
Soybean Elicited with Saccharomyces cerevisiae and Light Attenuates Stromal-Derived Factor-1 Associated with CXCR4 in High-Fat/Fructose Diet Mice
Yunita Diyah Safitri, Yuyun Ika Christina, Mochammad Fitri Atho illah, Galuh Wening Permatasari, Farida Dewi Nur aini, Didin Wahyu Agustina, Sri Widyarti, and Muhaimin Rifai*Published Date : 2023-01-05
DOI : https://doi.org/10.12982/NLSC.2023.014
Journal Issues : Number 1, January-March 2023
Abstract Soybean elicited with microorganisms transforms its phytochemical content which is rich in anti-inflammatory action. However, the potential effect of the elicited soybean extract (ESE) on the maintenance of the stromal-derived factor-1 (SDF-1) regulatory system in the spleen, thymus, hepar, and bone marrow in high-fat/fructose diet (HFFD) mice is yet to be investigated. In our study, the spleen, thymus, hepar, and femurs were collected and isolated at the end of the experiment. The expression of SDF-1 in hepar, CD4+, CD8+, T cells, and B220+ cells was measured using flow cytometry analysis. To determine the interaction between the active compound of ESE and CXCR4, docking analysis was conducted using PyRx 0.8. The expression of splenic and thymic CD4+SDF-1+, CD8+SDF-1+, and hepatic SDF-1+ was elevated in HFFD mice but not in normal diet (ND) mice. ESE had significantly declined (P < 0.05) in CD4+SDF-1+ and CD8+SDF-1+ T-cells subsets. ESE treatment improved the development of B cells in HFFD mice. The molecular docking analysis identified glyceollin I with the strongest affinity of −8.4 kcal/mol. The results suggest that glyceollin I has a positive effect as a CXCR4 inhibitor. ESE supplementation was significant in maintaining the regulatory system of SDF-1 expressed by CD4+, CD8+, hepatocyte, and B220+ cells in HFFD associated with obesity
Keywords: CXCR4, Glyceollin, High-fat, High-fructose, SDF-1, Soybean
Citation: Safitri, Y.D., Christina, Y.I., Atho illah, M.F., Permatasari, G.W., Nur aini, D.f., Agustina, D.W., Widyarti, S. and Rifai, M. 2023. Soybean Elicited with Saccharomyces cerevisiae and Light Attenuates Stromal-Derived Factor-1 Associated with CXCR4 in High-Fat/Fructose Diet Mice. Nat. Life Sci. Commun. 22(1): e2023014.
INTRODUCTION
Obesity is a lifestyle-related disease, although some genetic factors also contribute to obesity development. Over the past four decades, obesity and overweight have gradually increased in adults, children, and adolescents in most regions (Abarca-Gómez et al. 2017). High consumption of fat-rich diet or fructose-rich beverages increases the risk rate of obesity worldwide (Pereira et al. 2017; Wang et al. 2018). Evidence has proven that high-fat and high-fructose diets are linked to low-grade chronic inflammation due to excessive proinflammatory cytokines released in the adipose tissue (Wang et al., 2017). These proinflammatory mediators and chemokines will attract macrophages to infiltrate adipose tissue and initiate inflammation (Yoshida et al., 2014).
Stromal-derived factor-1 (SDF-1), also referred to as C-X-C motif chemokine 12 (CXCL12), is one of the chemokines that is a crucial regulatory element in the inflammation mechanism (Charo and Ransohoff 2006). The interaction between SDF-1 and its receptor, CXCR4, is crucial for hematopoiesis, stem cell mobilization, migration, homing, and immunoregulation (Asri et al. 2016). The SDF-1/CXCR4 axis has gained much attention recently for its role in obesity and diabetes progression (Wada et al. 2018; Shin et al. 2018; Kurita et al. 2019). Interestingly, SDF-1 in adipocytes decreases at the initial stages of high-fat diet (HFD) before increasing significantly at later stages (Girousse et al. 2019). It is essential to develop an anti-obesity agent that will target SDF-1/CXCR4 to overcome the high risk of obesity-associated systemic inflammation.
Herbal medicines are frequently used in developing countries for alternative healthcare after synthetic drugs. Herbal medicines such as plant extracts are attractive alternatives because of their familiar and hereditary use in society; moreover, they are nontoxic, inexpensive, readily available, and have minimum side effects due to their antioxidant presence (Djati and Christina 2019; Kumar et al. 2020). Soybean (Glycine max) is recognized as a healthful food due to its high-quality protein, dietary fiber, and isoflavones-rich content (Pabich and Materska 2019). Elicitation is the modification process by exposing a plant with the biotic or abiotic stressor to produce secondary metabolites. Recent investigations of soybean have focused on its secondary metabolite, glyceollin. Glyceollin is a phytoalexin synthesized in large amounts from daidzein as its precursor due to fungal infection or other elicit factors (Nwachukwu et al. 2013). In addition, Saccharomyces cerevisiae and light act synergistically to enhance quadrupled glyceollin synthesis (Atho’illah et al. 2017). Glyceollin is beneficial to health; it acts as an antioxidant (Kim et al. 2011), has anti-inflammatory properties (Atho’illah et al. 2018; Nur’aini et al. 2019), improves erythroid progenitor capacity (Safitri et al. 2017), and maintains the hematopoietic system (Safitri et al. 2018).
Although some biological activities and pharmacological functions of bioactive compounds from ESE have been studied, information about the direct effect of ESE on the SDF-1/CXCCR4 axis under HFFD is still limited. This study provides evidence that SDF-1 expression is present in the spleen, thymus, bone marrow (BM), and hepar, and that ESE can help regulate immune cell homeostasis in high-fat/fructose diet (HFFD) mice.
MATERIALS AND METHODS
Preparation of soybean extract
Soybean var. Anjasmoro was obtained from Indonesian Legumes and Tuber Crops Research Institute, Malang, Indonesia, and was further elicited by S. cerevisiae. Soybeans were briefly sterilized using ethanol 70% and then washed three times with distilled water. Soybeans were then sterilized for 24 hours with distilled water and subsequently germinated for three days. Based on a previous study, soybean was exposed to bulb light for 16 hours per day; it was then extracted using 80% ethanol (Atho’illah et al. 2019). The crude extract was obtained and concentrated using a rotary evaporator, freeze-dried, and kept at −20°C before use. The final yield of ESE obtained was 9.82%.
Animals and diet
Six-week-old male and female Balb/C mice (Mus musculus) of broodstock were obtained from The Integrated Research and Testing Laboratory, Gadjah Mada University, Indonesia. The offspring were bred until post-weaning. Three-week-old female mice were also selected and used in this study. The mice were divided into two major groups: ND and HFFD. The ND mice were retrieved from PT Galaxy Science, Jember, Indonesia. The ND content included carbohydrates (53.46%), protein (8.57%), and fat (21.06%). All mice in HFFD groups were fed with high-fat and fructose beverages for 24 weeks. All procedures in this study were approved by the University of Brawijaya Ethics Committee (Reg. No. 647-KEP-UB).
ESE treatment
After 20 weeks of diet induction, mice were randomly grouped into seven groups (n = 4): ND, ND + ESE-1 (ND treated with an ESE dose of 104 mg/kg BW), HFFD, HFFD + Simv (HFFD treated with a simvastatin dose of 2.8 mg/kg BW), HFFD + ESE-1 (HFFD treated with an ESE dose of 78 mg/kg BW), HFFD + ESE-2 (HFFD treated with an ESE dose of 104 mg/kg BW), and HFFD + ESE-3 (HFFD treated with an ESE dose of 130 mg/kg BW). The mice were then given ESE or simvastatin orally with a predetermined dose for four weeks, except for the HFFD control group. The HFFD control group was fed only with HFFD for 24 weeks without any additional treatment.
Cell isolation and immunostaining
After 24 weeks, the mice were killed by cervical dislocation, and the spleen, thymus, hepar, and femurs were isolated. Spleen, thymus, and hepar isolation were referred to in the previous study (Christina et al. 2015). The spleen was crushed, and the homogenate was collected into the centrifuge tube. Then, 5 ml of phosphate-buffered saline (PBS) was added to a homogenate tube and centrifuged at 2500 rpm, 4°C for 5 minutes. Bone marrow (BM) isolates were collected from the femur of mice. The BM isolation procedures outlined by Safitri et al. (Safitri et al. 2018) were followed. Femurs were cut on both ends and flushed-out using a 1-ml syringe filled with PBS. The homogenate was centrifuged at 2500 rpm, 4°C for 5 minutes. After centrifuging, pellets from the spleen and BM were stained with a specific antibody. The following antibody stains were used in this study: fluorescein isothiocyanate (FITC) anti-mouse CD4 (GK1.5, Biolegend, San Diego, CA), phycoerythrin (PE) anti-mouse CD8α, (53-6.7, Biolegend, San Diego, CA) PE anti-mouse B220 (RA3-6B2, BD Biosciences Pharmingen, San Diego, CA), and peridinin-chlorophyll-protein anti-human/mouse SDF-1 (79018, R&D Systems, Minneapolis). Pellets with specific antibodies from the spleen, thymus, BM, and hepar were incubated for 30 minutes at 4°C. After incubation, the stained cells were mixed with 5 ml of PBS, and the data was collected for each sample using FACS CaliburTM (BD Biosciences, San Jose, CA). The single-cell population was gated and analyzed for the expression of CD4+SDF-1+, CD8+SDF-1+, and B220+SDF-1+ using FlowJo v10 for Windows (FlowJo LLC, Ashland, OR).
Molecular interaction between the selected active compound of soybean and CXCR4
To understand the interaction between natural compounds and CXCR4 protein, docking analysis was conducted using PyRx 0.8 software. The grid box was set for center X: 20.091; Y: 5.587; Z: 29.312, and the dimensions (Angstrom) were set for center X: 12.402; Y: 13.033; Z: 16.896. A three-dimensional structure of the CXCR4 protein was obtained from the PDB databank (ID 3OE9), and a similar structure of soybean’s active compound was collected from the PubChem database: daidzein (5281708), genistein (5280961), glyceollin I (162807), glyceollin II (181883), glyceollin III (11954193), and simvastatin (54454). IT1t (25147749) was chosen as the control for CXCR4 because of its ability to bind as an antagonist (Wu et al. 2010). In comparison, simvastatin acts as the control for in vivo, as an anti-cholesterol agent (S. Antonopoulos et al. 2012). Interaction analysis and complex visualization were conducted using Discovery Studio R2017, Chimera 1.13.1rc, and PyMol.
Statistical analysis
The obtained data were displayed as mean and standard deviation. One-way analysis of variance with P < 0.05 was conducted to compare the differences between groups, followed by Tukey's honestly significant difference test for statistical significance. Data were statistically analyzed using GraphPad Prism 8 (GraphPad Software Inc., La Jolla, CA).
RESULTS
Elicited soybean extract restores and down-regulates splenic and thymic CD4+SDF-1+ and CD8+SDF-1+ expressions in HFFD mice
This study investigates CD4+SDF-1+ and CD8+SDF-1+ expressions in the spleen and thymus (Figures. 1A–1H). Our result suggests that splenic CD4+SDF-1+ (Figure. 1A) and CD8+SDF-1+ (Figure. 1B) expressions increased significantly (P < 0.05) in the HFFD group than in the normal diet (ND) group. Similarly, thymic CD4+SDF-1+ (Figure. 1C) and CD8+SDF-1+ (Figure. 1D) expressions also increased in HFFD mice. During the last four weeks of the experiment, ESE given to HFFD mice restored CD4+SDF-1+ and CD8+SDF-1+ T cells in the spleen and thymus close to the values in the ND group. ESE treatment decreased the splenic CD4+SDF-1+, but the decline was not significantly different (P > 0.05) from simvastatin (Figure. 1E). Meanwhile, an ESE dose of 130 mg/kg BW significantly declined CD8+SDF-1+ (P < 0.05) better than simvastatin (Figure. 1F). However, simvastatin showed the same effect as ESE in declining CD4+SDF-1+ (Figure. 1G) and CD8+SDF-1+ (Figure. 1H) significantly (P < 0.05) in the thymus of HFFD mice. Nevertheless, there was no significant difference between splenic and thymic CD4+SDF-1+ and CD8+SDF-1+ T-cell expressions between the HFFD group and ND group when treated with an ESE dose of 104 mg/kg BW.
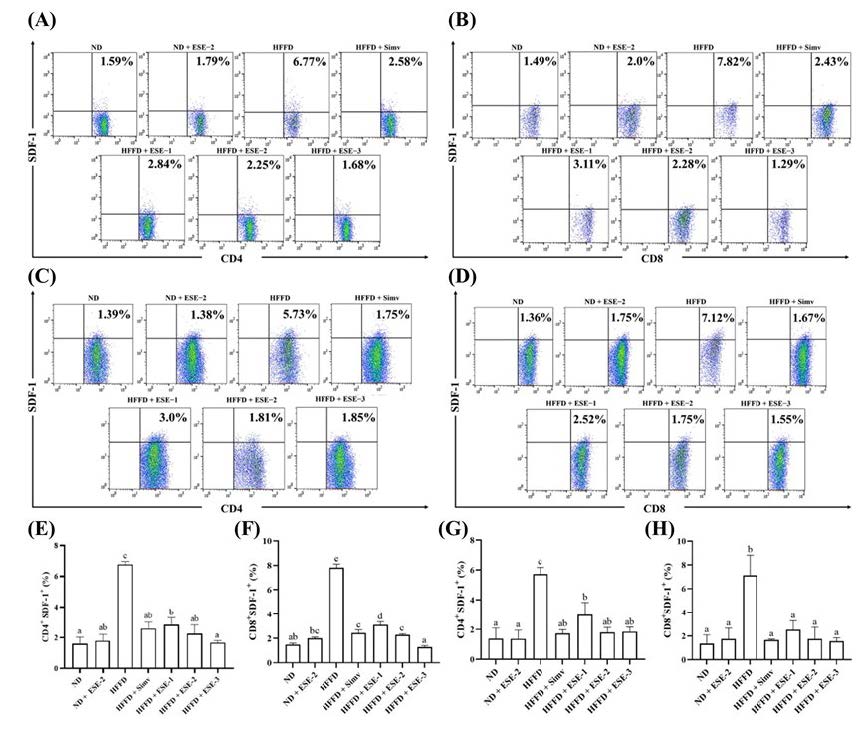
Figure 1. Effects of elicited soybean extract (ESE) on CD4+SDF-1+ and CD8+SDF-1+ subsets in the spleen and thymus of high-fat/fructose diet mice. (A–D) Flow cytometry analysis of CD4+SDF-1+ and CD8+SDF-1+ subsets in the spleen and thymus, respectively. The upright value in dot plot analysis indicates the proportion of CD4+SDF-1+ or CD8+SDF-1+. (E–F) CD4+SDF-1+ and CD8+SDF-1+ subsets in the spleen and thymus, respectively, with and without ESE treatment. The bar chart is displayed as mean ± standard deviation (n = 4). The different letters were significantly different between each group (P < 0.05) based on Tukey’s honestly significant difference test. SDF-1: stromal-derived factor-1.
Elicited soybean extract normalizes B220+SDF-1+ expression in the bm of HFFD mice
According to our results, ESE treatment had a beneficial effect on the development of B cells in HFFD mice. Based on dot plot analysis, B220+SDF-1+ expression decreased in mice fed with HFFD over 24 weeks than in ND mice (Figure. 2A). ESE treatment in HFFD mice enhanced the expression of B220+SDF-1+ B cells. Simvastatin declined B220+SDF-1+ expression significantly (P < 0.05) than in the normal groups (Figure. 2B). There was no significantly different B220+SDF-1+ expression between HFFD and ND groups when treated with an ESE dose of 104 mg/kg BW.
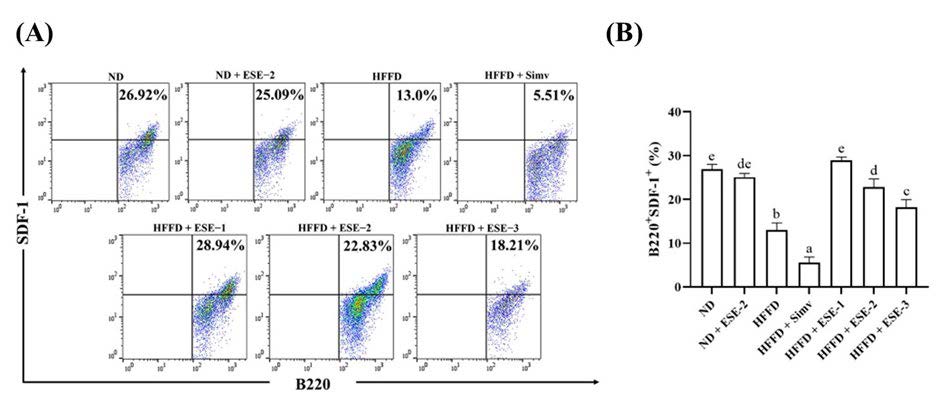
Figure 2. Effects of elicited soybean extract (ESE) on B220+SDF-1+ subsets in the bone marrow (BM) of high-fat/fructose diet (HFFD) mice. (A) Flow cytometry analysis of B220+SDF-1+ subsets in the BM. (B) ESE enhances B220+SDF-1+ subsets in the BM of HFFD mice. The upright value in dot plot analysis indicates the proportion of B220+SDF-1+. The bar chart is displayed as mean ± standard deviation (n = 4). The different letters were significantly different between each group (P < 0.05) based on Tukey’s honestly significant difference test. SDF-1: stromal-derived factor-1.
Elicited soybean extract down-regulates hepatic SDF-1+ expression in HFFD mice
ESE treatment also restores hepatic SDF-1+ expression in HFFD mice. According to dot plot analysis, SDF-1+ expression increased in mice fed with HFFD over 24 weeks but not in ND mice (Figure. 3A). The expression of hepatic SDF-1+ reduced (P < 0.05) after ESE administration in HFFD mice. An ESE dose of 104 mg/kg BW administered to ND mice did not alter hepatic SDF-1+ expression compared with the HFFD group.
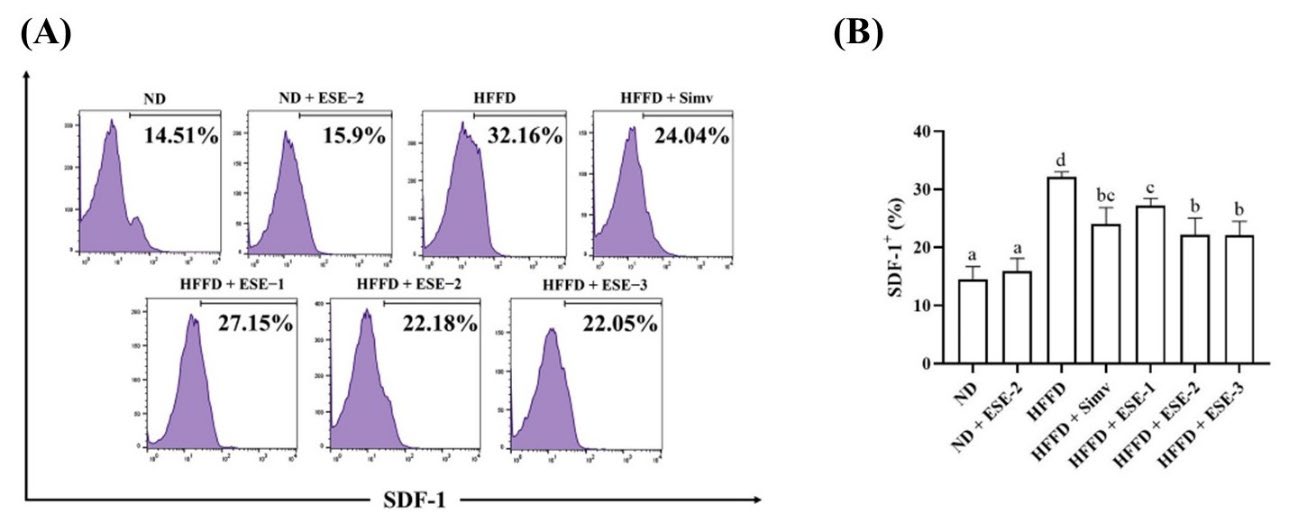
Figure 3. Effects of elicited soybean extract (ESE) on SDF-1+ subsets in high-fat/fructose diet (HFFD) mice hepatocyte. (A) Flow cytometry analysis of SDF-1+ subsets in hepatocytes. (B) ESE decreased the SDF-1+ expression in the hepatocytes of HFFD mice. The upright value in dot plot analysis indicates the proportion of SDF-1+. The bar chart is displayed as mean ± standard deviation (n = 4). The different letters were significantly different between each group (P < 0.05) according to Tukey’s honestly significant difference test. SDF-1: stromal-derived factor-1.
Docking analysis
The molecular docking analysis demonstrated that glyceollin I has the strongest binding affinity compared with other natural compounds (Table 1), with a score of −8.4 kcal/mol, followed by glyceollin II and III with a score of −8.3 and −8.0 kcal/mol, respectively. Simvastatin and isothiourea-1t (IT1t) as the control exhibited a moderate binding affinity score of −7.2 kcal/mol. After the control, daidzein and genistein have the weakest binding affinity. The docking analysis data strengthened the in vivo results, demonstrating that glyceollin-rich extract in ESE positively affected the SDF-1 expression in immune cells. Moreover, the binding site between active compounds and the CXCR4 receptor is in the same site (Figure. 4). The amino acid involved in the interaction is shown in Table 1 and Figure. 4. The key binding site, which appeared in all of the complexes excluding daidzein-CXCR4.
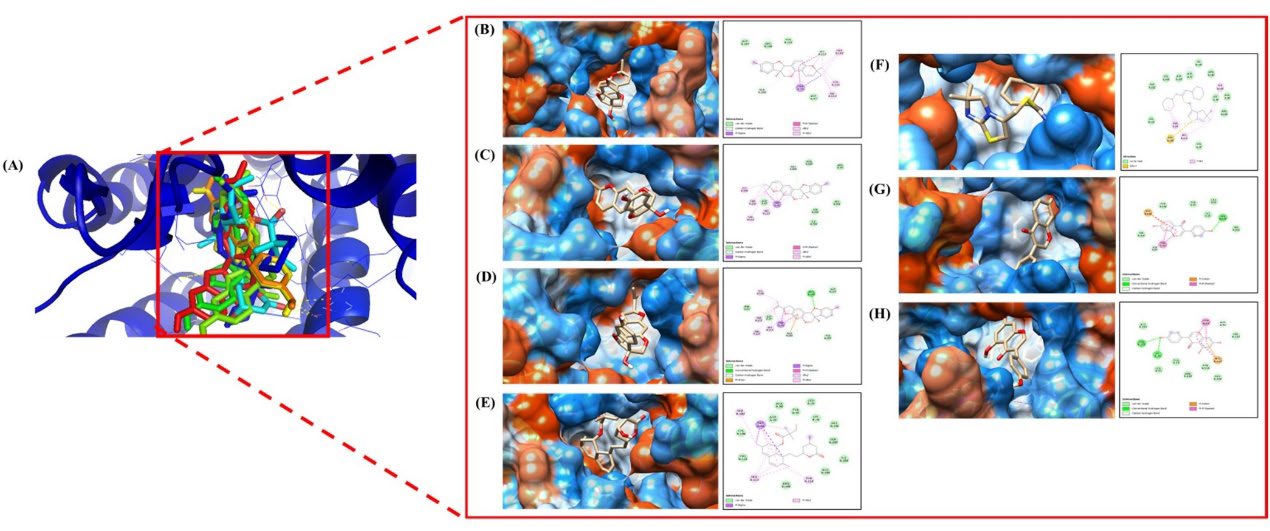
Figure 4. (A) The result of CXCR4 molecular docking shows every ligand bond at the same point. Visualization of the relationship between CXCR4, complex protein, and amino acids (B) Glyceollin I, (C) Glyceollin II, (D) Glyceollin III, (E) Simvastatin, (F) IT1t, (G) Daidzein, and (H) Genistein.
DISCUSSION
SDF-1 significantly correlated with the pathological factor in obese adipose tissue, and this resulted in inflammation (Leng et al. 2008; Kim et al. 2014). To understand the involvement of these chemokines in the effects of HFFD diet, we examined whether the HFFD diet affects the level of SDF-1 expression in various organs such as the spleen, thymus, BM, and hepar in mice. We found that ESE improved several pathological changes after HFFD induction by decreasing the expression of CD4+SDF-1+ and CD8+SDF-1+ T cells. It also upregulated the level of B220+SDF-1+ B cells.
Our study suggested that the expression of SDF-1 in CD4 T cells (CD4+SDF-1+) and CD8 T cells (CD8+SDF-1+) increased in the spleen and thymus after HFFD induction for 24 weeks in mice compared with healthy mice. A previous study suggested that HFD over 180 days increased the thymus size and promoted lymphopoiesis. Further results reported the absence of dramatic change in the number of mature single positive CD4+ and CD8+ T cells in the thymus (Trottier et al. 2012). Another report suggested that HFD impaired T-cell expansion in the thymus (Li et al. 2020) and that its number and function can be partially restored with natural dietary supplementation (Gulvady et al. 2013; Lewis et al. 2018).
The thymus aids cell-mediated immunity by ensuring that T cells differentiate and mature before being released into the bloodstream. The interaction between SDF-1 and CXCR4-regulating T cells migrates to the secondary lymphoid organ in normal homeostasis (Nanki and Lipsky 2000). Dynamic changes in CXCR4 expression during T-cells expansion are limited to pre-CD4+CD8+ phases (Lucas et al. 2017). The previous data suggested that ESE treatment restores naive T cells and regulatory T cells under HFFD (Atho’illah et al. 2017; Atho’illah et al. 2021). We assumed that ESE regulates CD4+ and CD8+ migration from the thymus, leading to improved immune response and reducing chronic inflammatory states caused by HFFD. However, we found that the effect of ESE is no from that of simvastatin on improving the SDF-1 expression in CD4 and CD8 T cells.
HFD could alter the CD4+ and CD8+ numbers in the spleen after 12-16 weeks of HFD (Surendar et al. 2019). Our previous study demonstrated that HFFD over 24 weeks still maintains CD4+ and CD8+ higher than ND mice (Atho’illah et al. 2021). Both CD4+ and CD8+ T-cells subsets induced the recruitment and numbers of M1 macrophages, which are essential to maintain low-grade chronic inflammation (Kiran et al. 2021). Interestingly, a previous study reported that CD4+ and CD8+ activation positively correlate with fasting plasma glucose and insulin resistance (Surendar et al. 2019). The elevated expression of SDF-1 in the spleen and hepar of HFFD mice is related to macrophage incorporation and inflammation induction, specifically in adipose tissue and insulin resistance. The study reveals the secretion of SDF-1/CXCL12 from hypertrophic adipocytes, including macrophages, via CXCR4 in obesity cases (Kim et al. 2014). The levels of SDF-1+ expression upregulated in obese mice induced by HFD showed that SDF-1/CXCR4 signaling triggered the accumulation of M1 macrophages. A previous study demonstrated that glyceollin reduces SDF-1 and CXCR4 mRNA (Choi et al. 2013). Further results indicated that glyceollin reduced CXCR4 expression by two-folds (Lecomte et al. 2017). The inhibition of SDF-1/CXCR4 signaling led to downregulated macrophage incorporation and reduced proinflammatory secretion, such as cytokines in white adipose tissue and insulin resistance improvement (Xu et al. 2015).
We also identified the expression of B220+SDF-1+ in the BM after HFFD induction. According to a previous study, B cells are more active in HFFD conditions (Atho’illah et al. 2018) and promote insulin resistance through excessive pathogenic immunoglobulin G antibody production (Winer et al. 2011). Hypercholesterolemia condition affects the BM microenvironment and homeostasis via the SDF-1/CXCR4 axis (Gomes et al. 2010). Our results revealed that the administration of ESE changed the B220+SDF-1+ population in the BM of HFFD mice. High-fat and fructose consumption causes BM dysregulation to produce hematopoietic cells and decreases B220+SDF-1+ cells. HFD alters hematopoietic stem cells (HSC) niches, and decreased SDF-1 leads to HSC mobilization and differentiation into myelopoiesis (Luo et al. 2015).
Our results support the findings of the above mentioned study that under HFFD condition, GR-1+ expression increased, whereas erythrocytes lineage expression, TER-119+CD34+, TER-119+VLA-4+, and TER-119+ decreased (Safitri et al. 2018). SDF-1 is a leading factor that supports pre-B-cell development, and the lack of SDF-1 in B-cell development affects the cell-maturation process itself (Abi-Younes et al. 2000). In the growth of B cells, genistein and daidzein were expected to have a protective effect on cell metabolism and proliferation and then delay the aging of endothelial cells. Furthermore, isoflavones in soybean reduced the impact of free radicals and increased antioxidant enzyme activity (Ibrahim et al. 2015). Daidzein is also known as an effective immune-stimulator of lymphocyte cell proliferation (Wang et al. 1997). Simvastatin is one of the most widely used statins for reducing cholesterol and preventing cardiovascular disease. The administration of simvastatin impacts the decline of SDF-1 in BM cells and triggers apoptosis in B cells through caspase-9, which indirectly activates caspase-3 and -8, which triggers B-cells apoptosis (Chapman-Shimshoni et al. 2003).
The high-affinity score also supports the effect of ESE on SDF-1 expression, as shown by molecular docking analysis. The glyceollin I, II, and III offer strong affinity binding to the CXCR4 and the G-coupled receptor for the SDF-1. The amino acids included in interaction, Asp187, Asp97, and Glu288, play an essential role in SDF-1 binding and signaling, as reported by Brelot et al. (Brelot et al. 2000). The paper confirmed the reduced binding affinity of SDF-1 to CXCR4 when there was a mutation in the Glu288 (E Q), Asp97 (N D), and Asp187 (D A) sites. This condition automatically abolished the signaling process in the pathway. Even though this finding was related to HIV-1 activity, the amino acid sites that appeared in our docking results were almost similar. This finding led to the hypothesis that there might be a similarity between binding sites in the hematopoietic case, including CXCR4. Thus, ESE has the potential to interrupt the signaling and binding activity of CXCR4.
Our findings demonstrated that glyceollin rich in ESE suppresses CXCR4 in silico study and SDF-1 in the spleen, thymus, and hepar, expressed by CD4+ and CD8+ T cells. The limitation of the current study was that we did not analyze the adipose tissue macrophages. During nutrient excess-induced obesity, adipocytes have been considered to orchestrate the imbalance between proinflammatory cytokines and anti-inflammatory cytokines secreted by adipose tissue (He et al. 2020). A previous study demonstrated that HFD impairs the AMPK-mTOR axis, which leads to dysregulated CD4+ T-cell glycolysis (Surendar et al. 2019). Moreover, the adipocytes from HFD-mice were reported to reduce insulin sensitivity through lowering the IRS-1 protein levels. Importantly, SDF-1 directly induced these mechanisms (Shin et al. 2018). Here we provide that the lymphoid organs also have a pivotal role in low-grade chronic inflammation by elevating SDF-1 secreted by CD4+ and CD8+ T-cells and B-cells during HFFD. Alternatively, ESE may maintain the balance of HSC and progenitor B cells in the BM. The dual action of ESE in primary and secondary lymphoid organs may have beneficial effects in preventing or delaying any deleterious effects caused by HFFD through the SDF-1/CXCR4 axis. To conclude, CD4+SDF-1+ and CD8+SDF-1+ expressions in HFFD mice were elevated when compared with ND mice. ESE significantly decreased the expression of CD4+SDF-1+ and CD8+SDF-1+ T cells. HFFD mice treated with ESE had improved B-cell development. Glyceollin I acts as a CXCR4 inhibitor with a binding of −8.4 kcal/mol. These findings suggest that ESE may help maintain the regulatory system of SDF-1 expressed by CD4+, CD8+, and hepatocytes.
CONCLUSION
In HFFD mice, CD4+SDF-1+ and CD8+SDF-1+ expression was elevated compared to ND mice. ESE has significantly declined the expression of CD4+SDF-1+ and CD8+SDF-1+ T-cells. HFFD mice treated with ESE had improved B-cell development. Glyceollin I act as a CXCR4 inhibitor with the strongest binding of −8.4 kcal/mol. These findings suggest that ESE may help maintain the regulatory system of SDF-1 expressed by CD4+, CD8+, and hepatocytes.
ACKNOWLEDGEMENTS
We thank Prof. Nashi Widodo, Ph.D Med.Sc., for granting the Saccharomyces cerevisiae culture.
AUTHOR CONTRIBUTIONS
Conceptualization: MR, SW; Methodology: YDS, MFA, FDN; Formal analysis and investigation: YDS, YIC, MFA, GWP, FDN, DWA; Writing - original draft preparation: YDS, YIC, MFA, GWP, FDN, DWA; Writing - review and editing: MR, SW; Funding acquisition: MR; Resources: MR; Supervision: MR, SW.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Abarca-Gómez, L., Abdeen, Z.A., Hamid, Z.A., Abu-Rmeileh, N.M., Acosta-Cazares, B., ..., Ezzati, M. 2017. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128•9 million children, adolescents, and adults. The Lancet. 390: 2627–2642.
Abi-Younes, S., Sauty, A., Mach, F., Sukhova, G.K., Libby, P., and Luster, A.D. 2000. The stromal cell–derived factor-1 chemokine is a potent platelet agonist highly expressed in atherosclerotic plaques. Circulation Research. 86: 131–138.
Antonopoulos, A.S., Margaritis, M., Lee, R., Channon, K., and Antoniades, C. 2012. Statins as anti-inflammatory agents in atherogenesis: molecular mechanisms and lessons from the recent clinical trials. Current pharmaceutical design. 18: 1519-1530.
Asri, A., Sabour, J., Atashi, A., and Soleimani, M. 2016. Homing in hematopoietic stem cells: Focus on regulatory role of CXCR7 on SDF1a/CXCR4 axis. EXCLI Journal. 15: 134–143.
Atho’illah, M.F., Safitri, Y.D., Nur’aini, F.D., Savitri, R.U., Rahayu, S., Widyarti, S., and Rifa’I, M. 2019. Evaluation of glyceollin accumulation and antioxidant properties on soybean (Glycine max L.) through combination of different biotic elicitor and light. Scientific Study & Research: Chemistry & Chemical Engineering, Biotechnology, Food Industry. 20: 199–208.
Atho’illah, M.F., Safi̇tri, Y.D., Nur’Ai̇ni̇, F.D., Wi̇dyarti,̇ S., Hi̇deo, T., and Ri̇fa’I, M. 2018. Soybean extract suppresses B cell activation through TLR3/TLR4 in high fat-high fructose diet mice. Turkish Journal of Immunology. 6: 95–103.
Atho’illah, M.F., Safitri, Y.D., Nur’aini, F.D., Widyarti, S., Tsuboi, H., and Rifa’I, M. 2021. Elicited soybean extract attenuates proinflammatory cytokines expression by modulating TLR3/TLR4 activation in high−fat, high−fructose diet mice. Journal of Ayurveda and Integrative Medicine. 12:43–51.
Atho’illah, M.F., Widyarti, S., and Rifa’I, M. 2017. Elicited soybean (Glycine max L.) extract improves regulatory T cell activity in high fat-fructose diet mice. AIP Publishing, Malang, Indonesia. pp 020004-1-020004–6.
Brelot, A., Heveker, N., Montes, M., and Alizon, M. 2000. Identification of residues of cxcr4 critical for human immunodeficiency virus coreceptor and chemokine receptor activities. Journal of Biological Chemistry. 275: 23736–23744.
Chapman-Shimshoni, D., Yuklea, M., Radnay, J., Shapiro, H., and Lishner, M. 2003. Simvastatin induces apoptosis of B-CLL cells by activation of mitochondrial caspase 9. Experimental Hematology. 31: 779–783.
Charo, I.F. and Ransohoff, R.M. 2006. The many roles of chemokines and chemokine receptors in inflammation. The New England Journal of Medicine. 354: 610–621.
Choi, J-H., Nguyen, M.P., Jung, S-Y., Kwon, S-M., Jee, J-G., Bae, J-S., Lee, S., Lee, M.Y., and Lee, Y-M. 2013. Inhibitory effect of glyceollins on vasculogenesis through suppression of endothelial progenitor cell function. Molecular Nutrition & Food Research. 57: 1762–1771.
Christina, Y.I., Ibrahim, M., and Rifa’i, M. 2015. Polyherbal EMSA ERITIN blocks nuclear factor-kappa B (NF-κB) and proinflammatory cytokines in irradiated mice. American Journal of Immunology. 11: 17–25.
Djati, M.S. and Christina, Y.I. 2019. Rempah-Rempah Indonesian traditional food flavor toward modern functional food and herbal medicine. Functional Foods in Health and Disease. 9: 241.
Girousse, A., Gil-Ortega, M., Bourlier, V., Bergeaud, C., Sastourné-Arrey, Q., Moro, C., Barreau, C., Guissard, C., Vion, J., Arnaud, E., Pradère, J-P., Juin, N., Casteilla, L., and Sengenès, C. 2019. The release of adipose stromal cells from subcutaneous adipose tissue regulates ectopic intramuscular adipocyte deposition. Cell Reports. 27: 323-333.
Gomes, A.L., Carvalho, T., Serpa, J., Torre, C., and Dias, S. 2010. Hypercholesterolemia promotes bone marrow cell mobilization by perturbing the SDF-1:CXCR4 axis. Blood. 115: 3886–3894.
Gulvady, A.A., Ciolino, H.P., Cabrera, R.M., and Jolly, C.A. 2013. Resveratrol inhibits the deleterious effects of diet-induced obesity on thymic function. The Journal of Nutritional Biochemistry. 24: 1625–1633.
He, M-Q., Wang, J-Y., Wang, Y., Sui, J., Zhang, M., Ding, X., Zhao, Y., Chen, Z-Y., Ren, X-X., and Shi, B-Y. 2020. High-fat diet-induced adipose tissue expansion occurs prior to insulin resistance in C57BL/6J mice. Chronic Diseases and Translational Medicine. 6: 198–207.
Ibrahim, M., Widodo, M.A., Sumitro, S.B., and Widjajanto, E. 2015. The protective effects of polyherbal extracts of soybeans, coconut water, and brown rice (EMSA eritin) on stromal cell-derived factor 1 production in radiated mice. Biomarkers and Genomic Medicine. 7: 147–153.
Kim, D., Kim, J., Yoon, J.H., Ghim, J., Yea, K., Song, P., Park, S., Lee, A., Hong, C-P., Jang, M.S., Kwon, Y., Park, S., Jang, M.H., Berggren, P-O., Suh, P-G., and Ryu, S.H. 2014. CXCL12 secreted from adipose tissue recruits macrophages and induces insulin resistance in mice. Diabetologia. 57: 1456–1465.
Kim, H.J., di Luccio, E., Kong, A-N.T., Kim, J-S. 2011. Nrf2-mediated induction of phase 2 detoxifying enzymes by glyceollins derived from soybean exposed to Aspergillus sojae. Biotechnology Journal. 6: 525–536.
Kiran, S., Kumar, V., Murphy, E.A., Enos, R.T., and Singh, U.P. 2021. High fat diet-induced CD8+ T cells in adipose tissue mediate macrophages to sustain low-grade chronic inflammation. Frontiers in Immunology. 12: 680944.
Kumar, P., Singh, S., and Ahmad, M.I. 2020. Synergistic effect of cinnamomum zeylanicum and murraya koenigii formulation for antiobesity and hypolipidemic activity on wistar albino rats. Advances in Traditional Medicine. 21: 553-563.
Kurita, K., Ishikawa, K., Takeda, K., Fujimoto, M., Ono, H., Kumagai, J., Inoue, H., Yokoh, H., and Yokote, K. 2019. CXCL12-CXCR4 pathway activates brown adipocytes and induces insulin resistance in CXCR4-deficient mice under high-fat diet. Scientific Reports. 9: 6165.
Lecomte, S., Chalmel, F., Ferriere, F., Percevault, F., Plu, N., Saligaut, C., Surel, C., Lelong, M., Efstathiou, T., and Pakdel, F. 2017. Glyceollins trigger anti-proliferative effects through estradiol-dependent and independent pathways in breast cancer cells. Cell Communication and Signaling. 15: 26.
Leng, Q., Nie, Y., Zou, Y., and Chen, J. 2008. Elevated CXCL12 expression in the bone marrow of NOD mice is associated with altered T cell and stem cell trafficking and diabetes development. BMC Immunology. 9: 51.
Lewis, E.D., Ren, Z., DeFuria, J., Obin, M.S., Meydani, S.N., and Wu, D. 2018. Dietary supplementation with blueberry partially restores T-cell-mediated function in high-fat-diet-induced obese mice. British Journal of Nutrition. 119: 1393–1399.
Li, H., Wetchapinant, C., Zhang, L., and Wu, K. 2020. High‐fat diet from weaning until early adulthood impairs T cell development in the thymus. Lipids. 55: 35–44.
Lucas, B., White, A.J., Parnell, S.M., Henley, P.M., Jenkinson, W.E., and Anderson, G. 2017. Progressive changes in CXCR4 expression that define thymocyte positive selection are dispensable for both innate and conventional αβT-cell development. Scientific Reports. 7: 5068.
Luo, Y., Chen, G-L., Hannemann, N., Ipseiz, N., Krönke, G., Bäuerle, T., Munos, L., Wirtz, S., Schett, G., and Bozec, A. 2015. Microbiota from obese mice regulate hematopoietic stem cell differentiation by altering the bone niche. Cell Metabolism. 22: 886–894.
Nanki, T. and Lipsky, P.E. 2000. Cutting edge: Stromal cell-derived factor-1 is a costimulator for CD4+ T cell activation. The Journal of Immunology. 164: 5010–5014.
Nur’aini, F.D., Rahayu, S., and Rifa’I, M. 2019. Anti-inflammatory activity of elicited soybean (Glycine max) extract on Balb/C mice (Mus musculus) with high-fat and -fructose diet. Central European Journal of Immunology. 44: 7–14.
Nwachukwu, I.D., Luciano, F.B., and Udenigwe, C.C. 2013. The inducible soybean glyceollin phytoalexins with multifunctional health-promoting properties. Food Research International. 54: 1208–1216.
Pabich, M. and Materska, M. 2019. Biological effect of soy isoflavones in the prevention of civilization diseases. Nutrients. 11: 1660.
Pereira, R., Botezelli, J., da Cruz Rodrigues, K., Mekary, R., Cintra, D., Pauli, J., da Silva, A, Ropelle, E., and de Moura, L. 2017. Fructose consumption in the development of obesity and the effects of different protocols of physical exercise on the hepatic metabolism. Nutrients. 9: 405.
Safitri, Y.D., Atho’illah, M.F., Nur’aini, F.D., Widyarti, S., and Rifa’I, M. 2018. The effects of elicited soybean (Glycine max) extract on hematopoietic cells of high fat-fructose diet Balb/C mice model. Jordan Journal of Biological Sciences. 11: 241–246.
Safitri, Y.D., Widyarti, S., and Rifa’I, M. 2017. Elicited soybean (Glycine max) extract effect on improving levels of Ter-119+Cd59+ in a mouse model fed a high fat-fructose diet. AIP Publishing, Malang, Indonesia. pp 020009-1−020009-4.
Shin, J., Fukuhara, A., Onodera, T., Kita, S., Yokoyama, C., Otsuki, M., and Shimomura, I. 2018. SDF-1 is an autocrine insulin-desensitizing factor in adipocytes. Diabetes. 67: 1068–1078.
Surendar, J., Frohberger, S.J., Karunakaran, I., Schmitt, V., Stamminger, W., Neumann, A-L., Wilhelm, C., Hoerauf, A., and Hübner, M.P. 2019. Adiponectin limits IFN-γ and IL-17 producing CD4 T cells in obesity by restraining cell intrinsic glycolysis. Frontiers in Immunology. 10:2555.
Trottier, M.D., Naaz, A., Li, Y., and Fraker, P.J. 2012. Enhancement of hematopoiesis and lymphopoiesis in diet-induced obese mice. Proceedings of the National Academy of Sciences. 109: 7622–7629.
Wada, T., Watanabe, E., Kotera, Y., Onogi, Y., Tsuneki, H., and Sasaoka, T. 2018. The SDF1-CXCR4 signals regulate adipose tissue expansion by modulating angiogenesis in diet-induced obesity in mice. Diabetes. 67(Supplement 1): 2005-P.
Wang, L., Du, S., Wang, H., and Popkin, B.M. 2018. Influence of dietary fat intake on bodyweight and risk of obesity among Chinese adults, 1991–2015: A prospective cohort study. The Lancet. 392: S20.
Wang, N., Guo, J., Liu, F., Wang, M., Li, C., Jia, L., Zhai, L., Wei, W., and Bai, Y. 2017. Depot-specific inflammation with decreased expression of ATM2 in white adipose tissues induced by high-margarine/lard intake. PLoS One. 12: e0188007.
Wang, W., Higuchi, C.M., Zhang, R. 1997. Individual and combinatory effects of soy isoflavones on the in vitro potentiation of lymphocyte activation. Nutrition and Cancer. 29: 29–34.
Winer, D.A., Winer, S., Shen, L., Wadia, P.P., Yantha, J., Paltser, G., Tsui, H., Wu, P., Davidson, M.G., Alonso, M.N., Leong, H.X., Glassford, A., Caimol, M., Kenkel, J.A., Tedder, T.F., McLaughlin, T., Miklos, D.B., Dosch, H-M., and Engleman, E.G. 2011. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nature Medicine. 17: 610–617.
Wu, B., Chien, E.Y.T., Mol, C.D., Fenalti, G., Liu, W., Katritch, V., Abagyan, R., Brooun, A., Wells, P., Bi, F.C., Hamel, D.J., Kuhn, P., Handel, T.M., Cherezov, V., and Stevens, R.C. 2010. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science. 330: 1066–1071.
Xu, L., Kitade, H., Ni, Y., and Ota, T. 2015. Roles of chemokines and chemokine receptors in obesity-associated insulin resistance and nonalcoholic fatty liver disease. Biomolecules. 5: 1563–1579.
Yoshida, H., Watanabe, H., Ishida, A., Watanabe, W., Narumi, K., Atsumi, T., Sugita, C., and Kurokawa, M. 2014. Naringenin suppresses macrophage infiltration into adipose tissue in an early phase of high-fat diet-induced obesity. Biochemical and Biophysical Research Communications. 454: 95–101.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand.
Yunita Diyah Safitri1, Yuyun Ika Christina2, Mochammad Fitri Atho illah2, Galuh Wening Permatasari3, Farida Dewi Nur aini2, Didin Wahyu Agustina2, Sri Widyarti2, and Muhaimin Rifai2 , 4, *
1 Medical Laboratory Technology Program, Sekolah Tinggi Ilmu Kesehatan Karya Putra Bangsa, 66291, Tulungagung, East Java, Indonesia
2 Department of Biology, Faculty of Mathematics and Natural Sciences, Brawijaya University, 65145, Malang, East Java, Indonesia.
3 Indonesian Oil Palm Research Institute (IOPRI), 16128, Bogor, West Java, Indonesia.
4 Center of Biosystem Study, LPPM of Brawijaya University, 65145, Malang, East Java, Indonesia5 Center of Biosystem Study, LPPM of Brawijaya University, 65145, Malang, East Java, Indonesia.
*Corresponding author: Muhaimin Rifai, E-mail: immunobiology@ub.ac.id
Total Article Views
Editor: Wasu Pathom-aree,
Chiang Mai University, Thailand
Article history:
Received: February 3, 2022;
Revised: November 15, 2022;
Accepted: November 21, 2022;
Published online: December 13, 2022

