
The Effect of Topical Epigallocatechin-3-gallate (EGCG) on Collagen type-I, MMP-1 Expression and Dermal Collagen Count in Photoaging Prevention
Damayanti, Cita Rosita Sigit Prakoeswa*, Djoko Agus Purwanto, Anang Endaryanto, Muhammad Yulianto Listiawan, Yohanes Widodo Wirohadidjoyo, Soetjipto, Siswandono, Budi Utomo, and WidjiatiPublished Date : 2023-01-05
DOI : https://doi.org/10.12982/NLSC.2023.011
Journal Issues : Number 1, January-March 2023
Abstract The increasing of life expectancy in world population, increases the risk of photoaging. Photoaging of the skin is a complex biologic process especially caused by ultraviolet irradiation. Photoaging becomes new concern issue, because of high public awareness of skin health and performance. Many agents had been tried to be an alternative prevention for photoaging, such as green tea. Epigallocatechin-3-gallate (EGCG) is the most abundant catechin in green tea, that showed its possibility to be an alternative agent in photoaging prevention. This study was aimed to evaluate the effect of topical EGCG in photoaging prevention by evaluating collagen type-I, MMP-1 expression and dermal collagen count in photoaging animal model. This was an animal in vivo study. Male Wistar rats aged 10-12 weeks were acclimated for 1 week and randomly divided into 6 groups: without intervention; ultraviolet irradiated group; ultraviolet irradiated group with topical EGCG (2.5%, 5%, 10%); and ultraviolet irradiated group with topical based cream (control group). The effect of topical EGCG in collagen type-I, MMP-1 expression and dermal collagen count were evaluated after 5 weeks of treatment. The mean of collagen type-I expression in group treated with topical EGCG were higher than control group, meanwhile the MMP-1 expression was lower than control group. Topical EGCG prevented the reduction of dermal collagen count significantly (P <0.05). It showed that topical EGCG plays role in photoaging prevention.
Keywords: Topical EGCG, Photoaging, Collagen type-I, MMP-1, Dermal collagen count, Life expectancy
Funding: This research was financially supported by Directorate of Research and Community Service - Directorate General of Research and Development - Ministry of Research, Technology and Higher Education (Direktorat Riset dan Pengabdian Masyarakat - Direktorat Jenderal Riset dan Pengembangan - Kementerian Riset, Teknologi dan Pendidikan Tinggi/Kemenristekdikti) Indonesia.
Citation: Damayanti, Prakoeswa, C.R.S., Purwanto, D.A., Endaryanto, A., Listiawan, M.Y., Wirohadidjoyo, Y.W., Soetjipto, Siswandono, Utomo, B., and Widjiati 2023. The effect of topical epigallocatechin-3-gallate (EGCG) on collagen type-I, MMP-1 expression and dermal collagen count in photoaging prevention. Nat. Life Sci. Commun. 22(1): e2023011.
INTRODUCTION
Photoaging is skin aging, that mostly caused by chronic ultraviolet exposure of the sunlight (Puizina, 2008). Ultraviolet irradiation promotes the inhibition of transforming growth factor β receptor II (TGFβ-RII) and collagen type-I. Ultraviolet also promotes the elevation of matrix metalloproteinase-1 (MMP-1), an enzyme that degrades dermal collagen. These mechanism play role in photoaging pathogenesis by degrading the collagen and inhibiting the collagen synthesis (Rittie and Fisher, 2002; Zouboulis and Makrantonaki, 2011; Lephart, 2016; Pittayapruek et al., 2016).
The increasing of life expectancy was described in demographic transition in the population. The increasing of life expectancy would increase the risk of skin aging, especially photoaging. Photoaging becomes new important health problem in the population, because high public awareness of skin health and performance. Photoaging may give high psychological and social impacts, that decrease the human’s quality of life (Krutmann and Gilchrest, 2006; Puizina, 2008; Jafferany et al., 2012; Kerns et al., 2019). Many agents had been tried to be alternative agents in photoaging prevention. The biggest problems of new agent’s development are effectivity and efficiency of the agent. The development of new agent that has effective target and is easy to get by the public, is needed. Some plants had been used in photoaging prevention studies, such as green tea plant. Topical green tea extract application to photoaging mice model was able to inhibit the elevation of MMP-1 expression and the reduction of dermal collagen count (Hsu, 2005; Cavinato et al., 2017; Widiyowati et al., 2017). Epigallocatechin-3-gallate (EGCG) is the highest extract component level in green tea (59%), and is the main source of green tea’s bioactivity (Krupkova et al., 2016). There is still no further study about the role of topical EGCG in photoaging prevention.
EGCG at 1 μM concentration decreased p38 MAPK level in ultraviolet-irradiated fibroblast and keratinocyte (Kim et al., 2005). EGCG inactivated extracelullar signal-regulated kinase (ERK)-1/2 pathway in ultraviolet-irradiated HaCaT cell line at 1, 10, 100 μM concentration (Huang et al., 2005). In silico study, showed that there was interaction of EGCG molecules and receptor on inhibitory kappa B kinase (IKK) cavity, that plays role in photoaging pathogenesis. It also showed that EGCG was predicted having good skin permeability and not causing skin sensitisation (Damayanti et al., 2020a). An animal study showed that EGCG cream 5% and 10% could penetrate into Wistar rat’s skin after one week of administration through high performance liquid chromatography (HPLC) examination of extracted rat’s skin (Damayanti et al., 2020b).
Topical EGCG is expected to be able to prevent photoaging. This study was aimed to evaluate the effect of topical EGCG in photoaging prevention by evaluating collagen type-I, MMP-1 expression and dermal collagen count in photoaging animal model. The result of this study was expected to be the basic research of the next study about topical EGCG in photoaging prevention in human subjects.
MATERIALS AND METHODS
Animal
Male Wistar rats aged 10-12 weeks with average weight 100-250 grams (n=60) was provided by Faculty of Veterinary Medicine, Universitas Airlangga Surabaya, Indonesia and acclimated for 1 week. The animal were fed a commercial food and allowed water ad libitum throughout the study. All animal experiments were conducted Approval of Ethical Committee in Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia (approval number 2.KE.145.07.2019). The animals were randomly divided into 6 groups: without intervention (Group I); ultraviolet irradiated group (Group II); ultraviolet irradiated group with topical EGCG 2.5% (Group III/EGCG 2.5% group); ultraviolet irradiated group with topical EGCG 5% (Group IV/EGCG 5% group); ultraviolet irradiated group with topical EGCG 10% (Group V/EGCG 10% group); and ultraviolet irradiated group with topical based cream (Group VI/control group).
Preparation of topical EGCG
EGCG powder (98.76% purity) was purchased from Xi’ An Rongsheng Biotechnology Co., LTD, China (batch number 190702). Preparation of topical EGCG cream was started from base cream preparation. The base cream consist of virgin coconut oil (VCO), cetacium, cera alba, olive oil, and aqua destilata. Cera alba and cetacium were boiled in the porcelain bowl above the water bath until the mixture was melting. Aqua destilata was added to the mixture and stir. VCO and olive oil were added to the mixture and stir until it became the base cream. Topical EGCG cream (2.5%) was prepared by adding the pure EGCG powder to the base cream (1:39). Topical EGCG cream (5%) was prepared by adding the pure EGCG powder to the base cream (1:19). Topical EGCG cream (10%) was prepared by adding the pure EGCG powder to the base cream (1:9) (Lestari and Binarjo, 2013).
Treatment of topical EGCG
The back part of the male Wistar rat was shaved carefully in 3x3 cm2 size. Topical EGCG cream was administered to rat skin twice a day for 5 weeks. The size of topical EGCG cream applied to the rat skin was 4 mg/cm2 body surface area (Scalia, 2014).
Ultraviolet irradiation
An ultraviolet lamp (Ultraviolet B Broadband TL lamps, Philips TL 20W/01RS) with emission spectrum between 290-315 nm was used to perform photoaging animal model (ultraviolet irradiated mice). An ultraviolet meter (UV light meter, UV 340B) was used to measure the ultraviolet irradiance. An ultraviolet light was applied to the rats for 5 weeks, and the amount of irradiance was gradually increased until the ultraviolet irradiance total reach dose of 3,100 mJ/cm2 (Kwon and Jung, 2013; Kim et al., 2015).
Histopathology analysis
The skin on the back part of the male Wistar rat were obtained from sacrifice animal under anesthesia at the end of the study. The skin specimens were fixed in 10% formalin buffer and cryosections were prepared.
Immunohistochemistry examination
To evaluate collagen type-I and MMP-1 expression in fibroblast, skin specimens were incubated with primary anti-collagen type I monoclonal antibody (Santacruz Biotechnology, USA) and primary MMP-1 polyclonal antibody (BIOSS, USA) in antibody dilution buffer and then washed with phosphat buffer saline (PBS). The expression of collagen type-I and MMP-1 was evaluated semi-quantitatively, as the mean of index scale in modified-Remmele or Immuno-Reactive Score (IRS) method. The IRS scale was counted by multiplying the score of positive cells percentage and the score of color reaction intensity. The score of positive cells percentage can be divided into 0 (no positive cell reaction), 1 (up to 10% positive cells reaction), 2 (11-50% positive cells reaction), 3 (51-80% positive cells reaction), and 4 (more than 80% positive cells reaction). The score of color reaction intensity can be divided into 0 (no color reaction), 1 (low color reaction), 2 (moderate color reaction), and 3 (intense color reaction). The total score of IRS can be between 0 to 12. The IRS method was evaluated in 5 different fields of view in 400x magnification. All of the evaluation used light microscopy Nikon H600L completed with digital camera DS Fi2 300 megapixel and image analyzer software Nikon Image System.
Masson’s Trichrome staining
Masson’s Trichrome staining is a staining technique using acid fuchsin and methyl blue in order to evaluate the dermal collagen. Dermal collagen will be blue stained. All of these evaluation used light microscopy Nikon H600L completed with digital camera DS Fi2 300 megapixel and image analyzer software Nikon Image System. Dermal collagen count was evaluated by calibrated Image J software version 1.8 (NIH, USA). The evaluation was performed in 200x magnification (Chen et al., 2017).
Data Analysis
Statistical analyses were conducted using SPSS software version 26 (IBM, USA). Significance was determined by Anova test followed by post hoc t-test or Mann whitney u-test for multiple comparisons. The results were considered significant at P < 0.05.
RESULTS
Effect of topical EGCG on inhibiting the reduction of collagen type I expression in UVB irradiated rats.
The mean of collagen type-I expression using index scale in modified-Remmele or Immuno-Reactive Score (IRS) method in Group I, II, III, IV, V, and VI were 3.20; 1.90; 2.60; 2.85; 2.95; and 1.85, respectively. Data analysis using Kruskal-Wallis test showed that collagen type-I expression data difference was significant (P =0.0014). The data showed that the mean of collagen type-I expression in group receiving topical EGCG (Group III, IV, and V) were higher than in control group and the ultraviolet irradiated group. Meanwhile, the mean of collagen type-I expression in the group without intervention was higher than in the ultraviolet irradiated group (Figure 1). Figure 2 showed that the group receiving topical EGCG (Group III, IV, V) have higher chromogenic brown colour of collagen type-I expression in immunohistochemistry staining than the control group. The high value of collagen type-I expression showed the low reduction of collagen production. This result showed that EGCG cream play role by preventing the reduction of collagen type-I expression in photoaging.
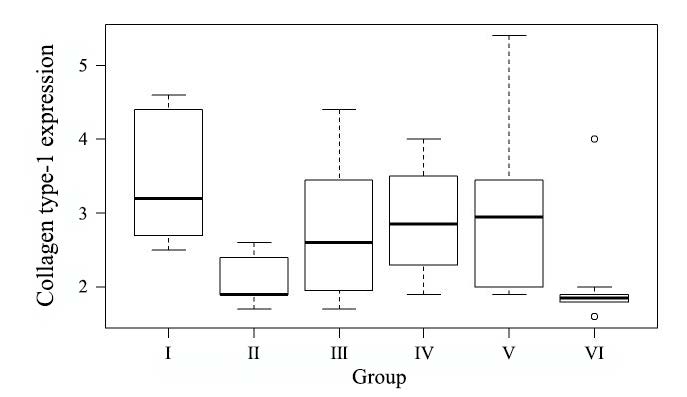
Figure 1. Collagen type-I expression value. Group I: without intervention, II: ultraviolet irradiated group, III: EGCG 2.5% group, IV: EGCG 5% group, V: EGCG 10% group, VI: control group.
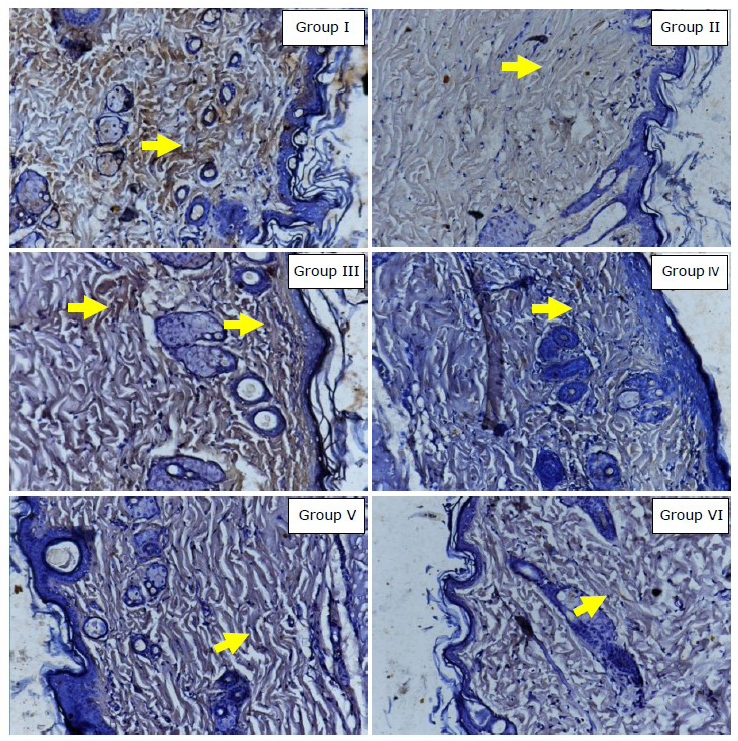
Figure 2. Immunohistochemistry result of collagen type-I expression (400x magnification, Nikon H600L microscope, DS Fi2 300-megapixel camera). Yellow arrow showed collagen type-I expression in fibroblast (chromogenic brown colour). Group I: without intervention, II: ultraviolet irradiated group, III: EGCG 2.5% group, IV: EGCG 5% group, V: EGCG 10% group, VI: control group.
Effect of topical EGCG on inhibiting the elevation of MMP-1 expression in UVB irradiated rats.
Data analysis using one-sample Komolgorov-Smirnov test showed that MMP-1 expression data was not normally distributed (P =0.013). The mean MMP-1 expression using index scale in modified-Remmele or Immuno-Reactive Score (IRS) method in Group I, II, III, IV, V, and VI were 3.15; 2.80; 2.10; 2.00; 1.85; and 3.60, respectively. The data showed that the mean of MMP-1 expression in group receiving topical EGCG (Group III, IV, V) were lower than in the group without intervention, ultraviolet irradiated group, and control group (Figure 3). Figure 4 showed that the group receiving topical EGCG (Group III, IV, V) have lower chromogenic brown colour of MMP-1 expression in immunohistochemistry staining than the control group. The low value of MMP-1 expression showed the low incidence of collagen degradation. This result showed that EGCG cream play role by preventing the elevation of MMP-1 expression in photoaging.
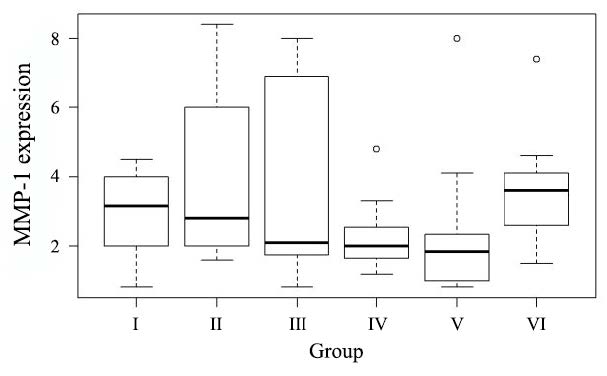
Figure 3. The MMP-1 expression value. Group I: without intervention, II: ultraviolet irradiated group, III: EGCG 2.5% group, IV: EGCG 5% group, V: EGCG 10% group, VI: control group.
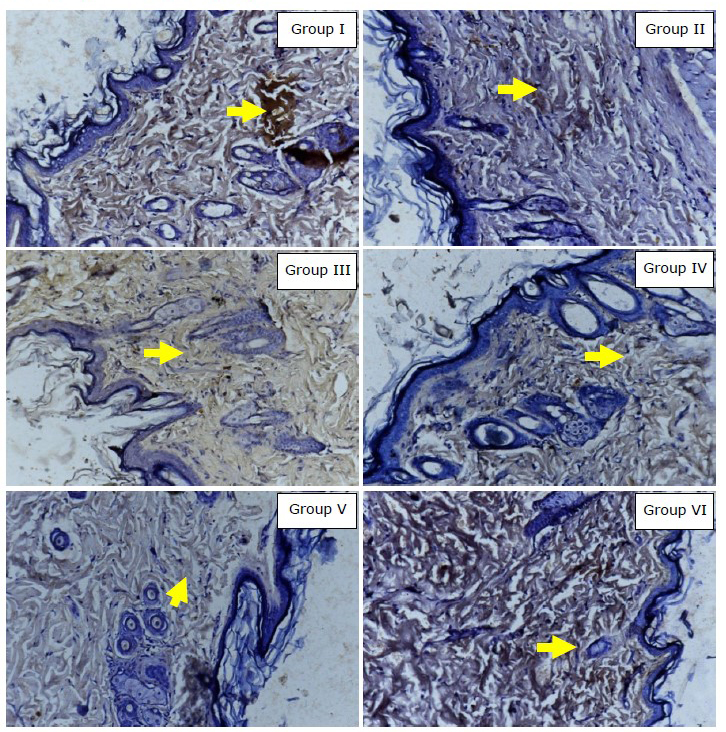
Figure 4. Immunohistochemistry result of MMP-1 expression (400x magnification, Nikon H600L microscope, DS Fi2 300-megapixel camera). Yellow arrow showed MMP-1 expression in fibroblast (chromogenic brown colour). Group I: without intervention, II: ultraviolet irradiated group, III: EGCG 2.5% group, IV: EGCG 5% group, V: EGCG 10% group, VI: control group.
Effect of topical EGCG on inhibiting the reduction of dermal collagen count in UVB irradiated rats
The result of Anova test in Table 1 showed there was significant mean different of dermal collagen count among the groups (P =0.000). The result of post hoc pairwise t-test showed that there were significant differences of mean of dermal collagen count between group without intervention with ultraviolet irradiated group (P <0.01), EGCG 5% group (P <0.05), EGCG 10% group (P <0.01), and control group (P <0.01); Ultraviolet irradiated group with EGCG 2.5% group (P <0.01); Control group with EGCG 2.5% group (P <0.01), EGCG 5% group (P <0.01), and EGCG 10% group (P <0.05); and there were significant differences between EGCG 5% group with group without intervention (P <0.05) and ultraviolet irradiated group (P <0.05). Figure 5 showed that the group receiving topical EGCG (Group III, IV, V) have higher dermal collagen density than control group. This result showed that EGCG cream play role by preventing the reduction of dermal collagen count in photoaging.
Table 1. The effect of EGCG on dermal collagen count.
|
Dermal collagen count |
Group I |
Group II |
Group III |
Group IV |
Group V |
Group VI |
P (Anova) |
|
Mean |
38.211 |
27.7265 |
33.793 |
32.932 |
32.153 |
27.917 |
0.000 |
|
SD |
2.667 |
0.770 |
3.715 |
3.969 |
3.632 |
2.532 |
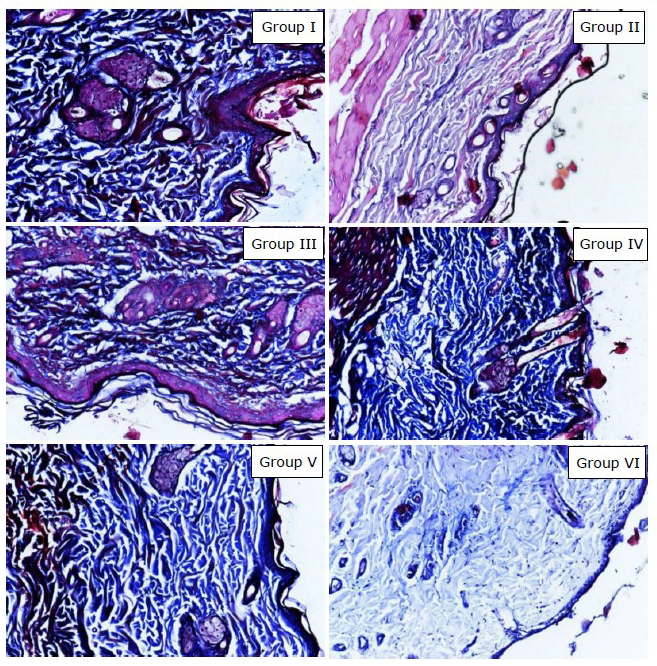
Figure 5. Histology examination result showed dermal collagen density with Masson’s trichrome staining (200x magnification, Nikon H600L microscope, DS Fi2 300-megapixel camera). Group I: without intervention, II: ultraviolet irradiated group, III: EGCG 2.5% group, IV: EGCG 5% group, V: EGCG 10% group, VI: control group.
DISCUSSION
Ultraviolet irradiation increases the production of reactive oxygen species (ROS). ROS precipitate the destruction of cell membrane, deoxyribonuclease acid (DNA) and protein. Ultraviolet may inhibit the activation of transforming growth factor β receptor II (TGFβ-RII) resulting the reduction of procollagen and collagen synthesis, especially procollagen type-I and collagen type-I; as well as the elevation of collagen degradation caused by activation of matrix metalloproteinase (MMP), especially MMP-1. All of these mechanism results in photoaging clinical manifestation (Zouboulis and Makrantonaki, 2011; Lephart, 2016; Pittayapruek et al., 2016; Ahmad and Damayanti, 2018).
Collagen is the most structural protein in dermal layer, that plays role in skin strength and elasticity. Two main regulators in collagen synthesis are TGFβ and Activator protein-1 (AP-1). TGFβ increases the synthesis of procollagen and collagen, especially procollagen type I and collagen type I. AP-1 inhibits the syntesis of procollagen and collagen. Ultraviolet irradiation increases the production of ROS, that activates AP-1 and inhibits the activity of TGFβ. These mechanisms reduce the synthesis of procollagen and collagen; and also increase the collagen degradation by MMP (Rittie and Fisher, 2002; Pittayapruek et al., 2016; Poon et al., 2014; Chiu et al., 2017).
AP-1 regulates the transcription of MMP; especially MMP-1, MMP-3 and MMP-9, that play role in photoaging pathogenesis. Fisher et al showed that gene expression of MMP-1, MMP-3 and MMP-9 would be induced after ultraviolet irradiation, as the activity of AP-1 was also induced by ultraviolet irradiation. The activity of MMP-1, MMP-3 and MMP-9 increases in 8 hours after ultraviolet irradiation. The induction of MMP plays role in skin tissue destruction caused by ultraviolet through collagen degradation (Fisher et al., 1997; Rittie and Fisher, 2002; Chiu et al., 2017).
Matrix metalloproteinase (MMP) was produced by keratinocyte and fibroblast. MMP degrades extracelullar matrix including collagen. The activity of MMP increases in photoaging process. The activity of MMP causes degradation of collagen and decreases the collagen count (Bosch et al., 2015; Agustyniak et al., 2016; Pittayapruek et al., 2016). The marker of collagen degradation evaluated in this study was MMP-1. MMP-1 is the most affected by ultraviolet irradiation and having great role in collagen degradation in photoaging. MMP-1 is also named as interstitial collagenase or collagenase I, which degrades collagen type I (Fisher et al., 1997; Zouboulis and Makrantonaki, 2011; Pittayapruek et al., 2016).
The MMP-1 expression in group receiving topical EGCG 2.5%, 5% and 10% were lower than in UV-irradiated group. The mean of MMP-1 expression on topical EGCG 2.5%, 5% and 10% groups were 2.1, 2.0 and 1.85. There was a decreasing of MMP-1 expression on higher concentration of topical EGCG. MMP-1 expression data showed, there was a dose-response relationship of topical EGCG in preventing photoaging. The dose-response relationship of topical EGCG in preventing the elevation of MMP-1 expresion showed the prediction of the higher role of EGCG in photoaging prevention in higher dose of EGCG. Although the mean of MMP-1 expression in the group without intervention was higher than the ultraviolet irradiated group, the MMP-1 expression in the ultraviolet irradiated group had wide standard deviation.
An in vitro study conducted by Won et al. evaluated the inhibitory effect of EGCG (10 and 20 μM) on MMP-1 gene expression in tumor necrosis factor-α (TNF-α) treated human dermal fibroblast cell (Hs 68 cells). Pre-treatment with EGCG was able to decrease the TNF-α-induced MMP-1 expression. It also suppressed the phosphorylation of extracellular signal regulated kinase (ERK) and mitogen-activated protein extracellular kinase (MEK) that are the main signal protein of ERK signal pathway. Similar to our study, Won et al. also reported that EGCG might have potential activity to inhibit the skin aging process through inhibition of collagen breakdown by MMP-1 (Won et al., 2021).
This study showed significant difference in collagen type I expression data among groups using the Kruskal-Wallis test analysis (P <0.05). There was a significant difference of collagen type I expression in the group receiving EGCG cream 5% and 10% compared to the control group given base cream (Group VI). Meanwhile, there was no significant difference between group receiving EGCG cream 2.5% compared to the control group, although the result of collagen count showed significant difference between two groups.
The collagen count data in this animal study showed that topical EGCG was able to prevent the reduction of dermal collagen count in photoaging significantly (Anova test; P <0.05). It may be caused by the activity of EGCG, that is be able to inhibit the activation of AP-1. The inhibition of AP-1 elevates the activity of TGFβ. This mechanism results in the elevation of collagen production, especially collagen type I (Kim et al., 2018).
In this study, EGCG appears to increase collagen type I expression, but higher concentration may be required to induce the signalling pathways. An in vitro study conducted by Bae et al., EGCG 1, 10, and 20 μM inhibit ultraviolet-B induced photoaging through inhibition of cJun, p53 and c-fos, downstream of JNK, p38 MAPK and ERK 1/2, that play role in photoaging pathogenesis (Bae et al., 2008). EGCG increased collagen type I production in keloid fibroblasts by interfering phosphatidylinositol-3-kinase signalling pathways (Zhang et al., 2006). Other study conducted by Vayalil et al. demonstrated that EGCG inhibit ultraviolet-B-induced phosphorylation of ERK 1/2, JNK and p38 proteins (Vayalil et al., 2003). Inhibition of ERK1/2 and MAPK signalling pathways lead to increase the activation of collagen type I and collagen production (Bae et al, 2008, Zouboulis and Makrantonaki, 2011).
The significant difference of collagen count between group receiveing EGCG cream (Group III, IV, V) with control group showed that EGCG cream 2.5%, 5%, and 10% had advantages in preventing photoaging compared to basic cream ingredients through the pathway of increasing the amount of collagen. EGCG is the most abundant component in green tea. In vitro and in vivo studies showed, it might prevent the oxidative damage caused by ultraviolet irradiation. EGCG was reported to be able to protect the skin from the suppression of immunity system caused by ultraviolet irradiation and prevent photoaging by inhibiting the elevation of MMP expression. EGCG has antioxidant effect, that plays role in the management of age-related diseases, including photoaging (Scalia, 2014; Krupkova et al., 2016).
The vehicle of topical agent and the penetration ability of active substance play important role in the action of active substance. The vehicle used in this study were virgin coconut oil (VCO), cetacium, cera alba, olive oil, and aqua destilata. The HPLC examination of this topical EGCG formula showed that EGCG as the active substance was able to penetrate into Wistar rat skin (Scalia, 2014; Damayanti et al., 2020b).
The result of this animal study was similar to the previous in vitro and in vivo studies. An in vitro study conducted by Kim et al. showed, that EGCG was able to inhibit the production of MMP-1 by dermal fibroblast after heat shock treatment. This result supported that EGCG is a potential alternative agent in photoaging therapy and management (Kim et al., 2013). An in vivo study conducted by Chen et al. showed, that EGCG subcutaneous injection once daily in aging mouse model for 8 months was able to decrease the dermal collagen degradation significantly (P <0.05). This study supported that EGCG inhibit the reduction of collagen type I expression, the elevation of MMP-1 expression and the reduction of dermal collagen count in photoaging (Chen et al., 2017).
CONCLUSION
Topical EGCG may play role in photoaging prevention, by inhibiting the reduction of collagen type I synthesis, elevation of collagen degradation by MMP-1, as well as inhibit the reduction of dermal collagen count in photoaging.
ACKNOWLEDGEMENTS
We gratefully thank Directorate of Research and Community Service - Directorate General of Research and Development - Ministry of Research, Technology and Higher Education Indonesia for supporting this research.
CONFLICT OF INTEREST
No conflict of interest regarding the publication.
REFERENCES
Agustyniak, A.M., Rotsztejn, H., Bartnicka, E., and Budzisz., 2016. Effects of reactive oxygen species on skin photoaging. Przeglad Dermatologiczny. 103: 233-239.
Ahmad, Z., and Damayanti, 2018. Skin Aging: Pathophysiology and Clinical Manifestation. Berkala Ilmu Kesehatan Kulit dan Kelamin. 30(3): 208-215.
Bae, J.Y., Choi, J.S., Choi, Y.J., Shin, S.Y., Kang, S.W., Han, S.J., et al., 2008. (-)Epigallocatechin gallate hampers collagen destruction and collagenase activation in ultraviolet-B-irradiated human dermal fibroblasts: Involvement of mitogen-activated protein kinase. Food and Chemical Toxicology. 46: 1298-1307.
Bosch, R., Philips, N., Suarez-Perez, J.A., Juarranz, A., Devmurari, A., Khaosaat, J.C., and Gonzalez, S., 2015. Mechanism of photoaging and cutaneous photocarcinogenesis, and photoprotective strategies with phytochemicals. Antioxidants. 4: 248-268.
Cavinato, M., Waltenberger, B., Baraldo, G., Grade, C.V.C., Stuppner, H., and Durr, P.J., 2017. Plant extracts and natural compounds used against UVB-induced photoaging. Biogerontology. 18: 499-516.
Chen, Y., Yu, Q., and Bao Xu, C., 2017. A convenient method for quantifying collagen fibers in atherosclerotic lesions by Image J software. International Journal of Clinical and Experimental Medicine. 10: 14904-14910.
Chiu, H.W., Chen, C.H., Chen, Y.J., and Hsu, Y.H., 2017. Far infrared suppresses in skin photoaging in ultraviolet B-exposed fibroblast and hairless mice. PLoS One. 12: 1-15.
Damayanti, Prakoeswa, C.R.S., Purwanto, D.A., Endaryanto, A., Siswandono, 2020. Mollecular docking, pharmacogenetics, and toxicity prediction of EGCG on IKK receptor in photoaging prevention. Indian Journal of Forensic Medicine and Toxicology. 14: 1392-1398.
Damayanti, Prakoeswa, C.R.S., Purwanto, D.A., Endaryanto, A., and Widjiati, W., 2020. Skin penetration of topical Epigallocatechin-3-Gallate (EGCG) as an alternative agent for photoaging prevention. Indian Journal of Forensic Medicine & Toxicology. 14: 882-6.
Fisher, G.J., Wang, Z.Q., Datta, S.C., Varani, J., Kang, S., and Voorhees, J.J., 1997. Pathophysiology of premature skin aging induced by ultraviolet light. The New England Journal of Medicine. 337: 1419-1428.
Hsu, S., 2005. Green tea and the skin. Journal of the American Academy of Dermatology. 52: 1049-1059.
Huang, C.C., Fang, J.Y., Wu, W.B., Chiang, H.S., Wei, Y.J., and Hung, C.F., 2005. Protective effect of epigallocatechin-3-gallate on UVA-induced damage in HaCaT keratinocyte. Archives of Dermatological Research. 296: 473-481.
Jafferany, M., Huynh, T.V., Silverman, M.A., and Zaidi, Z., 2012. Geriatric dermatoses: a clinical review of skin diseases in an aging population. International Journal of Dermatology. 51: 509-522.
Kerns, M.L., Chien, A.L., and Kang, S., 2019. Skin aging. p. 1779-91. In Kang S, Amagai M, Bruckner AL, Enk AH, Margolis DJ, McMichael AM, et al. (eds) Fitzpatrick’s in General Medicine, 9th ed. The McGraw Hill Companies, New York.
Kim, J.E., Shin, M.H., and Chung, J.H., 2013. Epigallocatechin-3-gallate prevents heat shock-induced MMP-1 expression by inhibiting AP-1 activity in human dermal fibroblast. Archives of Dermatological Research. 305: 595-602.
Kim, J., Hwang, K., Lee, J., Han, S.Y., Kim, E.M., Park, J. et al., 2018. Skin protective effect of epigallocatechin gallate. International Journal of Molecular Sciences. 19: 173.
Kim, S.Y., Kim, D.S., Kwon, S.B., Park, E.S., Huh, C.H., Youn, S.W. et al., 2005. Protective effect of EGCG on UVB-induced damage in living skin equivalents. Archives of Pharmacal Research. 28: 784-790.
Kim, Y.J., Kim, H.N., Shin, M.S., and Choi, B.Y., 2015. Thread embedding acupuncture inhibits ultraviolet B irradiation-induced skin photoaging in hairless mice. Evidence-Based Complementary and Alternative Medicine. 1-10.
Krupkova, O., Ferguson, S.J., and Kozak, K.W., 2016. Stability of epigallocatechin gallate and its activity in liquid formulations and delivery systems. Journal of Nutritional Biochemistry. 37: 1-12.
Krutmann, J. and Gilchrest, B.A., 2006. Photoaging of Skin. In Gilchrest, B.A., Krutmann, J. (eds) Skin Aging. Springer, Berlin, Heidelberg.
Kwon, S. and Jung, S.H., 2013. Topical administration of manuka oil prevents UV-B irradiation-induced cutaneous photoaging in mice. Evidence-based Complementary and Alternative Medicine. 1-10.
Lephart, E.D., 2016. Skin aging and oxidative stress: equol’s anti-aging effects via biochemical and molecular mechanism. Ageing Research Reviews. 31: 36-54.
Lestari, M. and Binarjo, A., 2013. Formulasi cold cream propanolol untuk penghantaran transdermal dengan basis emulsi yang mengandung VCO (virgin coconut oil). Pharmaciana. 3: 37-43.
Pittayapruek, P., Meephasan, J., Prapapan, O., Komine, M., and Ohtsuki, M., 2016. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. International Journal of Molecular Sciences. 17: 868(1-20).
Poon, F., Kang, S., and Chien, A.L., 2014. Mechanism and treatments of photoaging. Photodermatology, Photoimmunology and Photomedicine. 31: 65-74.
Puizina, I.N., 2008. Skin aging. Acta Dermatovenereol Alp Pannonica Adriat. 17: 47-54.
Rittie, L. and Fisher, G.J., 2002. UV-light-induced signal cascades and skin aging. Ageing Research Reviews. 1: 705-720.
Scalia, S., 2014. In vivo human skin penetration of epigallocatechin-3-gallate from topical formulation. Acta Pharmaceutica. 64: 257-265.
Vayalil, P.K., Elmets, C.A., and Katiyar, S.K., 2003. Treatment of green tea polyphenols in hydrophilic cream prevents UVB-induced oxidation of lipids and proteins, depletion of antioxidant enzymes and phosphorylation of MAPK proteins in SKH-1 hairless mouse skin. Carcinogenesis. 24: 927–936.
Widiyowati, H.S., Pangkahila, W.I., Wiraguna, A.A.G.P., Pangkahila, J.A., Adiputra, I.N., and Aman, I.G.M., 2017. Pemberian krim ekstrak teh hijau (Camellia sinesis) dapat mencegah penurunan jumlah kolagen dermis dan peningkatan kadar matriks metalloproteinase-1 pada mencit Balb-C yang dipapar sinar ultraviolet B. Indonesian Journal of Anti Aging Medicine. 1: 1-6.
Won, H.R., Lee, P., Oh, S., and Kim, Y.M., 2021. Epigallocatechin-3-gallate suppresses the expression of TNF-α-induced mmp-1 via mapk/erk signaling pathways in human dermal fibroblasts. Biological & Pharmaceutical Bulletin. 44: 18–24.
Zhang, Q., Kelly, A.P., Wang, L., French, S.W., Tang, X., Duong, H.S., et al., 2006. Green tea extract and (−)-epigallocatechin-3-gallate inhibit mast cell-stimulated type I collagen expression in Keloid fibroblasts via blocking PI-3K/Akt signaling pathways. Journal of Investigative Dermatology. 126: 2607-2613.
Zouboulis, C.C. and Makrantonaki, E., 2011. Clinical aspects and molecular diagnostics of skin aging. American Journal of Clinical Dermatology. 29: 3-14.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand.
Damayanti1,2, Cita Rosita Sigit Prakoeswa2, *, Djoko Agus Purwanto3, Anang Endaryanto4, Muhammad Yulianto Listiawan2, Yohanes Widodo Wirohadidjoyo5, Soetjipto6, Siswandono3, Budi Utomo7, and Widjiati8
1 Doctoral Study Program of Medical Science, Faculty of Medicine, Universitas Airlangga, Indonesia.
2 Department of Dermatology and Venereology, Faculty of Medicine, Universitas Airlangga, Indonesia.
3 Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Universitas Airlangga, Indonesia.
4 Department of Pediatrics, Faculty of Medicine, Universitas Airlangga, Indonesia.
5 Department of Dermatology and Venereology, Faculty of Medicine, Universitas Gajah Mada, Indonesia.
6 Department of Biochemistry, Faculty of Medicine, Universitas Airlangga, Indonesia.
7 Department of Community Medicine, Faculty of Medicine, Universitas Airlangga, Indonesia.
8 Department of Embriology, Faculty of Veterinary Medicine, Universitas Airlangga, Indonesia.
*Corresponding author: Cita Rosita Sigit Prakoeswa, E-mail: cita-rosita@fk.unair.ac.id
Total Article Views
Editor: Wipawadee Yooin,
Chiang Mai University, Thailand
Article history:
Received: June 23, 2022;
Revised: November 7, 2022;
Accepted: November 11, 2022;
Published online: December 27, 2022

