
Development of Sunscreen Containing Alpha-Mangostin Riched Extract with Anti-Tyrosinase Activities
Prasan Tangyuenyongwatana* and Wandee GritsanapanPublished Date : 2022-10-18
DOI : https://doi.org/10.12982/CMUJNS.2022.064
Journal Issues : Number 4, October-December 2022
Abstract This study focused on the development of extracts from mangosteen rinds for use as whitening and sunscreen agents. Mangosteen rinds were obtained from 9 locations in the East and the South of Thailand. Ethanol (95%) crude rind extracts were prepared and separated by column chromatography. The fractions were collected from top (F1-F4) and mid spots (F12-F15). These fractions were subjected to tyrosinase inhibitory activity assay and found that the top spot exhibited low activity with IC50 > 100 µg/mL while the mid spots inhibited tyrosinase enzyme at IC50 18.48 µg/mL which is more sensitive than a positive control, kojic acid (38.46 µg/mL). The mid spots fraction from the crude extract of Chanthaburi sample 1 was analyzed with HPLC and was assigned as MGS-1 extract which contained a high amount of α-mangostin. MGS-1 extract was formulated as oil in water cream with 2, 4, 6, and 9 %w/w extract. All creams were subjected to measure SPF and UVA/UVB ratio using an Optometrics SPF-290S analyzer. It was found that the cream with 6 %w/w MGS-1 extract showed the best results with SPF at 10.36 ± 1.07 and had a UVA/UVB ratio equal to 0.451. These sunscreen properties are close to the properties of Salisol-3 (benzophenone), a standard sunscreen. In conclusion, MGS-1 extract has a good potential to be used as a whitening and sunscreen agent in cosmetics with a lower cost of substrate because mangosteen rinds can be found as waste from eating mangosteen fruit every year in Thailand.
Keywords: Mangosteen rind, MGS-1 extract, Tyrosinase inhibition, Whitening agent, Sunscreen
Funding: The authors are grateful for the research funding (Fund No. 91/2562) provided by Research Institute, Rangsit University, Thailand.
Citation: Tangyuenyongwatana, P. and Gritsanapan, W. 2022. Development of sunscreen containing alpha-mangostin riched extract with anti-tyrosinase activities. CMUJ. Nat. Sci. 21(4): e2022064.
INTRODUCTION
At present, it has been proven that sunlight is probably one of the causes of skin problems such as freckles, premature aging, and immunity decreasing. The weakened skin’s immunity is one of the motives of skin cancer (Li et al., 2016). This is caused by many factors such as getting more sunlight due to lifestyle changes. Changing the style of clothing that focuses on revealing more skin to exposure to sunlight can cause skin diseases. In addition, the earth’s ozone layer has been declined, and skin contact with sunlight for a long period causes suppression of immunity (Garbe and Leiter, 2008).
Sunscreen products are divided into two types: reflection and ultraviolet (UV) absorption. The latter group of absorbents is organic compounds and expensive (Korać et al., 2011; Geoffrey et al., 2019). Herbal medications that provide protection against the harmful effects of UV radiation are increasingly popular. Herbal compounds may act as UV ray adsorbents and antioxidants with few potential negative effects. In vitro and in vivo studies have demonstrated that several phytochemicals have the potential to protect against UV radiation (Rabinovich and Kazlouskaya, 2018). Many groups of substances can protect against UV rays such as phenolic and hydrocinnamic compounds (Yarnell and Abascal, 2012; Saewan and Jimtaisong, 2015). Dutta et al. used polyherbal extracts as an active component for preparing microsponge formulations composed of polyherbal extracts, ethylcellulose as a polymer, and polyvinyl alcohol as an emulsifier presented in the formulation, and sunscreen was successfully obtained (Dutta et al., 2021). Ahmady et al. studied the phytochemical profile and sun protection potentials of the crude and purified extracts. Elaeagnus angustifolia pure extract (EAPE), sesame oil, and sea buckthorn oil were the herbal materials chosen for the sunscreen formulation based on their findings (Ahmady et al., 2020). Netto and Jose focused on developing sunscreen which contained silymarin, a flavonoid that has strong antioxidant effects, in solid lipid nanoparticles, a new type of drug carrier that improves medication stability while simultaneously increasing penetration (Netto and Jose 2018).
In Thailand, mangosteen rind has not been fully utilized. In some years, mangosteen fruit has a low price causing a lot of mangosteens to be discharged by the farmers. Mangosteen rind contains active substances in the group of xanthones especially α-mangostin (Muchtaridi et al., 2016). This group of substances has an absorption range of ultraviolet radiation at wavelengths of 230-400 nm, with high absorbance at wavelengths of 243, 317, and 352 nm (Ahmed et al., 2013). This wavelength range corresponds to the UVA-UVB range, which causes damage to the skin, make the skin wrinkled, and have a chance of skin cancer. In addition, there was a report on xanthones in mangosteen rind that can also inhibit the enzyme tyrosinase, the enzyme that produces melanin causing melasma and freckle (Tadtong et al., 2009). If we can find a natural whitening substance with high efficacy in Thailand, it will greatly reduce the importation of sunscreen from abroad. This work was intended to prepare the crude extract with high α-mangostin content from mangosteen rind for developing as a whitening and sunscreen cream. Tyrosinase inhibitory activities of the extracts and creams were demonstrated.
MATERIALS AND METHODS
Material
Mangosteen (Garcinia mangostana L.) fruit was obtained in May 2021 from 9 locations in the eastern and southern parts of Thailand i.e., Chanthaburi (3 samples), Rayong (2 samples), Prachuap Khiri Khan province (2 sample), Nakhon Si Thammarat (1 sample) and Phang Nga (1 sample). The fruit was cleaned with tap water and the rind was separated. The rind was sliced into small pieces, dried at 50°C for 15 hours in an oven and pulverized into course powder. The powder (500 g) was macerated with 95% ethanol (2,000 mL) for 7 days with frequently shaking and the solvent was filtered. The filtrate was concentrated by a rotary evaporator to obtain the dried crude extract. The crude extract was analyzed for the content of α-mangostin using HPLC. Validation for the HPLC method included linearity (0.9998, y = 4E+07x–122705), precision (%RSD = 0.99 for Intraday and 1.43 for Interday), accuracy (99.34 ± 2.78%), limit of detection (LOD = 0.15 µg/mL), and limit of quantitation (LOQ = 0.04 µg/mL). Alpha-mangostin (Sigma-Aldrich, USA) was used as a reference standard.
Preparation of MGS-1 extract
MGS-1 extract was prepared from crude mangosteen rind extract (600 mg) from Chanthaburi 1 sample, separated by column chromatography using silica gel as a stationary phase, and eluting with hexane: ethyl acetate (7 : 3) as a mobile phase. Fractions of 5 mL were collected and examined by thin-layer chromatography with same solvent system as column chromatography. Fractions with the same characteristics with Rf = 0.4-0.6 were combined and the solvent was evaporated using a rotary evaporator until the dry extract was obtained. The extract was dissolved in methanol and analyzed by the validated HPLC for the content of a main substance, α-mangostin. The percentage of α-mangostin in the extract was then calculated.
Tyrosinase inhibitory activity assay
The tyrosinase inhibitory activity of crude mangosteen peel extract and MGS-1 extract was tested by using kojic acid as the standard substance (Zhang et al, 2007). First the extracts were weighed 0.10 g and dissolved with 20% ethanol, shake for 30 min to aid dissolution, and adjusted volume to 10 mL. Next, the previous solution was diluted with 20% ethanol to obtain a concentration of 100, 75, 50, and 25 mg/mL. The sample solutions of various concentrations were subjected to test the inhibition of the enzyme tyrosinase in the test well plate (96-well plate) and used kojic acid as standard compound. 70 mL of 0.1M phosphate buffer pH 6.8 (PBS), 30 mL of mushroom tyrosinase were diluted in the phosphate buffer (167 units/mL) and various concentration of different test samples dissolved in 20mL dimethyl sulfoxide (DMSO) were inserted into 96-well plates for 5 min pre-incubation at 30°C. 100 mL L-DOPA was then added to start the enzymatic reaction. Optical density (OD) at 492 nm was measured on a Sunrise absorbance microplate reader (Tecan Trading AG, Switzerland) to observe dopachrome formation for 10 min. The percentage of inhibition was calculated as follows:
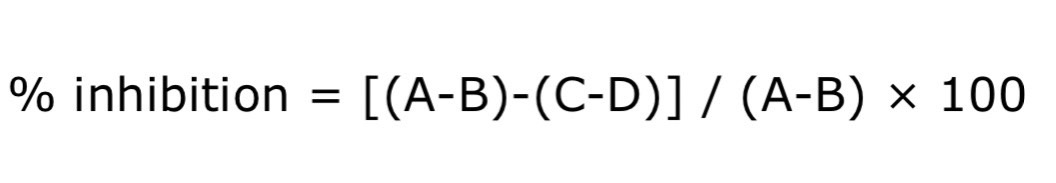
A: OD at 492nm with tyrosinase but without test substance; B: OD at 492 nm without test substance and tyrosinase; C: OD at 492 nm with test substance and tyrosinase; D: OD at 492 nm with test substance but without tyrosinase.
Preparation of MSG-1 cream and determination of SPF values
Preparation of the MGS-1 cream
The crude mangosteen rind extract and MGS-1 extract were separately prepared as oil in water (o/w) creams containing 2, 4, 6 and 9% w/w of the extract. Cream base was composed of Nikkomulesse 41, cetostearyl alcohol, caprylic/capric triglyceride, 1, 3-butylene glycol, carbopol 940, L-arginine, and water.
Determination of SPF values.
The SPF measurement was performed on the Optometrics SPF-290S analyzer as mentioned in preceding report (Choochana et al., 2015). First, the blank PMMA plate (Solar Light’s Sandblasted, USA) was measured, and the data were collected. Next, the sample was measured at six different points according to standard protocol and the data were analyzed for SPF, UVA/UVB ratio, and Boots Star Rating values. In this test, Salisol-3 (benzophenone) was used as a positive control.
RESULTS
Preparation of crude mangosteen rind extract and MGS-1 extract
Preparation of crude mangosteen rind extract and MGS-1 extract, and analysis for amount of a major marker α-mangostin in each extract by the validated HPLC method
Yields of crude rind extracts and amounts of α-mangostin in each extract from 9 locations are shown in Table 1. The highest percentage of α-mangostin amount in Chanthaburi 1 crude rind extract was chosen as a representative sample in this research. After that 600 mg of this crude extract was chromatographed by siliga gel column chromatography to yield 86.3 mg of mid spots which equal to 14.38%w/w of starting crude extract and the α-mangostin content in this portion was analyzed by the HPLC to yield 74.95%w/w. In summary, this mid spots portion was assigned as MGS-1 extract.
Table 1. Yields of mangosteen extracts and amounts of α-mangostin in the extracts from 9 locations.
|
Location |
Amount of extract (g) |
Yield (% dry wt.) |
Amount of |
|
Chanthaburi 1 |
2.59 ± 0.02 |
8.63 ± 0.05 |
11.70 ± 0.01 |
|
Chanthaburi 2 |
2.34 ± 0.05 |
7.80 ± 0.16 |
10.46 ± 0.04 |
|
Chanthaburi 3 |
4.74 ± 0.23 |
15.80 ± 0.75 |
10.24 ± 0.06 |
|
Rayong 1 |
3.24 ± 0.32 |
10.80 ± 1.05 |
8.43 ± 0.03 |
|
Rayong 2 |
5.10 ± 0.20 |
17.00 ± 0.67 |
3.81 ± 0.01 |
|
Prachuap Khiri Khan 1 |
4.95 ± 0.13 |
16.50 ± 0.44 |
4.92 ± 0.01 |
|
Prachuap Khiri Khan 2 |
3.56 ± 0.11 |
11.87 ± 0.37 |
7.96 ± 0.32 |
|
Nakhon Si Thammarat |
4.52 ± 0.18 |
15.07 ± 0.61 |
7.53 ± 0.15 |
|
Phang Nga |
1.61 ± 0.12 |
5.37 ± 0.38 |
9.95 ± 0.23 |
Note: n = 3
Tyrosinase inhibitory activity assay
Mangosteen rind extract from Chanthaburi 1 (600 mg) was separated by column chromatography, using silica gel as a stationary phase, and eluting with hexane: ethyl acetate (7 : 3) as a mobile phase. The silica gel TLC plate with the same solvent system was used to examine the separation under UV at 254 nm. The fractions were collected as top spot (F1-F4) and mid spots (F12-F15) and the organic solvent was evaporated. Amounts of 43.4 and 86.3 mg of top and mid spots, were obtained, respectively (Figure 1).
Figure 1. Thin-layer chromatography of mangosteen rind crude extract used. hexane: ethyl acetate (7 : 3) as solvent system and detected with UV at 254 nm..
The anti-tyrosinase activities of the crude extract, top spot, mid spots, and standard kojic acid are shown in Table 2.
Table 2. Anti-tyrosinase activity (IC50) of crude extract, top spot, mid spots, and standard kojic acid.
|
Sample |
Anti-tyrosinase activity (IC50, µg/mL) |
Correlation coefficient equation |
|
Crude extract |
102.67 |
Y= 0.3311x + 16.005 (r = 0.9364) |
|
Top spot |
>100.0 |
Y= 3.2394In(x) + 21.814 (r = 0.9864) |
|
Mid spots |
18.48 |
Y= 1.3443x + 25.148 (r = 0.9746) |
|
kojic acid |
38.46 |
Y= 1.2265x + 2.815 (r = 0.9959) |
Determination of SPF values
From the SPF evaluation of all compounds in this study using the Optometrics SPF-290S analyzer, the SPF values of a cream base, creams containing crude extract or mid spots extract (2, 4, 6, 9% w/w) are demonstrated in Table 3, and the SPF scanning graph from Optometrics SPF-290S analyzer was depicted in Figure 2. Table 3 shows SPF, UVA/UVB ratio, and Boot Star Rating values. Salisol-3 (SCCS, 2021) was used as a reference standard in this experiment.
Table 3. SPF, PA, UVA/UVB, and Boot Star Rating of the cream base, creams containing crude extract and MGS-1 extract.
|
Sample |
SPF |
UVA/UVB |
Boot Star Rating |
|
Cream base |
0.71 ± 0.01 |
0 |
NA |
|
Crude extract (5%w/w) cream |
15.69 ± 0.18 |
0.688 |
3 (good) |
|
MGS-1 (2%w/w) cream |
3.01 ± 0.22 |
0.226 |
1 (minimum) |
|
MGS-1 (4%w/w) cream |
4.68 ± 0.27 |
0.350 |
1 (minimum) |
|
MGS-1 (6%w/w) cream |
10.36 ± 1.07 |
0.451 |
2 (moderate) |
|
MGS-1 (9%w/w) cream |
9.24 ± 0.97 |
0.926 |
4 (superior) |
|
Salisol-3 (5%w/w) cream |
10.41 ± 1.74 |
0.525 |
2 (moderate) |
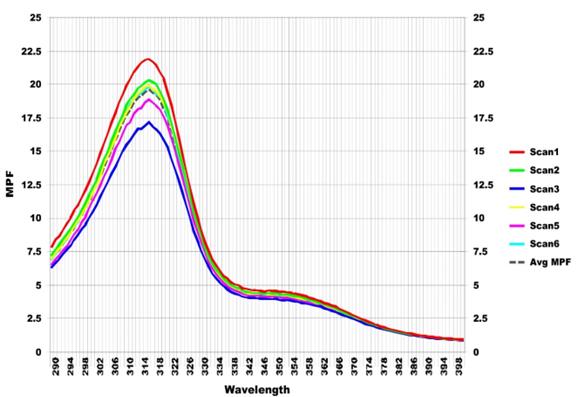
Figure 2. SPF scanning chart of MGS-1 (6%w/w) cream from Optometrics SPF-290S analyzer.
DISCUSSION
From the extracts of mangosteen rinds from 9 locations, it was found that amounts of the extracts obtained showed differences in a wide range of 5.37 – 17.00 %w/w (Table 1) with no special distinction of mangosteen from any region. Analysis of the amount of a marker α-mangostin by HPLC in the rind extracts from 9 locations showed that mangosteen from Phang-nga and Chanthaburi province (Chanthaburi 1, Chanthaburi 2, Chanthaburi 3) contained high α-mangostin contents in the range of 9.95 ± 0.23 to 11.70 ± 0.01 %w/w. Chanthaburi 1 rind sample contained the highest amount of α-mangostin at 11.70 ± 0.01% w/w and it was chosen for preparing MGS- 1 extract. The extracts that contained moderate α-mangostin contents were obtained from Nakhon si Thammarat, Prachuap Khiri Khan 2 and Rayong 1 of which the major marker content ranged from 7.53 ± 0.15 to 8.43 ± 0.03 %w/w. The last group provided low level of α-mangostin (3.81 ± 0.01 to 4.92 ± 0.01 %w/w) were the extracts from Rayong 2 and Prachuap Khiri Khan 1. The results of α-mangostin content differed from a previous report (Pothitirat and Gritsanapan, 2008). However, they used a different extraction method (Soxhlet extraction) and quantitative analysis with the High-Performance Thin Layer Chromatographic (HPTLC) method. The difference in the amount of α-mangostin in the mangosteen rind depends on various factors such as the cultivating location, harvesting period, weather conditions affecting the production of secondary metabolites, extraction method, and quantitative analysis method (Figueiredo et al., 2008). For MGS-1 extract, The highest percentage of α-mangostin amount in Chanthaburi 1 crude rind extract was picked to prepare the extract in this research. Using 600 mg of this crude extract for separation with siliga gel column chromatography yield 86.3 mg of mid spots equaling to 14.38%w/w of starting crude extract. The amount of α-mangostin in theses mid spots was analyzed by the HPLC to obtain 74.95%w/w which was a high content.
For the results of the anti-tyrosinase activity of the crude extract, top spot and mid spots fractions, and standard kojic acid, the crude extract and top spot expressed poor anti-tyrosinase activity with IC50 higher than 100.0 µg/mL. This low inhibition activity may result from the dark purple-brown material in the extract that might be decomposed of large compounds like tannins and resins which could not bind with the tyrosinase enzyme. On the other hand, mid spots where most components were α-mangostin and xanthone derivatives showed high anti-tyrosinase activity with IC50 at 18.48 µg/mL which was better than the activity of standard kojic acid (38.46 µg/mL). From these results, the mid spots fraction was chosen and assigned as MSG- 1 extract.
Table 3 showed the SPF value of crude mangosteen rind extract which demonstrated the highest value of SPF at 15.69 ± 0.18 and showed a UVA/UVB ratio equal to 0.688. However, this high value could come from the dark purple-brown color of the cream that comprised resins and tannins and promoted cream with no good appearances. For MGS-1 extract cream, the SPF value ranged from 3.01 ± 0.22 to 10.36 ± 1.07 while the cream with 6 -9 %w/w MGS-1 showed the highest SPF. When the concentrations of MGS-1 extract in the cream were increased from 6% to 9%w/w, the SPF values were not increased, this result might come from the saturation of UV absorption (Skoog et al., 2014). For UVA/UVB ratio that responds for UVA protection, the MGS-1 extract demonstrated low to moderate protection except for 9%w/w cream which showed a high UVA/UVB ratio.
MGS-1 at 6%w/w cream had pale yellow color (Figure 3) with the SPF and UVA/UVB ratio and Boot Star Rating equal to 10.36 ± 1.07, 0.451, and 2 (moderate), respectively which is close to the results of a reference UV protection chemical Salisol- 3 (benzophenone), with SPF at 10.41 ± 1.74, UVA/UVB ratio at 0.525 and a Boots Star rating equal to 2 (moderate). The results could be noticed that 6%w/w MGS-1 cream might be a good candidate for further development as commercial cosmetic products.

Figure 3. Cream with 6%w/w MGS-1 showing the cream texture.
CONCLUSION
This study revealed that the mangosteen rind extract could be used as a source of whitening and sunscreen ingredients for the production of commercial cosmetic products by semi-purifying as MGS-1 extract. The MGS-1 extract was composed of a major component α-mangostin showing high anti-tyrosinase activity with the IC50 at 18.48 µg/mL which is better than the activity of standard kojic acid. For the sunscreen activity, a standard equipment (Optometrics SPF-290S analyzer) was used to analyze SPF, UVA/UVB ratio values which a 6% w/w MGS-1 cream gave a high SPF at 10.36 ± 1.07 and had UVA/UVB ratio equal to 0.451. These sunscreen properties are close to the properties of Salisol-3 (benzophenone), a standard sunscreen. In conclusion, MGS-1 extract has a good potential to be used for a whitening product in general cosmetics. This allows for enhancing product quality with lower cost of substrate because a large quantity of mangosteen rind can be obtained every year in Thailand with low cost.
ACKNOWLEDGMENTS
The authors would like to thank the RSU Scientific and Technological Equipment Center (RSU-STREC) for supporting HPLC equipment and microplate reader instrument. We thank Dr. Panupon Khumsupan for proofreading the manuscript.
AUTHOR CONTRIBUTIONS
Prasan Tangyuenyongwatana conducted all the experiments, data interpretating and writing the manuscript. Wandee Gritsanapan performed data interpretation, critical revision, and final approval. All authors have read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Ahmad, M., Yamin, B.M., and Mat Lazim, A. 2013. A study on dispersion and characterisation of α-mangostin loaded pH sensitive microgel systems. Chemistry Central Journal, 7: 85
Ahmady, A., Amini, M.H., Zhakfar, A.M., Babak, G., and Sediqi, M.N. 2020. Sun protective potential and physical stability of herbal sunscreen developed from afghan medicinal plants. Turkish Journal of Pharmaceutical Sciences, 17: 285–292.
Choochana, P., Moungjaroen, J., Jongkon, N., Gritsanapan, W., and Tangyuenyongwatana, P. 2015. Development of piperic acid derivatives from Piper nigrum as UV protection agents. Pharmaceutical Biology, 53: 477–482.
Dutta, D., Goyal, N., and Kumar Sharma, D. 2021. Formulation and development of Figueiredo, A.C., Barroso, J.G., Pedro, L.G., and Scheffer, J.J.C. 2008. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour and Fragrance Journal, 23: 213–226.
Figueiredo, A.C., Barroso, J.G., Pedro, L.G., and Scheffer, J.J.C. 2008. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour and Fragrance Journal, 23: 213–226.
Garbe, C. and Leiter, U. 2008. Epidemiology of melanoma and nonmelanoma skin cancer--the role of sunlight. Advances in Experimental Medicine and Biology, 624: 89–103.
Geoffrey, K., Mwangi, A.N., and Maru, S.M. 2019. Sunscreen products: Rationale for use, formulation development and regulatory considerations. Saudi Pharmaceutical Journal , 27: 1009.
Korać, R.R., Khambholja, K.M., and Korać, M.R. (n.d.). Potential of herbs in skin protection from ultraviolet radiation. Pharmacognosy Reviews, 10: 164-173
Li, Q., Chen, Y., Ma, K., Zhao, A., Zhang, C., & Fu, X. 2016. Regenerative and reparative effects of human chorion-derived stem cell conditioned medium on photo-aged epidermal cells. Cell Cycle (Georgetown, Tex.), 15: 1144–1155.
Muchtaridi, M., Suryani, D., Qosim, W. A., and Saptarini, N. M. 2016. Quantitative analysis of Α-mangostin in mangosteen (Garcinia mangostana L.) pericarp extract from four district of west java by HPLC method. International Journal of Pharmacy and Pharmaceutical Sciences. 8: 232-236.
Netto MPharm, G. and Jose, J. 2018. Development, characterization, and evaluation of sunscreen cream containing solid lipid nanoparticles of silymarin. Journal of Cosmetic Dermatology, 17: 1073–1083.
Pothitirat, W. and Gritsanapan, W. 2008. Quantitative analysis of total mangostins in Garcinia mangostana fruit rind. Journal of Health Research, 22: 161–166.
Rabinovich, L. and Kazlouskaya, V. 2018. Herbal sun protection agents: Human studies. Clinics in Dermatology, 36: 369–375.
Saewan, N., and Jimtaisong, A. 2015. Natural product as photoprotection. Journal of Cosmetics and Dermatology. 14: 47-63.
SCCS (Scientific Committee on Consumer Safety), 2021. Opinion on benzophenone-3 (CAS No 131-57-7, EC No 205-031-5), European Commission.
Skoog, D.A., West, D.M., Holler, F.M., and Crouch, S.R. 2014. Fundamentals of Analytical Chemistry 8th Ed., Brooks/Cole, California.
Tadtong, S., Viriyaroj, A., Vorarat, S., Nimkulat, S., and Suksamrarn, S. 2009. Antityrosinase and antibacterial activities of mangosteen pericarp extract. Journal Health Research. 23: 99-102.
Yarnell, E. and Abascal, K. 2012. Herbal sunscreens and ultraviolet protectants. Alternative and Complementary Therapies, 18: 141–144.
Zhang, C., Lu, Y., Tao, L., Tao, X., Su, X., and Wei, D. 2007. Tyrosinase inhibitory effects and inhibition mechanisms of nobiletin and hesperidin from citrus peel crude extracts. Journal of Enzyme Inhibition and Medicinal Chemistry, 22: 83–90.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand.
https://cmuj.cmu.ac.th
Prasan Tangyuenyongwatana1, * and Wandee Gritsanapan2
1 College of Oriental Medicine, Rangsit University, Pathumthani, 12000, Thailand.
2 Phyto Product Research, 165 Soi Suwandee 3, Rimklongprapra Road, Bangsue, Bangkok 10800, Thailand.
Corresponding author: Prasan Tangyuenyongwatana, E-mail: prasan.t@rsu.ac.th
Total Article Views
Editor: Wipawadee Yooin,
Chiang Mai University, Thailand
Article history:
Received: June 4, 2022;
Revised: August 12, 2022;
Accepted: September 7, 2022;
Published online: September 13, 2022

