
Effect of Ultrasound-Assisted Extraction and Acid Type Extractant on Pectin from Industrial Tomato Waste. Nipaporn Sengkhamparn*, Patareeya Lasunon, and Prapaporn Tettawong
Published Date : 2019-04-1
DOI : https://doi.org/10.12982/CMUJNS.2019.0016
Journal Issues :
Number 2 , April-June 2019
ABSTRACT
Pectin can be extracted from many fruits and also by-product from industry. Tomato can be produced into many products led to large amount of waste. This work aims to extract pectin from tomato waste achieved by tomato paste industry using Ultrasound Assisted Extraction (UAE) method (temperature of 40, 60 or 80 oC and time of 10, 15, 20 or 30 min, at pH of 2.5 and solid: liquid ratio of 1:40 w/v) using citric acid as an extractant. Moreover, the extractant (citric acid, hydrochloric acid and nitric acid) for pectin extraction under best UAE condition were compared, as well as physical and chemical properties of obtained pectin were determined. The results showed that the temperature of 80 oC and time of 20 min providing the highest pectin yield with shorter time. Moreover, the citric acid gave the highest yield compare to the other acid extractant. Citric acid extraction gave pectin with higher anhydrouronic acid content (31.59%), however, it gave comparable methoxyl content compare to others. Furthermore, citric acid extraction pectin could be categorized as low DM pectin while the others gave the high DM pectin. All obtained pectin were highly color and the a* of pectin extracted with citric acid was the lowest. Nevertheless, the extractant was not significantly impact to lycopene content (6.65 – 9.35 mg/100g pectin). This suggest that UAE method together with citric acid extraction could be used as an efficient method for pectin extraction with interestingly lycopene content.
Keywords: Pectin, Tomato waste, Ultrasound assisted extraction, Citric acid, Lycopene
INTRODUCTION
Pectin has been widely used in food industry as the gelling agent and stabilizer (Pinheiro et al., 2008) as well as texturizing agent and emulsifier (Guo et al., 2017). It is a complex polysaccharide comprised of mostly galacturonic acid and presented in many plant tissue (Voragen et al., 1995). Generally, the galacturonic acid in pectin backbone can be esterified at C-6 and/or can be acetylated at O-2 and/or O-3 (Voragen et al., 1995). The percentage of methyl- esterified galacturonic acid can be expressed as the degree of methyl-esterification (DM) which affected to its gelling properties (Voragen et al., 1995; Sengkhamparn et al., 2009). Based on DM, pectin can be divided into 2 types, Low DM Pectin (LMP) and High DM Pectin (HMP). LMP can be gel under presence of divalent ion such as calcium ion while LMP can be gel under high soluble solid cooperated with acid condition (Voragen et al., 1995; Pereira et al., 2016).
Pectin can be extracted commercially by hot acid solvent at pH 1.3-3.0 and temperature of 60-100 oC for long time (Koubala et al., 2008). The acid extractant has been commonly used for pectin extraction was mineral acid namely hydrochloric acid, sulfuric acid, nitric acid (Wang et al., 2016). However, this mineral acid has toxicity and affect to environmental safety (Guo et al., 2017). Besides, the chelating agent has also been studied for extracting pectin such as CDTA, EDTA (Renard and Thibault, 1993; Sengkhamparn et al., 2009; Jamsazzadeh Kermani et al., 2014). Citric acid was non-toxicity organic acid and has a chelating property. Jamsazzadeh Kermani et al., (2014) reported that applying citric acid as an extractant provided higher pectin yield than that applying sulfuric acid. It has been used for pectin extraction studies in many plant materials for example sugar beet pectin (Guo et al., 2017), mango peel (Jamsazzadeh Kermani et al., 2014; Wang et al., 2016), passion fruit peel (Pinheiro et al., 2008) and banana peel (Oliverira et al., 2016).
Ultrasound technique has been used for bioactive compound extraction from plant due to the lower extraction time and temperature consuming for extraction (Grassino et al., 2016). Wang et al. (2016) reported that Ultrasound Assisted Extraction (UAE) method gave pectin yield comparable to conventional method at temperature of 80 oC with a shorter extraction time. Moreover, the UAE method has been also used for pectin extraction such as mango peel (Wang et al, 2016), tomato waste (Grassino et al., 2016), grapefruit (Bagherian et al., 2011), Jackfruit peel (Moorthy et al., 2017) and passion fruit peel (Freitas de Oliverira et al., 2016).
Tomato fruits are rich in nutrient and are widely processed in many products such as tomato juice, paste, ketchup or puree. (Schieber et al., 2001; Grassino et al., 2016). During processing in industry, tomato waste has been generated. Tomato waste from processing has been used as raw material for lycopene extraction (Kaur et al., 2008; Poojary and Passmonti, 2015) as well as pectin extraction (Grassino et al., 2016). Grassino et al. (2016) extracted pectin from tomato waste from canning industry using ammonium oxalate/oxalic acid under UAE method compare to conventional extraction. They found that pectin yield from UAE was in range of 15.2–17.2% and 16.3-18.5 % at extraction temperature of 60 oC and 80 oC, respectively. Moreover, they also reported that UAE method is an efficient method for pectin extraction.
Therefore, the purpose of this studied was to use UAE method with citric acid for pectin extraction from industrial tomato waste and compare to other acid extractant with the same UAE condition. Moreover, the chemical properties, namely degree of methyl-esterification, as well as lycopene concentration, and also some physical properties of obtained pectin were determined.
MATERIALS AND METHODS
Materials
The tomato wastes were obtained from Tomato Process tomato paste Industry at Nong Khai Province, Thailand. Then, they were dried at 60 oC for 24 h. The dried tomato wastes were ground and kept in plastic bag and stored at -18 oC before further experiment.
Ultrasound Assisted Extraction Method (UAE)
The UAE was carried out using ultrasound bath (Elmasonic S70H, ElmaHans Schmidbauer Gmbh & Co. KG, Singen, Germany). The tomato waste powder from industrial (about 5 g) was added with 200 mL of 0.05 M citric acid and then the pH of mixture was adjusted to 2.5. The mixture was then sonicated at different condition, including extraction temperature of 40, 60 or 80 oC and extraction time of 10, 15, 20 or 30 min. After cooling, the mixture was centrifuged at 8,000 rpm for 20 min, then the supernatant was poured into 2 times volume of 5% ethanol in order to precipitation of pectin and left it for 24 h at 4 oC. The floating precipitant were discarded and drying at 60 oC for an hour using vacuum oven. The obtained pectin yield was calculated.
Acid type extractant
For comparing the acid type extractant, the acid solution of 0.05 M HNO3, 0.0125 M HCl were used to pectin extraction under the UAE method. The other parameters namely solid/solvent ratio of 1:40 and initial pH of 2.5 were controlled. The pectin yield obtained from different acid type extractant was calculated and compared.
The color of pectin
The color of pectin obtained from different acid type extractant were determined using Chromameter (JS555) and expressed as L* (lightness) a* (redness) and b*(yellowness).
The chemical properties of pectin
Anhydrouronic acid content. The anhydrouronic acid (AUA) content of extracted pectin was determined according to Ismail et al. (2012) with some modification. Briefly, the pectin (about 0.1 g, W) was dissolved using 20 mL of carbon dioxide free water and the solution was titrated by 0.1 N NaOH(V1) using Phenol red as an indicator. The percentage of AUA content was calculated following the equation:
AUA (%) = [(17600) x (V1+V2)] / W(mg) (1)
Methoxyl content and degree of methyl-esterification (DM). The methoxyl (MeO) content and degree of methyl-esterification (DM) of pectin was determined by adding with 5 mL of 0.2 N NaOH to the titrated solution and left for 30 min. After that, it was added with 5 mL of 0.2 N of HCl. Later, the solution was titrated by 0.1 N NaOH (V2) using Phenol red as an indicator until the solution turn to pink. The percentage of MeO content and DM was calculated following the equation (2) and (3), respectively.
MeO (%) = [V2 x 31 x 100] / W (mg) (2)
DM (%) = (176 x MeO x 100) / (31 x AUA) (3)
Lycopene content. About 0.5 g of pectin was added with 5 mL of n-hexane and left for 15 hrs. in the dark at room temperature. The absorbance of its supernatant was determined at 503 nm. The lycopene content was then calculated using molar extinction coefficient (17.2x104mol cm-1) and expressed as mg/100 g of pectin (Poojary and Passmonti, 2015).
Statistical Analysis
All data measurements were done in triplicate with using a full factorial design and expressed as the mean with standard deviation. The treatment comparison was performed using Duncan’s New Multiple Range Test with a significance level of P≤ 0.05.
RESULTS
Ultrasound Assisted Extraction Method (UAE)
Ultrasonication has been applied for bioactive extraction as well as pectin extraction due to the shorter time consumption comparing to traditional extraction method. The pectin from industrial tomato waste was extracted by UAE method under different condition with citric acid as an extractant. The pectin yield obtained from each condition is shown in Figure 1A.
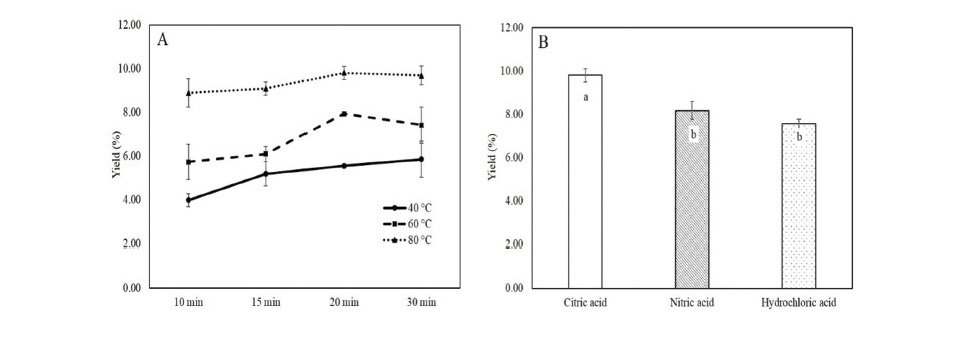
Figure 1. The pectin yield from different UAE condition (A) and acid type extractant (B).
Note: Different letters in Figure 1B indicate a significant difference (P ≤ 0.05).
The results showed that the higher temperature and the longer time of extraction gave a higher yield. However, the extraction time of 20 min was not significantly differ from that of 30 min. Therefore, the extraction condition under sonication at temperature of 80 oC for 20 min would be appropriate for pectin extraction from tomato waste powder. This condition provided a higher pectin yield of 9.80 %.
Acid type extractant
In order to compare the type of acid used for extraction, two type of acid extractant (HCl and HNO3) which commonly used for pectin extraction in commercial were performed under appropriate UAE condition (temperature of 80 oC and time of 20 min). The pectin yield form different acid extractant is shown in Figure 1B. The results revealed that the extractant affected to pectin yield. The highest pectin yield was obtained under citric acid extraction.
The color of pectin
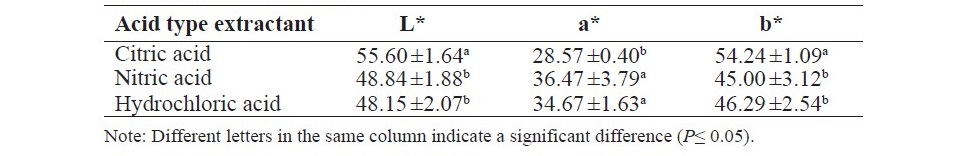
The color of pectin from different acid type extractant are shown in Table 1. Obviously, the all pectin has highly color. Moreover, the results exhibited that the acid extractant affected to the color of pectin. The citric acid extraction resulted in a pectin with the highest L* and b*, but the lowest a*.
Table 1. The color of pectin.
The chemical properties of pectin
The pectin mainly consisted of the galacturonic acid in which this galacturonic acid backbone can be methyl esterified (Voragen et al., 1995). The anhydrouronic acid (AUA) content of obtained pectin are showed in Table 2. The results showed that pectin extracted with citric acid contained higher AUA content than the others. On the other hand, the amount of methoxyl content of all extracted pectin was comparable.
The galacturonic acid backbone of pectin can be methyl esterified and the percentage of esterification is expressed as the DM which related to the gelling property (Voragen et al., 1995). The DM of obtained pectin are shown in Table 2. The DM of pectin extracted with citric acid was lowest (40.61%) and could be categorized as low DM pectin (DM< 50%). While the DM of pectin extracted with nitric acid and hydrochloric acid (72.59 % and 81.76%, respectively) was significantly comparable and could be categorized as high DM pectin (DM> 50%).
Table 2.The chemical properties of obtained pectin.
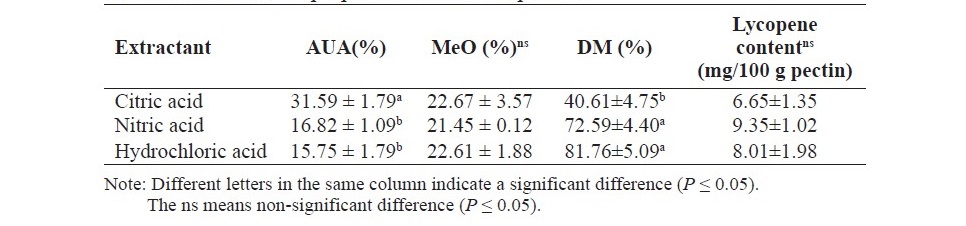
The pectin showed highly color which may be attributed to the lycopene content, therefore, the lycopene content in pectin was determined. The results are shown in Table 2. All pectin contained lycopene in the range of 6.65–9.35 mg/ 100 g pectin. Nevertheless, the acid type extractant was not significantly affected the lycopene amount in pectin.
DISCUSSION
The UAE condition (temperature and time) affected to the pectin yield. The higher temperature and longer time for extraction resulted in a higher yield. This could be explained by the higher extraction temperature as well as cavitation phenomenon during sonication enhanced the solubility of pectin from material into the solvent medium. However, the extraction time of 20 min was similar to that of 30 min. This indicated that the prolong extraction did not improve the pectin yield, hence the extraction time of 20 min would be statistically enough. Therefore, the extraction condition under sonication at temperature of 80 oC for 20 min would be a suitable condition for pectin extraction. This condition provided a pectin yield of 9.80 % which was lower than the pectin extracted from dried tomato waste that conducted by Grassino et al., 2016 which pectin yield was 15.2-17.2% and 16.3–18.5% which using ammonium oxalate/oxalic acid under first step sonication at 60 and 80 oC, respectively. This results probably due to the different of tomato source and the acid extractant as well as extraction parameter such as solid: liquid ratio and initial pH. In our study, the citric acid was used as an extractant with the ration 1:40 (g/mL) and pH of 2.5 and the tomato wasted obtained from tomato paste industry. However, the pectin yield obtained in this study was comparable to other sources such as pectin from banana peel (5.2–12.2% of its dried alcohol insoluble residue, Oliverira et al., 2016) and pomegranate peels (4-11 g/100g of alcohol insoluble residue, Pereira et al., 2016). However, the other extraction factors may also be affected to pectin yield such as pH, solid:liquid ratio, therefore, the vary of pH and solid:liquid ratio would be further studied.
Beside UAE condition, the acid type extractant also influenced to pectin yield which citric acid extraction revealed the highest pectin yield. This was probably due to citric acid contained a chelating property which might extract more strongly bound pectin in the cell wall (Oliverira et al., 2016). Wang et al (2016) reported that organic acid with chelating property could improve the pectin yield and physicochemical property. Jamsazzadeh Kermani et al. (2014) found that the pectin yield from mango peel which extracted with citric acid was higher than extracted with sulfuric acid. Moreover, citric acid was also less toxic and more environmental safety compare to hydrochloric acid and nitric acid. Therefore, the resulted pointed that the pectin extraction form tomato wasted achieved by tomato paste industry with citric acid was better than other organic acid and more efficiency based on its yield. However, the tomato waste in this study was composed of tomato peel as well as seed and some pulp in which other polysaccharides such as cellulose as well as hemicellulose could be found. The monosaccharide in extracted pectin should be studied.
The color of obtained pectin showed red-yellow color. This was probably due to the pigment in the tomato waste powder such as lycopene which could be extracted under pectin extraction. This physical property of pectin was also found in the pectin from the research conducted by Grassino et al. (2016). In addition, the acid type extractant influenced the color of pectin. The citric acid extractant provided a pectin with the highest L* and b*, but the lowest a*. It may be attributed to the lycopene content in the pectin.
The backbone of pectin mainly composed of galacturonic acid and C-6 of galacturonic acid can be esterified with methyl alcohol (Voragen et al., 1995). The galacturonic acid of obtained pectin was determined as the anhydrouronic acid (AUA) content. The results (Table 2) showed that the acid extractant affected to AUA content of obtained pectin. The pectin extracted with citric acid consisted of higher AUA content (31.59 %) than the others. This amount was closely to the AUA content of pectin from tomato waste reported by Grassino et al. (2016) which was in the range of 24.95–37.55 % depending on the extraction temperature of UAE used. However, this amount was lower than the EU regulation. Mierczyńska et al. (2017) also found that the uronic acid content in pectin from many plants such as peach, black currant, raspberry, plum, strawberry and carrot, extracted with citric acid was lower than specification of pectin commercial. The lower galacturonic acid content in extracted pectin might be due to the extraction condition. Guo et al. (2017) reported that the extraction at lower pH, higher temperature and longer time resulted the higher galacturonic acid content in sugar beet pectin. Moreover, they also mentioned that the galacturonic acid content in pectin was mainly affected by temperature. On the other hand, Denman and Morris (2015) reported that the initial pH was influenced on the galacturonic acid content. Furthermore, Methancanon et al. (2014) reported that the pectin extracted at lower pH contained higher galacturonic acid content. Therefore, the extraction condition such as pH and also purification procedure should be modified in order to obtained higher galacturonic acid content.
Besides, the methoxyl content was also determined. The results showed that the methoxyl content was not affected by acid extractant. All obtained pectin in our results were in the range of 21.45-22.67 % which was higher than the research conducted by Grassino et al. (2016), 3.38–5.56% depending on the UAE condition. This result was probably due to the extraction procedure such as acid type extractant, pH as well as raw material used in the experiment.
DM of pectin is an important parameter for pectin gelling property (Voragen et al., 1995). The acid type extracted may play a role in DM of obtained pectin. Citric acid extraction resulted in a low DM pectin (DM of 40.61%) while the hydrochloric acid and nitric acid extraction both provided the high DM pectin. This would be probably due to chelating property of citric acid which extracted pectin bound with calcium in the cell wall, hence, pectin expectedly contained low DM. The chelating soluble pectin has been reported that it presented a low DM such as from Okra cell wall (Sengkhamparn et al., 2009). The low DM pectin was also found in pectin used citric acid as an extractant from many raw materials such as from papaya peel (Koubala et al., 2014), banana peel (Oliverira et al., 2016) and also peach, black currant, raspberry, strawberry and carrot (Mierczyńska et al., 2017). The DM of pectin in this study was lower than that produced by tomato waste from food canning industry in research conducted by Grassino et al. (2016).
The lycopene content in obtained pectin was also determined. The results revealed that all obtained pectin contained lycopene. This indicated that lycopene including pectin could be extracted under UAE method and might be trapped during pectin precipitation. Jazaeri et al. (2018) mentioned that lycopene might be linked to cellulose, hemicellulose, pectin or peptides during tomato paste processing. Interestingly, this complex system might be found during pectin extraction. Nevertheless, the acid type extractant was not affected to lycopene content. However, the lycopene content seems to be lower in citric acid extraction. This result was in accordance with the lowest of a* in pectin extracted with citric acid. It can be explained by the citric acid have a lower hydrolyzing capacity compare to other acid extractants in this study, hence the extracted lycopene would be presented lower. However, the amount of lycopene content could be reduced by washing the pectin precipitant with more ethanol. Besides, the pectin has been reported that it can be linked with phenolic compound in the material (Caffall and Mohnen, 2009) so it would be more advantage to determine the phenolic compound content in the extracted pectin.
CONCLUSION
Ultrasound Assisted Extraction (UAE) has been recently performed for bioactive compound extraction from plant. Pectin from tomato waste from Industrial was extracted under UAE. The different extraction temperature (40, 60 or 80 oC) and time (10, 15, 20 or 30 min) under solid: liquid ratio of 1:40 w/v and pH of 2.5 with citric acid as an extractant were performed. The results indicated that the extraction temperature of 80 oC and time of 20 min was a suitable condition which gave the highest pectin yield. Additionally, three acid type extractant (citric acid, hydrochloric acid and nitric acid) beyond this UAE condition were compared. The results showed that the citric acid extractant could extract strongly bond pectin resulting in the highest pectin yield was obtained. Besides, pectin extracted with citric acid contained higher AUA content compare to the others but contained comparable methyl content. This pectin could be categorized as Low DM pectin, while the others categorized as High DM pectin. Moreover, due to the low hydrolyzing property of citric acid, the pectin extracted with citric acid exhibited a lower a* as well as lower lycopene content. In conclusion, the UAE with citric acid could be an efficiency method for pectin extraction with a higher pectin yield, low DM pectin with interestingly trapped lycopene. However, the extraction procedure should be improved in order to obtained higher AUA content.
ACKNOWLEDGEMENTS
The researchers gratefully thank the Research and Technology Transfer Affairs for their financial support (Code 61003002). Moreover, the researchers are also thankful to Natthaporn Wannasai and Theerapong Intaphad for their help in performing experimentation. The researchers are grateful to thank the Faculty of Applied Science and Engineering for supporting instruments.
REFERENCES
Bagherian, H., Ashtiani, F.Z., Fouladitajar, A, and Mohtashamy, M. 2011. Comparison between conventional, microwave- and ultrasound-assisted method for extraction of pectin form grapefruit. Chemical Engineering and Processing: Process Intensification. 50: 1237–1243. https://doi.org/10.1016/ j.cep.2011.08.002
Caffall, K.H., and Mohnen, D. 2009. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydrate Research. 344: 1879–1900. https://doi.org/10.1016/j.carres.2009.05.021
Denman, I.J., and Morris, G.A. 2015. An experimental design approach to the chemical characterisation of pectin polysaccharides extracted from Cucumidmelo Inodorus. Carbohydrate Polymers. 117: 364–369. https://doi.org/10.1016/j.carbpol.2014.09.081
Freitas de Oliveira, C., Giordani, D., Lutckemier, R., Gurak, P.D., Cladera- Olivera, F., and Ferreira Marczak, L.D. 2016. Extraction of pectin from passion fruit peel assisted by ultrasound. LWT - Food Science and Technology. 71: 110-115. https://doi.org/10.1016/j.lwt.2016.03.027
Grassino A.N., Brnčić, M., Vikić-Topić, D., Roca, S., Dent, M., and Brnčić, S.R. 2016. Ultrasound assisted extraction and characterization of pectin from tomato waste. Food Chemistry. 198: 93-100. https://doi.org/10.1016/ j.foodchem.2015.11.095
Guo, X., Guo, X., Meng, H., Zhang, B., and Yu, S. 2017. Using the high temperature resistant pH electrode to auxiliary study the sugar beet pectin extraction under different extraction condition. Food Hydrocolloids. 70: 105-113.
Jamsazzadeh Kermani, Z., Shpigelman, A., Kyomugasho, C., Van Buggenhout, S., Ramezani, M., Van Loey, A.M., and Hendrickx, M.E. 2014. The impact of extraction with a chelating agent under acidic conditions on the cell wall polymers of mango peel. Food Chemistry. 161: 199–207. https://doi.org/ 10.1016/j.foodchem.2014.03.131
Jazaeri, S., Mohammadi, A., Kermani, A.M.P., Paliyath, G., and Kakuda, Y. 2018. Characterization of lycopene hydrocolloidal structure induced by tomato processing. Food Chemistry. 245: 958–965. https://doi.org/10.1016/j.food chem.2017.11.077
Kaur, D., Wani, A.A., Oberoi, D.P.S., and Sogi, D.S. 2008. Effect of extraction conditions on lycopene extractions from tomato processing waste skin using response surface methodology. Food Chemistry. 108: 711–718. https://doi.org/10.1016/j.foodchem.2007.11.002
Koubala, B.B., Christiaens, S., Kansci, G., Van Loey, A.M., and Hendrickx, M.E. 2014. Isolation and structural characterisation of papaya peel pectin. Food Research International. 55: 215–221. https://doi.org/10.1016/j.foodres.2013.11.009
Koubala, B.B., Mbome, L.I., Kansci, G., Mbiapo, F.T., Crepeau, M.J., Thibault, J.F., and Ralet, M.C. 2008. Physicochemical properties of pectins from extraction conditions. Food Chemistry. 106: 1202–1207. https://doi.org/ 10.1016/j.foodchem.2007.07.065
Ismail, N.S.M., Ramli, N., Hani, N.M., and Meon, Z. 2012. Extraction and characterization of pectin from dragon fruit (Hylocereuspolyrhizus) using various extraction conditions. Sains Malaysiana. 41: 41–45.
Methancanon, P., Krongsin, J., and Gamonpillas, C. 2014. Pomelo (Citrus maxima) pecin: Effects of extraction parameters and its properties. Food Hydrocolloids. 35: 383–391. https://doi.org/10.1016/j.foodhyd.2013.06.018 Mierczyńska, J., Cybulska, J., and Zdunek, A. 2017. Rheological and chemical properties of pectin enriched fractions from different sources extracted with
citric acid. Carbohydrate Polymers. 156: 443-451. https://doi.org/10.1016/ j.carbpol.2016.09.042
Moorthy, I.G., Maran, J.P., Ilakya, S., Anitha, S.L., Sabarima, S.P., and Priya, B. 2017. Ultrasound assisted extraction of pectin from waste Artocarpus heterophyllus fruit peel. Ultrasonics Sonochemistry. 34: 525–530. https:// doi.org/10.1016/j.ultsonch.2016.06.015
Oliverira, T.Í.S., Rosa, M.F., Cavalcante, F.L., Pereira, P.H.F., Moates, G.K., Wellner, N., Mazzetto, S.E., Waldron, K.W., and Azeredo, N.M.C. 2016. Optimization of pectin extraction from banana peels with citric acid by using response surface methodology. Food Chemistry. 198: 113–118. https://doi.org/10.1016/j.foodchem.2015.08.080
Pereira, P.H.F., Oliverira, T.Í.S., Rosa, M.F., Cavalcante, F.L., Moates, G.K., Wellner, N., Waldron, K.W., and Azeredo, H.M.C. 2016. Pectin extraction from pomegranate peels with citric acid. International Journal of Biological Macromolecules. 88: 373–379. https://doi.org/10.1016/j.ijbio mac.2016.03.074
Pinheiro, E.R., Silva, I.M.D.A., Gonzaga, L.V., Amante, E.R., Teófilo, R.F., Ferreira, M.M.C., and Amboni, R.D.M.C. 2008. Optimization of extraction of high-ester pectin from passion fruit peel (Passiflora edulis flavicarpa) with citric acid by using response surface methodology. Bioresource Technology. 99: 5561–5566. https://doi.org/10.1016/j.biortech.2007.10.058
Poojary, M.M., and Passamonti, P. 2015. Optimization of extraction of high purity all-trans-lycopene from tomato pulp waste. Food Chemistry. 188: 84–91. https://doi.org/10.1016/j.foodchem.2015.04.133
Renard, C.M.G.C., and Thibault, J.F. 1993. Structure and properties of apple and sugar beet pectins extracted by chelating agent. Carbohydrate Research. 244: 99–114. https://doi.org/10.1016/0008-6215(93)80007-2
Schieber, A., Stintzing, F.C., and Carle, R. 2001. By-products of plant food processing as a source of functional compounds-recent development. Trends in Food Science and Technology. 12: 401–413. https://doi.org/10. 1016/S0924-2244(02)00012-2
Sengkhamparn, N., Verhoef, R., Schols, H.A., Sajjaanantakul, T., and Voragen, A.G.J. 2009. Characterization of Cell Wall Polysaccharide from Okra (Abelmoschus esculentus (L.) Moench). Carbohydrate Research. 344: 1824-1832. https:// doi.org/10.1016/j.carres.2008.10.012
Voragen, A.G.J., Pilnik, W., Thibault, J.F, Axelos, M.A.V., and Renard, C.M.G.C. 1995. Pectins. In Stephen A.M. (Ed). Food polysaccharide and their application (pp. 287–339) Marcel Dekker, New York.
Wang, M., Huang, B., Fan, C., Zhao, K., Hu, H., Xu, X., Pan, S. and Liu, F. 2016. Characterization and functional properties of mango peel pectin extracted by ultrasound assisted citric acid. International Journal of Biological Macromolecules. 91: 794–803. https://doi.org/10.1016/
Nipaporn Sengkhamparn*, Patareeya Lasunon, and Prapaporn Tettawong
Faculty of Applied Science and Engineering, Khon Kaen University, Nong Khai Campus, NongKhai 43000, Thailand
*Corresponding author. E-mail: nipaporn@kku.ac.th
Total Article Views
Article history:
Received: July 16, 2018;
Revised: October 24, 2018;
Accepted: November 1, 2018

