
Effect of Long-term Ketogenic Diet on Serum Alanine Transaminase Levels in Mice (Mus musculus)
Ismi Dian Meiliana, Purwo Sri Rejeki*, Muhammad Miftahussurur, and Minidian FasitasariPublished Date : April 3, 2023
DOI : https://doi.org/10.12982/NLSC.2023.024
Journal Issues : Number 2, April-June 2023
ABSTRACT
The prevalence of obesity is increasing worldwide over the years. One of the non-pharmacological therapy which is believed to be promote weight loss is ketogenic diet. Nevertheless, the long-term effects which may be caused by this diet are still debating, especially in the liver. This study aimed to determine the effect of long-term ketogenic diet (8 weeks) on serum alanine transaminase (ALT) levels. Twelve male mice were divided into basal diet (BD) and ketogenic diet (KD) group and given intervention for eight weeks adlibitum. Body weight was weighed in pre and post-intervention, while ALT levels were measured only once after eight weeks of intervention. As a result, pre-intervention body weight in the BD group was 25.170 ± 2.858 g and KD group was 27.170 ± 1.329 g (P =0.151). In the post-intervention, body weight in BD and KD group were 44.500 ± 5.244 g and 31.830 ± 5.707 g, respectively (P =0.003). BD group showed a significant difference between pre-and post-intervention body weight (P <0.0001); however, it was not significant in the KD group (P =0.096). After eight weeks, serum ALT levels in BD-fed mice were 90.672 ± 20.786 U/L and in KD-fed mice showed 117.037 ± 19.261 U/L (P =0.046). In conclusion, KD elevated serum ALT levels and attenuated weight gain after 8-week KD supplementation.
Keywords: Alanine transaminase, Ketogenic diet, Liver, Mice, Obesity
Funding: This study was funded by the Directorate of Research and Community Service (DRPM) Directorate General of Research and Development – Ministry of Research, Technology, and Higher Education of the Republic of Indonesia.
Citation: Meiliana, I.D., Rejeki, P.S., Miftahussurur, M., and Fasitasari, M. 2023. Effect of Long-term Ketogenic Diet on Serum Alanine Transaminase Levels in Mice (Mus musculus). Nat. Life Sci. Commun. 22(2): e2023024.
INTRODUCTION
Obesity is a multifactorial disease caused by unbalanced daily energy intake and energy elimination contribute to redundant weight gain. Obesity is also associated with diabetes mellitus, cardiovascular disease, dyslipidemia, fatty liver, and high mortality and morbidity rates (Panuganti et al., 2020). According to the World Health Organization, the prevalence of obesity is still increasing enormously to nearly tripling since 1975 worldwide. More than 1.9 billion adults were excessing body weight, and 650 million of them were obese in 2016 (WHO, 2020). In Indonesia, the prevalence of obesity in 2007, 2013, and 2018 increased by 10.5 %, 14.8 %, and 21.8 %, respectively (Ministry of Health of the Republic of Indonesia, 2018). To overcome the obesity problems, pharmacological and non-pharmacological therapies are needed, one of which is diet (Taghavi et al., 2017).
Ketogenic diet (KD) is believed to reduce body weight in obese patients (Bruci et al., 2020). KD is a high-fat diet, adequate-protein, and very low carbohydrate. The body will provide an alternative energy source, namely ketone bodies, and there will be nutritional ketosis (Masood et al., 2020). Apart from being used for obesity therapy, the ketogenic diet is also in demand by many groups, such as for overweight patients with type II diabetes mellitus, powerlifting and weightlifting athletes, adjuvant therapy in epilepsy and cancer (Saslow et al., 2017; Greene et al., 2018; Weber et al., 2018; Ulamek-Koziol et al., 2019). Besides having various benefits, the researchers also thought about the side effects that might be caused by KD, one of which is in the liver (Ma and Suzuki, 2018). It is well known that the liver has many functions, such as detoxification, stores blood, metabolizes carbohydrates, fats, and protein (Barrett et al., 2019). Then if there is damage or an abnormality in the liver, it can be indicated by an increase in liver enzymes, especially on alanine transaminase (ALT) because it is more specific than aspartate transaminase (AST) (Lala et al., 2020).
The advantages of using a ketogenic diet are that it can induce weight loss and increase glycemic control, but has a risk of hyperlipidemia, increased liver enzymes, and the incidence of fatty liver disease (Anekwe et al., 2020). Whereas giving Low Carbohydrate Diet (LCD) to patients with Nonalcoholic Fatty Liver Disease (NAFLD) was able to reduce intra hepatic lipid content significantly, but did not significantly affect liver enzyme levels such as AST and ALT (Haghighatdoost et al., 2016). The use of the long-term ketogenic diet in mice managed to lose body weight, even though. Moreover, mice also showed signs of lipid accumulation and glucose intolerance (Li et al., 2021). These statements are still contradicting. Elevated serum ALT levels in circulation indicate various conditions of the hepatocellular injury, such as viral hepatitis, autoimmune hepatitis, Wilson’s disease, hereditary hemochromatosis, or nonalcoholic fatty liver disease and alcoholic liver disease (Kwo et al., 2017).
A high-fat diet with a 30-60% fat proportion, if given within less than one week, is called a short-term diet, while more than one week can be called a long-term diet (Kakimoto and Kowaltowski, 2016). In the preliminary research, ketogenic diet composition that can prevent weight gain and result in low visceral fat mass and serum IGF-1 levels is 60% fat, 30% protein, and 10% fiber (Syahraya et al., 2020; Utami et al., 2021; Widiatmaja et al., 2021), but what about the effect of that composition on long-term use on liver enzymes, especially ALT, is still not clear and this opens up opportunities to do novelty research. This study aimed to determine the effect of the long-term ketogenic diet for eight weeks on serum ALT levels in ddY mice (Mus musculus).
MATERIALS AND METHODS
Ethical approval
This experimental study was approved by the Institutional Animal Care and Use Committee of Health Research Ethics Committee, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia (No. 237/EC/KEPK/FKUA/2020).
Animals and diets
This study was conducted with a post-test-only control group design. Twelve male ddY mice (Mus musculus) aged 2-3 months with 20-30 g were obtained from Pusvetma (Surabaya, Indonesia). Mice were given a basal diet with a composition of 62% carbohydrate, 20% protein, 12% fat, and 6% fiber and water adlibitum one week before the experiment for acclimatization (23 ± 3°C room temperature, 30-70% humidity and 12:12 dark-light cycle). Subsequently, mice were separated randomly into two groups, the control group (BD) given basal diet (n=6) and the intervention group (KD) given a ketogenic diet with a composition of 60% fat, 30% protein, and 10% fiber (n = 6) for eight weeks adlibitum. The diets were given at 11.00 a.m; daily. See Table 1 for the dietary composition obtained from Veterinary and Food Analysis Testing Unit, Faculty of Veterinary, Universitas Airlangga, Surabaya, Indonesia (No. 087/PT 5.10/SERT/TL/9/2020).
Table 1. Composition of the basal diet (BD) and ketogenic diet (KD).
|
Content |
Diets |
|
|
BD |
KD |
|
|
Carbohydrate |
62% |
0% |
|
Protein |
20% |
30% |
|
Fat |
12% |
60% |
|
Fiber |
6% |
10% |
|
Metabolic energy (Kcal/Kg) |
2993.558 |
4735.377 |
Abbreviation: BD, Basal Diet; KD, Ketogenic Diet
Animal housing
This experiment was conducted at the Laboratory of Biochemical Experimental Animal, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia. There were two cages, BD and KD group with 30x45x20 cm each size, made of plastic covered by wire mesh, free access to water and food (adlibitum). All mice were maintained under standard animal handling at 23 ± 3 °C room temperature, 30-70% humidity and 12:12 dark-light cycle.
Measurement of body weight and serum alanine transaminase levels
The body weight was measured pre and post-intervention using a digital scale. After eight weeks of intervention, mice were sacrificed. Blood samples were collected by cardiac puncture method at 2 p.m. (mice were fasted for 5hr before) then were centrifuged to collect serum and assayed using ALT colorimetric kits E-BC-K235-M (Elabscience Biotechnology, Wuhan, China).
Data analysis
All data were analyzed using Statistic Package for Social Science (SPPS) version 16 (SPSS Inc., Chicago, IL, USA). The data presented as the mean ± SD. This experiment used normality test by the Shapiro-Wilk test. The statistical difference were determined using Independent t-test and Paired t-test. P <0.05 was considered to show a statistically significant difference.
RESULTS
Body weight
This study results showed that the pre-intervention six mice body weight in KD was higher than the BD group, but no significant difference. Body weight varied from 25.170 ± 2.858 in the BD group to 27.170 ± 1.329 in KD-fed mice (P =0.151). After eight weeks of intervention, there was a significant difference (P =0.003) between body weight in both groups, from 44.500 ± 5.244 in the BD group to 31.830 ± 5.707 in the KD group. By using paired t-test, there was a significant difference of pre and post-dietary supplementation with P<0.0001 in the BD group, however, it was not significant in the KD group (Table 2).
Table 2. Difference of pre and post-intervention body weight.
|
Group |
n |
Body weight (g) (Mean ± SD) |
Paired t-test (P-value) |
|
|
Pre-intervention |
Post-intervention |
|
||
|
BD |
6 |
25.170 ± 2.858 |
44.500 ± 5.244 |
<0.0001* |
|
KD |
6 |
27.170 ± 1.329 |
31.830 ± 5.707 |
0.096 |
Note: Abbreviations: BD, Basal Diet; KD, Ketogenic Diet, *shows a significant difference
Change of pre and post-intervention body weight
This study also presented the body weight change after eight weeks intervention. The Δbody weight in the BD-fed mice was 19.330 ± 3.559 g, then in the KD-fed mice was 4.670 ± 5.574 g. There was a significant difference with P<0.001 in independent t-test, the change of body weight in KD presented lower than BD group significantly (Figure 1).
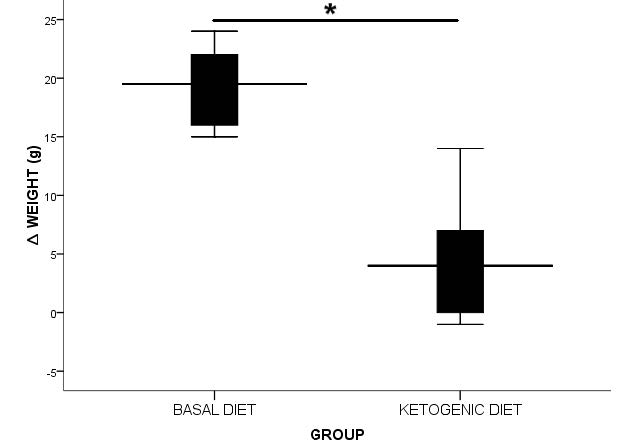
Figure 1. ∆ Body weight (g) present in mean ± SD.
* shows a significant difference
Post-intervention serum ALT levels
After eight weeks of intervention, serum ALT levels were significantly elevated in KD group with P<0.05 compared to the BD group as shown in Table 3, with the minimum and maximum ALT levels in BD group (71.965 U/L, 117.916 U/L), KD (95.317 U/L, 149.554 U/L), respectively.
Table 3. Post-intervention serum ALT levels.
|
Group |
n |
Serum ALT levels (U/L) (Mean ± SD) |
Independent t-test (P-value) |
|
BD |
6 |
90.672 ± 20.786 |
0.046* |
|
KD |
6 |
117.037 ± 19.261 |
|
Note: Abbreviation: BD, Basal Diet; KD, Ketogenic Diet; ALT, Alanine Transaminase, *shows a significant difference (P<0.05)
DISCUSSION
It is believed to have various benefits, one of which is therapy in obese patients, the ketogenic diet may also cause short-term and long-term side effects. Keto flu is a short-term side effect that includes nausea, vomiting, headaches, while long-term side effects can occur in human organs, such as the liver (Masood et al., 2020). Based on this research that conducted during the 8-week intervention, it was found that the mean serum Alanine transaminase levels post-intervention in KD-fed mice had a significant increase compared to BD-fed mice. This result is in line with the prior research that giving a ketogenic diet for 12 weeks in mice with a fat and carbohydrates ratio of 95.1% : 0.4% resulted in an increase in serum ALT levels and showed like NAFLD on its histopathology examination (Garbow et al., 2011). Administering KD for five weeks in mice also resulted in a significant increase in plasma ALT levels (Jornayvaz et al., 2010). Meanwhile, obese patients who were given ketogenic diet therapy showed lose weight but increased levels of the transaminase enzymes and showed fatty liver on ultrasound examination (Anekwe et al., 2020). In contrast to previous statements, the ALT levels and inflammatory markers decreased at 6wk KD-fed rat with the proportion of 69.5% fat and 10.3% carbohydrate, when compared to the control group (Holland et al., 2016). The difference in the results of this study may be due to the different types of rodents used.
The mechanism underlying this elevation in ALT levels may be due to the increased lipolysis that occurs during the administration of the ketogenic diet, resulting in increased free fatty acids, which triggers the accumulation of hepatic lipids (Zhang et al., 2016). Lipolysis caused by the ketogenic diet is known to induce hepatic Fibroblast Growth Factor 21 (FGF21) production through Peroxisome Proliferator-activated Receptor-alpha (PPARα) activation (Woo et al., 2013). FGF21 is known to be one of the potential biomarkers in determining NAFLD (Keuper et al., 2020; Tucker et al., 2020). Mice that given a high-fat diet for 4-month caused increased serum FGF21 levels and intrahepatic FGF21, which might be induced by an accumulation of hepatic lipids which had an impact on fatty liver (Yueh et al., 2020). Furthermore, in mice that were given a ketogenic diet for five weeks, hepatic lipids accumulated in the form of triglycerides, diacylglycerols (DAG), and ceramide (Jornayvaz et al., 2010). The histopathological features of the liver showed steatosis, hepatocellular mitosis, regeneration, picnotic hepatocytes, apoptosis, and hepatic parenchymal inflammatory foci, but no signs of hepatic fibrosis. These features indicate liver damage like NAFLD (Garbow et al., 2011). After the inflammation that is mediated by Tumor Necrosis Factor-alpha (TNFα) and Reactive Oxygen Species (ROS) in the fatty liver formed due to the provision of this diet, there will be hepatocyte damage (Zhang et al., 2016). Pinthong and Suanarunsawat’s (2020) study results also stated that a high-fat and high-fructose diet could induce oxidative stress that causes hepatocyte damage. This hepatocyte damage will cause the levels of serum transaminase enzymes, namely ALT and AST, to increase. It was found that serum ALT levels were significantly increased compared to serum AST levels (Jornayvaz et al., 2010).
Ketogenic diet is a very low carbohydrate, sufficient protein, and high-fat diet believed to treat obesity and type 2 diabetes mellitus (Joshi et al., 2019). Based on this study results, the mean body weight in the KD group experienced slower weight gain and lower body weight after eight weeks of intervention when compared to the control group (BD). The weight change between pre and post-intervention on KD was lower than BD and there was a significant difference between them. These results are in line with the previous study, which was an administration of a ketogenic diet for three months was able to slower weight gain of C5JBL/6J mice with a proportion of 90% fat, 10% protein (Huang et al., 2019). Likewise, KD-fed fisher rats with a dietary composition of 2.9% fiber and 67% fat can reduce weight gain compared to the control group (Kephart et al., 2017). While administering a ketogenic diet can lose body weight significantly in obese patients (Gomez-Arbelaez et al., 2017). Glucose levels become low when the body lacks carbohydrates, as a result, insulin levels will decrease and glucagon will increase (Poff et al., 2020). Decreased glucose stores become insufficient to provide glucose to the brain, then it will enter the ketogenic pathway (Dhillon and Gupta, 2021). Other hormonal changes can contribute to increased lipolysis which produces fatty acids (Masood et al., 2020). By the beta-oxidation process in the mitochondria, fatty acids are converted into acetyl-CoA (Ma and Suzuki, 2018), then converted to acetoacetate, β-hydroxybutyrate (BHB) and acetone. Acetoacetate, BHB, and acetone are rudimentary ketone bodies that amass in the body when the ketogenic diet is maintained. Ketone bodies replace glucose as the main energy source and this state is voiced as nutritional ketosis (Masood et al., 2020). Weight loss that occurs when consuming a ketogenic diet may be caused by increased lipolysis, the direct action of ketone bodies that can reduce appetite, as well as hormones that control appetite (Paoli, 2014). There was a relationship between an increase in leptin and cholecystokinin (CCK) and an increase in fatty acid oxidation and BHB levels on a decrease in food intake (Visinoni et al., 2012). Increased leptin plays a role in suppressing weight gain in KD-fed rats by reducing food intake and increasing energy expenditure (Thio et al., 2006). In addition to affecting the leptin, cholecystokinin, and insulin, the ketogenic diet also affects other neurohormones, reducing levels of ghrelin, which acts as a hunger hormone (Stubbs et al., 2018).
Therefore, this study had several limitations, i.e., this study use only serum ALT level which is one part of the liver function test which functions to detect liver damage. It is necessary to note that the elevation of the serum ALT has many confounding factors, temporary and reversible, so to find out if there is permanent liver injury, it is necessary to carry out a histopathological examination of the liver, which is the gold standard for detecting abnormalities in the liver, especially NAFLD. Hopefully, this research can add new knowledge about the effect of ketogenic diet, especially on the liver. It can be useful in clinical practice to regularly observe liver damage parameters such as ALT when administering KD.
In conclusion, serum ALT levels in the long-term KD-fed ddY mice is elevated compared to the basal diet group. The probable mechanism is through the accumulation of hepatic lipid that causes fatty liver. Also, KD can be used for obesity treatment because it can reduce body weight and maintain it. Further detailed studies are warranted to do a histopathology examination of the liver to confirm the presence or absence of fatty liver in KD-fed mice.
AUTHOR CONTRIBUTIONS
Conception or design: Purwo Sri Rejeki. Acquisition, analysis, or interpretation of data: Ismi Dian Meiliana, Purwo Sri Rejeki. Drafting the work or revising: Ismi Dian Meiliana, Purwo Sri Rejeki, Muhammad Miftahussurur. Final approval of the manuscript: Purwo Sri Rejeki, Muhammad Miftahussurur, Minidian Fasitasari. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Anekwe, C.V., Chandrasekaran, P., and Stanford, F.C. 2020. Ketogenic diet-induced elevated cholesterol, elevated liver enzymes and potential non-alcoholic fatty liver disease. Cureus. 12: e6605.
Barrett, K.E., Barman, S.M., Brooks, H.L., and Yuan, J. 2019. Ganong’s review of medical physiology (26th ed). McGraw-Hill Education, New York.
Bruci, A., Tuccinardi, D., Tozzi, R., Balena, A., Santucci, S., Frontani, R., Mariani, S., Basciani, S., Spera, G., Gnessi, L., et al. 2020. Very low-calorie ketogenic diet: A safe and effective tool for weight loss in patients with obesity and mild kidney failure. Nutrients. 12: 333.
Dhillon, K.K., and Gupta, S. 2021. Biochemistry, ketogenesis [online]. Website https://www.ncbi.nlm.nih.gov/books/NBK493179/ (accessed 20 February 2021).
Garbow, J.R., Doherty, J.M., Schugar, R.C., Travers, S., Weber, M.L., Wentz, A.E., Ezenwajiaku, N., Cotter, D.G., Brunt, E.M., and Crawford, P.A. 2011. Hepatic steatosis, inflammation, and ER stress in mice maintained long term on a very low-carbohydrate ketogenic diet. American Journal of Physiology-Gastrointestinal and Liver Physiology. 300: G956–G967..
Gomez-Arbelaez, D, Bellido, D., Castro, A.I., Ordoñez-Mayan, L., Carreira, J., Galban, C., Martinez-Olmos, M.A., Crujeiras, A.B., Sajoux, I., and Casanueva, F.F. 2017. Body composition changes after very-low-calorie ketogenic diet in obesity evaluated by 3 standardized methods. The Journal of Clinical Endocrinology and Metabolism. 102: 488–498.
Greene, D.A., Varley, B.J., Hartwig, T.B., Chapman, P., and Rigney, M. 2018. A low-carbohydrate ketogenic diet reduces body mass without compromising performance in powerlifting and olympic weightlifting athletes. Journal of Strength and Conditioning Research. 32: 3373–3382.
Haghighatdoost, F., Salehi-Abargouei, A., Surkan, P.J., and Azadbakht, L. 2016. The effects of low carbohydrate diets on liver function tests in nonalcoholic fatty liver disease: A systematic review and meta-analysis of clinical trials. Journal of Research in Medical Sciences : the Official Journal of Isfahan University of Medical Sciences. 21: 53.
Holland, A.M., Kephart, W.C., Mumford, P.W., Mobley, C.B., Lowery, R.P., Shake, J.J., Patel, R.K., Healy, J.C., McCullough, D.J., Kluess, H.A., et al. 2016. Effects of a ketogenic diet on adipose tissue, liver, and serum biomarkers in sedentary rats and rats that exercised via resisted voluntary wheel running. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 311: R337–R3351.
Huang, J., Li, Y.Q., Wu, C.H., Zhang, Y.L., Zhao, S.T., Huang, J., Li, Y.Q., Wu, C.H., Zhang, Y.L., Zhao, S.T., et al. 2019. The effect of ketogenic diet on behaviors and synaptic functions of naive mice. Brain and Behavior. 9: e01246.
Jornayvaz, F.R., Jurczak, M.J., Lee, H.Y., Birkenfeld, A.L., Frederick, D.W., Zhang, D., Zhang, X.M., Samuel, V.T., and Shulman, G.I. 2010. A high-fat, ketogenic diet causes hepatic insulin resistance in mice, despite increasing energy expenditure and preventing weight gain. American Journal of Physiology-Endocrinology and Metabolism. 299: E808–E815.
Joshi, S., Ostfeld, R.J., and McMacken, M. 2019. The ketogenic diet for obesity and diabetes—enthusiasm outpaces evidence. JAMA Internal Medicine. 179: 1163–1164.
Kakimoto, P.A., and Kowaltowski, A.J. 2016. Effects of high fat diets on rodent liver bioenergetics and oxidative imbalance. Redox Biology. 8: 216–225.
Kephart, W.C., Mumford, P.W., Mao, X., Romero, M.A., Hyatt, H.W., Zhang, Y., Mobley, C.B., Quindry, J.C., Young, K.C., Beck, D.T., et al. 2017. The 1-week and 8-month effects of a ketogenic diet or ketone salt supplementation on multi-organ markers of oxidative stress and mitochondrial function in rats. Nutrients. 9: 1019.
Keuper, M., Häring, H.U., and Staige, H. 2020. Circulating FGF21 levels in human health and metabolic disease. Experimental and Clinical Endocrinology & Diabetes : Official Journal, German Society of Endocrinology [and] German Diabetes Association. 128: 752–770.
Kwo, P.Y., Cohen, S.M., and Lim, J.K. 2017. ACG clinical guideline: Evaluation of abnormal liver chemistries. The American Journal of Gastroenterology. 112: 18–35.
Lala, V., Goyal, A., Bansal, P., and Minter, D.A. 2020. Liver function tests [online]. Website https://www.ncbi.nlm.nih.gov/books/NBK482489/ (accessed 1 January 2021).
Li, Y., Yang, X., Zhang, J., Jiang, T., Zhang, Z., Wang, Z., Gong, M., Zhao, L., and Zhang, C. 2021. Ketogenic diets induced glucose intolerance and lipid accumulation in mice with alterations in gut microbiota and metabolites. mBio. 12: e03601–e03620.
Ma, S., and Suzuki, K. 2018. Potensial application of ketogenic diet to metabolic status and exercise performance: A review. EC Nutrition. 13: 496–499.
Masood, W., Annamaraju, P., and Uppaluri, K.R. 2020. Ketogenic diet [online]. Website https://www.ncbi.nlm.nih.gov/books/NBK499830/ (accessed 29 December 2020).
Ministry of Health of the Republic of Indonesia. 2018. Hasil Utama RISKESDAS. Ministry of Health of the Republic of Indonesia, Jakarta.
Panuganti, K.K., Nguyen, M., and Kshirsagar, R.K. 2021. Obesity [online]. Website https://www.ncbi.nlm.nih.gov/books/NBK459357/ (accessed 29 December 2020).
Paoli, A. 2014. Ketogenic diet for obesity: Friend or foe? International Journal of Environmental Research and Public Health. 11: 2092–2107.
Pinthong, W., and Suanarunsawat, T. 2020. Tea seed oil alleviates metabolic derangement and oxidative stress in rats fed with high fat and high fructose diet. Chiang Mai University Journal of Natural Sciences. 19: 665–683.
Poff, A.M., Koutnik, A.P., and Egan, B. 2020. Nutritional ketosis with ketogenic diets or exogenous ketones: Features, convergence, and divergence. Current Sports Medicine Reports. 19: 251–259.
Saslow, L.R., Mason, A.E., Kim, S., Goldman, V., Ploutz-Snyder, R., Bayandorian, H., Daubenmier, J., Hecht, F.M., and Moskowitz, J.T. 2017. An online intervention comparing a very low-carbohydrate ketogenic diet and lifestyle recommendations versus a plate method diet in overweight individuals with type 2 diabetes: A randomized controlled trial. Journal of Medical Internet Research. 19: e36.
Stubbs, B.J., Cox, P.J., Evans, R.D., Cyranka, M., Clarke, K., and de Wet, H. 2018. A ketone ester drink lowers human ghrelin and appetite. Obesity (Silver Spring). 26: 269–273.
Syahraya, I., Novida, H., Herawati, L., and Rejeki, P.S. 2020. Effect of high fat diet on weight loss through the expression of uncouple protein 1 in mice visceral fat. Folia Medica Indonesiana. 56: 223–228.
Taghavi, S.A., van Wely, M., Jahanfar, S., and Bazarganipour, F. 2017. Pharmacological and non‐pharmacological strategies for obese women with subfertility. The Cochrane Database of Systematic Reviews. 3: CD012650.
Thio, L.L., Erbayat-Altay, E., Rensing, N., and Yamada, K.A. 2006. Leptin contributes to slower weight gain in juvenile rodents on a ketogenic diet. Pediatric Research. 60: 413–417.
Tucker, B., McClelland, R.L., Allison, M.A., Budoff, M.J., Wu, B.J., Barter, P.J., Rye, K.A., and Ong, K.L. 2020. Relationship of fibroblast growth factor 21 levels with inflammation, lipoproteins and non-alcoholic fatty liver disease. Atherosclerosis. 299: 38–44.
Ulamek-Koziol, M., Czuczwar, S.J., Januszewski, S., and Pluta, R. 2019. Ketogenic diet and epilepsy. Nutrients. 11: 2510.
Utami, D.M., Herawati, L., I’tishom, R., Al-Arif, M.A., Miftahussurur, M., and Rejeki, P.S. 2021. Ketogenic diet slows down weight gain in juvenile Mus musculus with benzopyrene as cancer inducer. Indian Journal of Forensic Medicine & Toxicology. 15: 2268–2274.
Visinoni, S., Khalid, N.F.I., Joannides, C.N., Shulkes, A., Yim, M., Whitehead, J., Tiganis, T., Lamont, B.J., Favaloro, J.M., Proietto, J., et al. 2012. The role of liver fructose-1,6-bisphosphatase in regulating appetite and adiposity. Diabetes. 61: 1122–1132.
Weber, D.D., Aminazdeh-Gohari, S., and Kofler, B. 2018. Ketogenic diet in cancer therapy. Aging. 10: 164–165.
WHO. 2020. Obesity and overweight [online]. Website https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed 29 December 2020).
Widiatmaja, D.M., Prabowo, G.I., and Rejeki, P.S. 2021. A long-term ketogenic diet decreases serum insulin-like growth factor-1 levels in mice. Journal of Hunan University (Natural Sciences). 48: 1-7.
Woo, Y.C., Xu, A., Wang, Y., and Lam, K.S. 2013. Fibroblast Growth Factor 21 as an emerging metabolic regulator: Clinical perspectives. Clinical Endocrinology. 78: 489–496.
Yueh, M.F., He, F., Chen, C., Vu, C., Tripathi, A., Knight, R., Karin, M., Chen, S., and Tukey, R.H. 2020. Triclosan leads to dysregulation of the metabolic regulator FGF21 exacerbating high fat diet-induced nonalcoholic fatty liver disease. Proceedings of the National Academy of Sciences of the United States of America. 117: 31259–31266.
Zhang, X., Qin, J., Zhao, Y., Shi, J., Lan, R., Gan, Y., Ren, H., Zhu, B., Qian, M., and Du, B. 2016. Long-term ketogenic diet contributes to glycemic control but promotes lipid accumulation and hepatic steatosis in type 2 diabetic mice. Nutrition Research. 36: 349–358.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand.
Ismi Dian Meiliana1, Purwo Sri Rejeki2, *, Muhammad Miftahussurur3, 4, and Minidian Fasitasari5
1 Medical Programme, Faculty of Medicine, Universitas Airlangga, Surabaya 60131, Indonesia.
2 Department of Physiology, Faculty of Medicine, Universitas Airlangga, Surabaya 60131, Indonesia.
3 Gastroentero-Hepatology Division, Department of Internal Medicine, Faculty of Medicine-Dr. Soetomo Teaching Hospital, Universitas Airlangga, Surabaya 60286, Indonesia.
4 Institute of Tropical Disease, Universitas Airlangga, Surabaya 60115, Indonesia.
5 Department of Nutrition, Faculty of Medicine, Universitas Islam Sultan Agung, Semarang 50112, Indonesia.
Corresponding author: Purwo Sri Rejeki, E-mail: purwo-s-r@fk.unair.ac.id; purwo_faal@yahoo.com
Total Article Views
Editor: Veerasak Punyapornwithaya,
Chiang Mai University, Thailand
Article history:
Received: September 25, 2021;
Revised: December 31, 2022;
Accepted: January 18, 2023;
Published online: January 25, 2023

