
Chemical Constituents Antioxidant and Antibacterial Activities of the Leaves and Flowers from Gardenia carinata Wallich
Nichthima Warinthip, Boonsom Liawruangrath, Surapol Natakankitkul, Narabhats Rannurags, Stephen G. Pyne and Saisunee Liawruangrath*Published Date : 2023-01-03
DOI : https://doi.org/10.12982/NLSC.2023.003
Journal Issues : Number 1, January-March 2023
Abstract The essential oil of the leaves was isolated by hydrodistillation and the ethanolic extract of the flowers from Gardenia carinata Wallich. were analyzed for the first time using GC and GC-MS. For the leaves essential oil: Seventeen compounds were detected and eleven compounds were identified, corresponding to 71.10% of the total chromatographical oil compounds. The major constituents were phytol (13.80%), 3-hexene-1-ol, benzoate (12.50%), vitispirane (8.00%), hexadecanal (7.90%), β-damascenone (3.90%) and (E,E)-α-farnesene (3.80%). For the flowers extract: Seventeen compounds were identified, constituting 37.5% of the total chromatographical components. The typical compounds were heneicosane (9.70%), ethyl linolenate (5.70%), squalene (5.70%), 4-allylphenol (5.40%), stigmasterol (2.10%), n-docosane (1.60%), lupenone (1.40%), hexadecanoic acid (0.90%) and compesterol (0.70%). The leaves essential oil showed antibacterial activity against both Gram positive S. aureus with the most potent activity at 18.7 µg/disc, and Gram negative E. coli with an activity of 17.6 µg/disc. The oil was not active against Gram negative P. aeruginosa. The antioxidant activity of the leave essential oil and the ethanolic flower extract were evaluated by using the DPPH and ABTS radical scavenging assay. The leave essential oil showed medium antioxidant activity with the IC50 value of 15.24 ± 0.08 and 10.88 ± 0.05 mg/mL. The ethanolic flower extract possessed high antioxidant activity with the IC50 value of 3.68 ± 0.03 and 2.53 ± 0.03 mg/mL.
Keywords: Gardenia carinata, GC-MS, Leave essential oil and ethanolic flowers extract, Antibacterial and antioxidant activities.
Citation: Warinthip, N., Liawruangrath, B., Natakankitkul, S., Rannurags, N., Pyne, S.G. and Liawruangrath, S. 2023. Chemical constituents antioxidant and antibacterial activities of the leaves and flowers from Gardenia carinata wallich. Nat. Life Sci. Commun. 22(1): e2023003.
INTRODUCTION
Medicinal plant is defined as any substance with one or more of its organ containing properties that can be used for therapeutic purposes on which can be used as precursors for the synthesis of various drugs. The Rubiaceae family is a large diversity of substances such as iridoids, indole alkaloids, anthraquinones, terpenoids, flavonoids and other phenolic derivatives. Phenolic compounds are a main class of secondary metabolites in medicinal plants. The beneficial effects of phenolic compounds is attributed to the antioxidant activity that mainly due to the capacity of scavenging free radicals, donating hydrogen atoms, electrons, or chelate metal cations [Bendjedid et al., 2020]. Gardenia carinata Wallich., belonging to the family Rubiaceae, is native from Malaysia and Indonesia and it has been found in the southern part of Thailand. The plant is commonly known as Pood Nam Boost in Thai. It is often grow in garden as an ornamental. The flowers are initially open white and turn yellow and then slowly turn golden orange over a several day period before falling off [Kahriman et al., 2012]. The methanol extracts of the leaves and the twigs from G. carinata showed activity against P-388, KB and MCF-7 cancer cell lines. In addition, the fraction showed potential activity against HIV-1 reverse with 100% inhibition transcriptase. New cycloartane triterpenoids were isolated from the leaves and the twigs extract from G. Carinata and their structure were determined the cytotoxic, anti-HIV-1 activities and topoisomerase IIα inhibitory [Kongkum et al., 2012].
There is no previous report on the compositions of leave essential oil and ethanolic flowers extract of G. carinata. The aim of this work was to isolate and identify the chemical constituents, antibacterial and antioxidant activities from Gardenia carinata. (Figure 1a, 1b).
Figure 1. (a) Leaves of Gardenia carinata Wallich and (b) flowers of Gardenia carinata Wallich.
MATERIALS AND METHODS
Chemicals
Ascorbic acid was obtained from Fluka (Buchs, Switzerland). ABTS (2,2’-Azino-bis(3-ethylbenzothaizoline-6-sulfonic acid), DPPH (2,2’-diphenyl-1-picrylhydrazyl) and Trolox (6-hydroxy-2,5,7,8-tetramethyl-chroman-2-carboxylic acid), from Sigma Aldrich (Germany). Potassium persulfate and anhydrous sodium sulfate from Merck (Darmstadt, Germany). Vancomycin and Amikacin from Oxoid, UK. 95% ethyl alcohol was purchased from RCI Labscan (Bangkok, Thailand).
Instruments
Hewlett-Packard GC 6850 equipped with a DB5-M5 column (30 m×0.25 mm, 0.25 µm film thickness) GC-MS on the HPGC-6850 coupled with a HP 5973N mass selective detector Spectrophotometer multimode detector, Beckman Coulter DT×880, USA. UV-Visible spectrophotometer, Shimadzu UV2450, Japan.
Plant materials
The fresh leaves and flowers of Gardenia carinata Wallich. were collected from the Saraphi Garden, Chiang Mai, Thailand, in November, 2016. The plant materials were identified by a botanist at the Herbarium of Biology Department, Faculty of Science, Chiang Mai University where a voucher specimen has been deposited under the code number N. Warinthip 01.
Isolation of the essential oil
Hydrodistillation Set 1:
Fresh leaves of G. carinata (1 kg) were cleaned and chopped into small pieces. The leaves were subjected to hydrodistillation in a Clevenger-type apparatus for 8 h. Then the essential oil was dried over anhydrous sodium sulfate and kept in a sealed from glass vial at 4°C for further analysis.
Hydrodistillation Set 2:
Fresh flowers of G. carinata (1 kg) were cleaned and chopped into small pieces. The flowers were subjected to hydrodistillation in a Clevenger-type apparatus for 8 h. There is no essential oil present in the distillate.
Extracted of G. carinata flowers
The flowers of G. carinata (500 g) were cleaned and dried in a hot air oven at 45°C for 6 h. Then the sample was ground and macerated in 1,000 mL of 95% ethanol for one week at room temperature with continuous shaking. The sample was filtered through Whatman No.1 filter paper. The filtrate was evaporated to dryness using a rotary evaporator and weighed. The percentage yield of the extract was 5.02%. The extract was stored at 4°C. This extract was subjected for further analysis.
Gas chromatography mass spectrometry (GC-MS) analysis
Analysis of the essential oil and the flower extract from G. carinata were analyzed by GC-MS. The composition of the essential oil was analyzed by means of GC (FID) and GC-MS. The GC analysis was performed on a Hewlett-Packard GC 6850 equipped with a DB5-M5 column (30 m × 0.25 mm, 0.25 µm film thickness) and helium was used as the carrier gas at a flow rate of 20.0 mL/min. The oven temperature was programmed from 40°C to 275°C at 6°C/min and the end temperature was held for 12 min. The injection volume was 1.0 µL in split mode 1:20. The injector and detector temperatures were 260°C and 280°C respectively, GC-MS analysis was performed on the HP GC 6850 coupled with a HP5973N mass selective detector under the same capillary column and conditions as described in GC program. The mass spectrometer was in the EI mode at 70 eV in m/z range 29-550 amu. The identification of the constituents in the essential oil was based on their GC retention indices (RI) relative to n-alkanes indices on a DB5-M5 column and computer matching of spectral MS data with the NISTREP and NIST libraries MS data and literature data.
Antibacterial activity
The minimal inhibitory concentration (MIC) of the essential oil against three bacteria (Staphylococcus aureus ATCC 25923, Pseudomonas aeruginosa ATCC 27853 and Escherichia coli ATCC 25922) was determined using the disc diffusion assay [Zaidan et al., 2005].
Each bacterial suspension which has been adjusted to 0.5 McFarland was uniformly spread using a cotton swab on nutrient agar Petri dish. Five sterile paper discs (5 mm filter paper disc, Whatman no.1) were placed on the surface of each agar plate and were impregnated with 10 µL of the diluted concentration of 9.40, 7.05, 4.70, 3.53, 2.35, 1.76 and 1.18 mg/mL for the essential oil. Plates were incubated for 24 h. at 36 ± 0.1°C under appropriate cultivation conditions, Antibacterial activity as MIC was determined as the lowest concentration of the essential oil which inhibits the growth of bacteria. A disc impregnated with ethanol served as a negative control and discs with vancomycin 30 µg and amikacin 30 µg (Oxoid, UK) served as positive controls. Test were performed in duplicate.
Antioxidant activity
DPPH method
The antioxidant activity of the leave essential oil of G. carinata and the ethanolic flower extract of this plant were evaluated spectrophotometrically using the DPPH radicals assay [Lakshmanashetty et al., 2010] with some modification. Briefly, 20 µL of each different sample concentrations was added to 180 µl of 0.004% DPPH (in ethanol). The samples were incubated for 30 min in the dark and the absorbance of the reaction mixture was measured at 517 nm spectrophotometrically (spectrophotometer multimode detector, Beckman Coulter DTX 880, USA). The DPPH solution without the test sample was used as a control. Trolox and ascorbic acid were used as the standards. Triplicate determination were performed. The percentage of the DPPH radical scavenging activity was calculated as [(Ac-As)/Ac]×100 where Ac is the absorbance of control and As is the absorbance of the samples. Then %inhibitions were plotted against respective concentrations used. Then the IC50 was calculated by reference to the calibration curve.
ABTS method
The antioxidant activity of the leave essential oils of G. carinata and the ethanolic flower extract were investigated using the ABTS radical cation scavenging assay [Roberta, et al., 1999]. The ABTS radical cation (ABTS•+) was prepared by reacting 7 mM of ABTS stock solution with 2.4 mM of potassium persulfate in darkness for 16 h at room temperature. The freshly ABTS•+ solution was diluted with deionized water to the absorbance at 734 nm of 0.700 ± 0.020 determined using UV-Visible spectrophotometer, Shimadzu UV2450, Japan. Different sample concentrations were prepared in ethanol. For the assay, 20 µL of sample was mixed with 2.0 mL of diluted ABTS•+ solution followed by the measurement of the absorbance after 5 min incubation at room temperature. Trolox and ascorbic acid were used as the standards. Triplicate determinations were performed. The percentage inhibition of free radical by ABTS•+ was calculated using the following equation as [(Ab-As)/Ab] x100 where Ab is the absorbance of the control reaction (containing all reagents except the test compound) and as is the absorbance of the test compound. Then % inhibitions were plotted against respective concentrations used. Then the IC50 was calculated by reference to the calibration curve.
RESULTS AND DISCUSSION
Fresh leaves of G. carinata were subjected to hydrodistillation for 8 h. using a modified Clevenger-type apparatus to yield 0.002% (w/w) of a light yellow-colored oil. The composition of the essential oil was analyzed by GC (FID) and GC-MS. The components of the leave essential oil were identified corresponding to 71.10% of the chromatographical oil components. The components identified from the essential oil with retention time (RT), percentage composition (%) and kovats retention indies (KI) are summarized in Table 1. Seventeen compounds were detected and eleven compounds of the leave essential oil were identified. The major compounds were phytol (13.80%), 3-hexene-1-ol, benzoate (12.50%), vitispirane (8.00%), hexadecanal (7.90%), β-damascenone (3.90%), (E,E)-α-farnesene (3.80%), palmitic acid (3.50%), n-nonacosane (3.60%), hexyl benzoate (2.70%) and the minor component was hexahydrofarnesyl acetone (0.90%).
Table 1. Chemical compositions of the essential oil from the leaves of G. carinata.
|
No. |
Compounds |
RT |
% area |
Kia(exp) |
KIb(lit) |
IDc |
Ref |
|
1 |
Unidentified |
13.12 |
2.6 |
1197 |
- |
- |
- |
|
2 |
Vitispirane |
15.46 |
8.0 |
1283 |
1286 |
KI,MS |
[Zaidan, et al., 2005] |
|
3 |
β-damascenone |
18.06 |
3.9 |
1381 |
1385.5 |
KI, MS |
[Kongkum, et al., 2013] |
|
4 |
Unidentified |
19.66 |
0.8 |
1444 |
- |
- |
- |
|
5 |
(E,E)-alpha-farnesene |
21.19 |
3.8 |
1504 |
1504.1 |
KI, MS |
[Kongkum, et al., 2013] |
|
6 |
3-hexene-1-ol, benzoate |
22.83 |
12.5 |
1574 |
1569.5 |
KI, MS |
[Kongkum, et al., 2013] |
|
7 |
Hexyl benzoate |
23.01 |
2.7 |
1581 |
1581.8 |
KI, MS |
[Kongkum, et al., 2013] |
|
8 |
Unidentified |
23.20 |
2.4 |
1589 |
- |
- |
- |
|
9 |
Unidentified |
23.40 |
1.2 |
1597 |
- |
- |
- |
|
10 |
Hexadecanal |
26.08 |
7.9 |
1716 |
1717.1 |
KI, MS |
[Kongkum, et al., 2013] |
|
11 |
Hexahydrofarnesyl acetone |
28.74 |
0.9 |
1842 |
1844.4 |
KI, MS |
[Kongkum, et al., 2013] |
|
12 |
Unidentified |
29.06 |
1.6 |
1857 |
- |
- |
- |
|
13 |
Palmitic acid |
31.30 |
3.5 |
1969 |
1968.4 |
KI, MS |
[Kongkum, et al., 2013] |
|
14 |
Ethyl hexadecanoate |
31.76 |
0.9 |
1993 |
1993 |
KI, MS |
[Kahriman, et al., 2012] |
|
15 |
Phytol |
33.91 |
13.8 |
2108 |
2107 |
KI, MS |
[Daines, et al., 2003] |
|
16 |
Unidentified |
34.47 |
0.9 |
- |
- |
- |
- |
|
17 |
n-nonacosane |
46.32 |
3.6 |
2902 |
2900 |
KI, MS |
[Santos, et al., 2013] |
|
|
Monoterpenes |
|
3.9 |
|
|
|
|
|
|
Diterpenes |
|
13.8 |
|
|
|
|
|
|
Sesquiterpenes |
|
4.8 |
|
|
|
|
|
|
Fatty aldehyde |
|
7.9 |
|
|
|
|
|
|
Fatty acid |
|
4.4 |
|
|
|
|
|
|
Flavour agent |
|
8.0 |
|
|
|
|
|
|
Flavor and fragrance agents |
|
15.2 |
|
|
|
|
|
|
Hydrocarbon |
|
3.6 |
|
|
|
|
|
|
Unidentified |
|
9.5 |
|
|
|
|
|
|
Total |
|
71.1 |
|
|
|
|
RT: Retention time on DB5-M5 column
KIa(exp): kovats retention indies on DB5-MS column relative to n-alkane
KIb(lit): values from literature data
IDc: method of identification MS: From a comparison of the mass spectrum with MS libraries
Note: Results from 3 determinations
The most abundant compounds consisted mainly of monoterpenes (3.90%), diterpene (13.80%), sesquiterpene (4.80%), fatty aldehyde (7.90%), fatty acid (4.40%), flavour agent (8.00%), flavor and fragrance agents (15.20%), hydrocarbon (3.60%) and unidentified (9.50%). GC-MS chromatogram are shown in Figure 2.
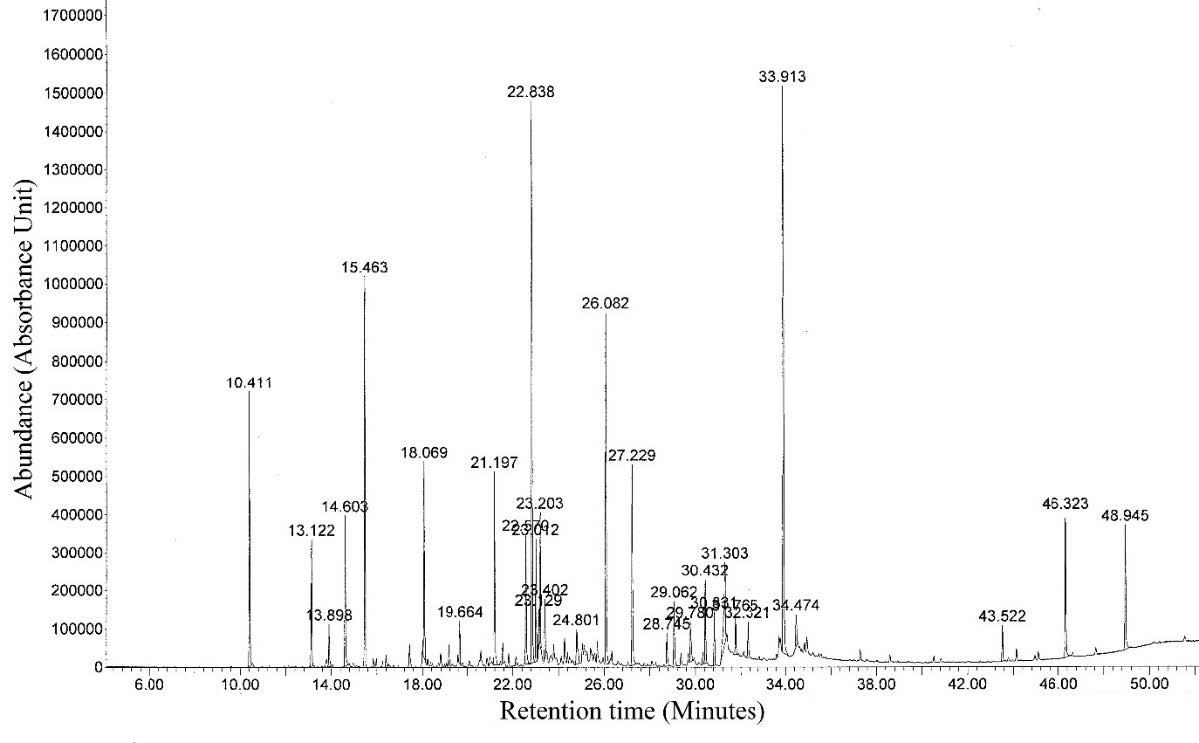
Figure 2. GC-MS chromatogram of the leave essential oil from G. carinata.
Phytol is a natural linear diterpene alcohol which is used in the precursor for the preparation of the synthetic forms of vitamin E [Thomas, 2007] and vitamin E [Daines, et al., 2003]. It is also a decomposition product of chlorophyll. In medicinal fields, phytol has shown antinociceptive and antioxidant activities [Santos, et al., 2013] as well as anti-inflammatory and antiallergic effects [Ryu, et al., 2011]. Phytol has been also shown antimicrobial activity against Mycobacterium tuberculosis [Rajab, et al., 1998; Saikia, et al., 2010] and Staphylococcus aureus [Inoue, et al., 2005]. In addition, phytol is widely used as a food additive and has potential addition to antischistosomal therapy [Moraes, et al., 2014]. The ingredient, phytol used in many fragrance compounds and it may be found in cosmetic, including soaps, detergents, shampoos and beauty care products and noncosmetic products [McGinty, et al., 2010]. cis-3-hexenyl benzoate or 3-hexene-1-ol, is a balsam, fatty, and floral tasting compound. It has been detected in several different foods, such as green tea, fruits, cauliflowers (Brassica oleracea var. botrytis), black tea, and red tea. Vitispirane is an important norisoprenoid odorant that occurs in many wines. It has two chiral carbons and consequently four stereoisomers [Georgilopoulos, et al., 1988]. β-damascenone are components of a variety of essential oils which known as rose ketones. β-damascenone is a major contributor to the aroma of roses, despite its very low concentration. It is an important fragrance chemical used in perfumery. (E,E)-α-farnesene is the most common isomer. It is found in the coating of apples, and other fruits, and it is responsible for the characteristic green apple odor [Huelin, et al., 1966]. Palmitic acid is also known as hexadecanoic acid, the most common saturated fatty acid are found naturally in animals, plants and can also be found in meats, cheeses, cocoa butter, olive oil, and other dairy products [Innis, 2016]. Palmitic acid is widely used in a variety of applications in cosmetic, it used to produce soaps and personal care products. The bioactivity of fatty acids is palmitic revealed in essential oil of Jacaranda obtusifolia has been reported the hexadecanoic acid has shown cytotoxicity to human leukemic cells [Harada, et al., 2002].
About 2 mg of the flower extract from G. carinata were accurately weighed and dissolved in ethanol in a 10 mL volumetric flask. This solution was analyzed by GC (FID) and GC-MS using the same procedure as described in the analysis of the essential oil. The chemical composition of the extract of G. carinata flowers was analyzed by GC (FID) and GC-MS. Seventeen components of the flower extract were identified, constituting 37.5% of the total chromatographical flower extract components. The components of the flower extract were identified by comparing of their retention indies (RI) relative to n-alkanes indices on DB5 column and by the comparison of mass spectra from NISTREP and NIST libraries, as well as by comparison of the fragmentation patterns of the mass spectra with the data reported in the literature. Results are presented in Table 2.
Table 2. Chemical compositions of the extract from the flowers of G. carinata.
|
No. |
Compounds |
RT |
% area |
Kia(exp) |
KIb(lit) |
IDc |
Ref |
|
1 |
4-vinylphenol |
13.62 |
1.3 |
1223 |
1224 |
KI,MS |
[Kahriman, et al., 2012] |
|
2 |
4-allylphenol |
17.04 |
5.4 |
1254 |
1254 |
KI,MS |
[Kahriman, et al., 2012] |
|
3 |
Phenol |
19.77 |
0.8 |
- |
- |
- |
- |
|
4 |
Unidentified |
22.81 |
0.4 |
- |
- |
- |
- |
|
5 |
Cyclohexene |
26.04 |
0.3 |
- |
- |
- |
- |
|
6 |
Hexadecanoic acid |
31.14 |
0.9 |
1991 |
1990 |
KI, MS |
[Kongkum, et al., 2013] |
|
7 |
11,15-Trimethyl-3-methylene |
32.29 |
0.3 |
- |
- |
- |
- |
|
8 |
Heneicosane |
33.77 |
9.7 |
2102 |
2100 |
KI, MS |
[Kahriman, et al., 2012] |
|
9 |
Linoleic acid |
34.44 |
0.6 |
2107 |
2107 |
KI, MS |
[Kahriman, et al., 2012] |
|
10 |
Ethyl linolenate |
34.92 |
5.7 |
2162 |
2163 |
KI, MS |
[Rajab, et al., 1998] |
|
11 |
Octadecnoic acid |
35.49 |
0.3 |
2172 |
2170 |
KI, MS |
[Kongkum, et al., 2013] |
|
12 |
Ethyl stearate |
41.93 |
0.5 |
2195 |
2195 |
KI, MS |
[Kahriman, et al., 2012] |
|
13 |
n-docosane |
43.49 |
1.6 |
2200 |
2200 |
KI, MS |
[Kongkum, et al., 2013] |
|
14 |
Squalene |
45.07 |
5.7 |
2790 |
2791 |
KI, MS |
[Kongkum, et al., 2012] |
|
15 |
Heptacosyl acetate |
46.35 |
0.2 |
3111 |
3112 |
KI, MS |
[Kahriman, et al., 2012] |
|
16 |
Stigmasterol |
50.70 |
2.1 |
3172 |
3172 |
KI, MS |
[Georgilopoulos, et al., 1988] |
|
17 |
Campesterol |
51.55 |
0.7 |
3305 |
3305 |
KI, MS |
[Kahriman, et al., 2012] |
|
18 |
Lupenone |
52.45 |
1.4 |
3384 |
3384.2 |
KI, MS |
[Thomas, 2007] |
|
19 |
Unidentified |
55.08 |
5.8 |
- |
- |
- |
- |
|
20 |
Unidentified |
55.81 |
41.5 |
- |
- |
- |
- |
|
21 |
Unidentified |
56.09 |
2.0 |
- |
- |
- |
- |
|
|
Alkane |
|
11.3 |
|
|
|
|
|
|
Phenolic compound, phenol |
|
7.5 |
|
|
|
|
|
|
Triterpene |
|
7.1 |
|
|
|
|
|
|
Unsaturated fatty acid and ester |
|
6.3 |
|
|
|
|
|
|
Steroid derivative |
|
2.8 |
|
|
|
|
|
|
Saturated fatty acid |
|
0.9 |
|
|
|
|
|
|
Stearic acid and ester |
|
0.8 |
|
|
|
|
|
|
Alkene |
|
0.6 |
|
|
|
|
|
|
Heptacosyl acetate |
|
0.2 |
|
|
|
|
|
|
Unidentified |
|
49.7 |
|
|
|
|
|
|
Total |
|
87.2 |
|
|
|
|
KIa(exp): kovats retention indies on DB5-MS column relative to n-alkane
KIb(lit): values from literature data
IDc: method of identification MS: From a comparison of the mass spectrum with MS libraries
Note: Results from 3 determinations
The major components were heneicosane (9.70%), ethyl linolenate (5.70%), squalene (5.70%), 4-allylphenol (5.40%), stigmasterol (2.10%), n-docosane (1.60%), lupenone (1.40%), hexadecanoic acid (0.90%) and compesterol (0.70%). The most abundant compounds consisted mainly of alkanes 11.30%, phenolic compound and phenol 7.50%, triterpenes 7.10%, unsaturated fatty acid and ester 6.30%, steroid derivative 2.80%, saturated fatty acid 0.90%, stearic acid and ester 0.80%, alkene 0.60%, heptacosyl acetate 0.20% and unidentified 49.70%. GC-MS chromatographic are shown in Figure 3.
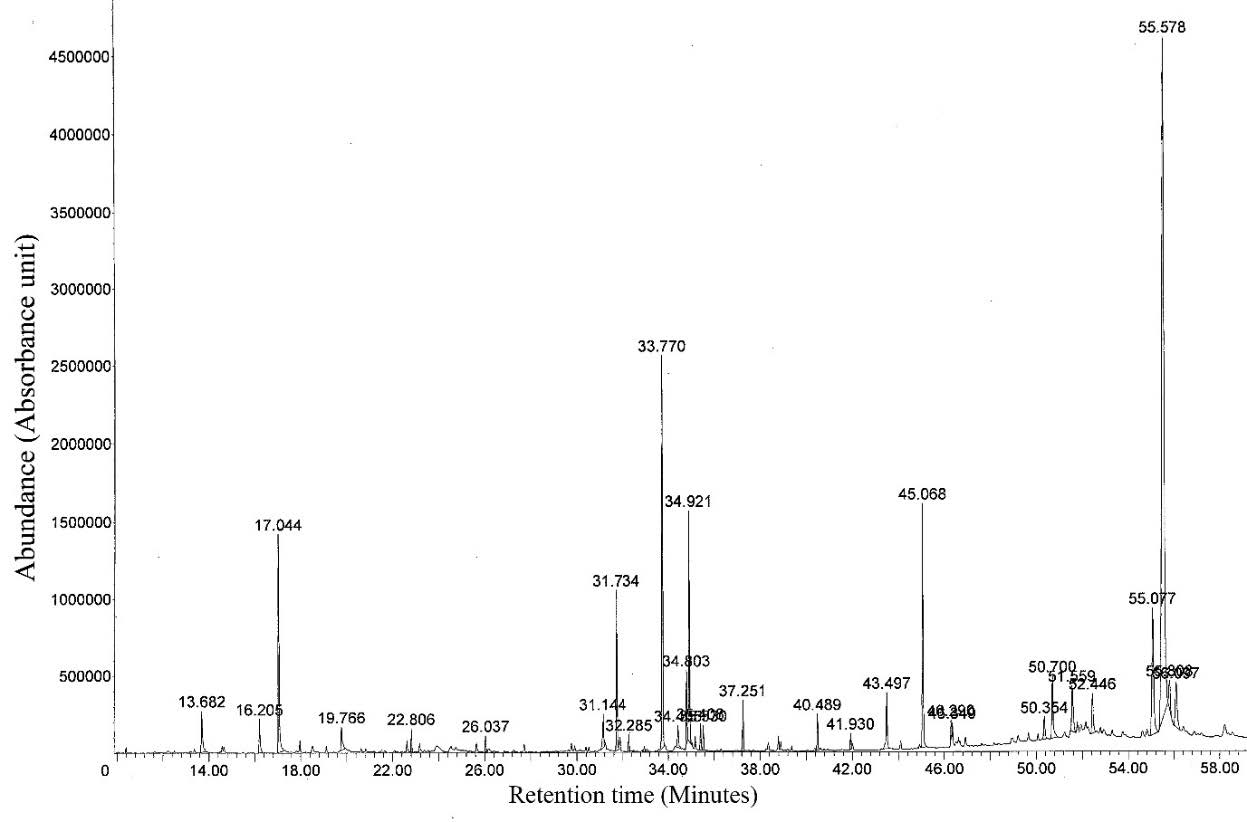
Figure 3. GC-MS chromatogram of flower extract from G. carinata.
Squalene is a linear triterpene. It is a natural compound, a precursor of various hormones in animals and sterols in plants. It is considered a molecule with pharmacological, cosmetic and nutritional potential. Scientific research has shown that squalene reduces skin damage by UV radiation, LDL levels, and cholesterol in the blood, prevents the suffering of cardiovascular diseases, and has antitumor and anticancer effects against ovarian, breast, lung and colon cancer. Several studies have confirmed the health benefits of squalene in nutritional, medicinal and pharmaceutical aspects. It is considered a potential chemopreventive and chemotherapeutic agent, which inhibits the tumor growth in the colon, skin, lung and breast and it stimulates the immune system for the application of drugs in the treatment of diseases such as HIV, HINI, leukemia, papilloma and herpes [Wolosik et al., 2013; Reddy et al., 2009]. Lupenone can stimulate melanogenesis in B16 murine melanoma cells, [Alberts et al., 1992] inhibit α-glucosidase (α-Glu), protein tyrosine phosphatase 1B (PTB1B) and adipogenic differentiation in vitro [Ahn et al., 2013;Villareal et al., 2013]. Lupenone shows a good anti-diabetic activity in vivo [Xu et al., 2014]. Phenolic compounds are a host of natural antioxidants. They are used as nutraceutical and are used to prevent heart ailments to an appreciable degree and sometimes are anti-inflammatory agents. Ethyl linolenate and hexadecanoic acid are lipidic derivatives which are used as flavour and fragrance agents, essential ingredient in making soap and shampoos [Sanseera, et al., 2012].
Stigmasterols likewise, other plant sterols have shown astonishing anticancer properties against various cancer cell lines through the different potential mechanisms of actions. Stigmasterol has been reported for its anticancer properties against a number of cancer cell lines including but not limited to lung, ovary, stomach and estrogen-dependent human breast cancers [Bradford, et al., 2007]. Stigmasterol is an important plant sterol that can inhibit the development of various cancerous cells by inhibiting the promotion and growth of apoptosis of cancer cells. This inhibition could be due to the activation of caspase enzymes (enzymes involved in cell regulatory networks that control cell death). Plant sterols, stigmasterol, when incorporates in the cell membrane increased the caspase enzyme’s activity by producing changes in membrane structure and fluidity by replacing the cholesterol of the membrane. These changes in membrane structure increased extra and intracellular protein efflux and affect the signal transduction pathway that activates the caspase enzyme. The other pathway which helps in the avoidance of cancer is the inhibition of cancer enhancing species or scavenging of free radicals. These free radicals are reactive oxygen species (ROS) produced in the body by the oxidation of stressed cells that can damage DNA, and consequently could initiate or proliferate cancer in the body, and breakage of this chain could be helpful to control cancer proliferation [Michelini, et al., 2016]. In addition, stigmasterol also possess anti-inflammatory activity [Chukwu et al., 2021]. Campesterol was evaluated for its anti-inflammatory activity and it was found to reduce carrageenan induced paw oedema and also inhibited ear oedema induced by 12-o-tetradecanoylphorbol acetate (TPA) after topical application [Antonio et al., 2001].
The chemical structures of some important compounds are found in the leave essential oil and the ethanolic flowers extract are presented in Figure 4.
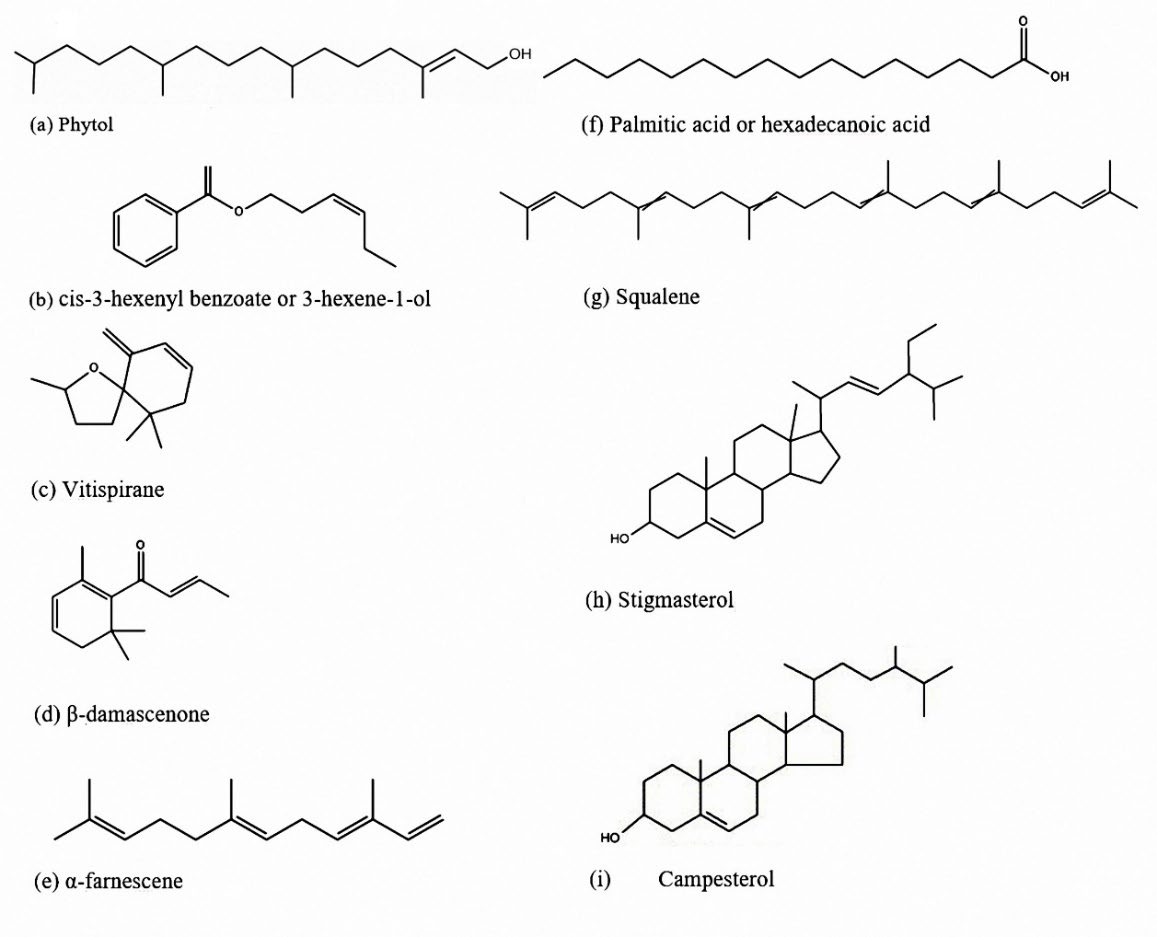
Figure 4. The chemical structures of some bioactive compounds found in the leave essential oil and the flowers extract from G. carinata.
The leave essential oil was analysed by GC and GC-MS, the main compound found was phytol. This compound possessed antibacterial activity. [Keawsa-ard et al., 2012].
The antibacterial activity of the essential oil against three bacterial strains was assessed by determination of MIC values using a disc diffusion assay. The results are shown in Table 3. The essential oil showed antibacterial activity against both Gram positive S. aureus with the most potent activity at 18.7 µg/disc, and Gram negative E. coli with an activity of 17.6 µg/disc. The oil was not active against Gram negative P. aeruginosa. Hexadecanoic acid and octadecanoic acid have been reported to possess antibacterial activity. In addition, the ethanolic flower extract was not active to antibacterial activity.
Table 3. Antibacterial activity of the leave essential oil of G. carinata.
|
Sample |
Gram positive bacteria Gram negative bacteria |
||
|
MIC (µg/disc) |
|||
|
S. aureus |
P. aeruginosa |
E. coli |
|
|
Essential oil(leaves) |
18.7 µg (10.0 mm) |
NA |
17.6 µg (10.0 mm) |
|
Positive control |
Vancomycin 30 µg (16 mm) |
Amikacin 30 µg (17 mm) |
Amikacin 30 µg (17 mm) |
|
Negative control(ethanol) |
NA |
NA |
NA |
Note: NA: No activity
The methanol extracts of the leaves from G. carinata had been reported to possess antibacterial activity against Bacilus subtitis, Staphylococcus aureus and Proteus vulgaris using agar streak dilution method [Kongkum et al., 2013]. The methanol extracts of the leaves and the twigs showed anti-cancer activity against P-388, KB and MCF-7 cancer cell lines. In addition, the fraction showed potential activity against HIV-1 reverse with 100% inhibition transcriptase [Kongkum, et al., 2012].
The antioxidant activity of the leave essential oil and the ethanolic flower extract of G. carinata were determined by DPPH and ABTS methods. In the DPPH assay, the ability of the examined samples act as donor of hydrogen atoms or electrons in the transformation of DPPH* into its reduced form DPPH-H was investigated. The examined samples can reduce the stable purple-colored radical DPPH into yellow-colored DPPH-H. The ABTS assay is a decolorization assay which starts with the generation of the greenish blue ABTS radical cation (ABTS•+) by oxidation of ABTS with potassium persulfate. The ABTS•+ is reduced by accepting an electron from the antioxidant. The leave essential oil showed medium antioxidant activity with the IC50 value of 15.24 ± 0.08 and 10.88 ± 0.05 mg/mL. The ethanolic flower extract possessed high antioxidant activity with the IC50 value of 3.68 ± 0.03 and 2.53 ± 0.03 mg/mL, respectively. Results are presented in Table 4-5.
Table 4. Antioxidant activity of the leave essential oil and the ethanolic flower extract from G. carinata by DPPH method.
|
Sample |
IC50 (mg/mL) |
|
Essential oil (leaves) |
15.24 ± 0.08 |
|
Ethanolic flower extract |
3.68 ± 0.03 |
|
Trolox* |
0.10 ± 0.01 |
|
Vitamin C* |
0.06 ± 0.03 |
Standards used as positive controls
Table 5. Antioxidant activity of the leave essential oil and the ethanolic flower extract from G. carinata by ABTS method.
|
Sample |
IC50 (mg/mL) |
|
Essential oil (leaves) |
10.88 ± 0.05 |
|
Ethanolic flower extract |
2.53 ± 0.03 |
|
Trolox* |
0.35 ± 0.04 |
|
Vitamin C* |
0.27 ± 0.05 |
*Standards used as positive controls
CONCLUSION
This is the first report that describes the chemical constituents of the leave essential oil and the ethanolic flower extract from G. carinata were analyzed by means of GC (FID) and GC-MS. The main compound in the leave essential oil was phytol. It is a natural linear diterpene alcohol which is used in the precursor for the preparation of the synthetic forms of vitamin E and vitamin K. In medicinal fields, phytol has shown antinociceptive and antioxidant activities as well as anti-inflammatory and anti-allergic effects. Phytol has been also shown antimicrobial activity against Mycobacterium tuberculosis and Staphylococcus aureus. The main compounds found in the ethanolic flower extract were squalene, lupenone and stigmasterol. Squalene is a polyunsaturated triterpene. In the cosmetics, it has been used as a solvent and a moisturizer. It is reported that squalene protects the skin damage from ultraviolet radiation. Squalene also prevents the suffering of cardiovascular diseases, and it has been reported to possessed antitumor and anticancer effects against ovarian, breast, lung and colon cancer. Stigmasterols likewise, other plant sterols have shown astonishing anticancer properties against various cancer cell lines through the different potential mechanisms of actions. Stigmasterol has been reported for its anticancer properties against a number of cancer cell lines including but not limited to lung, ovary, stomach and estrogen-dependent human breast cancers. Phenolic compounds are a host of natural antioxidants. They are used as nutraceutical and are used to prevent heart ailments to an appreciable degree and sometimes are anti-inflammatory agents. Ethyl linolenate and hexadecanoic acid are lipidic derivatives which are used as flavour and fragrance agents, essential ingredient in making soap and shampoos. Phytol was present in the essential oil and haxadecanoic acid, octadecanoic acid were present in the flower extract. They possessed antibacterial activity. The antibacterial activity of the essential oil against three bacterial strains was assessed by determination of MIC values using a disc diffusion assay. The results are shown in Table 3. The essential oil showed antibacterial activity against both Gram positive S. aureus with the most potent activity at 18.7 µg/disc, and Gram negative E. coli with an activity of 17.6 µg/disc. The oil was not active against Gram negative P. aeruginosa. The methanol extracts of the leaves from G. carinata had been reported to possess antibacterial activity against Bacilus subtitis, Staphylococcus aureus and Proteus vulgaris using agar streak dilution method. The methanol extracts of the leaves and the twigs showed anti-cancer activity against P-388, KB and MCF-7 cancer cell lines. In addition, the fraction showed potential activity against HIV-1 reverse with 100% inhibition transcriptase. The antioxidant activity of the leave essential oil and the ethanolic flower extract of G. carinata were determined by DPPH method. The results are present in Table 4. The leave essential oil showed medium antioxidant activity with the IC50 value of 15.24 ± 0.08 mg/mL. The ethanolic flower extract possessed high antioxidant activity with the IC50 value of 3.68 ± 0.03 mg/mL. The leave essential oil and the ethanolic flower extract were also possessed antioxidant activity using ABTS method. The results are present in Table 5. The leave essential oil showed medium activity with the IC50 value of 10.88 ± 0.05 mg/mL and the ethanolic flower extract possessed high antioxidant activity with the IC50 value of 2.53 ± 0.03 mg/mL respectively. Therefore, the leave essential oil may play an important role for the production of health supplement, suggests the oil could be used for the treatment of bacterial infections and has the potential to be developed as an accessible and relatively inexpensive alternative to synthetic antibiotic drug.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the Graduate School and Faculty of Pharmacy, Chiang Mai University for partial support and Central Diagnostic Laboratory, Faculty of Medicine, Chiang Mai University for their very kind partial support. Additionally, we wish to thank Center of Excellence in Materials Science and Technology, Chiang Mai University for financial support under the administration of Materials Science Research Center, Faculty of Science, Chiang Mai University.
REFERENCES
Ahn, E.K. and Oh, J.S. 2013. Lupenone isolated from Adenophora triphylla var. japonica extract inhibits adipogenic differentiation through the downregulation of PPARγ in 3T3-L1 cells. Phytotherapy Research. 27: 761-766.
Alberts, A.C., Sharp, T.R., Werner, D.I. and Weldon, P.J. 1992. Seasonal variation of lipids in femoral gland secretions of male green Iguanas (Iguana iguana). Journal of Chemical Ecology 18: 703-712.
Antonio, N., Beatriz, D.L.H. and Angel, V. 2001. Anti-inflammatory and immunomodulating properties of a sterol fraction from Sideritis foetens Clem. Biological and Pharmaceutical Bulletin. 24: 470-473.
Bendjedid, S., Djelloul, R., Tadjine, A., Bensouici, C. and Boukhari, A. 2020. In vitro assessment of total bioactive contents, antioxidant, anti-alzheimer and antidiabetic activities of leaves extracts and fractions of Aloe vera. Chiang Mai University Journal of Natural Sciences 19: 469-486.
Bradford, P.G. and Awad, A.B. 2007. Phytosterols as anticancer compounds. Molecular Nutrition & Food Research. 51: 161-170.
Chukwu, C.N., Onyedikachi, U.B., and Ejiofor, E. 2022. Evaluation of chemical constituents, antioxidant and anti-inflammatory properties of n-hexane extract of Viscum album L. (Mistletoe) leaves. Chiang Mai University Journal of Natural Sciences 21: 1-21.
Daines, A., Payne, R., Humphries, M., and Abell, A. 2003. The synthesis of naturally occurring vitamin K and vitamin K analogues. Current Organic Chemistry. 7: 1625-1634.
Georgilopoulos, D.N. and Gallois, A.N. 1988. Flavour compounds of a commercial concentrated blackberry juice. Food Chemistry. 28: 141-148.
Harada, H., Yamashita, U., Kurihara, H., Fukushi, E., Kawabata, J., and Kamei, Y. 2002. Antitumor activity of palmitic acid found as a selective cytotoxic substance in a marine red alga. Anticancer Research. 22: 2587-2590.
Huelin, F.F and Murry, K.E. 1966. α-Farnesene in the natural coating of apples. Nature. 210: 1260-1261.
Innis, S.M. 2016. Palmitic acid in early human development. Critical Reviews in Food Science and Nutrition. 56: 1952-1959.
Inoue, Y., Hada, T., Shiraishi, A., Hirose, K., Hamashima, H., and Kobayashi, H. 2005. Biphasic effects of geranylgeraniol, teprenone, and phytol on the growth of Staphylococcus aureus. Antimicrobial Agents and Chemotherapy. 49: 1770-1774.
Kahriman, N., Yayli, B., Yucel, M., Karaoglu, S.A., and Yayli, N. 2012. Chemical constituent and antimicrobial activity of the essential oil from Vicia Dadianorum extracted hydro and microwave distillations. Records of Natural Products. 6: 49-56.
Keawsa-ard, S., Liawruangrath, B., Liawruangrath, S., Teerawutgulrag, A., and Pyne, S.G. 2012. Chemical constituents and antioxidant and biological activities of the essential oil from leaves of Solanum spirale. Natural Product Communications. 7: 955-958.
Kongkum, N., Tuchinda, P., Pohmakotr, M., Reutrakul, V., Piyachaturawat, P., Jariyawat, S., Suksen, K., Yoosook, C., Kasisit, J., and Napaswad, C. 2012. DNA topoisomerase IIα inhibitory and anti HIV-1 flavones from leaves and twigs of Gardenia carinata. Fitoterapia. 83: 368-372.
Kongkum, N., Tuchinda, P., Pohmakotr, M., Reutrakul, V., Piyachaturawat, P., Jariyawat, S., Suksen, K., Akkarawongsapat, R., Kasisit, J., and Napaswad, C. 2013. Cytotoxic, antitopoisomerase IIα, and anti-HIV‑1 activities of triterpenoids isolated from leaves and twigs of Gardenia carinata. Journal of Natural Products 76: 530-537.
Lakshmanashetty, R.H., Nagaraj, V.B., Hiremath, M.N., and Kumar, V. 2010. In vitro antioxidant activity of Vitex negundo L. leaf extracts. Chiang Mai Journal of Science. 37: 489-497.
McGinty, D., Letizia, C.S., and Api, A.M. 2010. Fragrance material review on phytol. Food and Chemical Toxicology. 48: 59-63.
Michelini, G., Kitsune, G.L., Cheung, C.H.M., Brandeis, D., Banaschewski, T., Asherson, P., Mcloughlin, G., and Kuntsi, J. 2016. Attention-deficit/hyperactivity disorder remission is linked to better neurophysiological error detection and attention-vigilance processes. Biological Psychiatry. 80: 923-932.
Moraes, J., Oliveira, R.N., Costa, J.P., Junior, A.L.G., Sousa, D.P., Freitas, R.M., Allegretti, S.M., and Pinto, P.L.S. 2014. Phytol, a diterpene alcohol from chlorophyll, as a drug against neglected tropical disease Schistosomiasis mansoni. PLOS Neglected Tropical Diseases. 8: 1-12.
Rajab, M.S., Cantrell, C.L., Franzblau, S.G., and Fischer, N.H. 1998. Antimycobacterial activity of (E)-phytol and derivatives: a preliminary structure-activity study. Planta Medica. 64: 2-4.
Reddy, L.H. and Couvreur, P. 2009. Squalene: A natural triterpene for use in disease management and therapy. Advanced Drug Deliverry Reviews. 61: 1412-1426.
Roberta, R., Nicoletta, P., Anna, P., Annath, P., Min, Y., and Catherine, R.E. 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine. 26: 1231-1237.
Ryu, K.R., Choi, J.Y., Chung, S., and Kim, D.H. 2011. Anti-scratching behavioral effect of the essential oil and phytol isolated from Artemisia princeps Pamp. in mice. Planta Medica. 77: 22-26.
Saikia, D., Parihar, S., Chanda, D., Ojha, S., Kumar, J.K., Chanotiya, C.S., Shanker, K. and Negi, A.S. 2010. Antitubercular potential of some semisynthetic analogues of phytol. Bioorganic & Medicinal Chemistry Letters. 20: 508-512.
Sanseera, D., Niwatananun, W., Liawruangrath, B., Liawruangrath, S., Baramee, A., and Pyne, S.G. 2012. Chemical composition and biological activities of the essential oil from leaves of Cleidion javanicum Bl. Journal of Essential Oil-Bearing Plants. 15: 186-194.
Santos, C.C., Salvadori, M.S., Mota, V.G., Costa, L.M., de Almeida, A.A., de Oliveira, G.A., Costa, J.P., de Sousa, D.P., de Freitas, R.M., and de Almeida, R.N. 2013. Antinociceptive and antioxidant activities of phytol in vivo and in vitro models. Journal of Neuroscience. 1-9.
Thomas, N. 2007. Synthesis of Vitamin E. In: Vitamin & amp; Hormones, ed: Litwack Gerald. Academic Press, Philadelphia. 76: 155-202.
Villareal, M.O., Han, J., Matsuyama, K., Sekii, Y., Smaoui, A., Shigemori, H., and Isoda, H. 2013. Lupenone from Erica multiflora leaf extract stimulates melanogenesis in B16 murine melanoma cells through the inhibition of ERK1/2 activation. Planta Medica. 79: 236-243.
Wolosik, K., Knas, M., Zalewska, A., and Niczyporuk, M. 2013. The impotance and perspective of plant-based squalene in cosmetology. Journal of Cosmetic Science 64: 19-65.
Xu, F., Wu, H., Wang, X., Yang, Y., Wang, Y., Qian, H., and Zhang, Y. 2014. RP-HPLC characterization of lupenone and β-sitosterol in Rhizoma musac and evaluation of the anti-diabetic activity of lupenone in diabetic Sprague-Dawley rats. Molecules. 19: 14114-14127.
Zaidan, M.R.S., Noor Rain, A., Badrul, A.R., Adlin, A., Norazah, A. and Zakiah, I. 2005. In vitro screening of five local medicinal plants for antibacterial activity using disc diffusion method. Tropical Biomedicine. 22: 165-170.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand.
Nichthima Warinthip1, Boonsom Liawruangrath1, Surapol Natakankitkul1, Narabhats Rannurags2, Stephen G. Pyne3 and Saisunee Liawruangrath2, 4,*
1 Department of Pharmaceutical Sciences, Faculty of Pharmacy, Chiang Mai University, Chiang Mai 50200, Thailand.
2 Department of Chemistry, Faculty of Sciences, Chiang Mai University, Chiang Mai 50200, Thailand.
3 School of Chemistry, Faculty of Sciences, University of Wollongong, Wollongong, NSW 2522, Australia.
4 Center of Excellent in Materials Science and Technology, Chiang Mai University, Chiang Mai 50200, Thailand.
Corresponding author: Saisunee Liawruangrath, E-mail: scislwrn@gmail.com
Total Article Views
Editor: Wipawadee Yooin,
Chiang Mai University, Thailand
Article history:
Received: June 22, 2022;
Revised: October 7, 2022;
Accepted: October 7, 2022;
Published online: October 20, 2022

