
The Embryotoxicity of Alpha-Pinene to the Early Life Stages of Zebrafish (Danio Rerio Hamilton, 1822)
Turgay Şişman* and Zeynep CeylanPublished Date : April 3, 2023
DOI : https://doi.org/10.12982/NLSC.2023.020
Journal Issues : Number 2, April-June 2023
ABSTRACT
The Alpha-pinene (AP) is produced by pine trees and other plants. The AP exhibits various biological activities such as the development of antimicrobial and antiviral agents, flavors, fragrances, and fungicidal agents. The toxicity data for AP are limited. This study aimed to determine the embryotoxic effects of AP in zebrafish embryos. 1.5 hpf zebrafish embryos were exposed to different concentrations of AP for 72 hours. The LC50 and EC50 values for AP were determined as 441.360 mg/L and 367.795 mg/L, respectively. The embryos were not much affected by AP until hatching. In addition, AP showed teratogenic effects at high doses (320 and 640 mg/L). Typical lesions were an absence of somite (≤48 hpf), lordosis, yolk sac deformity, tail abnormality, cardiac edema, and eye shrinkage (≤ 72 hpf). However, survival rates of zebrafish embryos in the 20, 40, and 80 mg/L AP groups were greater than 80 % during the exposure period. Very low teratogenicity for zebrafish embryos was observed in the 160 mg/L AP group. The results show virtually no embryotoxicity and teratogenicity at 20 and 40 mg/L AP concentrations. No delay in developmental was also observed. Therefore, AP can be considered a safe compound in the concentrations.
Keywords: Alpha-pinene, Embryotoxicity, EC50, LC50, Zebrafish
Funding: This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Citation: Şişman, T. and Ceylan, Z. 2023. The Embryotoxicity of Alpha-Pinene to the Early Life Stages of Zebrafish (Danio rerio Hamilton, 1822). Nat. Life Sci. Commun. 22(2): e2023020.
INTRODUCTION
In recent years, interest in the use of medicinal and aromatic plants has been increasing. These plants contain essential oils rich in biologically active compounds (González-Burgos and Gómez-Serranillos 2012). These compounds can prevent cell damage caused by reactive oxygen by preventing the propagation of the oxidative chain reaction. Alpha-pinene (AP) is a natural compound found in the oils of many coniferous wood species, particularly pine (Zhou et al. 2004). Previous studies have shown that AP has a variety of pharmacological effects. It is also known that AP has a strong inhibitory effect on inflammation in pathological conditions (Bae et al. 2012; Kim et al. 2015; Borges et al. 2019). AP is used in various industrial processes such as the fragrance and flavor industry, pulp and paper industry, pharmaceutical industry, color printing and dye processing (Linares, Fontanille, and Larroche 2009; Rene et al. 2010; Montes, Veiga, and Kennes 2010; Bertouche et al. 2012; Wu et al. 2012). People can be exposed to the compound during the processing, use, storage of softwood or its by-products, and during the use of personal care products, cleaning products, or air fresheners that contain α-pinene as a fragrance component. Human toxicity of α-pinene or terpene mixtures containing α-pinene has been reported as potential respiratory and skin irritation. An animal experiment to determine the general toxicity, reproductive toxicity, or developmental toxicity of α-pinene has not been found in the literature (NTP 2016).
Zebrafish (Danio rerio) has been widely used as a model organism in various fields such as genetics, cancer research, and developmental biology (Nishimura et al. 2016; Aksakal and Sisman 2020). The embryo of zebrafish develops quickly and its development is easy to follow because they are transparent. Therefore, it is a valuable experimental animal for vertebrate development studies (Tran, Facciol, and Gerlai 2016). Developmental processes in zebrafish embryos are very similar to the embryogenesis of other higher vertebrates, including humans (Kimmel et al. 1995). In addition, zebrafish adults and larvae provide great advantages in toxicity studies. In this way, larger vertebrate models such as rats have become less useful in toxicity studies. The zebrafish embryo has been accepted as an alternative model for toxicological evaluation (Tran, Facciol, and Gerlai 2016; Nishimura et al. 2016; Ceylan et al. 2019) and new drug discovery (Falcão et al. 2018; Yang et al. 2018). In this study, embryotoxic and teratogenic evaluations of both medically and naturally exposed AP were determined using zebrafish embryos. There is no previous study of this type in the literature.
MATERIALS AND METHODS
Materials
Alpha-pinene (AP) was obtained from Sigma Aldrich. ((+)-α-pinene; C10H16, CAS No. 7785-70-8; Sigma-Aldrich®, USA). Different concentrations of AP were tested. The concentrations (20, 40, 80, 160, 320, and 640 mg/L) were selected according to Turkez and Aydin (2016). Wild-type zebrafish (3-6 months) were purchased from Atatürk University Fisheries Faculty. According to EU Directive 2010/63/EU on the protection of animals used for scientific purposes, the early life stages of animals are not defined as protected (EU 2010). Therefore, it does not fall within the regulatory frameworks regarding animal experiments. Zebrafish embryos/larvae up to 120 hours postfertilization are not free feeding. This study involved the embryo toxicity up to 72 hrs and ethical approval was not required for the study.
Methods
Maintenance, spawning and egg collection
Fish were kept in a glass aquarium providing sufficient space for swimming (1 L per 1 gr fish). Fish were maintained at 26 ± 1°C and 14:10 light/dark cycle. Fish were fed twice a day with tetramin flakes. Eggs were obtained by randomly mating zebrafish in a 3-2 male-female ratio. Glass balls and plastic sieves were used in the hatching aquarium to prevent the eggs from being eaten by the adults. The same dilution water was used for spawning, but no filter. Male and female fish were placed in the brood aquarium at the beginning of the dark cycle. Ovulation and fertilization occur within 30 minutes of the onset of morning light. Ovulation occurred within one hour of the start of the light cycle. About 30-60 minutes after spawning, adult fish were removed. Eggs were collected by siphoning and transferred to Petri dishes containing Hank's solution. Fertilized eggs were detected under the microscope. Fertilized eggs were placed in Petri dishes with test solution.
Fish embryotoxicity test
The six concentrations of AP (20, 40, 80, 160, 320, and 640 mg/L) were prepared as dilution with Hank’s solution. Twenty fertilized fish eggs were transferred into 50 mm petri dish containing different concentrations of AP. The control group did not contain the AP, only Hank’s solution. The Petri dishes were kept at 26 ± 1°C and 14:10 light/dark cycle. Exposure was initiated within 1.5 hours of fertilization (OECD 2013). The exposure was maintained for 72 hours. All solutions were renewed every 24 hours. The embryos and larvae were observed at 24, 48 and 72 hours. Some teratogenic abnormalities were captured using a microscope with the digital camera. Dead embryos were counted and recorded. According to the data, LC50 (median lethal concentration) and EC50 (median effective concentration) values were calculated. Teratogenic abnormalities were classified according to the grading reported by Padilla et al. (2011). The Malformation Index (MI) was also proposed by Padilla et al. (2011) and has been applied here with some modifications. This value is a semi-quantitative data. Embryos and larvae of zebrafish were visually graded for malformation at 24, 48, and 72 hours on a grading scale. In the scale, each embryo/larva was evaluated by scoring yes/no or 0 to 4 for various categories (e.g., curved spine, abnormal head, edema). Scores from each category are summarized to give a total malformation index for each embryo/larva. It was accepted that MI value 0-3 was normal; values 4-6 were considered mild abnormality and values above 7-8 clearly deformed fish embryo/larva.
Statistical analysis
Statistical analysis was performed using the SPSS 20.0 software program. 72 hours LC50 and EC50 values were calculated by probit analysis. Evaluation of abnormal embryos and larvae was made using the one-way ANOVA test. All data were given as mean ± standard error. Duncan’s test was performed to determine whether any treatment significantly differed from controls or each other and the statistical significance level was accepted as P < 0.05.
RESULTS
LC50 and EC50 values
In embryos exposed to AP for 72 hours, toxicity was particularly dose-related. LC50 and EC50 values at 72 h were 441.360 mg/L and 367.795 mg/L, respectively. The dose-response curves of AP for 24, 48 and 72 h were indicated in figures 1 and 2.
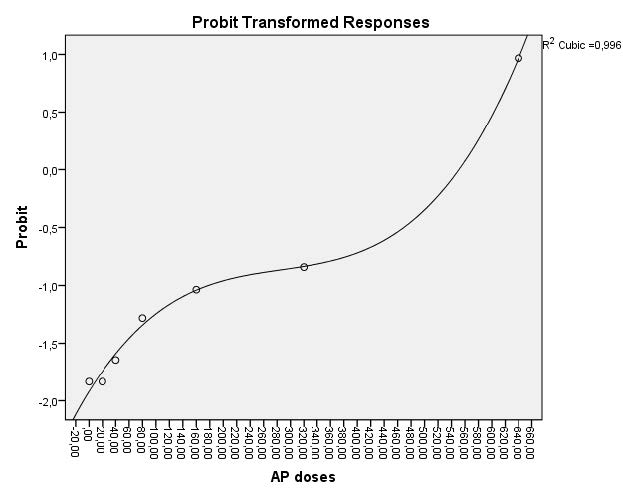
Figure 1. Dose-response curve used for calculation of LC50 value for AP (confidence interval 95%).
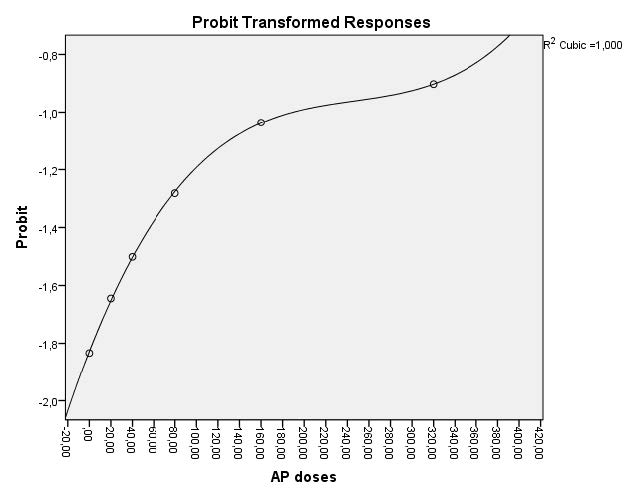
Figure 2. Dose-response curve used for calculation of EC50 value for AP (confidence interval 95%).
Mortality rates after 72 hours of exposure were quite interesting. When the deaths in the 20 and 40 mg dose groups were compared with the control, it was understood that there was no significant difference between them. In 80 and 320 mg/L doses, this ratio varies between 10% and 20%. The highest mortality rate was detected in embryos exposed to 640 mg/L AP. Embryotoxic effects appeared similarly. The highest frequency of teratogenic abnormalities was observed in the 320 mg/L AP group. In other groups, this ratio varied between 5% and 10%. The survival rates of zebrafish embryos in the 20, 40 and 80 mg/L AP groups were greater than 80 % during the exposure period (Table 1).
Table 1. Adverse effects in embryos caused AP at 72-hours exposure.
|
|
Control |
20 mg/L |
40 mg/L |
80 mg/L |
160 mg/L |
320 mg/L |
640 mg/L |
|
∑ Teratogenicity |
2 |
2 |
3 |
6 |
9 |
12 |
0 |
|
∑ Mortality |
2 |
3 |
4 |
6 |
9 |
11 |
60 |
|
∑ Affected embryos |
4 |
5 |
7 |
12 |
18 |
23 |
60 |
|
∑ Normal embryos |
56 |
55 |
53 |
48 |
42 |
37 |
0 |
|
% Teratogenicity |
3.2 ± 0.4 |
3.2 ± 0.6 |
5.2 ± 0.6 |
10.3 ± 0.9 |
15.0 ± 1.7 |
20.0 ± 0.8 |
0 |
|
% Mortality |
3.5 ± 0.2 |
5.5 ± 0.7 |
6.6 ± 0.5 |
10.3 ± 0.6 |
15.1 ± 0.9 |
18.3 ± 0.6 |
100 |
|
% Affected embryos |
6.6 ± 1.6 |
8.3 ± 0.8 |
11.3 ± 0.9 |
20.0 ± 0.7* |
30.3 ± 2.9* |
37.9 ± 1.8 |
100* |
|
% Normal embryos |
93.5 ± 1.7 |
91.2 ± 2.1 |
88.4 ± 1.7 |
80.1 ± 2.5 |
69.5 ± 4.1 |
61.3 ± 3.8 |
0 |
Note: *Shows differences at P < 0.05 level compared to control.
Embryotoxicity results
The embryos were not much affected by AP until hatching. Very low teratogenicity for zebrafish embryos was observed in the 160 mg/L AP group. However, AP showed teratogenic effects at high doses. Typical lesions were an absence of somite (≤48 hpf) (Figure 3), cardiac edema (48 hpf) (Figure 4), lordosis, tail abnormality, yolk sac deformity, cardiac edema, and eye shrinkage (≤ 72 hpf) (Figure 5).
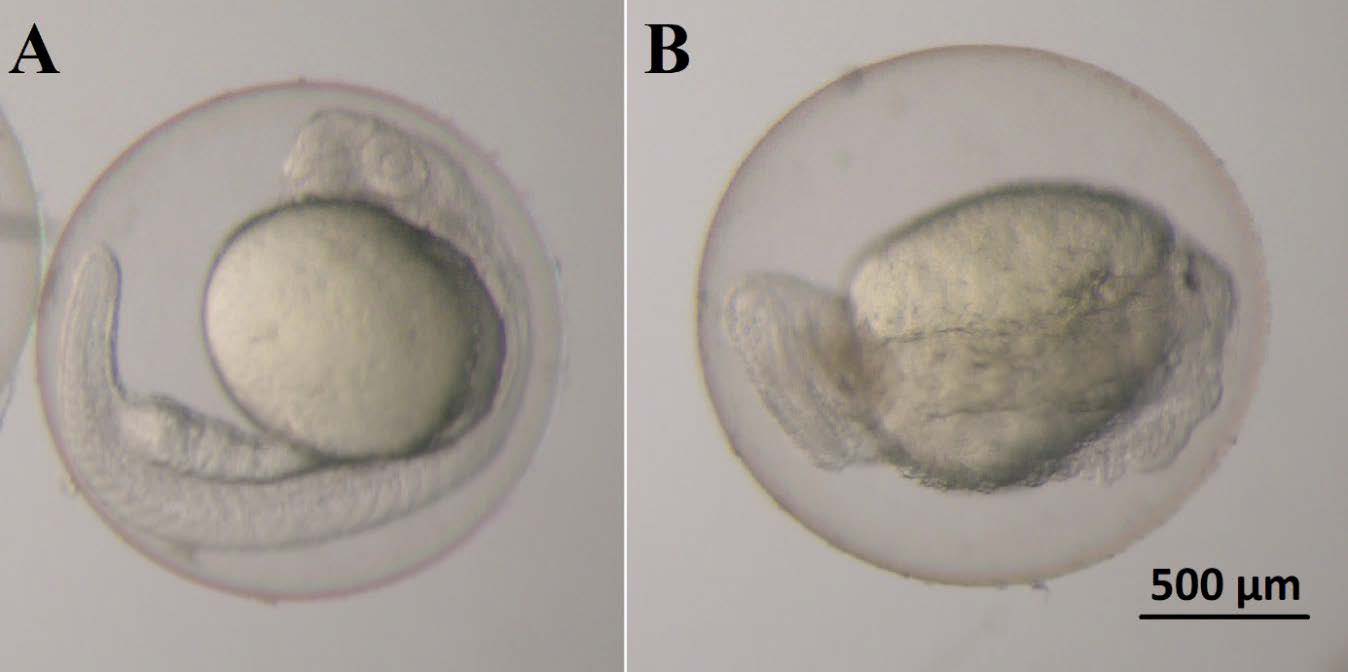
Figure 3. 24 hpf zebrafish embryos. A) normal embryo, B) abnormal embryo exposed to AP; absence of somite.

Figure 4. 48 hpf zebrafish larvae. A) normal larva, B) abnormal larva exposed to AP; cardiac edema deformity (arrow).
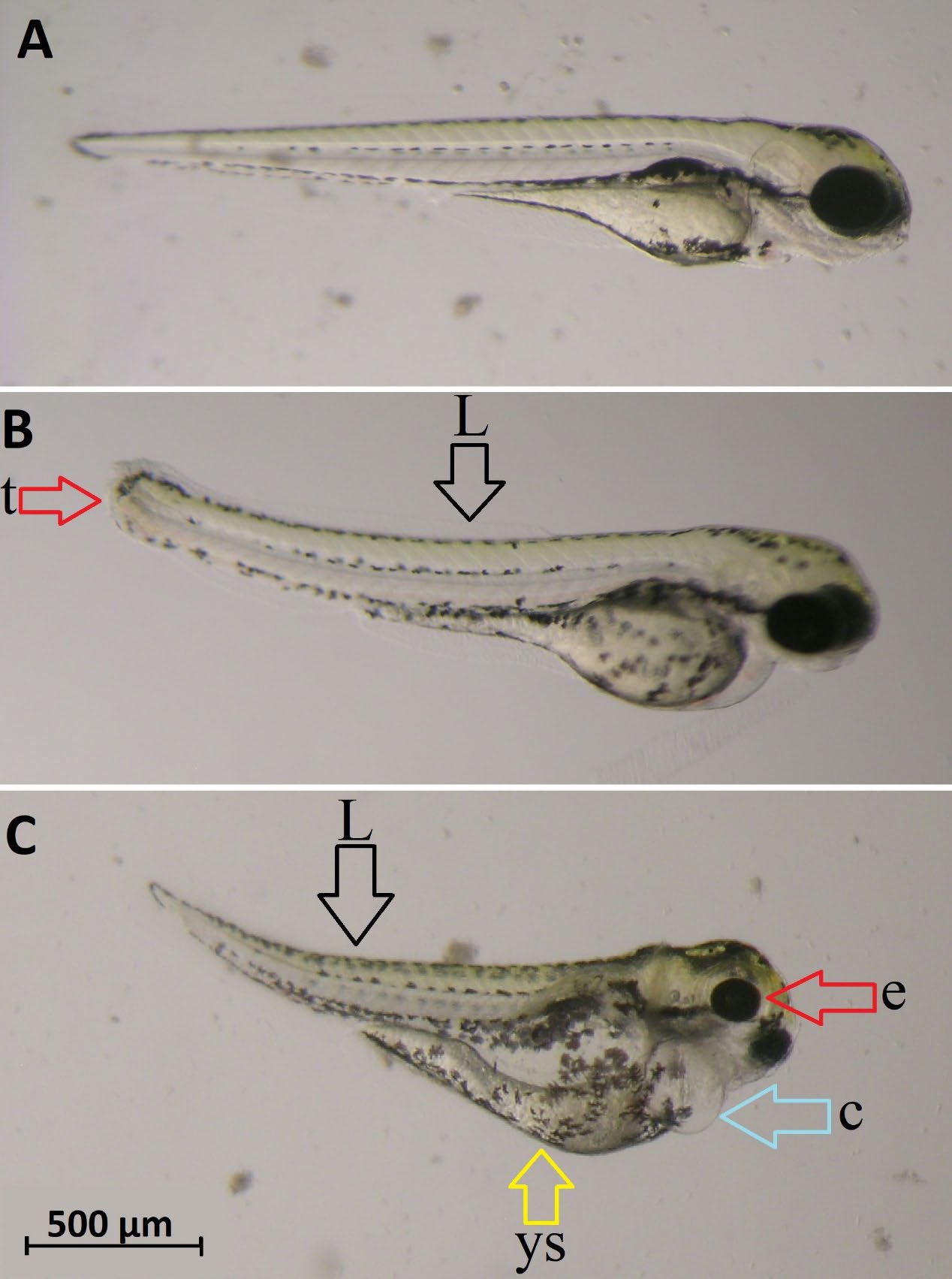
Figure 5. 72 hpf zebrafish larvae. A) normal larva, B) abnormal larva exposed to AP; lordosis (L), tail abnormality (t), C) abnormal larva exposed to AP; yolk sac deformity (ys) cardiac edema (c), eye shrinkage (e), and lordosis (L).
Total malformation index (MI) values calculated for AP were given in Table 2. The MI values for the 20 and 40 mg/L AP doses were not different from the control group. However, MI values increased for other AP concentrations. In particular, MI values for 320 mg/L of AP were above 4, indicating that mild abnormalities were observed in most embryos and larvae. MI values for 640 mg/L could not be calculated because all embryos died.
Table 2. Total malformation index (MI) values calculated for 72-hour fish larvae exposed to the AP.
|
Exposure time (hours) |
Control |
20 mg/L |
40 mg/L |
80 mg/L |
160 mg/L |
320 mg/L |
640 mg/L |
|
24 |
0.80 ± 0.02 |
0.60 ±0. 02 |
1.06 ± 0.06 |
0.9 ± 0.01 |
1.50 ± 0.03 |
0.90 ± 0.02 |
0 |
|
48 |
1.30 ± 0.06 |
1.50 ± 0.04 |
1.60 ± 0.04 |
1.90 ± 0.06* |
2.50 ± 0.04* |
3.30 ± 0.06* |
0 |
|
72 |
1.60 ± 0.06 |
1.30 ± 0.03 |
1.60 ± 0.01 |
2.00 ± 0.03* |
4.50 ± 0.04* |
6.30 ± 0.09* |
0 |
Note: *Shows differences at P < 0.05 level compared to control.
DISCUSSION
This is the first investigation for the embryotoxicity and teratogenicity of AP. Safe doses of AP alkaloid are also shown in the zebrafish model. AP at concentrations of 20 and 40 mg/L was not toxic to fish embryos. AP at a concentration of 80 mg/L showed a teratogenic effect of 10.3% and was not considered important in terms of embryotoxicity and teratogenicity. The LC50 and EC50 for AP were 441.360 mg/L and 367.795 mg/L, respectively. Our findings show virtually no embryotoxicity and teratogenicity at concentrations less than 80 mg/L. The zebrafish embryotoxicity assay is widely used in evaluating the toxicity of different plant extracts, compounds and components. Similar results were obtained in evaluating the toxicity of the active fractions of crude preparations and herbal medicines using the zebrafish embryotoxicity model. In a study, zebrafish embryos were exposed to a series of concentrations of green chireta (Andrographis paniculata; a herbal plant recommended various illness) leaves to assess toxicity. It was reported that the LC50 value (at 96 hpf) of the leaves was 520 mg/L, and abnormal organ development, enlarged yolk sac, pericardial edema, slow heartbeat, and delayed hatching were recorded in 72 hpf larvae (Ismail et al. 2017). The effect of the extract prepared from the root of the Turmeric plant, which has a lot of pharmaceutical properties, on zebrafish is as follows. The LC50 value (at 72 hpf) was 68.31 µg/mL, and several developmental abnormalities such as kink tail, bend trunk, and enlarged yolk-sac edema were reported in embryos treated with 62.5 µg/mL concentration (Alafiatayo et al. 2019). In another study, the 96 h LC50 of safflower (Carthamus tinctorius; a medicinal plant using blood stasis syndrome with dysmenorrhea, amenorrhea, postpartum abdominal pain and mass, and trauma and pain in the joints) to zebrafish embryos was calculated as 345.6 mg/L with delayed hatching rates (Xia et al. 2017). The LC50 and EC50 values (48 hpf) determined for zebrafish embryos of Martine, a type of alkaloid produced by Sophora flavescens, and possessing a variety of pharmacological effects such as antiinflammation, antivirus, antitumor, and antiarrhythmic activities, were 240 and 145 mg/L (Lu et al. 2014). It is seen that the embryotoxic effects of the active fractions of crude preparations and herbal medicines on zebrafish occur at very high doses. From this point of view, we can say that our results are compatible with the studies.
Previous studies have shown that AP has a variety of pharmacological effects. In addition, AP is effective in pathological conditions. It is known that the protective dose range varies in some studies. For example, Khoshnazar, Parvardeh, and Bigdeli (2020) reported that AP at doses of 50 and 100 mg/kg significantly improved sensorimotor function and reduced the volume of the infarct area in the rat brain. The study showed that AP is a promising molecule for attenuation of cerebral ischemia-reperfusion injury. In another study, it was reported that oral administration of AP at doses of 100 and 200 mg/kg for 14 days improved movement disorder and avoidance memory, accompanied by a decrease in malondialdehyde in the brain and blood (Goudarzi and Rafieirad 2017). A similar dose range gave best results in human cell culture studies. Turkez and Aydin (2016) demonstrated that AP had no mutagenic effects on human lymphocytes over the dose range of 10 to 200 mg/L, and AP exhibited antioxidant properties. AP can reach people in different concentrations depending on the places of use. However, the amount detected in such places is also quite low. Rastogi et al. (Rastogi et al. 2001) reported that they detected AP at 41.3 ± 51.5 ppm concentrations in 39% of 59 occupational and household products. After applying the floor varnish containing AP, the maximum terpene concentration measured in a small chamber was approximately 0.012 ppm (Colombo et al. 1991). Such studies show that the amount of AP released into the air is not high. The above doses are considerably lower than the LC50 (441.360 mg/L) and EC50 (367.795 mg/L) values obtained in the study.
CONCLUSION
The most abundant monoterpene in the atmosphere is AP. The monoterpene exhibits various biological activities. The embryotoxicity and teratogenicity of AP are not reported, yet. Thus, the current study was used zebrafish embryos to assess the toxicity. Our findings show virtually no embryotoxicity and teratogenicity at concentrations of 20, 40 and 80 mg/L of AP. Overall, results from the study, along with findings from other studies, suggest that AP develops low toxicity.
ACKNOWLEDGEMENTS
We would like to thank Dr. Hasan Türkez for AP supplying.
AUTHOR CONTRIBUTIONS
The authors confirm contribution to the paper as follows: study conception and design: T Şişman; data collection: T Şişman; analysis and interpretation of results: T Şişman; draft manuscript preparation: T Şişman, Z Ceylan; language editing: Z Ceylan. All authors reviewed the results and approved the final version of the manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Aksakal, F.I. and Sisman, T. 2020. Developmental toxicity induced by Cu(OH)2 nanopesticide in zebrafish embryos. Environmental Toxicology. 35: 1289-1298.
Alafiatayo, A.A., Lai, K.S., Syahida, A., Mahmood, M., and Shaharuddin, N.A. 2019. Phytochemical evaluation, embryotoxicity, and teratogenic effects of Curcuma longa extract on zebrafish (Danio rerio). Evidence-Based Complementary and Alternative Medicine. 2019: 3807207.
Bae, G.S., Park, K.C., Choi, S.B., Jo, I.J., Choi, M.O., Hong, S.H., Song, K., Song, H.J. and Park, S.J. 2012. Protective effects of alpha-pinene in mice with cerulein-induced acute pancreatitis. Life Sciences. 91: 866-871.
Bertouche, S., Tomao, V., Ruiz, K., Hellal, A., Boutekedjiret, C., and Chemat, F. 2012. First approach on moisture determination in food products using alpha-pinene as an alternative solvent for Dean-Stark distillation. Food Chemistry. 134: 602-605.
Borges, R.S., Ortiz, B.L.S., Pereira, A.C.M., Keita, H. and Carvalho, J.C.T. 2019. Rosmarinus officinalis essential oil: A review of its phytochemistry, anti-inflammatory activity, and mechanisms of action involved. Journal of Ethnopharmacology. 229: 29-45.
Ceylan, Zeynep, Sisman, T., Dane, H., and Adil, S. 2019. The embryotoxicity of some phenol derivatives on zebrafish, Danio rerio. Caspian Journal of Environmental Sciences. 17: 11-22.
Colombo, A., De Bortoli M., Knöppel, H., Schauenburg, H., and Vissers, H. 1991. Small chamber tests and headspace analysis of volatile organic compounds emitted from household products. Indoor Air. 1: 13-21.
EU. 2010. Directive 2010/63/EU of the European parliament and of the council of 22 September 2010 on the protection of animals used for scientific purposes. In, 79. Official Journal of the EU.
Falcão, M.A.P., de Souza, L.S., Dolabella, S.S., Guimarães, A.G., and Walker, C.I.B. 2018. Zebrafish as an alternative method for determining the embryo toxicity of plant products: A systematic review. Environmental Science and Pollution Research (international). 25: 35015-35026.
González-Burgos, E. and Gómez-Serranillos, M.P. 2012. Terpene compounds in nature: A review of their potential antioxidant activity. Current Medicinal Chemistry. 19: 5319-5341.
Goudarzi, S. and Rafieirad, M. 2017. Evaluating the effect of α-pinene on motor activity, avoidance memory and lipid peroxidation in animal model of Parkinson disease in adult male rats. Research Journal of Pharmacognosy. 4: 53-63.
Ismail, H.F., Hashim, Z., Soon, W.T., Rahman, N.S.A., Zainudin, A.N., and Majid, F.A.A. 2017. Comparative study of herbal plants on the phenolic and flavonoid content, antioxidant activities and toxicity on cells and zebrafish embryo. Journal of Traditional and Complementary Medicine. 7: 452-465.
Khoshnazar, M., Parvardeh, S., and Bigdeli, M.R. 2020. Alpha-pinene exerts neuroprotective effects via anti-inflammatory and anti-apoptotic mechanisms in a rat model of focal cerebral ischemia-reperfusion. Journal of Stroke and Cerebrovascular Diseases. 29: 104977.
Kim, D.S., Lee, H.J., Jeon, Y.D., Han, Y.H., Kee, J.Y., Kim, H.J., Shin, H.J., Kang, J., Lee, B.S., Kim, S.H. et al. 2015. Alpha-pinene exhibits anti-inflammatory activity through the suppression of MAPKs and the NF-κB pathway in mouse peritoneal macrophages. American Journal of Chinese Medicine. 43: 731-42.
Kimmel, C.B., Ballard, WW, Kimmel, S.R., Ullmann, B., and Schilling, T.F. 1995. Stages of embryonic development of the zebrafish. Developmental Dynamics. 203: 253-310.
Linares, Denis, Pierre Fontanille, and Christian Larroche. 2009. Exploration of α-pinene degradation pathway of Pseudomonas rhodesiae CIP 107491. Application to novalic acid production in a bioreactor. Food Research International. 42: 461-469.
Lu, Z.G., Li, M.H., Wang, J.S., Wei, D.D., Liu, Q.W., and Kong, L.Y. 2014. Developmental toxicity and neurotoxicity of two matrine-type alkaloids, matrine and sophocarpine, in zebrafish (Danio rerio) embryos/larvae. Reproductive Toxicology. 47: 33-41.
Montes, M., Veiga, M.C., and Kennes, C. 2010. Two-liquid-phase mesophilic and thermophilic biotrickling filters for the biodegradation of alpha-pinene. Bioresource Technology. 101: 9493-9499.
Nishimura, Y., Inoue, A., Sasagawa, S., Koiwa, J., Kawaguchi, K., Kawase, R., Maruyama, T., Kim, S., and Tanaka, T. 2016. Using zebrafish in systems toxicology for developmental toxicity testing. Congenital Anomalies (Kyoto). 56: 18-27.
NTP. 2016. National Toxicology Program (NTP) technical report on the toxicity studies of α-pinene (CAS no. 80-56-8) administered by inhalation to F344/N rats and B6C3F1/N mice. In. Research Triangle Park, NC: National Toxicology Program. Toxicity Report 81.
OECD. 2013. OECD guidelines for the testing of chemicals, section. In, 22. OECD.
Padilla, S., Hunter, D.L., Padnos, B., Frady, S., and MacPhail, R.C. 2011. Assessing locomotor activity in larval zebrafish: Influence of extrinsic and intrinsic variables. Neurotoxicology and Teratology. 33: 624-630.
Rastogi, S.C., Heydorn, S., Johansen, J.D., and Basketter, D.A. 2001. Fragrance chemicals in domestic and occupational products. Contact Dermatitis. 45: 221-225.
Rene, Eldon R., López, M.E., Veiga, M.C., and Kennes, C. 2010. Steady‐and transient‐state operation of a two‐stage bioreactor for the treatment of a gaseous mixture of hydrogen sulphide, methanol and α‐pinene. Journal of Chemical Technology & Biotechnology, 85: 336-348.
Tran, S., Facciol, A., and Gerlai, R. 2016. The zebrafish, a novel model organism for screening compounds affecting acute and chronic ethanol-induced effects. International Review of Neurobiology. 126: 467-484.
Turkez, H., and Aydin, E. 2016. In vitro assessment of cytogenetic and oxidative effects of alpha-pinene. Toxicology and Industrial Health. 32: 168-176.
Wu, C.S., Chen, Y.J., Chen, J.J., Shieh, J.J., Huang, C.H., Lin, P.S., Chang, G.C., Chang, J.T., and Lin, C.C. 2012. Terpinen-4-ol induces apoptosis in human nonsmall cell lung cancer in vitro and in vivo. Evidence-Based Complementary and Alternative Medicine. 2012: 818261.
Xia, Qing, Ma,Z., Mei, X., Luo, J., Wang, Y., Li, T., Feng, Y., Ni, Y., Zou, Q., and Lin, R. 2017. Assay for the developmental toxicity of safflower (Carthamus tinctorius L.) to zebrafish embryos/larvae. Journal of Traditional Chinese Medical Sciences, 4: 71-81.
Yang, J.B., Li, W.F., Liu, Y., Wang, Q., Cheng, X.L., Wei, F., Wang, A.G., Jin, H.T., and Ma, S.C. 2018. Acute toxicity screening of different extractions, components and constituents of Polygonum multiflorum Thunb. on zebrafish (Danio rerio) embryos in vivo. Biomedicine & Pharmacotherapy. 99: 205-213.
Zhou, J.-Y., Tang, F.-D., Mao, G.-G., and Bian, R.-L. 2004. Effect of alpha-pinene on nuclear translocation of NF-kappa B in THP-1 cells. Acta Pharmacologica Sinica. 25: 480-484.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand.
Turgay Şişman1, * and Zeynep Ceylan2
1 Department of Molecular Biology and Genetics, Faculty of Science, Atatürk University, Erzurum 25240, Turkey.
2 Department of Environmental Engineering, Faculty of Engineering, Atatürk University, Erzurum 25240, Turkey.
Corresponding author: Turgay Şişman, E-mail: tsisman@atauni.edu.tr
Total Article Views
Editor: Veerasak Punyapornwithaya,
Chiang Mai University, Thailand
Article history:
Received: February 16, 2022;
Revised: December 16, 2022;
Accepted: December 23, 2022;
Published online: January 12, 2023

