
Systematic Review-Meta Analysis Study the Effect of Cadmium and Copper Exposure on Children’s Cognitive
Leonardo Ferryanto M. Samadhi, Irwanto*, and Indri Safitri MukonoPublished Date : 2022-11-07
DOI : https://doi.org/10.12982/CMUJNS.2022.066
Journal Issues : Number 4, October-December 2022
Abstract Childhood neurodevelopment could be affected by various factors, such as heavy metal cadmium and copper. The aim of this study was to analyze the scientific evidence published to date on the potential effects on cognitive development in children exposed to cadmium and copper by pooling the results of the different studies. We conducted a systematic review and meta-analysis of original articles published from January 2012 until December 2020 in PubMed, Medline, and Cochrane which evaluate the effects on cognitive development due to pre or post-natal exposure to cadmium and copper in children. Meta-analysis was performed using Revman 5.3 and linear regression techniques were used to estimate the effect size. Publication bias was evaluated by Begg’s and Egger’s tests. Our search resulted in 6 articles that evaluated the effects of cadmium and copper on cognitive development met the inclusion criteria: 4 cadmium, 2 copper, and 1 both. Most studies evaluating cadmium exposure (4 of 5) reported a significant negative effect on cognitive development. Only two studies that evaluated exposure to copper found an association with neurodevelopment. The meta-analysis showed an increase of cadmium levels in urine would be associated with a 1.4 decrease in the full-scale intelligence quotient (IQ) (P =0.008) and a 1.03 decrease in verbal scale IQ of children (P =0.006). There were no enough studies of copper exposure to conduct a meta-analysis. Current evidence supports the finding that cadmium exposure has a detrimental effect on cognitive development in children but there is little information on copper.
Keywords: Cadmium, Child, Cognitive, Copper, Neurodevelopment
Citation: Ferryanto, M.L. Samadhi, Irwanto, and Mukono, I.S. 2022. Systematic review-meta analysis study the effect of cadmium and copper exposure on children’s cognitive. CMUJ. Nat. Sci. 21(4): e2022066.
INTRODUCTION
Heavy metals are known to have detrimental health effects on humans who are exposed to certain doses, examples of heavy metals are cadmium and copper (Järup, 2003). Anthropogenic sources of heavy metals in the environment come from fossil fuel combustion systems, waste fuel systems, consumer products and mining waste and metal smelting (Yáñ ez et al., 2002). Human exposure to cadmium and copper is generally comes from inhaling metal particles in the air, contaminated food or drinking water (WHO, 2004, 2011). Children are more susceptible than adults to poisoning from copper and cadmium exposure. This may result from increased exposure to copper and cadmium, mutations that interfere with normal homeostasis or epigenetic factors associated with the development of copper homeostasis functionality early in life (WHO, 2004, 2011). Environmental exposure in the uterus can have long-term effects on health and development. Prenatal and postnatal cadmium exposure have a detrimental effect on the neurodevelopment of the child and can decrease the mean head circumference at birth and the child's growth in the first 3 years of life (Lin et al., 2011).
One of the various factors affecting children's cognitive abilities is the exposure to common environmental pollutants, such as carbon monoxide, cadmium, copper, and other heavy metals (Liu and Lewis, 2014). Epidemiological studies have found controversial results regarding the relationship between children's exposure to cadmium and copper on children's cognitive development and children who already suffering from pathologies (Forns et al., 2014; Kippler et al., 2016; Polanska et al., 2017; Amoros et al., 2019; Fiore et al., 2020; Rashaid et al., 2021). To our knowledge, there was only one systematic review or meta-analysis to date that has examined the the effect of cadmium in neurovelopment (Rodríguez-Barranco et al., 2013) but an analysis was not possible due to the insufficient number of studies and none for copper. Therefore, the aim of this study was to systematically review recent epidemiological studies of the relationship between cadmium and copper exposure in children and children's cognitive development.
MATERIALS AND METHODS
Systematic reviews of observational studies were carried out with the PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) (Liberati et al., 2009).
Inclusion criteria
- Journal was published betweeen year 2012 to 2020
- From PubMed dan Medline database
- Prospective cohort and cross-sectional study
- The subjects of the study were children up to 18 years of age
- Study on evaluating the effect of cadmium and copper exposure on children's cognitive using the Wechsler Intelligence Scale (WISC) instrument and using linear regression techniques to estimate the effect size.
Exclusion criteria
Case study articles or case series, ecological designs, literature reviews and those that only evaluate indirect cadmium and copper exposure via a questionnaire.
Literature search strategy
A PubMed, Medline, and Cochrane search was carried out to identify related studies published in 2012 to the end of 2020. The keywords "child" AND "infant" AND "school" AND "prenatal" AND "postnatal" AND "intelligence" AND "IQ" AND "cognitive "AND ("cadmium" OR "copper") were used. Searches were limited to human studies and English language.
Study selection and data extraction
All considered publications were screened for relevance by three independent reviewer and any disagreement on the title and abstract of studies was resolved by discussion. The full text of the relevant studies were checked based on the inclusion criteria by researchers. A final list of eligible studies was prepared after a consensus between the three researchers. The data extracted from all eligible studies were as follows: year of publication, location, first author's last name, study design, sample size and method of cadmium and copper exposure assessment, and results expressed as β coefficients.
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist was used to assess the methodological quality of the study. This tool was originally developed to assess clarity in communicating research results in observational studies and has been used in recent systematic reviews to assess the methodological quality of observational studies (von Elm et al., 2008; Rodríguez-Barranco et al., 2013). Of the 22 items included in the checklist, 9 items related to the methods section were selected, which assessed different aspects of the methodology in the observational study (Table 1). After performing the assessment, the methodological qualities were classified as follows: articles meeting 0–3 out of 9 items were considered to have low methodological quality, 4-6 items as moderate methodological quality, and 7-9 items as high methodological quality (von Elm et al., 2008; Rodríguez-Barranco et al., 2013).
Meta analysis
A meta-analysis of the results reported by different studies was conducted to make estimates and an overall summary of the magnitude of the effects of cadmium and copper exposure on children's cognitive development. The meta-analysis was limited to studies evaluating the Full Scale IQ, Verbal IQ and Performance IQ using any version of the Wechsler scale and linear regression techniques to estimate their effects.
Table 1. STROBE Checklist.
|
Methods |
No item |
Recommendation |
|
Study Design |
4 |
Show the key elements of study design in the paper |
|
Setting |
5 |
The study describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection |
|
Participant |
6 |
(a) Cohort study—Give the eligibility criteria, and the sources and methods of selection of participants. Describe methods of follow-up Cross-sectional study—Give the eligibility criteria, and the sources and methods of selection of participants (b) Cohort study—For matched studies, give matching criteria and number of exposed and unexposed |
|
Variable |
7 |
Comprehensibly define outcomes, exposures, predictors, potential confounders, and effect modifiers. |
|
Data sources/ measurement |
8 |
For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group |
|
Bias |
9 |
Describe any endeavor to overcome potential sources of bias |
|
Study Size |
10 |
Explain how the study size was arrived at |
|
Quantitative Variable |
11 |
Explain how quantitative variables were picked up in the analyses. |
|
Statistical Methods |
12 |
(a) Describe all statistical methods, including those used to control for confounding (b) Describe any methods used to examine subgroups and interactions (c) Explain how missing data were addressed (d) Cohort study—If applicable, explain how loss to follow-up was addressed Cross-sectional study—If applicable, describe analytical methods taking account of sampling strategy (e) Describe any sensitivity analyses |
Statistical analysis
Heterogeneity across studies was evaluated using the Cochran Q-test and expressed using the I2 index (Whitehead and Whitehead, 1991). The pooled results for Fisher's z-transform were calculated by means of a fixed effect model (for I2 <50%) or a random effect model (for I2> 50%) (Thakkinstian et al., 2005). Publication bias was evaluated by Egger and Begg's test (Higgins and Thompson, 2002). Statistical analysis were conducted using RevMan 5.3 software
RESULTS
Study selection
Of the 226 articles originally identified in the literature search, 6 met the inclusion criteria: 4 articles evaluated the effect of cadmium, 1 article evaluated the effect of copper and 1 article evaluated the effects of the both metals on children's cognitive (Figure 1).
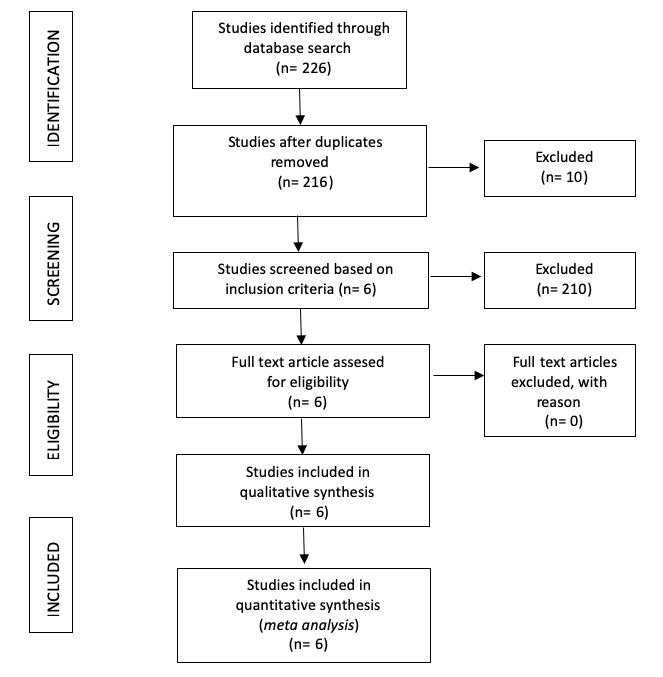
Figure 1. Flow chart of study selection for meta-analisis.
Design and study population
Most of the articles were prospective cohort study (5 articles) and cross-sectional (1 article). Randomized Clinical Trial (RCT) research articles were not found in the literature search for this study. The distribution of journal research took places showed that it was carried out in various countries such as Spain, Italy, China, and Bangladesh. If it is divided by continent, there are 3 journals conducted in Asia (China, Bangladesh) and 3 journals that conduct research in Europe (Spain, Italy).
The study publication year was from 2012 to 2020. The sample sizes ranged from 261 (Rodríguez-Barranco et al., 2014) to 1489 (Gustin et al., 2018) representing a total of 3651 participants for cadmium and 1,016 participants for copper for cognitive. STROBE scores and main characteristic of each studies included in the systematic review are shown in Table 2 and 3.
Table 2. Main characteristics of the study on cadmium were included in the systematic meta-analysis.
|
No. |
Location |
Title, Author & year of publication |
Age Range |
Sample size |
Study Design |
Confounding |
Mean ± SD (range) |
Psychological Test |
Result |
STROBE Score |
|
1. |
China |
Sex-Specific Differences in Cognitive Abilities Associated with Childhood Cadmium and Manganese Exposures in School-Age Children: a Prospective Cohort Study
Zhou et al.2020 |
6-8 years |
296 |
Prospective Cohort |
Gender of child, age of mother at childbirth, level of education, annual family income, area of residence and passive smoking |
0.18 ± 2.38 μg/L
|
WISC-R |
Cd urine ↓ Full Scale IQ, Verbal IQ and Performance IQ
|
9 (High) |
|
2. |
Italy |
Neurocognitive impact of metal exposure and social stressors among schoolchildren in Taranto, Italy
Lucchini et al. 2019 |
7–10 years |
299 |
Prospective Cohort |
Gender of child, age, mother's non-verbal intelligence and cognitive stimulation |
0.4 ± 1.4 ng/dL
|
WISC-IV
|
Cd urine ↓ Full Scale IQ
|
9 (High) |
|
3. |
Bangladesh
|
Cadmium exposure and cognitive abilities and behavior at 10 years of age: A prospective cohort study
Gustin et al. 2018 |
5- 10 years |
1489 |
Prospective Cohort |
Nutritional status, socioeconomic status, level of education of parents, IQ of mothers and level of stimulation at home |
0.24 ± 0.06 μg/L
|
WISC-IV
|
Cd urine ↓ Full Scale IQ
|
9 (High) |
|
4. |
Spain |
Cadmium exposure and neuropsychological development in school children in southwestern Spain
Rodríguez-Barranco et al. 2014
|
6 – 9 years |
261 |
Cross- Sectional |
Gender of child, child's age, body mass index, maternal age, IQ, father's education and occupation, family income, place of residence, family status, gestational age, body weight, height and head circumference at birth, intake of vegetables and cereals and IQ rater. |
0.29 μg/L
|
WISC-IV
|
Cd urine ↓ Full Scale IQ and Verbal IQ
|
9 (High) |
|
5. |
Bangladesh |
Early-Life Cadmium Exposure and Child Development in 5-Year-Old Girls and Boys: A Cohort Study in Rural Bangladesh
Kippler et al. 2012 |
5 tahun |
1305 |
Prospective Cohort |
Quality of stimulation, mother's IQ, birth order and socioeconomic status |
0.22 ± 0.06 μg/L |
WPPSI
|
Cd urine ↓ Full Scale IQ, Verbal IQ and Performance IQ |
9 (High) |
Table 3. Main characteristics of the study on copper were included in the systematic meta-analysis review.
|
No. |
Location |
Title, Author & year of publication |
Age range |
Sample size |
Study Design |
Confounding |
Sample |
Mean ± SD (range) |
Psychological Test |
Result |
STROBE Score |
|
1. |
China |
Sex-Specific Differences in Cognitive Abilities Associated with Childhood Cadmium and Manganese Exposures in School-Age Children: a Prospective Cohort Study
Zhou et al. 2020 |
7 years |
296 |
Prospective Cohort |
Gender of child, age of mother at childbirth, level of education, annual family income, area of residence and passive smoking |
Urine |
9.36 ± 2.23 μg/L
|
WISC-R |
Cu urine ↓ verbal IQ |
9 (High) |
|
2. |
Italy |
Associations of a Metal Mixture Measured in Multiple Biomarkers with IQ: Evidence from Italian Adolescents Living near Ferroalloy Industry
Bauer et al. 2020 |
10-14 years |
720 |
Prospective Cohort |
Age, gender of child, socioeconomic status, HOME (Home Observation Measurement of the Environment) score and hemoglobin levels |
Hair |
9.6 μg/g |
WISC-III |
Cu hair ↓ Verbal IQ |
9 (High) |
Association of cadmium with cognitive development in children
Cadmium exposure with the child's full scale IQ
The combined analysis of cadmium exposure was significantly associated with the child's Full Scale IQ with β = -1.4; 95% CI (- 2.44, - 0.36) using a random effects model. However, significant heterogenecity was detected for the meta-analysis of the relationship between cadmium exposure and cognitive (I2 = 64%, P = 0.008) (Figure 2). The p-value for Begg's test was 0.071 and Egger's test was 0.056, therefore there was no publication bias (P-value > 0.05).
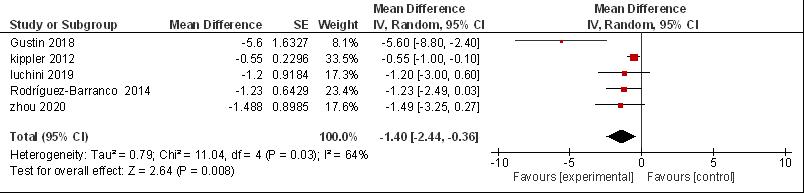
Figure 2. Forest plot urine cadmium in full scale IQ.
Cadmium exposure with the child's verbal scale IQ
The combined magnitude of the effect indicated that cadmium in the child's urine caused a decrease of -1.03 points (95% CI: -1.76, -0.29; P = 0.006). This shows that the P value <0.05, which means that in this study there is a significant relationship between cadmium exposure and Verbal IQ. This study is heterogeneous because it can be seen in the I2 results of 53%, which indicates the heterogeneity value of I2 > 50%, so a random effects model is used to combine the results (Figure 3). The p-value for Begg's test was 0.312 and Egger's test was 0.236, therefore there was no publication bias (P-value > 0.05).
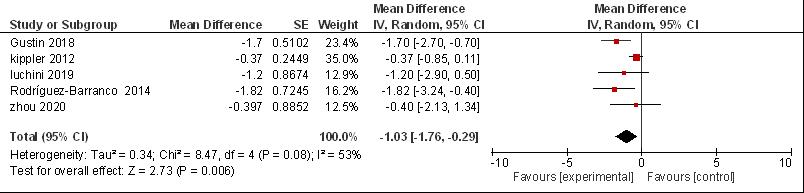
Figure 3. Forest plot urine cadmium in verbal IQ.
Cadmium exposure with the child's performance scale IQ
The combined analysis of cadmium exposure not significantly related to children's performance IQ was β = -1.3; 95% CI (-3.01, 0.42) using the random effects model. However, the heterogeneity was not significant for the meta-analysis result of the association between cadmium exposure and children’s cognitive (I2 = 68%, P = 0.14) (Figure 4). The P-value for Begg's test was 0.159 and Egger's test was 0.06, therefore there was no publication bias (P-value > 0.05).
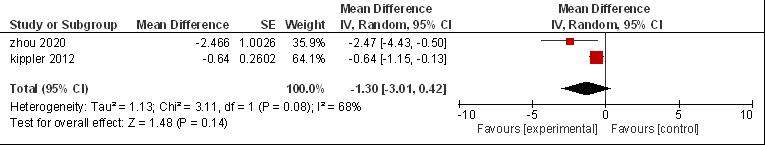
Figure 4. Forest plot urine cadmium in performance IQ.
Association of copper with cognitive development in children
There were not enough studies of copper exposure in children’s cognitive to conduct a meta-analysis.
DISCUSSION
In this study, we have systematically reviewed the recent evidence for the relationship between cadmium and copper exposure in children with children's cognitive develoment. These findings suggest that there was a significant association between cadmium exposure measured in children's urine and impaired cognitive of children. There was one previous systematic review or meta-analysis that has examined the the effect of cadmium in neurovelopment but an analysis was not possible due to the insufficient number of studies during that time (Rodríguez-Barranco et al., 2013).
In the meta-analysis results, the p-value was 0.008 for the Full Scale IQ and 0.006 for Verbal IQ. This result showed that there was a significant relationship between cadmium exposure in children's urine and cognitive development on the Full Scale IQ and Verbal IQ index. In the sample heterogeneity test, the sample used were heterogeneous. It was reported that the results of the Full Scale IQ measurement with cadmium exposure measured from children's urine with the combined magnitude of the effect showed that an increase in the level of cadmium levels in the urine caused a decrease of -1.4 points and the Verbal IQ caused a decrease of -1.03 points, but there were no effect on Performance IQ. The p-value for Begg's test and Egger's tests were > 0.05 for all studies therefore there were no publication bias among these three meta-analyzes.
Of the 5 articles reviewed, it was found that the effect sizes varied for the Full Scale IQ index ranging from -0.55 (Kippler et al., 2012) to the highest -5.6 (Gustin et al., 2018). The results of this study are in line with with research Gustin et al., 2018 which states that there was an association between cadmium exposure and a decrease in Full Scale IQ and a study by Rodríguez-Barranco et al., 2014 with a decrease in Verbal IQ (Rodríguez-Barranco et al., 2014; Gustin et al., 2018).
Cadmium exerts toxicity to various organ systems and tissues, such as the respiratory, cardiovascular, nervous, and bone, by affecting their function either directly or indirectly. This toxic effect induces degeneration or even transmutation of cells. At the cellular level, cadmium induces both destruction and repair processes in which cellular redox status plays an important role (Cuypers et al., 2010). There is clear evidence on the ability of cadmium to provoke indirect oxidative damage to DNA, through the induction of cell proliferation mechanisms, inhibition of the apoptotic mechanism, and block the DNA repair mechanism. Oxidative stress is assumed to be the main molecular basis underlying the cytotoxicity induced by cadmium (Cuypers et al., 2010). Cadmium could reaches to the central nervous system directly, which will cause a neurotoxic effect on child development and an impact on neurodevelopment. Much of the evidence regarding cadmium-induced neuronal toxicity was due to the induction of ROS, leading to oxidative stress. Cadmium induces the formation of ROS which can cause apoptosis of nerve cells through activation of the MAPK (Mitogen-Activated Protein Kinases) and mTOR (mammalian target of rapamycin) signaling pathways (Chen et al., 2011). Cerebral cortical neurons have been identified as a target for toxicity. Cadmium-induced apoptosis of cerebral cortical neurons occurs via mitochondrial Ca2+ signaling. Cadmium interferes with intracellular free calcium (Ca2+) homeostasis, leading to neuronal apoptosis. Calcium is an intracellular ion that acts as a signaling mediator in many cellular processes including proliferation, differentiation and cell survival (Xu et al., 2011). In vivo and in vitro studies revealed that exposure to cadmium at certain concentrations can produce cadmium dose-thyroid response relationships (Buha et al., 2018). It has been known for many years that thyroid hormones are very important in mammalian brain maturation, influencing many aspects related to neural cell migration, differentiation, signaling, and cognitive function (Rovet, 2014).
Vacchi-Suzzi et al., have proven the stability of cadmium in both spontaneous and morning urine samples thus showing that urine cadmium can be used as a biomarker of long-term cadmium exposure in research (Vacchi-Suzzi et al., 2016). The result of the study by Vacchi- Suzzi et al., could be as one explanation of the correlation between urine cadmium levels and children's cognitive in this meta-analysis study. Miura et al., analyzed genetic polymorphisms of people in Japan who have jobs exposed to cadmium in cadmium toxicity susceptibility. In populations with SNP A → G significantly reduced transcription of the metalothionein-2A gene so that people with the G / G genotype were more sensitive to cadmium toxicity compared to type A / A (Miura, 2009).
There were not enough cognitive studies on copper exposure to conduct a meta-analysis. Several studies published to date have evaluated the effects of copper exposure on children's cognitive development. Of all the studies included in this review, the relationship was observed in two studies. Two studies conducted in China and Italy, found that exposure can reduce children's cognitive abilities. From the 2 articles that were reviewed, it was found that the effect sizes varied for the Verbal Scale IQ from a study by Bauer et al., 2020 in Italy which found that an increase in copper levels in hair significantly led to a decrease in Verbal IQ of -2.9 points (CI 95 %: -5.2, -0.5, P = <0.05) (Bauer et al., 2020) and the research by Zhou et al., 2020 stated that there was a decrease in the Verbal Scale IQ of - 2.2 points (95% CI: -4.36, -0.039, P = 0.046) on increase in copper levels in children's urine (Zhou et al., 2020).
The results obtained from a number of experimental studies support the view that damage ROS-induced oxidative action plays an important role in copper toxicity. For example, excess copper can cause peroxidative damage to membrane lipids through the reaction of lipid and oxygen radicals to form peroxy radicals, and cause peroxidation in the lysosome membrane of liver cells (Bremner, 1998). In mice, copper could cause oxidative injury, decreased liver GSH level and α-tocopherol, increased levels of mitochondrial lipid peroxidation products, decreased respiratory control ratio in hepatic mitochondria, and decreased IV complex activity (cytochrome C oxidase) and increased levels of copper in the liver (Zhang et al., 2000). Copper also reduced cytochrome C oxidase activity and impaired hepatic mitochondrial respiration and increased rat liver chemiluminescence, while GSH catalase and peroxidase activity decreased significantly. With the increase of oxidation activity that exceeds the body's antioxidant system and induces DNA damage, lipid peroxidation, protein modification and other effects, ROS can lead to the development of degenerative diseases, including cancer, cardiovascular disease, diabetes, atherosclerosis, neurological disorders, and chronic inflammation (Jomova and Valko, 2011).
Strength and limitation of the current review
No systematic review and meta-analysis has ever reported an association between childhood Cadmium exposure and children's cognitive development. The main strength of this research is its novelty and applied findings that can be useful in preventing developmental retardation in children. The findings in this review also have several limitations, such as the number of journals that carry this theme was still very small.
CONCLUSION
This systematic review and meta-analysis revealed that there was a significant relationship between children's cadmium exposure measured from urine and children's cognitive abilities. The small number of studies that have evaluated cognitive problems due to copper exposure makes it impossible to draw clear conclusions regarding this compound. Therefore, future research is highly recommended to re-evaluate new data on the impact of heavy metals cadmium and copper on children's cognitive abilities
ACKNOWLEDGMENTS
The work described is original research that has not ben published previously and is not under consideration for publication elsewhere, in whole or in part. All of the authors listed have approved the enclosed manuscript.
REFERENCES
Amoros, R., Murcia, M., Gonzalez, L., Soler-Blasco, R., Rebagliato, M., Iniguez, C., Carrasco, P., Vioque, J., Broberg, K., Levi, M., Lopez-Espinosa, M.-J., Ballester, F., and Llop, S. 2019. Maternal copper status and neuropsychological development in infants and preschool children. International Journal of Hygiene and Environmental Health. 6–9.
Bauer, J. A., Devick, K. L., Bobb, J. F., Coull, B. A., Bellinger, D., Benedetti, C., Cagna, G., Fedrighi, C., Guazzetti, S., Oppini, M., Placidi, D., Webster, T. F., White, R. F., Yang, Q., Zoni, S., Wright, R. O., Smith, D. R., Lucchini, R. G. and Claus Henn, B. 2020. Associations of a metal mixture measured in multiple biomarkers with IQ: Evidence from Italian adolescents living near ferroalloy industry. Environmental Health Perspectives. 128: 97002.
Bremner, I. 1998. Manifestations of copper excess. American Journal of Clinical Nutrition. 67(5 Suppl): 1069–1073.
Buha, A., Matovic, V., Antonijevic, B., Bulat, Z., Curcic, M., Renieri, E.A., Tsatsakis, A.M., Schweitzer, A., and Wallace, D. 2018. Overview of cadmium thyroid disrupting effects and mechanisms. International Journal of Molecular Sciences 19.
Chen, L., Xu, B., Liu, L., Luo, Y., Zhou, H., Chen, W., Shen, T., Han, X., Kontos, C.D., and Huang, S. 2011. Cadmium induction of reactive oxygen species activates the mTOR pathway, leading to neuronal cell death. Free Radical Biology & Medicine. 50: 624–632.
Cuypers, A., Plusquin, M., Remans, T., Jozefczak, M., Keunen, E., Gielen, H., Opdenakker, K., Nair, A.R., Munters, E., Artois, T.J., et al. 2010. Cadmium stress: an oxidative challenge. Biometals. 23: 927–940.
Fiore, M., Barone, R., Copat, C., Grasso, A., Cristaldi, A., Rizzo, R. and Ferrante, M. 2020. Metal and essential element levels in hair and association with autism severity. Journal of Trace Elements in Medicine and Biology : Organ of the Society for Minerals and Trace Elements (GMS). 57: 126409.
Forns, J., Fort, M., Casas, M., Caceres, A., Guxens, M., Gascon, M., Garcia-Esteban, R., Julvez, J., Grimalt, J.O., and Sunyer, J. 2014. Exposure to metals during pregnancy and neuropsychological development at the age of 4 years. Neurotoxicology. 40: 16–22.
Gustin, K., Tofail, F., Vahter, M., and Kippler, M. 2018. Cadmium exposure and cognitive abilities and behavior at 10 years of age: A prospective cohort study. Environment International. 113: 259–268.
Higgins, J.P.T. and Thompson, S.G. 2002. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine. 21:1539–1558.
Järup, L. 2003. Hazards of heavy metal contamination. British Medical Bulletin 68(1): 167–182.
Jomova, K. and Valko, M. 2011. Advances in metal-induced oxidative stress and human disease. Toxicology. 283: 65–87.
Kippler, M., Bottai, M., Georgiou, V., Koutra, K., Chalkiadaki, G., Kampouri, M., Kyriklaki, A., Vafeiadi, M., Fthenou, E., Vassilaki, M., et al. 2016. Impact of prenatal exposure to cadmium on cognitive development at preschool age and the importance of selenium and iodine. European Journal of Epidemiology. 31: 1123–1134.
Kippler, M., Hossain, M.B., Lindh, C., Moore, S.E., Kabir, I., Vahter, M., and Broberg, K. 2012. Early life low-level cadmium exposure is positively associated with increased oxidative stress. Environmental Research. 112: 164–170.
Liberati, A., Altman, D.G., Tetzlaff, J., Mulrow, C., Gøtzsche, P.C., Ioannidis, J.P.A., Clarke, M., Devereaux, P.J., Kleijnen, J. and Moher, D. 2009. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. The BMJ. 339: b2700.
Lin, C.-M., Doyle, P., Wang, D., Hwang, Y.-H., and Chen, P.-C. 2011. Does prenatal cadmium exposure affect fetal and child growth? Occupational and Environmental Medicine. 68: 641–646.
Liu, J. and Lewis, G. 2014. Environmental toxicity and poor cognitive outcomes in children and adults. Journal of Environmental Health. 76: 130–138.
Lucchini, R.G., Guazzetti, S., Renzetti, S., Conversano, M., Cagna, G., Fedrighi, C., Giorgino, A., Peli, M., Placidi, D., Zoni, S., Forte, G., Majorani, C., Pino, A., Senofonte, O., Petrucci, F., and Alimonti, A. 2019. Neurocognitive impact of metal exposure and social stressors among schoolchildren in Taranto, Italy. Environmental Health. 18: 67.
Miura, N. 2009. Individual susceptibility to cadmium toxicity and metallothionein gene polymorphisms: With references to current status of occupational cadmium exposure. Industrial Health. 47: 487–494.
Polanska, K., Hanke, W., Krol, A., Gromadzinska, J., Kuras, R., Janasik, B., Wasowicz, W., Mirabella, F., Chiarotti, F., and Calamandrei, G. 2017. Micronutrients during pregnancy and child psychomotor development: Opposite effects of zinc and selenium. Environmental Research 158: 583–589.
Rashaid, A.H.B., Nusair, S.D., Alqhazo, M.T., Adams, J.B., Abu-Dalo, M.A., and Bashtawi, M.A. 2021. Heavy metals and trace elements in scalp hair samples of children with severe autism spectrum disorder: A case-control study on Jordanian children. Journal of Trace Elements in Medicine and Biology : Rgan of the Society for Minerals and Trace Elements (GMS) 67: 126790.
Rodríguez-Barranco, M., Lacasaña, M., Aguilar-Garduño, C., Alguacil, J., Gil, F., González-Alzaga, B. and Rojas-García, A. 2013. Association of arsenic, cadmium and manganese exposure with neurodevelopment and behavioural disorders in children: A systematic review and meta-analysis. Science of the Total Environment. 454-455: 562–577.
Rodríguez-Barranco, M., Lacasaña, M., Gil, F., Lorca, A., Alguacil, J., Rohlman, D.S., González-Alzaga, B., Molina-Villalba, I., Mendoza, R., and Aguilar-Garduño, C. 2014. Cadmium exposure and neuropsychological development in school children in southwestern Spain. Environmental Research. 134: 66–73.
Rovet, J.F. 2014. The role of thyroid hormones for brain development and cognitive function. Endocrine Development. 26: 26–43.
Thakkinstian, A., McElduff, P., D’Este, C., Duffy, D., and Attia, J. 2005. A method for meta-analysis of molecular association studies. Statistics in Medicine. 24: 1291–1306.
Vacchi-Suzzi, C., Kruse, D., Harrington, J., Levine, K., and Meliker, J.R. 2016. Is urinary cadmium a biomarker of long-term exposure in humans? A Review. Current Environmental Health Reports. 3: 450–458.
Von Elm, E., Altman, D.G., Egger, M., Pocock, S.J., Gøtzsche, P.C. and Vandenbroucke, J. P. 2008. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Journal of Clinical Epidemiology. 61: 344–349.
Whitehead, A. and Whitehead, J. 1991. A general parametric approach to the meta-analysis of randomized clinical trials. Statistics in Medicine. 10: 1665–1677.
WHO. 2004. Copper in drinking-water. Background document for development of WHO Guidelines for Drinking-water Quality. Geneva: World Health Organization.
WHO. 2011. Cadmium in drinking-water. Background document for development of WHO Guidelines for Drinking-water Quality. Geneva: World Health Organization.
Xu, B., Chen, S., Luo, Y., Chen, Z., Liu, L., Zhou, H., Chen, W., Shen, T., Han, X., Chen, L., and Huang, S. 2011. Calcium signaling is involved in cadmium-induced neuronal apoptosis via induction of reactive oxygen species and activation of MAPK/mTOR network. PloS One. 6: e19052.
Yáñ ez, L., Ortiz, D., Calderón, J., Batres, L., Carrizales, L., Mejía, J., Martínez, L., García-Nieto, E. and Díaz-Barriga, F. 2002. Overview of human health and chemical mixtures: Problems facing developing countries. Environmental Health Perspectives. 110 Suppl: 901–909.
Zhang, S.S., Noordin, M.M., Rahman, S.O., and Haron, J. 2000. Effects of copper overload on hepatic lipid peroxidation and antioxidant defense in rats. Veterinary and Human Toxicology 42: 261–264.
Zhou, T., Guo, J., Zhang, J., Xiao, H., Qi, X., Wu, C., Chang, X., Zhang, Y., Liu, Q. and Zhou, Z. 2020. Sex-specific differences in cognitive abilities associated with childhood cadmium and manganese exposures in school-age children: A prospective cohort study. Biological Trace Element Research. 193: 89–99.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand.
Leonardo Ferryanto M. Samadhi1, Irwanto1,*, and Indri Safitri Mukono2
1 Department of Child Health, Faculty of Medicine, Universitas Airlangga, Surabaya-Indonesia.
2 Department of Physiology and Medical Biochemistry, Faculty of Medicine, Universitas Airlangga, Surabaya-Indonesia.
Corresponding author: Irwanto, E-mail: irwanto.idris@gmail.com
Total Article Views
Editor: Korakot Nganvongpanit,
Chiang Mai University, Thailand
Article history:
Received: April 7, 2021;
Revised: September 1, 2021;
Accepted: September 13, 2021;

