test for phenols. To 1 ml of the extract, 2 ml of distilled water was added followed by a few drops of 10% ferric chloride. The formation of blue or green color indicates the presence of phenols. Scores were recorded as: no color development = (-); slight bluish-greenish color = (+); distinct blue or green color = (++); heavy blue or green color = (+++).
Test for coumarins. To 1 ml of the berry extract, 1 ml of 10% NaOH was added. The formation of yellow color indicates the presence of coumarins. Scores were recorded as: no color development = (-); slight yellow color = (+); distinct yellow color = (++); heavy yellow color = (+++).
Test for steroids and phytosteroids. To 1 ml of the extract, 1 ml of chloroform was added followed by a few drops of concentrated sulfuric acid. The formation of brown and bluish rings indicates the presence of steroids and phytosteroids, respectively. Scores were recorded as: no ring formation = (-); slight brown or bluish rings = (+); formation of distinct brown or bluish rings = (++); heavy brown or bluish rings = (+++).
Test for phlobotannins. To 1 ml of the plant extract, a few drops of 10% ammonia solution were added. Appearance of a red-colored precipitate confirms the presence of phlobotannins. Scores were recorded as: no precipitate = (-); slight formation of red precipitate = (+); distinct red precipitate = (++); heavy red precipitate = (+++).
Test for anthraquinones. To 1 m of the extract, a few drops of 10% ammonia solution were added. The appearance of a pink-colored precipitate indicates the presence of anthraquinones. Scores were recorded as: no color development = (-); slight pinkish color = (+); distinct pink color = (++); heavy pink color = (+++).
Statistical Analysis
All chemical analyses were performed in triplicates and results were expressed as means ± standard error of the mean (SEM). For the data on nutritional and functional properties of freeze-dried bignay fruits, independent-t-test was performed. Data were analyzed using Minitab 19 Statistical Software for Windows (Minitab 19.0, Minitab LLC).
RESULTS
Vitamin content of Bignay-Common and Bignay-Kalabaw freeze-dried fruits
In terms of vitamin A and E content, bignay-kalabaw had significantly higher concentrations (P <0.05) than bignay-common per 100 g freeze-dried fruit (Figure 1). Meanwhile, bignay-common had a higher ascorbic acid content than bignay-kalabaw. Nonetheless, differences in the vitamin C content of the two cultivars were not statistically significant.
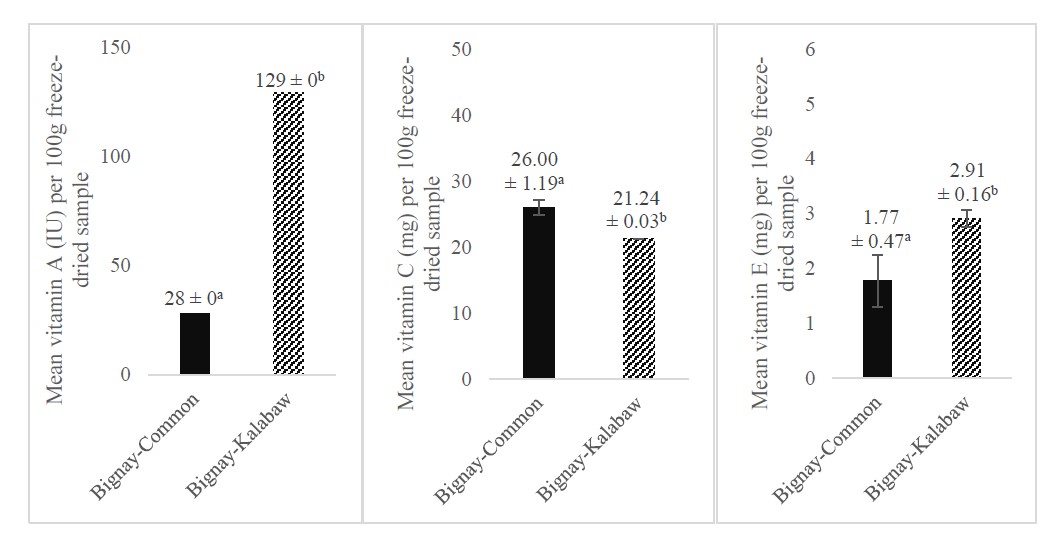
Figure 1. Mean vitamins A (IU), C (mg), and E (mg) contents of bignay-common and bignay-kalabaw per 100g freeze-dried fruit. Means with different superscripts indicate significant differences between the two cultivars at P <0.05. Error bars indicate SD.
Mineral content of Bignay-Common and Bignay-Kalabaw freeze-dried fruits
For this study, macrominerals composed of calcium, potassium, and magnesium while microminerals include boron, chromium, copper, iron, manganese, and zinc. This study also quantified two trace metals, namely, nickel and lead.
Among macrominerals, potassium had the highest concentration for both bignay cultivars wherein bignay-common had a significantly higher content than bignay-kalabaw per 100 g freeze-dried fruit at P <0.05 (Figure 2). On the other hand, bignay-kalabaw had a higher magnesium content of 119.792 ± 0.900 mg and a significantly higher calcium content of 203.665 ± 1.770 mg (P <0.05) than bignay-common (116.992 ± 0.792 mg and 170.117 ± 0.766 mg, respectively).
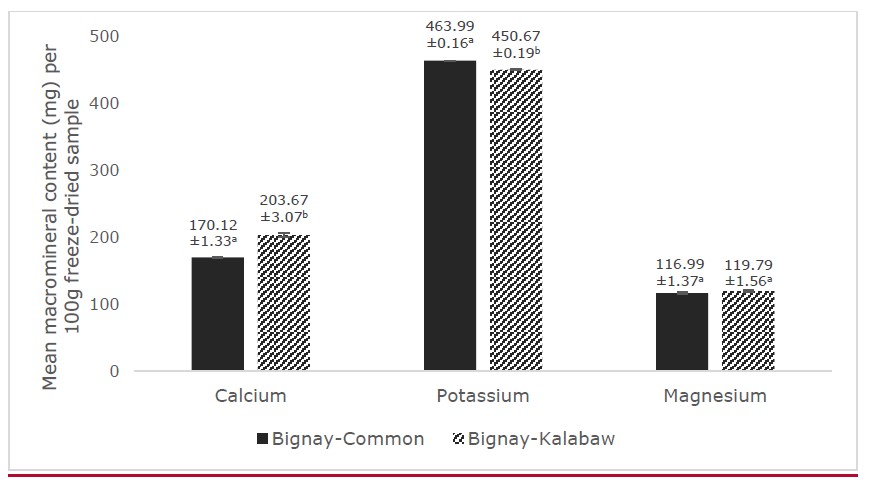
Figure 2. Mean macromineral content (calcium, potassium, and magnesium) of freeze-dried bignay-common and bignay-kalabaw (mg/100g). Means with different superscripts indicate significant differences between the two cultivars at P <0.05. Error bars indicate SD.
In terms of microminerals, iron was highest in concentration while zinc was lowest for both bignay cultivars. Bignay-common was found to be higher in boron (1.318 ± 0.014 mg), chromium (1.12 ± 0.010 mg), zinc (0.828 ± 0.008 mg), and iron (6.653 ± 0.011 mg) content while bignay-kalabaw was superior in terms of copper (2.806 ± 0.015 mg) and manganese (2.687 ± 0.020 mg) per 100 g freeze-dried fruit (Figure 3). Notably, significant differences at P <0.05 were detected between the two cultivars across all microminerals.
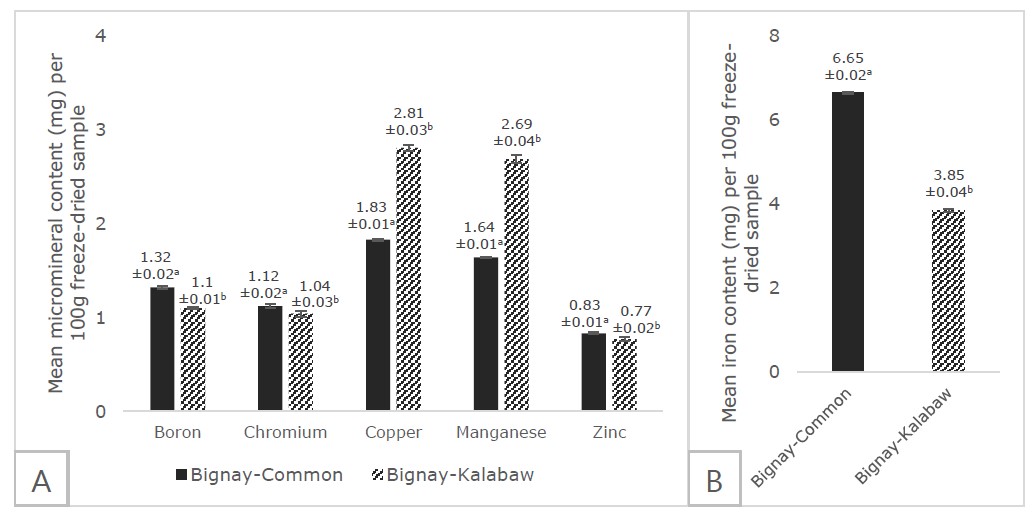
Figure 3. Mean micromineral content (A: boron, chromium, copper, manganese, and zinc; B: iron) of freeze-dried bignay-common and bignay-kalabaw (mg/100g). Means with different superscripts indicate significant differences between the two cultivars at P <0.05. Error bars indicate SD.
In Figure 4, results on the heavy metal analysis revealed that lead content was higher in bignay-common (0.075 ± 0.000 mg/100 g freeze-dried fruit) but bignay-kalabaw had a higher nickel content (0.311 ± 0.000 mg/100 g freeze-dried fruit). In the present study, the levels of these trace metals in both fruit cultivars were below the permissible limits of 0.05 mg/kg for lead (Afonne and Ifediba, 2020) and 0.5 mg/kg for nickel (Lanre-Iyande and Adekunle, 2012) indicating that the heavy metals were not absorbed in bignay-common and bignay-kalabaw fruits and thus, do not pose heavy metal toxicity.
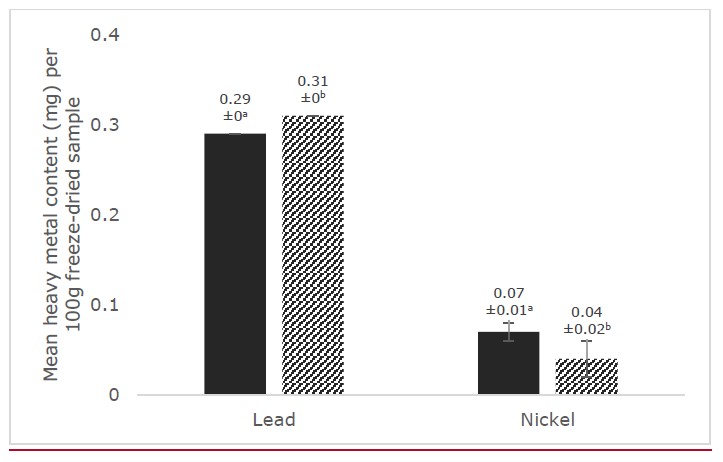
Figure 4. Mean heavy metal content (lead and nickel) of freeze-dried bignay-common and bignay-kalabaw (mg/100g). Means with different superscripts indicate significant differences between the two cultivars at P <0.05. Error bars indicate SD.
Table 2. Percent mineral component (%) provided by 100g freeze-dried bignay-common and bignay-kalabaw fruits for Filipino males and females aged 19 years and older based on the Philippine Dietary Reference Intakes 2015.
|
Mineral component |
Bignay-common (%) |
Bignay-kalabaw (%) |
||
|
Male |
Female |
Male |
Female |
|
|
Iron |
55 |
24 |
32 |
14 |
|
Zinc |
13 |
18 |
12 |
17 |
|
Calcium |
23 |
23 |
27 |
27 |
|
Magnesium |
49 |
56 |
50 |
57 |
|
Potassium |
23 |
23 |
23 |
23 |
Phytochemical profile of Bignay-common and Bignay-kalabaw freeze-dried fruits
Phytochemicals are non-nutritive, bioactive plant constituents that have the potential to be incorporated into food or food supplements as nutraceuticals because of their health benefits (Dillard and German, 2000). The screening of certain phytochemicals in bignay-common and bignay-kalabaw fruits showed that both cultivars have tannins, saponins, flavonoids, quinones, terpenoids, phenols, coumarins, steroids, and phlobatannins. Notably, a more intense color was observed in bignay-common compared to bignay-kalabaw in the phenol test, possibly suggesting a higher concentration of phenols in the ‘common’ cultivar.
Table 3. Qualitative phytochemical profile of freeze-dried bignay-common and bignay-kalabaw fruits.
|
Phytochemical |
Bignay-common |
Bignay-kalabaw |
|
Tannins |
+ |
+ |
|
Saponins |
+ |
+ |
|
Flavonoids |
+++ |
+++ |
|
Alkaloids |
- |
- |
|
Quinones |
+++ |
+++ |
|
Glycosides |
- |
- |
|
Cardiac glycosides |
- |
- |
|
Terpenoids |
+++ |
+++ |
|
Phenols |
++ |
+ |
|
Coumarins |
+++ |
+++ |
|
Steroids |
+ |
+ |
|
Phytosteroids |
- |
- |
|
Phlobatannins |
+ |
+ |
|
Anthraquinones |
- |
- |
Note: (+) = presence of phytochemicals, (-) = absence of phytochemicals
DISCUSSION
In this study, vitamin A, C, and E contents of bignay-common and bignay-kalabaw were analyzed. These are also termed as antioxidant vitamins, which are known to counteract the effects of reactive oxygen and nitrogen species that are mostly formed by stress conditions in inflammation, diseases, unsuitable heat conditions, and long-term exercises, among others (Koekkoek and van Zanten, 2016). The formation and accumulation of these ROS leads to oxidative stress in tissues which causes cell membrane damage (Erol et al., 2019). Hence, adequate supply of these nutrient-derived antioxidants may reduce the risk of developing chronic diseases (Khadim and Al-Fartusie, 2020).
Besides cultivar variations, differences in vitamin A content can be due to higher light intensity in the Bignay-kalabaw plant, as previous research in two goji berry cultivars showed that exposure to sunlight enhanced biosynthesis which can increase carotenoid content (Kulaitiene et al., 2020). Similarly, a positive correlation between sunlight exposure and carotenoid concentration was observed in apples (Merzlyak et al., 2002).
Findings showed that 100g of freeze-dried bignay-common fruit can provide approximately 1% of vitamin A, 37% of vitamin C, and 18% of vitamin E recommended nutrient intake (RNI) of a typical adult aged 19 years old and above (FNRI-DOST, 2015) while the same amount of freeze-dried bignay-kalabaw fruit will provide 6% of vitamin A, 33% of vitamin C, and 29% of vitamin E. With this, it is assumed that both cultivars are rich sources of the antioxidant vitamins C and E. According to the USFDA (2022), if a food meets 20% of the % DV per serving, it is considered high in that nutrient. Hence, with reference to the Philippine Dietary Reference Intakes (PDRI), both bignay cultivars are high in vitamin C, while bignay-kalabaw is also high in vitamin E.
From the mineral content analysis, it was noted that 100g of freeze-dried bignay-common and bignay-kalabaw fruits provide considerable percentages of the daily RNI of iron, zinc, calcium, magnesium, and potassium for Filipino adults aged 19 years and older (Table 2), making them good sources of these minerals. Both ‘common’ and ‘kalabaw’ bignay fruits are rich sources of calcium, magnesium, potassium, iron, and zinc based on the present study, implying that they have the potential to be developed as food supplements. However, extensive studies on their biochemical profile must still be done since it is possible that the bignay fruits contain other compounds that may affect the bioavailability or absorption of these vitamins and minerals.
The qualitative results of the phenol test in this study corroborates with quantitative studies on the total phenolic content (TPC) of the two cultivars, wherein the TPC of the ‘common’ cultivar was more than twice that of the ‘kalabaw’ cultivar (Castillo-Israel et al., 2020; Sartagoda et al., 2021). Furthermore, the phytochemicals detected in the methanolic extracts of bignay-common and bignay-kalabaw are known to have medicinal importance. For example, tannins were previously demonstrated to lower blood sugar levels (Kumari and Jain, 2015) and improve vascular health (Ashok and Upadhyaya, 2012) while phlobatannins and terpenoids have exhibited antimicrobial and antiviral activities, respectively (Zahara et al., 2019). Evidence suggests that saponins possess hypolipidemic properties by inhibiting cholesterol absorption from the intestinal lumen, which consequently reduces the concentration of plasma cholesterol (Khan et al., 2015). Supplementation of citrus flavonoids were also observed to have anti-atherogenic effects in rabbits fed a high-cholesterol diet (Lee et al., 2001). Meanwhile, quinones plays a crucial role in the conversion of carbohydrates and fatty acids into ATP (Crane, 2001) and acts as an antioxidant by protecting circulating lipoproteins including LDL from oxidation (Tsai et al., 2011). Coumarins, on the other hand, were previously documented to exert anti-inflammatory (Kontogiorgis and Hadjipavlou-Litina, 2005) and anticoagulant (Kidane et al., 2004) activities. These phytochemical compounds may have been responsible for the biological activities of bignay and the reason for their use as traditional medicine. With the various phytochemicals present in both cultivars, it may be useful to quantify these in order to determine the extent of the effects of their presence in bignay fruits. The study remains to only quantify vitamins and minerals present in bignay fruits.
CONCLUSION
The study presented significant nutritional and phytochemical content of bignay (Antidesma bunius (L.) Spreng) fruit cultivars, bignay-common and bignay-kalabaw. Vitamin analysis showed that both cultivars are rich sources of vitamins C and E where 100g of the freeze-dried fruits can supply 33-37% and 18-29% of the vitamins, respectively, of the daily RNI for Filipino adults aged 19 years and older. These bignay fruits are also good sources of minerals, specifically iron, zinc, calcium, potassium, magnesium, and potassium, providing 12-57% of the daily RNI of Filipino adults of the same age group. Qualitative phytochemical screening using methanolic fruit extracts revealed the presence of tannins, saponins, flavonoids, quinones, terpenoids, phenols, coumarins, steroids, and phlobatannins. Hence, the medicinal properties of bignay fruits may be attributed to these phytochemical compounds. Paired with existing quantitative studies on the phytochemical content of bignay-common and bignay-kalabaw, the present study concludes that these bignay fruit cultivars have high amounts of vitamins, minerals, and phytochemicals, and thus have the potential to be further developed as food supplements or used as a functional food ingredient. It is recommended that further studies be done to quantify the phytochemicals present and to determine their possible interactions with other compounds in the fruit which may affect its bioavailability and bioaccessibility.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Afonne, J. and Ifediba, E. 2020. Heavy metals risks in plant foods - Need to step up precautionary measures. Current Opinion in Toxicology. 22: 1-6.
Ashok, P.K. and Upadhyaya, K. 2012. Tannins as astringent. Journal of Pharmacognosy and Phytochemistry. 1(3): 45-50.
Belina-Aldemita, M.D., Sabularse, V.C., Dizon, E.I., Hurtada, W.A., and Torio, M.A.O. 2013. Physicochemical properties of bignay (Antidesma bunius (L.) Spreng) wine at different stages of processing. Philippine Science Letters. 6(2): 249-256.
Castillo-Israel, K.A.T., Sartagoda, K.J.D., Ilano, M.C.R., Flandez, L.E.L., Compendio, M.C.M., and Morales, D.B. 2020. Antioxidant properties of Philippine Bignay (Antidesma bunius (Linn.) Spreng cv. ‘Common’) flesh and seeds as affected by fruit maturity and heat treatment, Food Research. 4(6): 1980-1987.
Crane, F.L. 2001. Biochemical functions of coenzyme Q10. Journal of the American College of Nutrition. 20(6): 591-598.
Crieta, B.R.A., Tuaño, A.P.T., Torio, M.A.O., Villanueva, J.C., Gaban, P.J.V., Castillo-Israel, K.A.T. 2021. In vitro lipid-lowering properties of the fruits of two Bignay [Antidesma bunius (L.) Spreng] cultivars as affected by maturity stage and thermal processing. Food Chemistry: Molecular Sciences, 2: 100020.
Dillard, C.J. and German, J. 2000. Phytochemicals: nutraceuticals and human health. Journal of the Science of Food and Agriculture. 80(12): 1744–1756.
Erol, N., Saglam, L., Saglam, Y.S., Erol, H.S., Altun, S., Aktas, M.S., and Halici, M.B. 2019. The protection potential of antioxidant vitamins against acute respiratory distress syndrome: A rat trial. Inflammation. 42(5): 1585-1594.
[FNRI-DOST] Food and Nutrition Research Institute-Department of Science and Technology. 2007. Philippine Food Composition Tables. Department of Science and Technology: Taguig City, Philippines.
[FNRI-DOST] Food and Nutrition Research Institute-Department of Science and Technology. 2015. Philippine Dietary Reference Intakes. Department of Science and Technology: Taguig City, Philippines.
Harborne, I.B. 1973. Phytochemical methods: A guide to modern techniques of plant analysis. 2nd ed. Chapman and Hall, New York. 88-185.
Islam, M.S., Ahammed, S., Sukorno, F.I., Koly, S., Biswas, M., and Hossain, S. 2018. A review on phytochemical and pharmacological potentials of Antidesma bunius. Journal of Analytical and Pharmaceutical Research. 7(5): 602-604.
Jayapriya, G. and Shoba, F.G. 2014. Evaluation of Gambusia affinis and Bacillus thuringiensis var. israelensis as Culex quinquefasciatus Control Agents. Journal of Entomology and Zoology Studies. 2(3):121-125.
Khadim, R.M. and Al-Fartusie, F.S. 2020. Antioxidant vitamins and their effect on immune system. Journal of Physics: Conference Series. 1853(1): 1-13.
Khan, N., Akhtar, M.S., Khan, B.A., Braga, V.D.A., and Reich, A. 2015. Antiobesity, hypolipidemic, antioxidant, and hepatoprotective effects of Achyranthes aspera seed saponins in high cholesterol fed albino rats. Archives of Medical Science. 11(6): 1261-1271.
Kidane, A.G., Salacinski, H., Tiwari, A., Bruckdorfer, K.R., and Seifalian, A.M. 2004. Anticoagulant and antiplatelet agents: Their clinical and device application(s) together with usages to engineer surfaces. Biomacromolecules. 5(3): 798-813.
Koekkoek, W.A.C. and Van Zanten, A.R.H. 2016. Antioxidant vitamins and trace elements in critical illness. Nutrition in Clinical Practice. 31(4): 457-474.
Kontogiorgis, C.A. and Hadjipavlou-Litina, D. 2005. Synthesis anti-inflammatory activity of coumarin derivatives. Journal of Medicinal Chemistry. 48(20): 6400-6408.
Kulaitiene, J., Vaitkeviciene, N., Jariene, E., Cerniauskiene, J., Jeznach, M., and Paulauskiene, A. 2020. Concentrations of minerals, soluble solids, vitamin C, carotenoids and toxigenic elements in organic goji berries (Lycium barbarum L.) cultivated in Lithuania. Biological Agriculture & Horticulture, 36(2): 130-140.
Kumari, M. and Jain, S. 2012. Tannins: An antinutrient with positive effect to manage diabetes. Research Journal of Recent Sciences. 1(12): 1-8.
Lanre-Iyande, T.Y. and Adekunle, I.M. 2012. Assessment of heavy metals and their estimated daily intakes from two commonly consumed foods (Kulikuli and Robo) found in Nigeria. African Journal of Food, Agriculture, Nutrition and Development. 12(3): 6156-6169.
Lee, C.H., Jeong, T.S., Choi, Y.K., Hyun, B.H., Oh, G.T., Kim, E.H., Kim, J.R., Han, J.I., and Bok, S.H. 2001. Anti-atherogenic effect of citrus flavonoids, naringin and naringenin, associated with hepatic ACAT and aortic VCAM-1 and MCP-1 in high cholesterol-fed rabbits. Biochemical and Biophysical Research Communications. 284(3): 681–688.
Lizardo, R.C.M., Mabesa, L.B., Dizon, E.I., and Aquino, N.A. 2015. Functional and antimicrobial properties of bignay [Antidesma bunius (L.) Spreng.] extract and its potential as natural preservative in a baked product. Int. Food Research Journal. 22(1): 88-95.
Merzlyak, M.N., Solovchenko, A.E., and Chivkunova, O.B. 2002. Patterns of pigment changes in apple fruits during adaptation to high sunlight and sunscald development. Plant Physiology and Biochemistry. 40: 679-684.
Muñoz, M.N.M., Alvarado, U.G., Reyes, J.I.L., and Watanabe, K. 2021. Acute oral toxicity assessment of ethanolic extracts of Antidesma bunius (L.) Spreng fruits in mice. Toxicology Reports. 8: 1289-1299.
Sartagoda, K.J.D., Ilano, M.C.R., Flandez, L.E.L., and Castillo-Israel, K.A.T. 2021. Evaluation of the antioxidant activity of Bignay (Antidesma bunius (Linn.) Spreng var. Kalabaw) flesh and seeds as affected by maturity and processing method. Chiang Mai University Journal of Natural Sciences. 20(2): e2021042.
Shimada, K., Fujikawa, K., Yahara, K., Nakamura, T. 1992. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. Journal of Agricultural and Food Chemistry. 40: 945-948.
Sofowora, A. 1993. Phytochemical Screening of Medicinal Plants and Traditional Medicine in Africa Edition. Spectrum Books Ltd., Nigeria, 150-156.
Surmaghi, M.S., Amin, Y.A.G., and Mahmoodi, Z. 1992. Survey of Iranian plants for saponins, alkaloids, flavonoids, and tannins. IV. DARU Journal of Pharmaceutical Sciences. 2(2-3): 1-11.
Tsai, K.L., Chen, L.H., Chiou, S.H., Chiou, G.Y., Chen, Y.C., Chou, H.Y., Chen, L.K., Chen, H.Y., Chiu, T.H., Tsai, C.S., Ou, H.C., and Kao, C.L. 2011. Coenzyme Q10 suppresses oxLDL-induced endothelial oxidative injuries by the modulation of LOX-1-mediated ROS generation via the AMPK/PKC/NADPH oxidase signaling pathway. Molecular Nutrition & Food Research. 55(Suppl 2): S227–S240.
[USFDA] U.S. Food and Drug Administration. 2022. Daily Value on the New Nutrition and Supplement Facts Labels. Retrieved from https://www.fda.gov/food/new-nutrition-facts-label/daily-value-new-nutrition-and-supplement-facts-labels on 24 January 2023.
Zahara, K., Ahmad, N., Bibi, Y., Bibi, F., Sadaf, H.M., and Sardar, N. 2019. An insight to therapeutic potential and phytochemical profile of Solanum villosum (L.). Medicine in Drug Discovery. 2: 1-14.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand.
Jonina Marie J. Tengco1, 2, Liezl M. Atienza1, *, Dianne Jane A. Sunico1, 2, Ann C. Cayetano1, Aimee Sheree A. Barrion1, Maria Amelita C. Estacio3, and Katherine Ann T. Castillo-Israel4
1 Institute of Human Nutrition and Food, College of Human Ecology, University of the Philippines Los Baños (UPLB), Laguna 4031, Philippines.
2 Department of Science and Technology– Science and Education Institute, DOST Compound, Bicutan, Taguig City 1631, Philippines.
3 Department of Basic Veterinary Sciences, College of Veterinary Medicine, UPLB, Laguna 4031, Philippines.
4 Institute of Food Science and Technology, College of Agriculture and Food Science, UPLB, Laguna 4031, Philippines.
Corresponding author: Liezl M. Atienza, E-mail: lmatienza@up.edu.ph
Total Article Views
Editor: Pachara Sattayawat,
Chiang Mai University, Thailand
Article history:
Received: September 2, 2022;
Revised: February 3, 2023;
Accepted: February 20, 2023;
Published online: March 7, 2023

