
Formulation of Coconut Oil Mouthwash with Mixed Emulsifier and Its Growth Inhibition of Candida albicans Biofilms
Paween Chanpa, Darunee Owittayakul, Phenphichar Wanachantararak, Wantida Chaiyana, Siriwoot Sookkhee*Published Date : 2023-01-05
DOI : https://doi.org/10.12982/NLSC.2023.016
Journal Issues : Number 1, January-March 2023
Abstract Virgin coconut oil has been used for oil pulling, which helps to promote oral health and can inhibit oral fungi. Due to 15-20 minutes of rinsing time, oily taste, and instability of lately developed mouthwashes, this present study aimed to develop a mouthwash formula from virgin coconut oil with good stability and appropriate rinsing time to inhibit Candida albicans in biofilm. The formulation results showed that the mixed emulsifiers between tween 80 and span 80 at the ratio of 40:60 were appropriate to use as the mixed emulsifier of virgin coconut oil. After the formulation, seven mouthwash formulas including F5 (10:25:65), F21 (20:20:60), F36 (30:20:50), F49 (40:20:40), F59 (50:15:35), F67 (60:10:30) and F73 (70:5:25) which comprised the ratios among virgin coconut oil: mixed emulsifier: distilled water, respectively, exhibited the stable emulsion by using the lowest mixed emulsifier in each ratio. Results from the XTT assay at various exposure times demonstrated that a five-minute exposure with formula F73 exhibited the highest inhibitory activity against C. albicans biofilm with a significant difference compared to other formulas. To confirm the anticandidal activity, the F73 formula could inhibit the growth of C. albicans after enumerating the colonies with an exposure time of five minutes. It may be concluded that the appropriate virgin coconut oil mouthwash was formulated at the ratio of coconut oil: mixed emulsifier: distilled water at 70:5:25 and could inhibit C. albicans after five minutes of exposure.
Keywords: Formulation, Coconut oil mouthwash, Mixed emulsifier, Candida albicans, Biofilm
Funding: We are grateful for the research funding provided by the Faculty of Dentistry, Chiang Mai University, Chiang Mai, Thailand.
Citation: Chanpa, P., Owittayakul, D., Wanachantararak, P., Chaiyana, W., Sookkhee, S. 2023. Formulation of coconut oil mouthwash with mixed emulsifier and its growth inhibition of Candida albicans biofilms. Nat. Life Sci. Commun. 22(1): e2023016.
INTRODUCTION
Fungal infection is one of the main clinical problems resulting from the extensive use of broad-spectrum antibiotics and immunosuppressive therapy. Candidiasis is the most common opportunistic fungal infection in the oral cavity. It is primarily caused by the overgrowth of C. albicans (Kim et al., 2011). Currently, topical antifungal agents, such as polyenes, echinocandins, and azoles have been the standard treatment for oral candidiasis treatment (Garcia-Cuesta et al., 2014). However, these synthetic compounds also showed some adverse effects. Natural or herbal extracts may be alternative options to reduce oral infection with few adverse effects in the bacterial reduction (Sookkhee et al., 2001), inhibition of plaque development (Amith et al., 2007; Asokan et al., 2009), prevention of caries and gingivitis (An et al., 2008; Peedikayil et al., 2015), relief of dry mouth (Ludwar et al., 2022), reduction of bad breath (Asokan et al., 2011a), and inhibition of fungal growth (Ogbolu et al., 2007; Owittayakul et al., 2018). One of these options was vegetable oil pulling for promoting oral hygiene (Shanbhag, 2017). Coconut oil is popular in several trending diets and exhibits clinical action for preventing fat deposition in blood vessels and reducing oxidative stress (Carandang, 2008). Coconut oil pulling produces a clean feeling, pleasing scent, reducing oral malodor but it has an oily taste and requires a long duration of rinsing. Lastly, mouthwash formulated among virgin coconut oil, propylene glycol, and distilled water at the ratio of 60:30:10 was reported (Intarakaewsri et al., 2020). Its activity against the biofilm of C. albicans ATCC 10231 was not statistically different after being compared with nystatin at 100,000 U/ml for ten minutes (P >0.05). Virgin coconut oil from cold extraction has a nice fragrance and is tasty. Coconut oil contains medium-chain fatty acids (MCFA), tocopherols (Vitamin E), tocotrienol, phytosterols, phytostanols, flavonoids, and other polyphenols (Carandang, 2008). A saturated fatty acid is mainly in this oil. The important antimicrobial medium-chain fatty acids, lauric acid (C12:0), capric acid (C10:0), and caprylic acid (C8:0) are reported as the ingredients of this oil at 36.95, 4.67, and 0.54% (Wang et al., 2015). Bergsson et al. (Bergsson et al., 2001) reported the significant anticandidal activity of fatty acids, namely capric acid and lauric acid, and monoglycerides, namely monocaprin (10:0) contained in coconut oil after ten minutes of testing. They also reported the probable mechanism of 10 mM capric acid after incubating with candidal cells for 30 minutes. Transmitted electron microscope results demonstrated the disruption of the plasma membrane of candida cells, vacuole disorganization, and disappearances of the nucleus and mitochondria (Bergsson et al., 2001). Therefore, the advantages of coconut oil pulling to oral hygiene are the antifungal effect of the fatty acids, and its saponification as the cleansing action due to the conversion reaction of triglyceride to glycerine (Asokan et al., 2011a). Another advantage is caused by the emulsification effect that increased the surface reaction of oil particles. The study by Owittayakul et al. reported the satisfaction of users after rinsing orally with the formulated coconut oil mouthwash (Owittayakul et al., 2018). Feeling of oral cleanliness (80%), pleasant scent (60%), and reduction of oral malodor (60%) were the primary reasons for users who desired to continue use. On the other hand, the long rinsing time (76.5%), feeling of oily taste (16.7%) and the worry of the numbness of the tongue which may be an adverse reaction (11.8%) were the primary reasons for users who rejected continuing its use (Owittayakul et al., 2018). The application of emulsifiers is the difference of virgin coconut oil mouthwash from the coconut oil pulling. Emulsion status of coconut oil can increase the surface reaction of antimicrobial activity. The emulsifier also affects to decrease the surface tension and increase the emulsion status (Tadros, 2009), and also maintains the hydrophile-lipophile balance (HLB) of w/o emulsion of virgin coconut oil mouthwash approximately 8 ± 1 as well as Intarakaewsri et al. ’s study (Intarakaewsri et al., 2020). Although propylene glycol was utilized as the emulsifier and solvent in their study. The formulation was not stable. The finding of other emulsifiers for more stable mouthwash is required. The factors affecting the stable anticandidal mouthwash are also investigated in our study. In the present study, we hypothesized that the kind and percentage of applied emulsifier can affect the stability and anticandidal activity of coconut oil mouthwash, and the duration of rinsing mouthwash at 2-20 minutes did not affect anticandidal activity in the biofilm. Therefore, the aims of the present study were to formulate the stable anticandidal coconut oil mouthwash, investigate the appropriate time for inhibit the biofilm of one standard and three clinical isolates of C. albicans.
MATERIALS AND METHODS
Development of virgin coconut oil mouthwash
Virgin coconut oil preparation
Cold-pressed coconut oil (CoCo Delight, Lot NP630107, GPO, Pathumthani, Thailand) was chosen as the virgin coconut oil.
Emulsion preparation
Tween 80 (polysorbate 80; Batch D0013RA312, Krungthepchemi Ltd., Lad Prao, Bangkok, Thailand.) and Span 80 (Batch SMOML0321, Top Inno Tec Co., Ltd., Suan Luang, Bangkok, Thailand) with HLB 15 and 4.3 were the studied emulsifiers in our emulsion developments. Various ratios of these chemicals including 100:0, 80:20, 60:40, 40:60, 20:80, and 0:100 was established and then mixed as the emulsifier for preparing the emulsions. Distilled water, coconut oil and the mixed emulsifier at the combination ratio of 25:70:5 were mixed with a homogenizer (CDEKTM, FSH-2A, Pioway Medical Lab Equipment Co., Ltd., Nanjing, Jiangsu, China) at 10,000 rpm for five minutes.
Emulsion stability test
After the homogenization was completed, two ml of the emulsion was aliquoted into the tested bottle. The emulsion stability of each bottle was tested in five incubation cycles of the conversion between 35°C for 12 hours and 5°C for 12 hours.
% residual stability was calculated = the height of emulsion at the end of test * 100
the height of emulsion at the begin
The mixed ratio of two emulsifiers that exhibited the highest % stability of any emulsion was selected to use as the mixed emulsifier in further investigation. (Figure 1) Factors of ingredient composition affect the stability of the emulsion. Each ingredient composition in the emulsion was varied as shown in Table 1 for preparing each formulation before evaluating their stability as described above. The formulations which exhibited high stability were observed. In the same ratio of coconut oil, the stable formulations which contained the lowest quantity of emulsifier were selected for further investigation (Figure 2).
Determination of pH (Sanjeewani, 2013)
The pH value of each emulsion formulation was determined in triplicate at 28°C by using pH meter (pH-686, YieryiTM, Shen Zhen Yage Technology Co., Ltd., Shenzhen, Guangdong, China).
Determination of viscosity (Wiyani et al., 2016)
Fifty ml of each emulsion was evaluated for its viscosity by using a rotary viscometer (RM-1(NDJ-1) rotary viscometer, Wand J Instrument Co., Ltd., Mudu, Jiangsu, China) with the spindle at 28°C. Viscosity was demonstrated in millipascalseconds (mPa.s) with the coefficient at the spindle.
Table 1. Formulations of virgin coconut oil mouthwash.
|
Formula |
% w/v |
|
Formula |
% w/v |
||||
|
Coconut oil |
Mixed emulsifier |
Distilled water |
|
Coconut oil |
Mixed emulsifier |
Distilled water |
||
|
F1 |
10 |
5 |
85 |
|
F42 |
30 |
50 |
20 |
|
F2 |
10 |
10 |
80 |
|
F43 |
30 |
55 |
15 |
|
F3 |
10 |
15 |
75 |
|
F44 |
30 |
60 |
10 |
|
F4 |
10 |
20 |
70 |
|
F45 |
30 |
65 |
5 |
|
F5 |
10 |
25 |
65 |
|
F46 |
40 |
5 |
55 |
|
F6 |
10 |
30 |
60 |
|
F47 |
40 |
10 |
50 |
|
F7 |
10 |
35 |
55 |
|
F48 |
40 |
15 |
45 |
|
F8 |
10 |
40 |
50 |
|
F49 |
40 |
20 |
40 |
|
F9 |
10 |
45 |
45 |
|
F50 |
40 |
25 |
35 |
|
F10 |
10 |
50 |
40 |
|
F51 |
40 |
30 |
30 |
|
F11 |
10 |
55 |
35 |
|
F52 |
40 |
35 |
25 |
|
F12 |
10 |
60 |
30 |
|
F53 |
40 |
40 |
20 |
|
F13 |
10 |
65 |
25 |
|
F54 |
40 |
45 |
15 |
|
F14 |
10 |
70 |
20 |
|
F55 |
40 |
50 |
10 |
|
F15 |
10 |
75 |
15 |
|
F56 |
40 |
55 |
5 |
|
F16 |
10 |
80 |
10 |
|
F57 |
50 |
5 |
45 |
|
F17 |
10 |
85 |
5 |
|
F58 |
50 |
10 |
40 |
|
F18 |
20 |
5 |
75 |
|
F59 |
50 |
15 |
35 |
|
F19 |
20 |
10 |
70 |
|
F60 |
50 |
20 |
30 |
|
F20 |
20 |
15 |
65 |
|
F61 |
50 |
25 |
25 |
|
F21 |
20 |
20 |
60 |
|
F62 |
50 |
30 |
20 |
|
F22 |
20 |
25 |
55 |
|
F63 |
50 |
35 |
15 |
|
F23 |
20 |
30 |
50 |
|
F64 |
50 |
40 |
10 |
|
F24 |
20 |
35 |
45 |
|
F65 |
50 |
45 |
5 |
|
F25 |
20 |
40 |
40 |
|
F66 |
60 |
5 |
35 |
|
F26 |
20 |
45 |
35 |
|
F67 |
60 |
10 |
30 |
|
F27 |
20 |
50 |
30 |
|
F68 |
60 |
15 |
25 |
|
F28 |
20 |
55 |
25 |
|
F69 |
60 |
20 |
20 |
|
F29 |
20 |
60 |
20 |
|
F70 |
60 |
25 |
15 |
|
F30 |
20 |
65 |
15 |
|
F71 |
60 |
30 |
10 |
|
F31 |
20 |
70 |
10 |
|
F72 |
60 |
35 |
5 |
|
F32 |
20 |
75 |
5 |
|
F73 |
70 |
5 |
25 |
|
F33 |
30 |
5 |
65 |
|
F74 |
70 |
10 |
20 |
|
F34 |
30 |
10 |
60 |
|
F75 |
70 |
15 |
15 |
|
F35 |
30 |
15 |
55 |
|
F76 |
70 |
20 |
10 |
|
F36 |
30 |
20 |
50 |
|
F77 |
70 |
25 |
5 |
|
F37 |
30 |
25 |
45 |
|
F78 |
80 |
5 |
15 |
|
F38 |
30 |
30 |
40 |
|
F79 |
80 |
10 |
10 |
|
F39 |
30 |
35 |
35 |
|
F80 |
80 |
15 |
5 |
|
F40 |
30 |
40 |
30 |
|
F81 |
90 |
5 |
5 |
|
F41 |
30 |
45 |
25 |
|
|
|
|
|
Microscopic observation of emulsion particle (Wiyani et al., 2016)
Oil in water (O/W) emulsion appearance was observed under the microscope (Olympus BX41TF, Olympus Optical Co., Ltd., Tokyo, Japan). Two drops of the emulsion were placed on the glass slide before being mixed with methylene blue (water-soluble stain), and then covered with a glass cover slip.
Anticandidal activity against C. albicans biofilms of virgin coconut oil mouthwash formulas
C. albicans strains
The standard reference strain of C. albicans ATCC 10231 was purchased from the bacterial culture bank of the Sciences and Technology Institute of Thailand. Three tested strains were isolated from patients who were diagnosed with oral candidiasis at the Oral Diagnosis Clinic and confirmed as C. albicans on HiCromeTM Candida Differential agar (Himedia, Mumbai, India) at Medical Science Laboratory, Dental Hospital, Faculty of Dentistry, Chiang Mai University, Chiang Mai, Thailand. All strains were separately cultured in Sabouraud Dextrose Broth (DifcoTM; Bacton Dickinson, Sparks, MD) supplemented with 1% glucose (Lot BCCD2059, ACS reagent, Sigma-Aldrich Co., St.Louis, MO.) at 37°C for 24 hours under aerobic condition. At the end of incubation, one ml of culture was centrifuged at 2000 rpm/minute for ten minutes. Cell-free supernatant was then omitted. Candidal cells were resuspended with 1 ml of Phosphate Buffer Saline (PBS) and then adjusted the optical density at 600 nm to be equal to McFarland No.1 (106 CFU/ml) (Yuan et al., 2019).
Subjects and saliva collections
Ten ml of each unstimulated saliva was collected from three subjects who exhibited good oral hygiene with simple random sampling. Two hours before the collection, the subject received the guidelines to stop eating, drinking, and cleaning the oral cavity (Fenoll-Palomares, 2004; Muddugangadhar, 2015). Each collected saliva was centrifuged at 10,000 rpm, 4°C for 30 minutes (Thaweboon et al., 2011), and then the supernatant part of saliva was separately passed through 0.45 µm of the sterilized membrane (PuridiscTM 25mm, Lot. 17024407, GE Healthcare UK Ltd., Buckinghamshire, UK). To produce the pellicle, 50 µl sterilized saliva was dropped into each well of a 96-well plate with a flat bottom shape. The 96-well plate was incubated at 37°C for two hours, and then washed twice with 200 µl of PBS solution. The prepared pellicle of each well was conducted to confirm the missing bacterial and yeast contaminations by using the spreading technique on blood and Sabouraud Dextrose agars under the incubation condition at 37°C for 24 hours.
Biofilm formation of C. albicans on the bottom surface of 96-well plate
One hundred µl of each isolate of C. albicans which adjusted the optical density to McFarland No. 1 was dropped into each well of the pellicle-prepared 96-well plate. One hundred µl of Sabouraud Dextrose broth supplemented with 1% glucose was also separately dropped into each well. The tested 96-well plate was then incubated at 37°C for 1.5 hours (Santos, 2016) for initial adherence. After the end of incubation, the whole supernatant in each well was gradually drained out before being washed twice with 200 µl of PBS solution. One hundred µl of Sabouraud Dextrose broth supplemented with 1% glucose was immediately dropped into each well and replaced every 24 hours until the complete incubation at 48 hours. After the end of incubation, the whole supernatant in each well was completely drained before being washed twice with 200 µl of PBS solution. The growth of candida biofilm was considered before carried out to further experiments.
Inhibition of C. albicans in biofilm by chlorhexidine gluconate
Minimal fungicidal concentration (MFC) of chlorhexidine gluconate, the positive control of mouthwash, against C. albicans in biofilm was determined at 48 hours of incubation. Briefly, one hundred ml of 2% chlorhexidine gluconate was serially diluted into a 96-well plate until the concentration was at 0.003% with sterilized distilled water. Each well of the tested 96-well plate was then completely sealed with Breathe-Easier® sealing membrane (Breathe-Easy®, Sigma-Aldrich Co., St.Louis, MO.) for protecting the contamination of any wells (Gulati et al., 2018). The tested 96-well plate was vertically placed on the stand of an orbital shaker (orbital shaker KJ-201BD, Hinotel Co., Ltd., Ningbo, China), and then shaken at a rotating speed of 210 rpm (Gulati et al., 2018) for imitating the mechanical force in the oral cavity when used the mouthwash. The shaking time varied from 2, 5, 10, and 20 minutes. At the end of shaking time, the 96-well plate was normally placed on the bench. The sealing membrane was removed. The biofilm was washed twice with 200 µl of PBS solution. The biofilm in each well was completely removed and resuspended with Sabouraud Dextrose broth before enumerating on Sabouraud Dextrose agar at 37°C under aerobic condition for 24 hours. MFC of chlorhexidine gluconate was determined.
Inhibition of C. albicans in biofilm at the different times of exposure with each virgin coconut oil mouthwash formula and its base by XTT reduction assay
The selected formulas of coconut oil mouthwash and their bases from the above investigation were carried out to determine the inhibitory activity of C. albicans in biofilm at different times of exposure such as 2, 5, 10, and 20 minutes. Chlorhexidine at MFC and the PBS solution in a well of the 96-well plate were the positive and negative controls, respectively. XTT reduction assay was performed to determine the inhibitory activity against C. albicans. One hundred ml of each tested formula or each formula base was dropped into each well of the 96-well plate. All wells were completely sealed with sealing silicone membrane before being vertically placed on the orbital shaker. The exposures of biofilm to each tested formula or each formula base were shaken at 210 rpm at 2, 5, 10, and 20 minutes. After finishing, the tested plate was normally placed on the bench before removing the sealed membrane. The whole supernatant in each well was completely drained out and washed twice with PBS solution.
The XTT solution was freshly prepared as follows. XTT powder (XTT sodium salt, Lot R05H095, Thermo Fisher Scientific, Waltham, MA.) was solved in PBS to the final concentration of 0.5 mg/ml. Phenazine methosulphate powder (Sigma-Aldrich, Lot 079M4198V, St. Louise, MO.) was solved in distilled water to the final concentration of 0.32 mg/ml. The working solution was freshly prepared in the ratio of 9:1. One hundred µl of the working solution was dropped into the biofilm of each well. The tested 96-well plate was incubated at 37°C for 30 minutes. At the finish of incubation, the 96-well plate was carried out to determine the optical density at 492 nm by using a Sunrise Tecan® absorbance microplate reader (Tecan Group Ltd., Männedorf, Switzerland). Due to the XTT assay being the colorimetric technique for determining the enzymatic activity of viable candidal cells, the inhibition of each tested mouthwash formula was calculated by comparing with the positive and negative control utilizing the following formulars.
% inhibition of mouthwash formula = OD492tested formula-OD492PBS *100
OD492chlorhexidine-OD492PBS
% differential inhibition of mouthwash formula compared with its emulsion base
= %inhibition of tested formula-%inhibition of its base *100
%inhibition of tested formula\
Confirmation of anticandidal activity in biofilm by enumeration of Candidal colonies
Anticandidal activity in biofilm was performed in 96-well plates. The experiment was described above but not carried out to perform with an XTT working solution. Two hundred µl of PBS solution was dropped into each well before thoroughly resuspending with a micropipette until the complete disruption of biofilm. The whole suspension in each well was separately removed to a new Eppendorf tube. Eight hundred µl of PBS solution was mixed into this tube and then mixed with a vortex mixer for three minutes. Ten-fold dilution with PBS solution was performed in the new tube. One hundred µl of each tube was spread on Sabouraud Dextrose agar. All tested plates were incubated at 37°C under aerobic condition for 24 hours before the Candidal colonies were enumerated by using Image J software. The total amount of C. albicans in the original tube was calculated in CFU/ml. The percentage of anticandidal activity of the selected mouthwash formula was determined after compared with the experiment of chlorhexidine gluconate and PBS solution as the positive and negative control, respectively.
% inhibitory activity of mouthwash formula = CFU/ml of tested formula-CFU/ml of PBS *100
CFU/ml of chlorhexidine- CFU/ml of PBS
Statistical Analysis
Two-way ANOVA and Tukey’s multiple comparisons at P <0.05 were performed as the statistical analysis in the present study by using the software IBM SPSS Statistics for Windows Version 25 (IBM Corp., Armonk, NY, USA).
RESULTS
Results in Figure 1 showed the stabilities of these emulsifiers (Tween 80: Span 80) that mixed before formulating the mouthwash were varied from 28.25% to 100% at the ratio 60:40 (HLB = 10.72), and 40:60 (HLB = 8.58), respectively. From this result, the mixed ratio of these emulsifiers at 40:60 was used as the mixed emulsifier in further investigations.
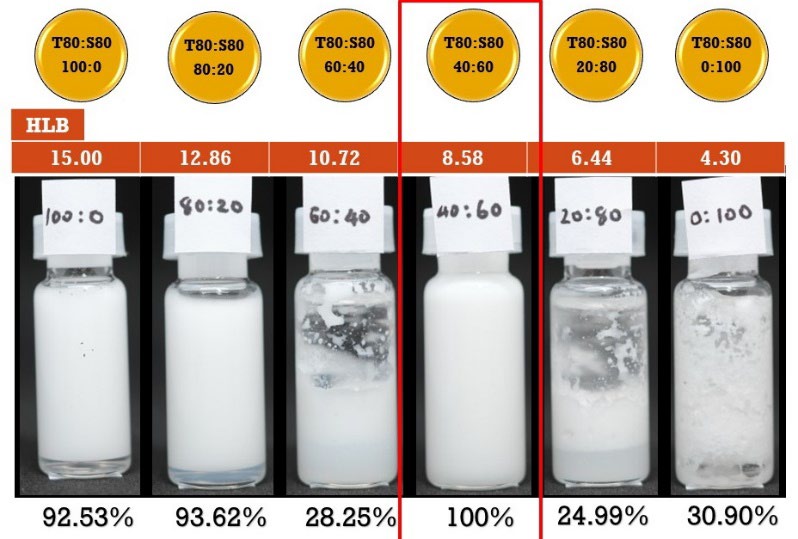
Figure 1. Percentage emulsion stability of various ratios between tween 80: span 80 which formulated in mouthwash formula and their HLB values.
After formulating all mouthwash formulas at the ratio of Tween 80: Span 80 at 40:60 with the varied ratio between coconut oil, distilled water, and mixed emulsifier, 81 mouthwash formulas were developed and also evaluated for their % stability (Figure 2). It was found that 23 mouthwash formulas demonstrated high stability at 100% of the emulsion. However, we selected only the formula that contained the lowest amount of emulsifier in the same amount of coconut oil as shown in Figure 3. Seven formulas including F5, F21, F36, F49, F59, F67, and F73, were selected as the high emulsion stability of mouthwash formulas that contained the lowest amount of mixed emulsifier after compared with the same amount of coconut oil at 10%, 20%, 30%, 40%, 50%, 60%, and 70%, respectively.

Figure 2. Emulsion stability evaluation of each coconut oil mouthwash formula.
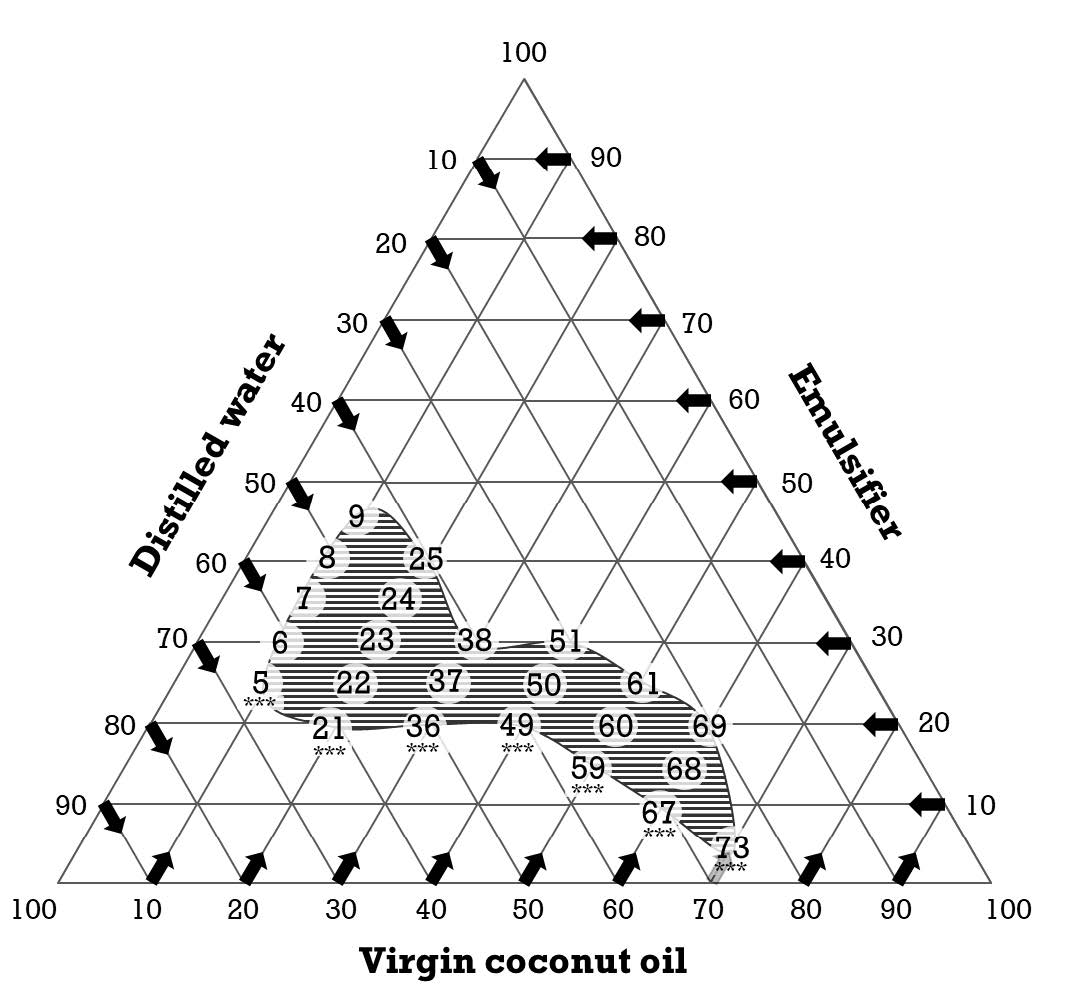
Figure 3. Ternary phase diagram of each coconut oil mouthwash formulation that exhibited % content of each ingredient. The numbers in the circle were the mouthwash formulas that demonstrated high emulsion stability. ***, the selected formula that contained the lowest amount of emulsifier after being compared with the same amount of coconut oil.
Their pH, viscosity, and microscopic appearance under the microscope were exhibited in Table 2. They were weak alkali. Their viscosities varied in a range of 81.00 to 1976.00 mPa.s. Their emulsion appearances were oil in-water (O/W) after being observed under a microscope (Figure 4). As the mouthwash control, MFC of chlorhexidine gluconate was determined at various exposure times against each C. albicans tested isolates. Results revealed MFC of chlorhexidine gluconate was gradually decreased after being exposed for a longer time (Table 3).
Table 2. Physical characteristics of each selected coconut oil mouthwash.
|
Formula |
Ratio of coconut oil (C): Mixed emulsifier (E): Distilled water (W) |
pH |
Viscosity (mPa.s) |
Microscopic appearance of emulsion |
|
F5 |
C10 E25 W65 |
7.34 ± 0.02 |
81.00 ± 7.42 |
O/W |
|
F21 |
C20 E20 W60 |
7.34 ± 0.02 |
183.00 ± 6.71 |
O/W |
|
F36 |
C30 E20 W50 |
7.35 ± 0.02 |
286.00 ± 8.94 |
O/W |
|
F49 |
C40 E20 W40 |
7.43 ± 0.01 |
982.00 ± 10.95 |
O/W |
|
F59 |
C50 E15 W35 |
7.47 ± 0.02 |
954.00 ± 16.73 |
O/W |
|
F67 |
C60 E10 W30 |
7.55 ± 0.01 |
952.00 ± 30.33 |
O/W |
|
F73 |
C70 E5 W25 |
7.76 ± 0.03 |
1976.00 ± 16.73 |
O/W |
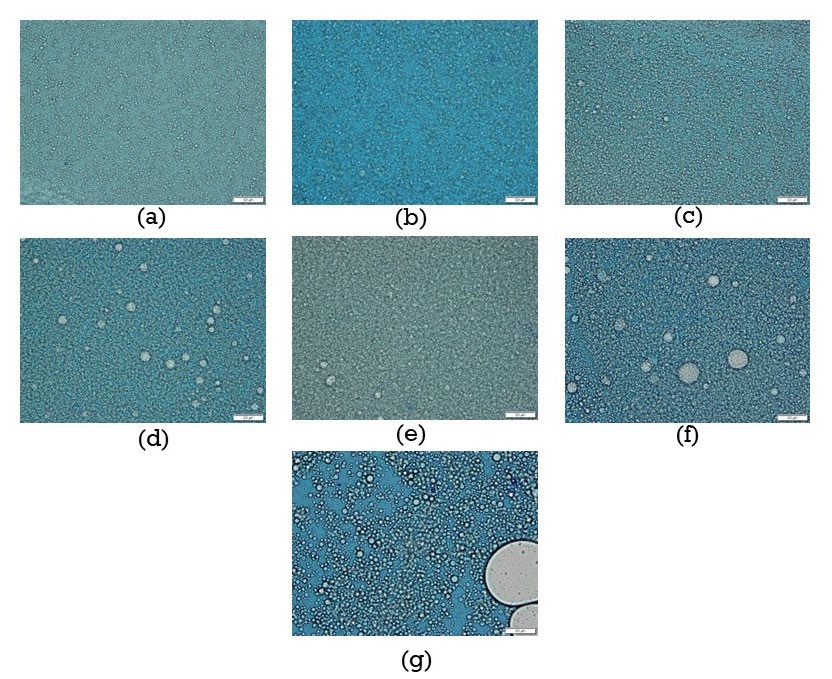
Figure 4. Oil-in-water emulsion appearance of each selected coconut oil mouthwash formula (a) F5 (b) F21 (c) F36 (d) F49 (e) F59 (f) F67 (g) F73 after observed under microscope.
Table 3. MFC of chlorhexidine gluconate, the positive control, against C. albicans in biofilm at various exposure times.
|
C. albicans isolates
|
MFC (%w/v) at the exposure time (minutes) |
|||
|
2 |
5 |
10 |
20 |
|
|
Reference standard ATCC 10231 |
0.25 |
0.25 |
0.125 |
0.125 |
|
Oral isolate from patient 1 |
0.25 |
0.25 |
0.062 |
0.031 |
|
Oral isolate from patient 2 |
0.25 |
0.25 |
0.125 |
0.062 |
|
Oral isolate from patient 3 |
0.25 |
0.125 |
0.062 |
0.031 |
To determine the inhibition of C. albicans biofilm of mouthwash formulas by XTT reduction assay, the activity of the mixed emulsifier base of each mouthwash formula was also determined. It was shown that the anticandidal activity of a 5% mixed emulsifier was detected as shown in the result of Figure 6. Therefore, the percentage of actual inhibition of mouthwash formulas after being calculated with the activity of mixed emulsifier was exhibited in Figure 5. It was revealed that the activity of mixed emulsifier bases in four formulas including F5, F21, F49, and F59 was higher than the actual activity of coconut oil with an exposure time of two minutes. Results were shown very low median values of %inhibition. It also detected the higher activity of mixed emulsifier bases of F5, F49, and F59 formulas after being exposed for 5 minutes. The activity of formula F49 was also lower than the activity of mixed emulsifiers as shown as the lowest inhibition at 10 and 20 minutes of exposure times. Most of the selected formulas demonstrated the actual activity of coconut oil at ten minutes. Due to the large inter quartile rank of F73 at the exposure time of two minutes, significant activity was not detected after compared with formula F5, F21, F36, F49, F59 and F67 (P = 0.11, 0.115, 0.15, 0.113, 0.107 and 0.139), respectively. At the exposure time of five minutes, the highest significant activity was detected in formula F73 after being compared with formula F5, F21, F36, F49, and F59 (P = 0.013, 0.010, 0.014, 0.005, 0.021) except F67 (P = 0.112), respectively. At the exposure time of ten minutes, the highest significant activity was detected in formula F73 after being compared with formula F5, F21, F49, F59, and F67 (P = 0.026, 0.031, 0.004, 0.007, 0.011) except F36 (P = 0.186), respectively. At the exposure time of 20 minutes, the highest significant activity was detected in formula F73 after being compared with formula F5, F36, F49, and F59 (P = 0.01, 0.047, 0.008, 0.007) except F21 and F67 (P = 0.181 and 0.247), respectively. It was found that this formula exhibited higher anticandidal activity than the activity of its emulsifier base. It may be due to the lowest percentage of mixed emulsifier base used in this formula (5%), and the highest percentage of coconut oil (70%).
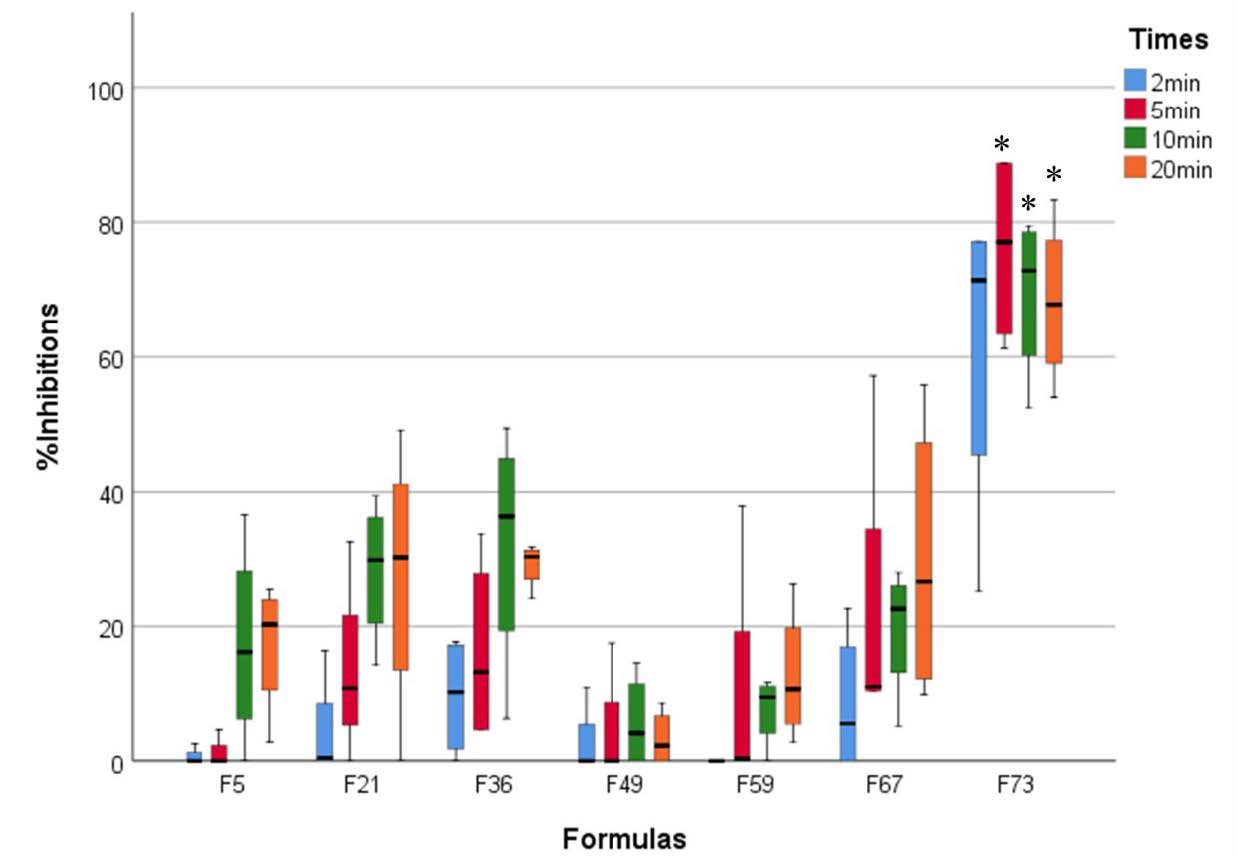
Figure 5. Percentage of inhibition against C. albicans in biofilm of each selected coconut oil mouthwash formula with exposure time at two minutes (blue), five minutes (red), ten minutes (green), and 20 minutes (orange). Interquartile rank at Q1 (lower bar), Q3 (upper bar), and the median value (inner bar) were also demonstrated. *, the significant difference at P < 0.05.
Anticandidal activity of coconut oil mouthwash formula f73 by using colony enumeration (CFU/ml)
To confirm the inhibition of C. albicans biofilm by colony enumeration, it was found that the activity of formula F73 was significantly different among the exposure time of two or five minutes after comparison with the activity at 20 minutes. Moreover, the anticandidal activities of formula F73 at the exposure time of 5, 10, and 20 minutes were significantly different from the activity of a 5% mixed emulsifier at the same exposure time. It could be suggested that the formula F73 which possessed the highest inhibitions at each exposure time against C. albicans biofilm, especially at five minutes was performed to investigate the anticandidal activity (Figure 6).
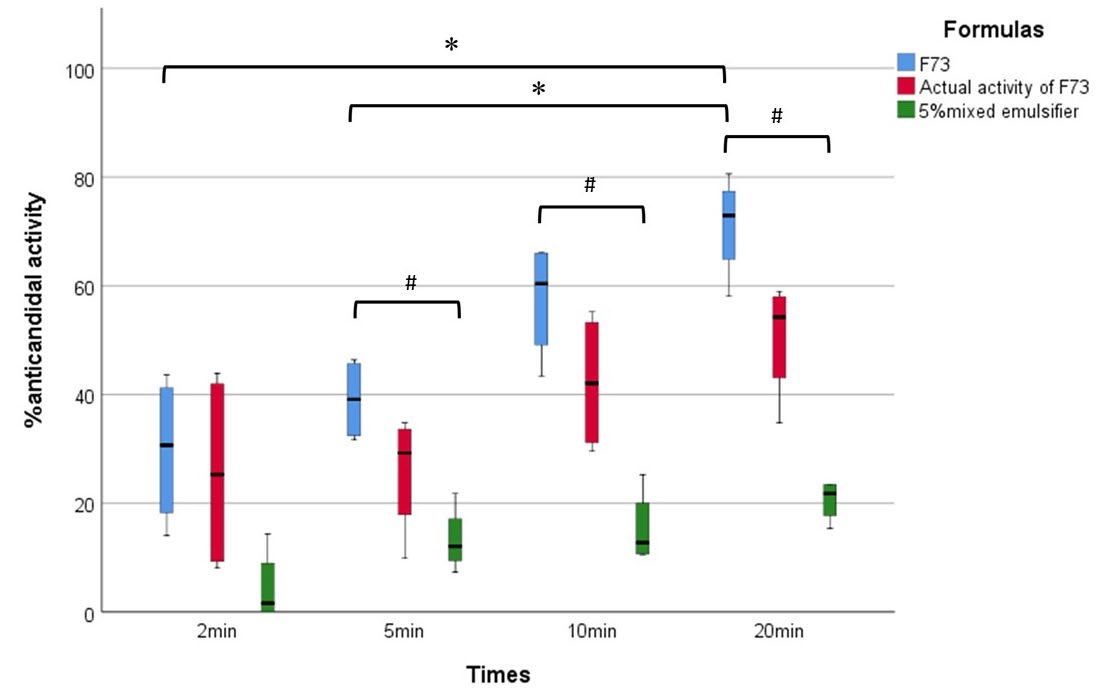
Figure 6. Box chart of anticandidal activity of F73 coconut oil mouthwash (blue box) after compared with its mouthwash base containing same ratio (5%) of mixed emulsifier (green box) at various exposure times. The actual anticandidal activity of coconut oil in F73 at various times was calculated and shown in the red box. Data shows their median values (middle bar in the box), Q1, (black lower bar), and Q3 (black upper bar). *, the significant difference at P < 0.05.
DISCUSSION
In the present study, we would like to focus on the emulsion formation of virgin coconut oil for preserving its anticandidal efficacy. Theoretically, the formation of emulsion is caused by three important compositions including virgin oil, emulsifier, and distilled water. The ratio between the virgin coconut oil and the mixed emulsifier may be affected by the different stabilities of the emulsion. However, the property of the emulsifier is important for the recruitment of emulsifiers in the present study. The first inclusion criterion was mentioned on the chemical that had been reported to apply as the stable emulsion. Secondly, it should be a non-ionic surfactant because of the little bubble production, no irritation, and no disturbance from the acid-base effect. Thirdly, the HLB of the formed emulsion should be proper for the objectives and utilization of the product. Additionally, the mixed ratio between Tween 80 and Span 80 was also considered for setting the HLB value of the mixed emulsifier at 8.58 because the different values of HLB were made from the other ratio of Tween 80 and Span 80 from the present study. Tubtimsri et al. (Tubtimsri et al., 2015) reported that the HLB value, 12 and 13, of these emulsifiers can be proper for formulating the stable emulsion of 20% modified coconut oil. While the stable emulsion was also reported by Wiyani et al. (Wiyani et al., 2016) after being formulated from 80% virgin coconut oil and a mixed emulsifier that possessed an HLB value of 8.58. From the last report, we would like to confirm that the mixed emulsifier with an HLB value of 8.58 can form the stable emulsion of a high percentage of virgin coconut oil and applied the mixed emulsifier at this HLB value for our formulation. As the compositions of emulsion in the present study, the sterilized distilled water that possessed a pH value of 6.24 was selected as well as virgin coconut oil that was extracted under cool conditions and resulted in the odoriferous smell and the preservation of chemical compounds.
According to the ternary phase diagram, we determined the effect of each component phase behavior on the stability of formulated emulsions as slightly modified from the study of Gani and Adisah (Gani et al., 2015). The narrow range of the virgin coconut oil in the earlier study gave large numbers of formulations and demonstrated similar results in a group of formulations. We modified the range of the oil phase in a ternary diagram from 5% to 10%. A reduction in the number of the formulation was observed. This extending range also resulted in decreasing the similarity of the formulation group. On the other hand, we aimed to discover the minimal ratio of mixed emulsifiers applied in the formulation of the stable emulsion. A narrower percentage of mixed emulsifiers in the formulation was required in our study from 10% to 5%. This modification of the mixed emulsifier resulted in 81 formulations in the present ternary phase diagram. According to our ternary phase diagram, the stable emulsions could be observed in 23 formulations (28.40% of total formulations) which consisted of the range of virgin coconut oil and mixed emulsifier between 10-70%, and 5-45%, respectively. After compared in each percentage of virgin coconut oil, the minimal use of a mixed emulsifier to form a stable emulsion was considered. Therefore, seven formulations contained different ratios of three phases. They were the stable emulsion carried out to further study the ability to inhibit the candida growth in the biofilm of these formulations.
In the present study, the in vitro inhibition of C. albicans growth in biofilm was determined to be slightly modified from the method described by Gulati et al. (Gulati et al., 2018). XTT reduction assay was performed to analyze the relationship between candidal cellular density and its metabolic activity by the observation of colorimetric spectrophotometry as studied by Hawser et al. (Hawser et al., 1998), and Honraet et al. (Honraet et al., 2005). The study by Agarwal et al (Agarwal et al., 2008) revealed that coconut oil possessed slightly lower anticandidal activity than the other tested plant oils, for example, eucalyptus oil, peppermint oil, ginger oil, and clove oil. To determine the inhibition of C. albicans growth in biofilm, an XTT reduction assay was performed to evaluate the activity of seven selected formulas of coconut mouthwash that exerted the stable emulsion in the present study.
In contrast to the results reported by Agarwal et al. (Agarwal et al., 2008), we found that the highest inhibition of candida growth was shown in the formula which contained 70% coconut oil, the largest amount of coconut oil in our study. Moreover, we found that the largest enhancement of activity was demonstrated in this formula. It may be suggested that the optimal formation of emulsion among coconut oil, mixed emulsifier and distilled water could result in the increase of small droplets of oil, increase the oil surface that reacts to the candida cells, and then affected the increase of candida growth inhibition as well as the report of Eshtiaghi et al. (Eshtiaghi et al., 2011). After the highest inhibition of candidal growth in biofilm was found in the test of formula 73 which consisted of 70% coconut oil, 5% mixed emulsifier, and 25% distilled water, this inhibition was confirmed by counting the residual colony-forming units. Results revealed the confirmation of candidal inhibition with the reduction of colony forming unit of tested candida. Wang et al. (Wang et al., 2015) hypothesized that the inhibition of coconut oil may be due to the anticandidal effect of lauric acid, the most common fatty acid found in coconut oil. The report of Bergsson et al. (Bergsson et al., 2001) described that lauric acid exhibited higher anticandidal activity than other fatty acids. The study of Asokanet al. (Asokan et al., 2011b) reported that coconut oil pulling within five minutes can result in the saponification and dissociation of coconut oil. Depending on the time exposure, the sizes of fat cells were decreased and formed the complete emulsion. Moreover, the increase of mechanical force from shaking and the increase in exposure time can result in the production of homogenous small oil molecules. It may be suggested that the increase of oil surface from homogenous small oil molecules with longer exposure can enhance the inhibition of C. albicans growth in biofilm. The result obtained from our study may be applied as the implementation of coconut oil mouthwash for inhibiting the oral candida infection by using about 10-15 ml with five minutes of washing. We found that the emulsion of coconut oil mouthwash was more viscous than the virgin coconut oil. This viscosity may affect the increase of pulling force. Therefore, further study to reduce the viscosity of the formulation will be considered. This formulation impacts the implementation of coconut oil mouthwash for use with the patients. However, the production of this mouthwash on a large scale will be of concern to the compatibility of oil-water emulsion of this mouthwash.
CONCLUSION
The results of our present study revealed that the ratio of three phase components (coconut oil: mixed emulsifier: distilled water), and the exposure time of mouthwash were the affecting factors towards the inhibition of candida growth in biofilm. After formulation of coconut oil mouthwash, formula F73 gave the stable emulsion with the lowest mixed emulsifier used and possessed the highest anticandidal activity especially at least five minutes of exposure time. This formulation was made from the ratio of three phase components at 75: 5: 25. The research about safety tests and clinical trials in the volunteers will be further done soon for controlling the oral candida infection and improving oral hygiene in the elderly.
ACKNOWLEDGMENTS
We would like to express our sincere thanks to Dr. Thanapat Sastraruji, Dental Research Center, Faculty of Dentistry, Chiang Mai University, for his invaluable guidance. We wish to thank the technicians and all volunteers at the Comprehensive Dental Clinic, Faculty of Dentistry. This research would have been impossible without the approval of the Faculty of Dentistry Human Experimentation Committee, Faculty of Dentistry, Chiang Mai University.
AUTHOR CONTRIBUTIONS
Paween Chanpa wrote the research proposal, prepared the IBC document, designed the experiment, did the experiments, analyzed the statistical data, and wrote the manuscript draft. Darunee Owittayakul consulted regarding the research proposal, submitted the research funding, and proofread the research work. Phenphichar Wanachantararak consulted the IBC document, provided the candidal isolates, equipment and techniques, and consultation for the experiment. Wantida Chaiyana designed the experiment and provided the chemical and technique for formulating mouthwash. Siriwoot Sookkhee provided the conception and designed the experiment, consulted the research proposal, analyzed the statistical data, and wrote the manuscript. All authors approved the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Agarwal, V., Lal, P., and Pruthi, V. 2008. Prevention of Candida albicans biofilm by plant oils. Mycopathologia, 165: 13-19.
Amith, H. V., Ankola, A. V., and Nagesh, L. 2007. Effect of oil pulling on plaque and gingivitis. Journal of Oral Health and Community Dentistry 1(1): 12-18.
An, T. D., Pothiraj, C., Gopinath, R. M., and Kayalvizhi, B. 2008. Effect of oil-pulling on dental caries causing bacteria. African Journal of Microbiology Research. 2: 63-66.
Asokan, S., Emmadi, P., and Chamundeswari, R. 2009. Effect of oil pulling on plaque induced gingivitis: A randomized, controlled, triple-blind study. Indian Journal of Dental Research. 20: 47-51.
Asokan, S., Kumar, R. S., Emmadi, P., Raghuraman, R., and Sivakumar, N. 2011a. Effect of oil pulling on halitosis and microorganisms causing halitosis: A randomized controlled pilot trial. Journal of Indian Society of Pedodontics and Preventive Dentistry. 29: 90-94.
Asokan, S., Rathinasamy, T.K., Inbamani, N., Menon, T., Kumar, S.S., Emmadi, P., et al. 2011b. Mechanism of oil-pulling therapy - in vitro study. Indian Journal of Dental Research. 22: 34-37.
Bergsson, G., Arnfinnsson, J., Steingrímsson, O., and Thormar, H. 2001. In vitro killing of Candida albicans by fatty acids and monoglycerides. Antimicrobial Agents and Chemotherapy. 45: 3209-3212.
Carandang, E.V. 2008. Health benefits of virgin coconut oil. Indian Coconut Journal. 38: 8.
Eshtiaghi, M.N., Phurahong, K., and Kuldiloke, G.P.J. 2011. Nano-particle emulsion from coconut oil using high pressure jet. Paper presented at the TIChE International Conference 2011.
FenolI-Palomares, C., Muñoz-Montagud, J.V., Sanchiz, V., Herreros, B., Hernández, V., Mínguez, M., and Benages, A. 2004. Unstimulated salivary flow rate, pH and buffer capacity of saliva in healthy volunteers. Revista Española de Enfermedades Digestivas 96(11): 773-783.
Gani, S.S.A., and Adisah, S.Z. 2015. Phase behaviour study of swiftlet nest using virgin coconut oil with non-ionic surfactants. Malaysian Journal of Analytical Sciences. 19: 184-193.
Garcia-Cuesta, C., Sarrion-Pérez, M.G., and Bagán, J.V. 2014. Current treatment of oral candidiasis: A literature review. Journal of Clinical and Experimental Dentistry. 6: e576-582.
Gulati, M., Lohse, M.B., Ennis, C.L., Gonzalez, R.E., Perry, A.M., Bapat, P., et al. 2018. In vitro culturing and screening of Candida albicans biofilms. Current Protocols in Microbiology. 50: e60.
Hawser, S.P., Norris, H., Jessup, C.J., and Ghannoum, M.A. 1998. Comparison of a 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-t etrazolium hydroxide (XTT) colorimetric method with the standardized National Committee for Clinical Laboratory Standards method of testing clinical yeast isolates for susceptibility to antifungal agents. Journal of Clinical Microbiology. 36: 1450-1452.
Honraet, K., Goetghebeur, E., and Nelis, H. J. 2005. Comparison of three assays for the quantification of Candida biomass in suspension and CDC reactor grown biofilms. Journal of Microbiological Methods. 63: 287-295.
Intarakaewsri, T., Owittayakul, D., and Wanachantararak, P. 2020. Development of virgin coconut oil mouthwash against Candida albicans Biofilms. Chiang Mai Dental Journal. 41: 55-64.
Kim, J. and Sudbery, P. 2011. Candida albicans, a major human fungal pathogen. Journal of Microbiology. 49: 171-177.
Ludwar, L., Mannel, H., Hamacher, S., Noack, M.J., and Barbe, A.G. 2022. Oil pulling to relieve medication-induced xerostomia: A randomized, single-blind, crossover trial. Oral Diseases. 28: 373-383.
Muddugangadhar, B.C., Sangur, R., Rudraprasad, I.V., Nandeeshwar, D.B., and Kumar, B.H.D. 2015. A clinical study to compare between resting and stimulated whole salivary flow rate and pH before and after complete denture placement in different age groups. Journal of Indian Prosthodontic Society. 15: 356-366.
Ogbolu, D. O., Oni, A. A., Daini, O. A., and Oloko, A. P. 2007. In vitro antimicrobial properties of coconut oil on Candida species in Ibadan, Nigeria. Journal of Medicinal Food, 10(2): 384-387.
Owittayakul, D., Palee, K., Khongkhunthian, S., Langkapin, W., and Wanachantararak, P. 2018. Effect of coconut oil and 0.12% chlorhexidine mouthrinses in reduction of plaque and gingivitis: A two-week randomized clinical trial. Journal of the Dental Association of Thailand. 68: 360-369.
Peedikayil, F.C., Sreenivasan, P., and Narayanan, A. 2015. Effect of coconut oil in plaque related gingivitis - A preliminary report. Nigerian Medicine Journal. 56: 143-147.
Sanjeewani, N.A. and Sakeena, M.H.F. 2013. Formulation and characterization of virgin coconut oil (VCO) based emulsion. International Journal of Science and Research. 3: 1-6.
Santos, J.D.D., Piva, E., Vilela, S.F.G., Jorge, A.O.C., and Junqueira, J.C. 2016. Mixed biofilms formed by C. albicans and non-albicans species: A study of microbial interactions. Brazilian Oral Research. 30.
Shanbhag, V.K. 2017. Oil pulling for maintaining oral hygiene - A review. Journal of Traditional and Complementary Medicine. 7: 106-109.
Sookkhee, S., Chulasiri, M., and Prachyabrued, W. 2001. Lactic acid bacteria from healthy oral cavity of Thai volunteers: Inhibition of oral pathogens. Journal of Applied Microbiology. 90: 172-179.
Tadros, T.F. 2009. Emulsion science and technology: A general introduction. Emulsion Science and Technology 1-56.
Thaweboon, S., Nakaparksin, J., and Thaweboon, B. 2011. Effect of oil-pulling on oral microorganisms in biofilm models. Asia-Pacific Journal of Public Health. 2: 62-66.
Tubtimsri, S., Limmatvapirat, C., Sriamornsak, P., and Limmatvapirat, S. 2015. Determination of required hydrophile-lipophile balance value of modified coconut oil. Paper presented at the Advanced Materials Research.
Wang, J., Wang, X., Li, J., Chen, Y., Yang, W., and Zhang, L. 2015. Effects of dietary coconut oil as a medium-chain fatty acid source on performance, carcass composition and serum lipids in male broilers. Asian-Australasian Journal of Animal Sciences. 28: 223-230.
Wiyani, L., Aladin, A., Yani, S., and R., N. 2016. Stability of virgin coconut oil emulsion with mixed emulsifiers Tween 80 and Span 80. Journal of Engineering and Applied Science. 11: 5198-5202.
Yuan, Y., Zhou, F., Su, H., and Zhang, Y. 2019. Structural design of microbicidal cationic oligomers and their synergistic interaction with azoles against Candida albicans. Sciences Report. 9: 1-11.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand.
Paween Chanpa1, Darunee Owittayakul1, Phenphichar Wanachantararak2, Wantida Chaiyana3, Siriwoot Sookkhee4, *
1 Department of Family and Community Dentistry, Chiang Mai University, Chiang Mai, Thailand.
2 Dental Research Center, Faculty of Dentistry, Chiang Mai University, Chiang Mai, Thailand.
3 Department of Pharmaceutical Sciences, Faculty of Pharmacy, Chiang Mai University, Chiang Mai, Thailand.
4 Department of Microbiology, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand.
*Corresponding author: Siriwoot Sookkhee, E-mail: siriwoot.s@cmu.ac.th
Total Article Views
Editor: Anak Iamaroon,
Chiang Mai University, Thailand
Article history:
Received: August 11, 2022;
Revised: November 22, 2022;
Accepted: November 24, 2022;
Published online: December 8, 2022

