
Phytochemical Properties, In Vitro Antimicrobial, and Bioactive Compounds of Banana Peel Extractions Using GC-MS
Wirot Likittrakulwong*, Sanipon Chanburee, Thanapon Kitpot, Padarat Ninjiaranai and Pornkanok PongpamornPublished Date : April 3, 2023
DOI : https://doi.org/10.12982/NLSC.2023.021
Journal Issues : Number 2, April-June 2023
ABSTRACT
The purpose of this study was to investigate the phytochemical content, antioxidant activity, in vitro antimicrobial activity, DNA damage activity, and bioactive compound identifications in three different banana peels, namely Musa acuminata (Kluai Hom Thong; HT), Musa sapientum L. (Kluai Nam Wa; NW), and Musa balbisiana (Kluai Ta Nee; TN). The extraction was accomplished through maceration with 95% ethanol. Antioxidant capacity was determined to confirm the antioxidant and phytochemical contents. Their antibacterial activity against pathogenic bacteria (Bacillus subtilis, Staphylococcus aureus, and Escherichia coli) that commonly infect livestock. ANOVA analysis was used to statistically analyze the results. The amount of ethanolic extractive yield in NW peel extracts was the highest value (9.80 ± 0.12% of dry material weight). In addition, NW had the highest total phenolic content than that of other species, which may be related to its high FRAP, DPPH-antiradical, and DNA damage activity. Furthermore, the antibacterial activity of NW peel extracts was more effective against B. subtilis, S. aureus, and E. coli with the mean inhibition zone of 13, 15 and 13 mm respectively. The bioactive compounds were identified using GC-MS. Several antioxidant compounds included n-hexadecanoic acid, hexadecanoic acid, ethyl ester, and squalene. While phytol and squalene were found to possess antibacterial activity. The extracts of banana peels contained 9,19-cyclolanost-25-en-3-ol, 24-methyl-, (3.beta.,24S)- which exhibited antibacterial activity against E.coli. Phytochemical characteristics, antibacterial activity, and bioactive components of NW banana peel extracts were superior to those of HT and TN.
Keywords: GC-MS, Antioxidant, Antimicrobial, Musa acuminata, Musa sapientum L., Musa balbisiana
Funding: This research was financially supported by the Research and Development Institute (RDI) of Pibulsongkram Rajabhat University (PSRU) Grant No. RDI-1-64-2.
Citation: Likittrakulwong, W., Chanburee, S., Kitpot, T., Ninjiaranai, P., and Pongpamorn, P. 2023. Phytochemical properties, in vitro antimicrobial, and bioactive compounds of banana peel extractions using GC-MS. Nat. Life Sci. Commun. 22(2): e2023021.
INTRODUCTION
Bananas are one of the most popular fruits in the world, and they are an economically important tropical fruit for both domestic and export markets (Rajkumar et al., 2012). The banana fruits are cultivated complex based on two wild diploid species from South-East Asia: Musa acuminata Colla (AA), a highly polymorphous plant with spindly plants that grow in clumps, and Musa balbisiana Colla (BB), a homogenous hardy plant with a massive pseudo-trunk (Aurore et al., 2009). There are many commercial cultivars of banana in Thailand, including Musa acuminata (Kluai Hom Thong; HT), Musa sapientum L. (Kluai Nam Wa; NW), and Musa balbisiana (Kluai Ta Nee; TN) (Rajkumar et al., 2012). Banana peels are a common agricultural waste that have been utilized as medicine, animal feed, leather blacking, and rubber fillers. Banana peels, an underappreciated source of phenolic content, are considered a useful source of antioxidant and functional antibiotic for foods (Baskar et al., 2011; Moukamnerd et al., 2020). These bioactive molecules reported in banana peel extracts have been shown to have pharmacological properties, including anti-diabetic, anti-inflammatory, and antibacterial properties (Chabuck et al., 2013). Many medicines contain secondary metabolites, which can be found in bananas. Despite the fact that bananas are frequently used as an active ingredient in cosmetic products, there have only been a few scientific studies that support their use (Noysang et al., 2018). The aim of this research was to investigate the antioxidant and phytochemical properties and, in vitro antimicrobial activities of ethanolic extracts from three varieties of banana cultivars: HT, NW and, TN. Furthermore, the major bioactive compounds from banana peel extractions were identified using GC-MS.
MATERIALS AND METHODS
Collection of plant samples
Three fresh banana fruits from different families were purchased from the local markets in Phitsanulok, Kamphaeng Phet, and Sukhothai provinces of Thailand. Three varieties were selected for investigation in this study: “Kluai Hom Thong; HT” (Musa acuminata), “Kluai Nam Wa; NW” (Musa sapientum L), and “Kluai Ta Nee; TN” (Musa balbisiana). Banana fruits were washed in the laboratory with running tap water, surface sterilized with 70% alcohol, and rinsed with sterile distilled water. The peels were removed and cut into small pieces (Supplementary Figure 1). The banana peels were dried in the oven at 40°C for 24 hours (Dahham et al., 2010). The dried banana peels were ground using household grinder to obtain a fine powder and stored in sealed plastic bags at 4°C until further use.
Preparation of banana peel extractions
Banana peels were extracted using a 1:10 maceration methods (Zhang et al., 2018; Noysang et al., 2018), in which 500g of banana peel powder was macerated for 3 weeks at room temperature in 5 L of 95% ethanol stirred daily for 5 min. Following that, the mixture was filtered to obtain a clarified extracted and concentrated under reduced pressure at 40°C. The filtrates were concentrated by evaporating the solvents in a rotary evaporator. The concentrated extracts from each banana variety were dissolved in 10% dimethyl sulfoxide (DMSO), stored in dark vials, and kept refrigerated at 4°C until further use (Hanafy et al., 2021). Each extraction was carried out at least in triplicate. The percentage yield of the extractions was calculated.
Phytochemical properties and antioxidant capacity
Total phenolic compounds
Phenolic content of each sample was determined using the previously described method (Singleton et al., 1999) with some modifications. The DMSO-diluted extract (0.5 mL) was mixed with 0.5 mL of distilled water. Following, 0.5 mL of Folin-Ciocalteu reagent (1:1 with water) and 2.5 mL of 2% Na2CO3 solution in distilled water were added. The mixture was thoroughly mixed before being placed in the dark for 40 min. The absorbance of the incubated samples was measured at 765 nm. The results expressed as gallic acid equivalent (GAE)/g extract, using calibration curves of gallic acid as the standard (Likittrakulwong et al., 2021).
FRAP assay
The ferric reducing antioxidant power (FRAP) was determined as previously described (Likittrakulwong et al., 2021). The results expressed as Trolox equivalent (TE)/g extract, using calibration curves of Trolox as the standard.
DPPH radical scavenging activity assay
The DPPH radical scavenging activity assay was determined using the method described previously (Nuengchamnog et al., 2009). Briefly, adding 75 µL of different concentrations (0.09 – 20 mg/mL) of the test extract to the 150 µL of 0.2 mM methanolic DPPH solution to achieve a final DPPH in each well of a 96 well plate. After 30 min of incubation in the dark at room temperature, the absorbance at 515 nm was measured using a microplate reader. The DPPH scavenging percentage was calculated using the following equation (L-ascorbic acid was used as a control):
% Scavenging effect = [1 – (Absorbance of sample/Absorbance of control)] × 100
The percentage of antioxidant activity against log concentration was plotted. The results were expressed in mg/mL extract as the half-inhibitory concentration (IC50).
DNA damage activity
DNA damage activity was investigated using previously described the method (Srikaeo et al., 2019). Extracted banana peels at varied concentrations (0, 1, 3, 6 and 9 mg/mL DMSO) were used. DNA from banana leaves were extracted using a modified cetyl trimethylammonium bromide (CTAB) method for DNA extraction of plants (Porebski et al., 1997). Briefly, 0.5 µg DNA was incubated with 1 µL of 1 mM FeSO4, 1 µL of 6% H2O2, and 3 µL of extracts of banana peels, and the final volume was made up to 15 µL with 50 mM phosphate buffer (pH7.0). The mixture was then incubated in a water bath at 37 ± 2°C for 30 min. Following incubation, the sample was immediately loaded into 1.5% agarose gel along with 3 µL ethidium bromide, which contained 40 mM Tris, 20 mM sodium acetate, and 2 mM EDTA, and electrophoresed in a horizontal slab apparatus in Tris/boric/EDTA gel buffer. After that, the gel was imaged under the UV light.
In vitro antimicrobial activity
Microorganisms used
The antimicrobial activity of banana peel extracts was tested for in vitro antimicrobial activity using three microorganisms; Gram-positive bacteria (Bacillus subtilis and Staphylococcus aureus) and Gram-negative bacteria (Escherichia coli). These bacteria were obtained from the Microbiology Program at the Faculty of Science and Technology at Pibulsongkram Rajabhat University in Phitsanulok, Thailand.
Minimum inhibitory concentration (MIC)
The MIC is defined as the lowest concentration of an extracting sample that greatly inhibits the visible growth of a microorganism (Sirajudin et al., 2014). The inoculum extract stock solution (100 µL) was added to all sterile 96 well plates.
A volume of 100 µL of test material in 1% (V/V) DMSO was pipetted into the plate's first row. Dilutions of 100, 50, 25, 12.5, 6.25, 3.13, 1.56, 0.78, and 0.39 mg/mL were achieved using serial two-fold dilution of the sample.
Minimum bactericidal concentration (MBC)
A loopful of broth was collected from those plates wells that did not show any visible signs of growth and streaked on sterile TSA to determine the MBC for each set of wells in the MIC determination. The plates were then incubated at 37°C for 24 hours. Following incubation, the concentration at which no visible bacterial growth was observed and recorded as the MBC (Sirajudin et al., 2014).
Disc diffusion assay
Loopful growths of bacterial isolates were inoculated into nutrient broth and incubated for 18 hours at 37°C. Normal saline was used to dilute the bacterial suspensions. Adjust the turbidity and compare the results to the standard tube (McFarland number 0.5). Dip a cotton swab into the adjustment suspension and streak the entire Mueller-Hinton agar surface of the plates for 20 min. at room temperature. Filter paper discs (Whatman No.3, 5mm diameter) were sterilized in a hot air oven after being placed in glass Petri dishes. Each disc received a 20 µL portion of extract stock solution (100mg/mL), resulting in a crude extract concentration of 1.66 mg/mL per disc for B. subtilis and S. aureus. Furthermore, to provide a crude extract concentration of 3.13 mg/mL per disc for E. coli. Ampicillin 10 µg/disc (Amp.), Norfloxacin 10/disc µg (Nor.), Ceftriaxone 30 µg/disc (Cro.), Cephalothin 30 µg/disc (Cep.), Tetracycline 30 µg/disc (Tet.), and Kanamycin 1 mg/disc (Kan.) discs were manufactured in a similar manner. The extracts and antibiotics discs were incubated for 24 hours at 37°C. The negative controls were 1% DMSO while ampicillin, norfloxacin, ceftriaxone, cephalothin, tetracycline, and kanamycin were employed as positive controls. A transparent ruler was used to manually measure the diameter of any clear zone of inhibition around the discs. Each experiment was independently performed in triplicate and repeated three times (Chabuck et al., 2013).
Bioactive components using GC-MS
The banana peel extracts (10 mg/mL) was dissolved in absolute methanol, sonicated for 30 min, and centrifuged for 5 min 10,000 rpm (9391 rcf). Then, the supernatant was subjected to GC-MS analysis using a GCMS – QP2020 NX (Shimadzu Co., Japan) equipped with SH-Rxi-5Sil MS column (0.25 µm df x 0.25 mm ID x 30 m length). Helium (99.9%) was used as carrier gas at a flow rate of 1 mL/min. Sample (0.4 µl) was injected in split mode with a split ratio of 1:5. The ion source and interface temperatures of the mass spectrometer, as well as the injector temperature, were maintained at 250°C. Mass spectra were obtained by electron ionization at 70 eV, using a mass scan range of m/z 45-700, with a scan speed of 2500. The column oven temperature program was set as follows: started at 80°C (held for 2 min), raised at a rate of 5°C/min to 120°C (held for 2 min), raised at a rate of 10°C/min. to 240°C (held for 6 min), and finally raised at a rate of 10°C/min to 300°C (held for 10 min). The compounds were identified by matching with the mass spectra in the NIST 17 database. Compound having a spectrum that is more than 80% similarity to the database will be annotated.
Statistical analysis
For statistical analysis, three replicates of each sample were used. The original data were analyzed using one-way analysis of variance (ANOVA) with SPSS statistical software version 21 (Chicago, IL, USA).
RESULTS
Banana peel extractions
The extracts obtained were continually evaporated to dryness by vacuum evaporator at 40 °C. The appearances of the extracts are shown in Supplementary Figure 1. The yields of the extractions from three varieties of banana peels ranged from 6.67 – 9.80%. The polarities of different compounds present in the peels were responsible for the variation in the extract yields. NW had the highest concentration of ethanolic extractives (9.80±0.12%). While TN showed the lowest concentration (6.67±0.51%), as detailed in Supplementary Table 1.
Phytochemical properties
Antioxidant properties
Antioxidant properties of the three banana peel extracts, as indicated by total phenolic compounds, FRAP assay and DPPH radical scavenging activity assay, are shown in Table 1. The total phenolic contents of NW peels were the highest value (34.90 mg GAE/g extract). These results were found to be similar to those previously reported. The results of the present study indicated that NW peel extracts demonstrated significantly higher the total phenolic content than other varieties, which could be related to its antioxidant potential. In addition, the total phenolic content of NW was five times higher than that of HT and TN. Similar trend, as found in total phenolic content, was also observed for DPPH radical scavenging activity assay and FRAP.
Table 1. Antioxidant properties of the banana peel extracts.
|
Varieties |
Total phnolic contents (mg GAE/g extract) |
FRAP (mg TE/g extract) |
DPPH-antiradical (IC50 mg/mL extract) |
|
HT |
7.31 ± 0.02b |
5.17 ± 0.06b |
1.07 ± 0.03a |
|
NW |
34.90 ± 0.10a |
37.30 ± 0.36a |
0.18 ± 0.02b |
|
TN |
7.07 ± 0.06c |
2.39 ± 0.01c |
1.44 ± 0.25a |
Note: HT: Musa acuminata (Kluai Hom Thong), NW: Musa sapientum L (Kluai Nam Wa), TN: Musa balbisiana (Kluai Ta Nee). All measurements were taken in triplicate and the results reported as mean±standard deviation (S.D.). a-c Means with different superscripts in a column differ significantly (P ≤ 0.05).
DNA damage activity
The gel patterns of DNA exposed to FeSO4 and H2O2 in the presence and absence of banana peel extracts are shown in Figure 1. It was clear that higher concentrations of the extracts provided more effective protection against DNA damage, as evidenced by shorter or smaller smear bands. NW peels extract provided better protection against DNA damage than HT and TN peel extracts (Supplementary Figure 2). This damage can be reduced in the presence of banana peel extracts. According to the findings of this study, NW peel extract with high level of antioxidant activity effectively induced DNA protecting activity (Figure 1). This could be due to the higher levels of phytochemicals in NW than those found in HT and TN.
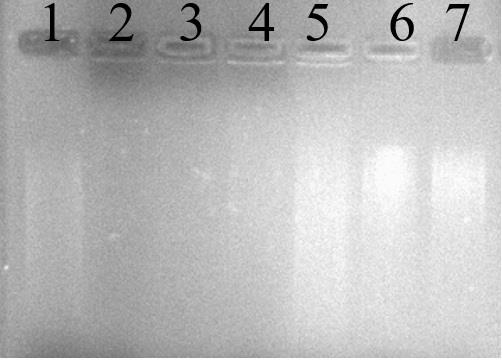
Figure 1. DNA damage activity induced by FeSO4 and H2O2 in the presence of banana peel extracts from banana peel extracts of NW: Musa sapientum L. The lanes (1-2) are positive and negative controls, lane 3 is without the addition of NW peel extracts, and lane 4 – 7 are with the addition of NW peels extraction at 1, 3, 6 and 9 mg/mL, respectively.
Antimicrobial activity and disc diffusion assay
Antimicrobial activity of the three banana peel extracts as determined by the MIC and MBC values against the test organisms are shown in Table 2. The MIC and MBC values were in the ranges of 1.66 – 3.13 and 1.66 – 12.5 mg/mL, respectively. Moreover, the MIC and MBC values of banana peel extracts against Gram-positive bacteria were lower than those in Gram-negative bacteria. The ethanolic extract of the banana peels displayed the highest potency against B. subtilis and S. aureus.
Table 2. Antibacterial activity of the banana peel extracts against test organisms.
|
Bacteria |
HT |
NW |
TN |
|||
|
|
MIC (mg/mL) |
MBC (mg/mL) |
MIC (mg/mL) |
MBC (mg/mL) |
MIC (mg/mL) |
MBC (mg/mL) |
|
Gram-positive |
|
|
|
|
|
|
|
Bacillus subtilis |
1.66 |
3.13 |
1.66 |
3.13 |
1.66 |
3.13 |
|
Staphylococcus aureus |
1.66 |
1.66 |
1.66 |
1.66 |
1.66 |
1.66 |
|
Gram-negative |
|
|
|
|
|
|
|
Escherichia coli |
3.13 |
12.5 |
3.13 |
12.5 |
3.13 |
12.5 |
In terms of the disc diffusion assay, the diameters of the zone of inhibition produced by banana peel extracts are shown in Table 3 and Figure 2. NW had the highest antibacterial activity against B. subtilis, S. aureus and E. coli, with means inhibition zone of 13, 15 and 13 mm, respectively. While HT showed the lowest antibacterial activity against all test microorganisms.
Table 3. Diameters of inhibition zone of banana peel extracts against test organisms.
|
Varieties |
Antibiotic
|
Diameter zone of inhibition (mm) |
||
|
Gram-positive |
Gram-negative |
|||
|
Bacillus subtilis |
Staphylococcus aureus |
Escherichia coli |
||
|
HT |
|
8.50 ± 0.50c |
8.83 ± 1.04b |
9.67 ± 1.15b |
|
NW |
|
13.00 ± 1.00a |
15.00 ± 1.00a |
13.00 ± 1.00a |
|
TN |
|
11.00 ± 1.00b |
9.67 ± 1.15b |
8.67 ± 1.15b |
|
P-value |
|
0.002 |
0.001 |
0.007 |
|
|
Amp.10µg/disc |
39.33 ± 1.15 |
13.33 ± 2.31 |
23.00 ± 1.00 |
|
|
Nor. 10µg/disc |
20.67 ± 1.15 |
21.33 ± 1.15 |
39.00 ± 1.00 |
|
|
Cro. 30µg/disk |
31.67 ± 2.08 |
21.67 ± 1.53 |
39.33 ± 1.15 |
|
|
Cep. 30µg/disk |
41.00 ± 1.00 |
22.33 ± 0.58 |
29.67 ± 1.53 |
|
|
Tet. 30µg/disk |
29.67 ± 0.58 |
20.67 ± 1.15 |
21.67 ± 2.08 |
|
|
Kan. 1mg/disk |
21.67 ± 2.08 |
13.67 ± 1.53 |
36.00 ± 2.00 |
Note: HT: Musa acuminata (Kluai Hom Thong), NW: Musa sapientum L (Kluai Nam Wa), TN: Musa balbisiana (Kluai Ta Nee), Amp: Ampicillin; Nor: Norfloxacin, Cro: Ceftriaxone, Cep: Cephalothin, Tet: Tetracycline, Kan: Kanamycin. The negative controls were 1% DMSO. Amp, Nor, Cro, Cep, Tet, and Kan were employed as positive controls. All measurements were taken in triplicate and the results reported as mean±standard deviation (S.D.). a,b Means with different superscripts in a column differ significantly (P ≤ 0.05).
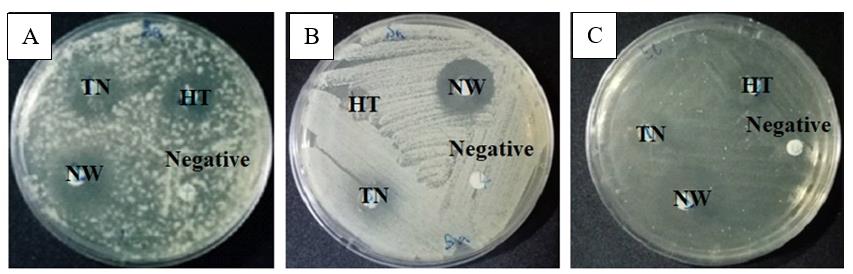
Figure 2. Diameters of inhibition zone of banana peel extracts against pathogenic bacteria, (A) Bacillus subtilis; (B) Staphylococcus aureus, and (C) Escherichia coli. HT: Musa acuminata (Kluai Hom Thong), NW: Musa sapientum L (Kluai Nam Wa), TN: Musa balbisiana (Kluai Ta Nee). Negative control was 1% DMSO.
From Table 3, it can be seen that all banana peel extracts showed potency against pathogenic bacteria. Unfortunately, they exhibited less potency when compared to the positive controls (antibiotic disc) as detailed in Table 3 and Supplementary Figure 3. Results of this study revealed that banana peel extracts contained a variety of antibacterial compounds. Furthermore, these compounds of NW had inhibitory zones of 13.00 mm (the highest), 15.00 mm (the highest), and 13.00 mm (the highest) against B. subtilis, S. aureus, and E. coli, respectively.
Identification of bioactive components from banana peel extracts by GC-MS technique.
According to the GC-MS analysis, forty-one compound were identified from the ethanol extract of three different banana peel extracts. A total of 26 compounds can be identified in HT, 38 compounds in NW, and 30 compounds in TN (Table 4).
Table 4. Compounds were identified in the banana peel extracts.
|
No. |
Compound Name |
RT. |
FM. |
MW. |
Peak Area |
||
|
1HT |
2NW |
3TN |
|||||
|
1 |
2-hydroxy-gamma-butyrolactone |
4.646 |
C4H6O3 |
102 |
89796 |
58494 |
320525 |
|
2 |
4h-pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- |
8.008 |
C6H8O4 |
144 |
214611 |
n.d. |
65002 |
|
3 |
L-glutamic acid |
10.421 |
C5H9NO4 |
147 |
124960 |
145055 |
n.d. |
|
4 |
tetradecane |
15.503 |
C14H30 |
198 |
19473 |
44763 |
51347 |
|
5 |
DL-prolinate, 5-oxo-, ethyl ester |
15.616 |
C7H11NO3 |
157 |
90980 |
n.d. |
n.d. |
|
6 |
tetradecanoic acid, ethyl ester |
20.723 |
C16H32O2 |
256 |
n.d. |
99847 |
n.d. |
|
7 |
lidocaine |
21.834 |
C14H22N2O |
234 |
169602 |
64322 |
90432 |
|
8 |
methyl palmitate |
22.158 |
C17H34O2 |
270 |
34164 |
112799 |
59061 |
|
9 |
n-hexadecanoic acid |
22.505 |
C16H32O2 |
256 |
311673 |
603693 |
288135 |
|
10 |
hexadecanoic acid, ethyl ester |
22.845 |
C18H36O2 |
284 |
747494 |
3093720 |
725282 |
|
11 |
methyl oleate |
23.884 |
C19H36O2 |
296 |
n.d. |
167087 |
n.d. |
|
12 |
phytol |
23.983 |
C20H40O |
296 |
230730 |
43910 |
87886 |
|
13 |
linoleic acid ethyl ester |
24.468 |
C20H36O2 |
308 |
208703 |
1039747 |
303921 |
|
14 |
ethyl oleate |
24.532 |
C20H38O2 |
310 |
733044 |
3257874 |
667089 |
|
15 |
octadecanoic acid, ethyl ester |
24.801 |
C20H40O2 |
312 |
82009 |
411299 |
87072 |
|
16 |
glycidyl palmitate |
26.014 |
C19H36O3 |
312 |
178483 |
469373 |
183974 |
|
17 |
9-octadecenoic acid (9Z)-, oxiranylmethyl ester |
28.892 |
C21H38O3 |
338 |
179007 |
577935 |
165740 |
|
18 |
hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester |
29.617 |
C19H38O4 |
330 |
565542 |
1655253 |
762094 |
|
19 |
behenic alcohol |
32.914 |
C22H46O |
326 |
86228 |
n.d. |
n.d. |
|
20 |
squalene |
34.345 |
C30H50 |
410 |
58470 |
102863 |
910130 |
|
21 |
decane, 1,1-diethoxy- |
36.699 |
C14H30O2 |
230 |
155261 |
57268 |
256568 |
|
22 |
vitamin E |
37.467 |
C29H50O2 |
430 |
144482 |
159229 |
n.d. |
|
23 |
campesterol |
38.657 |
C28H48O |
400 |
212468 |
425928 |
475103 |
|
24 |
stigmasterol |
38.997 |
C29H48O |
412 |
872794 |
1184257 |
1443356 |
|
25 |
9,19-cyclolanostan-3-ol, acetate, (3.beta.)- |
40.800 |
C32H54O2 |
470 |
12401722 |
12426450 |
8806718 |
|
26 |
9,19-cyclolanostan-3-ol, 24-methylene-, (3.beta.)- |
40.644 |
C31H52O |
440 |
23594431 |
55723179 |
46018049 |
|
27 |
9,19-cyclolanost-25-en-3-ol, 24-methyl-, (3.beta.,24S)- |
41.046 |
C31H52O |
440 |
1568112 |
5242264 |
4428872 |
|
28 |
glyceraldehyde |
2.606 |
C3H6O3 |
90 |
n.d. |
58859 |
371337 |
|
29 |
oleic acid |
24.234 |
C18H34O2 |
282 |
n.d. |
462508 |
n.d. |
|
30 |
octadecanoic acid, 2,3-dihydroxypropyl ester |
33.167 |
C21H42O4 |
358 |
n.d. |
116074 |
n.d. |
|
31 |
1-heptacosanol |
32.912 |
C27H56O |
396 |
n.d. |
423765 |
389520 |
|
32 |
2,3-butanediol, [R-(R*,R*)]- |
2.281 |
C4H10O2 |
90 |
n.d. |
182988 |
n.d. |
|
33 |
glycerin |
4.191 |
C3H8O3 |
92 |
n.d. |
173148 |
30629 |
|
34 |
ethyl 9-hexadecenoate |
22.635 |
C18H34O2 |
282 |
n.d. |
100527 |
n.d. |
|
35 |
(E)-9-octadecenoic acid ethyl ester |
24.593 |
C20H38O2 |
310 |
n.d. |
151375 |
32682 |
|
36 |
1,8,11-heptadecatriene, (Z,Z)- |
28.778 |
C17H30 |
234 |
n.d. |
154882 |
n.d. |
|
37 |
9-octadecenoic acid (Z)-, 2,3-dihydroxypropyl ester |
32.822 |
C21H40O4 |
356 |
419934 |
1574810 |
442793 |
|
38 |
ethyl tetracosanoate |
34.145 |
C26H52O2 |
396 |
n.d. |
63193 |
64044 |
|
39 |
nonacosanal |
34.624 |
C29H58O |
422 |
n.d. |
57080 |
523909 |
|
40 |
cholesterol |
37.406 |
C27H46O |
386 |
n.d. |
109991 |
100945 |
|
41 |
obtusifoliol |
39.365 |
C30H50O |
426 |
n.d. |
756141 |
1757197 |
|
Note: 1 HT:Musa acuminata (Kluai Hom Thong), 2NW:Musa sapientum L (Kluai Nam Wa), 3TN: Musa balbisiana (Kluai Ta Nee). No.: Number, RT.: Retention Time, FM.: Molecular Formula, MW.: Molecular Weight, n.d.: Not Detected. |
|||||||
Their chromatograms were illustrated in Figure 3. The HT contained 7 major compounds, which belonged to the triterpenoid and the fatty acid ester group: 9,19-cyclolanostan-3-ol, 24-methylene-, (3.beta.)- (41.8%) (26), 9,19-cyclolanostan-3-ol, acetate, (3.beta.)- (41%) (25), 9,19-cyclolanost-25-en-3-ol, 24-methyl-, (3.beta.,24s)- (3.6%) (27), stigmasterol (2.0%) (24), hexadecanoic acid, ethyl ester (1.7%) (10), ethyl oleate (1.7%) (14), and hexadecanoic acid, and 2-hydroxy-1-(hydroxymethyl)ethyl ester (1.3%) (18); and 19 minor compounds having abundances lower than 1%. The NW contained 9 major compounds, which belonged to the triterpenoid and the fatty acid ester group: 9,19-cyclolanostan-3-ol, 24-methylene-, (3.beta.)- (60.9%) (26), 9,19-cyclolanostan-3-ol, acetate, (3.beta.)- (13.6%) (25), 9,19-cyclolanost-25-en-3-ol, 24-methyl-, (3.beta.,24s)- (5.7%) (27), ethyl oleate (3.6%) (14), hexadecanoic acid, ethyl ester (3.4%) (10), hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester (1.8%) (18), 9-octadecenoic acid (z)-, 2,3-dihydroxypropyl ester (1.7%) (37), stigmasterol (1.3%) (24), and linoleic acid ethyl ester (1.1%) (13); and 29 minor compounds. The TN contained 9 major compounds, which belonged to the triterpenoid and the fatty acid ester group: 9,19-cyclolanostan-3-ol, 24-methylene-, (3.beta.)- (65.8%) (26), 9,19-cyclolanostan-3-ol, acetate, (3.beta.)- (12.6%) (25), 9,19-cyclolanost-25-en-3-ol, 24-methyl-, (3.beta.,24s)- (6.3%) (27), obtusifoliol (2.5%) (41), stigmasterol (2.1%) (24), squalene (1.3%) (20), hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester (1.1%) (18), hexadecanoic acid, ethyl ester (1.0%) (10), and ethyl oleate (1.0%) (14); and 21 minor compounds (Figure 3).
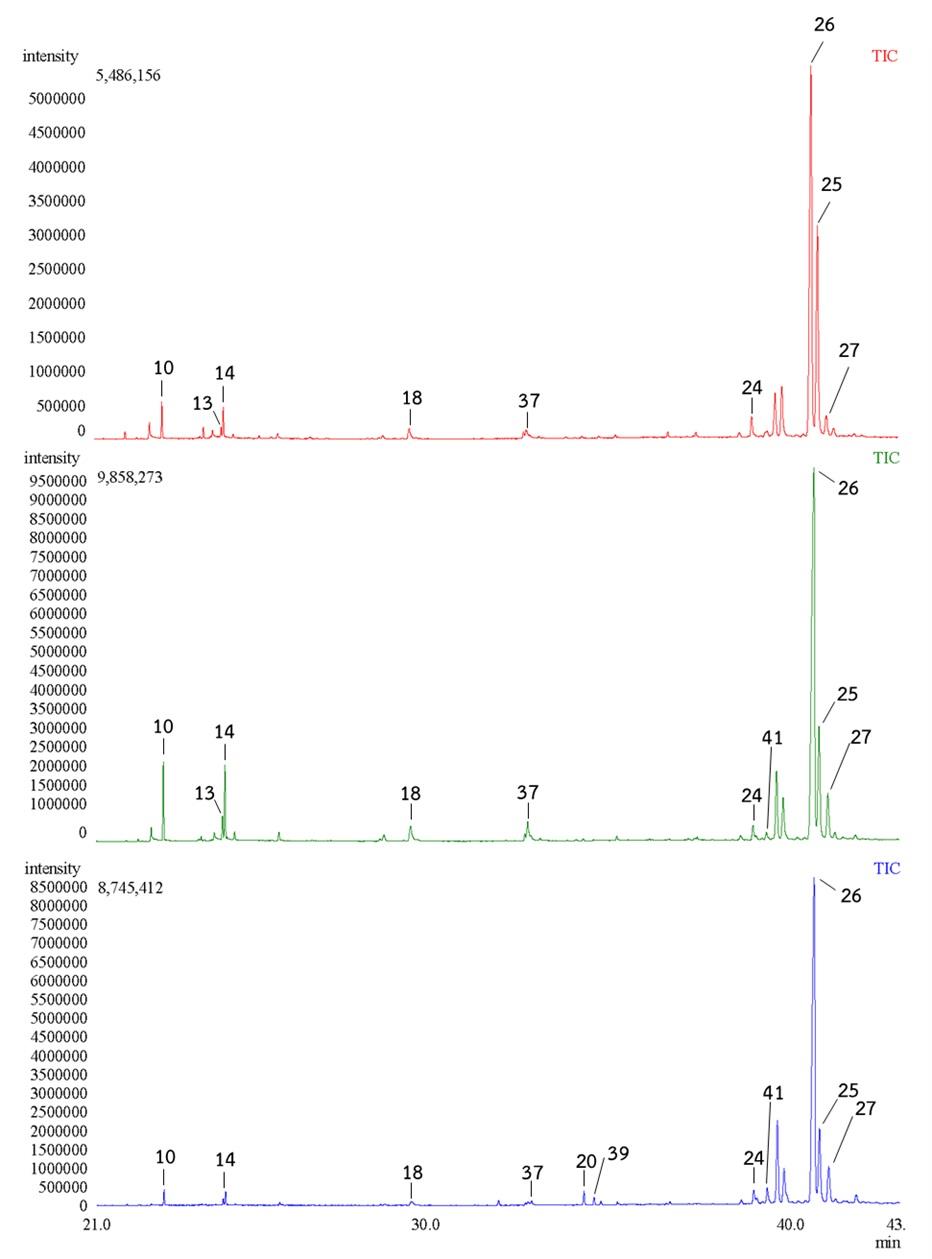
Figure 3. GC-MS chromatograms of the HT (Top), NW (Middle) and TN (Bottom) banana peel extracts at 21-43 min. HT:Musa acuminata (Kluai Hom Thong), NW: Musa sapientum L (Kluai Nam Wa), TN: Musa balbisiana (Kluai Ta Nee).
In the HT banana peel extracts, the relative amount of compound 25 was almost similar to that of compound 26, whereas in the NW and TN banana peel extracts, the relative amount of compound 25 was much lower than that of compound 26. In the NW banana peel extracts, the relative amount of compounds 10 and 14 were almost similar to that of compound 27, whereas in the HT and TN bananas peel extracts, the relative amount of compounds 10 and 14 were much lower than compound 27 (Figure 3).
DISCUSSION
The antioxidant potential, in vitro antimicrobial activity, and bioactive compounds of three different banana peel extracts were evaluated in this research. In the antioxidant properties, the total phenolic content of NW was higher than those found in the other species. This could be related to its high FRAP, DPPH-antiradical and DNA damage activity. According to González-Montelongo, et al. (2010), banana peels contained a high concentration of phenolic content ranging from 4.95 to 47 mg GAE/g. This level was 1.5-3 times higher than that level measured in the flesh (Sulaiman et al., 2011). The phenols are plant secondary metabolites with a wide range of medicinal applications, including antioxidant, antimutagenic, anticarcinogenic, free radical-scavenging, and cardiovascular problem reduction (Yen et al., 1993). Several studies have found a substantial association between the level of phenolic content and oxygen radical absorbance capacity, free radical scavenging, and ferric reducing ability, with banana peel having great radical scavenging activity and reducing ability (Vu et al., 2018). The majority of phytochemicals identified in banana peel extracts were alkaloids, flavonoids, tannins and, polyphenols (Noysang et al., 2018; Prommajak et al., 2020). The HT peel ethanolic extract had the highest antioxidant activity, with an IC50 = 3.25 ± 0.52 µg/mL.
For DNA damage activity, the site-specific DNA damage assay was used to investigate the protective effects of banana peel extracts on hydroxyl radical-mediated DNA strand break. Incubating DNA isolated from banana leaves with FeSO4 and H2O2 for 30 min in a water bath, hydroxyl ions were produced, showing FeSO4/H2O2 at the indicated concentrations and incubation duration can induce both single-single strand and double-strand DNA breaks. The Fenton reaction produced hydroxyl radicals, which caused oxidative induced breaks in DNA strands, resulting in fragmented forms. On genomic DNA, the free radical scavenging activities of banana peel extracts were investigated. The treatment of supercoiled DNA with Fenton's reagent caused the DNA to change into a circular form. The addition of the extracts to the reaction mixture significantly reduced DNA strand scission while retaining the supercoiled form, effectively protecting the DNA. The reaction between O2 and H2O2 in the presence of metal ions produced OH, which caused most of oxidative damages in biological systems (Gutteridge, 1984; Likittrakulwong et al., 2020).
For in vitro antimicrobial activity, NW peel extracts may be effective as an antibacterial agent against both Gram-positive and Gram-negative bacteria. The largest inhibitory zone was achieved against B. subtilis (Figure 2A) and S. aureus (Figure 2B), while the smallest zone was obtained against E. coli (Figure 2C). The susceptibility of bacteria to antibacterial substances varied according to the types of microorganism (Balouiri et al., 2016). E.coli is a Gram-negative bacteria with three cell wall layers, namely lipoprotein, outer membrane, and lipopolysaccharide, and a very high fat or lipid contents ranging from 11 – 22%. As a result, antibacterial substances found it difficult to penetrate. Gram-positive bacteria (B. subtilis and S. aureus) have a fat or lipid content of 1 – 4%, which may make it easier for antibacterial substances to penetrate the cell wall (Fajrih et al., 2022).
The bioactive compounds discovered by GC-MS suggested that banana peel extracts could be a valuable source of medicinal ingredients. An intriguing aspect of this research is the identification of chemical compounds from different banana peel extracts that are known to have biological activities. Some similar compounds can be found in all three different banana species (compound 9, 10, 12, 14, 20, 25, 26, and 27, Figure 4).
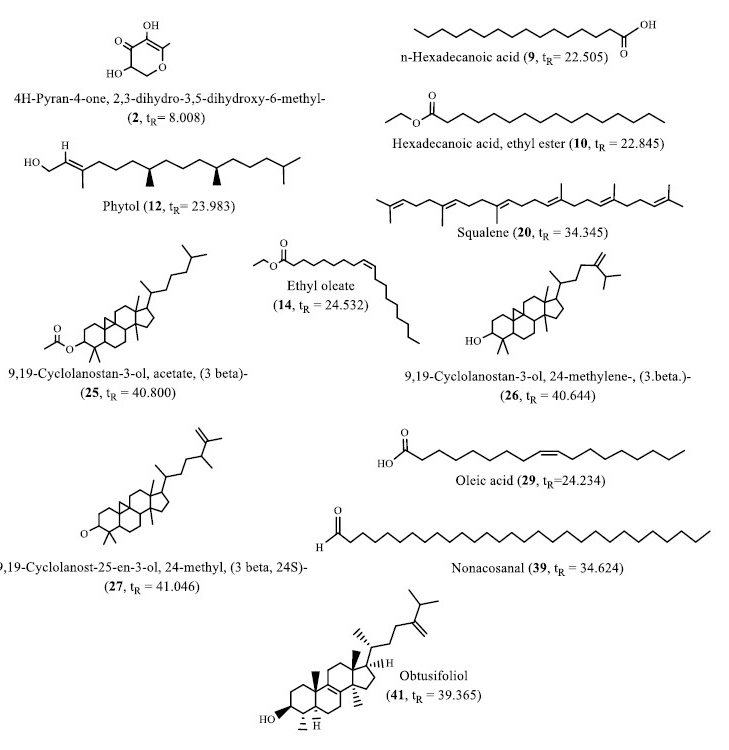
Figure 4. Structures of selected compounds identified in the banana peel extracts.
Compounds 9 and 10 were a fatty acid (palmitic acid ester) that was an anticancerous and antioxidative, hypocholesterolemic, nematicide, anti-androgenic, pesticide and antipsychotic (Ameachi and Chijioke, 2018; Pascual
et al., 2017; Tyagi and Agarwal, 2017). Accordingly, compound 12 contained antibacterial, anti-cancer, anti-inflammatory, anti-diuretic, immune-stimulatory and antidiabetic properties (Ameachi and Chijioke, 2018). Compound 14 is used for vehicle for intramuscular drug delivery (Elaiyaraja and Chandramohan, 2016). Compound 20 was an antibacterial, antioxidant, pesticide, antitumor, cancer preventive, immunostimulant, chemo preventive and lipoxygenase-inhibitor (Sermakkani and Thangapandian, 2012). Compound 26 was also referred to as 24 methylenecycloartanol. This compound had a variety of bioactivities such as anti-inflammatory, lipid inhibitor and antiobesity (Ferdosi et al., 2021). Compound 27 was commonly used in the pharmaceutical, food, and chemical industries.
It was reported to be an effective the potent antibacterial against E. coli (Fajrih et al., 2022), while compounds 39 and 41 were found in the NW and TN, respectively (Figure 4). Compound 39 (nonacosanal), in terms of biological potential, medium chain aliphatic aldehydes exhibited antimicrobial activity, whereas triacontanal showed hepatoprotective activity (Kubo et al., 1995; Ramos et al., 2019). The compound 41 (obtusifoliol) had a high potential as a compound that can inhibit MCF-7 and MDA-MB231 breast cancer cell proliferation through the cell cycle, thereby halting development and inducing of apoptosis (Aghaei et al., 2016). Compound 2 was a flavonoid that was found in both HT and TN species (Figure 4). It was a powerful antioxidant with antibacterial, anti-inflammatory and anti-proliferative properties (Yu et al., 2013). Whereas compound 29 was only found in the NW (Figure 4). It was oleic acid which had anti-cancer, anti-autoimmune, and anti-inflammatory properties in addition to promoting wound healing. The role of oleic acid in immune response is still debated, as the successful elimination of pathogens such as bacteria and fungi (Sales-Campos et al., 2013). Thus, this type of GC-MS analysis is the first step in understanding the nature of active principles in medicinal plants, and it will be useful for further detailed research. It is possible to conclude that the different banana peel extracts contain a variety of bioactive compounds and NW banana peels can be a potential source of useful nutrients.
CONCLUSION
The banana peel extracts from NW, HT, and TN exhibited antioxidant potential, in vitro antimicrobial activity, and bioactive compounds. NW contained the most ethanolic extractives. The total phenolic content of NW was higher than that found in the other varieties. Therefore, NW also exhibited high FRAP, DPPH-antiradical and DNA damage activity. Furthermore, NW peel extracts may be effective as an antibacterial agent against both Gram-positive and Gram-negative bacteria. The bioactive compounds as identified by GC-MS suggested that banana peel extracts are rich in phytochemicals. The presence of these compounds supports the use of banana peel extract in pharmaceutical, drug delivery, and anti-inflammatory applications.
ACKNOWLEDGEMENTS
Appreciation is extended to Science Center of PSRU for providing facilities and laboratory supports. This research was financially supported by the Research and Development Institute (RDI) of Pibulsongkram Rajabhat University (PSRU) Grant No. RDI-1-64-2.
AUTHOR CONTRIBUTIONS
Wirot Likittrakulwong contributed to the concept and design of this work. Sanipon Chanburee and Thanapon Kitpot performed the banana peel extractions. Wirot Likittrakulwong and Pornkanok Pongpamorn identified bioactive compounds using GC-MS. Wirot Likittrakulwong and Padarat Ninjiaranai contributed to the analysis and interpretation data. Wirot Likittrakulwong drafted the manuscript. Wirot Likittrakulwong and Padarat Ninjiaranai contributed to the final approval of the version for publication. All authors have read and agreed to the published version of manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Aghaei, M., Yazdiniapour, Z., Ghanadian, M., Zolfaghari, B., Lanzotti, V., and Mirsafaee, V. 2016. Obtusifoliol related steroids from Euphorbia sogdiana with cell growth inhibitory activity and apoptotic effects on breast cancer cells (MCF-7 and MDA-MB231). Steroids. 115: 90–97.
Ameachi, N.C., and Chijioke, C.L. 2018. Evaluation of bioactive compounds in Pseudarenthemum tunicatum leaves using gas chromatography-mass spectrometry. Evaluation. 18: 53-59.
Aurore, G., Parfait, B., and Fahrasmane, L. 2009. Bananas, raw materials for making processed food products. Trends in Food Science and Technology. 20: 78–91.
Balouiri, M., Sadiki, M., and Ibnsouda, S.K. 2016. Methods for in vitro evaluating antimicrobial activity: A review. Journal of Pharmaceutical Analysis. 6: 71–79.
Baskar, R., Shrisakthi, S., Sathyapriya, B., Shyampriya, R., Nithya, R., and Poongodi, P. 2011. Antioxidant potential of peel extracts of banana varieties (Musa sapientum). Food and Nutrition Sciences. 2: 1128-1133
Chabuck, Z.A.G., Al-Charrakh, A. H., Hindi, N.K. K., and Hindi, S.K. K. 2013. Antimicrobial effect of aqueous banana peel extract, Iraq. Research Gate Pharmaceutical Science. 1: 73–75.
Dahham, S.S., Ali, M.N., Tabassum, H., and Khan, M. 2010. Studies on antibacterial and antifungal activity of pomegranate (Punica granatum L.). American-Eurasian Journal Agriculture and Enviroment Science. 9: 273–281.
Elaiyaraja, A., and Chandramohan, G. 2016. Comparative phytochemical profile of Indoneesiella echioides (L.) Nees leaves using GC-MS. Journal of Pharmacognosy and Phytochemistry. 5: 158-171.
Fajrih,N., Wiryawank, K.G., Sumiati, S., Syahpura, S.K., and Winarsih, W. 2022. Identification of bioactive compounds of banana corm (Musa paradisiaca) using GC-MS and its inhibitory effect against pathogenic bacteria. Biodiversitas Journal of Biological Diversity. 23: 195-204.
Ferdosi, M.F.H., Khan, I.H., Javaid, A., Nadeem, M., and Munir, A. 2021. Natural pesticidal compounds of Euphorbia prostrata. Pakistan Journal of Phytopathology. 33: 349–355.
González-Montelongo, R., Lobo, M. G., and González, M. 2010. Antioxidant activity in banana peel extracts: Testing extraction conditions and related bioactive compounds. Food Chemistry. 119: 1030–1039.
Gutteridge, J.M.C. 1984. Reactivity of hydroxyl and hydroxyl-like radicals discriminated by release of thiobarbituric acid-reactive material from deoxy sugars, nucleosides and benzoate. Biochemical Journal. 224: 761–767.
Hanafy, S.M., Abd El-Shafea, Y.M., Saleh, W.D., and Fathy, H.M. 2021. Chemical profiling, in vitro antimicrobial and antioxidant activities of pomegranate, orange and banana peel-extracts against pathogenic microorganisms. Journal of Genetic Engineering and Biotechnology. 19: 1–10.
Kubo, A., Lunde, C.S., and Kubo, I. 1995. Antimicrobial activity of the olive oil flavor compounds. Journal of Agricultural and Food Chemistry. 43: 1629–1633.
Likittrakulwong, W., Srikaeo, K., Poolprasert, P., Laorodphan, N., Incharoen, T., and Koonawootrittriron, S. 2020. Chemical composition, nutrient digestibility and metabolizable energy of germinated paddy rice. Animal Nutrition and Feed Technology. 20: 333–343.
Likittrakulwong, W., Poolprasert, P., and Srikaeo, K. 2021. Effects of extraction methods on protein properties obtained from paddy rice and germinated paddy rice. PeerJ. 9: e11365.
Moukamnerd, C., Ounmuang, K., Konboa, N., and Insomphum, C. 2020. Bacterial cellulose production by Komagateibacter nataicola TISTR 2661 by agro-waste as a carbon source. Chiang Mai Journal of Science. 47: 16-27.
Noysang, C., Buranasukhon, W., and Khuanekkaphan, M. 2019. Phytochemicals and pharmacological activities from banana fruits of several Musa species for using as cosmetic raw materials. Applied Mechanics and Materials. 891: 30-40.
Nuengchamnong, N., Krittasilp, K., and Ingkaninan, K. 2009. Rapid screening and identification of antioxidants in aqueous extracts of Houttuynia cordata using LC–ESI–MS coupled with DPPH assay. Food Chemistry. 117: 750–756.
Pascual, G., Avgustinova, A., Mejetta, S., Martín, M., Castellanos, A., Attolini, C.S.O., Berenguer, A., Prats, N., Toll, A., and Hueto, J.A. 2017. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature. 541: 41–45.
Porebski, S., Bailey, L.G., and Baum, B.R. 1997. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Molecular Biology Reporter. 15: 8–15.
Prommajak, T., Leksawasdi, N., and Rattanapanone, N. 2020. Tannins in fruit juices and their removal. Chiang Mai University Journal of Natural Sciences. 19: 76-90.
Rajkumar, P., Wang, N., EImasry, G., Raghavan, G.S.V, and Gariepy, Y. 2012. Studies on banana fruit quality and maturity stages using hyperspectral imaging. Journal of Food Engineering. 108: 194–200.
Ramos, P.A.B., Moreirinha, C., Santos, S.A.O., Almeida, A., Freire, C. S. R., Silva, A.M.S., and Silvestre, A.J.D. 2019. Valorisation of bark lipophilic fractions from three Portuguese Salix species: A systematic study of the chemical composition and inhibitory activity on Escherichia coli. Industrial Crops and Products. 132: 245–252.
Sales-Campos, H., Reis de Souza, P., Crema Peghini, B., Santana da Silva, J., and Ribeiro Cardoso, C. 2013. An overview of the modulatory effects of oleic acid in health and disease. Mini Reviews in Medicinal Chemistry. 13(2): 201–210.
Sermakkani, M., and Thangapandian, V. 2012. GC-MS analysis of Cassia italica leaf methanol extract. Asian Journal of Pharmaceutical and Clinical Research. 5(2): 90–94.
Singleton, V.L., Orthofer, R., and Lamuela-Raventós, R.M. 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods in Enzymology. 299: 152–178.
Sirajudin, Z.N.M., Ahmed, Q.U., Chowdhury, A.J.K., Kamarudin, E.Z., Khan, A. V., and Uddin, A. 2014. Antimicrobial activity of banana (Musa paradisiaca L.) peels against food borne pathogenic microbes. Journal of Pure and Appled Microbiology. 8: 3627–3639.
Srikaeo, K., Sangkhiaw, J., and Likittrakulwong, W. 2019. Productions and functional properties of palm sugars. Walailak Journal of Science and Technology. 16: 897–907.
Sulaiman, S.F., Yusoff, N.A.M., Eldeen, I.M., Seow, E.M., Sajak, A.A.B., and Ooi, K. L. 2011. Correlation between total phenolic and mineral contents with antioxidant activity of eight Malaysian bananas (Musa sp.). Journal of Food Composition and Analysis. 24: 1–10.
Tyagi, T., and Agarwal, M. 2017. Phytochemical screening and GC-MS analysis of bioactive constituents in the ethanolic extract of Pistia stratiotes L. and Eichhornia crassipes (Mart.) solms. Journal of Pharmacognosy and Phytochemistry. 6: 195–206.
Vu, H.T., Scarlett, C. J., and Vuong, Q.V. 2018. Phenolic compounds within banana peel and their potential uses: A review. Journal of Functional Foods. 40: 238–248.
Yen, G.C., Duh, P.D., and Tsai, C.L. 1993. Relationship between antioxidant activity and maturity of peanut hulls. Journal of Agricultural and Food Chemistry. 41: 67–70.
Yu, X., Zhao, M., Liu, F., Zeng, S., and Hu, J. 2013. Identification of 2, 3-dihydro-3, 5-dihydroxy-6-methyl-4H-pyran-4-one as a strong antioxidant in glucose–histidine Maillard reaction products. Food Research International. 51: 397–403.
Zhang, Q.W., Lin, L.G., and Ye, W.C. 2018. Techniques for extraction and isolation of natural products: A comprehensive review. Chinese Medicine. 13: 1–26.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand.

Figure 1. Appearance of banana fruits from several banana cultivars belonging to the Musa acuminata, Musa sapientum L and Musa balbisiana species. (namely “ Kluai Hom Thong” (HT), “Kluai Nam Wa” (NW) and “Kluai Ta Nee” (TN), (I): (HT-peels), (I-A): (HT-powder); (I-B): (HT-extraction), (II): (NW-peels), (II-A): (NW-powder), (II-B): (NW-extraction), (III): (TN-peels), (III-A): (TN-powder) and (III-B): (TN-extraction).
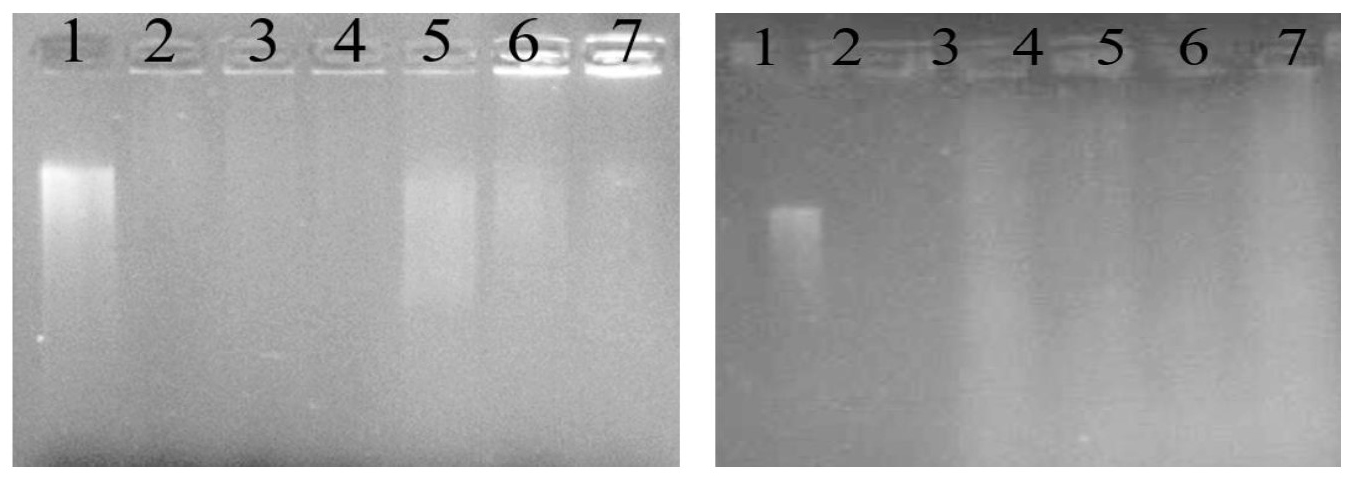
Figure 2. DNA damage activity induced by FeSO4 and H2O2 in the presence of banana peel extracts from various banana peels. (A) HT: Musa acuminata (Kluai Hom Thong), (B): TN: Musa balbisiana (Kluai Ta Nee). The lanes (1-2) are positive and negative controls, lane 3 is without the addition of peel extracts, and lane 4 – 7 are with the addition of peels extraction at 1, 3, 6 and 9 mg/ml, respectively
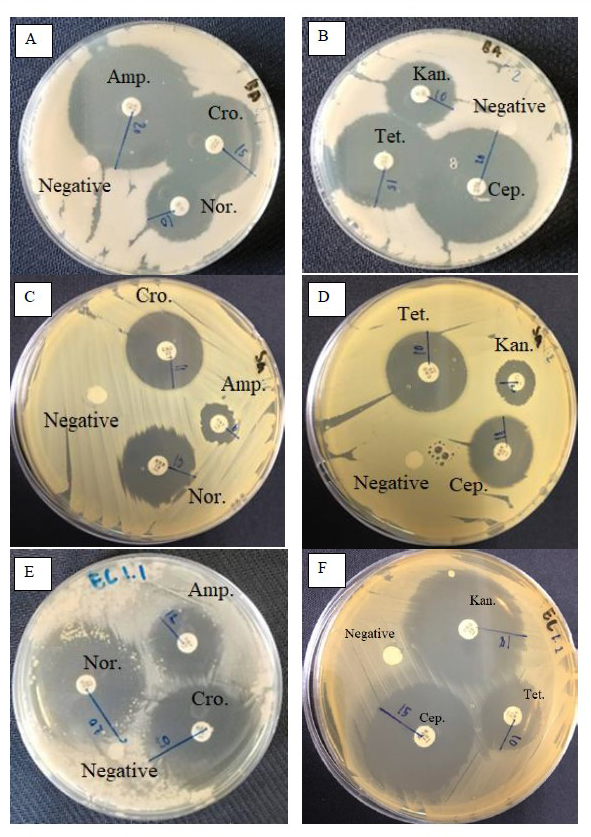
Figure 3. Disc diffusion tests against the some pathogenic bacteria (A,B): Bacillus subtilis, (C,D): Staphylococcus aureus, (E,F): Escherichia coli. Amp: Ampicillin, Nor: Norfloxacin, Cro: Ceftriaxone, Cep: Cephalothin, Tet: Tetracycline, Kan: Kanamycin. The negative controls were 1 % DMSO. Amp, Nor, Cro, Cep, Tet, and Kan were employed as positive controls.
Table 1. Physical properties and yield percentages of banana fruit extracts.
|
Varieties |
Color of extraction |
%Yield of extraction |
|
HT |
Light green |
7.18±0.13 |
|
NW |
Dark brown |
9.80±0.12 |
|
TN |
Dark green |
6.67±0.51 |
Note: HT: Musa acuminata (Kluai Hom Thong); NW: Musa sapientum L (Kluai Nam Wa) and TN: Musa balbisiana (Kluai Ta Nee). All measurements were taken in triplicate and the results reported as mean±standard deviation (S.D.)
Wirot Likittrakulwong1,*, Sanipon Chanburee2, Thanapon Kitpot2, Padarat Ninjiaranai3 and Pornkanok Pongpamorn4
1Animal Science Program, Faculty of Food and Agricultural Technology, Pibulsongkram Rajabhat University, Phitsanulok 65000, Thailand.
2Agro-Industrial Product Development Program, Faculty of Food and Agricultural Technology, Pibulsongkram Rajabhat University, Phitsanulok 65000, Thailand.
3Chemistry Program, Faculty of Science and Technology, Pibulsongkram Rajabhat University, Pibulsongkram Rajabhat University, Phitsanulok 65000, Thailand.
4National Omics Center (NOC), National Science and Technology Development Agency (NSTDS), Pathumthani 12120, Thailand.
Corresponding author: Wirot Likittrakulwong, E-mail: wirotliki@psru.ac.th
Total Article Views
Editor: Sirasit Srinuanpan,
Chiang Mai University, Thailand
Article history:
Received: October 12, 2022;
Revised: December 21, 2022;
Accepted: December 23, 2022;
Published online: January 13, 2023

