
Heavy Metals and Metalloid Effects on Cytogenetics in Frogs (Sylvirana nigrovittata) around the Sepon Gold-Copper Mine, Lao PDR
Nuntita Ruksachat, Bundit Tengjaroensakul, Lamyai Neeratanaphan, Alongklod Tanomtong, Nikom Srikacha, Thonglom Phommavong and Latsamy Soulivongsa*Published Date : 2023-01-05
DOI : https://doi.org/10.12982/NLSC.2023.007
Journal Issues : Number 1, January-March 2023
Abstract The objectives of the present study were to determine the concentrations of heavy metals and metalloids in frogs (Sylvirana nigrovittata) and to assess chromosome aberrations (CAs) in frogs collected from the Nam Kok River near the Sepon gold-copper mine, Lao PDR, as compared with those from a reference site. The results showed that the concentrations of As, Cd, Cr, and Mn in the frog muscle samples exceeded the standard values, and the accumulation of heavy metals in the frogs from the two sampling sites was significantly different (P <0.05), particularly Ba, Cd, Cr, Cu, Fe, Mn and Ni. Chromosome assessment of S. nigrovittata from the study site revealed 13 types of CAs, and the highest total number of CAs was isochromatid gap with a percentage of 31.80%. Furthermore, the total number of CAs, cell number with CAs and percentage of CAs in the frogs from both sites were significantly different (P <0.05). The results of this study suggest that heavy metal and metalloid from the Sepon gold-copper mine area negatively affected S. nigrovittata in terms of chromosomal defects.
Keywords: Aberration, Abnormality, Amphibian, Animal, Toxicant
Funding: This research was funded by Khon Kaen University, Thailand.
Citation: Ruksachat, N., Tengjaroensakul, B., Neeratanaphan, L., Tanomtong, A., Srikacha, N., Phommavong, T., and Soulivongsa, L. 2023. Heavy Metals and Metalloid Effects on Cytogenetics in Frogs (Sylvirana nigrovittata) around the Sepon Gold-Copper Mine, Lao PDR. Nat. Life Sci. Commun. 22(1): e2023007.
INTRODUCTION
At present, the government of Lao PDR estimates that an export of mineral resources of approximately 30% of gross domestic product is one of the main contributions to development in the country (Ngangnouvong, 2019; Owusu-Prempeh et al., 2022). The country’s total gold exports increased from 502 million US$ in 2018 to 520 million US$ in 2022 (US geological survey, 2022). The two largest gold mines in Lao PDR accounted for 90% of the country’s mining production, including the Sepon gold-copper mine in Savannakhet Province and the Phu Kham gold mine in Vientiane Province (ICMM, 2011). Generally, heavy metals and metalloids that leach out during the gold-extraction step in mining processes lead to detrimental pollution in water and aquatic animals as well as affecting human health due to their potential toxicities and tendency toward bioaccumulation (Zhang et al., 2012: Pejman et al., 2015). Several reports have confirmed potentially toxic heavy metals among the essential and nonessential metal elements, such as Cd, Cr, As and Pb (Fashola et al., 2016: Aydin and Tunca, 2022). Researchers have also mentioned that toxic metals can adversely affect the metabolism, structure and genetics of cells, including chromosome and DNA damage (Chandanshive, 2013; Govind and Madhuri, 2014; B’ey, 2015).
Frogs are species of amphibians that feed mostly on insects and other invertebrates. They are sensitive to environmental pollution owing to the high permeability of their unshelled eggs, larvae and skin (Burlibaşa and Gavrilă, 2011; Thammachoti et al., 2012). They are scientifically accepted as good indicators for toxicant or polluted-substance contamination and accumulation in aquatic ecosystems (Tengjaroenkul et al., 2018). Soulivongsa et al. (2020, 2021) reported cytotoxicity as chromosome aberrations of heavy metals in two fish species (Osteochilus vattatus and Hampala macrolepidota) from the Nam Kok River near the Sepon gold–copper mine area, Lao PDR. However, information related to chromosomal studies of amphibians near the Sepon gold–copper mine with regard to heavy metal point sources is very limited. Therefore, this study aims to investigate the concentration of heavy metals in water, sediment, and frogs (S. nigrovittata) and to assess frog chromosome aberrations compared to uncontaminated areas.
MATERIALS AND METHODS
Study areas and collection of samples
The reference site without electronic and mining activities is located on the Nam Souang River in Naxaythong district, Vientiane Capital, Lao PDR (18°14'52.88"N 102°27'54.78"E) (Figure 1). The study site was carried out in the Nam Kok River near the Sepon gold–copper mine, Viraboury district, Savannakhet Province, Lao PDR (16°52'06.32"N 106°02'22.88"E). Five sediment and water samples were randomly collected from each site during March 2022. The sediments were collected in plastic bags and dried at room temperature before sieving using a 2 mm sieve shaker. The water sample was contained in polyethylene bottles, acidified by HNO3 and stored at 4°C. Five samples of adult frogs (S. nigrovittata) were caught at both sites to measure the bioaccumulation of heavy metals and to investigate chromosome aberrations (CAs).
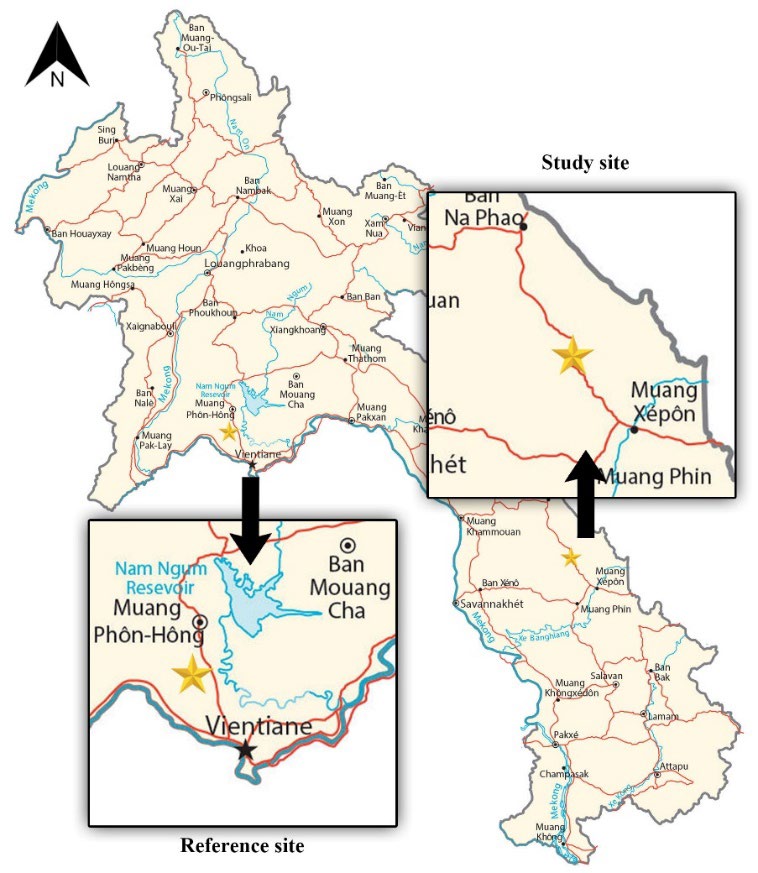
Figure 1. Location of study areas (star symbol), including the Nam Souang River (reference site) and Nam Kok River (study site).
Source: GIS Geography (n.d.)
Preparation of samples and heavy metal and metalloid concentration measurements
Sample preparation and digestion methods used were described by Chand and Prasad (2013). First, the digestion of each water sample was performed by mixing a 25 mL sample with 1.25 mL of 30% HNO3, which was then heated for 1 h on a hot plate at 95°C. Deionized water (DI) was added to the cooling sample to 25 mL, and then it was filtered through polycellulose paper No. 1. Second, the 1.0 g sediment sample was mixed with 5 mL nitric acid, 15 mL hydrochloric acid and 10 mL hydrogen peroxide and then digested on a hot plate at 95°C for 2 h. The cooling sample was adjusted to a volume of 50 mL with DI water and filtered through filter paper No. 42. Third, the digestion of frog muscle was performed by mixing a 1.0 g sample (dry weight) with sulfuric and nitric acid, which was then heated on a hot plate at 60°C for 30 min. Each sample was added with 10 mL hydrogen peroxide and was continuously digested on a hot plate at 95°C for 1 h. Then, DI water was added to the cooling sample to 25 mL and filtered by paper No. 1. Last, all samples were measured for heavy metals and metalloid concentrations by inductively coupled plasma‒optical emission spectrometry (ICP-OES). The ICP‒OES wavelengths and detection limits of elements are summarized in Table 1, while the quality control during the measurement step followed APHA (2012) and Chand and Prasad (2013).
Table 1. The wavelengths and detection limits of elements of the ICP‒OES.
|
Elements |
Wavelengths (nm) |
Detection limits (mg/L) |
|
As |
188.979 |
0.006 |
|
Ba |
233.527 |
0.002 |
|
Cd |
226.502 |
0.001 |
|
Cr |
267.716 |
0.001 |
|
Cu |
324.752 |
0.002 |
|
Fe |
259.939 |
0.002 |
|
Mn |
259.327 |
0.002 |
|
Ni |
231.604 |
0.001 |
|
Pb |
220.353 |
0.005 |
|
Se |
196.022 |
0.023 |
|
Zn |
213.857 |
0.001 |
Chromosome preparation and chromosome aberration (CA) assessment
Chromosome preparation of frog samples was described by Rooney (2001) and Maneechot et al. (2015) and is briefly explained as follows. Live frog samples were injected with 0.05% colchicine. After 8 h, the bone marrow was cut out and chopped into small pieces and then soaked in 7 mL of 0.075 M KCl for 30 min in a 15 mL tube. Approximately 5 mL of solution was discarded and replaced with cool fixative (3 methanol: 1 glacial acetic acid). This step was repeated 4 times or until the supernatant was clear, and the cell suspension was then dropped onto a glass slide. The slide was air-dried and stained with 20% Giemsa solution for 30 min. The clear metaphase cells were photographed under a light microscope at a magnification of 1,000X, after which 100 metaphase cells of individual samples were selected to investigate CAs.
Statistical analyses
The heavy metal and metalloid concentrations, including CAs, were statistically analyzed using the Mann‒Whitney U test in SPSS program Version 24. The 95% confidence level was statistically considered for significance between the Nam Souang River (reference site) and the Nam Kok River (study site).
RESULTS
Heavy metal and metalloid concentrations in water, sediment and frog samples
The heavy metals and metalloids were compared to determine the significant differences between both sites, and the average concentrations were compared to the standard values. Eleven heavy metals and metalloids were observed in the water samples, including As, Ba, Cd, Cr, Cu, Fe, Mn, Ni, Pb, Se and Zn (Table 2). However, only Fe and Zn exceeded the standard values at both sites, while Mn and Ni in the Nam Kok River were higher than the standard. The statistical analyses revealed that only As, Ba, Cu, Mn, Ni, Si and Zn in the Nam Kok River were statically higher than those at the reference site (P <0.05).
The results of heavy metal contamination in sediment samples revealed that 10 elements in the Nam Kok River were observed, and all of them were statistically higher than those at the reference site (P <0.05). Additionally, only As, Cd, Cu and Zn in the Nam Kok River exceeded the standard values (Table 3).
The bioaccumulation of heavy metals in the frog sample presented the lowest number of heavy metals among all samples (Table 4). As, Ba, Cd, Cr, Cu, Fe, Mn, Ni and Zn were observed in frogs that inhabited the Nam Kok River, while all of them except As were also found at the reference site. Seven elements, including Ba, Cd, Cr, Cu, Fe, Mn and Ni, were significantly different between both sites (P <0.05). Four elements (As, Cd, Cr and Mn) in the Nam Kok River presented higher concentrations than standard values.
Table 2. Concentrations of heavy metals and metalloids in water samples (mean ± S.D.).
|
Heavy metals
|
Concentration in water (mg/L) |
P value |
Standard value |
|
|
Nam Kok River(Study site) |
Nam Souang River(Reference site) |
|||
|
As |
0.0055 ± 0.0180 |
0.0009 ± 0.0008 |
0.002** |
0.010a |
|
Ba |
5.6874 ± 0.6884 |
1.9177 ± 1.3812 |
0.002** |
- |
|
Cd |
0.0021 ± 0.0006 |
0.0014 ± 0.0011 |
0.110 |
0.05b, 0.01c |
|
Cr |
0.0145 ± 0.0061 |
0.0179 ± 0.0150 |
0.903 |
0.050b,d |
|
Cu |
0.0399 ± 0.0205 |
0.0104 ± 0.0051 |
0.002** |
0.050d |
|
Fe |
0.8239 ± 0.3963* |
0.6103 ± 0.1650* |
0.017** |
0.300e |
|
Mn |
0.1896 ± 0.1276* |
0.0117 ± 0.0030 |
0.002** |
0.050f |
|
Ni |
0.3354 ± 0.3200* |
0.0107 ± 0.0018 |
0.002** |
0.020d |
|
Pb |
0.0287 ± 0.0224 |
0.0226 ± 0.0190 |
0.462 |
0.050b |
|
Se |
0.0074 ± 0.0030 |
0.0033 ± 0.0026 |
0.047** |
- |
|
Zn |
3.4930 ± 0.5766* |
1.7969 ± 0.9777* |
0.010** |
0.200c |
Note: * Metal or metalloid concentrations exceed the standard value.
** Statistically significant difference between both sites (P <0.05).
Water standard references: WHO (2011)a; Thailand Standard (TPCD, 1994)b; Standard of FAO (1994)c; FAO/WHO (1989)d; WHO (2003)e; USA Standard (UNEPGEMS, 2008)f
Table 3. Concentrations of heavy metals and metalloids in sediment samples (mean ± SD.)
|
Heavy metals
|
Concentration in sediment (mg/kg) |
P value |
Standard value |
|
|
Nam Kok River(Study site) |
Nam Souang River(Reference site) |
|||
|
As |
10.414 ± 6.318* |
0.030 ± 0.017 |
0.002** |
3.90a |
|
Ba |
420.230 ± 24.579 |
12.418 ± 16.067 |
0.001** |
- |
|
Cd |
2.399 ± 0.510* |
0.073 ± 0.061 |
0.002** |
0.16b |
|
Cr |
19.395 ± 6.122 |
0.482 ± 0.218 |
0.001** |
45.50b |
|
Cu |
39.053 ± 18.195* |
0.422 ± 0.276 |
0.002** |
21.50b |
|
Fe |
8,069.309 ± 7,036.865 |
214.938 ± 59.492 |
0.001** |
- |
|
Mn |
266.655 ± 119.057 |
2.951 ± 1.060 |
0.001** |
1,800.00a |
|
Ni |
36.875 ± 6.824 |
1.891 ± 1.790 |
0.002** |
75.00c |
|
Pb |
59.604 ± 18.691 |
1.437 ± 0.927 |
0.001** |
400.00a |
|
Se |
LDL |
LDL |
|
- |
|
Zn |
338.590 ± 34.095* |
9.630 ± 8.232 |
0.002** |
300.00c |
Note: * Metal or metalloid concentrations exceed the standard value.
** Statistically significant difference between both sites (P <0.05).
LDL is the lower detection limit.
Sediment standard references: TPCD (2004)a; TPCD (2018)b; EU (2002)c
Table 4. The concentrations of heavy metals and metalloids in S. nigrovittata samples (mean±S.D.).
|
Heavy metals
|
Concentration in frog (mg/kg) |
P value |
Standard value |
|
|
Nam Kok River(Study site) |
Nam Souang River(Reference site) |
|||
|
As |
2.867 ± 0.610* |
LDL |
- |
2.00 a |
|
Ba |
0.824 ± 0.130 |
1.868 ± 0.720 |
0.009** |
|
|
Cd |
0.113 ± 0.030* |
0.044 ± 0.010 |
0.010** |
0.05b |
|
Cr |
3.198 ± 0.090* |
1.656 ± 0.100 |
0.010** |
2.00c |
|
Cu |
7.543 ± 0.610 |
2.761 ± 0.710 |
0.009** |
|
|
Fe |
167.775 ± 12.610 |
88.489 ± 6.260 |
0.009** |
- |
|
Mn |
32.216 ± 1.040* |
14.826 ± 3.130* |
0.010** |
1.00c |
|
Ni |
13.583 ± 8.250 |
1.175 ± 0.190 |
0.009** |
- |
|
Pb |
LDL |
LDL |
- |
0.20d |
|
Se |
LDL |
LDL |
- |
- |
|
Zn |
98.248 ± 2.860 |
83.504 ± 15.890 |
0.117 |
100.00e |
Note: * Metal or metalloid concentrations exceed the standard value.
** Statistically significant difference between both sites (P <0.05).
LDL is the lower detection limit.
Standard references: Australia and New Zealand Food Standard (2011)a; EC (2005)b; WHO (1989)c; EC (2001)d; TPCD (1986)e
Chromosome assessment in S. nigrovittata
The chromosome number (2n) in the diploid model of S. nigrovittata that inhabited both sites was 2n = 26 or 13 pairs per metaphase cell (Figure 2). Table 5 and Figure 3 show the different types of CAs in the metaphase cells. The results found 13 types of CAs in S. nigrovittata from the Nam Kok River near the Sepon gold–copper mine area: CF = centric fragmentation, CG = centromere gap, DC = dicentric chromosome, T = translocation, R= ring chromosome, P = polyploidy, D = deletion, SCB = single chromatid break, ISCB = isochromatid break, F = fragmentation, SCG = single chromatid gap, ISCG = isochromatid gap, and ISAF = isoarm fragmentation with numbers of CAs of 39, 38, 23, 8, 4, 3, 45, 33, 84, 36, 42, 113, and 6, respectively. The total number of CAs, cell numbers with CAs and cell percentages of CAs of S. nigrovittata in the study and the reference sites were 474 and 46, 159 and 30, and 31.80 and 6.00, respectively. The statistical analyses indicated that the total number of CAs, cell numbers with CAs and percentages of CAs from both sites were significantly different (P <0.05).
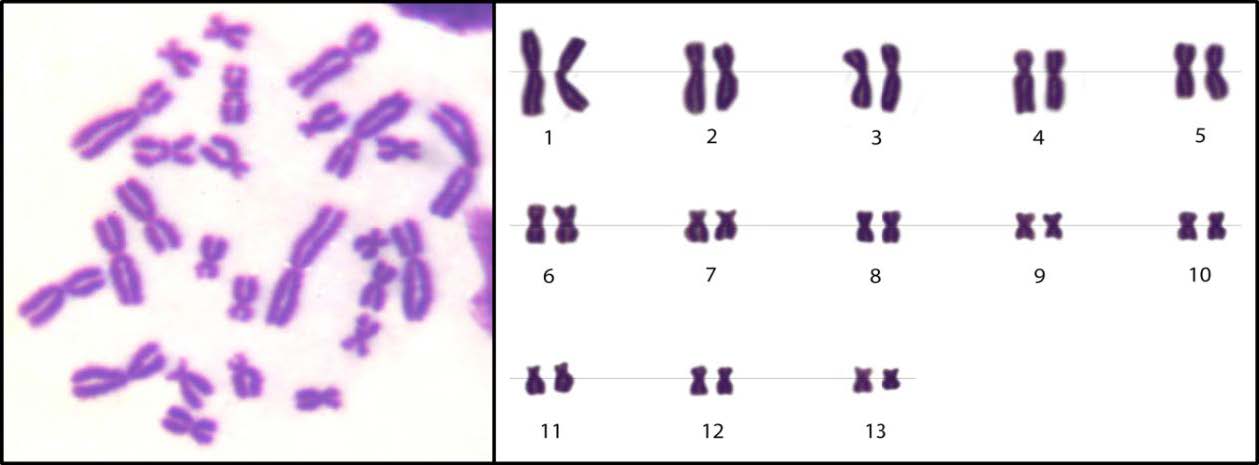
Figure 2. Karyotype of the normal chromosome of S. nigrovittata (2n= 26).
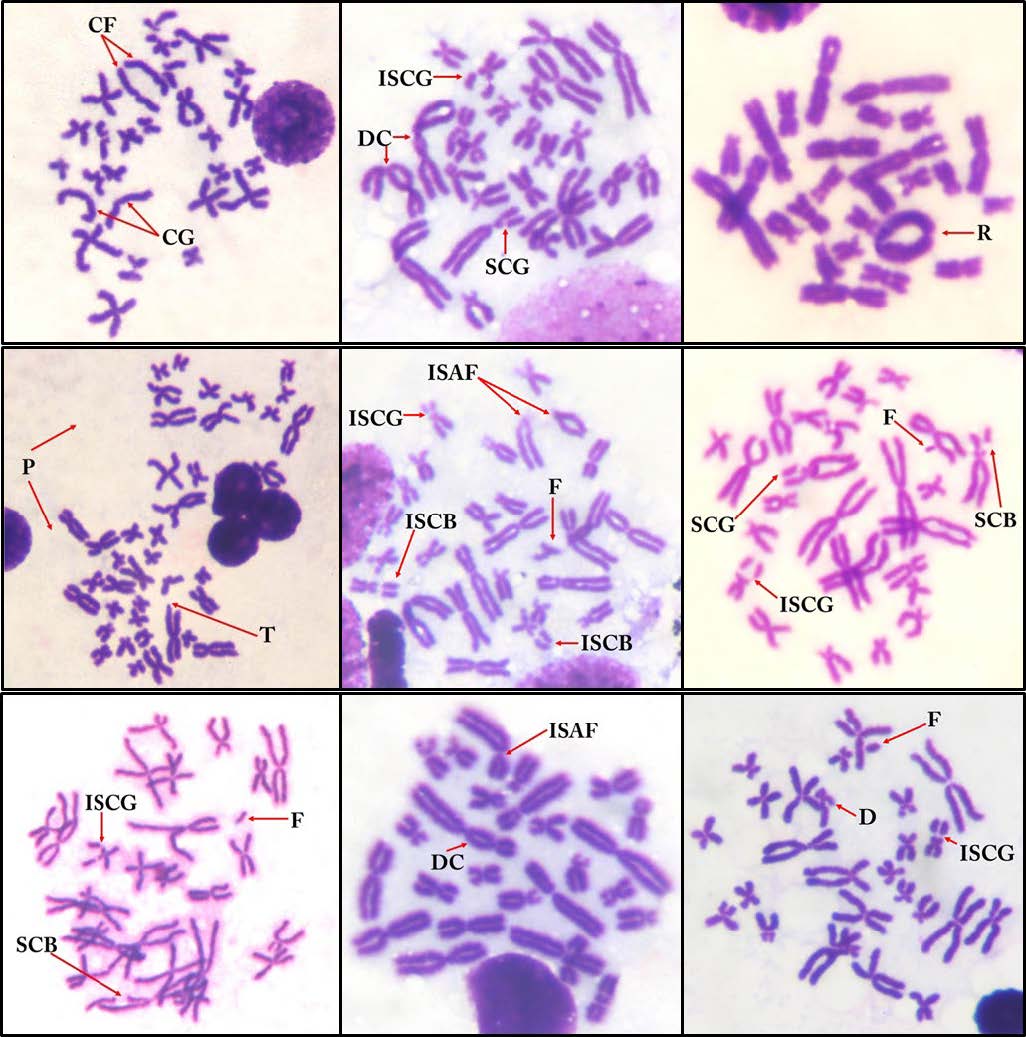
Figure 3. Types of chromosome aberrations in the metaphase spread cells of S. nigrovittata; CF = centric fragmentation, CG = centromere gap, DC = dicentric chromosome, T = translocation, R = ring chromosome, P = polyploidy, D = deletion, SCB = single chromatid break, ISCB = isochromatid break, F = fragmentation, SCG = single chromatid gap, ISCG = isochromatid gap, and ISAF = isoarm fragmentation.
Table 5. Total number of CAs, cell numbers with CAs and percentages of CAs of S. nigrovittata from the study site and the reference site.
|
S.nigrovittata |
Number metaphase cell |
|
|
|
|
|
Number of chromosomal aberrations |
|
Total number of CAs |
Cell number with CAs |
Percentage cell of CAs(%) |
|||||||
|
CF |
CG |
CD |
T |
R |
P |
D |
SCB |
ISCB |
F |
SCG |
ISCG |
ISAF |
||||||
|
Study site |
|
|
|
|
|
|
|
|||||||||||
|
Individual 1 |
100 |
9 |
8 |
6 |
2 |
1 |
0 |
12 |
12 |
21 |
6 |
7 |
22 |
1 |
107 |
37 |
37.00 |
|
|
Individual 2 |
100 |
8 |
8 |
3 |
2 |
1 |
2 |
9 |
7 |
18 |
13 |
12 |
16 |
2 |
101 |
33 |
33.00 |
|
|
Individual 3 |
100 |
7 |
6 |
5 |
2 |
0 |
0 |
10 |
5 |
15 |
7 |
6 |
29 |
1 |
93 |
31 |
31.00 |
|
|
Individual 4 |
100 |
7 |
9 |
5 |
1 |
0 |
1 |
7 |
4 |
13 |
6 |
6 |
21 |
2 |
82 |
28 |
28.00 |
|
|
Individual 5 |
100 |
8 |
7 |
4 |
1 |
2 |
0 |
7 |
5 |
17 |
4 |
11 |
25 |
0 |
91 |
30 |
30.00 |
|
|
Total/ Average* |
|
39 |
38 |
23 |
8 |
4 |
3 |
45 |
33 |
84 |
36 |
42 |
113 |
6 |
474 |
159 |
31.80* |
|
|
Reference site |
|
|
|
|
|
|
|
|||||||||||
|
Individual 1 |
100 |
1 |
4 |
0 |
0 |
0 |
0 |
2 |
0 |
0 |
1 |
2 |
1 |
0 |
11 |
8 |
8.00 |
|
|
Individual 2 |
100 |
3 |
5 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
4 |
0 |
14 |
11 |
11.00 |
|
|
Individual 3 |
100 |
4 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
2 |
0 |
9 |
6 |
6.00 |
|
|
Individual 4 |
100 |
1 |
2 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
1 |
0 |
1 |
0 |
6 |
3 |
3.00 |
|
|
Individual 5 |
100 |
2 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
0 |
1 |
0 |
6 |
2 |
2.00 |
|
|
Total/ Average* |
|
11 |
14 |
0 |
0 |
0 |
0 |
3 |
0 |
0 |
5 |
4 |
9 |
0 |
46 |
30 |
6.00* |
|
|
P value |
|
|
|
|
|
|
|
0.008** |
0.009** |
0.009** |
||||||||
|
Note: * Average percentage of chromosome aberrations ** Statistically significant difference (P <0.05) Remarks: CF = centric fragmentation, CG = centromere gap, DC= dicentric chromosome, T= translocation, R= ring chromosome, P= polyploidy, D = deletion, SCB = single chromatid break, ISCB= isochromatid break, F= fragmentation, SCG = single chromatid gap, ISCG = isochromatid gap, and ISAF= isoarm fragmentation |
||||||||||||||||||
DISCUSSION
Heavy metal and metalloid concentrations in water, sediment and S. nigrovittata samples
The heavy metal concentration values in water at the study site were in the order Ba > Zn > Fe > Ni > Mn > Cu > Pb > Cr > Se > As > Cd. However, the levels of Zn, Fe, Ni and Mn at this site exceeded the water standards of the FAO (1994), FAO/WHO (1989), WHO (2003) and UNEPGEMS (2008).
Heavy metals could be leached out by rainwater, and more chemicals may also be washed out to water bodies (Dorleku et al., 2018). The measurement of water samples from the study and reference sites found that the concentrations of Cd, Cr, and Pb were not significantly different (P >0.05), whereas the other metals, including As, Ba, Cu, Mn, Ni, Se, Fe and Zn, were significantly different between both sites (P <0.05) (Table 3).
The study of heavy metal contamination in water around a gold mine area in Oman was found to be similar to this study in that Zn, Fe, Ni and Mn were higher than the standard (Abdul-Wahab and Marikar, 2012), and Kumar et al. (2022) reported that these four elements in the Republic of Fiji (Wainivesi gold mine) were relatively high when compared to the same standard. A study in Thailand (Wang Saphung gold mine) revealed that only Fe and Mn were higher than the standard (Intamat et al., 2016a). All previous studies revealed that the levels of Zn, Fe, Ni and Mn were 0.12-21.1, 1.62-31, 0.01-3.05 and 0.05-23 mg/L, respectively.
The concentrations of some heavy metals in water may finally precipitate with sediment content. Additionally, some heavy metals in sediment may remix into the water body; consequently, sediment is called a secondary source of heavy metal pollution. Therefore, sediment has remarkable significance in the assessment of aquatic pollution, specifically in elemental pollution studies (Aydin and Tunca, 2022). The sediment sample results revealed that all heavy metal concentrations at the study site were statistically higher than those at the reference site (P <0.05), except for Se, which was lower than the detection limit at both sites. The heavy metal concentration values in sediment at the study site were in the order Fe > Ba > Zn > Mn > Pb > Cu > Ni > Cr > As > Cd. The average concentrations of Zn, Cu, As and Cd at this site were higher than the standard values reported by TPCD (2004), TPCD (2018), and EU (2002), while all heavy metal concentrations at the reference site were lower than standard values.
The appearance of 10 elements in sediment samples was similar to previous studies that reported that Cr, Mn, Fe, Co, Ni, Cu, Zn, Cd, Pb, As, Hg and Pb around gold mine areas in the Republic of Fiji, China and Papua New Guinea were discovered in high concentrations (Kumar et al., 2022; Tang et al., 2021; Kapia et al., 2016). In particular, the concentrations of Cd, Cr, Cu, Zn and As were 0.33-30, 58-281, 28-100, 118-1013 and 9 mg/kg, respectively.
The contamination of heavy metals in ecological systems directly affects natural habitats, especially aquatic animals that consume contaminated food. The bioaccumulation of heavy metal concentrations in frog muscle at the study site was in the order Fe > Zn > Mn > Ni > Cu > Cr > As > Ba > Ba > Cd. From the results, the frogs from the study site contained significantly different concentrations of Ba, Cd, Cr, Cu, Fe, Mn and Ni compared to the reference site (P<0.05). The concentrations of As, Cd, Cr and Mn in frog samples from the study site were higher than the standard values (WHO, 1989; EC, 2001; EC, 2005; Australia and New Zealand Food Standard, 2011). Sylvirana nigrovittata is a carnivorous frog and can take heavy metals into its body after consuming contaminated food through the digestive tract and by absorbing them via the skin. Consequently, due to the limitation of the study of heavy metal bioaccumulation in frogs around gold mine areas, this study will compare the results with the similar habits of fish. Compaore et al. (2020) reported bioaccumulation in fish, including Hydrocynus forskahlii, Oreochromis niloticus, Bagrus bajad and Clarias anguillaris, from 2 study sites around a gold mine in Burkina Faso. The results showed that As, Cr and Mn were reported at concentrations of 9-23, 7-24 and 200-1,000 mg/kg, respectively. Additionally, Kiser et al. (2010) revealed concentrations of As and Cd between 1.6 and 10 and 0.2 and 1.1 mg/kg, respectively, from bull trout (Salvelinus confluentus) in a gold mine in Idaho, USA. These results were similar to those of this study.
When compared among all samples in this study, the heavy metal concentrations revealed that the sediment sample had the highest heavy metal concentration. This result suggests that the heavy metals in the ecosystem finally precipitated and accumulated in sediment samples, and they were hardly eliminated by natural methods. The total heavy metals in all samples of this study that exceeded the standards were 8 elements, including Fe, Mn, Ni, Zn, As, Cd, Cu and Cr. This result was similar to previous studies that reported that these metals were found around gold mines because they were the main component in the gold ore and parent rock. Cd, Zn and Fe were the components of gold ore bodies in the form of sphalerite. Cd is highly mobile in soil-plant systems and can also exert a great effect on the proper functioning of ecosystems. The average natural level of Zn in the Earth’s crust is 70 mg/kg (dry weight). Fe is the primary mineral compound that is the dominant element in weathered rocks (Fashola et al., 2016; Nurcholis et al., 2017). Cr is widely distributed in soils and rocks where it occurs in minerals such as chromite, and its concentration ranges below 60 mg/kg in natural soil. The Ni level in soil greatly depends on the concentration in the parent rocks, and its concentration has been estimated to be approximately 100 mg/kg for natural soil. Ni exists in gold-bearing ore as pyrrhotite, which contains up to 5% Ni, and pentlandite. As occurs as arsenopyrite, realgar and orpiment in gold-bearing rock. This result has been attributed to the richness of arsenopyrite mineralization in the gold-bearing ore. Cu is widely distributed in sulfides, arsenites, chlorides and carbonates in gold ores. Gold mines have greatly increased the Cu concentration in the environment, which upon release binds to particles of organic matter, clay minerals and sesquioxides, leading to great accumulation in the soil (Fashola et al., 2016). Mn in mineral soil derived from weathering rocks forms Mn oxides when the reaction in soil is oxidative in nature. In the soil environment, Mn is generally concentrated in part of the soil profile (Nurcholis et al., 2017).
Assessment of chromosome aberrations in S. nigrovittata
The heavy metals during gold extraction leach out in an uncontrolled manner into surrounding environments upon exposure to water, plants and aquatic animals (Fashola et al., 2016). Evaluating the levels of potentially hazardous metals and metalloids in exposed environmental areas and local biodiversity is important (Moldovan et al., 2022). In this study, a representative habitant (frog) in the gold mine area was selected to evaluate cytotoxicity as a biomarker.
Structural abnormalities of chromosomes in aquatic animals from previous studies have been reported at both study sites and in laboratory research. Neeratanaphan et al. (2017, 2020) researched chromosome aberrations in fish (Esomus metallicus) and Asian swamp eel (Monopterus albus) from a gold mine site in the Wang Saphung District of Loei Province, Thailand. They found six types of CAs, including CF, CG, SCG, F, D, and P, and seven types of CAs, including SCG, SSCG, SCB, CF, F, SSCF and D. Additionally, Tengjaroenkul et al. (2018) and Intamat et al. (2016b) studied the effect of As on chromosome aberrations in fish (O. niloticus) and frogs (F. limnocharis) at the laboratory scale. They revealed five types of CAs, including SCG, SCB, CG, F and D in fish and five types of CAs, including SCG, ISCG, SCB, D, F, CF and P, in frogs. Boonmee et al. (2018) reported that the concentration of Cd induced 10 types of CAs, including SCG, ISCG, SCB, ISCB, CF, D, F, CG, ISAF and SCD, in F. limnocharis.
Gbadebo et al. (2022) reported that contamination by Pb, Cd, Cr, Cu, Zn and Fe in underground water significantly induced micronuclei and cytotoxicity in rat bone marrow. Bakare et al. (2013) suggested a possible mechanism for heavy metal pollution inducing toxicity via oxidative damage. Heavy metals can bind to DNA by interacting with phosphate and base residues and then change the primary and secondary structures of DNA. They can also interfere with protein structure and function to cause DNA damage (Bakare et al., 2013). Additionally, Cr, Cd, and As cause genomic instability and defects in DNA repair following the induction of oxidative stress and DNA damage (Balali-Mood et al., 2021). All previous reports mentioned above suggested that DNA damage and defects in DNA repair led to chromosome aberrations in frogs.
The results of chromosome analysis in frogs (S. nigrovittata) samples near the Sepon gold-copper mine area indicated that the chromosome aberrations were detected. The accumulation of heavy metal in the frog near the Sepon gold-copper mine can lead for concern because of its potential effects on breeding, conservation and chromosome evolution of living organisms in aquatic ecosystems, and can cause population decreases, mutations and diversity loss in amphibians (Serrano et al., 2012). The toxic heavy metals from Sepon gold-copper mine leached out to nearby river can cause many risks to aquatic animals as well as human health. The government should be concerned with toxicant management before they spread into the environment.
CONCLUSION
The Nam Kok River around the Sepon gold-copper mine as the study site contained relatively high concentrations of heavy metals, especially As, Ba, Cd, Cr, Cu, Fe, Mn, Ni, and Zn, as well as in frog samples (S. nigrovittata) when compared with the reference site. The concentrations of As, Cd, Cr and Mn in frogs at the study site exceeded the food quality of Australia New Zealand Food, EC and WHO standards. Moreover, the average concentration of heavy metals and metalloids affected S. nigrovittata by causing chromosome abnormalities in metaphase cells, especially ISCG, ISCB, D, CD, F, and ISAF. The results of this study revealed information that can apply to human health risks and environmental management and can contribute to improvements in standards, legislation, regulations, guidelines, and policies for the people around mining areas in the future.
ACKNOWLEDGEMENTS
The authors would like to thank the Research Project on Ecotoxicology, Natural Resources and Environment, Khon Kaen University, Khon Kaen, Thailand.
AUTHOR CONTRIBUTIONS
All authors read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
Abdul-Wahab, S. and Marikar, F. 2012. The environmental impact of gold mines: Pollution by heavy metals. Open Engineering. 2: 304-313.
American Public Health Association (APHA). 2012. Standard methods for the examination of water and waste-water; American public health association/American water works association/Water environment federation. Washington DC, USA: American Public Health Association.
Australia and New Zealand Standards. 2011. Contaminants and natural toxicants, Code-Standard 1.4.1 Issue 124; Federal register of legislative instruments F2011C00542. Canberra, Australia: Standards Australia.
Aydın, M. and Tunca, E. 2022. Ecological risk assessment of elemental accumulation under the impact of gold mine. International Journal of Environmental Science and Technology. 19: 7093-7112.
B’ey, T.D. 2015. Effects of chronic, sublethal ferric iron exposure on the critical swim speed of rainbow trout (Oncorhynchus mykiss) and critical thermal maximum of cutthroat trout (Oncorhynchus clarkii) [Thesis]. Colorado, USA: Colorado State University. p.88.
Bakare, A.A., Alabi, O.A., Gbadebo, A.M., Ogunsuyi, O.I., and Alimba, C.G. 2013. In vivo cytogenotoxicity and oxidative stress induced by electronic waste leachate and contaminated well water. Challenges. 4: 169-187.
Balali-Mood, M., Naseri, K., Tahergorabi, Z., Khazdair, M.R., and Sadeghi, M. 2021. Toxic mechanisms of five heavy metals: Mercury, lead, chromium, cadmium, and arsenic. Frontiers in Pharmacology. 12: 643972.
Boonmee, S., Thitiyan, T., Tanomtong, A., Tengjaroenkul, B., and Neeratanaphan, L. 2018. Cytotoxicity in the frog (Fejervarya limnocharis) after acute cadmium exposure in vivo. International Journal of Environmental Studies. 75: 978-989.
Burlibaşa, L., and Gavrilă, L. 2011. Amphibians as model organisms for study environmental genotoxicity. Applied Ecology and Environmental Research. 9: 1-5.
Chand, V., and Prasad, S. 2013. ICP-OES assessment of heavy metal contamination in tropical marine sediments: A comparative study of two digestion techniques. Microchemical Journal. 111: 53-61.
Chandanshive, N.E. 2013. The seasonal fluctuation of physico chemical parameters of river Mula-Mutha at Pune, India and their impact of fish biodiversity. Research Journal of Animal, Veterinary and Fishery Sciences. 1: 11-16.
Compaore, W.F., Dumoulin, A., and Rousseau, D.P. 2020. Metals and metalloid in gold mine pit lakes and fish intake risk assessment, Burkina Faso. Environmental Geochemistry and Health. 42: 563-577.
Dorleku, M.K., Nukpezah, D., and Carboo, D. 2018. Effects of small-scale gold mining on heavy metal levels in groundwater in the Lower Pra Basin of Ghana. Applied Water Science. 8: 1-11.
European Commission (EC). 2001. Commission regulation (EC) No 466/2001 of 8 March 2001 setting maximum levels for certain contaminants in foodstuffs. Luxembourg City, Luxembourg: Official Journal of the European Communities.
European Commission (EC). 2005. Muscle meat of fish as regards cadmium (Cd); commission regulation (EC) No 78/2005 amending regulation (EC) No 466/2001 as regards heavy metals, L 16/43–45. Luxembourg City, Luxembourg: Official Journal of the European Communities.
European Union (EU). 2002. Heavy metals in wastes; European commission on environment. Copenhaken, Denmark: European Commission.
Fashola, M.O., Ngole-Jeme, V.M., and Babalola, O.O. 2016. Heavy metal pollution from gold mines: Environmental effects and bacterial strategies for resistance. International Journal of Environmental Research and Public Health. 13: 1047.
Food and Agriculture Organization (FAO). 1994. Water quality for agriculture; FAO irrigation and drainage paper, 29 Rev. 1. Rome, Italy: Food and Agriculture Organization of the United Nations.
Food and Agriculture Organization/World Health Organization (FAO/WHO). 1989. National research council recommended dietary. Washington, DC, USA: National Academy Press.
Gbadebo, A.M., Alabi, O.A., Alimba, C.G., and Bakare, A.A. 2022. Metal bioaccumulation, cytogenetic and clinico-biochemical alterations in Rattus norvegicus exposed in situ to a municipal solid waste landfill in Lagos, Nigeria. Biological Trace Element Research. 200: 1287-1302.
GIS Geography. n.d. Laos Political Map; [Accessed 19 June 2022]. Retrieved From https://gisgeography.com/laos-map/
Govind, P., and Madhuri, S. 2014. Heavy metals causing toxicity in animals and fishes. Research Journal of Animal, Veterinary and Fishery Sciences. 2: 17-23.
Intamat, S., Phoonaploy, U., Sriuttha, M., Patawang, I., Tanomtong, A., and Neeratanaphan L. 2016a. Cytotoxic evaluation of rice field frogs (Fejervarya limnocharis) from gold mine area with arsenic contamination. The Nucleus. 59: 181-189.
Intamat, S., Phoonaploy, U., Sriuttha, M., Tengjaroenkul, B., and Neeratanaphan, L. 2016b. Heavy metal accumulation in aquatic animals around the gold mine area of Loei province, Thailand. Human and Ecological Risk Assessment. 22: 1418-1432.
International Council on Mining and Metals (ICMM). 2011. Utilizing mining and mineral resources to foster the sustainable development of the Lao PDR. Vientiane, Laos: National University of Laos.
Kapia, S., Rao, B.K., and Sakulas, H. 2016. Assessment of heavy metal pollution risks in Yonki Reservoir environmental matrices affected by gold mining activity. Environmental Monitoring and Assessment. 188: 1-10.
Kiser, T., Hansen, J., and Kennedy, B. 2010. Impacts and pathways of mine contaminants to bull trout (Salvelinus confluentus) in an Idaho watershed. Archives of Environmental Contamination and Toxicology. 59: 301-311.
Kumar, S., Islam, A.R.M.T., Hasanuzzaman, M., Salam, R., Islam, M., Khan, R., Rahman, M.S., Pal, S.C., Ali, M.M., Idris, A.M., Gustave, W., and Elbeltagi, A. 2022. Potentially toxic elemental contamination in Wainivesi River, Fiji impacted by gold-mining activities using chemometric tools and SOM analysis. Environmental Science and Pollution Research. 29: 42742-42767.
Maneechot, N., Supiwong, W., Jumrusthanasan, S., Siripiyasing, P., Pinthong, K., and Tanomtong, A. 2015. Chromosomal characteristics of the royal knifefish, Chitala blanci (Osteoglossiformes, Notopteridae) by conventional and Ag-NOR staining techniques. Cytologia. 80: 159-66.
Moldovan, A., Török, A.I., Kovacs, E., Cadar, O., Mirea, I.C., and Micle, V. 2022. Metal contents and pollution indices assessment of surface water, soil, and sediment from the Arieș river basin mining area, Romania. Sustainability 14: 8024.
Neeratanaphan, L., Kanjanakunti, A., Intamat, S., and Tengjaroenkul, B. 2020. Analysis of chromosome abnormalities in the Asian swamp eel (Monopterus albus) affected by arsenic contamination near a gold mine area. International Journal of Environmental Studies. 77: 815-829.
Neeratanaphan, L., Khamlerd, C., Chowrong, S., Intamat, S., Sriuttha, M., and Tengjaroenkul, B. 2017. Cytotoxic assessment of flying barb fish (Esomus metallicus) from a gold mine area with heavy metal contamination. International Journal of Environmental Studies. 74: 613-624.
Ngangnouvong, I. 2019. Mining in Laos, economic growth, and price fluctuation. in Proceedings of the United Nations Conference on Trade and Development, the 11th Multi-year Expert Meeting on Commodities and Development 15-16 April 2019. Geneva, Switzerland.
Nurcholis, M., Yudiantoro, D.F., Haryanto, D., and Mirzam, A. 2017. Heavy metals distribution in the artisanal gold mining area in Wonogiri. Indonesian Journal of Geography. 49: 133-144.
Owusu-Prempeha, N., Awuahb, O.K., Abebresec, I.K., and Amaninga, E.N. 2022. Analysis of the status and ecological risks of heavy metals contamination in artisanal and small-scale gold mine-spoils at the Atewa forest landscape, Ghana. Scientific African. 16: e01235.
Pejman, A., Bidhendi, G.N., Ardestani, M., Saeedi, M., and Baghvand, A.A. 2015. New index for assessing heavy metals contamination in sediments: A case study. Ecological Indicators. 58: 365-373.
Rooney, D.E. 2001. Human cytogenetics constitutional analysis, a practical approach. London, UK: Oxford University Press.
Serrano, A.E., Relyea, R.A., Tejedo, M., and Torralva, M. 2012, Understanding of the impact of chemicals on amphibians: A meta-analysis review. Impact of Pollution on Amphibians 2: 1382-1397.
Soulivongsa, L., Tengjaroenkul, B., and Neeratanaphan, L. 2020. Effects of contamination by heavy metals and metalloids on chromosomes, serum biochemistry and histopathology of the bonylip barb fish near Sepon gold-copper mine, Lao PDR. International Journal of Environmental Research and Public Health. 17: 9492.
Soulivongsa, L., Tengjaroenkul, B., Patawang, I., and Neeratanaphan, L. 2021. Cytogenetic, serum liver enzymes and liver cell pathology of the hampala barb fish (Hampala macrolepidota) affected by toxic elements in the contaminated Nam Kok river near the Sepon gold-copper mine, Lao PDR. International Journal of Environmental Research and Public Health. 18: 5854.
Tang, L., Zhang, Y., Ma, S., Yan, C., Geng, H., Yu, G., Ji, H., and Wang, F. 2021. Potentially toxic element contaminations and lead isotopic fingerprinting in soils and sediments from a historical gold mining site. International Journal of Environmental Research and Public Health. 18: 10925.
Tengjaroenkul, B., Intamat, S., Thanomsangad, P., Phoonaploy, U., and Neeratanaphan, L. 2018. Cytotoxic effect of sodium arsenite on Nile tilapia (Oreochromis niloticus) in vivo. International Journal of Environmental Studies. 75: 580-591.
Thailand Pollution Control Department (TPCD). 1986. The Standard levels of heavy metals in tissues of aquatic animals No. 98. Bangkok, Thailand: Thailand Pollution Control Department.
Thailand Pollution Control Department (TPCD). 1994. Surface water quality standard, notification of the national environmental board No. 8. Bangkok, Thailand: Thailand Pollution Control Department.
Thailand Pollution Control Department (TPCD). 2004. Soil quality standard for residential and agricultural use according; notification of the national environment board No. 25. Bangkok, Thailand: Ministry of Natural Resource and Environment.
Thailand Pollution Control Department (TPCD). 2018. Criteria for sediment quality standard in surface water source net. No.2. Bangkok, Thailand: Ministry of Natural Resource and Environment.
Thammachoti, P., Khonsue, W., Kitana, J., Varanusupakul, P., and Kitana, N. 2012. Morphometric and gravimetric parameters of the rice frog Fejervarya limnocharis living in areas with different agricultural activity. Journal of Environmental Protection. 3: 1403-1408.
United Nations Environment Programme Global Environment Monitoring System (UNEPGEMS). 2008. Water quality for ecosystem and human health, 2nd ed.; Burlington, Ontario, L7R 4A6 CANADA: prepared and published by united nations environment programme global environment monitoring system (GEMS)/Water Programme. Ontario, Canada: National Water Research Institute.
US geological survey. 2022. Laos gold production; [Accessed 19 June 2022]. Retrieved from https://www.ceicdata.com/en/indicator/laos/gold-production
World Health Organization (WHO). 1989. Heavy metals environmental aspects; environment health criteria. Geneva, Switzerland: World Health Organization.
World Health Organization (WHO). 2003. Iron in drinking-water. In background document for preparation of who guidelines for drinking-water quality. Geneva, Switzerland: World Health Organization.
World Health Organization (WHO). 2011. Arsenic in drinking-water. In background document for preparation of WHO guidelines for drinking-water quality. Geneva, Switzerland: World Health Organization.
Zhang, L., Campbell, L.M., and Johnson, T.B. 2012. Seasonal variation in mercury and food web biomagnification in Lake Ontario, Canada. Environmental Pollution. 161: 178-84.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand.
Nuntita Ruksachat1, Bundit Tengjaroensakul1, Lamyai Neeratanaphan2, Alongklod Tanomtong2, Nikom Srikacha3, Thonglom Phommavong4 and Latsamy Soulivongsa 4, *
1 Faculty of Veterinary Medicine, Khon Kaen University, Khon Kaen 40002, Thailand.
2 Faculty of Science, Khon Kaen University, Khon Kaen 40002, Thailand.
3 Faculty of Natural Resources, Rajamangala University of Technology Isan, Sakon Nakhon 47160, Thailand.
4 Faculty of Agriculture, National University of Laos, Vientiane 0604, Lao PDR.
Corresponding author: Latsamy Soulivongsa, E-mail: soulivongmee@gmail.com
Total Article Views
Editor: Veerasak Punyapornwithaya,
Chiang Mai University, Thailand
Article history:
Received: September 9, 2022;
Revised: October 13, 2022;
Accepted: October 21, 2022;
Published online: November 17, 2022

