
The Antioxidant Activity and the Anti-inflammatory Effect of Citrus sinensis L. Fruit on Intestinal Inflammation Induced by Hyperhomocysteinemia in Mice
Sara Khelfi, Sakina Zerizer*, Amina Foughalia, Souraya Tebibel, Chawki Bensouici, and Zahia KabouchePublished Date : 2023-01-05
DOI : https://doi.org/10.12982/NLSC.2023.009
Journal Issues : Number 1, January-March 2023
Abstract Hyperhomocysteinemia is considered to be one of the risk factors for inflammatory bowel disease (IBD), it is a chronic, relapsing, and remittent inflammatory disease of the gastrointestinal tract. Citrus sinensis L. has been used traditionally to treat bowel disorders. The present study aims to quantify the phenolic and flavonoid contents of the Citrus sinensis L. fruit (ORF) extract and to evaluate in vitro the antioxidant activity and the anti-inflammatory effect of ORF in vivo on intestinal inflammation induced by hyperhomocysteinemia. The total phenolic and flavonoid contents of the extract were determinated using the spectrophotometric method and the evaluation of antioxidant activity was performed by four methods: DPPH, ABTS, CUPRAC, and reducing power. The inflammatory marker (plasma homocysteine), the reduced glutathione (GSH) content in the liver tissue were measured and the histological sections of the intestines of the mice used were examined to assess the anti-inflammatory activity of ORF. The results of this study showed that the ethanolic extract of ORF possessed high phenolic content and exhibited good antioxidant activity. The use of ORF powder in the in vivo study showed an increase in GSH levels, a decrease in plasma homocysteine levels, and a restoration of the integrity of the intestinal epithelium.
Keywords: Hyperhomocysteinemia, Intestinal Inflammation, Citrus sinensis L. fruit, Antioxidant activity, Anti-inflammatory
Citation: Khelfi, S., Zerizer, S., Foughalia, A., Tebibel, S., Bensouici, C., and Kabouche, Z. 2023. The antioxidant activity and the anti-inflammatory effect of Citrus sinensis L. fruit on intestinal inflammation induced by hyperhomocysteinemia in mice. Nat. Life Sci. Commun. 22(1): e2023009.
INTRODUCTION
Homocysteine (Hcy) is a non-protein sulfur amino acid derivative of demethylated methionine, the accumulation of which may be caused by genetic defects or vitamin B deficiency. A serum level above 15 μmol/L is defined as hyperhomocysteinemia (HHcy) (Ledda et al., 2020; Moretti et al., 2021). HHcy, activate NFκB, a transcription factor that regulates the transcription of various genes involved in inflammatory and immune responses inducing the increase of pro-inflammatory cytokines and down-regulation of anti-inflammatory cytokines. Moreover, high levels of Hcy have been detected in various inflammatory diseases such as inflammatory bowel disease (Elsherbiny et al., 2020; Al Mutairi, 2020).
Inflammatory Bowel Disease (IBD), including Crohn's disease (CD) and ulcerative colitis (UC), is a group of disorders involving alterations in gastrointestinal physiology and chronic inflammation of the mucous membranes (Gampierakis et al., 2021; Lohning et al., 2021)
Previous studies have confirmed that HHcy is a risk factor associated with cardiovascular disease and possibly an important independent risk factor for inflammatory bowel disease (Vezzoli et al., 2020; Chen et al., 2021).
Inflammation and oxidative stress are closely related to pathophysiological events (Pepe et al., 2018). Oxidative stress results from an imbalance between an excess production of reactive oxygen species and antioxidant defenses, which has been linked to numerous pathologies (Liguori et al., 2018; Hayes et al., 2020).
Citrus sinensis L., known as orange or sweet orange belongs to the Rutaceae family, which is the most cultivated and most traded species in the world (Sathiyabama et al., 2018; Juibary et al., 2021). C. sinensis is an excellent source of secondary metabolites which have been identified in the fruits, peel, leaves, juice, and roots that contribute to the pharmacological activities attributed to this plant (Favela-Hernández et al., 2016). Eminently, Citrus sinensis L. fruit (ORF) is a rich source of vitamin C known to have beneficial effects on health (Liu et al., 2012; Oikeh, 2020).
C. sinensis identified metabolites include: flavonoids, steroids, hydroxyamides, alkanes and fatty acids, coumarins , peptides, carbohydrates, carbamates, alkylamines, carotenoids, volatile compounds, and nutritional elements such as potassium, magnesium, calcium and sodium (Grosso et al., 2013; Favela-Hernández et al., 2016). Additional nutrients are shown in Table 1 (Etebu and Nwausoma, 2014).
Table 1. Nutrient composition of Citrus sinensis.
|
Composition |
Amount |
Composition |
Amount |
|
Energy |
197 kJ (47 kcal) |
Vitamin B6 |
0.06 mg (5%) |
|
Sugars |
9.35 g |
Folate (vit. B9) |
30 μg (8%) |
|
Dietary fiber |
2.4 g |
Choline |
8.4 mg (2%) |
|
Protein |
0.94 g |
Vitamin C |
53.2 mg (64%) |
|
Fat |
0.12 g |
Vitamin E |
0.18 mg (1%) |
|
Water |
86.75 g |
Calcium |
40 mg (4%) |
|
Vitamin A equiv. |
11 μg (1%) |
Iron |
0.1 mg (1%) |
|
Thiamine (vit. B1) |
0.087 mg (8%) |
Magnesium |
10 mg (3%) |
|
Riboflavin (vit. B2) |
0.04 mg (3%) |
Manganese |
0.025 mg (1%) |
|
Niacin (vit. B3) |
0.282 mg (2%) |
Phosphorus |
14 mg (2%) |
|
Pantothenic acid |
(B5) 0.25 mg (5%) |
Potassium |
181 mg (4%) |
|
- |
- |
Zinc |
0.07 mg (1%) |
The nutritional benefits of citrus fruit consumption on human health are well demonstrated (Campone et al., 2020). C. sinensis has been used in traditional medicine to treat medical conditions such as constipation, cramps, colic, diarrhea, bronchitis, tuberculosis, cough, cold, menstrual disorder, angina, hypertension, anxiety, depression and stress (Favela-Hernández et al., 2016; Bentahar et al., 2020). In addition, these fruits are used also to treat bowel disorders, respiratory disorders, cardiovascular disease, and stress (Mannucci et al., 2018). The genus Citrus has been recognized for its antibacterial, antioxidant, and anti-inflammatory properties (Huang et al., 2010; Nawrin et al., 2021). In addition, it has demonstrated a protective role against oxidative stress and gastric ulcer (Selmi et al., 2017; Nawrin et al., 2021).
Several studies have asserted that Citrus fruits and their derivatives can be effective in the prevention or treatment of inflammatory diseases such as IBD (Ferlazzo et al., 2016; Musumeci et al., 2020).
Studies have demonstrated the beneficial effects of Citrus and other extracts on different experimental models of colitis (Impellizzeri et al., 2015; Abe et al., 2018), whereas others have questioned their real applicability (Farzaei et al., 2015), emphasizing the need for further investigation.
In this perspective, considering the content of ORF in biologically active constituents, the present study aims to provide new research on the possible mechanism of action of the protective effect of C. sinensis fruit against experimental intestinal inflammation caused by L-methionine-induced hyperhomocysteinemia in a mouse model to confirm its pharmacological use as a remedy for IBD.
MATERIALS AND METHODS
Reagents and solvent
Folin-Ciocalteu reagent, sodium carbonate, aluminium nitrate, potassium acetate, 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2'-azino-bis (3-ethylbenzothiazoline6sulfonicacid) diammonium salt (ABTS), butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), α-tocopherol, ethanol, methanol. Ethylenediaminetetraacetic acid (EDTA), 5,50-dithiobis (2- nitrobenzoic) acid (DTNB), 5-sulfosalicylic acid, potassium persulfate, copper (II) chloride, neocuproine, ammonium acetate, trichloroacetic acid, ferric chloride, potassium ferricyanide, 2-Amino-2-hydroxymethyl-propane-1, L- methionine. All chemicals were purchased from Sigma Aldrich, Merck and Fluka Chemika.
Extraction and preparation of samples
The ripe of Citrus sinensis L. fruits were obtained from the local market in Constantine (North-East Algeria) in November 2019. The fruits were fresh, of taste quality, and free from damage. Two methods of ORF sample preparation were used. The fruits were washed, peeled with a knife, then divided into small pieces, then ground before being macerated with the mixture of ethanol (80%) for 48 hours at room temperature. The solvent was removed under reduced pressure using a rotor evaporator (Buchi R-215) at 40°C. The extract obtained was stored in an airtight bottle for use in vitro experiments. A portion of crushed ORF was frozen for two days and dried using a laboratory freeze-dryer (ALPHA 1-4 LD plus, Martin Christ) then made into a fine powder and kept in an airtight flask for use in the in vivo experiment.
The determination of total phenolic and flavonoid contents and evaluation of the antioxidant activity
Determination of total phenolic content (TPC)
The determination of polyphenols in the extract using the Folin-Ciocalteu reagent (Singleton and Rossi, 1965) according to a microplate assay method described by Muller et al. (2010) with some modifications. A volume of 20 μL of ORF extract (1 mg/mL) was mixed with 100 μL of Folin-Ciocalteu reagent (2 N) and 75 μL of sodium carbonate (7% w/v) in each well of the microplate. The mixture was incubated in the dark at room temperature for 2 h. The absorbance was measured at 740 nm in the microplate reader (Perkin Elmer Enspire, Singapore). Gallic acid was used as a standard for the calibration curve and construction of a linear regression line. Results are expressed as μg of gallic acid equivalent (GAE)/mg extract.
Determination of total flavonoid content (TFC)
TFC of the extract was determined using the modified method described by (Topçu et al., 2007). Briefly, 50 μL of ORF extract (1 mg/mL) was added to 10 μL of 10% aluminum nitrate, 10 μL of 1 M potassium acetate, and 130 μL of methanol. The mixture was then allowed to stand for 40 min at room temperature. The absorbance was measured at 415 nm. TFC is expressed as quercetin equivalent (QE)/mg extract.
DPPH free radical scavenging assay
The scavenging activity of the extract against DPPH • radical was measured according to the modified method of Blois (1958). 160 µL of the DPPH solution (6 mg DPPH dissolved in 100 mL of methanol) was added to 40 µL of ORF extract at different concentrations in all wells. The mixture was incubated in dark for 30 min at room temperature before taking the absorbance readings at 517. DPPH solution in methanol was used as a control. α-tocopherol, BHT, and BHA are used as antioxidant standards.
The results are expressed as IC50 (µg/mL). IC50 value corresponding to the concentration of the extract, which inhibits 50% of the radical DPPH, and the % inhibition was calculated by applying the following formula:
Inhibition (%) = [(A Control − A Sample) ∕ A Control] × 100
A Control: absorbance of the control; A sample: absorbance of the extract or standard.
ABTS scavenging activity assay
The ABTS scavenging activity was determined according to the modified method of Re et al. (1999). In all wells, a mixture of 160 μL of diluted ABTS•+ solution (7 mM ABTS in water and 2.45 mM potassium persulfate, stored in the dark at room temperature for 12 h) was added to 40 μL of ORF extract in ethanol at different concentrations. The absorbance was measured at 734 nm after 10 min. ABTS•+ solution in ethanol was used as a control, BHT and BHA are used as antioxidant standards.
The results were given as IC50 value (mg/mL) corresponding the concentration of 50% inhibition using the following equation:
Inhibition (%) = [(A Control − A Sample) ∕ A Control] × 100
A Control: absorbance of the control; A sample: absorbance of the extract or standard.
Cupric reducing antioxidant capacity (CUPRAC)
The cupric reducing antioxidant capacity was measured according to Apak et al. (2004). A volume of 50 μL of copper (II) chloride solution (10 mM), 50 μL neocuproine (7.5 mM), and 60 μL ammonium acetate buffer (1 M, pH 7.0) solutions were added to each well. Then, 40 μL of ORF extract at different concentrations was added to the mixture. The absorbance was measured at 450 nm after 1h. The results were calculated as A0.5 (µg/mL) corresponding to the concentration indicating 0.50 absorbance. BHT and BHA are used as antioxidant standards.
Reducing power assay
The reducing power activity was determined according to the modified method by Oyaizu (1986). A mixture of 10 μL of ORF extract at various concentrations, 40 μL of 0.2 M phosphate buffer and 50 μL of potassium ferricyanide (1%) were added to each well of the plate and incubated for 20 min at 50°C in the water bath. After 50 μL of 10% trichloroaceticacid (TCA) and 10 μL of ferric chloride (0.1%) were added to the mixture and completed with 40 μL of distilled water. The absorbance was read at 700 nm. Ascorbic acid and α-tocopherol were used as standards. A0.5 values were calculated from the absorbance curves.
Evaluation of anti-inflammatory effect of Citrus sinensis L. fruit in vivo
Anti-inflammatory activity was tested in vivo, evaluating the effect of
C. sinensis fruits on homocysteine levels during L-methionine-induced intestinal inflammation in 28 adult male Mus musculus weighing (20–35 g). The mice were obtained from the Faculty of Pharmacy, University Constantine, Algeria. They were maintained under standard laboratory conditions with free access to water and food. The animal study was conducted according to the procedure of the research project code number (F00920140076) obtained from our Ministry of Scientific Research, Algeria, and the ethical principles and guidelines provided by the committee for the purpose of control and supervision of the experiments on animals (CPCSEA). Each treatment dose was prepared in distilled water and administered by oral gavage for 21 consecutive days at a dose of (200 mg/kg/day). The animals were divided into four experimental groups: the control group (C) received only distilled water; the second group (M) received (200 mg/kg) of L-methionine; the third group (OM) received (200 mg/kg) of L-methionine and was treated with (200 mg/kg) of ORF powder; the last group (O) received (200 mg/kg) of ORF powder only. The blood samples were taken after 21 days of treatment, it was immediately centrifuged for 15 minutes at 3,000 /rpm. Plasma homocysteine values are measured by immunoassay using the analyzer (IMMULITE 2000 XPi) and the homocysteine values were expressed in (µmol/L).
Reduced Glutathione assay (GSH)
Preparation of the homogenate
The animals were sacrificed and 0.5 g of liver from each mouse was homogenized in a volume of 2 mL of TBS (50 mM Tris, 150 mM NaCl, pH 7.4). The obtained homogenates were centrifuged for 15 min at 4°C at 9,000 g and the supernatant was used for the measurement of glutathione reduced (GSH).
GSH measurement
Reduced glutathione (GSH) concentration was performed according to Sakhri et al. (2021) method. First 800 μL of the liver homogenate was added to 200 μL of the sulfosalicylic acid (0.25%) solution, and then the mixture was incubated in an ice bath for 15 min. Next, centrifugation at 1000 tours/min for 5 min was realized. After that, 500 μL of the supernatant and 1 ml of the buffer Tris-EDTA (pH 9.6) were added to 25 μL ml of DTNB of (0.01 M) and after 5 min the absorbance was measured at 412. The GSH concentration was expressed as nmol of GSH nmol/mg protein.
Histological examination
Intestine fragments were removed after sacrifice the mice and were fixed in 10% formalin, sections were stained with hematoxylin-eosin and examined under a digital photographic microscope (OPTECH MICROSCOPE).
Statistical analyses
All results are given as mean ± standard errors of the mean (S.E.M). The in vitro experiments were performed in triplicate. The data from in vivo study was analyzed by SPSS 20.0 statistics software with one-way ANOVA followed by Turkey’s post hoc test for multiple comparisons and P < 0.05 was considered as statistically significant.
RESULTS
The determination of total phenolic and flavonoid contents and the antioxidant activity of Citrus sinensis L. fruit extract
The contents of total phenol and flavonoid in the extract were quantified. The results showed that the ethanolic extract of ORF exhibited the highest total phenolic content (173.41 ± 3.06µg GAE/mg extract), the extract gave the value of 17.01 ± 0.96µg QE/mg for the total flavonoidcontent. The results are shown in Table 2.
Table 2. Antioxidant activity of Citrus sinensis L. fruit extract.
|
Activity |
TPC (μg GAE/mg extract) |
TFC (μg QE/mg extract) |
|
ORF extract |
173.41 ± 3.06 |
17.41 ± 0.96 |
Note: Values are expressed as means ± SEM of three parallel measurements.
The evaluation of the antioxidant activity of the ORF extract showed the strongest antioxidant activity in the ABTS and in the reducing power assays with (IC50 = 76.27 ± 0.07 µg/mL and A0.5 = 80.16 ± 0.89 µg/mL) respectively. According to the Table 3, the extract showed good activity in DPPH (153.48 ± 0.98 µg/mL) and in the CUPRAC (161.81 ± 1.50 µg/mL).
Table 3. Total phenolic and flavonoid contents of Citrus sinensis L. fruit extract.
|
|
DPPH assay
|
ABTS assay |
CUPRAC assay |
Reducing power assay |
|
|
IC50 (µg/mL) |
A0.5 (µg/mL) |
||
|
ORF extract |
153.48 ± 0.98 |
76.27 ± 0.07 |
161.81 ± 1.50 |
80.16 ± 0.89 |
|
BHA |
6.14 ± 0.41 |
5.35 ± 0.71 |
5.35 ± 0.71 |
NT |
|
BHT |
12.99 ± 0.41 |
1.29 ± 0.30 |
8.97 ± 3.94 |
NT |
|
α-Tocopherol |
12.99 ± 0.41 |
NT |
NT |
34.93 ± 2.38 |
|
AA |
NT |
NT |
NT |
6.77 ± 1.15 |
Note: Values were expressed as means ± (n=3).
The anti-inflammatory effect of Citrus sinensis L. fruit powder
The results of this study showed that there is a significant difference in the concentration of homocysteine between groups (C, M, OM and O) at P = 0.01. In addition, we have detected that the level of homocysteine has decreased significantly in goup (O) when it is compared with group (M) administered by L- methionine, and the concentration of the homocysteine in the group (M) is increased significantly when it is compared to the control group (P <0.05). On the other hand, we have obtained that the level of homocysteine is decreased in group (OM) but not significantly (Figure 1).
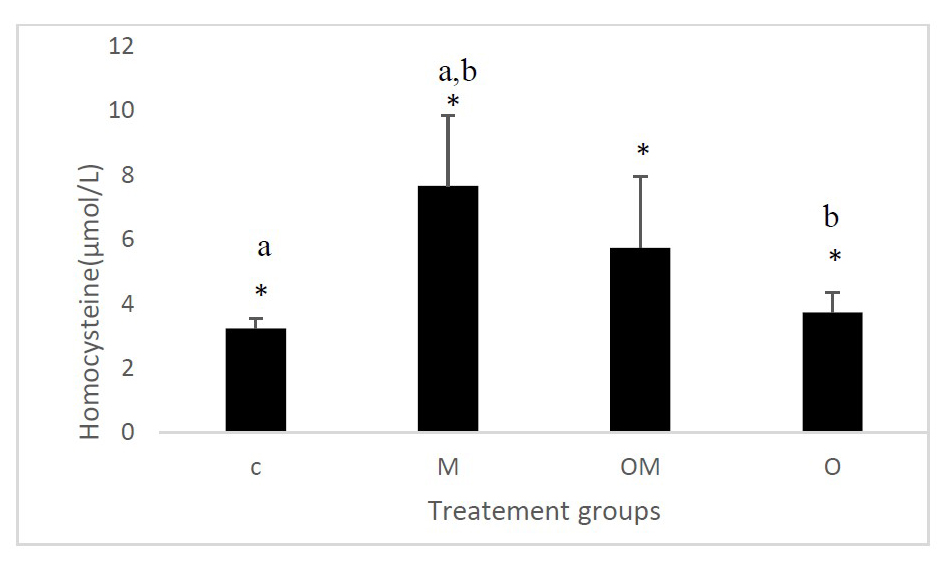
Figure 1. Effect of Citrus sinensis L. fruit powder on homocysteine values
Results are shown as mean ± S.E.M (n = 4) and significant between groups is shown as *P = 0.01. The letters a and b indicate a significant difference between (C, M) and (M, O) respectively.
(C): control group; (M): group administered with L-methionine (200 mg/kg); (OM): group administered with L- methionine (200 mg/kg) and treated with ORF (200 mg/kg); (O): group treated with ORF (200 mg/kg).
Effect of Citrus sinensis L. fruit powder on glutathione reducing values
Our results showed that there are very highly significantly in the GSH values between groups at P =0.001 (Figure 2). The Tukey test showed that the GSH values was decreased highly and significantly in group (M) when it is compared to the groups (C and OM) (P < 0.01) and significantly when it is compared to the group (O) (P < 0.05).
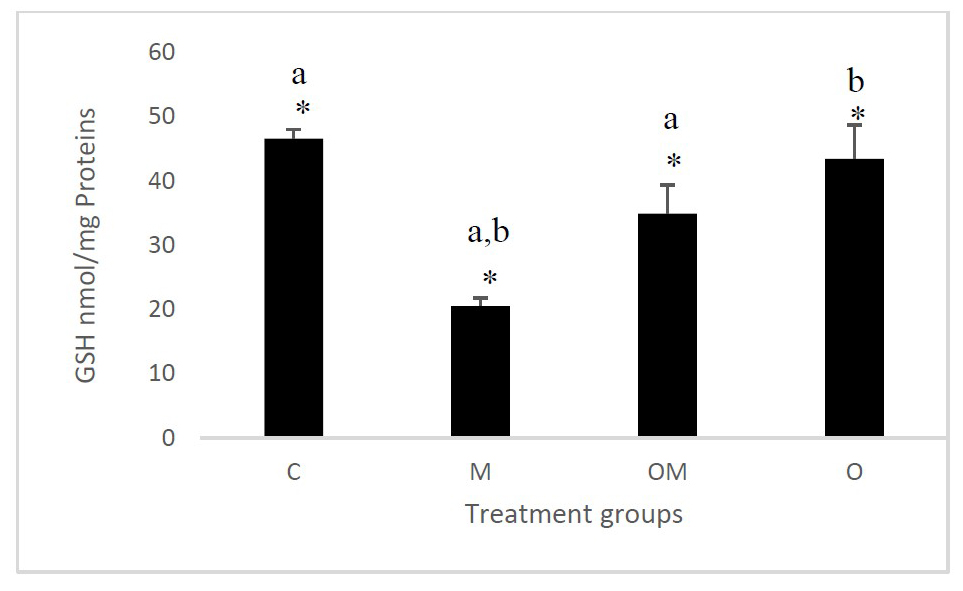
Figure 2. Effect of Citrus sinensis L. fruit powder on glutathione reducing values.
Results are shown as mean ± S.E.M (n = 4) and significant between groups is shown as *P = 0.001. The letters a and b indicate a significant difference between the groups.
(C): control group; (M): group administered with L-methionine (200 mg/kg); (OM): group administered with L- methionine (200 mg/kg) and treated with RCF (200 mg/kg); (O): group treated with RCF (200 mg/kg).
Histological estimation
The results obtained from the histological study of the intestines of the experimental groups are represented in figure 3. The groups (C) and (O) showed normal architecture of the intestinal membrane (Figures 3 A and D). Whereas in the group (M) which have administered with L-methionine (200 mg/kg), showed a granuloma with significant lymphocytic infiltration and degeneration in the enterocytes membrane cells (Figures 3 B). However, the group animal (OM) showed a normal structure with distinct villi, markedly reduced lymphocyte infiltration, and restoration of enterocyte membrane cell integrity (Figure 3 C).
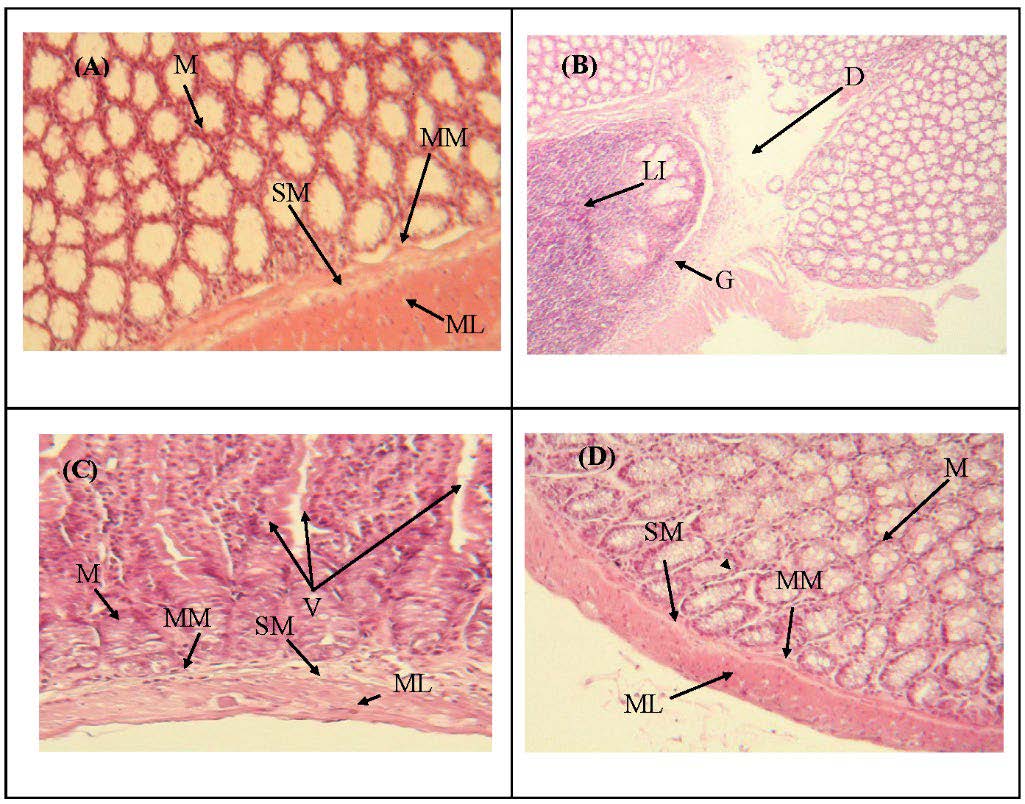
Figure 3. Histological sections of intestine of (A) received distilled water, (B) administered with L-methionine (200 mg/kg), (C) administered with L- methionine (200 mg/kg) and treated with ORF powder (200 mg/kg), (D) received ORF powder (200 mg/kg). Hematoxylin - eosin staining (A, B, C and D × 100). Mucosa (M), Muscularis Mucosa (MM), Submucosa (SM), Muscularis (ML), Granuloma (G), Degeneration (D), Lymphocytic Infiltration (LI), Villi (V).
DISCUSSION
The ORF extract revealed a high content of total phenolic with a moderate quantity of total flavonoids. Our results are in agreement with Campone et al. (2020) and AbdGhafar et al. (2021).
Antioxidant capacity tests can be broadly classified as tests based on electron transfer (ET) and hydrogen atom transfer (HAT). ET based tests measure an antioxidant's ability to reduce an oxidant, which changes color when reduced (Apak et al., 2007). In vitro antioxidant activities were measured in ethanol extract obtained using ABTS, DPPH, CUPRAC, and reducing power assays.
DPPH• is a free radical that accepts electrons or hydrogen radicals from donor compounds. On the other hand, ABTS• assay based on the inhibition of the formation of ABTS• by one-electron oxidants (Sridhar and Charles, 2018). The results showed that the ethanolic extract of C. sinensis fruits exhibited the highest activity in the ABTS assay and good activity against the DPPH assay. Our results are in agreement with the study of (Campone et al., 2020).
The reducing power is based on the capacity of substances, which have reduction potential, react with potassium ferricyanide to form potassium ferrocyanide, which then reacts with ferric chloride to form a ferric complex (Singhal et al., 2014). The extract showed the strongest reducing power, this agrees with the study of Bentahar et al. (2020) who showed that the ethanolic extract of C. sinensis fruits exhibited a strong reducing power. CUPRAC method is one of the most widely used antioxidant methods. It is based on the reduction of the copper (II) -neocuproine to copper (I) -neocuproine chelate complex (Akar and Burnaz, 2019). Our data showed that the extract exhibited a good copper reducing antioxidant power.
The above results may be due to the richness of ORF extract in total phenolic and the presence of flavonoids which have the principal contribution to the antioxidant capacity of extract. Previous studies showed that a good correlation significant was found between antioxidant activity and polyphenols and flavonoids contents (Canan et al., 2016; Bentahar et al., 2020).
Glutathione reductase (GR) is an important antioxidant enzyme essential for maintaining the GSH / GSSG ratio by catalyzing the recovery of reduced glutathione (GSH) from oxidized glutathione (GSSG) (Güller et al., 2021; Robbins et al., 2021).
In the present study, treatment with C. sinensis fruit and L-methionine showed a significant increase in the level of hepatic GSH, however, it is suggested that C. sinensis fruits increased glutathione reductase enzymatic activity and GSH levels and enhanced ROS scavenging capacity by inhibiting ROS over expression and oxidative damage.
Abdelghffar et al. (2021) worked on the extract of orange fruit peel (Citrus sinensis) and reported that the extract revealed increased GSH levels in tissue homogenates and confirmed its protective efficacy against chemotherapy-induced toxicity in male rats.
Our research confirmed that the treatment with ORF powder significantly reduced plasma homocysteine levels in animals administered with high dose of L-methionine. Numerous studies have confirmed that Citrus fruits have an anti-inflammatory potential (Leguizamón et al., 2019; Denaro et al., 2021) and inhibited NF-κB (Impellizzeri et al., 2015).
Citrus fruits are a good source of vitamin C, folate, vitamins B6 and, flavonoids (Rauf et al., 2014; Rao et al., 2021). Through this, their consumption has beneficial effects on human health due to the antioxidant and anti-inflammatory properties (Ma et al., 2020; Rao et al., 2021) and their gross use reduces the risk of gastric and colorectal diseases (Roussos et al., 2011; Rauf et al., 2014).
Previous work links increased levels of homocysteine to IBD, indicating a major role for vitamin B deficiency in intestinal damage and inflammation (Lurz et al., 2020). We suggest that ascorbic acid, flanovoids and phenolic contents prevent the oxidation process thereby protecting the intestine from damage caused by ROS and vitamin B supplementation provided by C sinensis corrected the deficiency of B vitamins resulting in lower levels of homocysteine.
Activation of the intestinal immune system and recruitment of inflammatory cells in the intestine help maintain inflammation and damage in the intestines (Wera et al., 2016). Histological examination revealed that the treatment with ORF powder restored the integrity of the intestine and the histopathological changes caused by the elevated homocysteine levels. Khan et al. (2016) reported that there is a marked reduction in histopathological damage and a protective role against inflammation in intestinal tissue in rat colitis treated with Citrus sinensis L.
The study of Gholap et al. (2012) also reported that C. sinensis fruit peel extract is effective in the treatment of UC in mice who showed less ulceration in histopathological observation. On the basis of these results, we can confirm that C. sinensis fruits exhibit protective role against intestinal inflammation.
A study carried out by Fusco et al. demonstrated that Orange juice decreased oxidative stress and inflammatory response in a murine model of IBD (Fusco et al., 2017).
Gholap et al. (2012) reported that the combination of Moringa oleifera root and a peel extract of Citrus sinensis fruit is effective in mice with acetic acid (AA)-induced UC, by decreasing malondialdehyde (MDA) content and myeloperoxidase (MPO) activity.
Khan and collaborators tested the effect of Citrus sinensis L., Citrus paradisi L. and their combinations in rats subjected to experimental trinitrobenzene sulfonic acid (TNBS)-induced colitis. By histological and biochemical analyses, they have found that these fruit juices exerted antioxidant and anti-inflammatory activities (Khan et al., 2016).
On the other hand, it was reported that the use of Citrus aurantium L. and its flavonoids have proved to exhibit anti-inflammatory activity, reduce weight loss and diarrhea as well as suppress isolated jejunal contraction in TNBS induced IBD rats (He et al., 2018).
Previous studies have confirmed that HHcy is a risk factor for several pathological disorders (Cordaro et al., 2021; Ji et al., 2022). On the grounds of this fact, we have planned to evaluate the impact of hyperhomocysteinemia induced by L- methionine to provoke intestinal inflammation and at the same time to estimate the protective ability of ORF, this work is an original never done before. Based on results, induced hyperhomocysteinemiahas showed significant intestinal alterations where the inflammatory process was well manifested. Besides, ORF has exhibited an important anti-hyperhomocysteinemic and anti-inflammatory effect.
CONCLUSION
The results of this study confirmed that hyperhomocysteinemia induced intestinal inflammation and the ethanolic extract of ORF had a high content of phenols, contained flavonoids, and exhibited good antioxidant activity. The use of C. sinensis fruit powder in the in vivo study showed increased GSH levels, decreased plasma homocysteine levels, and restored the integrity of the intestinal epithelium. The C. sinensis fruit is therefore recommended for the prevention of inflammatory bowel diseases due to its antioxidant and anti-inflammatory power.
ACKNOWLEDGEMENTS
The authors are grateful to the MESRS (Ministry of Scientific Research, Algeria).
AUTHOR CONTRIBUTIONS
Sara Khelfi performed the experiments, the statistical analysis and data visualization and wrote the manuscript. Sakina Zerizer was the supervisor of this work and contributed to its technical and academic realization and followed the revision of the manuscript. Soraya TEBIBEL was the second supervisor, followed the revision of the manuscript. Amina FOUGHALIA help ın pratıcal work. Chawki BENCOUICI and Zahia KABOUCHE provıded materials of the part of extraction and in vitro antioxidant activities for the experimental study.
REFERENCES
Abe, H., Ishioka, M., Fujita, Y., Umeno, A., Yasunaga, M., Sato, A., Ohnishi, S., Suzuki, S., Ishida, N., and Shichiri, M. 2018. Yuzu (Citrus junos Tanaka) peel attenuates dextran sulfate sodium-induced murine experimental colitis. Journal of Oleo Science. 67: 335–344.
Abdelghffar, E.A., El-Nashar, H.A., Al-Mohammadi, A.G., and Eldahshan, O.A. 2021. Orange fruit (Citrus sinensis) peel extract attenuates chemotherapy-induced toxicity in male rats. Food & Function. 12: 9443-9455.
Akar, Z. and Burnaz, N.A. 2019. A new colorimetric method for CUPRAC assay with using of TLC plate. LWT. 112: 108212.
Al Mutairi, F. 2020. Hyperhomocysteinemia: Clinical insights. Journal of Central Nervous System Disease. 12: 1179573520962230.
Apak, R., Guclu, K., Ozyurek, M., and Karademir, SE. 2004. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. Journal of Agricultural and Food Chemistry. 52: 7970-7981.
Apak, R., Güçlü, K., Demirata, B., Özyürek, M., Çelik, S.E., Bektaşoğlu, B., Berker, K.I., and Özyurt, D. 2007. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules. 12: 1496-1547.
Bentahar, A., Bouaziz, A., Djidel, S., and Khennouf, S. 2020. Phenolic content and antioxidant activity of ethanolic extracts from Citrus sinensis L. and Citrus reticulata L. fruits. Journal of Drug Delivery and Therapeutics. 10: 308-313.
Blois, M.S. 1958. Antioxidant determinations by the use of a stable free radical. Nature. 4617: 1119-1200.
Canan, I., Gündoğdu, M., Seday, U., Oluk, C.A., Karaşahin, Z., Eroğlu, E.Ç. Yazıcı, E., and Ünlü, M., 2016. Determination of antioxidant, total phenolic, total carotenoid, lycopene, ascorbic acid, and sugar contents of Citrus species and mandarin hybrids. Turkish Journal of Agriculture and Forestry. 40: 894-899.
Chen, X., Huang, X., Jie, D., Zheng, C., Wang, X., Zhang, B., Shao W., Wang G., and Zhang, W. 2021. Combining genetic risk score with artificial neural network to predict the efficacy of folic acid therapy to hyperhomocysteinemia. Scientific Reports. 11: 21430.
Campone, L., Celano, R., Rizzo, S., Piccinelli, A.L., Rastrelli, L., and Russo, M. 2020. Development of an enriched polyphenol (natural antioxidant) extract from orange juice (Citrus sinensis) by adsorption on macroporous resins. Journal of Food Quality. 2020:1-9.
Cordaro, M., Siracusa, R., Fusco, R., Cuzzocrea, S., Di Paola, R., and Impellizzeri, D. 2021. Involvements of hyperhomocysteinemia in neurological disorders. Metabolites. 11: 37.
Denaro, M., Smeriglio, A., and Trombetta, D. 2021. Antioxidant and anti-inflammatory activity of citrus flavanones mix and its stability after in vitro simulated digestion. Antioxidants. 10: 140.
Elsherbiny, N.M., Sharma, I., Kira, D., Alhusban, S., Samra, Y.A., Jadeja, R., Martin, P., Al-Shabrawey, M., and Tawfik A. 2020. Homocysteine induces inflammation in retina and brain. Biomolecules. 10: 393.
Etebu, E., and Nwauzoma, A.B. 2014. A review on sweet orange (Citrus sinensis L Osbeck): Health, diseases and management. American Journal of Research Communication. 2: 33-70.
Farzaei, M.H., Rahimi, R., and Abdollahi, M. 2015. The role of dietary polyphenols in the management of inflammatory bowel disease. Current Pharmaceutical Biotechnology. 16: 196–210.
Favela-Hernández, J.M.J., González-Santiago, O.,Ramírez-Cabrera, M.A., Esquivel-Ferriño, P.C., and Camacho-Corona, M.D.R. 2016. Chemistry and pharmacology of Citrus sinensis. Molecules. 21: 247.
Ferlazzo, N., Cirmi, S., Calapai, G., Ventura-Spagnolo, E., Gangemi, S., and Navarra, M. 2016. Anti-inflammatory activity of Citrus bergamia derivatives: Where do we stand? Molecules. 21: 1273.
Fusco, R., Cirmi, S., Gugliandolo, E., Di Paola, R., Cuzzocrea, S., and Navarra, M. 2017. A flavonoid-rich extract of orange juice reduced oxidative stress in an experimental model of inflammatory bowel disease. Journal of Functional Foods. 30: 168–178.
Gampierakis I.A., Koutmani Y., Semitekolou M., Morianos I., Polissidis A., Katsouda A., Charalampopoulos I., Xanthou G., Gravanis A., and Karalis K.P. 2021. Correction: hippocampal neural stem cells and microglia response to experimental inflammatory bowel disease (IBD). Molecular Psychiatry. 26: 3662.
Gholap, P.A., Nirmal, S.A., Pattan, S.R., Pal, S.C., and Mandal, S.C. 2012. Potential of Moringaoleifera root and Citrus sinensis fruit rind extracts in the treatment of ulcerative colitis in mice. Pharmaceutical Biology. 50: 1297-1302.
Grosso, G., Galvano, F., Mistretta, A., Marventano, S., Nolfo, F., Calabrese, G., Buscemi, S., Drago, F., Veronesi, U., and Scuderi, A. 2013. Red orange: experimental models and epidemiological evidence of its benefits on human health. Oxidative Medicine and Cellular Longevity. 2013: 157240.
Güller, P., Karaman, M., Güller, U., Aksoy, M., and Küfrevioğlu, Ö.İ. 2021. A study on the effects of inhibition mechanism of curcumin, quercetin, and resveratrol on human glutathione reductase through in vitro and in silico approaches. Journal of Biomolecular Structure and Dynamics. 39: 1744-1753.
Hayes, J.D., Dinkova-Kostova, A.T., and Tew, K.D. 2020. Oxidative stress in cancer. Cancer cell. 38: 167-197.
He, W., Li, Y., Liu, M., Yu, H., Chen, Q., Chen, Y., Ruan, J., Ding, Z., Zhang, Y., and Wang, T. 2018. Citrus aurantium L. and its flavonoids regulate TNBS-induced inflammatory bowel disease through antiinflammation and suppressing isolated jejunum contraction. International Journal of Molecular Sciences. 19: 3057.
Huang, Y.S., and Ho, S.C. 2010. Polymethoxy flavones are responsible for the anti-inflammatory activity of citrus fruit peel. Food Chemistry. 119: 868-873.
Impellizzeri, D., Bruschetta, G., Di Paola, R., Ahmad, A., Campolo, M., Cuzzocrea, S., Esposito, E., Navarra, M. 2015. The anti-inflammatory and antioxidant effects of bergamot juice extract (BJe) in an experimental model of inflammatory bowel disease. Clinical Nutrition. 34: 1146–1154.
Ji, D., Luo, C., Liu, J., Cao, Y., Wu, J., Yan, W., and Wang, W. 2022. Insufficient s-sulfhydration of methylenetetrahydrofolate reductase contributes to the progress of hyperhomocysteinemia. Antioxidants & Redox Signaling. 36: 1-14.
Juibary, P.L., Seyedmehdi, F.S., Sheidai, M., Noormohammadi, Z., and Koohdar, F. 2021. Genetic structure analysis and genetic finger printing of sweet orange cultivars (Citrus sinensis (L.) Osbeck) by using SCoT molecular markers. Genetic Resources and Crop Evolution. 68: 1645-1654.
Khan, R.A., Mallick, N., and Feroz, Z. 2016. Anti-inflammatory effects of Citrus sinensis L., Citrus paradisi L. and their combinations. Pakistan Journal of Pharmaceutical Sciences. 29: 843-852.
Ledda, C., Cannizzaro, E., Lovreglio, P., Vitale, E., Stufano, A., Montana, A., Li Volti, G., and Rapisarda, V. 2020. Exposure to toxic heavy metals can influence homocysteine metabolism?. Antioxidants. 9: 30.
Liu, Y., Heying, E., and Tanumihardjo, S.A. 2012. History, global distribution, and nutritional importance of Citrus fruits. Comprehensive Reviews in Food Science and Food Safety. 11: 530–45.
Liguori, I., Russo, G., Curcio, F., Bulli, G., Aran, L., Della-Morte, D., Gargiulo, G., Testa, G., Cacciatore, F., Bonaduce, D., and Abete, P. 2018. Oxidative stress, aging, and diseases. Clinical Interventions in Aging. 13: 757–772.
Leguizamon, N.D.P., Rodrigues, E.M., de Campos, M.L., Nogueira, A.V.B., Viola, K. S., Schneider, V.K., and Cirelli, J.A. et al. 2019. In vivo and in vitro anti-inflammatory and pro-osteogenic effects of citrus cystatin CsinCPI-2. Cytokine. 123: 154760.
Lurz, E., Horne, R.G., Määttänen, P., Wu, R.Y., Botts, S.R., Li, B., and Sherman, P.M. 2020. Vitamin B12 deficiency alters the gut microbiota in a murine model of colitis. Frontiers in Nutrition. 7: 83.
Lohning, A., Kidachi, Y., Kamiie, K., Sasaki, K., Ryoyama, K., and Yamaguchi, H. 2021. 6-(methylsulfinyl) hexyl isothiocyanate (6-MITC) from Wasabia japonica alleviates inflammatory bowel disease (IBD) by potential inhibition of glycogen synthase kinase 3 beta (GSK-3β). European Journal of Medicinal Chemistry. 216: 113250.
Mannucci, C., Calapai, F., Cardia, L., Inferrera, G., D’Arena, G., Di Pietro, M., and Calapai, G. 2018. Clinical Pharmacology of Citrus aurantium and Citrussinensis for the treatment of anxiety. Evidence-Based Complementary and Alternative Medicine. 2018: 1–18.
Ma, G., Zhang, L., Sugiura, M., Kato, M. 2020. Citrus and health. In The Genus Citrus (p. 495-511). Woodhead Publishing.
Moretti, R., Giuffré, M., Caruso P., Gazzin, S., and Tiribelli, C. 2021. Homocysteine in neurology: a possible contributing factor to small vessel disease. International Journal of Molecular Sciences. 22: 2051.
Müller, L., Gnoyke, S., Popken, A.M., and Böhm, V. 2010. Antioxidant capacity and related parameters of different fruit formulations. LWT - Food Science and Technology. 43: 992-999.
Musumeci, L., Maugeri, A., Cirmi, S., Lombardo, G. E., Russo, C., Gangemi, S., and Navarra, M. 2020. Citrus fruits and their flavonoids in inflammatory bowel disease: An overview. Natural Product Research. 34: 122-136.
Nawrin, K., Billah, M.M., Ahmed, F., Hossin, A., Tushar, R.R., and Islam, M.N. 2021. Protective potential of C. sinensis fruit peel aqueous extract on in vitro inflammation. Pharmacotherapy and Pharmascience Discovery. 1: 10-18.
Oikeh, E.I., Oviasogie, F.E. and Omoregie, E.S. 2020. Quantitative phytochemical analysis and antimicrobial activities of fresh and dry ethanol extracts of Citrus sinensis (L.) Osbeck (sweet orange) peels. Clinical Phytoscience. 6: 46.
Oyaizu, M. 1986. Studies on products of browning reactions: antioxidative activities of browning reaction prepared from glucosamine. Japanese Journal of Nutrition. 44: 307-315.
Pepe, G., Sommella E., Cianciarulo D., Ostacolo C., Manfra M., Di Sarno. V., Musella S., Russo, M., essore A., Parrino, B., Bertamino A., Autore G., Marzocco S., and Campiglia, P. 2018. Polyphenolic extract from Tarocco (Citrus sinensis L. Osbeck) Clone “Lempso” exerts anti-Inflammatory and antioxidant effects via NF-kB and Nrf-2 activation in murine macrophages. Nutrients. 10: 1961.
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., and Rice-Evans, C. 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine. 26: 1231-1237.
Robbins, M.E., Cho, H.Y., Hansen, J.M., Luchsinger, J.R., Locy, M.L., Velten, M., and Tipple, T. E. (2021). Glutathione reductase deficiency alters lung development and hyperoxic responses in neonatal mice. Redox Biology. 38: 101797.
Rauf, A., Uddin, G., and Ali, J. 2014. Phytochemical analysis and radical scavenging profile of juices of Citrus sinensis, Citrus anrantifolia, and Citrus limonum. Organic and Medicinal Chemistry Letters. 4: 1-3.
Rao, M.J., Wu, S., Duan, M., and Wang, L. 2021. Antioxidant metabolites in primitive, wild, and cultivated citrus and their role in stress tolerance. Molecules. 26: 5801.
Roussos, P.A. 2011. Phytochemicals and antioxidant capacity of orange (Citrus sinensis (l.) Osbeck cv. Salustiana) juice produced under organic and integrated farming system in Greece. Scientia Horticulturae. 129: 253-258.
Sakhri, F.Z., Zerizer, S., and Bensouici, C. 2021. Evaluation of the antioxidant, antidiabetic and immunomodulatory activity of Cydonia oblonga fruit extract. Chiang Mai University Journal of Natural Sciences. 20: e2021052.
Sathiyabama, R.G., Rajiv Gandhi, G., Denadai, M., Sridharan, G., Jothi, G., Sasikumar, P., and Gurgel, R.Q. 2018. Evidence of insulin-dependent signalling mechanisms produced by Citrus sinensis (L.) Osbeck fruit peel in an insulin resistant diabetic animal model. Food and Chemical Toxicology. 116: 86–99.
Selmi, S., Rtibi, K., Grami, D., Sebai, H., and Marzouki, L. 2017. Protective effects of orange (Citrus sinensis L.) peel aqueous extract and hesperidin on oxidative stress and peptic ulcer induced by alcohol in rat. Lipids in Health and Disease. 16:152.
Singleton, V.L., and Rossi, J.A.J. 1965. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture. 16: 144-158.
Singhal, M., Paul, A., and Singh, H. P. 2014. Synthesis and reducing power assay of methyl semicarbazone derivatives. Journal of Saudi Chemical Society. 18: 121-127.
Sridhar, K., and Charles, A.L. 2018. Application of multivariate statistical techniques to assess the phenolic compounds and the in vitro antioxidant activity of commercial grape cultivars. Journal of Chemometrics. 32: e3073.
Topçu, G., Ay, A., Bilici, A., Sarıkürkcü, C., Öztürk, M., and Ulubelen, A. 2007. A new flavone from antioxidant extracts of Pistaciaterebinthus. Food Chemistry. 103: 816-822.
Vezzoli, A., Dellanoce, C., Maria Caimi, T., Vietti, D., Montorsi, M., Mrakic-Sposta, S., and Accinni, R. 2020. Influence of dietary supplementation for hyperhomocysteinemia treatments. Nutrients. 12: 1957.
Wéra, O., Lancellotti, P., and Oury, C. 2016. The dual role of neutrophils in inflammatory bowel diseases. Journal of Clinical Medicine. 5: 118.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand.
Sara Khelfi1, 2, Sakina Zerizer1, 2,*, Amina Foughalia1, 2, Souraya Tebibel1, Chawki Bensouici3, and Zahia Kabouche2
1 Université des Frères Mentouri-Constantine 1, Département de Biologie Animale, Facultè des Sciences de la Nature et de la Vie, Constantine, Algeria.
2 Université des Frères Mentouri-Constantine 1, Laboratoire d’Obtention de Substances Thérapeutiques, 25000 Constantine, Algeria.
3 Centre de Recherche en Biotechnologie Ali Mendjli Nouvelle Ville UV 03 BP E73 Constantine, Algeria.
Corresponding author: Sakina Zerizer, E-mail: zerizer.sakina@umc.edu.dz
Total Article Views
Editor: Wasu Pathom-aree,
Chiang Mai University, Thailand
Article history:
Received: May 5, 2022;
Revised: October 29, 2022;
Accepted: November 4, 2022;
Published online: November 21, 2022

