
Anti-Adipogenic Effects of Unripe Green Banana Flour Extracts (Musa abb cv. Kluai “namwa”) in 3T3-L1 Adipocytes
Tantip Boonsong*, Wanida Chawnawa, Panatda Janneoy, and Suchada PhimsenPublished Date : 2023-01-05
DOI : https://doi.org/10.12982/NLSC.2023.004
Journal Issues : Number 1, January-March 2023
Abstract This study aimed to investigate anti-adipogenesis effects of unripe banana (Musa abb cv. Kluai “namwa”) flour which were extracted by organic solvents (methanol, ethanol, hexane) and distilled water in 3T3-L1 adipocytes in vitro. Adipocytes were treated by all extracts for 7 to 12 days from the starting of adipocyte differentiation. Caffeine, anti-adipogenic bioactive compound, was used as a positive control. After treatments, intracellular lipid contents were measured by Oil Red O staining and triglyceride assay kit, respectively. The underlying mechanisms were examined by measuring changes of mRNA expression level of adipogenesis-related genes using RT-PCR technique. The results showed that the methanolic, ethanolic and hexane extracts significantly decreased (P<0.05) lipid contents in 3T3-L1 adipocytes after 7 days of incubation in a dose-dependent manner while the water extract had a significant effect after a 12-day treatment. In addition, the expression level of genes involved in adipogenesis and lipid synthesis (PPARγ, SREBP-1c, FAS) was significantly decreased (P<0.05) in all treatment groups in accordance with the lipid contents. The present study suggests that the capability of unripe banana green flour extracts on lowering lipid accumulation in adipocytes in vitro may have further implication in in vivo anti-obesity effects. Moreover, green banana flour may be a potential ingredient for beneficial health diets.
Keywords: Musa abb cv. Kluai “namwa”, unripe green banana flour, 3T3-L1 adipocytes, adipogenesis
Funding: The authors are grateful for the research funding provided by the National Science, Research and Innovation Fund; NSRF (Code R2563B001).
Citation: Boonsong, T., Chawnawa, W., Janneoy, P., and Phimsen, S. 2023. Anti-adipogenic effects of unripe green banana flour extracts (Musa abb cv. Kluai “namwa”) in 3T3-L1 adipocytes. Nat. Life Sci. Commun. 22(1): e2023004.
INTRODUCTION
High fat diet consumption, sedentary lifestyle, and overeating causes an imbalance of energy intake and energy expenditure; and it is directly associated with obesity (Sarnali and Moyenuddin, 2010). Obesity is also known to relate with chronic diseases such as diabetes, hypertension, and cancer (Haslam and James, 2005). Energy intake affects whole body compositions, particularly adipose tissue. In a lean body, adipocytokines derived from adipose tissue such as leptin and adiponectin will regulate energy homeostasis and physiological status (Coelho et al., 2013). However, high energy intake can induce an excessive number of adipocytes (hyperplasia), and adipocyte size (hypertrophy) in adipose tissue, which will increase fat accumulation and cause pathological conditions including obesity, insulin resistance, and diabetes. Fat accumulation in adipocytes is modulated by lipogenesis and lipolysis (Coelho et al., 2013). As a result, the increasing of lipolysis and inhibiting adipogenesis may reduce adipose tissue mass and obesity. At present, lifestyle modifications, drug treatment and surgical interventions are used for obesity treatment. However, an inconsistently in the successfulness and side effects have been reported (Rodgers et al., 2012). Currently, natural products and their bioactive compounds are of interest to aid in the prevention of obesity and other chronic diseases. Many studies have shown anti-obesity properties of several plant extracts which can inhibit adipogenesis (Liou et al., 2015; Maki et al., 2017; Kim et al., 2020) and stimulate lipolysis in adipocytes (Pheiffer et al., 2013; Duangjai et al., 2018; Castillo et al., 2019). Banana (Musa sp.), a tropical fruit, is widely cultivated. Green bananas (Musa paradisiaca L.) are rich of starch (20-25%) in the pulp of the fruits (Cordenunsi and Lajolo, 1995) which can be utilized as banana flour combining into food products (Juarez-Garcia et al., 2006; Aparicio-Saguilan et al., 2007; Hernández-Nava et al., 2009; Utrilla-Coello et al., 2013; Flores-Silva et al., 2017; Khoozani et al., 2020). High fiber and slowly digestible starch contents in banana flour can sustain glucose release which will reduce insulin response, maintain satiety level, control energy intake (Aller et al., 2011), and decrease absorption of macronutrients (Dayib et al., 2020). This suggests that banana flour may have potentially prevent and/or treatment diabetes and obesity. In addition, phytochemicals composition in banana pulp extraction (Musa sp.) have been investigated (Maity et al., 2016; Sidhu and Zafar, 2018). The banana pulps which were extracted with different types of solvents (water, methanol, acetone, and hexane) contained various compounds such as tannins, alkaloids, phytosterols, flavonoids and phenols. It has been shown that these components were associated with pharmacological activities such as antioxidant, anti-inflammatory, anti-cancer (Apu et al., 2013), and antimicrobial (Raju et al., 2013). A previous study presented that flavonoids such as leucocyanidin and leucodelphinidin were the major compounds in the aqueous extract of unripe banana pulps (Musa sapientum L., var.paradisiaca (Lewis et al., 1999). The beneficial health effects of flavonoids were shown including anti-allergic, anti-inflammatory, antimicrobial, anticancer activities (Apu et al., 2010), and anti- obesity (Murase et al., 2002; Tsuda, 2008). This study, therefore, aimed to investigate the effect of unripe green banana flour extracts on intracellular lipid contents and mRNA expression of adipogenesis-related genes in 3T3-L1 adipocytes in vitro. The unripe green banana flour was extracted by different types of solvents including methanol, ethanol, hexane, and distilled water. All treatments were assessed by treating 3T3-L1 adipocytes with the non-toxic concentrations of the extracts for 7 or 12 days from the beginning of adipocyte differentiation. Subsequently, intracellular lipid contents and mRNA levels of potential marker genes for adipogenesis were then measured at the end of treatments.
MATERIALS AND METHODS
Sample preparation
Unripe green banana flour extraction
Unripe green bananas (Musa abb cv. Kluai “namwa”) were provided by the Looktung banana frying factory, Nongtoom, Sukhothai, Thailand. The banana flour extract was produced from the pulp of the fruits using the procedure described by Jannoey et al., 2021. Briefly, the banana pulp was separated from its peel and cut into small slices, rinsed in water. The slices were then dried in hot air oven at 50°C, ground using flour milling machine at 200 mesh pore size and stored at 4°C in refrigerator for further analyzing. The banana flour was initially analyzed chemical compositions. The unripe green banana flour examined in this study had a moisture content of 13.14 % comprised with 0.2% lipids, 2.29 % ash, 2.72% protein, and 40 % resistant starch. The banana flour was then extracted with different organic solvents which included ethanol, methanol, hexane, and distilled water at the ratio of 1:10. The banana flour samples (10 g) were macerated with 100 ml of all solvents at room temperature for 1 week. The extracts were filtered through Whatman No.1 paper, the solvent in each filtrate was then removed by rotary evaporation at 57°C and the concentrates were stored at 4°C. Regarding the water extraction samples, the aqueous extracts were freeze-drying and stored at 4°C until used.
Phytochemical and total phenolic content analysis
The phytochemical in all extracts were analyzed by Jannoey and colleagues (2022) using the Liquid Chromatography-Mass Spectrometry-Electrospray Ion (LC-MS-ESI) method. The C18 column (Luna 4.6x150 mm, 5 µm Phenomenex,USA) of HPLC (1260 infinityAgilent Technologies, Germany) and the mixing of the mobile phase including solvent A (0.1%Formic acid/H2O) and solvent B (0.1% formic acid /acetonitrile) were used in the analysis process. The gradient condition between mobile phase A : B was used to separate the phytochemical content (95% : 5% to 80% : 20%). Mass analyzer was established using the 6,540 UHD Accurate Mass Q-TOF LC-MS (Agilent Tecnologies, Singapore) in range 50-1,000 Da. Ionization was achieved with an electrospray source utilizing a cone voltage of 10,20,40 eV using negative and positive mode or determination of phenolic compounds. Desolvation gas was Nitrogen at a flow rate of 10 L/min, temperature of 350°C, nebulizer 30 psig Vcap 3,500V fragmentor 100V (positive mode), 250 (negative mode) skimmer1 65 V Octapole RFP 650V. The identification of phytochemical in each unripe banana flour extract was then examined using mass analyzer compared with mass spectrum database. From the analysis, various phytochemical compositions of the extracts were found due to the extractant type. In the methanolic and ethanolic extracts, the phenolic compounds were found such as gallic acid, glutaric acid, 2-hydroxyvaleric acid, protocatechuic acid, 1,4-Ipomeadiol, 5,6-dimethoxyflavone. Whereas, the hexane fraction presented the short chain hydrocarbons, fatty acids and its derivative including piperine and phytosphingosine. Though, various organic acids such as gluconic acid, succinic acid, 3-hydroxy-cis, cis-muconic acid, trans,cis-aconitic acid dibutyl succinic acid were presented in the water extract.
Total phenolic content measurement
The total phenolic content was assessed using Folin-Ciocalteu method with slight modification (Majhenic et al, 2007). Briefly, each extract was mixed with the Folin-Ciocalteu reagent containing phosphomolybdic phosphotungstic acid reagents and the reaction products were measured at 765 nm using a UV spectrophotometer microplate. Total phenolics were then calculated as gallic acid standard equivalent (mg GAE/100 g dry weight.)
For cell treatments, the weight of each extract was recorded before being re-dissolved with 100% DMSO and then sterile filtered through 0.2 µm syringe filter and diluted in 0.1% DMSO. All banana flour extracts were then diluted to various concentrations in 0.1% DMSO-containing culture media to examine intracellular lipid contents and mRNA expression of adipogenesis-related genes (PPARγ, SREBP-1c, FAS), using β-actin as a normalization control.
3T3-L1 cell culture and treatments
3T3-L1 pre-adipocytes were cultured in basal medium (BM) containing Dulbecco’s modified Eagle’s medium, 10% fetal bovine serum, and 1% antibiotic-antimycotic at 37°C in a humidified atmosphere of 5% CO2 until 100 % confluence which was designed as day “0”. Cells were then differentiated in differentiation medium I (DM I) containing 1 mM 3-isobutyl- 1-methylxanthine, 2 µM dexamethasone and 1 µg/ml insulin in basal medium (BM) for 3 days (day “3”). The culture medium was then replaced with differentiation medium II (DM II) containing 1 µg/ml insulin in the basal medium (BM) for 2 days (day “5”). On day “5”, cells were further cultured to mature adipocytes in BM containing 1 µg/ml insulin for 7 days (day “12”) or 12 days (day “17”) and the culture media were changed every 2-3 days during adipocyte differentiation induction. For the treatments, cells were treated with unripe banana flour extracts for 7 or 12 days from the starting of adipocyte differentiation at day “0” as described above. Caffeine, an anti- adipogenic bioactive compound (Kim et al., 2016) was used as a positive control.
Cell viability assay
3T3-L1 pre-adipocytes were cultured into a 96-well plate (2x103 cells/well). At the initial of adipocyte differentiation (day “0”), cells were co-treated with 62.5, 125, 250, 500, and 1,000 µg/ml of unripe green banana flour extracts for 7 days and with 350 µg/ml for 12 days. Afterwards, 180 µl of serum-free medium and 20 µl of 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-Diphenyltetrazolium Bromide (MTT) (5 mg/ml) was added, and then incubated at 37°C in a humidified atmosphere of 5% CO2 for 2 hours. The formazan crystal in adipocytes was dissolved by 100 µl of 100% DMSO. The cell viability was measured at 595 nm using a micro-plate reader, and the percentage of the cell viability was calculated by implementing the following array: % Cell viability = Absorbance of sample/Absorbance of control × 100.
Lipid accumulation assay
Cells were treated with unripe green banana flour extracts at 250, 350, 450 µg/ml for 7 or 12 days from the beginning of differentiation at day “0”. Intracellular lipid levels were then measured by Oil Red O staining and triglyceride quantification assay kit. Briefly, after dye staining, 3T3-L1 adipocytes were washed with phosphate buffered saline and fixed with 10% formalin for 1 h. Next, cells were incubated with 60% isopropanol for 5 minutes and stained with 0.5% (w/v) Oil Red O dye solution for 5 minutes. After the cells were photographed, the stained lipid droplets were dissolved by 100% isopropanol. The lipid levels were measured at 510 nm. For kit assay, cells were homogenized in RIPA buffer (Boster, USA) with protease inhibitors (Boster, USA). The lipid accumulation was calculated by the following formular; lipid accumulation (% of control) = Absorbance of sample/Absorbance of control × 100. (sample = lipid contents of treated adipocytes; control = lipid contents of untreated adipocytes)
The triglyceride levels were measured according to manufacturer’s protocol (Triglycerides liquicolormono, Germany) at 500 nm. The triglyceride levels were then normalized with total protein concentrations.
mRNA expression assay
The mRNA expression level was measured by Reverse Transcription-Polymerase Chain Reaction (RT-PCR). Briefly afterwards, total RNA was extracted using Ribozol reagent (Amresco, USA) following the manufacturer’s recommendations. RNA concentration and purity were assessed by a NanoDrop spectrophotometer (Thermo Scientific™ Nanodrop 2000 spectrophotometer, Thermo Fisher Scientific, USA). A 1000 ng of total RNA was then subjected to first-stand cDNA synthesis (Solis BioDyne, Germany) used for the PCR reaction. The PCR cycling condition included pre-denaturation (95°C) for 2 minutes, 30 cycles of denaturation (94°C, 30 seconds), annealing (58°C, 1 minute), and extension (72°C, 1 minute), and a cycle of final extension (72°C, 5 minutes). The PCR products were then examined employing agarose gel electrophoresis and visualized using a Gel Document (Image Quant LAS 500, GE Healthcare Life Sciences, Sweden). The expression levels of target genes (Table 1.) were normalized with β-actin gene.
Table 1. Primers for the measurement of mRNA expression.
|
Genes |
|
Primer sequence 5’ 3’ |
Size (bp) |
References |
|
PPARγ |
Forward |
CGCTGATGCACTGCCTATGA |
101 |
Ho et al. (2012) |
|
|
Reverse |
AGAGGTCCACAGAGCTGATTCC |
|
|
|
SREBP-1c |
Forward |
CTGCAGACCCTGGTGAGTG |
170 |
NM_011480.3 |
|
|
Reverse |
GACCGGTAGCGCTTCTCAAT |
|
|
|
FAS |
Forward |
TTTGTAAACGTCCTCACCCGA |
127 |
NM_007988.3 |
|
|
Reverse |
CGGAAGTTCAGAGAGGCGTAGTAG |
|
|
|
β-actin |
Forward |
TGTCCACCTTCCAGCAGATGT |
101 |
Ho et al. (2012) |
|
|
Reverse |
AGCTCAGTAACAGTCGCGCTAGA |
|
|
Statistical analysis
The statistical analysis was assessed by SPSS (version 17 SPSS Inc, Chicago. USA). Results were presented as mean ± SD. The analysis was performed in triplicate in each experiment. Differences among experimental groups were assessed by using one-way analysis of variance (ANOVA). The different between means were tested for significance using Tukey’s HSD test at P
RESULTS
After a 7 and 12 day of incubation period, banana flour extract at concentrations of 62.5 to 450 µg/ml did not reveal cytotoxic effect on 3T3-L1 adipocytes (Figure 1 and 2). This, therefore, was applied to examine anti-adipogenic effects throughout adipogenesis process for 7 or 12 days. The following biological parameters were assessed: the intracellular lipid contents by Oil Red O staining, the triglyceride assay kit, and the changes of mRNA expression of adipogenesis-related genes by RT-PCR technique, compared with control cells.
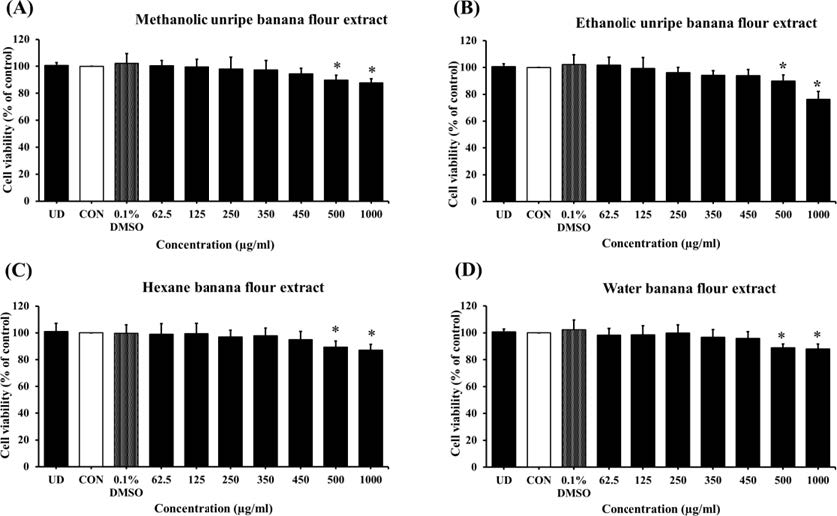
Figure 1. The effect of unripe banana flour extracts at concentrations of 62.5 to 1,000 µg/ml on 3T3-L1 adipocyte viability after 7 days of incubation. *P <0.05 compared with control. UD = Undifferentiated cells; CON = Differentiated cells. (N=8-16).
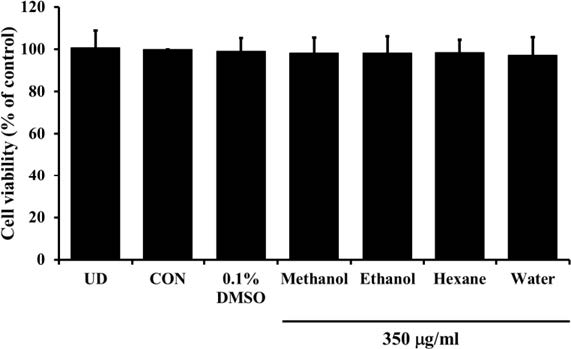
Figure 2. The effect of unripe banana flour extracts at a concentration of 350 µg/ml on 3T3-L1 adipocyte viability after 12 days of incubation.
*P <0.05 compared with control (D). UD = Undifferentiated cells; D = Differentiated cells. (N=16).
From the results, it was demonstrated that after treating with the solvent extracts (250 and 350 µg/ml) for 7 days, lipid contents in 3T3-L1 adipocytes significantly decreased (P<0.05) in a dose-dependent manner (Figure 3). However, when treating the cells with the water extract (250, 350 and 450 µg/ml) (Figure 3 and 4) for 7 days, lipid levels did not statistically differ from that in control cells. Nevertheless, after increasing the incubation time to 12 days, lipid contents were reduced significantly (P <0.05) in all groups (Figure 5). This indicated that the water extract may exhibit low efficacy to suppress adipogenesis less than other extracts.
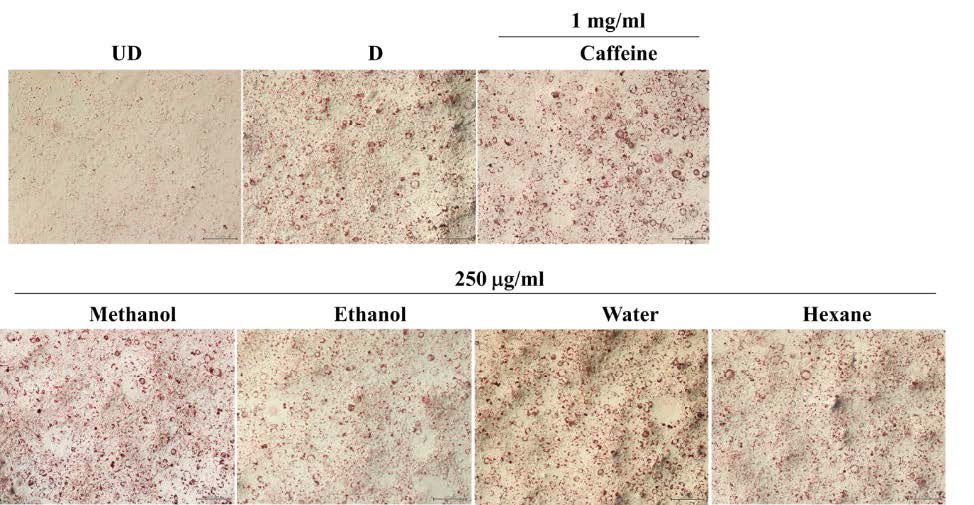
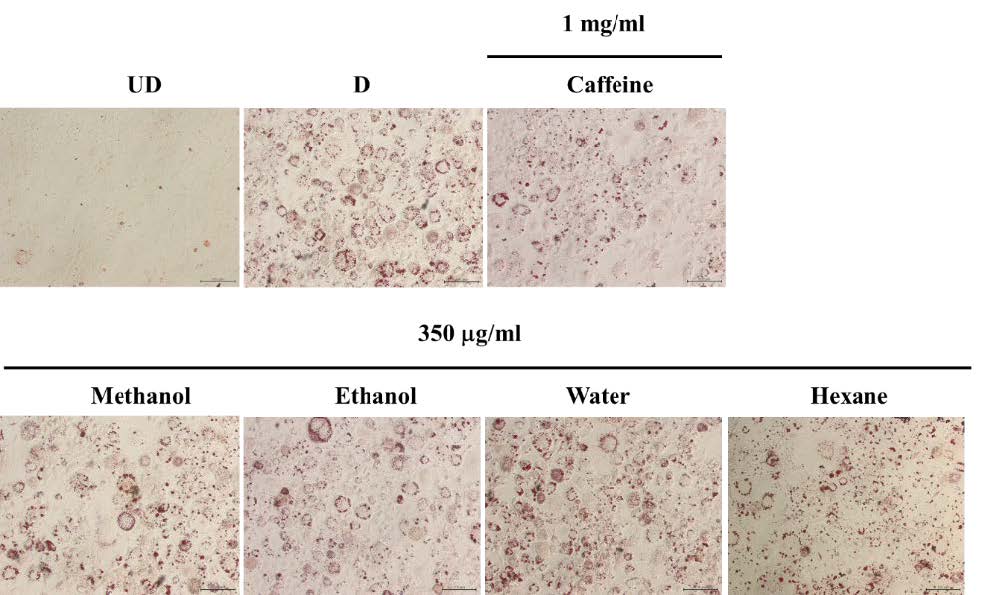
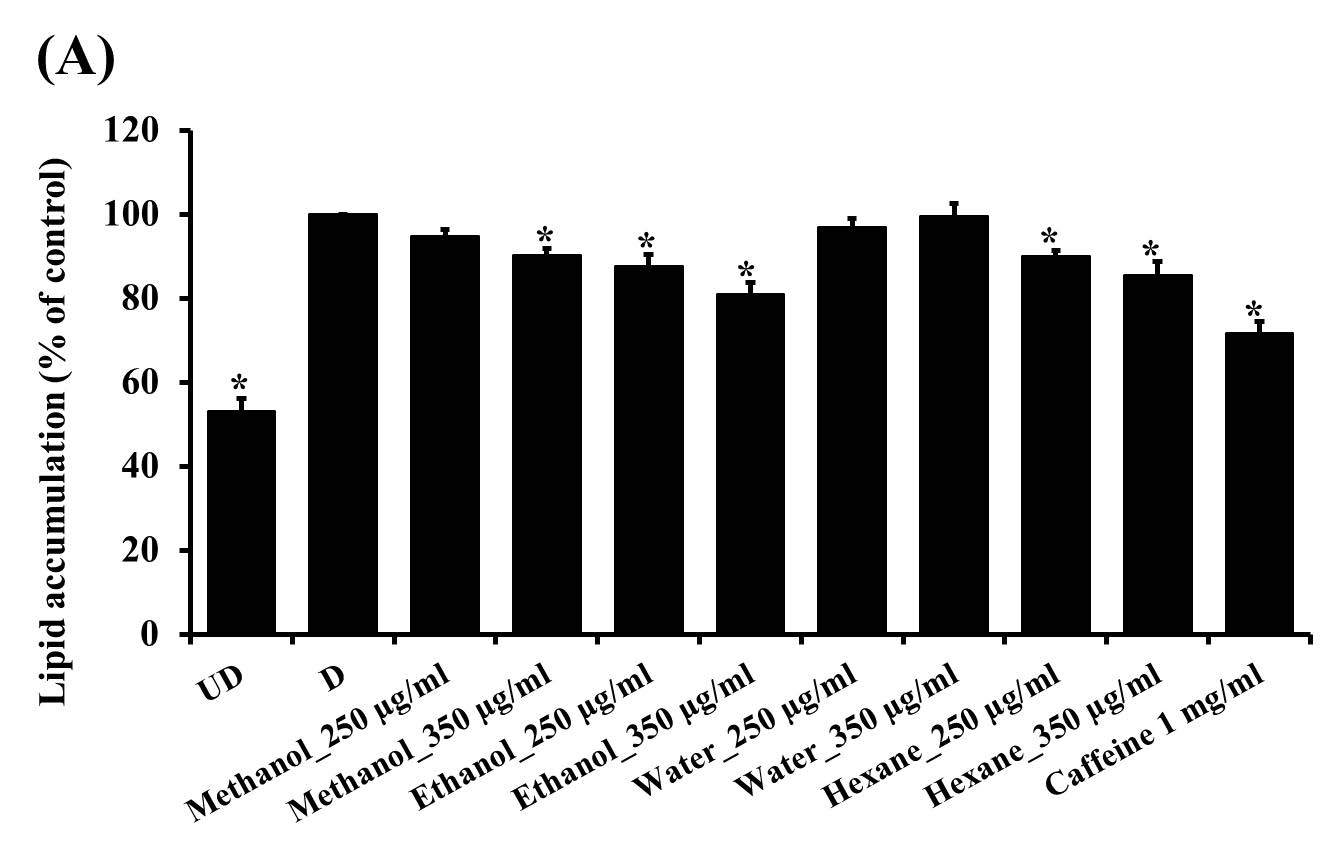
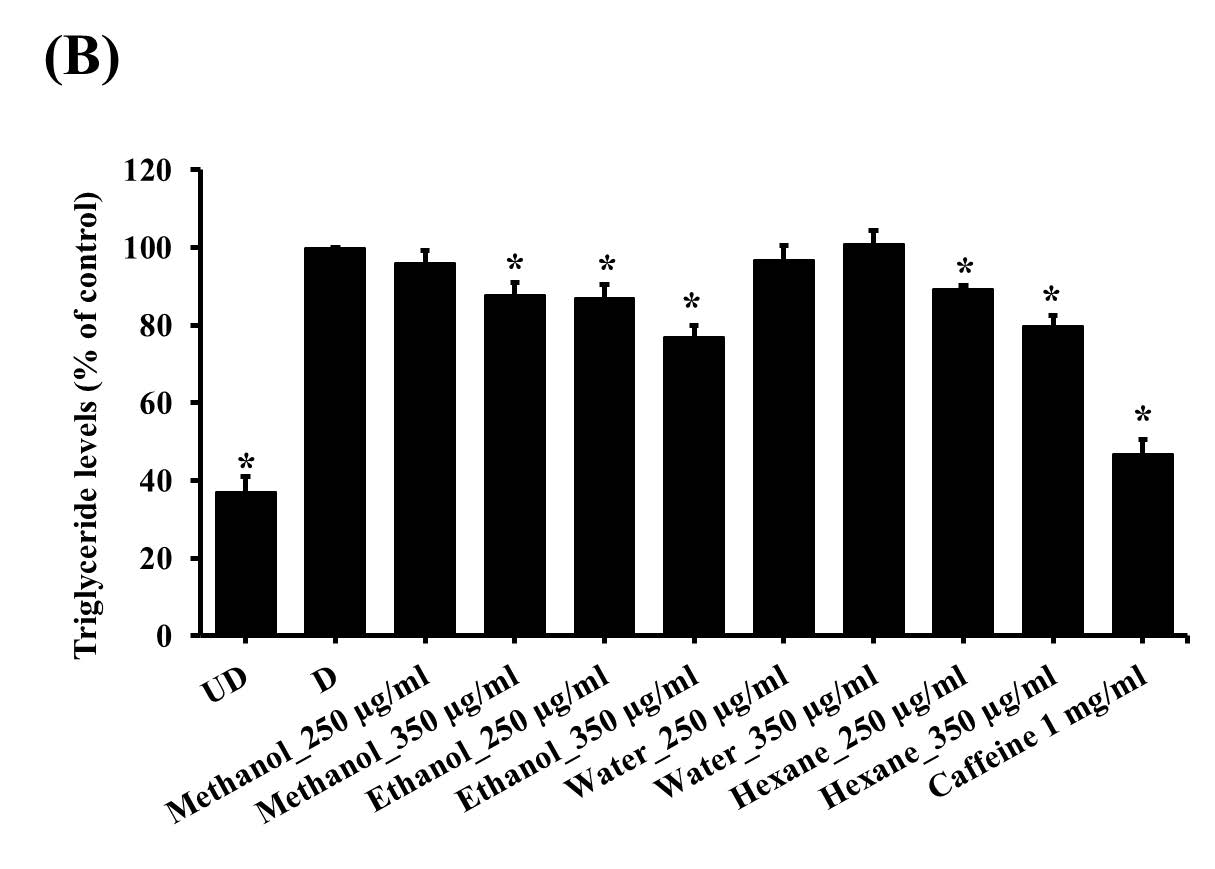
Figure 3. The lipid contents in 3T3-L1 adipocytes were treated with unripe banana flour extracts at concentrations of 250 and 350 µg/ml for 7 days. Total lipid contents were measured by Oil Red O staining (A) and triglyceride assay kit (B). *P <0.05 compared with control (D). UD = Undifferentiated cells; D = Differentiated cells. (N=3-6).
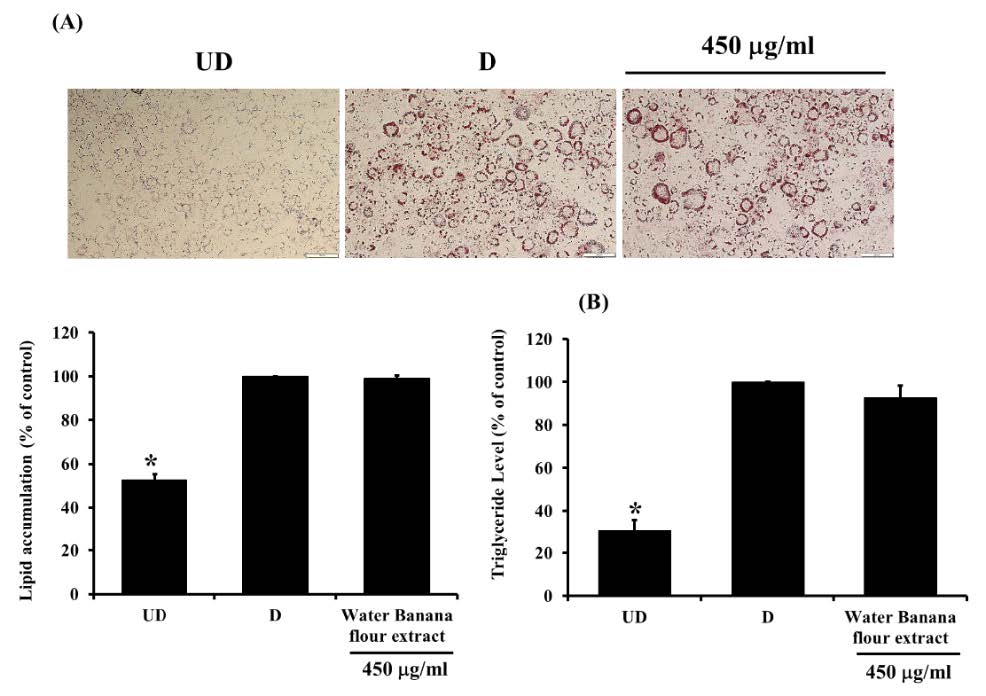
Figure 4. The lipid contents in 3T3-L1 adipocytes which treated with water unripe banana flour extract at a concentration of 450 g/ml for 7 days.
Total lipid contents were measured by Oil Red O staining (A) and triglyceride assay kit (B). *P <0.05 compared with control (D). UD = Undifferentiated cells; D = Differentiated cells. (N=4).
Figure 5. The lipid contents in 3T3-L1 adipocytes which treated with unripe banana flour extracts at a concentration of 350 µg/ml for 12 days.
Total lipid contents were measured by Oil Red O staining (A) and triglyceride assay kit (B). *P <0.05 compared with control (D). UD = Undifferentiated cells; D = Differentiated cells. (N=3).
Furthermore, in the present study, the underlying mechanisms of anti-adipogenic effects of all extracts were also determined. The results showed that, in consistent to the lipid contents measurement, the solvent extracts at a concentration of 350 µg/ml significantly decreased (P <0.05) the expression of PPARγ, SREBP-1c, and FAS genes after 7 days of treatment whereas the water extract did not show a significant effect (Figure 6). Nevertheless, after 12 days of treatment, all extracts showed anti-adipogenesis ability via suppressing adipogenic gene expression (Figure 7).
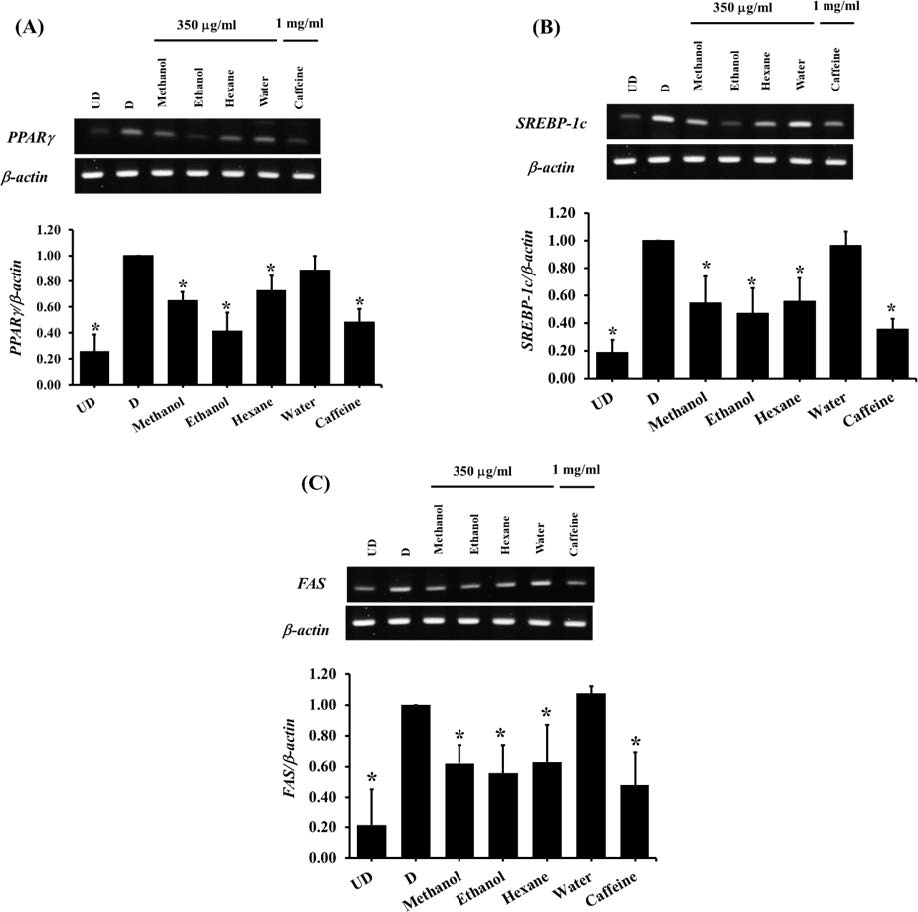
Figure 6. The expression of adipogenic genes in 3T3-L1 adipocytes which treated with unripe banana flour extracts at a concentration of 350 µg/ml for 7 days. The gene expression presented as relative expression normalized to β-actin gene. (A) PPARγ; (B) SREBP-1c; (C) FAS. *P<0.05 compared with control cells (D). UD = Undifferentiated cells; D = Differentiated cells. (N=4-5).
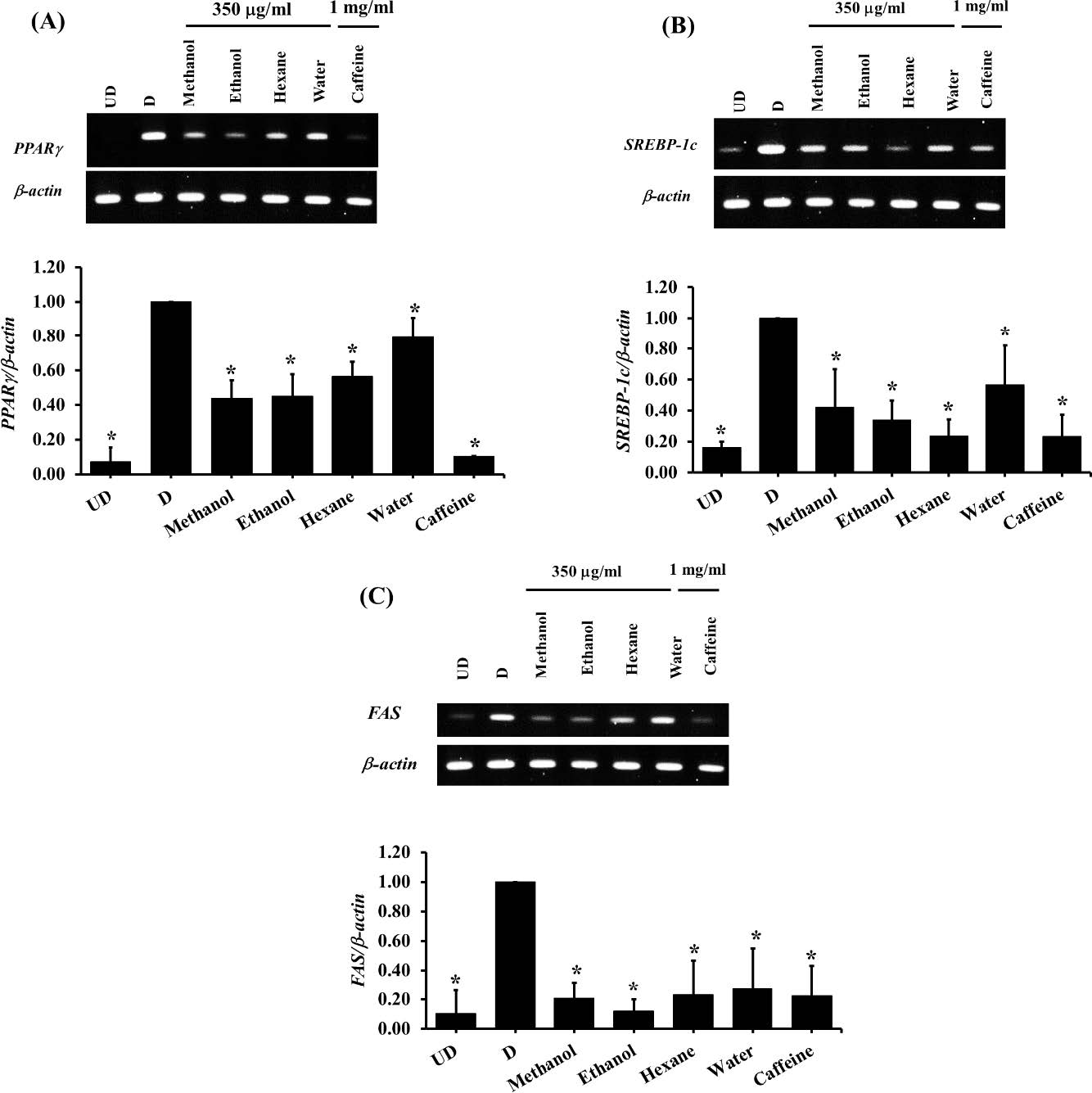
Figure 7. The expression of adipogenic genes in 3T3-L1 adipocytes which treated with unripe banana flour extracts at a concentration of 350 µg/ml for 12 days. The gene expression presented as relative expression normalized to β-actin gene. (A) PPARγ; (B) SREBP-1c; (C) FAS. *P<0.05 compared with control cells (D). UD = Undifferentiated cells; D = Differentiated cells. (N=4-7).
DISCUSSION
In the present study, we determined anti-adipogenic effects of unripe green banana flour which was extracted with methanol, ethanol, hexane, and distilled water in 3T3-L1 adipocytes in vitro. The results showed that all extracts can reduce intracellular lipid contents via inhibiting the expression of adipogenic and lipogenic genes (PPARγ, SREBP-1c, FAS). Moreover, the ethanolic, methanolic, and hexane extracts showed greater anti-adipogenic ability than the water extract. As shown in Figure 3, these organic solvent extracts affected the differentiation process by reducing lipid contents within 7 days of incubation, whereas anti-adipogenic effect of the water extract was shown in long-term treatment (12 days) (Figure 5). This may show the efficacy of the extracts on adipocyte differentiation, at least, due to their polarity. It has been reported that methanol, ethanol, acetone, chloroform, hexane, and water can isolate different amounts of bioactive compounds and their biological activities (lloki‑Assanga et al., 2015; Truong et al., 2019). Previous studies demonstrated that water dissolves soluble molecules (e.g., carbohydrates, proteins) and some natural phenolic compounds (e.g., gallic acid, salicylic acid, ferulic acid, caffeic acids) (Mota et al., 2008). Whereas semi-polar solvents such as methanol and ethanol and a non-polar solvent hexane can dissolve semi-polar compounds (e.g., phenols, flavonoids, alkaloids) and non-polar compounds (e.g., sterols, terpenoids, tannins, glycosides), respectively (Maity et al., 2016). All unripe green banana flour extracts used in this study were analyzed using the Liquid Chromatography-Mass Spectrometry-Electrospray Ion (LC-MS-ESI) (Jannoey et al., 2021). The results revealed that phytochemical constituents of unripe banana flour extraction were found to be different due to the polarity of solvent extraction. From the analysis, the major components such as malic acid, citric acid, lactic acid, fructose derivative, dimethylmalonic acid, and fatty acid derivatives were found in all extractants. Furthermore, phytosphingosine, 2,4-dimethylpimelic acid, C16-sphinganine, and piperine were also detected in methanolic, ethanolic, and hexane extracts. While phenolic compounds such as gallic acid, glutaric acid, 2-hydroxyvalericacid, protocatechuic acid and 1,4-ipomeadiol were only obtained in methanolic and ethanolic extracts. Additionally, choline, 5,6-dimethoxyflavone, 9,12,13,trihode, and 2-hexyl-1,3-dioxan-5-ol, were uniquely found in the methanolic extract. Moreover, gluconic acid, succinic acid, 3-hydroxy-cis, cis-muconic acid, cis-aconitic acid, and choline were obtained when extracting with distillated water. From the LC-MS-ESI analysis (Jannoey et al., 2021), various phytochemicals were found in the extracts, and they may play a role for affecting 3T3-L1 adipocyte differentiation. For example, choline, which was detected in water and methanolic extracts, has been shown to slowly increased body weight gain, reduced fat mass, decreased plasma glucose level, and improved insulin tolerance in mice fed diet-containing choline (Wu et al., 2012). In addition, a recent study by Korsmo et al., 2020 revealed that excess adiposity was inhibited after choline supplementation for 6 weeks in high fat-fed mice. Moreover, choline was also noted to stimulate adipocyte lipolysis by activating specific lipases (e.g., ATGL, LPL, HSL); reduced the expression of lipogenic genes (e.g., FAS, SREBP-1c); and upregulated the genes for Fatty acid oxidation (e.g., CPT1, PPARα, PGC-1α) in mice (Schenkel et al., 2015; Taylor et al., 2017). This suggests that food-containing choline may be useful for regarding prevention or Treatment of obesity. Furthermore, piperine and gallic acid, which were found only in methanol, ethanol, and hexane extracts, have been shown to provide an anti-obesity effect (Brahmanaidu et al., 2014; Park et al., 2012; Diwan et al., 2013; Pandey et al., 2014; Doan et al., 2015; Hsu et al., 2006). From in vitro study, gallic acid was shown to inhibit pre-adipocyte proliferation (Hsu et al., 2006) and adipocyte differentiation (Pandey et al., 2014). A recent study by Park et al., 2019 reported that piperine markedly reduced intracellular lipid accumulation through inhibiting the expression of transcription factors, PPARγ, SREBP-1c, and C/EBPβ in 3T3-L1 cells. In addition, among various components, malic acid, which was found in all extractants, has been reported lipolytic activity via stimulating glycerol release in 3T3-L1 adipocytes (Duangjai et al., 2018). Therefore, from the present study, various phytochemicals comprised in unripe banana flour extracts may play a vital role in anti-adipogenesis ability through lowering intracellular lipid contents and inhibiting adipogenic gene expression in 3T3-L1 adipocytes. Although the expression level of lipolysis-related genes was not assessed in this study, some phytochemicals comprised in water and methanolic unripe banana flour extracts such as choline which has been shown to modulate lipolytic genes (Schenkel et al., 2015; Taylor et al., 2017) may influence anti-adipogenesis. Moreover, a study carried out by Sardá et al., 2016 demonstrated that unripe banana flour can decrease energy intake via modulating gastrointestinal hormones such as ghrelin and peptide YY hormones in human. Taken together, it suggests that unripe green banana flour may possibly use as a food supplement to prevent or treat obesity and highlight new perspectives on future pharmacological use. The biological and pharmacological activities of phytochemicals in unripe banana (Musa paradisiaca) flour (Iroaganachi et al., 2015) and methanolic extract of unripe banana (Ojewole and Adewunmi, 2003) have also been reported including decreasing blood glucose and body weight in diabetic rats. A recent study carried out by Escobar and Rocha-Gomes, 2020 indicated that unripe banana flour (Musa cavendishii) decreased weight in Wistar rats after ingestion 20% of unripe banana flour for 49 days. In addition, it has been shown that unripe banana extract (Musa sapientum L.) enhanced glucose uptake in yeast cells (Bhinge et al., 2017). From a recent study, the ethanolic and methanolic unripe banana flour extracts used in this study were shown antioxidation activity greater than those the other extractants (Jannoey et al., 2021).
CONCLUSION
The present in vitro study demonstrated the potential effects of unripe green banana flour extracts on anti-adipogenesis ability via lowering intracellular lipid contents and inhibiting the expression of adipogenesis-related genes (PPARγ, SREBP-1c and FAS) compared to control cells. In addition, from the recent LC-MS-ESI analysis (Jannoey et al., 2021), it was demonstrated that some phytochemicals comprised in unripe banana green flour extracts such as piperine, gallic acid, malic acid, and choline may play a vital role in anti-adipogenesis effects. Therefore, this study suggests that green banana flour may be used as a potential ingredient for beneficial health diets and may have further implication in in vivo anti-obesity effects.
ACKNOWLEDGEMENTS
The authors thank the Faculty of Medical Science, Naresuan University for providing instruments.
AUTHOR CONTRIBUTIONS
Wanida Chawnawa, Panatda Janneoy, and Suchada Phimsen assisted in conducting the experiments, performed the statistical analysis and data visualization. Tantip Boonsong designed and conducted all the experiments and wrote the manuscript. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Aller, E.E.J.G., Abete, I., Astrup, A., Martinez, J.A., and van Baak, M.A. 2011. Starches, sugars and obesity. Nutrients. 3: 341–369.
Aparicio-Saguilan, A., Sayago-Ayerdi, S.G., Vargas- Torres. A., Tovar, J., Ascencio-Otero, T.E., and Bello-Perez, L.A. 2007. Slowly digestible cookies prepared from resistant starch-rich lintnerized banana starch. Journal of Food Composition and Analysis. 20: 175-181.
Apu, A.S., Chowdhury, F.A., Khatun, F., Jamaluddin, A.T.M., Pathan, A.H., and Pal, A. 2013. Phytochemical screening and in vitro evaluation of pharmacological activities of Aphanamixis polystachya (wall) parker fruit extracts. Tropical Journal of Pharmaceutical Research. 12: 111-116.
Apu, A.S., Muhit, M.A., Tareq, S.M., Pathan, A.H., Jamaluddin, A.T.M., and Ahmed, M. 2010. Antimicrobial activity and brine shrimp lethality bioassay of the leaves extract of Dillenia indica Linn. Journal of Young Pharmacists. 2: 50-53.
Bhinge, S.D., Bhutkar, M.A., Randive, D.S., Wadkar, G.H., and Hasabe, T.S. 2017. In vitro hypoglycemic effects of unripe and ripe fruits of Musa sapientum. Journal of Pharmaceutical Sciences. 53: 1-6.
Brahmanaidu, P., Nemani, H., Meriga, B., Mehar, S. K., Potana, S., and Ramgopalrao, S. 2014. Mitigating efficacy of piperine in the physiological derangements of high fat diet induced obesity in Sprague Dawley rats. Chemico-Biological Interactions. 221: 42–51.
Castillo, F., Gonza´lez, D.R., and Moore-Carrasco, R. 2019. Effects of phaseolus vulgaris extract on lipolytic activity and differentiation of 3T3-L1 preadipocytes into mature adipocytes: A strategy to prevent obesity. Journal of Nutrition and Metabolism. 1-8.
Coelho, M., Oliveira, T., and Fernandes, R. 2013. Biochemistry of adipose tissue: An endocrine organ. Archives of Medical Science. 9: 191-200.
Cordenunsi, B.R. and Lajolo, F.M. 1995. Starch breakdown during banana ripening: Sucrose synthase and sucrose phosphate synthase. Journal of Agricultural and Food Chemistry. 43: 347–351.
Dayib, M., Larson, J., and Slavin, J. 2020. Dietary fibers reduce obesity-related disorders: Mechanisms of action. Current Opinion in Clinical Nutrition and Metabolic Care. 23: 445-450.
Diwan, V., Poudyal, H., and Brown, L. 2013. Piperine attenuates cardiovascular, liver and metabolic changes in high carbohydrate, high fat-fed rats. Cell Biochemistry and Biophysics. 67: 297–304.
Doan, K.V., Ko, C.M., Kinyua, A.W., Yang, D.J., Choi, Y-H., Oh, I.Y., Nguyen, N.M., Ko, A., Choi, J.W., Jeong, Y., Jung, M.H., Cho, W.G., Xu, S., Park, K.S., Park, W.J., Choi, S.Y., Kim, H.S., Moh, S.H., and Kim, K.W. 2015. Gallic acid regulates body weight and glucose homeostasis through AMPK activation. Endocrinology. 156: 157–168.
Duangjai, A., Nuengchamnong, N., Suphrom, N., Trisat, K., Limpeanchob, N., and Saokaew, S. 2018. Potential of coffee fruit extract and quinic acid on adipogenesis and lipolysis in 3T3-L1 adipocytes. Kobe Journal of Medical Sciences. 64: 84-92.
Escobar, A. and Rocha-Gomes, A. 2020. Unripe banana flour (Musa cavendishii) promotes decrease in weight gain and elimination of fecal cholesterol in Wistar rats. Nutrition & Food Science. 50: 157-167.
Flores-Silva, P. C., Tovar, J., Reynoso-Camacho, R., and Bello-Pérez, L. A. 2017. Impact of chickpea- and raw plantain-based gluten-free snacks on weight gain, serum lipid profile, and insulin resistance of rats fed with a high-fructose diet. Cereal Chemistry. 94: 124-127.
Giri, D.P. and Rajbhandari, M. 2018. Phytochemical analysis and constituents of hexane extract of Melastoma Malabathricum L. Journal of Institute of Science and Technology. 3: 18-25.
Haslam, D.W. and James, W.P. 2005. Obesity. The Lancet. 366(9492): 1197-209.
Hernández-Nava, R. G., Berrios, J. D. J., Pan, J., Osorio-Díaz, P., and Bello-Pérez, L. A. 2009. Development and characterization of Spaghetti with high resistant starch content supplemented with banana starch. Food Science and Technology International. 15: 73–78.
Ho, J.N., Son, M.E., Lim, W.C., Lim, S.T., and Cho, H.Y. 2012. Anti-obesity effects of germinated brown rice extract through down-regulation of lipogenic genes in high fat diet-induced obese mice. Biosci. Biotechnol. Biochem. 76: 1068-1074.
Hsu, C.L., Huang, S.L., and Yen, G.C. 2006. Inhibitory effect of phenolic acids on the proliferation of 3T3-L1 preadipocytes in relation to their antioxidant activity. Journal of Agricultural and Food Chemistry. 54: 4191−4197.
Iloki‑Assanga, S.B., Lewis‑Luján, L.M., Lara‑Espinoza, C.L., Gil‑Salido, A.A., Fernandez‑Angulo, D., Rubio‑Pino, J.L., and Haines, D.D. 2015. Solvent effects on phytochemical constituent profiles and antioxidant activities, using four different extraction formulations for analysis of Bucida buceras L. and Phoradendron californicum. BMC Research Notes. 8: 1-14.
Iroaganachi, M., Eleazu, C.O., Okafor, P.N., and Nwaohu, N. 2015. Effect of unripe plantain (Musa paradisiaca) and ginger (Zingiber officinale) on blood glucose, body weight and feed intake of Streptozotocin-induced diabetic rats. The Open Biochemistry Journal. 9: 1-6.
Jannoey, P., Channei, D., Boonsong, T. Pimsen, S., and Nueangjumnong, N. 2021. Phytochemical screening and Antioxidant activity of Unripe Banana flour. NU. International Journal of Science. 18: 80-102.
Juarez-Garcia, E., Agama-Acevedo, E., Sayago-Ayerdi, S.G., Rodriguez-Ambriz, S.L., and Bello-Perez, L.A. 2006. Composition, digestibility and application in breadmaking of banana flour. Plant Foods for Human Nutrition. 61: 131-137.
Khoozani, A.A., Kebede, B., Birch, J., and Bekhit, A-D. 2020. The Effect of bread fortification with whole green banana flour on its physicochemical, nutritional and in vitro digestibility. Foods. 9: 152.
Kim, H.J., Yoon, B.K., Park, H., Seok, J.W., Choi, H., Yu, J.H., Choi, Y., Song, S.J., Kim, A., and Kim, J-w. 2016. Caffeine inhibits adipogenesis through modulation of mitotic clonal expansion and the AKT/GSK3 pathway in 3T3-L1 adipocytes. BMB Rep. 49: 111-115.
Kim, H-Y., Kim, J.H., Zuo, G., and Lim, S.S. 2020. Anti-obesity effect of Melandrium firmum Rohrbach extract in 3T3-L1 cells and high-fat diet-induced obese C57BL/6N mice. Food Science & Nutrition. 8: 2251–2261.
Korsmo, H.W., Edwards, K., Dave, B., Jack-Roberts, C., Yu, H., Saxena, A., Salvador, M., Dembitzer, M., Phagoora, J., and Xinyin Jiang. 2020. Prenatal choline supplementation during high-fat feeding improves long-term blood glucose control in male mouse offspring. Nutrient. 12: 1-17.
Lewis, D.A., Fields, W.N., and Shaw, G.P. 1999. A natural flavonoid present in unripe plantain banana pulp (Musa sapientum L. 6ar. paradisiaca) protects the gastric mucosa from aspirin-induced erosions. Journal of Ethnopharmacology. 65: 283–288.
Liou, C.J., Lai, X.Y., Chen, Y.L., Wang, C.L., Wei, C.H., and Huang, W.C. 2015. Ginkgolide C suppresses adipogenesis in 3T3-L1 adipocytes via the AMPK signaling pathway. Evidence-Based Complementary and Alternative Medicine. 1-11.
Maity, S., Das, K., and Mandal, N. 2016. To investigate which solvent extract (aqueous, methanol, acetone and hexane) of Apple banana is more potent content of phytocompound. International Journal of Physiology, Nutrition and Physical Education. 1: 33-45.
Maki, C., Funakoshi-Tago, M., Aoyagi, R., Ueda, F., Kimura, M., Kobata, K., Tago, K., and Tamura, H. 2017. Coffee extract inhibits adipogenesis in 3T3-L1 preadipocytes by interrupting insulin signalling through the downregulation of IRS1. PLoS One. 12: 1-19.
Majhenic, L., Skerget, M., and Knez, Z. 2007. Antioxidant and antimicrobial activity of quarana seed extracts. Food Chemistry. 104: 1258-1268.
Mota, F.L., Queimada, A.J., Pinho, S.P., and Macedo, E.A. 2008. Aqueous solubility of some natural phenolic compounds. Industrial & Engineering Chemistry Research. 47: 5182–5189.
Murase, T., Nagasawa, A., Suzuki, J., Hase, T., and Tokinitsu, I. 2002. Beneficial effects of tea catechins on diet induced obesity: stimulation of lipid metabolism in the liver. International Journal of Obesity and Related Metabolic Disorders. 26: 1459-1464.
Ojewole, J.A. and Adewunmi, C.O. 2003. Hypoglycemic effect of methanolic extract of Musa paradisiaca (Musaceae) green fruits in normal and diabetic mice. Methods and Findings in Experimental and clinical Pharmacology. 25: 453-456.
Pandey, A., Bani, S., and Sangwan, P.L. 2014. Anti-obesity potential of gallic acid from Labisia pumila, through augmentation of adipokines in high fat diet induced obesity in C57BL/6 mice. Advances in Research. 2: 556-570.
Park, U. H., Jeong, H. S., Jo, E. Y., Park, T., Yoon, S. K., Kim, E. J., Jeong, J.-C., and Um, S.-J. 2012. Piperine, a component of black pepper, inhibits adipogenesis by antagonizing PPARγ activity in 3T3-L1 cells. Journal of Agricultural and Food Chemistry. 60: 3853–3860.
Park, U.H., Hwang, J.K., Youn, H., Kim, E.J., and Um, S.J. 2019. Piperine inhibits adipocyte differentiation via dynamic regulation of histone modifications. Phytotherapy Research. 33: 2429-2439.
Pheiffer, C, Dudhia, Z., Louw, J., Muller, C., and Joubert, E. 2013. Cyclopia maculata (honeybush tea) stimulates lipolysis in 3T3-L1 adipocytes. Phytomedicine. 20: 1168-71.
Raju, G.S., Moghal, M.M.R., Dewan, S.M.R., Amin, M.H., and Billah, M.M. 2013. Characterization of phytoconstituents and evaluation of total phenolic content, anthelmintic, and antimicrobial activities of Solanum violaceum Ortega. Avicenna Journal of Phytomedicine 3: 313-320.
Rodgers, R.J., Tschöp, M.H., and Wilding, J.P.H. 2012. Anti-obesity drugs: Past, present and future. Disease Models & Mechanisms. 5: 621-626.
Sardá, F.A.H., Giuntini, E.B., Gomez, M.L.P.A., Lui, M.C.Y., Negrini, J.A.E., Tadini, C.C., Lajolo, F.M., and Menezes, E.W. 2016. Impact of resistant starch from unripe banana flour on hunger, satiety, and glucose homeostasis in healthy volunteers. Journal of Functional Foods. 24: 63–74.
Sarnali, T.T. and Moyenuddin, P.K. 2010. Obesity and disease association: A review. Anwer Khan Modern Medical College Journal. 1: 21-24.
Schenkel, L.C., Sivanesan, S., Zhang, J., and Wuyts, B. 2015. Choline supplementation restores substrate balance and alleviates complications of Pcyt2 deficiency. The Journal of Nutritional Biochemistry. 26: 1221–1234.
Sidhu, J.S. and Zafar, T.A. 2018. Bioactive compounds in banana fruits and their health benefits. Food Quality and Safety. 2: 183-188.
Taylor, A., Schenkel, L.C., Yokich, M., and Bakovic, M. 2017. Adaptations to excess choline in insulin resistant and Pcyt2 deficient skeletal muscle. Biochemistry and Cell Biology. 95: 223–231.
Truong, D-H., Nguyen, D.H., Ta, N.T.A., Bui, A.V., Do, T.H., and Nguyen, H.C. 2019. Evaluation of the use of different solvents for phytochemical constituents, antioxidants, and in vitro anti-Inflammatory activities of Severinia buxifolia. Journal of Food Quality.
Tsuda T. 2008. Regulation of adipocyte functions by anthocyanins: possibility of preventing the metabolic syndrome. Journal of Agricultural and Food Chemistry. 56: 642-646.
Utrilla-Coello, R.G., Agama-Acevedo, E., Osorio-Díaz, P., Reynoso-Camacho, R., and BelloPérez, L.A. 2013. Glycemic response in healthy rats fed with composite cereal bars. Starch – Stärke. 65: 354–359.
Wu, G., Zhang, L., Li, T., Lopaschuk, G., Vance, D.E., and Jacobs, R.L. 2012. Choline deficiency attenuates body weight gain and improves glucose tolerance in ob/ob Mice. Journal of Obesity. 1-7.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand.
Tantip Boonsong*, Wanida Chawnawa, Panatda Janneoy, and Suchada Phimsen
Faculty of Medical Science, Naresuan University, Phitsanulok 65000, Thailand.
Corresponding author: Tantip Boonsong, E-mail: tantipb@nu.ac.th
Total Article Views
Editor: Wasu Pathom-aree,
Chiang Mai University, Thailand
Article history:
Received: May 1, 2022;
Revised: October 7, 2022;
Accepted: October 20, 2022;
Published online: November 24, 2022

