
Development of Gummy Jelly Incorporated with Lysiphyllum strychnifolium Leaf Extract and Its Antioxidant and α-Glucosidase Inhibitory Activities
Thavaree Thilavech, Achira Sutiyaporn, Pimpikar Kanchanadumkerng, Vilasinee Hirunpanich Sato, Warisara Parichatikanond, Phennapa Charoenwiwattanakij, and Savita Chewchinda*Published Date : April 3, 2023
DOI : https://doi.org/10.12982/NLSC.2023.019
Journal Issues : Number 2, April-June 2023
ABSTRACT
The reduction of sugar and the supplementation of bioactive ingredients in gummy jelly help to improve the healthy characteristics of a product that is normally devoid of any nutritional value. Therefore, the objective of this study was to develop a reduced calorie gummy jelly supplemented with Lysiphyllum strychnifolium leaf extract (LS gummy jelly). The gummy jelly was formulated by total substitution of sucrose with a sugar alcohol, xylitol. The antioxidant activities of LS gummy jelly determined by DPPH radical scavenging activity assay was 0.07 ± 0.02 g ascorbic acid equivalent/100 g gummy jelly. The results of ABTS radical scavenging activity and ferric reducing power (FRAP) assay were found to be 0.21 ± 0.02 g TEAC/100 g gummy jelly and 0.98 ± 0.07 mmol FeSO4/100 g gummy jelly, respectively. The percentage inhibition of α-glucosidase activity of LS gummy jelly (at concentration 10 mg/ml) was 46.1 ± 7.9%. The LS gummy jelly was successfully developed with good physical characteristics and negative results for microbiological tests. HPLC quantitative analysis of gallic acid, the major active compound in L. strychnifolium leaf extract, was found to be 0.45 ± 0.04 mg/g of gummy jelly. According to preference ranking test (n=50), the results showed that berry flavor was preferred for LS gummy jelly. Thus, LS gummy jelly could be considered as a promising antioxidant functional food product that could provide health benefits to consumers.
Keywords: α-glucosidase, Antioxidant, Gummy jelly, Lysiphyllum strychnifolium
Citation: Thilavech, T., Sutiyaporn, A., Kanchanadumkerng, P., Sato, V.H., Parichatikanond, W., Charoenwiwattanakij, P., and Chewchinda, S. 2023. Development of gummy jelly incorporated with Lysiphyllum strychnifolium leaf extract and its antioxidant and α-glucosidase inhibitory activities. Nat. Life Sci. Commun. 22(2): e2023019.
INTRODUCTION
Lysiphyllum strychnifolium (Craib) A. Schmitz which belongs to the family Fabaceae, is commonly known as Khayan or Yanang Dang in Thai (Larsen and Larsen, 1984). Its synonyms are Lysiphyllum strychnifolia and Bauhinia strychnifolia (Hao et al., 2003). The plant widely distributed in the North and Northeast of Thailand. In Thai traditional medicine, L. strychnifolium is one of Thai longevity medicinal plants. Dried stems and leaves of L. strychnifolium were prepared as herbal tea for detoxification and health promotion (Bunleupuech and Tewtrakul, 2011). Many chemical constituents had been reported such as astilbin, epicatechin, phloretin 4’-O-β-D-glucoside, phloretin 4’-O-(6”-O-galloyl)-β-D-glucoside, resveratrol and gallic acid (Kidruangphokin et al., 2022; Praparatana et al., 2022). L. strychnifolium leaf extract exhibited several biological activities including antioxidant, anticancer, and anti-malarial activities (Yuenyongsawad et al., 2013; Somsak et al., 2015; Itharat et al., 2016).
Functional foods are foods containing bioactive ingredients, which intended to provide health benefits beyond the nutritional value. They are slowly emerging on supermarket shelves worldwide (Lang, 2007). Most dietary bioactive compounds found in supplements are natural products having antioxidants and/or other positive activities for consumer’s health (Cencic and Chingwaru, 2010). The ideas to fortify natural plant extracts into a candy product have been developed. Gummy jelly candies represented approximately 50% of candy market value. Customers preferred gummy jelly candies due to their unique texture and chewability (Garcia, 2000). Gummy jellies are obtained from concentrated sucrose solutions, and gelling agents with minor ingredients such as food acids, flavoring, and coloring agents. Among ingredients used for making gummy, sweeteners are an important factor for achieving consumer acceptance. However, excessive sugar consumption-mediated reactive oxygen species (ROS) production has been shown to increase the risk of metabolic syndrome, diabetes mellitus, obesity, hypertension, and cardiovascular diseases (Agarwal, 2010). For this reason, artificial sweeteners have been used for sugar substitution in many confectionery products. Although artificial sweeteners contain few or no calories, there is a concern of its harm when consumed. An example of artificial sweetener widely used in food industry is xylitol. It is a natural sweetener derived from plants and agricultural materials. The energy contributed by xylitol is 2.4 kcal per gram which is lower than typical carbohydrates which can contribute 4 kcal per gram. Moreover, the advantage of xylitol can help in combating dental caries by reducing plaque formation, inhibiting enamel demineralization, and inhibiting Streptococcus mutans which caused tooth decay (Gupta, 2018). Therefore, the aim of this study was to formulate a reduced calorie gummy jelly incorporated with L. strychnifolium leaf extract. The antioxidant and α-glucosidase inhibitory activities of the gummy jelly were also investigated for its health benefits for the consumers.
MATERIALS AND METHODS
Plant materials
The leaves of L. strychnifolium were collected in Bangkok, Thailand. It was identified by Asst. Prof. Dr. Bhanubong Bongcheewin, Department of Pharmaceutical Botany, Faculty of Pharmacy, Mahidol University, Bangkok, Thailand in which the voucher specimen was deposited (voucher no. PBM-005667).
Extraction of L. strychnifolium leaf extract
L. strychnifolium leaves were dried at 50°C for 48 h and further ground into powder. Dried powdered leaves (100 g) were extracted using decoction method by boiling the leaves in water (1 L) for 15 min in triplicate then filtered through a Whatman membrane filter No.4. The filtrates were pooled and lyophilized by freeze dryer. The obtained powder was stored in a sealed container at -20°C until further used.
Preparation of reduced calorie gummy jelly incorporated with L. strychnifolium leaf extract (LS gummy jelly)
Calculation the dosage for LS gummy jelly
According to conversion of animal to human dose (Nair and Jacob, 2016), the formula of human equivalent dose (HED) is the animal no-observed-adverse-effect level (NOAEL) multiplied by an exponent for body surface area, which difference in metabolic rate, to convert doses between animals and humans. Thus, HED is determined by the equation:
HED (mg/kg) = Animal NOAEL (mg/kg) x (Weightanimal [kg]/Weighthuman[kg])(1-0.67)
NOAEL of L. strychnifolium leaf extract showed no toxic effect or any sign of toxicity (Somsak et al., 2015). Moreover, NOAEL result with high beneficial effect from animal test led to the selection of dose 200 mg/kg (Sato et al., 2019). While the weight of mice was approximately 35 g and the weight of human was reported to 60 kg. Hence, the optimal dose of HED was 1.63 mg/kg. Thereby, LS gummy jelly should contain approximately 97.80 mg of L. strychnifolium leaf extract per one serving (25 g).
Effect of replacing sucrose by xylitol on the qualities of gummy jelly
Preparation of gummy jelly was slightly modified according to Kanpairo (2018) method. The formulations consisted of edible gelatin (240 Bloom), citric acid solution at 50% concentration, glucose syrup (40 dextrose equivalent), sucrose, xylitol and water as shown in Table 1. For the cooking procedure, glucose syrup, sucrose, xylitol, and water were mixed, and then heated to about 116°C. The mixture was cooled down to 70°C, then gelatin solution and citric acid concentration were added into the previous mixture. Air bubbles were removed from the mixture before pouring into corn starch mold with dimension 1.5 x 1.5 x 1.5 cm3. Gummy jelly solution was allowed to be settled for at least 4 hours or until the firmness of the formulations were completely detected. The gummy jelly was kept in a closed container and protected from light at room temperature. These formulations were prepared by four different ratios of sucrose to xylitol. The appropriate ratio of sucrose to xylitol of gummy jelly was evaluated by texture profile analysis, and the suitable formula was further developed into LS gummy jelly.
Table 1. Formulations of gummy jelly in different ratio of sucrose to xylitol.
|
Formulation |
Percentage (% w/w) |
|||
|
F1 |
F2 |
F3 |
F4 |
|
|
Gelatin (240 Bloom) |
8 |
8 |
8 |
8 |
|
50% citric acid solution |
3 |
3 |
3 |
3 |
|
Glucose syrup |
20 |
20 |
20 |
20 |
|
Sucrose |
20 |
10 |
5 |
0 |
|
Xylitol |
0 |
10 |
15 |
20 |
|
Water |
q.s.100 |
q.s.100 |
q.s.100 |
q.s.100 |
Note: q.s.; quantum satis
Formulation of LS gummy jelly
From four gummy jelly formulations, F4 was the most suitable formulation which was chosen as standard formula for further development of LS gummy jelly. From the dosage calculation, LS gummy jelly should contain approximately 97.80 mg of L. strychnifolium leaf extract per one serving (25 g). Therefore, L. strychnifolium leaf extract was added 391.2 mg (⁓ 0.4 g) per 100 g of gummy jelly in the standard formula (Table 2).
Table 2. Formulation of LS gummy jelly.
|
Ingredients |
Percentage (% w/w) |
|
Gelatin (240 Bloom) |
8 |
|
50% Citric acid solution |
3 |
|
Glucose syrup |
20 |
|
Xylitol |
20 |
|
L. strychnifolium leaf extract |
0.4 |
|
Water |
q.s.100 |
Note: q.s.; quantum satis
Physical property of LS gummy jelly
Texture profile analysis
Texture properties of LS gummy jelly were evaluated using texture analyzer (Ametek, Inc., Berwyn, PA, USA). The experiment was conducted with texture profile analysis (TPA) mode using a back extruder probe, 50% height, and test speed at 1 mm/s (Charoen et al., 2015). Hardness, gumminess, chewiness, and springiness of the products were determined.
Chemical property of LS gummy jelly
Determination of total phenolic content
Total phenolic content of LS gummy jelly was evaluated according to the method of Singleton et al. (1999). The sample solution (20 µl of 1 g/ml) and Folin-Ciocalteu’s reagent (50 µl) (diluted in 1:10 ratio with deionized water) were mixed for 3 min before adding 7.5% w/v sodium carbonate (80 µl). After 2 h of incubation in the darkness at room temperature, the absorbance was measured at 765 nm using microplate reader (Tecan Group Ltd., Männedorf, Switzerland). Total phenolic contents of each sample were calculated from calibration curve of gallic acid which expressed as gram of gallic acid equivalent per 100 g of gummy jelly (g GAE/100 g gummy jelly).
Determination of total flavonoid content
Total flavonoid content was carried out following the previous study (Stankovic, 2011). The sample solution (100 µl) at concentration of 1 g/ml was mixed with 2% w/v aluminium chloride solution (100 µl) in methanol. The mixture was incubated for 10 min at room temperature. The absorbance of the reaction was measured at 415 nm. The content of flavonoids was expressed in term of gram of quercetin equivalent per 100 g of gummy jelly (g QE/100 g gummy jelly).
Determination of gallic acid content in LS gummy jelly by HPLC
LS gummy jelly (25 g) was accurately weighed. It was heated in a water bath with 20 ml of 12% HCl for 30 min, and then cooled to room temperature. The sample solution was triplicately extracted with 10 ml of diethyl ether using a separatory funnel. The supernatant was dried and re-dissolved with methanol, then adjusted the volume to 5-ml using volumetric flask. Prior to the injection, each solution was filtered through a 0.45 µm nylon membrane filter and then analyzed in triplicate. For standard gallic acid, stock solution of standard gallic acid was accurately weighed and dissolved in methanol to obtain the final concentration of 1,000 µg/ml. Working solutions of standard compound were obtained by diluting the stock standard solution with methanol to achieve the desired concentrations (1.56 – 50 µg/ml). The analysis of gallic acid content in LS gummy jelly by HPLC method was done according to a previous study (Deshmukh and Prabhu, 2011). The mobile phase contained 0.05% ortho-phosphoric acid (solvent A) and methanol (solvent B) at ratio of solvent A: solvent B of 90:10. HPLC analysis was carried on using a BDS Hypersil C18 column (150 x 4.6 mm, i.d. 5 µm) with a guard column. HPLC chromatograms were detected using a UV detector at wavelength of 271 nm. The flow rate and injection volume were 1.0 ml/min and 20 µl, respectively. The content of gallic acid in LS gummy jelly was determined at 0, 1st, 2nd, 3rd, and 4th week after storage at room temperature.
Biological properties of LS gummy jelly
DPPH radical scavenging assay
DPPH scavenging activity was analyzed by a method provided by Sithisarn
et al. (2015). DPPH radical was freshly prepared in methanol with final concentration of 152 μM. In 96-well plate, 100 μl of each sample was added to each well followed by adding 100 μl of methanolic DPPH solution. The mixtures were placed at room temperature in dark condition for 30 min. The absorbance was recorded at 517 nm. Ascorbic acid was used to establish the standard curve. The results were expressed as gram ascorbic acid equivalent (AAE)/100 g gummy jelly.
ABTS radical scavenging assay
The procedure for ABTS assay was modified from Thaipong et al. (2006) study. The stock solutions included 7 mM ABTS solution and 2.45 mM potassium persulfate solution. The working solution was prepared by mixing two stock solutions in equal quantities and allowing them to react for 12-16 h in dark condition at room temperature. The solution was then diluted by mixing 1 ml ABTS solution with 25 ml of methanol to obtain an absorbance of 1.100 ± 0.020 at wavelength 734 nm. Fresh ABTS▪+ solution was prepared for each assay. The assay was performed by mixing 10 µl of sample with 200 µl of ABTS▪+ radical cation solution. The mixture solution was incubated at room temperature for 6 min and read at wavelength 734 nm. Trolox was used to establish the standard curve. The results were expressed as gram Trolox equivalent antioxidant capacity per 100 g of gummy jelly (g TEAC/100 g gummy jelly).
Ferric reducing power (FRAP) assay
Ferric reducing power assay was carried out according to the method of Vongsak et al. (2013). The sample solution (500 µl of 1 g/ml) was mixed with 500 µl of potassium phosphate buffer (0.2 M, pH 6.6) and 500 µl of 1% w/v potassium ferricyanide solution. The mixture was incubated at 50°C for 20 min and further added with 2 ml of trichloroacetic acid to stop the reaction. Then, 100 µl of supernatant was mixed with 100 µl of deionized water and then added 20 µl of 0.1% w/v ferric chloride solution. The procedure was carried out in triplicate and allowed the reaction to occur for 30 min before measuring the absorbance at 700 nm. The results were expressed as mmol ferrous sulfate equivalent per 100 g of gummy jelly (mmol FeSO4/ 100 g gummy jelly).
In vitro α-glucosidase inhibitory activity
The method was determined according to spectrophotometric method described by Supasuteekul et al. (2016) with some modifications. Briefly, 10 μl of sample
(at concentration of 10 mg/ml) was gently mixed with 40 μl of α-glucosidase enzyme (0.1 U/ml) in 0.1 M phosphate buffer (pH 6.9). After incubation at 37°C for 10 min, 50 μl of 2 mM p-nitrophenyl α-D-glucopyranoside (pNPG) was added to initiate the reaction and further incubated at 37°C for 20 min. The reaction mixture was terminated by adding 100 μl of 1 M Na2CO3. The p-nitrophenol released from the pNPG turned the solution to be yellow which was detected at wavelength 405 nm. For the reaction blank, pNPG was replaced by phosphate buffer. The percentage of inhibition was calculated using the equation below.
α-glucosidase inhibitory activity (%) = [(Ac-As)/Ac] × 100
Where Ac and As were the absorbance of control and sample, respectively.
Microbiological analysis of LS gummy jelly
According to Ministry of Public Health Notification No.364 B.E. 2556 Re: Standards for pathogenic microorganisms in food have limitation as following: Clostridium spp. is less than 100 cfu/g, Staphylococcus aureus is not detected in
0.1 g of sample while Salmonella spp. is not detected in 25 g of sample. The microbiological analysis of LS gummy jelly was investigated by Center of Analysis for Product Quality (MUPY-CAPQ), Faculty of Pharmacy, Mahidol University, Bangkok, Thailand. The specimen number was RM180-069.
Sensory evaluation of LS gummy jelly
To evaluate the impact of flavor on the acceptability of food product, LS gummy jelly with no added flavor, pineapple flavor, and berry flavor were examined by sensory evaluation (Meilgaard et al., 1991). The study was exempted by Institutional Review Board of Faculty of Dentistry and Faculty of Pharmacy, Mahidol University, with COE.No.MU-DT/PY-IRB 2018/016.1603. Sensory evaluation was performed using 50 untrained panelists of both genders and ages ranged from 18-70 years. Each sample contained 3 pieces of gummy jelly given in plastic container at room temperature. Three digits represented three different flavors which were randomly picked to avoid bias. The ranking for preference model was used for the evaluation of the developed gummy jelly product. Three samples were examined by ranking by numbers, 1=1st order, 2=2nd order, and 3=3rd order. The ranking test was analyzed by Friedman’s test using SPSS program (version 18.0).
Statistical analysis
All experiments were performed in triplicate. Data were expressed as mean ± SD. Statistical analysis was performed using SPSS program (version 18.0). One-way ANOVA followed by LSD’s post-hoc analysis was conducted to compare the difference of each group whereas the student’s t test was used to compare the difference of texture profile analysis between standard and LS gummy jelly. Differences in results were considered significant at P < 0.05.
RESULTS
Physical properties of LS gummy jelly
Physical appearance
The shape of LS gummy jelly was individually molded into a square shape using corn flour as base. The characteristics of LS gummy jelly were brownish-yellow color (Figure 1), elastically firm, with sour smell. The taste was sweet and sour without after taste. LS gummy jelly was found physically stable for at least 4 weeks.

Figure 1. The appearance of LS gummy jelly
Texture profile analysis
The standard formula of gummy jelly was developed by substitution of sucrose with xylitol at various ratios to minimize the sucrose content for reducing calorie intake. The texture profile analysis of different formulations is shown in Table 3. Hardness, gumminess, and chewiness of F3 and F4 significantly decreased when compared to F1 and F2. However, no difference was observed for springiness. The results revealed that the elevation of xylitol to sucrose ratio influenced the hardness and gumminess of the product. The formula with the highest proportion of xylitol (F4) was selected as the standard formula. The LS gummy jelly was developed by supplemented with 0.4% L. strychnifolium leaf extract in the standard formula. Texture profile analysis showed that the hardness, springiness, and chewiness of LS gummy jelly were similar to standard formulation (F4) whereas the incorporation of L. strychnifolium leaf extract significantly increased the gumminess of the reduced calorie gummy jelly when compared to standard formula.
Table 3. Texture profile analysis of the reduced calorie gummy jelly with different ratio of sucrose to xylitol and the gummy jelly supplemented with L. strychnifolium leaf extract.
|
Formula
|
Texture profile analysis |
|||
|
Hardness (N) |
Springiness |
Gumminess (N) |
Chewiness (N) |
|
|
F1 |
5.65 ± 1.11a |
0.94 ± 0.06a |
4.15 ± 0.83a |
3.90 ± 0.80a |
|
F2 |
4.85 ± 1.26a |
1.00 ± 0.09a |
3.76 ± 1.01a |
3.80 ± 1.20a |
|
F3 |
3.68 ± 0.83b |
0.95 ± 0.08a |
2.83 ± 0.54b |
2.70 ± 0.63b |
|
F4 |
3.62 ± 0.49b |
0.97 ± 0.08a |
2.61 ± 0.34b |
2.52 ± 0.30b |
|
LS gummy jelly |
3.47 ± 0.33 |
0.94 ± 0.07 |
3.31 ± 0.31* |
2.41 ± 0.28 |
Note: Data are expressed as mean ± SD in Newton (N) for hardness, gumminess, and chewiness and in ratio of a product's original height for springiness. Dissimilar letters in the same column indicated significantly different (P < 0.05). * P < 0.05 when compared to standard formula (F4).
Chemical properties of LS gummy jelly
Total phenolic and total flavonoid contents in LS gummy jelly
L. strychnifolium leaf extract from decoction method has been reported to contain total phenolic and total flavonoid contents by 197.82 ± 5.78 mg GAE/g extract and 32.22 ± 1.23 mg QE/g extract, respectively (Sato et al., 2019). In the present study, the addition of 0.4% L. strychnifolium leaf extract increased total phenolic and total flavonoid contents when compared to standard gummy jelly (Table 4). The total phenolic content of LS gummy jelly was 0.17 ± 0.01 g GAE/100 g LS gummy jelly and total flavonoid content was 0.003 ± 0.0003 g QE/100 g LS gummy jelly. The results showed that the incorporation of L. strychnifolium leaf extract as a source of bioactive compounds into gummy jelly may help to improve its functional property.
Gallic acid content in LS gummy jelly by HPLC method
Previous phytochemical studies revealed that gallic acid was a major compound in L. strychnifolium leaf extract (Kongkiatpaiboon et al., 2020; Maitree et al., 2018). The HPLC chromatogram of gallic acid in LS gummy jelly was corresponded with gallic acid reference standard with the retention time at 3.9 min (Figure 2). Gallic acid contents in LS gummy jelly at 0, 1st, 2nd, 3rd, and 4th week were 0.45 ± 0.04, 0.51 ± 0.03, 0.49 ± 0.02, 0.50 ± 0.05, and 0.47 ± 0.04 mg/g of gummy jelly, respectively (Figure 3). The amount of gallic acid at 4th week was found no significant difference from day 0, which indicated the stability of gallic acid content in gummy jelly maintaining at room temperature for at least 4 weeks.
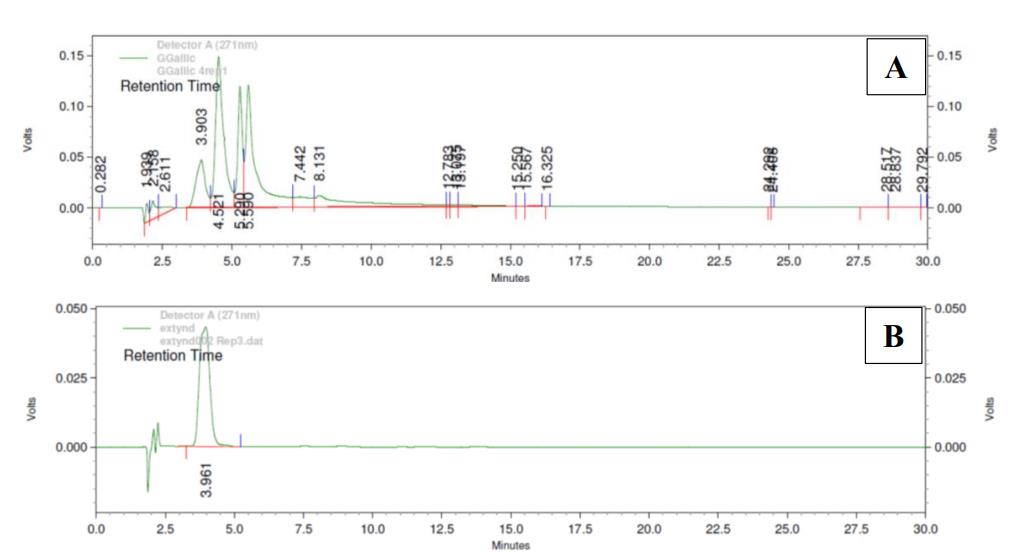
Figure 2. HPLC chromatogram of (A) LS gummy jelly and (B) gallic acid reference standard
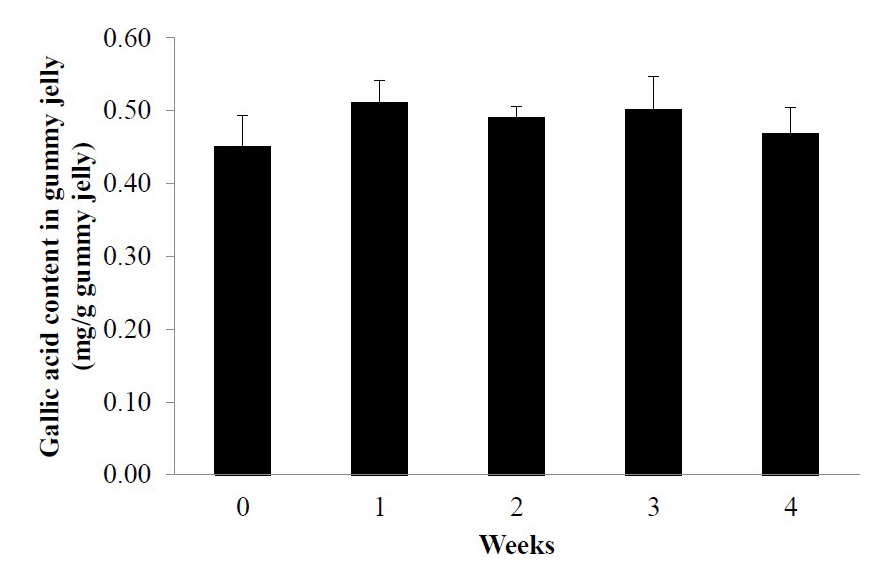
Figure 3. Gallic acid contents in LS gummy jelly at 0, 1st, 2nd, 3rd, and 4th week after storage. Data are expressed as mean ± SD (n = 3).
Biological properties of LS gummy jelly
Antioxidant activities of LS gummy jelly
The fortification of L. strychnifolium leaf extract increased the antioxidant activities of the standard gummy jelly gummy jelly (Table 4). The DPPH radical scavenging activity was 0.07 ± 0.02 g AAE/100 g gummy jelly. The results of ABTS and FRAP assays were found to be 0.21 ± 0.02 g TEAC/100 g gummy jelly and 0.98 ± 0.07 mmol FeSO4/100 g gummy jelly, respectively.
In vitro α-glucosidase inhibitory activity
Inhibitory activity of LS gummy jelly against α-glucosidase was 46.09 ± 7.9% at concentration of 10 mg/ml which was greater than standard gummy jelly at equal concentration by 4.7-fold (Table 4).
Table 4. Total phenolic, total flavonoid content, antioxidant, and α-glucosidase inhibitory activities of gummy jelly.
|
|
Standard gummy jelly |
LS gummy jelly |
|
TPC (g GAE/100 g gummy jelly) |
0.03 ± 0.01 |
0.17 ± 0.01 |
|
TFC (g QE/100 g gummy jelly) |
N.D. |
0.003 ± 0.0003 |
|
DPPH (g AAE/100 g gummy jelly) |
N.D. |
0.07 ± 0.02 |
|
ABTS (g TEAC/100g gummy jelly) |
N.D. |
0.21 ± 0.02 |
|
FRAP (mmol FeSO4/100 g gummy jelly) |
0.07 ± 0.01 |
0.98 ± 0.07 |
|
α-glucosidase inhibitory activity (%) |
9.8 ± 1.0 |
46.1 ± 7.9 |
Note: Data are expressed as mean ± SD (n = 3). N.D.; not detectable, GAE; gallic acid equivalent, QE; quercetin equivalent, AAE; ascorbic acid equivalent, TPC; total phenolic content, TFC; total flavonoid content, TEAC; trolox equivalent antioxidant capacity.
Microbiological analysis of LS gummy jelly
Clostridium spp., Staphylococcus aureus, and Salmonella spp. are pathogenic bacteria which are the most common cause of food product contamination and foodborne diseases (Bintsis, 2017). The microbiological test for LS gummy jelly exhibited negative results of Clostridium spp., Staphylococcus aureus, and Salmonella spp. which fall under the legislation of Ministry of Public Health, Thailand for processed gelatin and jelly desserts.
Sensory evaluation of LS gummy jelly
Three flavors of LS gummy jelly (no added flavor, pineapple flavor, and berry flavor) were examined by sensory evaluation with ranking by preference method. The results showed that the berry was preferred flavor for LS gummy jelly according to the lowest ranking score. The pineapple flavor and no added flavor were found to be second and third rank, respectively (Table 5).
Table 5. Ranking test for preference of three different flavors in LS gummy jelly (n=50).
|
Score |
Ranking score |
||
|
No added flavor |
Pineapple flavor |
Berry flavor |
|
|
Total ranking score |
124 |
101 |
75 |
|
Ranking of mean score |
2.48c |
2.02b |
1.50a |
Note: Ranking score; 1=1st order, 2=2nd order, and 3=3rd order. Dissimilar letters in the same row indicated significantly different (P < 0.05).
DISCUSSION
Physical properties of LS gummy jelly
The reduced calorie gummy jelly was formulated by replacing sucrose with xylitol. The optimal ratio of sucrose to xylitol was evaluated by texture profile analysis in terms of hardness, springiness, gumminess, and chewiness since these characteristics had the most impact on the rigidness of the gummy jelly formulating by the combining of sucrose into gelatin (Jiamjariyatam, 2018; Oakenfull and Scott, 1986). Moreover, glucose syrup was another ingredient which could improve the textural properties by reducing the haziness, enhancing thermal stability, and supporting gel structure. Additionally, the ratio of this component barely affected the formulation (Burey et al., 2009; Holm et al., 2009). The findings revealed that xylitol influenced loosen cohesion in gummy jelly. Previous studies supported that polyol (sugar alcohol) could obstruct the occupation of gelatin and water and caused the gel network structure to be closer. Moreover, xylitol concentration might cover up the binding sites and inhibit the cross-linking of gelatin molecules to form the network as well as weaken the gel strength (Gekko et al., 1992; Shimizu and Matubayasi, 2014). However, the gummy jelly with total substitution of sucrose with xylitol (F4 formula) was formed with good physical property and accepted as standard formula for developing LS gummy jelly. In addition, as xylitol provides only 2.4 kcal/gram, the replacing of sucrose with xylitol also helps to reduced calorie obtained from the standard gummy jelly. Although the gumminess of standard gummy jelly affected by the replacement of sucrose with xylitol, it was improved by the incorporation of 0.4% L. strychnifolium leaf extract in standard gummy jelly with no impact on the hardness, springiness, and chewiness.
Chemical properties of LS gummy jelly
Previous phytochemical study reported that gallic acid, trilobatin (phloretin-4’- β-D-glucoside) and yanangdaengin (phloretin 4’-O-(6”-O-galloyl)-β-D-glucoside) were isolated from the leaves of L. strychnifolium (Kongkiatpaiboon et al., 2020). In accordance with the study of Kidruangphokin et al. (2022) which isolated quercetin 3-O-β-D-arabinoside, phloretin, phloretin 4’-O-β-D-glucoside, phloretin 4’-O-(6”-O-galloyl)-β-D-glucoside and pinitol from L. strychnifolium leaf extract.
From the results of HPLC analysis, gallic acid was found as a major compound in LS gummy jelly. The results were related to previous studies which found that gallic acid was one of the bioactive components in L. strychnifolium leaf and stem extract (Praparatana et al., 2022; Nammatra and Photong, 2017). Additionally, gallic acid content could be enhanced by acid hydrolysis of yanangdaengin during sample preparation step (Kongkiatpaiboon et al., 2020). Structurally, gallic acid (3,4,5-trihydroxybenzoic acid) consist of an aromatic ring, three phenolic hydroxyl groups and a carboxylic acid group. The phenolic group could be served as a source of readily available hydrogen atoms such that the subsequent radicals produced can be delocalized over the phenolic structure (Robards et al., 1999; Nikolic, 2006). The phenolic group was found to be interested in its pharmacological activity as radical scavengers which demonstrated preventive and therapeutic effects in many diseases such as diabetes, cardiovascular and neurodegenerative diseases (Karamac et al., 2005; Kaur et al., 2005). In the present study, the content of gallic acid in LS gummy jelly was stable for 4 week-study. Long-term stability testing should be conducted to determine the shelf-life and storage conditions.
Biological properties of LS gummy jelly
Previous study from Itharat et al. (2016) reported strong DPPH radical scavenging activity of L. strychnifolium stem and leaf extract with the IC50 value range from 4.21 to 8.74 µg/ml. The ferric reducing antioxidant power (FRAP) values of both extracts ranged from 1481.21 to 627.58 mg Fe (II)/g sample. Moreover, the antioxidant activities of three isolated compounds from L. strychnifolium leaves, gallic acid, trilobatin and yanangdaengin, showed the DPPH IC50 value of 5.99, 51.59 and 5.03 mmol/L, respectively (Kongkiatpaiboon et al., 2020). Thus, these results supported the folklore knowledge for the use of L. strychnifolium stems and leaves for detoxification and prevention of chronic diseases as recommended by Thai traditional medicine practitioners.
The results of antioxidant activity of LS gummy jelly were consistent with previous study indicating the significant correlation between content of phenolic compounds and good antioxidant capacities in medicinal plants (Guo et al., 2008; Safdar et al., 2022). For example, the date bars supplemented with the polyphenolic extracts of moringa leaf and tamarind seed showed greater DPPH radical scavenging activity which was attributed to the elevated total polyphenolic content in the date bars (Safdar et al., 2022).
α-glucosidase is a key enzyme that is responsible for the breakdown of carbohydrates to monosaccharides, in order to be absorbed to enterocytes. Therefore, the inhibition of α-glucosidase is a therapeutic strategy to diminish postprandial hyperglycemia by modifying the intestinal absorption of carbohydrate (Dirir et al., 2022). The antidiabetic property of L. strychnifolium stem extract through α-glucosidase inhibition has recently been reported. The IC50 value of the crude extract to inhibit α-glucosidase activity was 2.37 ± 0.13 µg/ml (Praparatana et al., 2022). Another recent study also reported the DPPH radical scavenging activity and α-glucosidase inhibitory activity of L. strychnifolium leaf extract at the IC50 values of 3.44 ± 0.53 and 21.95 ± 2.95 µg/ml, respectively (Kidruangphokin et al., 2022). The α-glucosidase activities of L. strychnifolium might be due to the presence of high amount of flavonoids and phenolic compounds in the leaf extract. According to the previous in vitro studies, gallic acid exhibited a potent inhibition of α-glucosidase activity (Oboh et al., 2016, Adefegha et al., 2015). Moreover, gallic acid could produce synergistic effects with acarbose, an α-glucosidase inhibitor used to treat diabetes, to reduce postprandial rise in blood glucose level (Oboh et al., 2016). In addition to the inhibitory activity against α-glucosidase, the recent study of Variya et al. (2020) demonstrated that the isolated gallic acid from medicinal plant offered the potent antidiabetic effect by regulating the activation of peroxisome proliferation-activated receptor gamma (PPAR-γ) and Akt to promote the upregulation and translocation of GLUT4 in T2DM mice.
Inhibitory activity of LS gummy jelly against α-glucosidase was 46.09 ± 7.9% at concentration of 10 mg/ml which was greater than standard gummy jelly at equal concentration by 4.7-fold. However, further studies are needed to evaluate the effect of LS gummy jelly consumption together with foods or beverages high in carbohydrates and sugar on postprandial blood glucose.
Sensory evaluation of LS gummy jelly
According to the results, the flavoring agent indicated the impact on product preference. Berry flavor is synthetic flavoring agent, providing citrus-like smell which correlated to sour taste of gummy jelly. On the contrary, the flavor in no added flavor sample was natural flavor from L. strychnifolium leaf extract which may lead to disapproval of the panelists.
CONCLUSION
L. strychnifolium leaf extract was successfully incorporated into the reduced calorie gummy jelly product. LS gummy jelly was physically and chemically stable in the period of 4 weeks with negative results for microbiological analysis. Administration of this product could provide health benefits to consumers in term of its antioxidant and α-glucosidase inhibitory activities. For sensory evaluation, the results showed that LS gummy jelly with berry flavor was preferred. Additionally, the stability test of LS gummy jelly should be further conducted in long-term storage condition to ensure the quality of the product throughout their shelf life.
AUTHOR CONTRIBUTIONS
Thavaree Thilavech wrote original draft, conducted research methodology, investigation, and data analysis. Achira Sutiyaporn conducted the experiments. Pimpikar Kanchanadumkerng assisted in conceptualization, writing – review & editing, conducted research methodology, data analysis, research summary and recommendation. Vilasinee Hirunpanich Sato, Warisara Parichatikanond and Phennapa Charoenwiwattanakij assisted in writing – review & editing and recommendation. Savita Chewchinda supervised, conducted research methodology, writing – review & editing, data analysis, research summary and recommendation. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Adefegha, S.A., Oboh, G., Ejakpovi, I.I., and Oyeleye, S.I. 2015. Antioxidant and antidiabetic effects of gallic and protocatechuic acids: A structure-function perspective. Comparative Clinical Pathology. 24: 1579-1585.
Agarwal, V., Kochhar, A., and Sachdeva, R. 2010. Sensory and nutritional evaluation of sweet milk products prepared using stevia powder for diabetics. Studies on Ethno-Medicine. 4: 9-13.
Bintsis, T. 2017. Foodborne pathogens. AIMS Microbiology. 3: 529–563.
Bunluepuech, K., and Tewtrakul, S. 2011. Anti-HIV-1 integrase activity of Thai medicinal plants in longevity preparations. Warasan Songkhla Nakharin. 33: 693-697.
Burey, P., Bhandari, B.R., Rutgers. R.P.G., Halley, P.J., and Torley, P.J. 2009. Confectionery gels: A review on formulation rheological and structural aspects. International Journal of Food Properties. 12: 176-210.
Cencic, A., and Chingwaru, W. 2010. The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients, 2: 611-625.
Charoen, R., Savedboworn, W., Phuditcharnchnakun, S., and Khuntaweetap, T. 2015. Development of antioxidant gummy jelly candy supplemented with Psidium guajava leaf extract. KMUTNB International Journal of Applied Science and Technology. 8: 145-151.
Deshmukh, H., and Prabhu, P.J. 2011. Development of RP-HPLC method for qualitative analysis of active ingredient (gallic acid) from stem bark of Dendrophthoe falcate Linn. International Journal of Pharmaceutical Sciences and Drug Research. 3: 146-149.
Dirir, A.M., Daou, M., Yousef, A.F., and Yousef, A.L. 2022. A review of alpha-glucosidase inhibitors from plants as potential candidates for the treatment of type-2 diabetes. Phytochemistry Reviews. 21:1049-1079.
Garcia, T. 2000. Analysis and gelatin-based confections. The Manufactoring Confectioner. 80: 93-101.
Gekko, K., Xuan, L., and Mano, S. 1992. Effects of polyols and sugars on the sol-gel transition of gelatin. Bioscience, Biotechnology, and Biochemistry. 56: 1297-1284.
Guo, D.J., Cheng, H.L., Chan, S.W., and Yu, P.H. 2008. Antioxidative activities and the total phenolic contents of tonic Chinese medicinal herbs. Inflammopharmacology. 16: 201-207.
Gupta, M., 2018. Sugar substitutes: Mechanism, availability, current use and safety concerns-an update. Open Access Macedonian Journal of Medical Sciences. 6: 1888–1894.
Hao, H., Zhang, D.X., Zhang, M.Y., Guo, L.X., and Li, S.J. 2003. Phylogenetics of Bauhinia subgenus Phanera (Leguminosae: Caesalpinioideae) based on ITS sequences of nuclear ribosomal DNA. Botanical Bulletin of Academia Sinica. 44: 223-228.
Holm, K., Wendin, K., and Hermansson, A. 2009. Sweetness and texture perceptions in structured gelatin gels with embedded sugar rich domain. Food Hydrocolloid. 23: 2388-2393.
Itharat, A., Sayompark, S., Hansakul, P., and Dechayont, B. 2016. In vitro antioxidant activities of extracts of Bauhinia strychnifolia stems and leaves: Comparison with activities in green tea extracts. Medicinal & Aromatic Plants. 5: 243.
Jiamjariyatam, R. 2018. Influence of gelatin and isomaltulose on gummy jelly properties. International Food Research Journal. 25: 776-783.
Kanpairo, K. 2018. Effect of different sweeteners on the quality of Torch ginger. Burapha Science Journal. 23: 944-958.
Karamac, M., Kosiñska, A., and Pegg, R.B. 2005. Comparison of radical–scavenging activities of selected phenolic acids. Polish Journal of Food and Nutrition Sciences. 14: 165-170.
Kaur, S., Michael, H., Arora, S., Harkonen, P.L., and Kumar, S. 2005. The in vitro cytotoxic and apoptotic activity of Triphala-an Indian herbal drug. Journal of Ethnopharmacology. 97: 15-20.
Kidruangphokin, M., Suphrom, N., and Boonphong, S. 2022. α-glucosidase inhibitory and antioxidant activities of ethanolic extracts of different parts of Lysiphyllum strychnifolium and their constituents, Journal of Herbs, Spices & Medicinal Plants.
Kongkiatpaiboon, S., Duangdee, N., Tayana, N., Schinnerl, J., Bacher, M., and Chewchinda, S. 2020. Yanangdaengin, a dihydrochalcone glucoside galloyl ester as active antioxidative agent from leaves of Lysiphyllum strychnifolium (syn. Bauhinia strychnifolia). Chinese Herbal Medicines. 12: 452-455.
Lang, T. 2007. Functional foods. BMJ. 334: 1015-1016.
Larsen, K., and Larsen, S.S. 1984. Leguminosae-Caesalpinioideae. Flora of Thailand. vol. 4, TISTR Press, Bangkok.
Maitree, M., Sato, V.H., Sithisarn, P., and Chewchinda, S. 2018. Evaluation of antioxidant activities and HPTLC analysis of Lysiphyllum strychnifolium (Craib) A. Schmitz leaf extract. Thai Journal of Pharmaceutical Sciences. 42: 22-26.
Meilgaard, M., Civille, G.V., Carr, and B.T. 1991. Sensory Evaluation Techniques. 2nd, CRC Press, Boca Raton.
Nair, A.B., and Jacob, S. 2016. A simple practice guide for dose conversion between animals and human. Journal of Basic and Clinical Pharmacy. 7: 27-31.
Nammatra, R., and Photong, C. 2017. Chemical compositions and antioxidant capacities of Bauhinia strychnifolia Craib: Grilling with a double delt conveyor dryer. Thai Society of Agricultural Engineering Journal. 23: 44-51.
Nikolic, K. 2006. Theoretical study of phenolic antioxidants properties in reaction with oxygen-centered radicals. Journal of Molecular Structure: THEOCHEM. 774: 95-105.
Oakenfull, D., and Scott, A. 1986. Stabilization of gelatin gels by sugars and polyols. Food Hydrocolloids. 1: 163-175.
Oboh, G., Ogunsuyi, O.B., Ogunbadejo, M.D., and Adefegha, S.A. 2016. Influence of gallic acid on α-amylase and α-glucosidase inhibitory properties of acarbose. Journal of Food and Drug Analysis. 24: 627–634.
Praparatana, R., Maliyam, P., Barrows, L.R., and Puttarak, P. 2022. Flavonoids and phenols, the potential anti-diabetic compounds from Bauhinia strychnifolia Craib. stem. Molecules. 27: 2393.
Robards, K., Prenzler, P.D., Tucker, G., Swatsitang, P., and Glover, W. 1999. Phenolic compounds and their role in oxidative processes in fruits. Food Chemistry. 66: 401-436.
Safdar, M.N., Baig, U.Y., Riaz, M.M., Mumtaz, A., Jabbar, S., Ur-Rehman, N., Ahmad, Z., Malok, H., and Yousef, S. 2022. Extraction of polyphenols from different herbs for the development of functional date bars. Food Science & Technology. 42: e43521.
Sato, V.H., Chewchinda, S., Nuamnaichati, N., Mangmool, S., Sungthong, B., Lertsatitthanakorn, P., Ohta, S., and Sato, H. 2019. Pharmacological mechanisms of the water leaves extract of Lysiphyllum strychnifolium for its anti-inflammatory and anti-hyperuricemic actions for gout treatment. Pharmacognosy Magazine. 15: 98-106.
Shimizu, S., and Matubayasi, N. 2014. Gelation: the role of sugars and polyols on gelatin and agarose. Journal of Physical Chemistry B. 118: 13210-13216.
Singleton, V.L., Orthofer, R., and Lamuela-raventos, R.M. 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-ciocalteu reagent. Methods in Enzymology. 299: 152-178.
Sithisarn, P., Rojsanga, P., Sithisarn, P., and Kongkiatpaiboon, S. 2015. Antioxidant activity and antibacterial effects on clinical isolated Streptococcus suis and Staphylococcus intermedius of extracts from several parts of Cladogynos orientalis and their phytochemical screenings. Evidence-Based Complementary and Alternative Medicine. 2015: 908242.
Somsak, V., Noilod, J., Chachiyo, S., and Kraithep, S. 2015. Antimalarial activity of ethanolic leaf extract of Bauhinia strychnifolia in mice infected with Plasmodium berghei. Malaria Control & Elimination. 4: 1000131.
Stankovic, M.S. 2011. Total phenolic content, flavonoid concentration and antioxidant activity of Marrubium peregrinum. L. extracts. Kragujevac Journal of Science. 33: 63-72.
Supasuteekul C, Nonthitipong W, Tadtong S, Likhitwitayawuid K, Tengamnuay P, and Sritularak B. 2016. Antioxidant, DNA damage protective, neuroprotective, and α-glucosidase inhibitory activities of a flavonoid glycoside from leaves of Garcinia gracilis. Revista Brasileira de Farmacognosia. 26: 312-320.
Thaipong, K., Boonprakob, U., Crosby, K., Cisneros-Zevallos, L., and Hawkins, B.D. 2006. Comparison of ABTS, DPPH, FRAP, and Orac assays for estimating antioxidant activity from guava fruit extracts. Journal of Food Composition and Analysis. 19: 669-675.
Variya, B.C., Bakrania, A.K., and Patel, S.S. 2020. Antidiabetic potential of gallic acid from Emblica officinalis: Improved glucose transporters and insulin sensitivity through PPAR-gamma and Akt signaling. Phytomedicine. 73: 1-11.
Vongsak, B., Sithisarn, P., Mangmool, S., Thongpraditchote, S., Wongkrajang, Y., and Gritsanapan, W. 2013. Maximizing total phenolics, total flavonoids contents and antioxidant activity of Moringa oleifera leaf extract by the appropriate extraction method. Industrial Crops and Products. 44: 566-571.
Yuenyongsawad, S., Bunluepuech, K., Wattanapiromsakul, C., and Tewtrakul, S. 2013. Anti-cancer activity of compounds from Bauhinia strychnifolia stem. Journal of Ethnopharmacology. 150(2): 765-769.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand.
Thavaree Thilavech1, Achira Sutiyaporn1, Pimpikar Kanchanadumkerng1, Vilasinee Hirunpanich Sato2, Warisara Parichatikanond2, Phennapa Charoenwiwattanakij3, and Savita Chewchinda1,*
1Department of Food Chemistry, Faculty of Pharmacy, Mahidol University, Bangkok, 10400, Thailand.
2Department of Pharmacology, Faculty of Pharmacy, Mahidol University, Bangkok, 10400, Thailand.
3Faculty of Pharmacy, Siam University, Bangkok, 10160, Thailand.
Corresponding author: Savita Chewchinda, E-mail: savita.che@mahidol.ac.th
Total Article Views
Editor: Veerasak Punyapornwithaya,
Chiang Mai University, Thailand
Article history:
Received: August 24, 2022;
Revised: December 13, 2022;
Accepted: December 16, 2022;
Published online: December 27, 2022

