
Effect of Dietary Lasia (Lasia spinosa (L.) Thwaites) Extract on––Growth Performance and Intestinal Histology in Hybrid Catfish (Clarias macrocephalus x Clarias gariepinus).
Phukphon Munglue*, Khwanduean Rattana, Supavee Sangchanjiradet, and Kajohnpong DasriPublished Date : 2019-04-1
DOI : https://doi.org/10.12982/CMUJNS.2019.0017
Journal Issues : Number 2 , April-June 2019
ABSTRACT
Lasia (Lasia spinosa (L.) Thwaites) has long been used since ancient time for the treatment of various ailments in man. However, the use of this plant in fish culture as a natural feed additive in fish diets to improve growth rate has not yet been studied. Thus, the aims of this research were conducted to investigate the effects of lasia extract (LSE) on growth performance and intestinal histology of hybrid catfish (Clarias macrocephalus x C. gariepinus). Arial parts of lasia were extracted with 75% ethanol. Preliminary phytochemical screening was examined by using colorimetric assay. Fish with an average of initial weight of 7.00±1.00 g were divided into 4 treatments of 3 replications before being fed diets incorporated with different levels (0, 1, 3 and 5% based on dried weight) of LSE. Fish were fed ad libitum two times a day with a rate of 3% of live body weight for 8 weeks. Phytochemical prospecting of LSE exhibited the presence of flavonoids, terpenoids, phenolic compounds, steroids, saponins, coumarins, glycosides and anthraquinones. After a feeding trial, growth performance and feed utilization efficiencies of fish fed with the experimental diets were significantly improved when compared to the basal diet (P<0.05). Survival rate, intestinosomatic index and condition factor were not affected by the diets (P>0.05). Villi height, villi width, muscularis thickness, microvilli height and goblet cell number of treated fish were significantly enhanced when compared to control fish (P<0.05). The heights of enterocyte, supranucleus and subnucleus were significantly decreased in middle and distal part of intestines (P<0.05). The results demonstrated that LSE could be useful as a natural feed additive in fish diets in order to enhance growth and intestinal histology of hybrid catfish. The optimal level of LSE observed in this present research was 3%.
Keywords: Hybrid Catfish, Lasia, Growth performance, Intestinal histology, Feed additive
INTRODUCTION
Medicinal herbs and their products have long been used since ancient time for the treatment of various ailments in man (Elvin-Lewis, 2001). However, their uses as growth promoters, immunostimulants and other purposes in aquaculture operations are still being elucidated (Citarasu, 2010; Chakraborty et al., 2014). Several synthetic compounds such as hormones and antibiotics have led to the development of residual effects in aquaculture products and the creation of resistant strains of bacteria (Citarasu, 2010; Chakraborty et al., 2014; Yang et al., 2015). Thus, natural plant products seem to be more attractive alternatives to enhance growth, productivity and healthiness of fish (Heidarieh et al., 2012; Munglue, 2014, 2015, 2016).
Many herbal plants containing phytohormones have been used in fish cultures in order to enhance sex-reversal, growth and production of fish, while the success is still questionable (Turan and Akyurt, 2005a; Turan, 2006; Chakraborty et al., 2014). It was found that tilapia (Oreochromis aureus L.) received 100 mg/kg red clover (Trifolium pratense), a rich source of phytoestrogens, enriched diet for 90 days significantly increased growth rate, feed utilization efficiency and carcass crude protein composition when compared to the control fish (Turan, 2006). In addition, diets supplemented with Tribulus terrestris extract at the concentration of 400 mg/kg had a positive effect on weight gain, specific growth rate and feed conversion ratio in Nile tilapia (Oreochromis niloticus) (Gültepe et al., 2014). Turan and Akyurt (2005b) reported that African catfish (Clarias gariepinus) fed diets supplemented with red clover extract at the level of 75 mg/kg significantly increased in growth performances and carcass protein and lipid contents when compared with the control fish. From the above it can be appreciated that plants containing phytohormones such as phytoestrogens and phytoandrogens could act as natural growing promoting agents to improve fish growth and production (Pace et al., 2006; Chakraborty et al., 2014).
Lasia (Lasia spinosa (L.) Thwaites) is a perennial herb belonging to the family of Araceae and is widely distributed throughout the tropical and subtropical countries in Asia (Hong Van et al., 2006; Deb et al., 2010). For being traditional medicine, it has been used to treat body pains (Deb et al., 2010) helminthic diseases (Vijaya and Yadav, 2016), diarrhea (Deb et al., 2010), habitual constipation (Maneenoon et al., 2015), skin diseases (Maneenoon et al., 2015) and rheumatic diseases (Ueda et al., 2002). Additionally, antimicrobial, antioxidant and anticancer properties of lasia have been demonstrated (Nanasombat and Teckchuen, 2009; Deb et al., 2010; Alam et al., 2011). Studies on acute and subchronic toxicity tests of lasia rhizome extract exhibited that the plant extract did not produce any signs of toxicity, mortality or behavioral changes in male rats (Kaewamatawong et al., 2013). These data indicated that lasia is apparently safe for using as a medicinal plant to treat several disorders (Kaewamatawong et al., 2013). Phytochemical investigations showed that lasia contains triglochinin, (Nahrstedt, 1975), ß-sitosterol acetate, stigmasterol, flavonoids, flavone C-glycosides (Hong Van et al., 2006; Maisuthisakul et al., 2007), terpenoids, phenolic compounds, coumarins and alkaloids (Napiroon et al., 2013). Suthikrai et al. (2006) reported that rhizome, leave and root of lasia contain testosterone and estrogen, suggesting that lasia is served as a natural source of phytoestrogens and phytoandrogens. Kaewamatawong et al. (2013) showed that male rats administrated with lasia rhizome extract orally produced a significant increase in testosterone levels, testicular weights and sperm count when compared to control rats. Interestingly, lasia has been used as a feed additive to enhance growth and feed utilization efficiency in cattle and buffalo (Suthikrai et al., 2007; Jintana et al., 2013). Therefore, these data could support the growth promoting potential of lasia in animal production sections.
As the ethnopharmacological relevance described to lasia is widely based on scientific data, more research is required to experimentally confirm its safety and efficacy (Kaewamatawong et al., 2013). In addition, the use of this plant in fish culture as a natural feed additive in fish diets to enhance growth has not yet been studied. Thus, the aims of this present research were conducted to investigate the effects of lasia extract (LSE) on growth performance and intestinal histology of hybrid catfish (Clarias macrocephalus x C. gariepinus).
MATERIALS AND METHODS
Plant preparation and extraction
Arial parts of lasia were collected locally from Kut Khao Pun District, Ubon Ratchathani, Thailand during August to October, 2016. The plant was identified by a herbal specialist and the voucher specimen (P.Munglue 001) was kept at the Program of Biology, Ubon Ratchathani Rajabhat University, Thailand. The plant samples were then cleaned using tab water, cut into small pieces and dried in a hot air oven at 60oC for 7 days. Dried samples (100 g) were macerated with 75% ethanol (300 mL) (1:3 w/v) for 7 days. The extract was filtered through Whatman paper No. 1 and dried by rotary evaporator. The yield was expressed as 10.43±0.04 mg/g based on dried plant weight.
Phytochemical screening
Preliminary phytochemical screening of LSE including alkaloids, flavonoids, terpenoids, phenolic compounds, steroids, tannins, saponins, coumarins, glycosides and anthraquinones was determined by using colorimetric assay (Harborne, 1998; Evans et al., 2002; Kar, 2007). The interpretation of the results were demonstrated as presence (+) or absence (-) of each secondary metabolite found in LSE.
Diet preparation
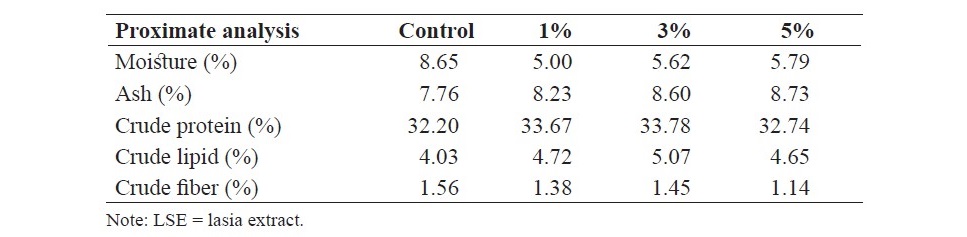
The fish diet containing 30% protein and 4% lipid was obtained from a commercial fish feed and mixed with different levels of (0, 1, 3 and 5% based on dry basis) of LSE by using a meat mincer to produce extruded string forms. Then, the diets were dried in hot air oven at 50oC for 24 h and kept in the plastic bag until used for the experiment. Proximate composition of the experimental diets including moisture, ash, crude protein, crude lipid and crude fiber was quantified using standard methods as described by the Association of Official Analytical Chemists (AOAC International, 2012) and the results are presented in Table 1.
Table 1. Proximate composition of the diets supplemented with different levels of LSE (0, 1, 3 and 5%).
Fish preparation
The animal procedures were conducted in accordance with the Institutional Animal Care and Use Committee, Ubon Ratchathani Rajabhat University, Thailand. Hybrid catfish (weighing 7.00±1.00 g) were obtained from Ubon Ratchathani Fishery Cooperative and acclimatized in the laboratory conditions for 2 weeks. Fish were randomly distributed in four treatments, with three replicates each treatment and cultivated in the circular concrete tanks (90 cm in a diameter and 50 cm in height). Fish wastes were removed by siphoning one half of tank’s water and replaced by aerated tap water from storage tank.
Fish were fed ad libitum two times a day with a rate of 3% of live body weight for 8 weeks. Every week, fish in each tank were weighed. Dead fish once appeared in tanks were noted and removed. Fish were observed daily for signs of toxicity. Also, feeding behavior, palatability and acceptability of feed were recorded. Water quality was maintained at pH of 6.5-7.5, a dissolved oxygen of 7 mg/L and a temperature of 25-30oC (Munglue, 2016).
Effects on growth performance
At the end of the experiment, fish were fasted for 24 h before study. The mean body weight and growth parameters were calculated as follows:
Weight gain (WG, g) = final fish weight (g) – initial fish weight (g).
Specific growth rate (SGR, %/day) = 100× [In final wet weight (g) – In
initial wet weight (g)]/experimental days.
Average daily growth (ADG, g/day) = (final wet weight (g) - initial wet weight (g)) /experimental days.
Feed conversion ratio (FCR) = feed intake (g)/ weight gain (g). Feed conversion efficiency (FCE) = weight gain (g)/feed intake (g). Condition factor (CF) = 100× (weight (g)/length3 (cm)).
Survival rate (SR, %) = 100× (final number of fish/initial number of fish).
Effects of intestinal histology
Three fish per each tank were collected and anesthetized by using clove oil dissolved in water to obtain the final concentration of 5 ppm. Abdominal wall was carefully opened. The intestines were removed, cleared from other organs and weighted. The relative organ weight was calculated by using the following equation:
Intestinosomatic index (ISI, %) = 100× (weight of intestine (g)/weight of fish (g)).
The samples of fish intestine were divided into proximal, middle and distal parts. They were cut transversely into small pieces (1 cm in length) and kept in 10% neutral buffered formalin for histological preparations and stained by Haematoxylin & Eosin (Ferrara et al., 2015). Histological slides were observed by a light microscope and recorded by a computer running DinoCapture 2.0 software (Munglue, 2016; Thummek et al., 2016). The specimens were observed as previously reported by Escaffre et al. (2007) and Couto et al. (2014).
Statistical analysis
In this study, Completely Randomized Design (CRD) was used. Data are expressed as mean±standard error of the mean (SEM). The significance of difference was analyzed using one-way analysis of variance (ANOVA). The significant differences among the treatments were assessed by Duncan’s multiple-range test. P value
RESULTS
Phytochemical screening
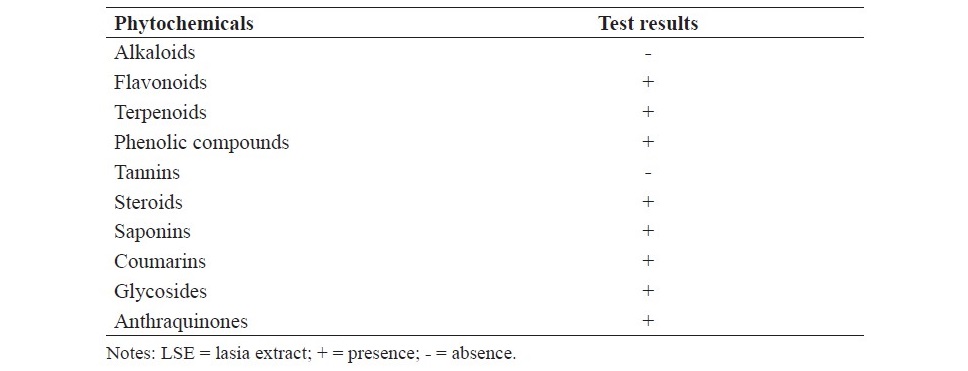
Preliminary phytochemical screening showed the presence of flavonoids, terpenoids, phenolic compounds, steroids, saponins, coumarins, glycosides and anthraquinones in LSE. However, alkaloids and tannins were not detected in LSE as presented in Table 2.
Table 2. Preliminary phytochemical screening of LSE.
Effects on growth performance
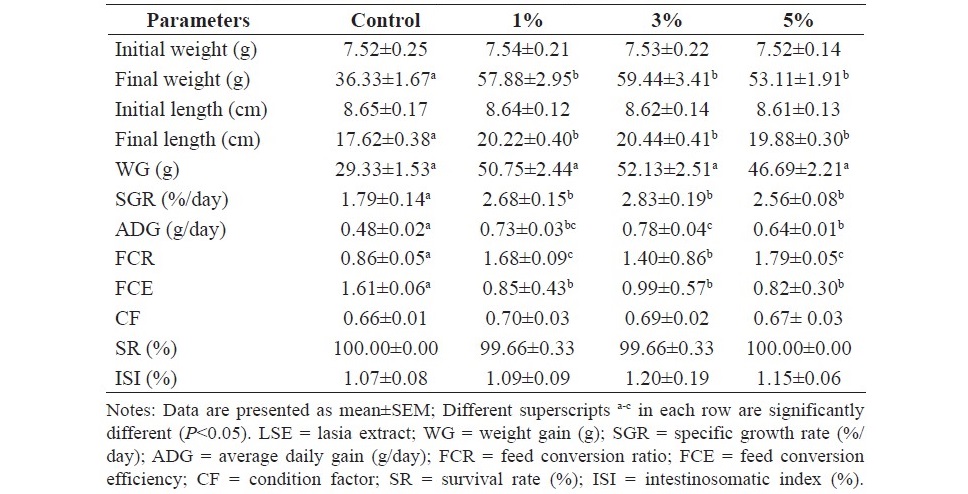
At the end of 8 weeks of feeding trial, the results showed that fish fed the diets supplemented with LSE produced a significant increase in final weight, final length, weight gain (WG), specific growth rate (SGR), average daily gain (ADG) and feed conversion ratio (FCR) (P<0.05) and caused a significant decrease in feed conversion efficiency (FCE) when compared to the control (P<0.05). No significant differences on condition factor (CF), survival rate (SR) and intestinosomatic index (ISI) were observed in the treated fish when compared to the control fish (P>0.05). In addition, feeding behavior, palatability and acceptability of fish fed the experimental diets were the same as the control fish. The experimental data of the effects of dietary supplementation of LSE on growth performance are shown in Table 3.
Table 3. Growth performance of hybrid catfish fed diets supplemented with different levels of LSE (0, 1, 3 and 5%) for 8 weeks.
Effects on intestinal macromorphology
Table 4 shows the effects of diets supplemented with LSE on macromorphology of intestines. Histological studies revealed that villi height, villi width, the thicknesses of muscularis and laminar propria were significantly increased in fish fed the experimental diets containing 3 and 5% LSE when compared to the control diet (P<0.05). The highest values of villi height were observed in all parts of intestines of fish fed 3% LSE in the diet. Vacuolization of intestinal villi was observed and generally distributed in all parts of intestines. Normal villi structures were observed in both tested fish and control fish. Some villi fused together. Enterocytes are arranged continuously throughout the villi segments with a single layer. Numerous goblet cells were observed and these cells are normally located between enterocytes. No pathological signs such as the rupture of epithelial cell and sloughing of epithelial cell layer were observed in all specimens.
Table 4. Intestinal macromorphological values of hybrid catfish fed diet supplemented with different levels of LSE (0, 1, 3 and 5%) for 8 weeks.
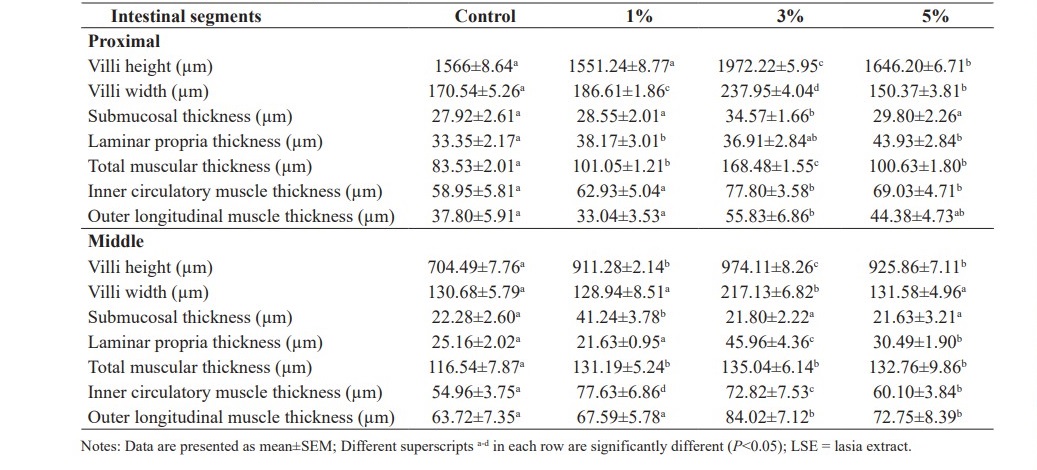
Table 4. Continued.
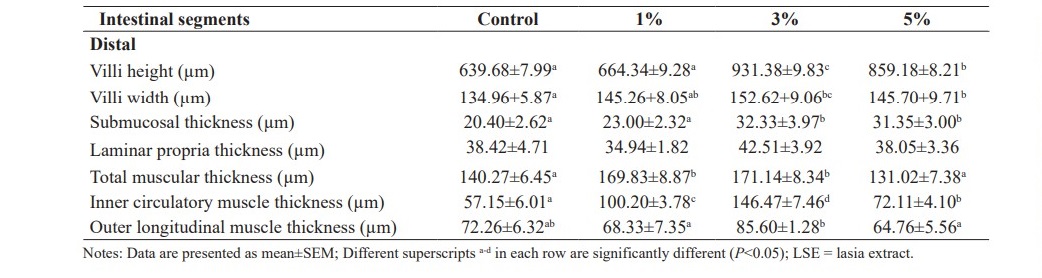
In the proximal segment of fish intestines, villi height, villi width, muscular thicknesses of fish fed 3% LSE in the diets were significantly higher than those of fish fed with the basal diet (P<0.05). There were no changes in submucosal thickness of fish fed the diets containing 1 and 5% LSE (P>0.05). Thicker laminar propria layers were observed in fish fed 1 and 5% LSE in the diets when compared to the control fish (P<0.05). Outer longitudinal smooth muscle thickness of fish fed the diet mixed with 3% LSE was significantly increased when compared to the control (P<0.05) (Figure 1).
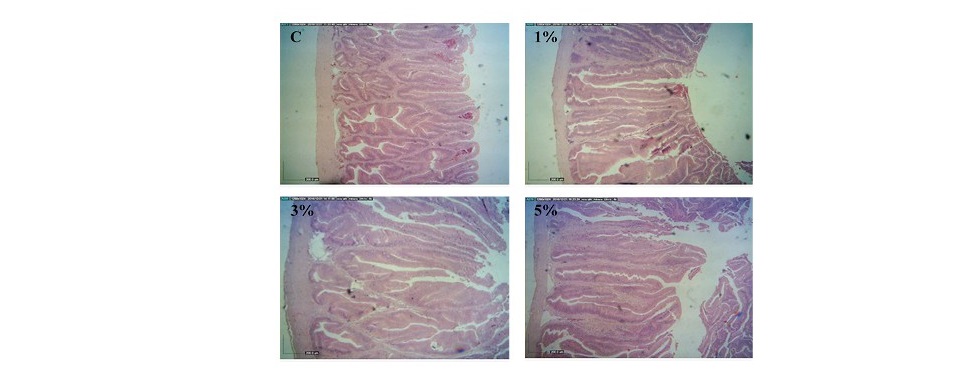
Figure 1. Histology of proximal segment of intestines obtained from hybrid catfish fed the diets supplemented with different levels (0, 1, 3 and 5%) of lasia extract for 8 weeks. (Scale bar = 200 µm).
In the middle segment of fish intestines, villi height values of fish fed the experimental diets were significantly better than those of the control fish (P<0.05). The thicknesses of submucosa, laminar propria and muscularis were significantly increased in fish fed 3 and 5% LSE in the diets in comparison with the control fish (P<0.05) (Figure 2).
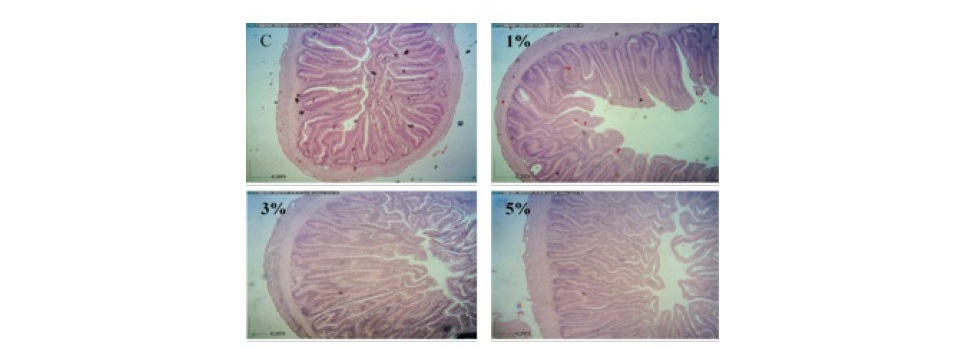
Figure 2. Histology of middle segment of intestines obtained from hybrid catfish fed the diets supplemented with different levels (0, 1, 3 and 5%) of lasia extract for 8 weeks. (Scale bar = 200 µm).
In the distal segment of fish intestines, villi height, villi width and submucosal thickness of fish fed 3 and 5% LSE enriched diets were significantly increased when compared to the control fish (P<0.05). No significant differences were observed in laminar propria thicknesses in the intestine tract from fish fed the basal diet or the diets supplemented with LSE (P>0.05). Total muscular thicknesses of fish fed diets supplemented with 1 and 3% LSE were significantly higher than those of fish fed the control diet and 5% LSE enriched diet (P<0.05). Outer longitudinal smooth muscle was significantly enhanced in fish fed diet mixed with 3% LSE when compared to the basal diet or 1 and 5% LSE enriched diets (P<0.05) (Figure 3).
Effects on intestinal micromorphology
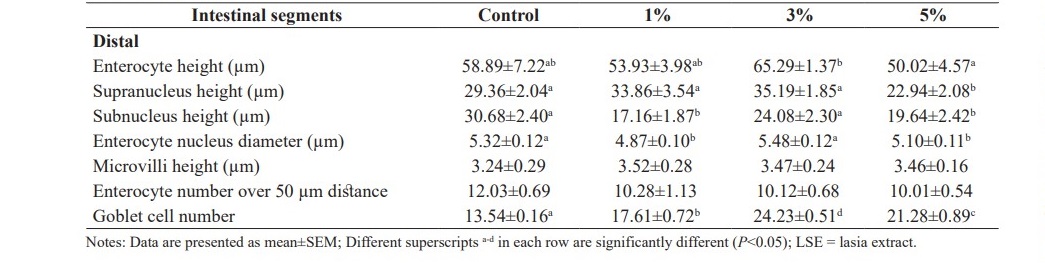
Table 5 demonstrates intestinal micromorphological studies of hybrid catfish fed the diets supplemented with different levels (0, 1, 3 and 5%) of LSE. Goblet cells observed in all parts of fish intestines were significantly increased in fish fed the experimental diets when compared to the control diet (P<0.05). It was observed that the structures of enterocyte and goblet cell as well as microvilli arrangement of fish fed diets supplemented with LSE did not show any changes or signs of intestine enteritis.
In the proximal segment of fish intestines, fish fed the diet supplemented with 5% LSE significantly decreased subnucleus height and enterocyte nucleus diameter and significantly increased microvilli height when compared to the control fish (P<0.05). No significant differences were observed in enterocyte height, supranucleus height and enterocyte number over 50 µm distance among the treatments (P>0.05).
In the middle segment of fish intestines, enterocyte height and subnucleus height observed in fish fed the diets supplemented with 1 and 3% LSE were significantly lower than those of fish fed the basal diet (P<0.05). In addition, supranucleus heights of fish fed 1 and 5% LSE enriched diets were significantly decreased when compared to control fish (P<0.05). Enterocyte number over 50 µm distance was significantly decreased in fish fed 1% LSE enriched diet when compared to control fish (P<0.05). No significant differences were observed in enterocyte nucleus diameter and microvilli height among dietary treatments (P>0.05).
In the distal segment of fish intestines, supranucleus height was significantly increased in fish fed 1 and 3% LSE enriched diets when compared to the control (P<0.05). Additionally, fish fed 1 and 5% LSE enriched diets significantly decreased in subnucleus height when compared to control fish (P<0.05). No significant differences were observed in enterocyte height and enterocyte number over 50 µm distance among dietary treatments (P>0.05).
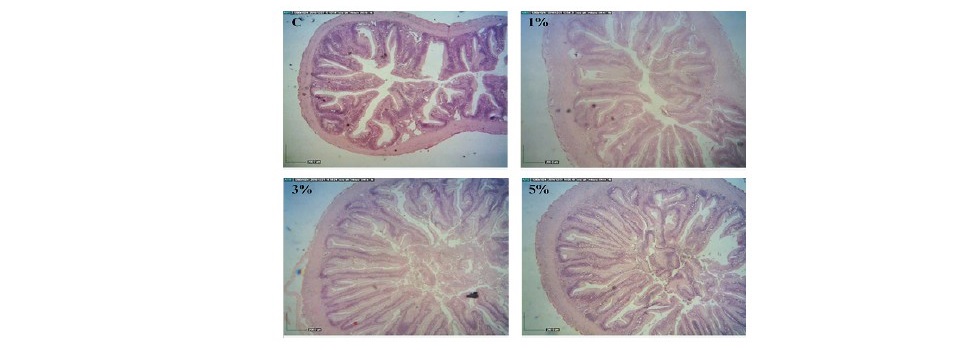
Figure 3. Histology of distal segment of intestines obtained from hybrid catfish fed the diets supplemented with different levels (0, 1, 3 and 5%) of lasia extract for 8 weeks. (Scale bar = 200 µm).
Table 5. Intestinal micromorphological values of hybrid catfish fed diets supplemented with different levels of LSE (0, 1, 3 and 5%) for 8 weeks.
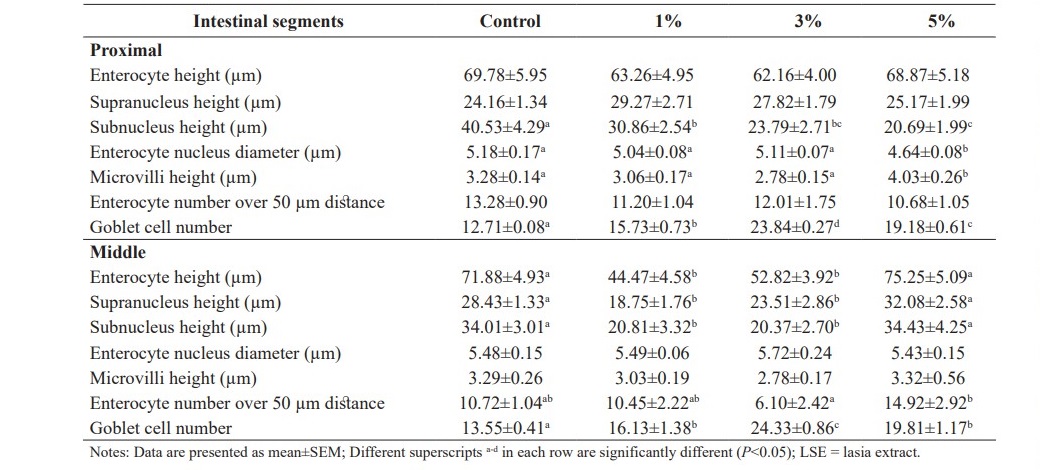
Table 5. Continued.
DISCUSSION
Medicinal plants serve as the great sources of the novel compounds not only for the treatment of various ailments but also for the synthesis of the con- ventional drugs (Elvin-Lewis, 2001). In addition, herbal biomedicines have also been used in the aquaculture production as growth promoters, immunostimulants, antimicrobial agents, anti-stress agents and appetite stimulators (Citarasu, 2010; Zahran et al., 2014; Munglue, 2014, 2015, 2016). However, the effects of LSE on growth and intestinal histology of fish have not yet been elucidated. Thus, the aims of this current study were to investigate the effects of the diet supplement- ed with LSE on growth performance and intestinal histology of hybrid catfish.
It has been accepted that oral administration is a non-stressful procedure to study the diets containing medicinal herbs or their products in aquaculture (Citarasu, 2010; Munglue, 2016). This present study demonstrated that dietary LSE supplementation produced a significant increase in final weight, final length, WG, SGR, ADG and FCR and caused a significant decrease in FCE when compared to the control diet. Furthermore, fish fed diets supplemented with LSE did not significantly alter ISI, SR and CF when compared to the control fish. In this study, dietary of 3% LSE is the effective level because it exhibited significantly the highest values for final weight, WG and SGR when compared to fish fed the diets containing 1 and 5% LSE. Phytochemical prospecting of LSE showed the presence of flavonoids, terpenoids, phenolic compounds, steroids, saponins, coumarins, glycosides and anthraquinones.
Some studies have been reported the herbal growth promoting agents in many species of aquatic animals (Munglue, 2014, 2016; Kamatit et al., 2016; Thummek et al., 2016). The herbal active compounds such as flavonoids, phenolic compounds, terpenoids, steroids, play an important role in antistress, growth stimulation, appetite activation and immunomodulation in aquatic animals (Citarasu, 2010; Chakraborty et al., 2014; Couto et al., 2014). The results of this research showed that diets supplemented with LSE enhanced growth and feed utilization efficiencies. Similar results were found in male swamp buffalo and Murrah × Swamp buffalo crossbreed calves fed with a concentrated mixed with a dry powder of lasia at the dose of 30 g/head/day for 7 months significantly improved growth rate (Suthikrai et al., 2007). Interestingly, several phytohormones found in medicinal plants may be used as natural feed additives in animal feeds in order to increase growth performance and feed efficiency. Turan (2006) found that tilapia fed the diet containing 100 mg/kg red cover, a rich source of isoflavones, for 90 days significantly increased growth parameters, feed utilization and protein content of carcass when compared to fish fed the basal diet. African catfish fed the diets supplemented with androstenedione, a phytoandrogen, at the level of 50 mg/kg for 120 days significantly improved growth rates as well as carcass protein and lipid composition when compared to the control fish (Turan and Akyurt, 2005a). Previous report showed that rhizome, leave, and root of lasia contain testosterone at the concentrations of 0.19, 0.15 and 0.92 pg/g dry weight, respectively (Suthikrai et al., 2006). Also, the levels of estrogen found in leave and root of lasia were 10.76 and 14.55 pg/g dry weight, respectively (Suthikrai et al., 2006). The underlying mechanisms of action of LSE as a growth promoting agent are not well understood. Recent data indicate that lasia contains natural phytoestrogens and phytoandrogens (Suthikrai et al., 2006). Therefore, it is hypothesized that phytohormones may act as regulators to modulate the production of growth hormone and insulin-like growth factor-I in order to increase fish growth (Moorby et al., 2004; Kangsen et al., 2012; Kumar et al., 2017). In addition, phytoandrogens may produce anabolic effects by the activation of androgen receptor (AR)-mediated anabolism to enhance muscle mass, protein synthesis, bone development and fertility in fish (Ong and Tan, 2007; Chakraborty et al., 2014). It is also assumed that the mechanisms of action of herbal plants with growth promoting activity seem to be due to an increase of the transcription rate and subsequently the enhancement of the production of RNA, amino acids and proteins in virtual animal cells (Moorby et al., 2004; Kangsen et al., 2012; Kumar et al., 2017). In this present research, phytochemical determination of LSE exhibited the presence of various classes of compounds such as flavonoids, terpenoids, phenolic compounds, steroids, saponins, coumarins, glycosides and anthraquinones. These results are consistent with previous reports indicating that the extracts of lasia were excellent sources of alkaloids, flavonoids, terpenoids, phenolic compounds, steroids, saponins, coumarins, tannins, glycosides and anthraquinones (Hong Van et al., 2006; Maisuthisakul et al., 2007; Napiroon et al., 2013). In contrast to other research reports, the absence of alkaloids and tannins observed in this present study may be related to environmental conditions, soil nutrients as well as plant extraction methods (Harborne, 1998; Evans et al., 2002; Kar, 2007). It has long been established that the applications of bioactive compounds to fish feeds are believed to be responsible for the improvement of fish growth and health (Francis et al., 2002; Zhang et al., 2014; Aanyu et al., 2018). It was found that common carp (Cyprinus carpio L.) fed the diets supplemented with Quillaja saponin significantly increased growth rate and metabolic efficiency (Francis et al., 2002). In addition, Zhang et al. (2014) indicated that juvenile Wuchang bream (Megalobrama amblycephala) fed diets supplemented with emodin (1, 3, 8-trihydroxy-6-methyl-anthraquinone) at the concentration of 30 mg/kg for 4-week feeding duration significantly enhanced final body weight, %WG and non-specific immune responses when compared to the control. Moreover, Aanyu et al. (2018) showed that increased final weight and %WG were observed in Nile tilapia fed the diets mixed with limonene, a natural cyclic monoterpene, through the up-regulation of specific genes which were associated with the modulation of growth, nutrient digestion and absorption in fish. It was found that phytosterols, flavonoids, phenolic compounds, glycosides, saponins and terpenoids have naturally found in lasia (Hong Van et al., 2006; Maisuthisakul et al., 2007; Napiroon et al., 2013) and these metabolites have been reported to have beneficial effects on growth and feed efficiency of fish (Francis et al., 2002; Zhang et al., 2014; Aanyu et al., 2018). Thus, the data can be taken to suggest that growth promoting property of LSE may possibly be attributed to its phytochemical constituents such as flavonoids, terpenoids, steroids, saponins and anthraquinones (Citarasu, 2010; Chakraborty et al., 2014; Yang et al., 2015).
It is well accepted that intestinal villi play an important role in digestion and absorption of the available nutrients (Merrifield et al., 2011; Fang et al., 2015). Increased intestinal villus height is associated with an increase in absorptive surface area in the gastrointestinal tract (Merrifield et al., 2011; Fang et al., 2015; Munglue and Dasri, 2015). In this present research, it was found that fish fed the diets supplemented with LSE significantly increased villi height, villi width and the thicknesses of submucosa, laminar propria and muscularis when compared to the control fish. Thus, these findings suggest that LSE enriched diets affected intestinal morphology and function. Vacuolization of intestinal villi was generally distributed in all parts of intestines. This is consistent with the recent findings of Munglue (2016) noted that villi height, villi width and muscularis thicknesses of catfish (C. gariepinus) fed the diets supplemented with lotus stamen extract for 8 weeks were significantly higher than those of control fish. In addition, juvenile tilapia (O. niloticus) fed diet enriched Ergosan significantly increased villi length, mucosal fold height and absorptive area of the gut (Merrifield et al., 2011). Rainbow trout (Oncorhynchus mykiss) fed dietary Ergosan for 50 days tended to increase villi length and fold length (Heidarieh et al., 2012). Functions of muscular layers of fish intestines are to support digestion and absorption of moisture and nutrients as well as defecation of fish (Ostaszewska et al., 2005; Munglue, 2016; Thummek et al., 2016). It is possible that herbs and their compounds may interact with specific population of intestinal cells directly or interact with gut endocrine cells indirectly in order to increase cell division and cell proliferation or decrease cell apoptosis (Gupta and Sandhu, 1998; Citarasu, 2010; Chakraborty et al., 2014). Thus, these could be the reasons why LSE enriched diets increased villi height and muscular thicknesses in this study (Kamatit et al., 2016; Munglue, 2016; Thummek et al., 2016).
Goblet cells were significantly increased in fish fed LSE enriched diets when compared to the control diet. There is evidence that goblet cells play an important role in the production of polymeric mucin glycoprotein to protect the gut epithelium from foreign substances and pathogenic microorganisms (Specian and Oliver, 1991). Thus, increased the number of goblet cell could lead to support intestinal defense in fish (Specian and Oliver, 1991). Sloughing off of the villus structure, cell damage and cell rupture were not found in the dietary treatments in this present investigation. Increased microvilli height was observed in the proximal part of intestines of fish fed LSE enriched diet. It is generally accepted that microvilli play a functional role in secretion, digestion and absorption of essential nutrients (Dimitroglou et al., 2010). Zahran et al. (2014) found that dietary supplementation of Astragalus polysaccharide caused a slight increase in microvilli height in Nile tilapia (O. niloticus) when compared to the control. Thus, longer microvilli found in this research could support absorptive capacity in the gastrointestinal tract of fish (Dimitroglou et al., 2010). Decreased values of enterocyte height, supranucleus height and subnucleus height were also observed. However, the decrease of these micromophological values did not affect body weight or length of fish fed dietary LSE. The modes of action of LSE on the changes of micromorphological values are not fully understood. Ferrara et al. (2015) reported that supranucleus height and enterocyte height in the proximal intestine and supranucleus height, nucleus height, nucleus width and enterocyte height in the distal intestine of sharpsnout seabeam (Diplodus puntazzo) fed soybean meal-based diets mixed with mannanoligosaccharides and inulin for 114 days were significantly decreased and these alterations did not have any effects on growth of fish. Escaffre et al. (2007) revealed that the decline of supranucleus height in the proximal intestine and enterocyte height in the distal intestine of rainbow trout fed the complete replacement of fish meal by a soy protein concentrate diet was not related to body weight or length of fish. Thus, it is possible to speculate that others factors could also modulate growth of hybrid catfish in this present study (Shabanzadeh et al., 2016; Thummek et al., 2016).
The results also stated that dietary supplementation of LSE did not produce unwanted side-effects during the treatment period. General behavior, palatability and acceptability of feed observed in the experimental fish were similar to the control fish. There are numerous reports on antimicrobial, anti- inflammatory and anti-oxidant therapeutic potentials of lasia (Maisuthisakul et al., 2007; Nanasombat and Teckchuen, 2009; Deb et al., 2010). Thus, these properties could have positive effects on the improvement of growth performance, nutrient utilization and well-being of fish in this study (Munglue, 2015, 2016).
CONCLUSION
In summary it seems from these experiments that LSE could be used as a growth promoting agent in fish feed to improve fish growth and feed utiliza- tion efficiency as well as intestinal histology. The data also demonstrated that the effective level of LSE was 3%. Preliminary phytochemical analysis of LSE showed the presence of flavonoids, terpenoids, phenolic compounds, steroids, saponins, coumarins, glycosides and anthraquinones. Therefore, it would be of interest to purify and characterize its active phytochemical(s) that would help to obtain a natural growth promoter for sustainable aquaculture farming.
ACKNOWLEDGEMENTS
We gratefully acknowledge Ubon Ratchathani Rajabhat University, Thailand for providing all the facilities to carry out this research. We also thank to Worarat Chalomphong and Siriwan Khamsrisuk for their help and support during the experiment. We would like to thank Assistant Profesoor Dr. Makabodee Ruaysap for the manuscript editing.
REFERENCES
Aanyu, M., Berancor, M.B., and Monroig, O. 2018. Effects of dietary limonene and thymol on the growth and nutritional physiology of Nile tilapia (Oreochromis niloticus). Aquaculture. 488: 217-226. https://doi.org/10.1016/ j.aquaculture.2018.01.036
Alam, F., Haque, M., Sohrab, M.H., Monsur, M.A., Choudhury, M.H., and Ahmed, N. 2011. Antimicrobial and cytotoxic activity from Lasia spinosa and isolated lignin. Latin American Journal of Pharmacy. 30(3): 550-553.
Association of Official Analytical Chemists (AOAC). 19th ed. 2012. Official methods of analysis of official analytical chemists international. Association of Official Analytical Chemists, Maryland, USA.
Chakraborty, S.B., Horn, P., and Hancz, C. 2014. Application of phytochemicals as growth-promoters and endocrine modulators in fish culture. Reviews in Aquaculture. 6(1): 1-19. https://doi.org/10.1111/raq.12021
Citarasu, T. 2010. Herbal biomedicines: a new opportunity for aquaculture industry. Aquaculture International. 18: 403-414. https://doi.org/10.1007/ s10499-009-9253-7
Couto, A., Kortner, T.M., Penn, M., Bakke, A.M., Krogdahl, Å., and Oliva-Teles, A. 2014. Effects of dietary phytosterols and soy saponins on growth, feed utilization efficiency and intestinal integrity of gilthead sea bream (Sparus aurata) juveniles. Aquaculture. 432: 295-303. https://doi.org/10.1016/ j.aquaculture.2014.05.009
Deb, D., Dev, S., Das, A.K., Khanam, D., Banu, H., Shahriar, M., Ashraf, A., Choudhuri, M.S.K., and Basher, S.A.M.K. 2010. Antinociceptive, anti- inflammatory and anti-diarrheal activities of the hydroalcoholic extract of Lasia spinosa Linn. (Araceae) roots. Latin American Journal of Pharmacy. 29(8): 1269-1276.
Dimitroglou, A., Merrifield, D.L., Spring, P., Sweetman, J., Moate, R., and Davies, S.J. 2010. Effects of mannan oligosaccharide (MOS) supplementation on growth performance, feed utilisation, intestinal histology and gut microbiota of gilthead sea bream (Sparus aurata). Aquaculture. 300(1-4): 182-188.https://doi.org/10.1016/j.aquaculture.2010.01.015
Elvin-Lewis, M. 2001. Should we be concerned about herbal remedies.
Journal of Ethnopharmacology. 75: 141-164.
Escaffre, A.-M., Kaushik, S., and Mambrini, M. 2007. Morphometric evaluation of changes in the digestive tract of rainbow trout (Oncorhynchus mykiss) due to fish meal replacement with soy protein concentrate. Aquaculture. 273(1): 127-138. https://doi.org/10.1016/j.aquaculture.2007.09.028
Evans, W.C., Evans, D., and Trease, G.E. 15th ed. 2002. Trease and Evans pharmacognosy. Saunders/Elsevier, Edinburgh.
Fang, C., Ma, M., Ji, H., Ren, T., and Mims, S.D. 2015. Alterations of digestive enzyme activities, intestinal morphology and microbiota in juvenile paddlefish, Polyodon spathula, fed dietary probiotics. Fish Physiology and Biochemistry. 41(1): 91-105. https://doi.org/10.1007/s10695-014-0008-7 Ferrara, E., Gustinelli, A., Fioravanti, M.L., Restucci, R., Quaglio, F., Marono, S., and Piccolo, G. 2015. Histological and micro-/macro-morphological evaluation of intestine in sharpsnout seabream (Diplodus puntazzo) fed soybean meal-based diets added with MOS and inulin as prebiotics. Aquaculture International. 23(6): 1525-1537. https://doi.org/10.1007/ s10499-015-9902-y
Francis, G, Makkar, H.P.S., and Becker, K. 2002. Dietary supplementation with a Quillaja saponin mixture improves growth performance and metabolic efficiency in common carp (Cyprinus carpio L.). Aquaculture. 203(3-4): 311-320. https://doi.org/10.1016/S0044-8486(01)00628-7
Gültepe, N., Acar, Ü., Kesbiç, O.S., Yılmaz, S., Yıldırım, Ö., and Türker, A. 2014. Effects of dietary Tribulus terrestris extract supplementation on growth, feed utilization, hematological, immunological, and biochemical variables of Nile tilapia Oreochromis niloticus. The Israeli Journal of Aquaculture–Bamidgeh. 60: 1024-1031.
Gupta, A., and Sandhu, R.S. 1998. Effect of garlic agglutinin and garlic extracts on the rat jejunum. Nutrition Research. 18(5): 841-850. https://doi.org/10.1016/S0271-5317(98)00069-4
Harborne, J.B. 1998. Phytochemical methods: a guide to modern techniques of plant analysis. Chapman and Hall, Ltd, London.
Heidarieh, M., Mirvaghefi, A.R., Akbari, M., Farahmand, H., Sheikhzadeh, N., Shahbazfar, A.A., and Behgar, M. 2012. Effect of dietary Ergosan on growth performance, digestive enzymes, intestinal histology, hematological parameters and body composition of rainbow trout (Oncorhynchus mykiss). Fish Physiology and Biochemistry. 38(4): 1169-1174. https://doi.org/10.1007/ s10695-012-9602-8
Hong Van, N.T., Minh, C.V., De Leo, M., Siciliano, T., and Braca, A. 2006. Secondary metabolites from Lasia spinosa (L.) Thw. (Araceae). Biochemical Systematics and Ecology. 34(12): 882-884. https://doi.org/10.1016/j.bse.2006.04.011
Jintana, R., Suthikrai, W., Sophon, S., Hengtrakulsin, R., Usawang, S., and Kamonpatana, M. 2013. Effects of Lasia spinosa Thw. and season on plasma leptin and glucose of weaned female murrah × swamp buffalo calves. Buffalo Bulletin. 32 (Special Issue 2): 947-950.
Kaewamatawong, T., Suthikrai, W., Bintvihok, A., and Banlunara, W. 2013. Acute to subchronic toxicity and reproductive effects of aqueous ethanolic extract of rhizomes of Lasia spinosa Thw. in male rats. The Thai Journal of Veterinary Medicine. 43(1): 69-74.
Kamatit, W., Aoki, S., and Munglue, P. 2016. Effects of dietary waterlily (Nymphaea pubescens) stamen extract on growth performance and intestinal morphology of common lowland frog (Rana rugulosa). KKU Research Journal. 22(2): 30-41. https://doi.org/10.14456/kkurj.2016.28
Kangsen, M., Yanjiao, Z., Wei, C., Wei, X., Qinghui, A., and Wenbing, Z. 2012. Effects of dietary soy isoflavones on feed intake, growth performance and digestibility in juvenile Japanese flounder (Paralichthys olivaceus). Journal of Ocean University of China. 11(4): 511-516. https://doi.org/10.1007/ s11802-012-2146-9
Kar, A. 2007. Pharmacognosy and Pharmacobiotechnology. New Age International
(P) Ltd, New Delhi.
Kumar, S., Sahu, N.P., Gupta, S., Deo, A.D., Shamna, N., and Ranjan, A. 2017. Inclusion level of deoiled rice bran (DORB) in the diet of Labeo rohita (Hamilton, 1882) fingerlings: Effect on growth and gene expression of IGF-I and IGF-II. Aquaculture. 481(1): 211-217. https://doi.org/10.1016/j. aquaculture.2017.08.025
Maisuthisakul, P., Suttajit, M., and Pongsawatmanit, R. 2007. Assessment of phenolic content and free radical-scavenging capacity of some Thai indigenous plants. Food Chemistry. 100(4): 1409-1418. https://doi.org/ 10.1016/j.foodchem.2005.11.032
Maneenoon, K., Khuniad, C., Teanuan, Y., Saedan, N., Prom-In, S., Rukleng, N., Kongpool, W., Pinsook, P., and Wongwiwat, W. 2015. Ethnomedicinal plants used by traditional healers in Phatthalung Province, Peninsular Thailand. Journal of Ethnobiology and Ethnomedicine. 11: 43. https://doi.org/10.1186/ s13002-015-0031-5
Merrifield, D.L., Harper, G.M., Mustafa, S., Carnevali, O., Picchietti, S., and Davies, S.J. 2011. Effect of dietary alginic acid on juvenile tilapia (Oreochromis niloticus) intestinal microbial balance, intestinal histology and growth performance. Cell and Tissue Research. 344(1): 135-146. https://doi.org/10. 1007/s00441-010-1125-y
Moorby, J., Fraser, M., Theobald, V., Wood, J., and Haresign, W. 2004. The effect of red clover formononetin content on live-weight gain, carcass characteristics and muscle equol content of finishing lambs. Animal Science. 79(2): 303-313. https://doi.org/10.1017/S1357729800090160
Munglue, P. 2014. Effects of dietary Nelumbo nucifera (lotus) peduncle extract on growth performance of Nile tilapia (Oreochromis niloticus). The first Environment and Natural Resources International Conference (ENRIC 2014); 2014 Nov; Bangkok, Thailand. Bangkok: Mahidol University. 279-285 p.
Munglue, P. 2015. Effect of Nelumbo nucifera Gaertn. leaf extract on growth performance of Nile tilapia (Oreochromis niloticus). SDU Research Journal. 8(1): 45-56.
Munglue, P. 2016. Effects of lotus (Nelumbo nucifera Gaertn.) stamen extract on growth performance, feed utilization and intestinal morphology of catfish (Clarias gariepinus). KKU Research Journal. 21(2): 7-17. https://doi. org/10.14456/kkurj.2016.31
Munglue, P., and Dasri, K. 2015. Effects of Bauhinia strychnifolia Craib leaf extract on growth parameters and intestinal morphology of catfish (Clarias gariepinus). Sakon Nakhon Rajabhat University International Conference; 2015 Jul; Sakon Nakhon, Thailand, Sakon Nakhon: Sakon Nakhon Rajabhat University. 50-57 p.
Nahrstedt, A. 1975. Triglochinin in Araceen (III). Phytochemistry. 14(12): 2627-2628. https://doi.org/10.1016/0031-9422(75)85237-X
Nanasombat, S., and Teckchuen, N. 2009. Antimicrobial, antioxidant and anticancer activities of Thai local vegetables. Journal of Medicinal Plants Research. 3(5): 443-449.
Napiroon, T., Sookchaloem, D., Vajrodaya, S., and Pachkong, T. 2013. Taxonomy and phytochemistry of Terrestrial Aroids (Araceae) in Saiyok National Park, Kanchanaburi province. Thai Journal of Forestry. 32(Supplementary Issue): 71-84.
Ong, V.Y., and Tan, B.K. 2007. Novel phytoandrogens and lipidic augmenters from Eucommia ulmoides. BMC Complementary and Alternative Medicine. 7: 3. https://doi.org/10.1186/1472-6882-7-3
Ostaszewska, T., Dabrowski, K., Palacios, M.E., Olejniczak, M., and Wieczorek, M. 2005. Growth and morphological changes in the digestive tract of rainbow trout (Oncorhynchus mykiss) and pacu (Piaractus mesopotamicus) due to casein replacement with soybean proteins. Aquaculture. 245(1-4): 273-286. https://doi.org/10.1016/j.aquaculture.2004.12.005
Pace, V., Carbone, K., Spirito, F., Iacurto, M., Terzano, M.G., Verna, M., Vincenti, F., and Settineri, D. 2006. The effects of subterranean clover phytoestrogens on sheep growth, reproduction and carcass characteristics. Meat Science. 74(4): 616-622. https://doi.org/10.1016/j.meatsci.2006.05.006
Shabanzadeh, S., Shapoori, M., Sheikhzadeh, N., Nofouzi, K., Oushani, A.K., Enferadi, M.H., Mardani, K., and Shahbazfar, A.A. 2016. Growth performance, intestinal histology, and biochemical parameters of rainbow trout (Oncorhynchus mykiss) in response to dietary inclusion of heat-killed Gordonia bronchialis. Fish Physiology and Biochemistry. 42(1): 65-71. https://doi.org/10.1007/s10695-015-0117-y
Specian, R.D., and Oliver, M.G. 1991. Functional biology of intestinal goblet cells. American Journal of Physiology. 260(2): C183-193. https://doi.org/ 10.1152/ajpcell.1991.260.2.C183
Suthikrai, W., Jintana, R., Hengtrakulsin, R., Sophon, S., Usawang, S., and Kamonpatana, M. 2006. Effects of Lasia spinosa Thw. on growth rate of weaned Swamp buffalo and Murrah × Swamp buffalo calves. p. 183-195. Proceedings of Seminar Enhancement of Reproductive Efficiency and Production of Livestock in Thailand; 2005 Dec 14-16; Chiang Mai. Thailand. Chiang Mai. 183-195 p.
Suthikrai, W., Jintana, R., Sophon, S., Hengtrakulsin, R., Usawang, S., and Kamonpatana, M. 2007. Effects of Lasia spinosa Thw. on growth rate and reproductive hormone of weaned Swamp buffalo and Murrah × Swamp buffalo calves. Italian Journal of Animal Science. 6: 532-535. https://doi.org/10. 4081/ijas.2007.s2.532
Thummek, P., Aoki, S., and Munglue, P. 2016. Growth performance and intestinal morphology of Common lowland frog (Rana rugulosa) fed diets supplemented with lotus (Nelumbo nucifera Gaertn.) stamen extract. KKU Research Journal. 22(2): 18-29. https://doi.org/10.14456/kkurj.2016.29 Turan, F. 2006. Improvement of growth performance in tilapia (Oreochromis aureus Linnaeus) by supplementation of red clover Trifolium pratense
in diets. The Israeli Journal of Aquaculture-Bamidgeh. 58(1): 34-38. Turan, F., and Akyurt, I. 2005a. Effects of androstenedione, a phytoandrogen, on growth and body composition in the African catfish Clarias gariepinus. The Israeli Journal of Aquaculture-Bamidgeh. 57(1): 62-66.
Turan, F., and Akyurt, I. 2005b. Effects of red clover extract on growth performance and body composition of African catfish Clarias gariepinus. Fisheries Science. 71: 618-620. https://doi.org/10.1111/j.1444-2906.2005.01006.x
Ueda, J., Tezuka, Y., Banskota, A.H., Tran, Q.L., Tran, Q.K., Harimaya, Y., Saiki, I., and Kadota, S. 2002. Antiproliferative activity of Vietnamese medicinal plants. Biological and Pharmaceutical Bulletin. 25(6): 753-760. Vijaya, and Yadav, A.K. 2016. In vitro anthelmintic assessment of selected phytochemicals against Hymenolepis diminuta, a zoonotic tapeworm. Journal of Parasitic Diseases. 40(3): 1082-1086. https://doi.org/10.1007/s12639-014-0560-1
Yang, C., Chowdhury, M.A., Huo, Y., and Gong, J. 2015. Phytogenic compounds as alternatives to in-feed antibiotics: potentials and challenges in application. Pathogens. 4(1): 137-156. https://doi.org/10.3390/pathogens4010137
Zahran, E., Risha, E., AbdelHamid, F., Mahgoub, H. A., and Ibrahim, T. 2014. Effects of dietary Astragalus polysaccharides (APS) on growth performance, immunological parameters, digestive enzymes, and intestinal morphology of Nile tilapia (Oreochromis niloticus). Fish & Shellfish Immunology. 38(1): 149- 157. https://doi.org/10.1016/j.fsi.2014.03.002
Zhang, Y.-y., Liu, B., Ge, X.-p., Liu, W.-b., Xie, J., Ren, M., Cui, Y.-t., Xia, S.-l., Chen, R., Zhou, Q., et al. 2014. The influence of various feeding patterns of emodin on growth, non-specific immune responses, and disease resistance to Aeromonas hydrophila in juvenile Wuchang bream (Megalobrama amblycephala). Fish & Shellfish Immunology. 36(1): 187-193. https://doi.org/ 10.1016/j.fsi.2013.10.028
Phukphon Munglue1*, Khwanduean Rattana1, Supavee Sangchanjiradet1, and Kajohnpong Dasri2
1Program of Biology, Faculty of Science, Ubon Ratchathani Rajabhat University, Ubon Ratchathani 34000, Thailand
2Program of Microbiology, Faculty of Science, Ubon Ratchathani Rajabhat University, Ubon Ratchathani 34000, Thailand
*Corresponding author. E-mail: phukphon.m@ubru.ac.th
Total Article Views
Article history:
Received: July 16, 2018;
Revised: October 24, 2018;
Accepted: November 1, 2018

