
Production, Characterization and Optimization of Red Pigment Echinenone Produced by Micrococcus sp., Isolated from Soil
Savinay K Jain, Dhamodhar Prakash*, Akash S., and Hema J NPublished Date : April 3, 2023
DOI : https://doi.org/10.12982/NLSC.2023.025
Journal Issues : Number 2, April-June 2023
ABSTRACT
In recent years, natural pigments are replacing synthetic colourants due to their undesirable side effects. Microorganisms are a major source of natural colours and can produce a variety of pigments that have high value in food and textile industries. In the present work, the isolation of a novel microbe capable of producing a stable and high intense red pigment and its optimization is attempted. In this study, purification, characterization and optimization of pigment production by a bacterium isolated from the anthill soil are reported. The bacterial isolate was biochemically identified as Micrococcus sp., and was confirmed with 16S rRNA gene sequencing. The effects of carbon and nitrogen source, temperature, pH and oxygen availability on pigment production were investigated. Optimum growth was observed with a pH 6 and 5% glycerol and incubation at 28°C at 220 RPM. Three pigments were extracted and purified by column chromatography. The extracted red pigment was characterized using UV-visible, Mass and NMR spectroscopy and was confirmed as the carotenoid, echinenone (beta, beta-Caroten-4-one). After optimization, a yield of 25.98 mg/L of total carotenoid was produced by Micrococcus sp. The extracted carotenoid pigment can be used as natural colouring agent in food and textile industry.
Keywords: Microbial pigments, Carotenoids, Micrococcus, Natural dyes, Eco-friendly
Citation: Savinay, K.J., Prakash, D., Akash, S., and Hema, J.N. 2023. Production, characterization and optimization of red pigment Echinenone produced by Micrococcus sp., isolated from soil. Nat. Life Sci. Commun. 22(2): e2023025.
INTRODUCTION
Synthetic dyes are commonly used globally in food, cosmetics, pharmaceutical and textile industries because they are cheap, easy to produce and exhibit superior colouring properties (Aman Mohammadi et al., 2021). Especially in the food industry, synthetic colour additives are added to many products which make them look more attractive and tastier. However, various synthetic dyes and pigments used in these industries are harmful, have deleterious side effects and are carcinogenic in nature (Azman et al., 2018; Sen et al., 2019; Chatragadda and Dufosse, 2021; Dey and Nagababu, 2022). Because of these disadvantages of synthetic dyes, people are moving towards natural colours and dyes which are safer than synthetic dyes (Rostami et al., 2016). Several plants, bacteria, fungi, algae and insects produce natural colours and pigments such as canthaxanthin, astaxanthin, prodigiosin, phycocyanin, violacein, riboflavin, beta-carotene, melanin and lycopene, which are non-toxic, edible and eco-friendly (Azman et al., 2018; Bhagwat et al., 2020). In addition, these natural pigments have been reported to exhibit antibacterial, antiviral, antioxidant and anti-carcinogenic activities. Natural pigments extracted from many plants and microalgae are widely employed in various food industries, but have some disadvantages like low stability and solubility, and limited availability due to seasonal variations (Shetty et al., 2021). Alternatively, microorganisms are associated with food and are responsible for certain special high value industrial products from fermentation like single cell protein, pigments, vitamins and enzymes (Kushwaha et al., 2014).
Microbial pigments are advantageous over the natural colours and pigments obtained from other sources because their production is not affected by climatic changes and seasonal variations, are easy to grow and exhibits high growth rate, downstream processing and extraction of pigments are comparatively easier and grows in cheap substrates (Dawoud et al., 2020; Chaudhary et al., 2022). Ideally, a pigment producing microbe must be capable of utilizing a wide range of carbon and nitrogen sources, must be tolerant to pH, temperature and minerals and give a better yield (Kushwaha et al., 2014; Butnariu, 2016). The fermentation process involved in microbial pigment production is much faster and has more productivity compared to any chemical process. Due to these advantages, the wide varieties of pigments produced by the microorganisms have a high scope, especially in food, cosmetic and textile industries as natural dyes. Bacterial pigments include melanin, quinones, carotenoids, indigo, violacein, and prodigiosin, of which carotenoids are widely studied (Tran et al., 2020). Carotenoids are widely used in the food, cosmetic, healthcare, pharmaceutical and nutraceutical industries (Sathasivam and Ki, 2018; Saini and Keum, 2019; Venil et al., 2020, Maoka, 2020). The microbes like Streptomyces aureofaciens, Xanthomonas oryzae, Pacilmycesfarinosus, Allochromatium vinosum, Allochromatium warmingii produces a different variety of carotenoids in different colour shades (Kushwaha et al., 2014; Galasso et al., 2017). Carotenoids are a group of natural tetraterpenoid pigments which are classified into the carotenes and the xanthophylls. Echinenone and canthaxanthin are important carotenoid xanthophylls pigments with food and industrial applications. These natural pigments serve as an effective alternate for synthetic colourants.
The wide range of applications and increasing use of carotenoids, especially in food industry for improving food quality has led to a surge in their demand in the global market (Chronis et al., 2021). Consequently, microorganisms isolated from various sources and their ability for carotenoid and other pigment production and their wide applications are being regularly explored. Soil and marine sources are the most commonly explored in search of these novel pigment producing microorganisms (Velmurugan et al., 2020). Presently the researchers are looking for novel strains of pigment producing microorganisms, improved extraction and purification methodologies and new applications of these microbial pigments (Chatragadda and Dufosse, 2021). In the present study the extraction, purification, optimization and characterization of the pigment carotenoid obtained from a novel Micrococcus sp. isolated from soil is reported.
MATERIALS AND METHODS
Isolation and identification
A total of 3 soil samples were collected from different locations on a same anthill. The soil was taken at a depth of 5 cm. One gram of soil sample was mixed with 9 ml of 0.9 % NaCl solution and serially diluted up to 10-10 and further inoculated on nutrient agar and incubated for 24 h. After growth, a loop full of pure colony was then sub cultured and plated on Trypticase soy agar (TSA) plates. Pure colonies from TSA plates were picked and subjected to biochemical tests such as Gram staining, catalase, indole and starch hydrolysis tests for identification (Shivaji et al., 1988). The isolate was stored in glycerol stock at −80 °C, until further studies.
Further the presumptively identified microorganism was subjected to 16S rRNA gene sequencing. The DNA extraction was carried out using Phenol-chloroform extraction method and amplified using PCR Thermocycler. The fragment of the 16S rRNA gene was amplified (Church et al., 2020). The PCR amplicon was further subjected to purification in order to remove contaminants. Using 16S rRNA-F and 16S rRNA-R primers, the amplified DNA fragment was sequenced with a BDT v3.1 Cycle sequencing kit on Applied Bio systems 3730xl Genetic Analyzer. Aligner software was used to generate the consensus sequence of the 16S rRNA gene.
Growth kinetics and pigment production
One litre of TSB adjusted to a pH of 6.5 was prepared and autoclaved at
121 °C, 15 PSI for 20 min and inoculated with 5 % of overnight culture (prepared in 50 ml TSB flask) and incubated at 28 °C for 72 hours. At a periodic interval of one hour, a sample volume of 10 ml was taken for further analysis. Out of 10 ml, 1 ml of sample was taken for measuring the optical density of the broth at 600 nm. Wet cell weight and total carotenoid were calculated (Hornero and Mínguez, 2001).
X = X0eµt
τ = ln2/µ
X = biomass concentration of the cell.
T = time for growth.
τ = Doubling time
µ=Specific growth rate
ln2=0.69314
Determination of wet cell weight
3 ml of sample was taken in a dry weighed tube and weight of the sample was recorded and centrifuged at 12,000 rpm for 5 min and the weight the of pellet was noted. Wet cell weight was calculated as follows.
Wet cell weight = ([Pellet weight] / [Broth weight]) x 1000
Determination of total carotenoid (TC)
The carotenoid was measured spectrophotometrically.6 ml of sample was subjected for solvent extraction. The crude methanol fraction extracted from microbial fermentation was used for the estimation of total carotenoid. The absorbance was measured at 472 and 508 nm and total carotenoid was determined as follows.
CY = (Abs472 x 1724.3)-(Abs508 x 2450.1)
270.9
CR = (Abs508 x 2144.0)-(Abs472 x 403.3)
270.9
Total Carotenoid (µg/ml) =CY+CR
Where CY and CR represent the yellow and red isochromatic families, respectively.
Optimization of parameters influencing pigment production
The effects of carbon and nitrogen source, temperature, pH and oxygen availability on pigment production were studied and optimized. One-factor-at-a-time (OFAT) was adopted for optimization.
Pigment production, extraction and purification
Trypticase soy broth (TSB) was used as media for the routine cultivation of microorganisms isolated from soil. One litre of TSB media was inoculated with seed culture and incubated at 28 °C for 48 h. Cells were harvested, by centrifugation at 4,700 rpm for 30 min. To the pellet, an equal volume of methanol was added and mixed for 30 min for higher pigment extraction, followed by centrifugation at 4,700 rpm for 30 min (Athira et al., 2016). Crude methanol extracts were partitioned against petroleum ether (1:1) v/v mixing was done for 3h for complete extraction of pigments and were left undisturbed for about 5 h. The (ether) epiphase was dried, concentrated by a rotary evaporator and applied to columns of silica gel for pigment extraction (Surekha et al., 2016). From hypophase (Methanol) the moisture content was removed by lyophilisation method and purified by thin layer chromatography for pigment extraction (Jagannadham et al., 1991).
Purification by column chromatography
The purification of the extracted pigment was carried out in column chromatography using silica gel (60-120 mesh size) and was eluted initially with petroleum ether (flow rate was 1 ml/min). The polarity of the solvent was decreased subsequently by the addition of 3-5 % of acetone in petroleum ether. The percentage of acetone was increased by 6-100 %, followed by polar solvent methanol. The coloured fraction was collected.
Purification by preparative thin layer chromatography
Thin layer chromatography was employed for the analysis and identification of the hypophase pigment. Purification was carried on silica gel of 10 micron which was cast on preparative TLC plates and oven-dried. A suitable solvent system containing ethyl acetate: methanol (9:1) (v/v) was designed by the trial-and-error method. The retention factor was determined.
Characterization of pigment
The pigment extracted and purified was further characterized. UV/Visible spectroscopy was used for the characterization of the pigments. Absorption spectra of pigments were taken using a UV-Vis bio spectrophotometer (V-730 double band UV-Vis spectrophotometer) between 250 – 700 nm. Mass spectra of pigments were recorded on MS-Shimadzu 2020 signal Quadrupole mass spectrometer. AV400-Burker 400 MHz High Resolution Multinuclear FT-NMR spectrometer was used for analysis.1H nucleus was observed and CDCl3 (Deuterated chloroform) was used as solvent.
RESULTS
Isolation and identification
The soil samples taken from the ant hills were serially diluted (10-1 to 10-10) and plated on Trypticase soy agar. Within 24 h, red coloured distinct colonies were observed in 10-4 dilution plate. From the 10-4 dilution plate a loop of red coloured colony was selected based on more intensity by visual observation and was taken and plated on TSA plates. TSA media promotes the growth of organism and increases the intensity of the pigment as it contains 0.5 % NaCl which promotes pigment production (Jagannadham et al., 1991). Biochemical tests carried out for the identification of microorganism isolated from soil revealed that the microorganism was Gram positive. It was positive for catalase and starch hydrolysis tests but negative for indole test. From the biochemical tests, the bacteria isolated from soil was presumptively identified as Micrococcus species. Further using 16S rRNA gene sequencing the isolated bacterial DNA was identified to be Micrococcus species which showed similarity based on nucleotide homology and phylogenetic analysis (Figure 1). The evolutionary history was inferred by using the Maximum Likelihood method. Hence the isolated microorganism from soil sample was confirmed as belonging to Micrococcus sp.
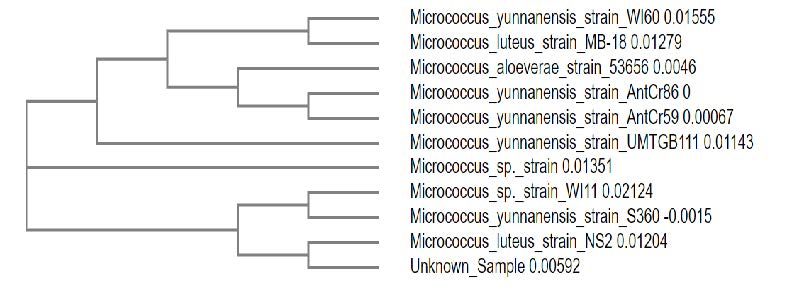
Figure 1. Molecular Phylogenetic tree depicting the relationship between the isolate and related Micrococcus species.
Pigment production and growth kinetics
In our study, trypticase soy broth was used as media for the routine cultivation of Micrococcus sp. The wet cell weight and total carotenoid was calculated. After 48 h of incubation 59.69 g/l of biomass was produced and 10.89 mg/l of total carotenoid was produced. The exponential growth rate is the first order reaction. The rate of biomass is correlated with the specific growth rate (µ) and the biomass concentration of the cell or cell number, X.T is the time for growth (Figure 2).
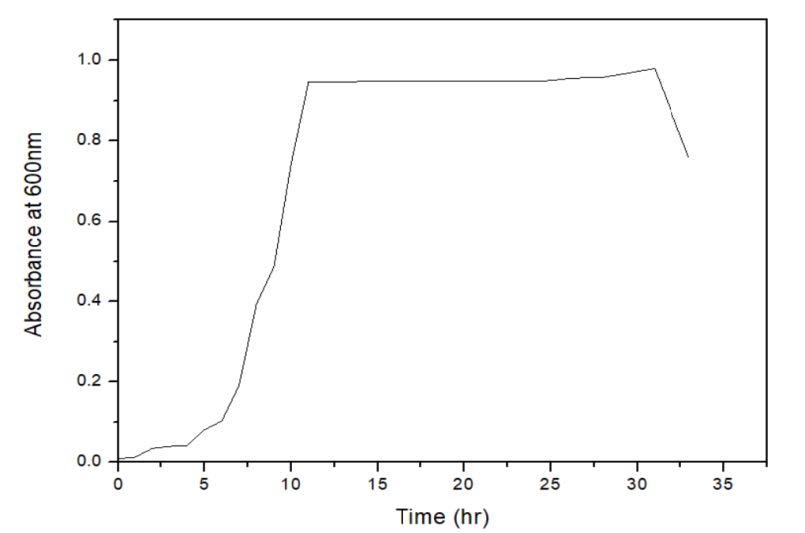
Figure 2. The growth curve for bacterial population.
After 48 h of incubation the growth kinetics data was plotted taking time on X-axis and natural log of wet cell weight on y-axis. The specific growth rate was found to be 0.344/h and the doubling time was 120 min. The studies on growth kinetics revealed that the Micrococcus sp. showed growth till 26 h from inoculation. Following that, decline in the bio mass was observed (Figure 3). It was evident that the pigment production depends on the biomass concentration. At the 26th hour, maximum pigment production (0.0788 µg/l) was observed. The initial value of total carotenoid was high because of the inoculum. Along with the exponential phase the pigment production was increasing and with the beginning of stationary phase pigment production was high.
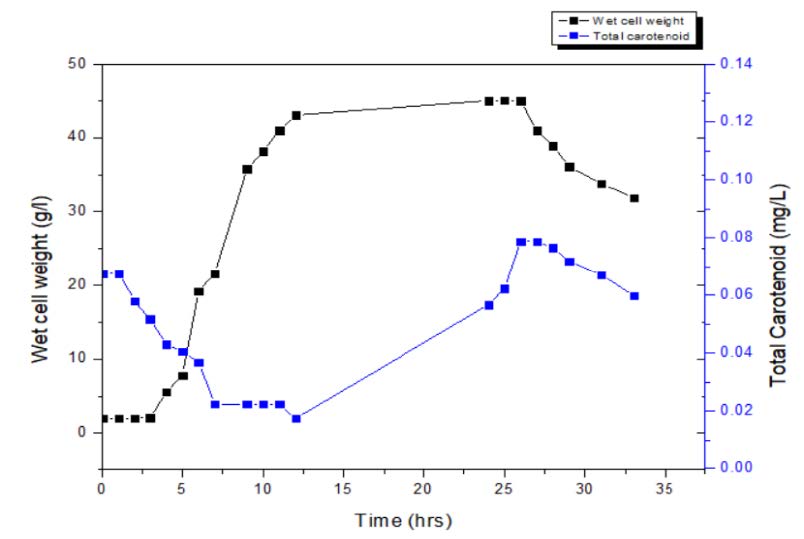
Figure 3. Time course of carotenoid production.
Optimization of parameters influencing pigment production
Effect of Carbon source
The optimization of carbon source was carried out using dextrose, glycerol, starch and sucrose, used at different concentrations ranging from 2.5 % to 10 %. The rate at which the carbon source is metabolized can often influence the formation of product. Fast growth due to high concentration of rapidly metabolized sugar is often associated with low productivity of pigment. This is apparent in case of dextrose and sucrose. The dextrose showed slightly better result than starch and sucrose. Maximum pigment production was observed with 5 % glycerol in media (Figure 4). Glycerol could decrease the fermentation broth pH value but increases in wet cell weight. The intensity of pigment was very high and the colour and property of pigment was stable for more than 25 days in its crude state. This was well in agreement with other studies where, it was observed that glycerol improves gene expression of regulatory and structural gene for pigments synthesis (Feng et al., 2015).
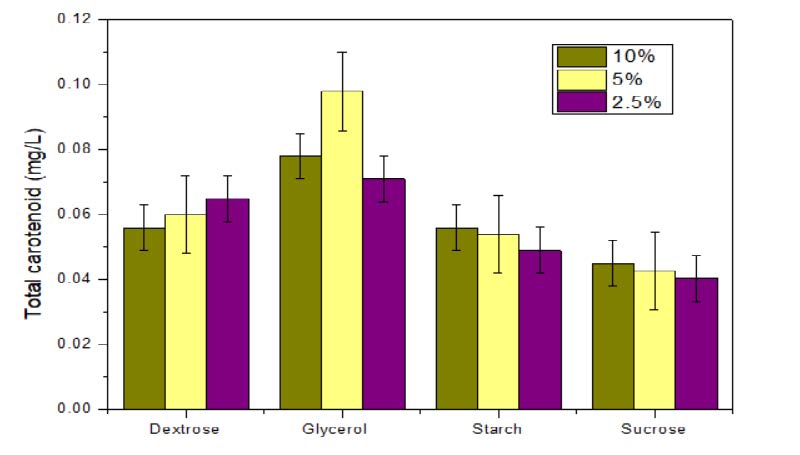
Figure 4. Effect of carbon source on pigment production.
Effect of nitrogen source
The effect of the nitrogen source in pigment production was attempted. Varying the nitrogen source did not show any significant change in the growth and pigment production (Figure 5). Hence it was observed that adding up the nitrogen source to TSB media will not be economical for pigment production.
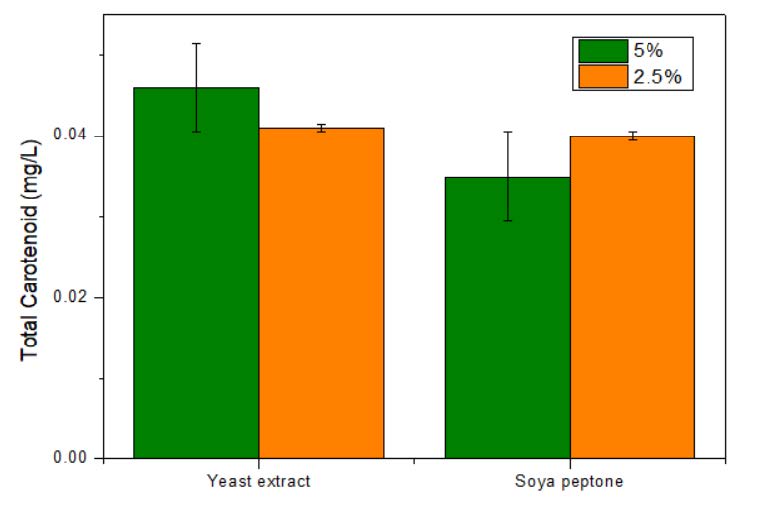
Figure 5. Effect of nitrogen source on pigment production.
Effect of temperature
The effect of temperature on pigment production was studied (Figure 6). It was observed that temperature at 28 °C was optimum for the pigment production whereas pigment content was reduced at 20 °C. Further increasing the temperature to 37 °C resulted in decreased growth and pigment production.
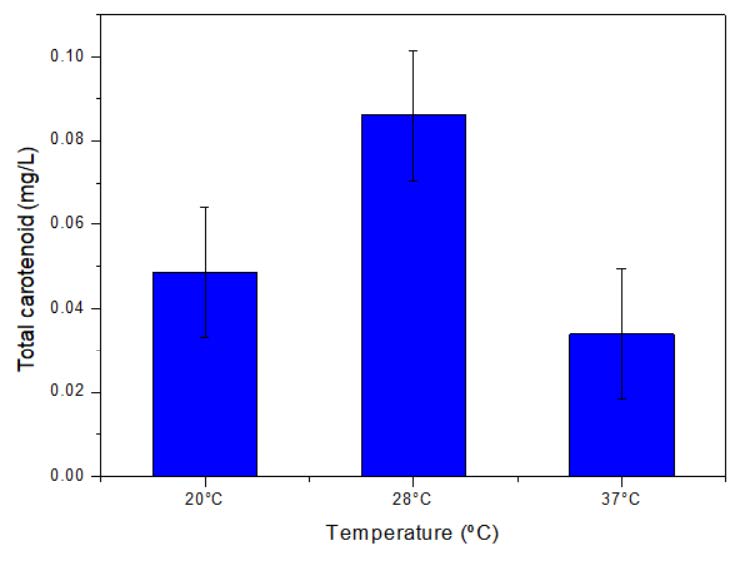
Figure 6. Effect of temperature on pigment production.
Effects of initial pH
Optimum growth and pigment synthesis were observed at pH range from 6 to 7 (Figure 7). Any further variation from optimum pH affected the growth and pigment production. Due to the negligible change in total carotenoid production from pH 6 to 7, pH 6 was considered as optimum pH.
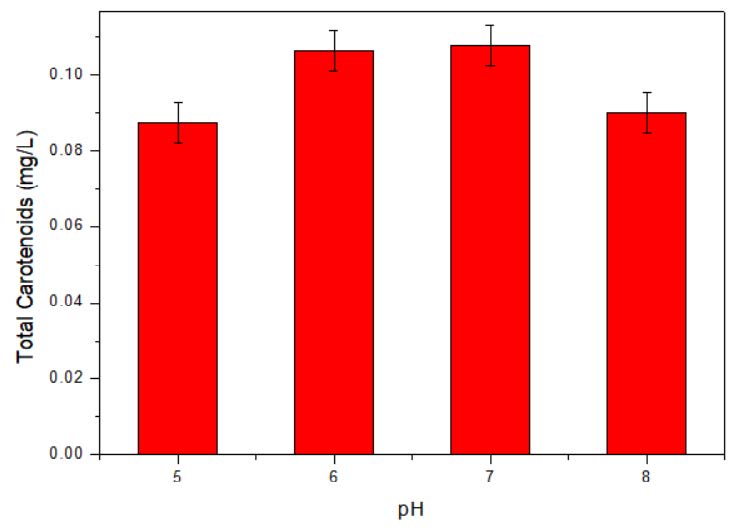
Figure 7. Effect of initial pH on pigment production.
Effect of oxygen availability
Oxygen is not directly added to the medium as such; nevertheless, a very important component of medium and its availability play a crucial role in growth and pigment production. In the batch shake flask study the oxygen availability is easily controlled by increasing the RPM. The higher RPM up to 220 will help to increase the dissolved oxygen. In the figure 8, the data from two different shaking speed of 180 and 220 RPM respectively are plotted and at 220 RPM the maximum of 25.98 mg/l pigment production was observed.
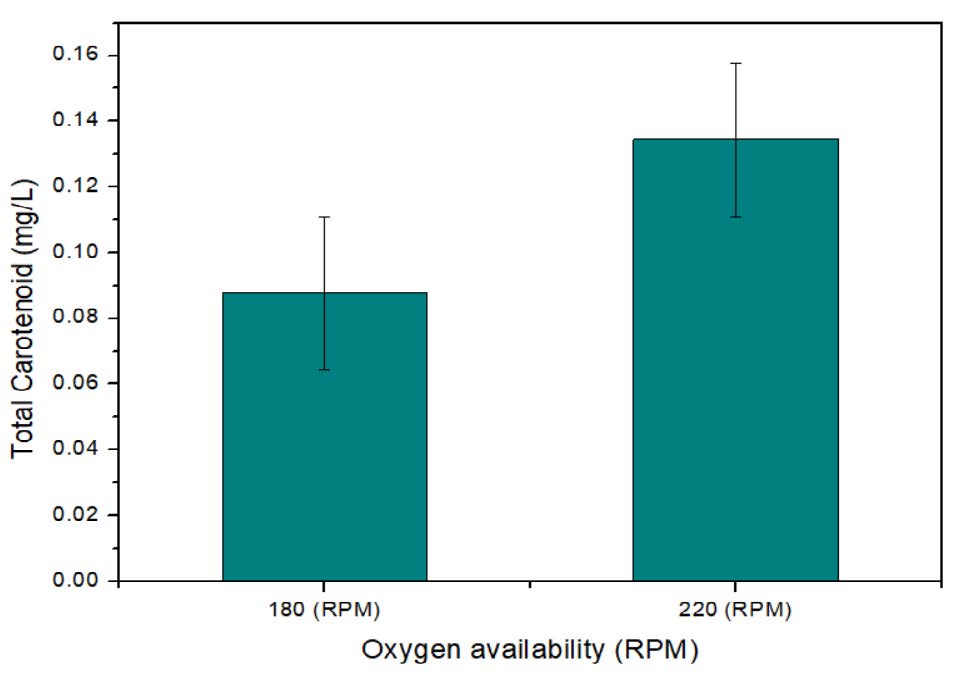
Figure 8. Effect of oxygen availability (RPM) on pigment production.
Pigment production, extraction and purification
Micrococcus sp. was inoculated in to tryptic soy broth and the cells were harvested and subjected to pigment extraction. After optimization, 102.5734 g of the biomass was obtained, which was subjected for pigment extraction. Concentrated epiphase sample when subjected to column chromatography resulted in isolation of two pigments. 17.93 mg/l of petroleum ether dissolved pigment and 1.009 mg/l of methanol dissolved pigment were extracted. 4.67 mg/l of the lyophilized hypophase sample were subjected to thin layer chromatography and 4.13 mg/l of pigment was isolated. On employing the optimized parameters in the pigment production the maximum intense and stable pigments were produced. The pigment isolated from the hypophase and methanol dissolved pigment from epiphase were very low in concentration, and hence were not subjected for characterization. The epiphase petroleum ether dissolved pigment was subjected to characterization study. TLC was carried out for the analysis and identification of the pigment extracted from hypophase. The spots with 0.694 Rf values were obtained after developing on the silica gel plates (Sinha et al., 2017).
Characterization of pigment
The pigment extracted was characterized using UV-Visible spectrophotometer, Mass spectrometer and NMR spectroscopy. UV-Visible absorption spectrum of the extracted pigment is of importance in detecting the structure. UV-Visible spectra exhibited a fine structure, with absorption maxima at 493 and 523nm (Figure 9). The maximum absorbance between the wavelengths of 300-600nm point the presence of carotenoids (Gross, 1991). The peaks are characteristic of carotenoid. The absorption spectrum of the pigment was typical of chromophore containing 12 conjugated double bonds.
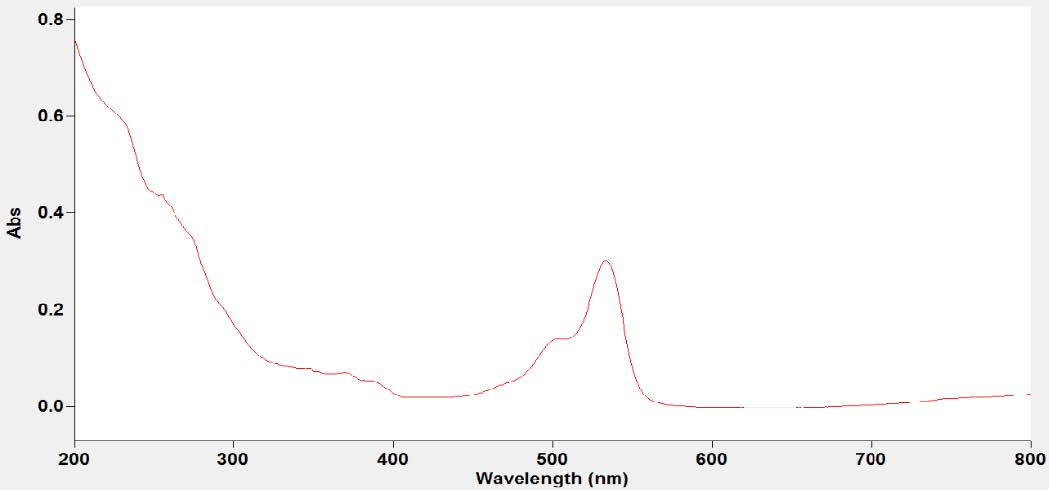
Figure 9. Absorption spectrum of pigment.
Mass spectrum and the proposed structure of pigment is depicted in figure 10. The mass spectrum of pigment displayed an [M+] at m/z 551, corresponding to molecular formula C40H54O. A peak at m/z 573 (M-14) indicates the ionization of the molecule by removal of double bonded oxygen group, indicating the presence of ketone group. The ionization peak arising from methyl group M-30 and all further ionization peaks are similar to β-carotene. The proposed tentative structure beta, beta-caroten-4-one (echinenone) is considered with mass and UV data.
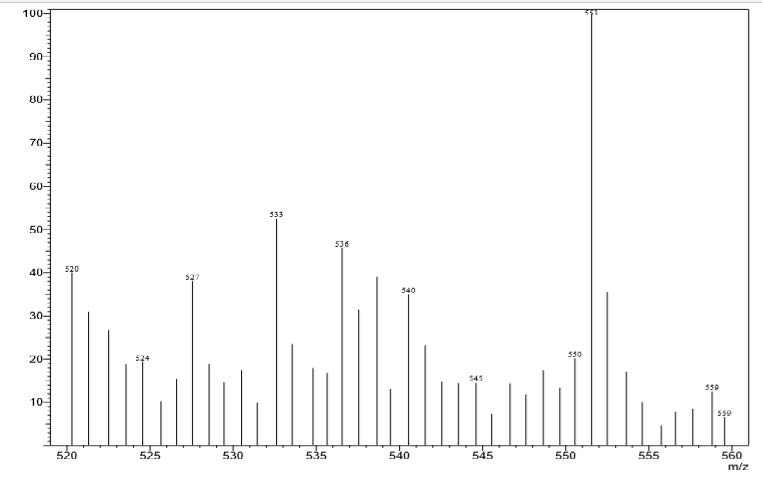
Figure 10. Mass spectrum of pigment indicating the fragmentation.
Further proposed structure is supported by NMR. The 1H-NMR spectrum of the pigment (Figure 11) showed 1-ethylene protons at 6.51 and 6.23 δ region, methylene protons at 3.16 and 1.36 δ region, methyl and cyclohexane protons in the range 2.43 to 1.57 δ regions respectively. The 3 sets of 6 Cyclohexene hydrogens and their chemical shifts are as follows: 1.57 for 2 hydrogen, 1.74 for 2 hydrogen and 1.96 for 2 hydrogen. The 4 sets of 30 methyl hydrogens and their chemical shifts are as follows: 2.41 for 3 hydrogen, 2.21 for 12 hydrogen, 1.82 for 3 hydrogen and1.25 for 12 hydrogen. The 2 set of 4 methylene hydrogen and their chemical shifts are as follows: 1.36 for 2 hydrogen and 3.16 for 2 hydrogen. The 2 set of 14 1-ethylene hydrogens and their chemical shifts are as follows: 6.23 for 4 hydrogen and 6.51 for 10 hydrogen.
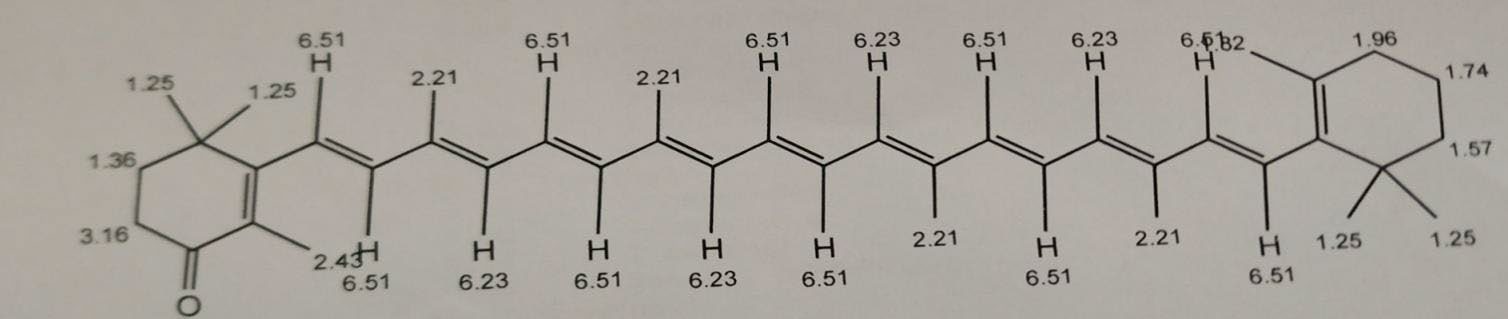
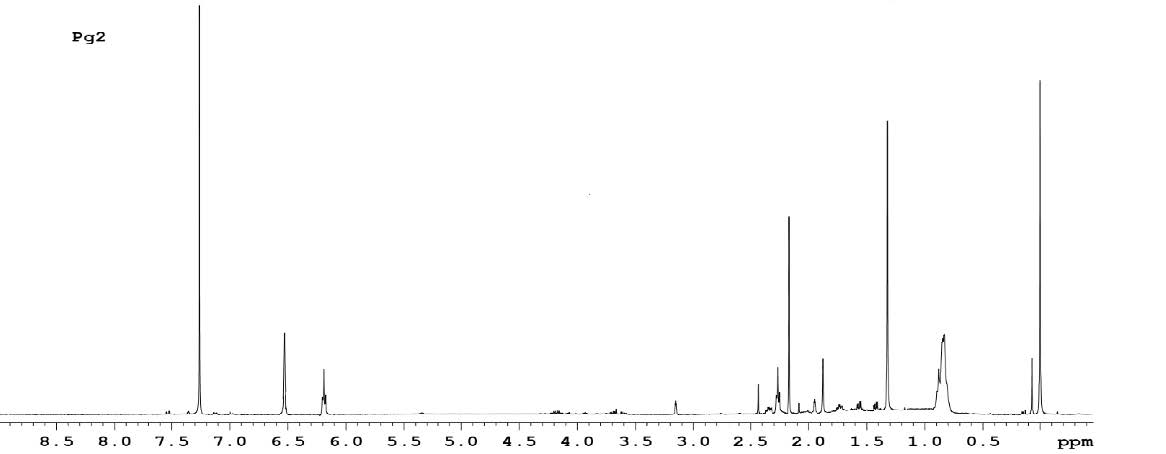
Figure 11. 1H-NMR spectrum of the pigment.
The pigment was identified as echinenone (beta, beta-Caroten-4-one). Echinenone is a carotenone that is beta-carotene in which the 4th position has undergone formal oxidation to afford the corresponding ketone. The molecular mass was found to be 550.87 g/mol.
DISCUSSION
Colours, wonderful creation of nature are one of the primary characteristics perceived by human senses (Kushwaha et al., 2014). They are the edge between the individuals and nature. Colour can sway thinking, change actions and cause reaction. Colour is perceived as an indication of quality and purity of the product. Colours are produced from different sources ranging from synthetic to natural. Synthetic colours are more attractive and are employed worldwide. But the synthetic colours have huge disadvantages like many of them are non-bio-degradable leading to their accumulation in environment, some of them are carcinogenic and cause health disorders (Rana et al., 2021; Sen et al., 2019). Consequently, current focus area of many researchers is finding various alternate physical, chemical, and biological methods for synthetic dye degradation and overcome the harmful effects caused by them to humans and the environment (Chatragadda and Dufosse,2021). Additionally, problems pertaining to environmental conservation and the choice of the customers have stimulated the interests of researchers in exploring pigments and colours from various natural sources. Such natural pigments are generally environment friendly, non-toxic, biodegradable and offers numerous benefits to the mankind and environment.
Microbes are invaluable source for natural pigments. Although in the past few decades, many pigment producing bacteria have been reported, but the search for novel microorganisms producing various coloured pigments are still going on, as these natural pigments are widely employed in many industries including the food industry. Soil and the marine environment are the common sources exploited in search of these novel pigment producing microbes (Nawaz
et al., 2020). The bacteria produce a variety of pigments in these hostile environments for its defence and survival. Hence finding such novel microorganisms and exploring their potential for pigment production would offer novel and potential known pigment molecules for multifaceted applications (Chatragadda and Dufosse, 2021). Considering the above facts, the present study involved collection of soil sample from an anthill, followed by isolation and identification of bacterium from soil and exploring its potential for pigment production.
In this study, the microorganism was identified by biochemical tests as Micrococcus species and further confirmed as belonging to Micrococcus sp. by 16S rRNA gene sequencing. In 2020, M Styczynski reported two microorganisms, Planococcus sp. and Rhodococcus sp. capable of producing several secondary metabolites including pigments. Planococcus are aerobic, Gram-positive, motile cocci which belong to Microcococcae family (Styczynski et al., 2020). This was in agreement with the present study reporting the production of red pigment carotenoids from the Micrococcus species, isolated from ant hill in soil. In 2016, Rostami explored the potential of Micrococcus sp. and Rhodotorula glutinis, for carotenoid production and evaluated their biological properties (Rostami et al., 2016). Shahin et al., reported Micrococcus lylae as the most promising pigment producer. The echinenone pigment with potent pharmacological activities, was optimized and produced to maximize the biomass production, pigment concentration, and the antimicrobial activity (Shahin et al., 2022).
The carotenoids are widely used in various industries and especially in food industry as colouring agents (Ramesh et al., 2019). In the last few decades, researchers and the industries have paid much focus to the microbes that can produce significant amounts of carotenoids, as these are obtained from natural resources and have a huge demand against the synthetic ones. This demand has in turn encouraged the search for novel carotenoid-producing microbes, extraction and optimization of carotenoid production, which is seen as an alternate for chemical synthesis (Torregrosa et al., 2018).
The isolation and characterization of compounds from non-pigmented microbes is laborious and time-consuming (Chatragadda and Dufosse, 2021). The synthesis of natural pigments from microbes is easier and convenient by optimizing their growth parameters (Galasso et al., 2017). Hence, many industries are now developing methods for efficient extraction and optimization of natural colours and pigments as an alternative to synthetic colourants (Nawaz et al., 2020). Hence the current study further involved the optimization of parameters for pigment production. The effects of carbon and nitrogen source, temperature, pH and oxygen availability on pigment production were studied and optimized.
From these studies, all the optimum conditions influencing pigment production were considered. 5 % glycerol was added as the carbon source to one litre TSB media and the pH was maintained at 6.5. Overnight culture of the Micrococcus species was inoculated at 28 °C for 30 hrs and 220 RPM was maintained for pigment production. The wet cell weight and total carotenoid before and after optimization of parameters was documented. The optimization of parameters resulted in increased wet cell weight and total pigment production. Montero et al., reported carotenoid producing Haloferax mediterranei and its optimization using response surface methodology (Montero et al., 2018). Various parameters like pH, temperature and salinity were optimized on the growth and carotenoid content by H. mediterranei, to produce 3.34 ± 0.29 mg/L and 3.74 ± 0.20 mg/L of total carotenoids by theoretical and experimental method respectively. In 2019, Zhao reported the isolation of Rhodotorula sp., from a marine environment, capable of red pigment production. After optimizing the parameters, 987 µg/L of total carotenoid was produced (Zhao et al., 2019). Whereas in the present study, after optimization of the parameters, a yield of 25.98mg/Lof total carotenoid was obtained.
From the present study, it was observed that the isolated Micrococcus species can be explored for carotenoid production, as the yield of total carotenoids are high. Therefore, natural pigments produced from microorganisms are invariably seen as a viable alternate to synthetic colours (Galasso et al., 2017; Shetty et al., 2021). Thus, the red carotenoid pigment echinenone obtained from this study may be useful in various biotech, food, pharma and healthcare industries.
CONCLUSION
Carotenoids are very valuable compounds in the present global market. In the current study a red carotenoid pigment producing microorganism was isolated from soil. The microorganism was presumptively identified and was confirmed by 16S rRNA gene sequencing, as belonging to Micrococcus genus. The effects of carbon and nitrogen source, temperature, pH and oxygen availability on pigment production were studied and optimized. From the optimization studies, it was concluded that TSB media alone was not efficient to yield a stable pigment. When 5 % glycerol was added to TSB media, increased yield of the pigment with greater stability was observed. The addition of nitrogen source did not alter the pigment production. Other factors like temperature at 28 °C, pH at 6 and oxygen availability with a RPM at 220 had a larger impact on pigment production. All the optimized parameters were adapted and a yield of 25.98 mg/Lof total carotenoid was produced. From the purification and characterization studies, the pigment extracted from the Micrococcus sp., isolated from soil was confirmed as echinenone (beta, beta-Caroten-4-one). The extracted microbial pigment has a high value in food and textile industry as an eco-friendly natural dye.
AUTHOR CONTRIBUTIONS
Savinay K Jain, Akash S and Dhamodhar Prakash conceived and designed the research and did Data acquisition. Savinay K Jain, Akash S, Hema JN did data analysis. Savinay K Jain and Hema JN drafted the manuscript work. Dhamodhar Prakash supervised the research work and revised the manuscript. All authors have read and approved the manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Aman Mohammadi, M., Ahangari, H., Mousazadeh, S., Hosseini, S.M., and Dufossé, L. 2022. Microbial pigments as an alternative to synthetic dyes and food additives: A brief review of recent studies. Bioprocess and Biosystems Engineering. 45(1): 1–12.
Athira, M., Haritha, V.S., Thangavel, M., and Nisha, P. 2016. Pigment production by Micrococcus sp. from Polluted water source. European Journal of Pharmaceutical and Medical Research. 3: 274-276.
Azman, A.S., Mawang C.I., and Abubakar S. 2018. Bacterial pigments: The bioactivities and as an alternative for therapeutic applications. Natural Product Communications. 13: 1747-1754.
Bhagwat, A., and Padalia, U. 2020. Optimization of prodigiosin biosynthesis by Serratia marcescens using unconventional bioresources. Journal of Genetic Engineering & Biotechnology. 18: 26.
Butnariu, M. 2016. Methods of Analysis (Extraction, Separation, Identification and Quantification) of Carotenoids from Natural Products. Journal of Ecosystem & Ecography. 6: 193.
Chatragadda, R., and Dufossé, L. 2021. Ecological and biotechnological aspects of pigmented microbes: A way forward in development of food and pharmaceutical grade pigments. Microorganisms. 9(3): 637.
Chaudhary, V., Katyal, P., Poonia, A. K., Kaur, J., Puniya, A. K., and Panwar, H. 2022. Natural pigment from Monascus: The production and therapeutic significance. Journal of Applied Microbiology. 133: 18–38.
Chronis M., Christopoulou V.M., Papadaki S., Stramarkou M., Krokida M. 2021. Optimization of mild extraction methods for the efficient recovery of astaxanthin, a strong food antioxidant carotenoid from microalgae. Chemical Engineering Transactions. 87: 151-156.
Church, D.L., Cerutti, L., Gürtler, A., Griener, T., Zelazny, A., & Emler, S. 2020. Performance and application of 16S rRNA gene cycle sequencing for routine identification of bacteria in the clinical microbiology laboratory. Clinical Microbiology Reviews. 33: e00053-19.
Dawoud, T.M., Alharbi, N.S., Theruvinthalakal, A.M., Thekkangil, A., Kadaikunnan, S., Khaled, J.M., Almanaa, T.N., Sankar, K., Innasimuthu, G.M., Alanzi, K. F., andRajaram, S.K. 2020. Characterization and antifungal activity of the yellow pigment produced by a Bacillus sp. DBS4 isolated from the lichen Dirinaria agealita. Saudi Journal of Biological Sciences. 27: 1403–1411.
Dey, S. and Nagababu, B.H. 2022. Applications of food color and bio-preservatives in the food and its effect on the human health. Food Chemistry Advances. 1: 100019.
Feng, Y., Shao, Y., Zhou, Y., and Chen, F. 2015. Effects of glycerol on pigments and monacolin K production by the high-monacolin K-producing but citrinin-free strain, Monascus pilosus MS-1. European Food Research and Technology. 240: 635-643.
Galasso, C., Corinaldesi, C., and Sansone, C. 2017. Carotenoids from marine organisms: Biological functions and industrial applications. Antioxidants. 6: 96.
Gross, J. 1991. Pigments in vegetables: Chlorophylls and carotenoid. Van Nostrand Reinhold, New York.
Hornero-Méndez, D. and Mínguez-Mosquera, M. I. 2001. Rapid spectrophotometric determination of red and yellow isochromic carotenoid fractions in paprika and red pepper oleoresins. Journal of Agricultural and Food Chemistry. 49: 3584–3588.
Jagannadham, M.V., Rao, V.J., and Shivaji, S. 1991. The major carotenoid pigment of a psychrotrophic Micrococcus roseus strain: Purification, structure, and interaction with synthetic membranes. Journal of Bacteriology. 173: 7911–7917.
Kushwaha, K., Saini, A., Saraswat, P., Agarwal, M.K., and Saxena, J. 2014. Colorful world of microbes: Carotenoids and their applications. Advances in Biology. 4: 1-13.h
Maoka T. 2020. Carotenoids as natural functional pigments. Journal of Natural Medicine. 74: 1–16.
Montero-Lobato, Z., Ramos-Merchante, A., Fuentes, J.L., Sayago, A., Fernández-Recamales, Á., Martínez-Espinosa, R.M., Vega, J.M., Vílchez, C., and Garbayo, I. 2018. Optimization of growth and carotenoid production by Haloferax mediterranei using response surface methodology. Marine Drugs. 16: 372.
Nawaz, A., Chaudhary, R., Shah, Z., Dufossé, L., Fouillaud, M., Mukhtar, H., and Haq, I. U. 2020. An overview on industrial and medical applications of bio-pigments synthesized by marine bacteria. Microorganisms. 9(1): 11.
Ramesh, C., Vinithkumar, N.V., Kirubagaran, R., Venil, C.K., and Dufossé, L. 2019. Multifaceted applications of microbial pigments: Current knowledge, challenges and future directions for public health implications. Microorganisms. 7(7): 186.
Rana B., Bhattacharyya M., Patni B., Arya M., and Joshi G.K. 2021. The realm of microbial pigments in the food color market. Frontiers in Sustainable Food Systems. 5:54.
Rostami, H., Hamedi, H., and Yolmeh, M. 2016. Some biological activities of pigments extracted from Micrococcus roseus (PTCC 1411) and Rhodotorula glutinis (PTCC 5257). International Journal of Immunopathology and Pharmacology. 29: 684–695.
Saini, R.K. and Keum, Y.S. 2019. Microbial platforms to produce commercially vital carotenoids at industrial scale: An updated review of critical issues. Journal of Industrial Microbiology & Biotechnology. 46: 657–674.
Sathasivam, R., and Ki, J.S. 2018. A review of the biological activities of microalgal carotenoids and their potential use in healthcare and cosmetic industries. Marine Drugs. 16: 26.
Sen, T., Barrow, C.J., and Deshmukh, S.K. 2019. Microbial pigments in the food industry-challenges and the way forward. Frontiers in Nutrition. 6: 7.
Shahin Y.H., Elwakil B.H., Ghareeb D.A., and Olama Z.A. 2022. Micrococcus lylae MW407006 pigment: Production, optimization, nano-pigment synthesis, and biological activities. Biology. 11:1171.
Shetty, A.V.K., Dave, N., Murugesan, G., Pai, S., Pugazhendhi, A., Varadavenkatesan, T., Vinayagam, R., and Selvaraj, R. 2021. Production and extraction of red pigment by solid-state fermentation of broken rice using Monascus sanguineus NFCCI 2453. Biocatalysis and Agricultural Biotechnology. 33: 101964.
Shivaji S., Rao N.S., Saisree L., Sheth V., Reddy G.S., Bhargava P.M. 1988. Isolation and identification of Micrococcus roseus and Planococcus sp. from Schirmacher oasis, Antarctica. Journal of Biosciences. 13:409-414.
Sinha S., Choubey S., Ajay Kumar A., and Bhosale P. 2017. Identification,characterization of pigment producing bacteria from soil and water and testing of antimicrobial activity of bacterial pigments. International Journal of Pharmaceutical Sciences Review and Research. 42: 119-124.
Styczynski, M., Rogowska, A., Gieczewska, K., Garstka, M., Szakiel, A., and Dziewit, L. 2020. Genome-based insights into the production of carotenoids by antarctic bacteria, Planococcus sp. ANT_H30 and Rhodococcus sp. ANT_H53B. Molecules. 25: 4357.
Surekha, P.Y., Dhanya, P.R., Mk, S.J., Pradeep, S., and Benjamin, S. 2016. Micrococcus luteus strain baa2, a novel isolate produces carotenoid pigment. Electronic Journal of Biology. 12: 83-89.
Torregrosa-Crespo, J., Montero, Z., Fuentes, J.L., Reig García-Galbis, M., Garbayo, I., Vílchez, C., and Martínez-Espinosa, R.M. 2018. Exploring the valuable carotenoids for the large-scale production by marine microorganisms. Marine Drugs. 16: 203.
Tran, T., Dawrs, S.N., Norton, G.J., Virdi, R., and Honda, J.R. 2020. Brought to you courtesy of the red, white, and blue-pigments of nontuberculous mycobacteria. AIMS Microbiology. 6: 434–450.
Velmurugan, P., Venil, C.K., Veera Ravi, A. and Dufosse, L. 2020. Marine bacteria is the cell factory to produce bioactive pigments: A prospective pigment source in the ocean. Frontiers in Sustainable Food Systems. 4: 589655.
Venil C.K., Dufossé L., and Renuka Devi, P. 2020. Bacterial pigments: Sustainable compounds with market potential for pharma and food industry. Frontiers in Sustainable Food Systems. 4: 100.
Zhao, Y., Guo, L., Xia, Y., Zhuang, X., and Chu, W. 2019. Isolation, identification of carotenoid-producing Rhodotorula sp. from marine environment and optimization for carotenoid production. Marine Drugs. 17: 161.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand.
Savinay K Jain1, Dhamodhar Prakash1, *, Akash S 1, 2, and Hema J N 1
1 Department of Biotechnology, M S Ramaiah Institute of Technology, Bangalore, India.
2 Biopol Biosciences, Bangalore, India.
Corresponding author: Dhamodhar Prakash, E-mail: dhamu.bio@gmail.com
Total Article Views
Editor: Sirasit Srinuanpan,
Chiang Mai University, Thailand
Article history:
Received: October 5, 2021;
Revised: January 14, 2022;
Accepted: January 19, 2023;
Published online: January 26, 2023

