
Non-Polar Fraction Constituents, Phenolic Acids, Flavonoids and Antioxidant Activity in Fruits from Florina Apple Variety Grown under Different Agriculture Management
Nadezhda Petkova*, Tatyana Bileva, Ekaterina Valcheva, Galya Dobrevska, Neli Grozeva, Mima Todorova, and Vladislav PopovPublished Date : 2023-01-05
DOI : https://doi.org/10.12982/NLSC.2023.012
Journal Issues : Number 1, January-March 2023
Abstract The effect of agricultural management on non-polar compounds, phenolic acids, flavonoids and antioxidant properties in apple fruit of Florina variety was studied. Antioxidant activity was evaluated using four assays (DPPH, ABTS, FRAP and CUPRAC), based on different mechanisms. The current research demonstrated that organically grown apple fruits yielded significantly higher levels of total phenolics, unsaturated fatty acids and antioxidant activity than apples from the conventional conditions. Seven representatives of phenolic acids (gallic, p-coumaric, chlorogenic, vanillic, caffeic, syringic and salicylic) and four flavonoids (quercetin, rutin, catechin and epicatechin) were found in fruits. Chlorogenic, vanillic, salicylic, caffeic acids and catechin were dominated in apples grown in organic orchards. Epicatechin, gallic and syringic acids were in the highest content in conventionally grown sward apples. The highest correlation (r2>0.99) was found between total polyphenols and antioxidant activity by three methods (DPPH, ABTS and FRAP methods). As a result of the organically grown conditions, the quality of the fruits of the Florina apple variety is improved based on the accumulation of bioactive compounds with antioxidant activity. Moreover, thirty-nine volatile components (saturated and unsaturated fatty acids, fatty alcohols, phytosterols and triterpenes) were detected in non-polar apple fraction. Palmitoleic, palmitelaidic, oleic, elaidic, (13E)-octadecenoic, (13Z)-octadecenoic, (11Z)-eicosenoic and (13Z)-eicosenoic, linoleic acid and linolenic acid and ursolic and oleanolic acid acetates dominated in organic apple orchards. Therefore, organic apple provided more phenolic acids, unsaturated fatty acids, ursolic and oleanolic acid acetates and catechin for healthy human nutrition.
Keywords: Apples, Antioxidants, Anthocyanins, Organical And Conventional Growing Conditions, Polyphenolic and non-polar compounds
Funding: The authors are grateful for the research funding provided by the Bulgarian Ministry of Education and Science under the National Research Programme “Healthy Foods for a Strong Bio-Economy and Quality of Life” approved by DCM # 577/17 .08.2018.
Citation: Petkova, N., Bileva, T., Valcheva, E., Dobrevska, G., Grozeva, N., Todorova, M., and Popov, V. 2023. Non-polar fraction constituents, phenolic acids, flavonoids and antioxidant activity in fruits from florina apple variety grown under different agriculture management. Nat. Life Sci. Commun. 22(1): e2023012.
INTRODUCTION
Apple (Malus domestica Borkh.) occupied third place as the most consumed fruits, worldwide (FAOSTAT, 2021). Apple production in Bulgaria increased, as in 2018 reached 50 298 tons (Manolova, 2021). Five apple varieties were among the most popular for growing - “Golden Delicious”, “Red Delicious”, “Granny Smith”, “Florina” and “Melrose”. Among them “Florina” is the widespread and preferred variety with 1023.3 ha, which presented 19.9% of the total apple orchards, followed by “Golden Delicious”, with 18.0% and “Granny Smith” – with 11.7% (Blagoeva and Krishkova, 2020). “Florina” is French variety and it is among the most preferred apple varieties for fresh consumption (Vasile et al, 2021). The reason for that is the resistance of this apple variety against the most significant disease on the apples – scab. The “Florina” apple fruits are yellow-green, and all surfaces are covered by red to dark red stripes. The typical features for the fruit flesh are creamy-colour, medium firmness, juiciness, and a blend of sweet and tart taste (Petkova et al., 2020). The representatives of bioactive compounds, such as polyphenols, organic acids and carbohydrates caused a serious positive impact on the taste and apple quality (Wojdyło et al., 2008; Mılosevıc et al., 2019; Petkova et al., 2019). The values of these compounds varied in different ranges according to the apple varieties, maturity stage, agriculture management practices and environmental conditions (Tarola et al., 2019).
Apple fruits are rich sources of polyphenols, as flavonoids, flavonols, dihydrochalcones, anthocyanins, and phenolic acids, especially hydroxybenzoic and hydroxycinnamic acids. Phenolic substances are important for human nutrition because of the positive effect on health as a result of their high antioxidant activity. Polyphenols have a serious influence on the quality characteristics of fresh fruits. The concentration of phenolic compounds in apples varied and depends on the cultivar, fruit maturity, cultivation method, soil and climatic conditions, and other factors (Vasile et al, 2021). Apple peel is a rich source of flavonols and anthocyanins, while flavanols, dihydrochalcones and hydroxycinnamic acids are the main phenolic compounds located in the apple flesh. It was reported that phenolic compounds and triterpene acids in apples possessed anti-inflammatory properties, reduce the risk of cardiovascular disease, type II diabetes, and protective effect against lung and colorectal cancers, neurodegeneration and Alzheimer’s disease (Gean˘a et al., 2021). However, the detailed study about the presence of phenolic compounds in Florina apple is still not sufficient. The impact of agriculture management on phenolic acids and antioxidant potential of apple fruits were not evaluated in details.
Therefore, the aim of this research was to evaluate the effect of different agricultural practices on phenolic acids, total phenols, anthocyanins and flavonoids content. Moreover, antioxidant activity in apple fruit of Florina variety was studied.
MATERIALS AND METHODS
Materials
All the reagents and solvents were of the analytical grade. They were purchased from Sigma-Aldrich (St. Louis, MO, USA) and Fillab (Plovdiv, Bulgaria) and used as they were received.
The apple fruits from Florina variety were obtained from the training and experimental fields of Agriculture University, Plovdiv, from nine years old apple orchards from Plovdiv (42.133845 N, 24.807315 E) and Brestnik village. The apple orchards were cultivated under organic and conventional agricultural practices. Two technological approaches are applied, which are the main technologies for growing apples in our country - black fallow and mulching (sward orchards). Black fallow is a technology that combines a number of tillage treatments aimed at destroying weeds and preserving soil moisture. In addition to soil maintenance, plant protection, fertilization and irrigation operations are also included. In the other technological solution, the soil is covered with grass, and the main technological operations are mowing, irrigation, fertilization and plant protection measures. The conventional growing conditions included treatment with the following fungicides: Tiram 80 VG - 0.3%, Follicur 250 EV - 0.04% (broad spectrum system), Delan 700 VDG - 0.05%, Score 250 EC - 0.02%, Horus 50 EC - 0.03%, Bayfidan 250 EC (systemic) - 0.015% and Shavit F 72 VDG - 0.2% during May. The orchard was treated with Nurele D - 0.04%, Fury 10 EC - 0.125% (contact synthetic pyrethroid of a new generation) and Coragen 20 SC - 16 ml/ha. against cross moth, lice and mites. Organic orchard treatment included the uses of Funguran ON – 0.3% (contact broad-spectrum, containing 77% Cu-hydroxide) with colloidal sulfur – 1:400 and champion VP – 0.15% (at the end of November). Against apple worms were used Trifolio S Forte 0.3% (50% vegetable oil + 50% emulsifier) and Acarzin 3% (85% mineral oil + 15% emulsifier). Against apple diseases and pests the antifungal agent Kuore 200g/dka (that contains 10% Cu and 1.1% Zn, S – 1:400, Nimazal T/C - 200 ml/dka (a bio insecticide) + Trifolio S Forte - 0.3% were used. Pheromone traps were also applied to control apple fruit worm.
Florina Apple variety were collected during the first week of October 2021, transferred to the lab, stored at room temperature for three days before analysis (Figure 1).

Figure 1. Florina apple variety grown under different agriculture practices.
Sample preparation
The apple fruits were cleaned with tab water, then the seeds were removed. The samples were cut and chopped into pieces. The peel was not removed and apple was analyzed whole. Then the samples were finely ground to the puree in laboratory homogenizer (Gorenje). The samples (2.5 g) were weighted in 50 ml plastic tubes and extracted with 50 ml 70% (v/v) ethanol using an ultrasonic bath (Isolab, Germany) at 40 kHz frequency for 20 minutes. The extraction procedure was repeated three times. The obtained extracts were collected, the volume was measured. The extract was analyzed for phenolic compounds and antioxidant activity.
Total phenolic content
The total phenolic content was measured using the Folin–Ciocalteu’s reagent diluted five times (Sansomchai et al., 2021). Apple extract 0.2 ml was put into a disposable plastic cuvette, then 1 ml Folin–Ciocalteu reagent and 0.8 ml 7.5% Na2CO3 was added. The absorbance was measured after 20 min at 765 nm against a blank sample, prepared with ethanol instead of extract. The results were expressed in mg equivalent of gallic acid (GAE) per g dry weight (dw) (Nikolova et al., 2022).
Total flavonoids content
The total flavonoids content was defined by Al(NO3)3 reagent. The values were expressed as mg equivalents quercetin (QE) per g dw (Ivanov et al., 2014).
Total monomeric anthocyanins content
pH differential method was used as the measurement was performed at wavelengths 520 and 700 nm, respectively. The results were presented as mg cyanidin-3-glycoside per g dry weight (Lee et al., 2005).
Antioxidant activity
The DPPH radical-scavenging ability.
Apple extract (0.15 ml) was mixed with 2.85 ml 0.1 mM DPPH methanol solution. The reduction of absorbance was measured at 517 nm against methanol. The percent inhibition was also calculated. The results were expressed in mM Trolox® equivalents (TE)/g dw (Ivanov et al., 2014).
ABTS+ radical scavenging ability.
The ABTS+ solution (2.85 ml) was added to 0.15 M apple extract. The absorbance was measured at 734 nm after 15 min at 37°C. The percent inhibition was also calculated. The results were expressed in mM Trolox® equivalents (TE)/g dw (Ivanov et al., 2014).
FRAP assay.
The FRAP reagent was prepared before analysis and consisted of 0.3M acetate buffer (pH 3.6), 10 mM 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ) in 40 mm HCl and 20 mm aqueous solutions of FeCl3×6H2O in the ratio 10:1:1 (v/v/v). FRAP reagent (3.0 ml) was added to 0.1 ml apple extract. The absorbance of the sample was measured at 593 nm (Benzie and Strain, 1996). The results were expressed in mM Trolox® equivalents (TE)/g dw apple.
CUPRAC assay.
Apple extract (0.1 ml) was added to 1 ml CuCl2 × 2H2O, 1 ml ethanol solution of Neocuproine, 1 ml 0.1M ammonium acetate buffer and 1 ml distilled H2O. The absorbance was measured at 450 nm after 20 min at 50°С. The results were expressed in mM Trolox® equivalents (TE)/g dw (Ivanov et al., 2014).
HPLC analysis of phenolic acids and flavonoids.
Phenolic acids and flavonoids were analyzed on HPLC system Waters 1525 Binary Pump (Waters, Milford, MA, USA), equipped with Waters 2484 Dual Absorbance Detector (Waters, Milford, MA, USA) and a C18 column (Supelco Discovery HS, 5 µm, 25cm × 4.6mm), and Breeze 3.30 software. Mobile phase consisted of 2% (v/v) acetic acid (solvent A) and 0.5% (v/v) acetic acid: acetonitrile (50:50) (solvent B) was used in a gradient mode according to Marchev et al. (2011). Gallic, protocatehuic, chlorogenic, vanillic, caffeic, syringic, p-coumaric, ferulic and salicylic acids were used for preparation of the standard calibration curves. Mobile phase consisted of 2.0% (v/v) acetic acid (solvent A) and methanol (solvent B) was used for separation of flavonoids in gradient mode (Marchev et al., 2011). Kaempferol, catechin, epicatechin, quercetin, hesperidine and rutin were used for calibration curve.
GC-MS of volatile compounds from non-polar fraction.
Dried and finely ground apple fruits (without seeds) were extracted with n-hexane in a Soxhlet apparatus for 6 h. The extracts were evaporated to dryness and then they were saponified under a reflux for 1.5 h with 2 mol/L KOH (dissolved in 50% ethanol) The liquid-liquid extraction with n-hexane was performed and then the extract was evaporated. The GC-MS analysis was done with a gas chromatograph Agilent Technology, Hewlett Packard 7890 A, coupled with mass detector Agilent Technology 5975 C inert XL EI/CI MSD (Agilent, USA) at 70 eV. Under conditions mentioned by Ivanov et al. (2018). The identification of compounds was done using 2.64 AMDIS (Automated Mass Spectral Deconvolution and Identification System, National Institute of Standardization and Technology, NIST, Gaithersburg, MD, USA).
Statistical analysis
All the experiments were performed in triplicate, and the results were expressed as mean ± SD (standard deviation).Statistical analysis was performed using and Excel 2015.
RESULTS
The total phenols, total flavonoids and total monomeric anthocyanins were evaluated in Florina apple grown in organic and conventional conditions. Moreover, the antioxidant activity of apples was evaluated by four methods based on two different mechanisms SET (single electron transfer) – FRAP and CUPRAC assays; and HAT-type (hydrogen atom transfer (DPPH radical-scavenging ability and ABTS+ radical scavenging ability). The results were summarized in table 1. The high antioxidant activity was demonstrated by copper reducing method (CUPRAC) that is based on single electron transfer, followed by ABST method that is based on mix mode. The highest antioxidant potential demonstrated organically grown apple especially sward orchard (Table 1).
Table 1. Phenolic content and antioxidant capacity of apple variety Florina.
|
|
Conventional orchard |
Conventional sward orchard |
Organic orchard |
Organic sward orchard |
|
Total phenols, mg GAE1/g |
2.49 ± 0.11d |
3.13 ± 0.44b |
3.93 ± 0.23c |
4.84 ± 0.54a |
|
Total flavonoids, mg QE2/g dw |
0.65 ± 0.10a |
0.56 ± 0.05c |
0.79 ± 0.05c |
0.94 ± 0.08b |
|
Total monomeric anthocyanins, mg cyn-3-glc/ g dw |
4.57 ± 1.03a |
24.72 ± 1.03a |
26.43 ± 0.52c |
31.22 ± 0.70b |
|
Antioxidant capacity, mM TE/ g dw |
|
|
|
|
|
DPPH |
27.71 ± 0.12d |
36.21 ± 1.20a |
44.57 ± 1.15b |
45.46 ± 0.61c |
|
ABTS |
59.79 ± 0.28d |
63.14 ± 0.35b |
81.66 ± 0.75a |
103.09 ± 0.33c |
|
FRAP |
15.53 ± 0.13c |
22.02 ± 0.65b |
30.06 ± 1.42a |
38.15 ± 0.65b |
|
CUPRAC |
62.53 ± 0.20c |
157.35 ± 0.19d |
170.68 ± 1.15b |
220.48 ± 2.38a |
Note: 1GAE- gallic acid equivalent, 2QE- quercetin equivalent, Values followed by different letters in the same column are significantly different as pairwise comparisons were done with the Duncan post-hoc test (P = 0.05)
Compositions of the individual phenolic compounds, as phenolic acids and flavonoids were summarized in table 2. Seven phenolic acids (gallic, chlorogenic, vanillic, caffeic, syringic and p-coumaric acids) were detected in samples, mainly in organic orchards. From representatives of flavonoids, two flavan-3-ols ((+)-catechin and (-)- epicatechin), one flavonol (quercetin), one quercetin glycoside (rutin) were detected in Florina apples.
Table 2. HPLC analysis of polyphenols content in apple variety Florina, µg/g dw.
|
|
Retention time, min |
Conventional orchard |
Conventional sward orchard |
Organic orchard |
Organic sward orchard |
|
|||||
|
Phenolic acids |
|
|
|
|
|
|
|||||
|
Gallic acid |
6.63 |
0.41 |
1.01 |
0.52 |
nd |
||||||
|
Chlorogenic acid |
24.13 |
109.22 |
140.42 |
493.41 |
130.73 |
||||||
|
Vanillic acid |
27.51 |
127.26 |
140.08 |
274.27 |
90.69 |
||||||
|
Caffeic acid |
29.42 |
nd |
nd |
nd |
34.52 |
||||||
|
Syringic acid |
33.20 |
18.86 |
30.36 |
26.29 |
18.31 |
||||||
|
p-coumaric acid |
43.56 |
nd |
nd |
nd |
5.65 |
||||||
|
Salicylic acid |
49.55 |
13.54 |
24.76 |
35.33 |
21.97 |
||||||
|
Total phenolic acids |
|
269.30 |
336.63 |
829.82 |
301.87 |
||||||
|
fl avan-3-ols |
|
|
|
|
|
|
|||||
|
(+)-Catechin |
20.95 |
37.87 |
31.68 |
36.35 |
78.56 |
|
|||||
|
(-)- Epicatechin |
36.10 |
78.84 |
173.21 |
97.63 |
125.58 |
|
|||||
|
Flavonols |
|
|
|
|
|
|
|||||
|
Quercetin |
62.68 |
nd |
nd |
1.36 |
nd |
|
|||||
|
Quercetin glycoside |
|
|
|
|
|
||||||
|
Rutin |
52.33 |
10.49 |
24.03 |
87.99 |
91.17 |
|
|||||
Note: n.d. - not detected
The correlation between total phenolic contents, total flavonoid content, total monomeric anthocyanins, and total antioxidant activity were evaluated (Table 3).
Table 3. Correlation (r2) coefficient between phenolic compounds and antioxidant activity.
|
|
DPPH |
ABTS |
FRAP |
CUPRAC |
Total phenols |
Total flavonoids |
|
Total phenols |
0.9344 |
0.9801 |
0.9994 |
0.9326 |
- |
0.8848 |
|
Total flavonoids |
0.7413 |
0.9541 |
0.8768 |
0.6571 |
0.8848 |
- |
|
Total monomeric anthocyanin |
0.6909 |
0.0315 |
0.2520 |
0.5515 |
0.8613 |
0.5316 |
Additionally, the non-polar fraction of the fruits of apple variety "Florina" was investigated. Thirty-nine components were found, in which saturated fatty acids, beneficial for the human body unsaturated (omega-9, 6 and 3 fatty acids) such as oleic, linolenic, linoleic, waxes, phytosterols and esters of oleanolic acid and ursolic acid were detected (Table 4). Unsaturated fatty acids and fatty alcohols predominated in organic apple orchards, while saturated fatty acids, phytosterols, and terpenoids were highest in the conventionally grown fruit. Oleanolic and ursolic acid acetates dominated in organic apples. The detected compounds in the non-polar fraction of apple fruits demonstrated the nutritional lipid composition, with fatty acids dominating (40-59% TIC). Monounsaturated (palmitoleic, palmitelaidic, oleic, elaidic, (13E)-octadecenoic, (13Z)-octadecenoic, (11Z)-eicosenoic and (13Z)-eicosenoic acids) and polyunsaturated fatty acids and linoleic acid (linolenic acid) dominated in organic apple orchards. α-Amyrin and β-amiryn, which are precursors of oleanolic and ursolic acids were also detected in Florina apples, as the levels of β-amiryn was twice higher than α-amyrin and it dominated in organically grown apple fruits. However, triterpene betulin was higher in conventionally grown ones. Additionally, the distribution of volatile compounds by chemical groups in non-polar fraction of Florina apple fruits was shown (Figure 2).
Table 4. Content of volatile components in non-polar fraction of harvested apples.
|
Peak |
RT |
RI |
Name |
% of TIC |
|||
|
Organic sward orchard |
Conventional sward orchard |
Organic orchard |
Conventional |
||||
|
1 |
11,86 |
1258 |
n-Octanoic acid tms |
0.20 |
0.09 |
0.12 |
0.26 |
|
2 |
16,79 |
1447 |
n-Decanoic acid tms |
0.11 |
0.10 |
0.14 |
0.22 |
|
3 |
21,41 |
1652 |
Dodecanoic acid tms |
0.62 |
0.75 |
0.60 |
0.70 |
|
4 |
25,62 |
1847 |
n-Tetradecanoic acid tms (Myristic acid) |
0.95 |
0.95 |
0.67 |
1.01 |
|
5 |
27,16 |
1943 |
n-Pentadecanoic acid tms |
0.36 |
0.28 |
0.23 |
0.18 |
|
6 |
28,53 |
2017 |
9Z-Hexadecenoic acid tms (Palmitoleic acid) |
0.19 |
0.15 |
0.12 |
0.10 |
|
7 |
28,66 |
2026 |
9E-Hexadecenoic acid tms (Palmitelaidic acid) |
0.30 |
0.24 |
0.19 |
0.16 |
|
8 |
29,56 |
2038 |
n-Hexadecanoic acid tms (Palmitic acid) |
16.56 |
13.39 |
12.85 |
15.52 |
|
9 |
31,27 |
2122 |
n-Heptadecanoic acid tms |
0.67 |
0.46 |
0.33 |
0.63 |
|
10 |
32,49 |
2205 |
(9Z,12Z)-Octadecadienoic acid tms (Linoleic acid) |
11.65 |
1.40 |
5.72 |
10.37 |
|
11 |
32,58 |
2209 |
(9Z)-Octadecenoic acid tms (Oleic acid) |
5.63 |
0.77 |
2.01 |
3.73 |
|
12 |
32,66 |
2214 |
(9E)-Octadecenoic acid tms (Elaidic acid) |
0.35 |
0.12 |
0.10 |
0.39 |
|
13 |
32,90 |
2218 |
(9Z,12Z,15Z)-Octadecatrienoic acid tms |
0.16 |
0.14 |
0.13 |
0.11 |
|
14 |
32,40 |
2225 |
(13E)-Octadecenoic acid tms |
0.14 |
0.13 |
0.11 |
0.10 |
|
15 |
32,51 |
2232 |
(13Z)-Octadecenoic acid tms |
0,21 |
0,19 |
0,17 |
0,15 |
|
16 |
32,63 |
2240 |
n-Octadecanoic acid tms (Stearic acid) |
4.95 |
3.77 |
4.22 |
4.88 |
|
17 |
35,35 |
2409 |
n-Heneicosanol tms |
2.25 |
0.75 |
1.51 |
1.60 |
|
18 |
35,53 |
2419 |
(11Z)-Eicosenoic acid tms |
3.76 |
3.38 |
1.93 |
2.27 |
|
19 |
35,86 |
2425 |
(13Z)-Eicosenoic acid |
4.17 |
2.81 |
2.38 |
3.50 |
|
20 |
36,30 |
2436 |
n-Eicosanoic acid tms (Arachidic acid) |
2.97 |
2.72 |
3.15 |
3.67 |
|
21 |
37,81 |
2531 |
n-Heneicosanoic acid tms |
0.70 |
0.50 |
0.49 |
0.60 |
|
22 |
37,97 |
2544 |
n-Docosanol tms |
0.43 |
0.63 |
0.60 |
0.49 |
|
23 |
39,32 |
2637 |
Docosanoic acid tms (Behenic acid) |
2.73 |
3.92 |
3.82 |
3.07 |
|
24 |
40,88 |
2740 |
n-Tetracosanol tms |
0.45 |
2.71 |
2.76 |
1.50 |
|
25 |
42,14 |
2828 |
n-Tetracosanoic acid tms (Lignoceric acid) |
1.42 |
3.17 |
3.07 |
1.92 |
|
26 |
43,01 |
2900 |
n-Nonacosane |
1.69 |
1.38 |
0.66 |
0.58 |
|
27 |
44,76 |
2936 |
n-Hexacosanol tms |
1.73 |
3.28 |
3.12 |
1.93 |
|
28 |
45,16 |
3032 |
n-Hexacosanoic acid tms (Cerotic acid) |
0,49 |
2,50 |
1,37 |
1,49 |
|
29 |
46,12 |
3134 |
n-Octacosanol tms (Octacosyl alcohol) |
0,84 |
1,80 |
1,59 |
0,87 |
|
30 |
47,27 |
3261 |
Campsterol tms |
0,87 |
1,75 |
1,49 |
0,96 |
|
31 |
48,40 |
3302 |
β-Sitosterol tms |
7,61 |
7,00 |
6,03 |
8,30 |
|
32 |
48,52 |
3314 |
Stigmastanol tms |
0,47 |
1,24 |
1,34 |
0,55 |
|
33 |
49,66 |
3327 |
β-Amyrin tms |
1,20 |
2,77 |
2,81 |
1,42 |
|
34 |
50,09 |
3351 |
α-Amyrin tms |
0,68 |
0,57 |
0,49 |
0,34 |
|
35 |
50,64 |
3416 |
Cycloartenyl acetate |
1,24 |
2,05 |
1,51 |
1,36 |
|
36 |
51,14 |
3440 |
Lupenyl acetate |
1,18 |
1,44 |
1,40 |
1,60 |
|
37 |
51,23 |
3455 |
Betulin tms |
6,32 |
11,37 |
9,91 |
7,81 |
|
38 |
51,49 |
3502 |
Oleanolic acid acetate |
1,97 |
1,44 |
1,62 |
1,51 |
|
39 |
52,05 |
3625 |
Ursolic acid acetate |
10,25 |
16,56 |
17,70 |
13,36 |
RT-retention time, min; RI-retention index (Kovach’s index), TIC– total ion current.
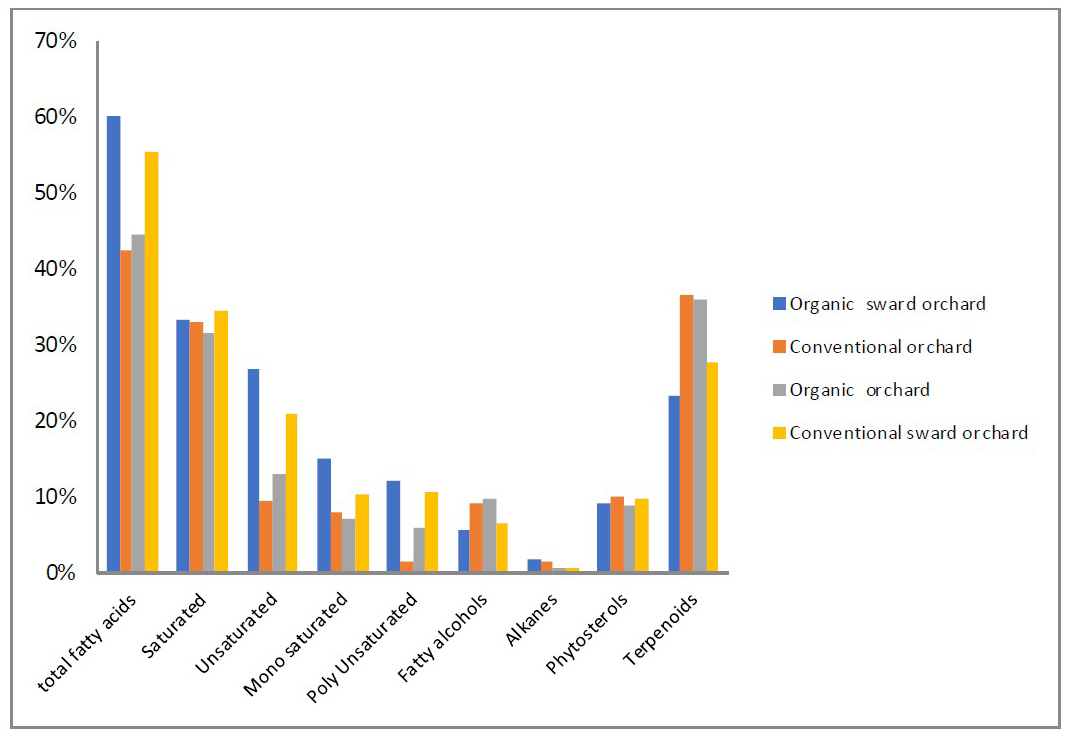
Figure 2. Distribution of volatile compounds by chemical groups in non-polar fraction of Florina apple fruits
DISCUSSION
In general, apples from all organic orchards demonstrated the highest antioxidant activity by all methods (Table 1). Total phenolic content was between 2.49 to 4.38 mg GAE/g dry weight. In general, organic apple orchard demonstrated higher polyphenols content (Table 1). The highest total phenols were found in organic sward orchard (4.84 mg GAE/g dw). A report of Wojdyło et al. (2008) who showed values for Florina apple 3.75 ± 0.15 GAE g/fw. Dobrevska et al. (2022) reported values between 50 – 100 mg GAE/100 g for organic Florina apples, while Bouayed et al. (2011) found 130.0 – 2.7 mg gallic acid equivalents/100 g fw. Our data were in a good agreement with that reported in literature data for Florina apple fruits (Petkova et al., 2019) and higher than a report of Leccese et al. (2009) - 0.3 to 0.84 mg GAE/g fresh weight and Tarola et al., (2019) - 9.6 ± 0.8 µg chlorogenic acid/g dw, respectively. The levels of total flavonoids were the highest in organic apple orchard 0.79 to 0.94 mg QE/g. The same tendency was observed for the total monomeric anthocyanins, where its content in organic apple was 26.43 to 31.22 mg cyn-3-glc/g dw. The presence of cyanidin-3-galactoside and 3-glucoside explained the characteristic red or partially dark red peels of Florina apples (Wojdyło et al., 2008). Our values were higher than that reported by Tarola et al., (2019) who found antioxidant potential in the peel of organic apple Florina 377.3 ± 0.6 µmol/g dw (DPPH assay), while Dobrevska et al. (2022) reported data for organic Florina apple 250 to 420 µmol/100 g (DPPH assay). Iordănescu et al. (2021) showed antioxidant activity evaluated by the DPPH· method at various time ranges that reveal significant differences between organic and non-organic samples, as well as apple parts. They found that organic apple extracts had higher antioxidant activities in comparisons of non-organic apples from Romania.
Leccese et al. (2009) reported that total antioxidant activity in peel varied from 17.5 to 41 μmol TE/g and it was 3 times higher than in flesh. Wojdyło et al., (2008) reported that Florina apple possessed antioxidant activity evaluated by FRAP, ABTS and DPPH in the range from 15.4 to 85.9 (μMTE/100 g dry weight). Our values of antioxidant potential were near to Wojdyło et al., (2008) and higher than Italian organic Florina apple (Tarola et al., 2019).
Tarola et al. (2019) reported the presence of two phenolic acids (gallic and chlorogenic acids) and three flavonoids in Florina apple in Italy. The results in our research showed that chlorogenic acid was the major phenolic acid, especially in organic apple, followed by vanilic acid, where its content was the highest in conventionally grown fruits. In all examined apples chlorogenic acid varied between 109.22 and 493.41 μg/g, as its content in organic apple orchards was four times higher in comparison to conventional orchards. However, the content of chlorogenic acid in sward orchards were not differ strongly (Table 2). Caffeic acid and p-coumaric acids were detected only in organic sward orchards, while Gallic acid was completely absent in them. Gallic acid was in the highest content in conventional sward orchard 1.01 µg/g. The level of this acid in organic and conventional orchard was twice lower (Table 2). Tarola et al. (2019) reported twice to four times higher results for gallic acid in Florina organic apples grown in Italy. However, the levels of chlorogenic acid in 493.41 μg/g organic apple orchard were more than four times higher in comparison to its content in Italian organic Florina apple (203 μg/g in peels) (Tarola et al., 2019). The amounts of garlic and syringic acids dominated in conventionally grown sward apple orchards. According to Ferrentino et al. (2018) in various apple cultivars the main phenolic acids were from benzoic acid derivatives - gallic, p-hydroxybenzoic, protocatechuic and syringic acids. Contrary to Gean˘a et al. (2021) in our study resveratrol were no detected in Florina apples.
Flavan-3-ols dominated apples are rich in catechin, epicatechin. In Florina apple were found (+)-catechin and (-)-epicatechin, as the levels of epicatechin were twice higher than catechin in most of the samples. It was reported that flanonols and flavonoids were influenced by fertilization (Mılosevıc et al., 2019). Li et al. (2002) found an increase in flavonols in apples sprayed with Senifos (a mixture of N, P and K). In our case conventionally grown apple sward orchard demonstrated epicatechin content - 173.21 µg/g dw (Table 2). Catechin was twice higher in organically sward apple in comparison to other fruits - 78.56 µg/g dew. Vrhovsek et al. (2004) reported for different cultivar apples also high values of epicatechin (5.2-18.40 mg/100 g) while the presence of catechin was lower (0.45-3.40 mg/100 g). Our data for catechin were lower, while the values for epicatechin were near to reported for Italian organic Florina apple (Tarola et al., 2019). From quercetin glycoside only rutin was detected similarly to Tarola et al., (2019). Rating content in organic orchard was 8 to 9 times higher in comparison to the conventional orchard (Table 2). In our case the detected return values in organic orchard were twice lower in a comparison of organic Florina apple from Italy (Tarola et al., 2019). Therefore, the growing conditions caused a strong effect of bioactive phenolic compounds in apple fruits. According to Gean˘a et al. (2021) ferulic acid and epicatechin represent polyphenolic markers for some apple cultivars as Idared, Richard and Florina.
The results (Table 3) demonstrated positive linear correlations between the total antioxidant activities and total phenolic contents (coefficient of correlation r2 = 0.9801 and 0.9994 for ABTS and FRAP values, respectively). Wojdyło et al. (2008) also reported for the linear regression between antioxidant activity evaluated by three methods (DPPH, ABTS and FRAP) and total phenolic content. The results showed strong correlation between total flavonoids and ABTS method (r2 = 0.9541). Low correlation was observed between total monomeric anthocyanin and all methods, except DPPH. Wojdyło et al. (2008) found even lower positive correlations between total monomeric anthocyanin and DPPH, ABTS and FRAP methods. In our case, results can be concluded that total phenols, total flavonoids and total monomeric anthocyanins correlated well with the radical scavenging method DPPH based on hydrogen transfer, while flavonoids correlated better with ABTS which is based on mix mode mechanisms. Total phenols demonstrated the highest correlation with FRAP method which is based on electron transfer. Other studies also confirm our observations that total phenolic compounds content was positively correlated with antioxidant activity (DPPH) in apple pulp (r2= 0.870) and peel (r2=0.699) respectively. It was found that in apple, phenolic compounds have a significant contribution to the antioxidant capacity (Lee et al., 2003).
The detected volatile compounds in non-polar fraction comprised mainly fatty acids (60-40%) and 23-37 % triterpenes (Figure 2). It was reported that in the waxy apples, massive contents of the pentacyclic triterpenes, ursolic and oleanolic acids were present with values 30–60% of the total content depending on the variety (Legay et al. 2017). The main fatty acids in apple surface layers are palmitic acid, stearic acid, linoleic acid, and oleic acid (Duroňová et al., 2012). Linoleic and oleic were the main fatty acids identified in some apple varieties, while palmitic and stearic acid were the representant of saturated ones (Fărcaș et al. 2022). In our case saturated fatty acids was 33% from total non-volatile compounds with palmitic acid as a major representative in organic sward apples (16% of TIC), while monounsaturated fatty acids occupied 10-15% of total non-polar components with linoleic acid as the dominating representatives up to 11% of TIC (Figure 2 and Table 4). The presence of very long chain fatty acids, primary and secondary alcohols, alkanes, and triterpenes could be explain with the fact that they are major component of apple waxes found in the cuticle, which are deposited on the cutin surface (epicuticular waxes), or embedded within the polymer matrix (intraculticular waxes). β-sitosterol was found similarly to the previous report of Verardo et al. (2003) who found it in the apple peel of the “Florina” variety, but its role in the cuticle remains unclear (Verardo et al., 2003).
1-octacosanol was also found and it is reported to possess cholesterol-lowering effects, antiaggregatory properties, cytoprotective use, and ergogenic properties. It has been studied as a potential therapeutic agent for the treatment of Parkinson's disease. (Fernandez-Moreno et al., 2016). Heneicosane is a naturally occurring, waxy tasting compound.
Phenolic compounds and triterpene acids in apples showed protective effects against Alzheimer’s disease and anti-inflammatory properties (Fărcaș et al. 2022). Triterpenes, especially ursolic and oleanolic acids, are one of the predominant compounds in apple cuticular wax and participated in the defense and communication between plants and the environment. The plant can synthesize many different types of terpenoids as part of primary or secondary metabolism under different conditions, or in different environments. It was demonstrated that the values ursolic and oleanolic acids was mainly influenced by cultivar and side subjected to sun exposure, and to a minor extent by storage and seasonal year (Lv et al., 2015). The results obtained in our study showed that acetyl esters of ursolic and oleanolic acids dominated in organically grown Florina apple orchard – 17.70% of TIC and 1.62 % of TIC, respectively (Table 4). Oleanolic acid possessed anti-inflammatory, antitumor, antimicrobial, hepato-protective, antidiabetic and antihypertensive properties (Gean˘a et al., 2021). Oleanolic acid acetate content was the highest in organically sward apple orchard – 1.97 % of TIC (Table 4). Gean˘a et al., (2021) reported the values of oleanolic acid (10.88–82.53 mg/g) and ursolic acid (54.68 to 435.57 mg/g extract) from different apple cultivar, as urolic acid in Florina apple dominated above oleanolic acid content. In our study, the same trend was observed, as ursolic acid acetate reached 10 times higher values in comparison to oleanolic acid acetates. Moreover, the content of urolic and oleanolic acetate dominated in organically grown apples in comparison to conventional ones that could be explained with the natural prevention of apple orchards against environmental factors.
Apple polyphenols (as procyanidins, epicatechin, phloridzin) and ursolic acid has a serious impact on prevention of cancer, in comparison to the simple phenolic acids (Gean˘a et al., 2021). The structure of phenolic compounds in apple defines the strength and the mechanism of anticancer activity. Quercetin (a polyphenol with high antioxidant activity) mainly contributed to the chemopreventive effect through reduction of the oxidative damage, flavonoids such as quercitin, epicatechin and procyanidins B2 determined the anticancer activity of apple extracts (Kalinowska et al., 2014), while the triterpenoids, especially ursolic acid isolated from the skins of apples were responsible for the anticancer properties (Yamaguchi et al. 2008).
CONCLUSION
The present research demonstrated the accumulation of polyphenolic compounds in apple Florina grown under organic and conventional conditions. Seven phenolic acids (gallic, chlorogenic, vanillic, caffeic, syringic and p-coumaric acids) and flavonoids ((+)-catechin, (-)-epicatechin quercetin and rutin were detected in Florina apples. The results showed that at the organically grown conditions, the quality of the fruits of the Florina apple variety is improved based on the accumulation of bioactive compounds with antioxidant activity. Thirty-nine volatile components (saturated and unsaturated fatty acids, fatty alcohols, phytosterols and triterpenes) were detected in non-polar apple fraction. Apples from an organic orchard sward possessed the highest level of secondary metabolites as total monomeric anthocyanins, total phenols, total flavonoids, phytosterols and triterpenes, especially derivatives of urolic acid. Total phenols demonstrated the highest correlation with ABST, FRAP and FRAP method. Therefore, Florina apples grown from organic and organic sward orchard are a valuable source of flavonoids and phenolic acid with antioxidant potential, as well as urolic and oleanolic acids acetates and unsaturated fatty acids.
ACKNOWLEDGEMENTS
The authors thank to the supported of the Bulgarian Ministry of Education and Science under the National Research Programme “Healthy Foods for a Strong Bio-Economy and Quality of Life” approved by DCM # 577/17 .08.2018, as well as to Assoc. Prof. Vasil Georgiev for HPLC assistance during analyses.
AUTHOR CONTRIBUTIONS
Nadezhda Petkova conduct all of the experiments, performed the statistical analysis and data visualization and wrote the manuscript. Tatyana Bileva and Ekaterina Valcheva help in conducting the statistical analysis and participate in writing of manuscript. Galya Dobrevska provided samples for analyses. Neli Grozeva, Mima Todorova, and Vladislav Popov coordinate project and manage the work. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
REFERENCES
Benzie, F., and Strain, J. 1996. Ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Analytical Biochemistry. 239: 70–77
Blagoeva, T., and Krishkova, I. 2020. Efficiency of pruning on growth manifestations of apple cultivars. Rastenievadni nauki. 57: 41–48.
Bouayed, J., Hoffmann, L., and Bohn, T. 2011. Antioxidative mechanisms of whole-apple antioxidants employing different varieties from Luxembourg. Journal of Medicinal Food. 14: 1631-1637.
Dobrevska, G., Ivanova, P. and Dallev, M. 2022. Influence of agricultural cultivation methods on the physicochemical and colour parameters of Florina variety apples immediately after harvest. Bulgarian Journal of Agricultural Science. 28: 279–283.
FAOSTAT. Crops and livestock products; Food and agriculture organization. Rome, Italy, 2021
Fărcaș, A. C., Socaci, S. A., Chiș, M. S., Dulf, F. V., Podea, P., and Tofană, M. 2022. Analysis of Fatty Acids, Amino Acids and Volatile Profile of Apple By-Products by Gas Chromatography-Mass Spectrometry. Molecules (Basel, Switzerland), 27(6), 1987.
Fernandez-Moreno, J., Malitsky, S., Lashbrooke, J., Biswal, A.K., Racovita, R.C., Mellerowicz, E.J., Jetter, R., Orzaez, D., Aharoni, A., and Granell, A. 2016. An efficient method for medium throughput screening of cuticular wax composition in different plant species. Metabolomics. 12: 73
Ferrentino, G., Morozova, K., Mosibo, O.K.., Ramezani, M., and Scampicchio, M. 2018. Biorecovery of antioxidants from apple pomace by supercritical fluid extraction. Journal of Cleaner Production. 186: 253–261.
Gean˘a, E.-I., Ciucure, C.T., Ionete, R.E., Ciocârlan, A.; Aricu, A., Ficai, A. & Andronescu, E. 2021. Profiling of phenolic compounds and triterpene acids of twelve apple (Malus domestica Borkh.) Cultivars. Foods. 10: 267.
Iordănescu, O.A., Băla, M., Iuga, A.C., Gligor Pane, D., Dascălu, I., Bujancă, G.S., David, I., Hădărugă, N. G., and Hădărugă, D. I. 2021. Antioxidant activity and discrimination of organic apples (Malus domestica Borkh.) cultivated in the western region of Romania: A DPPH· Kinetics-PCA Approach. Plants (Basel, Switzerland), 10: 1957.
Ivanov, I.G., Vrancheva, R.Z., Marchev, A.S., Petkova, N.T., Aneva, I.Y., Denev, P.P., Georgiev, V.G., and Pavlov, A.I. 2014. Antioxidant activities and phenolic compounds in Bulgarian Fumaria species. International Journal of Current Microbiology and Applied Sciences. 3: 296–306.
Ivanov, I., Petkova, N., Tumbarski, J., Dincheva, I., Badjakov, I., Denev, P. & Pavlov, A. 2018. GC-MS characterization of n-hexane soluble fraction from dandelion (Taraxacum officinale Weber ex F.H. Wigg.) aerial parts and its antioxidant and antimicrobial properties. Zeitschrift für Naturforschung C. 73: 41-47.
Kalinowska, M., Bielawska, A., Lewandowska-Siwkiewicz, H., Priebe, W., & Lewandowski, W. 2014. Apples: Content of phenolic compounds vs. variety, part of apple and cultivation model, extraction of phenolic compounds, biological properties. Plant Physiology and Biochemistry. 84: 169-188.
Leccese, A., Bartolini, S., and Viti, R. 2009. Antioxidant properties of peel and flesh in GoldRush and Fiorina scab‐resistant apple (Malus domestica) cultivars, New Zealand Journal of Crop and Horticultural Science, 37: 71-78.
Lee, J., Durst, R.W., and Wrolstad, R.E. 2005. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. Journal of AOAC International, 88: 1269–1278.
Lee, K., Kim, Y., Kim, D. and Lee, C. 2003. Major phenolics in apple and their contribution in the total antioxidant capacity. Journal of Agricultural and Food Chemistry. 51: 6516.
Li, Z., Gemma, H., and Iwahori, S. 2002. Stimulation of ‘Fuji’ apple skin color by ethephon and phosphorus-calcium mixed compounds in relation to flavonoid synthesis. Scientia Horticulturae. 94: 193-199.
Legay, S., Cocco, E., André, C.M., Guignard, C., Hausman, J.F., and Guerriero, G. 2017. Differential lipid composition and gene expression in the semi-russeted "cox orange pippin" apple variety. Frontiers in Plant Science, 8: 1656.
Lv, Y., Tahir, I.I., & Olsson, M.E. (2015). Factors affecting the content of the ursolic and oleanolic acid in apple peel: Influence of cultivars, sun exposure, storage conditions, bruising and Penicillium expansum infection. Journal of the Science of Food and Agriculture, 96: 2161–2169.
Manolova, V. 2021. Evaluation of the development of fruit growing in Bulgaria (II part). Bulgarian Journal of Agricultural Science, 27 (Suppl. 1):23–30.
Marchev, A., Georgiev, V., Ivanov, I.,Badjakov, I., and Pavlov, A. 2011. Two-phase temporary immersion system for Agrobacterium rhizogenes genetic transformation of sage (Salvia tomentosa Mill.). Biotechnology letters. 33: 1873-1878.
Mılosevıc, T., Mılosevıc, N., and Mladenovıc, J. 2019. Tree vigor, yield,fruit quality, and antioxidant capacity ofapple (Malus × domestica Borkh.) influenced by different fertilization regimes: preliminary results. Turkish Journal of Agriculture and Forestry. 43: 48-57 .
Nikolova, M., Ilinkin, V., Yankova-Tsvetkova, E., Stanilova, M. and Berkov, S. 2022. GC/MS based metabolite profiling and biological activity of leaves and flower heads of Tanacetum cinerariifolium (Trevir.) Sch.Bip.. Chiang Mai University Journal of Natural Sciences. 21: e2022038
Petkova, N., Bileva, T., Valcheva, E., Dobrevska, G., Grozeva, N., Todorova, M., and Popov, V. 2019. Bioactive compounds and antioxidant activity in apple fruits cultivar Florina. Bulgarian Journal of Agricultural Science, 25 (Suppl. 3): 13–18.
Petkova, N., Ognyanov, M., Kuzmanova, S., Bileva, T., Valcheva, E., Dobrevska, G. and Grozeva, N., 2020. Carbohydrate content of “Florina” apples grown under organic and conventional farming systems, Proceedings of the 16th International Conference on Polysaccharides-Glycoscience Prague 4th - 6th November 2020, 98-101.
Sansomchai, P., Jumpatong, K., Lapinee, C., and Utchariyajit, K. 2021. Melientha suavis Pierre. Extract: antioxidant and sunscreen properties for future cosmetic development. Chiang Mai University Journal of Natural Sciences. 20: e2021008
Tarola, A.M., Girelli, A.M., and D’ascenzo F. 2019. Bioactive polyphenol profiles and antioxidant activity in Italian apples varieties. Italian Journal of Food Science. 31: 243-252.
Vasile, M., Bunea, A., Ioan, C.R., Ioan, B.C., Socaci, S., and Viorel, M. 2021. Phytochemical content and antioxidant activity of Malus domestica Borkh peel extracts. Molecules. 26: 7636.
Verardo, G., Pagani, E., Geatti, P., and Martinuzzi, P. 2003. A thorough study of the surface wax of apple fruits. Analytical and Bioanalytical Chemistry, 376: 659–667.
Vrhovsek, U., Rigo, A., Tonon, D., and Mattivi, F. 2004. Quantitation of polyphenols in different apple varieties. Journal of Agricultural and Food Chemistry. 52: 6532–6538.
Wojdyło, A., Oszmian´Ski, J., and Laskowski P. 2008. Polyphenolic compounds and antioxidant activity of new and old apple varieties. Journal of Agricultural and Food Chemistry. 56: 6520-6530.
Yamaguchi, H., Noshita, T., Kidachi, Y., Umetsu, H., Hayashi, M., Komiyama, K., and Ryoyama, K. 2008. Isolation of ursolic acid from apple peels and its specific efficacy as a potent antitumor agent. Journal of Health Science, 54: 654–660.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand.
Nadezhda Petkova1,*, Tatyana Bileva2, Ekaterina Valcheva2, Galya Dobrevska3, Neli Grozeva4, Mima Todorova4, and Vladislav Popov2
1Departament of Organic Chemistry and Inorganic Chemistry, Technological Faculty, University of Food Technologies, 26 Maritza Blvd., 4002, Plovdiv, Bulgaria
2Department of Agroecology and Environmental Protection, Agriculture University Plovdiv, 4000, Plovdiv, Bulgaria
3Department of Fruit Growning Agriculture University, 4000 Plovdiv, Bulgaria
4Trakia University, Faculty of Agriculture, 6000 Stara Zagora, Bulgaria.
Corresponding author: Nadezhda Petkova, E-mail: petkovanadejda@abv.bg
Total Article Views
Editor: Tonapha Pusadee,
Chiang Mai University, Thailand
Article history:
Received: October 4, 2022;
Revised: November 8, 2022;
Accepted: November 11, 2022;
Published online:November 17, 2022

