
Effects of Pre-Heating Between Controlled Temperature Microwave Heating and Hot Air Oven Heating in Protein Extraction Process of Soybeans
Pakorn Suwannasopon*, Viboon Changrue*, Tanachai Pankasemsuk, Nattasak Krittigamas and Kunakorn KatsriPublished Date : 2022-10-18
DOI : https://doi.org/10.12982/CMUJNS.2022.063
Journal Issues : Number 4, October-December 2022
Abstract Heating is an important treatment before protein extraction process. Rapid heating of microwave heating (MW) was investigated to compare with hot air oven (HA) heating prior to protein extraction in soybeans. Heating temperature was controlled at 50°C, with the heating time of 15, 30, 45, 60 and 75 min. The control treatment was no heat treatment. The treated samples were analyzed i) the total protein using Kjeldahl method, ii) the protein soluble by Bradford method and iii) cells microscope investigated by scanning electron microscope (SEM). The results were found that the pretreatment of 75 min microwave (MW75) heating provided the highest protein with the amount of absorbance value and total protein of 3.0365 ± 0.004 mg mL-1 and 3.70 ± 0.01% respectively. The lowest protein extraction of 2.7707 ± 0.020 mg mL-1 and 3.38 ± 0.005% total protein value occurred in the condition of 15 mines hot air oven (HA15) heating. The investigation using scanning electron microscope (SEM) revealed that protein extraction correlated with the enlargement and breakage of cell wall and endosperm caused by microwave heating rather than hot air oven heating. The rapid heating of microwave and heating time influenced the amount of extracted protein.
Keywords: Soybean, Protein, Microwave, Hot air, Heating
Funding: The authors are grateful for the research grants received from Chiang Mai University, Chiang Mai, Thailand, and thanks to the Agricultural Research Development Agency (ARDA) for the main funding support of the research.
Citation: Suwannasopon, P., Changrue, V., Pankasemsuk, T., Krittigamas, N. and Katsri, K. 2022. Effects of pre-heating between controlled temperature microwave heating and hot air oven heating in protein extraction process of soybeans. CMUJ. Nat. Sci. 21(4): e2022063.
INTRODUCTION
As a cash crop, soybean is also known to be good alternative source of protein. Soybean production has increased over the years but not enough for domestic demand (Somchai, 2015). Imported soybean in Thailand increased 63.59 % from year 2001 to 2021 (Office of Agricultural Economics, 2021). In the agricultural-food sector, protein extraction by alkaline solutions and isoelectric precipitation is one of the most common methods reported in the literature (Lim et al., 2016; Deng et al., 2019; Mir et al., 2019). The lowest solubility at the isoelectric point is the basis of this method. However, many factors are involved, such as pH, temperature, extraction time, and the ratio of raw materials to solvents, which may affect the solubility of proteins (Wani et al., 2006). Therefore, the alkali extraction capability is interested and simplicity in terms of extraction methods as well as considerations of safety in practice (Zhang et al., 2009). Nevertheless, the pre-heat of raw material is reported to reduce the process time.
Microwaves are non-ionizing radiation that generates heat by using the result of the interaction of electromagnetic fields alternating with the chemical composition of food (Mullin, 1995). For microwave heating, Paiboon, (1989) has described the microwave energy is absorbed into the food pieces and then the molecules are moving rapidly by spinning back and forth in microwave frequencies of 915 or 2450 million times per second. Microwave energy is electromagnetic energy that can transmit through waves and penetrates into the food and this energy has absorbed and converted into heat energy (Fellows, 2009 and Shivhare et al., 1993). The most applied microwave heating is microwave-assisted drying utilized by the effect of volumetric heating (Murthy and Prasad, 2005) to create a common evaporation level with relative humidity and temperature causes an acceleration of moisture removal from the material. However, the major disadvantage of microwave assisted drying (MWD) is its unstable heating. In particular, the edge of the sample causes overheating (Nijhuis et al., 1998). Therefore, methods have been applied to eliminate the disadvantages of microwave drying, for example, vacuum and freeze-drying. These combinations create high quality dry products (Lin et al., 1998; Yanyang et al., 2004; Xu et al., 2006; Bondaruk et al., 2007; Cui et al., 2008; Nahimana and Zhang, 2011). In addition, Sedani et al., (2021) applied the low-wattage microwave drying to eliminate the complexity of the combination method and found that the use of low-wattage (180 W) microwaves increased the efficiency by 30 % of the extracted protein in the solution. In addition, Gohi et al., (2019) gave 57°C of microwave heating temperature and found that pretreatment of protein powder in solution for enzymolysis resulted in protein yield and degree of hydrolysis. The mechanism of microwave heating increases the distribution of moisture. While large air convection conveys both moisture and heat from the material. This energy will help to accelerate the chemical reaction of the substance.
In previous articles, most of microwave heating are non-controlled temperature. The studies use a fixed microwave watt power for the whole process. This research investigates the heating scheme of 600 W controllable temperature microwave, with a temperature of 50°C of soybean. The high wattage of microwaves supposes to rapid molecular vibrate and heat, resulting in cell breakdown and increased efficiency of the protein extraction process.
MATERIALS AND METHODS
Material and preparation
Thai soybeans (Glycine max {L.} Merr. cv. ‘Chiang Mai 60’) were used in this research. Soybeans were obtained from Chiang Mai Field Crops Research Center. All other chemicals used were of analytical grade. Then, 5 g of soybeans were determined for moisture at 105°C for 24 hr or until stable weight (AOAC 2000) and recorded prior to experimentation and raw materials were cleaned and divided to 200 g in a vacuum bag, then store at 4°C with the refrigerator for the whole study.
Methods
Using 200 g of soybeans were heated by microwave heating and hot air oven heating under the temperature of soybeans at 50°C (Miranda et al., 2010). Each method will be stored at 15, 30, 45, 60 and 75 min with 5 replications and control samples were no heat treatment. The treated samples and soybean meal were analyzed, i) stability of heating temperature, ii) protein content by Kjeldahl method and Bradford method, iii) cells change by scanning electron microscope. Finally, all experimental design was statistical software (SPSS Inc., Chicago, IL, USA) was used to do data analysis.
Microwave and hot air oven setup
In this research, the microwave (NN-DF383B, Panasonic, Japan) was modified to be able to control the temperature with an on-off system and measure the temperature with a Fiber Optic Thermometer (FOTS100, OpSens, Canada) as shown in Figure 1. The fiber optic sensor was inserted to the center of soybean kernel and the temperature was controlled at 50°C (Figure 2). In the hot air oven, select the oven button, then select the baking temperature range at 50°C. The oven mode of microwave oven was used for hot air oven study.

Figure 1. Microwave setting laboratory.

Figure 2. Soybean heating inside microwave chamber.
Soybean extraction
Protein was extracted by weighing 20 g of crushed soybean seeds and adding 125 mL of 0.4% sodium hydroxide (Sigma-Aldrich, Germany) solution, then extracted for 45 min using a magnetic stirrer (MGS-1001, LMS, Japan), stirring level 6, at room temperature 25°C. The solution was separated by a centrifugation at 10,000 rpm by centrifugation machine (universal 320R, HETTICH, Germany) for 15 min and filtered with filter paper No.1 (Grade 1 circles diam. 25 mm, Whatman, United Kingdom) for reduced error of absorbance value. The extracted solution was stored under -70°C by adapted from Lee el al. (2015) and used freezer (MDF-792, SANYO, Japan) before the solution was processed for analysis. The results of protein extracts were analyzed by Kjeldahl method and Bradford method.
Kjeldahl method
The Kjeldahl method was performed according to method 981.10 of the AOAC International (Latimer, 2016). Approximately 1 g of raw material was hydrolyzed with 15 mL concentrated sulfuric acid (Anapure, New Zealand) containing two copper catalyst tablets (Merck, Germany) in a heat block (Kjeltec system 2020 digestor, Tecator Inc., Herndon, VA, USA) at 420°C for 2 hr. After cooling, water was added to the hydrolysates before neutralization and titration. The amount of total nitrogen in the raw materials were multiplied with both the traditional conversion factor of 6.25 (Kjeldahl, 1883) and species-specific conversion factors (Fellows, 2009 and Mariotti et al., 2008) in order to determine total protein content. The species-specific conversion factors were 6.25 for soybean.
Bradford method
Bradford's assay was described by Bradford (Bradford, 1976). Briefly, 100 mg coomassie brilliant blue G-250 (Sigma-Aldrich, Germany) was dissolved in 50 mL 95 % ethanol (RCI Labscan, Ireland). Thereafter, 100 mL of 85% phosphoric acid (Merck, Germany) was carefully added under stirring, before H2O was added to a total volume of 1 L. The solution was filtered and kept at 4°C. For the measurements, 100 µL extract and 5 mL Bradford solution were mixed and incubated for 5 min. A standard curve was made of BSA (Sigma-Aldrich, United States) at 0, 0.0625, 0.125, 0.25, 0.5 and 1 mg mL-1 then was read at 595 nm of absorbance value.
Cells microscope
Soybeans seeds that were heated by microwave heating technique and hot air oven heating were cut crosswise at the center of the seeds. Microstructural analysis of cross-section soybean seed was carried out using scanning electron microscope (JMS 5410LV, JEOL, Japan) with a magnification of 350X and 500X.
Statistics description
All results are presented as the arithmetic means of 5 parallels ± standard deviation (SD), consisting of two factors (heating type), and four levels (heating time) of the experiment. Statistical software (SPSS Inc., Chicago, IL, USA) was used to perform statistical analyses. The data should be analyzed in a one-way analysis of variance (ANOVA) was performed. Means were considered significantly different at P < 0.05.
RESULTS
Effects of heating temperature and heating technique
The comparison between microwave heating and hot air oven heating for soybean with 10.13% (w.b.) of moisture showed that microwave heating in soybeans increased more rapidly and less when heated by hot air oven heating in terms of heating time. The microwave heating time of soybeans to the target of 50°C was 47 sec, while hot air oven heating required 35 min. However, temperature fluctuations of the control system showed that heating soybeans with hot air oven heating were less accurate than microwave heating. The above shows the efficiency of the heating mechanism resulting from vibrate of molecules under microwave waves over hot air. As shown in Figure 3.
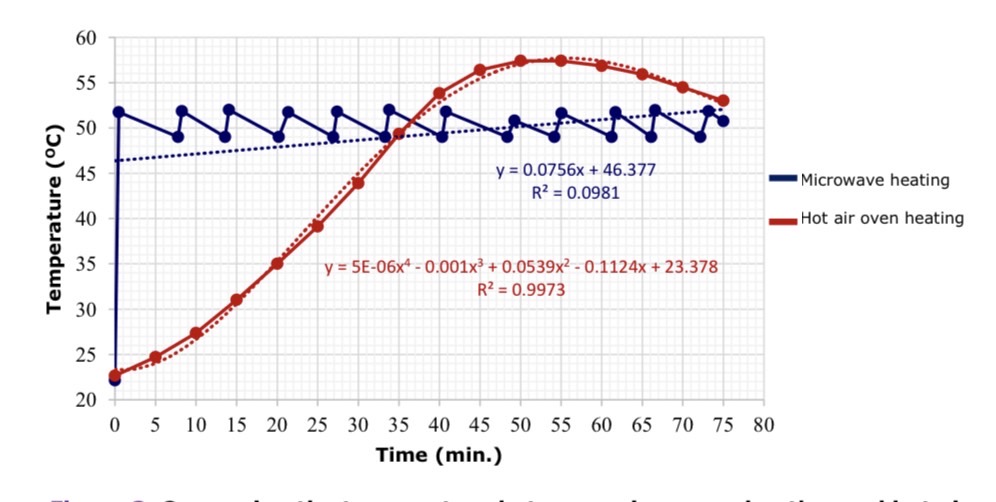
Figure 3. Comparing the temperature between microwave heating and hot air oven heating in soybeans at 50°C during 75 min.
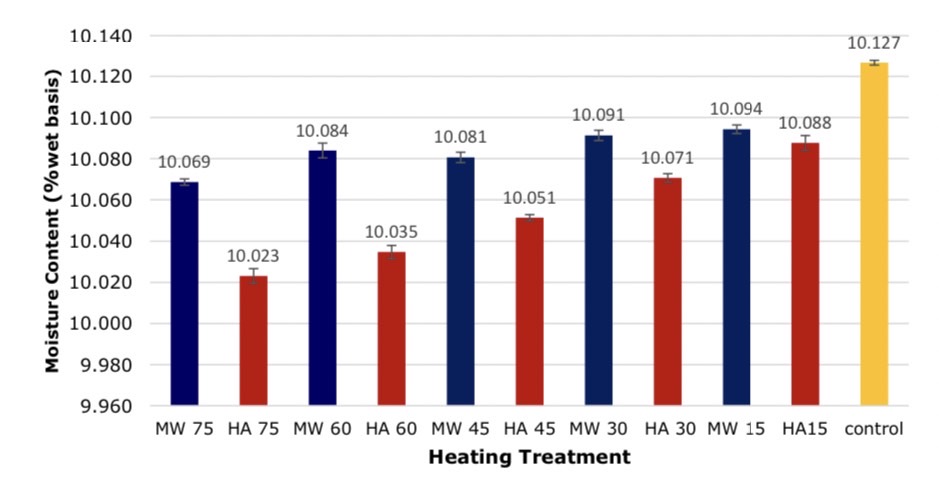
Figure 4. Comparison of moisture loss in soybean after heating, *significant at P < 0.05 by Duncan test.
The heating technique and heating time affected the moisture content of soybean after heating. The results showed that microwave heating had a statistically significant effect on moisture loss than the hot air oven heating technique statistically significant at P
Protein extraction
The results of different heating techniques found the heating technique and extraction time had a statistically significant effect on the protein content after extracted solution at 95 % confidence level as shown in Figures 5 and 6. Soybeans were heated by 2 techniques and the solution was taken for protein values by absorption method and overall protein was found. The long heating times encourages higher extraction of proteins than short extraction times at the same heating temperature. In addition, microwave heating still produces a higher protein extraction more than hot air oven heating. These methods for analyzing solution proteins Bradford method and the Kjeldahl method supported the maximum value of MW75 with 3.036 ± 0.004 mg mL-1 and 3.70 ± 0.010%, whereas HA15 was the lowest extraction solubility with 2.771 ± 0.020 mg mL-1 and 3.38 ± 0.005% respectively.
The analysis of protein content in soybean after the protein extraction showed that the MW75 heating technique results in the lowest protein of soybean meal and the HA15 maintain the highest protein content as the result shown in Figure 7.
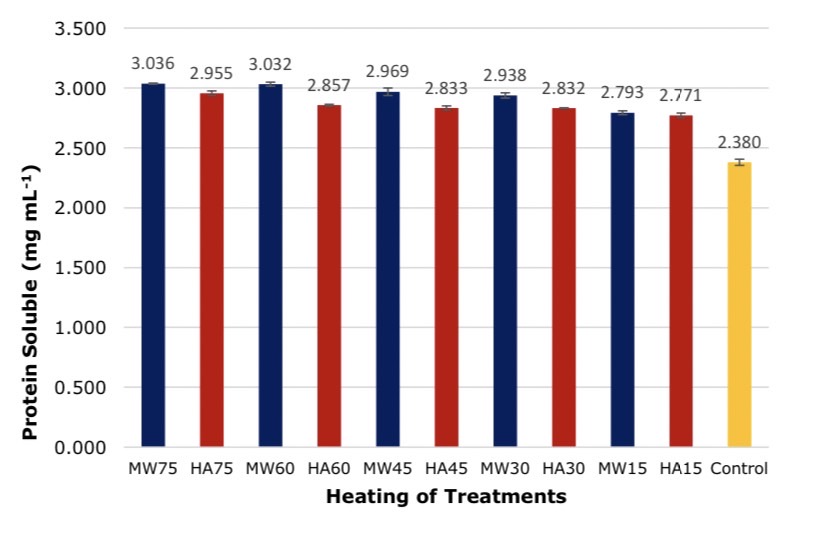
Figure 5. Comparison of proteins in solutions by absorbance measurement method, *significant at P < 0.05 by Duncan test.
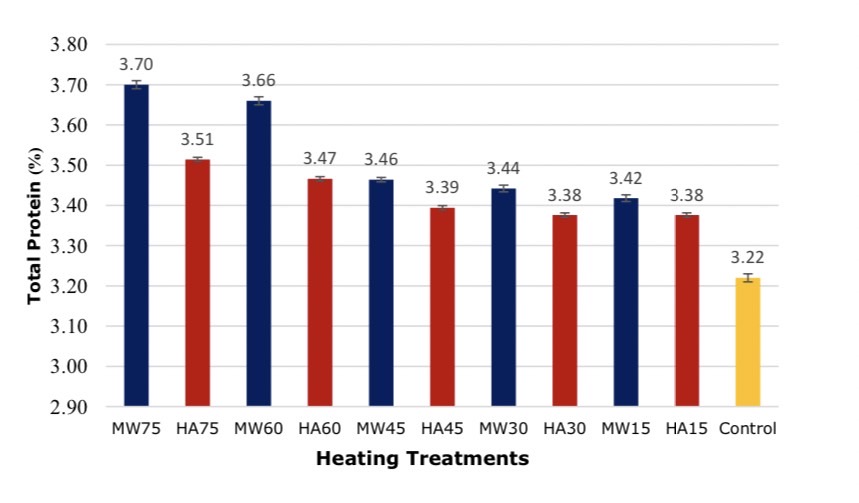
Figure 6. Comparison of proteins in solutions by Kjeldahl method, *significant at P < 0.05 by Duncan test.
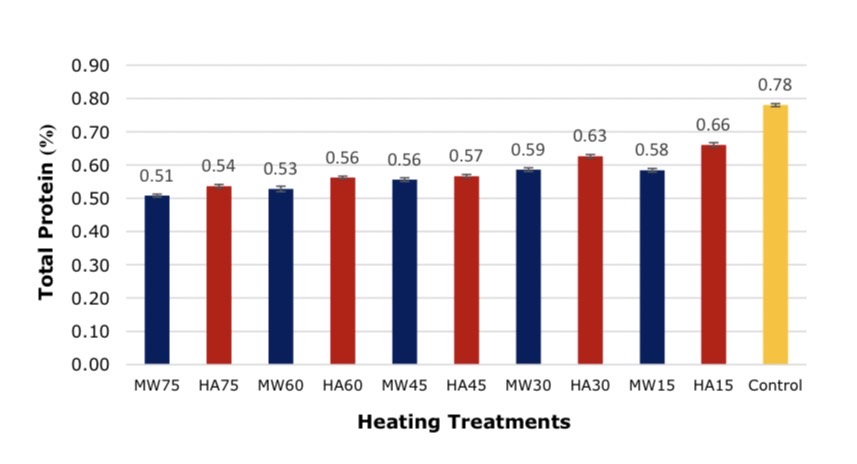
Figure 7. Comparison of proteins in soybean after the protein extraction, *significant at P < 0.05 by Duncan test.
Cell microscope
The results of a cross-sectional analysis of soybean in microwave heating and hot air oven heating seeds with cells microscope showed that the cell wall tissue has enlarged compared to the unheated group. The enlarged tissue was also found in the endosperm and is shown in Figure 8, 9 and 10.
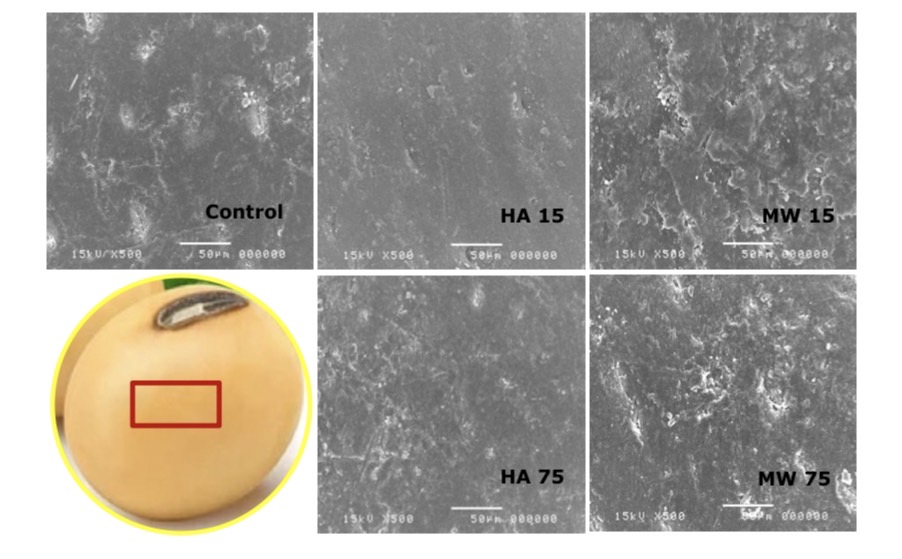
Figure 8. Difference of seed coat tissue by microwave heating and hot air oven heating techniques using Scanning electron microscope (SEM).
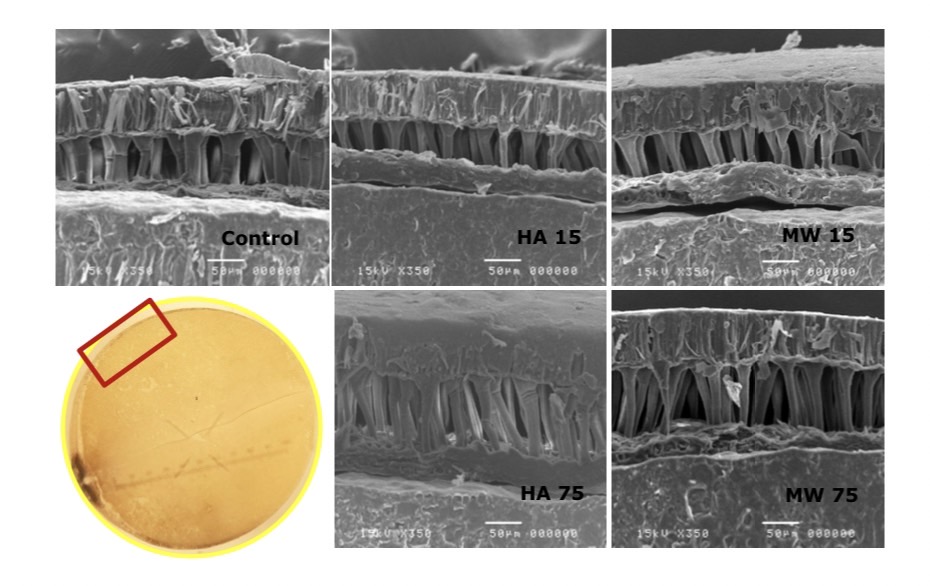
Figure 9. Difference of cell wall tissue by microwave heating and hot air oven heating techniques using Scanning electron microscope (SEM).
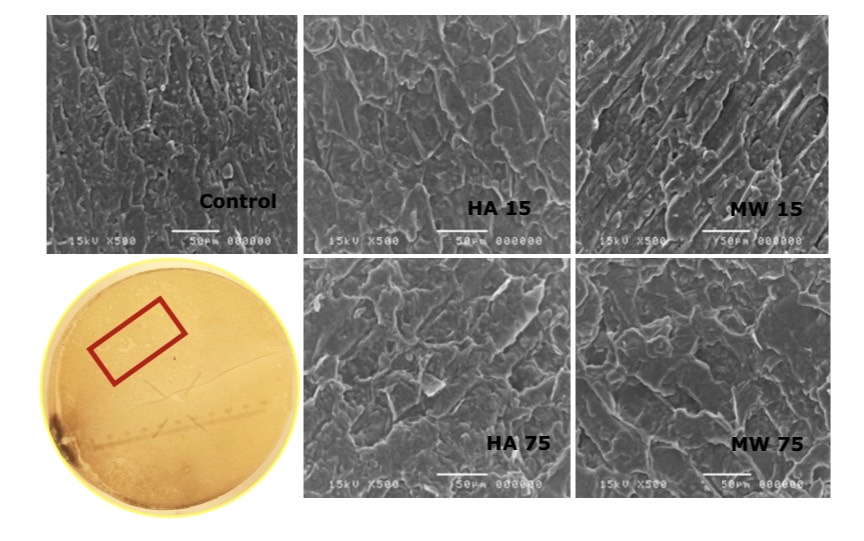
Figure 10. Difference of endosperm tissue by microwave heating and hot air oven heating techniques using Scanning electron microscope (SEM).
DISCUSSION
Effects of heating temperature and heating technique
The different results are due to the mechanism of electromagnetic heating the molecules move rapidly, rotating back and forth at the microwave frequency 2450 million times per second. The effect of friction on rotation friction causes heating to the whole seed (Nahimana and Zhang, 2011). While the hot air oven heating technique heats the air as the medium to carry energy to the soybean. The heat is diffused from the outside to the center of the seed (Zhong et al., 2015).
In general, microwave heating are not equipped with an airflow system, so in some cases, the samples scorch very quickly. This effect can be attributed to the thermal radiation resulting from the internal heat generation of soybeans (Bouraoui. et al., 1993; Digman et al., 2012). Results can be affected by uneven power distribution during heating, and drying of microwave heating error often requires a lot of tests to avoid the common problem of uneven heating (Tassi et al., 2019). These results due to the non-uniform of energy in soybean seed content. A summary of the study by Inchuen and Nakrugsa, (2010) comparing microwave heating and hot air oven heating in Thai red-curry powder found that heating to the final moisture content of raw materials at 8.00-8.70%. The microwave heating used 8 min at 540 w while hot air oven heating takes 130 min, and 80°C of temperature for reduced moisture in materials. That result supporting of the temperature of soybean increased rapidly, by the rapid increase in temperature results of higher rate of water loss in soybean. However, high-wattage microwaves have a decreasing effect on the protein content of raw materials and is the result of decomposition of organic matter at high temperature inside the raw material (Inchuen and Nakrugsa, 2010). The results of the soybean moisture loss supported controlled temperature microwave heating that was more efficient than hot air oven heating for the same period time and 50°C of temperature. This is because the efficiency of the heating mechanism with microwave heating is better than with hot air oven heating. The conclusions, consistent with Nisoa et al., (2021), found that microwave heating in stink bean seed was more effective at reducing moisture loss than hot air oven heating at 6 hr and that included to the quality of the stink bean seed after heating. The high moisture loss in hot air oven heating is a result of the mechanism of heat generation caused by heat energy in the air absorbed by the soybean and heat conduction to the core of the seed. Therefore, during the first period of thermal energy exposure, heat is generated higher on the seed surface than inside the seed (Orphanides et al., 2016). The heat conduction from the pellet surface to the core takes a long time to absorb energy and the internal seed center measured to measure temperature. Which results in a higher surface temperature than a predetermined temperature. The effect was that the seeds have a high moisture loss and can be shown in Figures 4.
Protein extraction
The results of comparison of microwave heating and hot air oven heating modes, as well as different heating times, influenced the protein content in the solution. However, the phenomenon occurred as a result of the different heating mechanisms of the two techniques, namely microwave heating and hot air oven heating. The conclusion remains that MW75 has the highest protein extraction properties and is also greater than HA75, and is in line with Kumar et al., (2018) who found microwave heating in peanuts support protein extraction than hot air oven heating. In terms of time, microwave heating had a higher effect on protein extraction estimates and matched that with Mohseni et al., (2019), it was found that microwave time resulted in an increase in protein content in canola seeds. Massive acquisition of proteins in solution by microwave heating technique is a form of electromagnetic energy, and their interaction with charged particles and polar molecules causes turbulence, which means heat (Aladjadjiyan, 2010). Through this mechanism, there is an increased degradation effect on the cell wall, which may result in increased degradation of protein reserves present in the intact soy structure (Phongthai et al., 2016; Varghese and Pare, 2019) and the result that microwave heating can extract proteins more effectively than hot air oven heating.
Cells microscope
It was found that microwave heating resulted in enlargement of seed coat tissue, cell wall and endosperm cell than hot air oven heating. However, when considering the tissues that were heat at the same temperature, it was found that the microwave heating technique resulted in high tissue cell enlargement and breakdown, hot air oven heating and long heating time also had a greater effect on cell enlargement than short-term seed heating. Consistency of these results was also found in the research by Hemis et al., (2016) and Nisoa et al., (2021) that the heating time had an effect on cell enlargement and breakdown of the tissue, as well as the watt boost, is one reason for the increase in stink bean of poor condition. This effect can be caused by the microwave heating mechanism or may be combined with the hydrolysis reaction inside the soybean. This explains that microwave heating result in molecular oscillation in a contractile and torsional form, resulting in cell breakdown (Reynosa et al., 2017). It was reported that the use of microwave heating resulted in the reduction of trypsin inhibitors, so this effect influenced the activity of protein-digesting enzymes, and in soybeans, the moisture content was suitable for the reaction (Pays et al., 2012; Vagadia et al., 2018.)
CONCLUSION
Comparative study of heating techniques between controllable temperature microwave and hot air oven per 200 g of soybeans at 50°C of temperature as the follows.
The microwave heating can increase the temperature of the soybean faster than the hot air oven heating technique, and it also had a greater effect on the moisture loss in the soybeans than the hot air oven heating technique.
The correlation of the heating technique was also on the amount of extracted proteins was found that microwave heating technique provided higher support for protein extraction than hot air oven heating technique. The 75 min of microwave heating (MW75) was the best choice for pre-heating before the protein extraction process.
In the cross-section analysis, soybean cells in the seed coat tissue, cell wall tissue and endosperm tissue showed the most different characteristics from the control in MW75. The cell enlargement also corresponded to the results of protein extraction indicating that The MW75 heating can extract more proteins with every treatment. The comparison of heating techniques during the same period time found that microwave heating is also superior to hot air oven heating in terms of protein extraction.
The overall results of the research explain that the heating technique and duration affected the extracted proteins by microwave heating in soybean protein extraction using 600 W of microwave power at 50 °C of temperature, and 75 min extraction time have contained maximum extraction of protein from soybeans. In terms of protein extraction time, it was found that the extraction time of 75 min was more able to extract protein than extraction at 60, 45, 30, and 15 min due to an increase in cell breakdown of heating times. The comparison of the microwave heating technique and the hot air oven heating technique gave different results and at the same time, the microwave heating technique promoted the yield of extracted protein more than the hot air oven heating technique.
ACKNOWLEDGMENTS
The authors thank the graduate school Chiang Mai University to provide the opportunity to get research fund and thank to Agricultural Research Development Agency (ARDA) for supporting research fund. Also thanks to Postharvest Technology Research Center, Faculty of Agriculture, Chiang Mai University and Postharvest Technology Innovation Center for providing facilities for laboratory operation.
AUTHOR CONTRIBUTIONS
Pakorn Suwannasopon and Viboon Changrue applied electromagnetic heating in the condition of controlled temperature to extraction process. In addition, the authors designed and performed experiment works statistical analysis, visualization and writing manuscript. All authors have read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Aladjadjiyan, A. 2010. Effect of microwave irradiation on seeds of lentils (Lens Culinaris, Med.). Romanian Journal of Biophysics, 20: 213-221.
Bradford, M.M. 1976. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Analytica Biochemistry. Journal of Biochemical Technology, 72: 248–254.
Bondaruk, J., Markowski, M., and Błaszczak, W. 2007. Effect of drying conditions on the quality of vacuum-microwave dried potato cubes. Journal of Food Engineering, 81: 306–312.
Bouraoui, M., Richard, P., and Fichtal, J.A. 1993. Review of moisture content determination in foods using microwave oven drying. Food Research International, 26: 49–57.
Cui, Z. W., Li, C.Y., Song, C.F., and Song, Y. 2008. Combined microwave-vacuum and freeze drying of carrot and apple chips. Drying Technology, 26: 1517–1523.
Deng, Y., Huang, L., Zhang, C., Xie, P., Cheng, J., Wang, X. and Li, S. 2019. Physicochemical and functional properties of Chinese quince seed protein isolate. Food Chemistry, 283: 539–548.
Digman, M.F., Conley, S.P., and Lauer, J.G. 2012. Evaluation of a microwave resonator for predicting grain moisture independent of bulk density. Applied Engineering in Agriculture, 28: 611–617.
Fellows, P.J. 2009. Food Processing Technology. 22nd Ed. Woodhead Publishing UK, 982 pp
Gohi, B.F.C.A., Du, J., Zeng, H.Y. Cap, X.J., and Zou, K.M. 2019. Microwave pretreatment and enzymolysis optimization of the lotus seed protein. Bioengineering, 6: 1-13.
Hemis, M., Choudhary, R., Becerra-Mora, N., Kohli, P., and Raghavan, V. 2016. Modelling of microwave assisted hot-air drying and microstructural study of oilseeds. International Journal of Agricultural and Biological Engineering, 9: 167-177.
Inchuen, S. and Narkrugsa, W. 2010. Effect of drying methods on chemical composition, color and antioxidant properties of thai red curry power. Kasetsart Journal Natural Science, 44: 142-151.
Lam, A.C.Y., Can Karaca, A., Tyler, R.T., and Nickerson, M.T. 2016. Pea protein isolates: Structure, extraction, and functionality. Food Reviews International, 34: 126–147.
Latimer, G.W. 2016. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA.
Lee, Y., Choi, H., and Eun, J.B. 2015. Protein extraction and purification of soybean flakes and meals using a lime Treatment followed by ultrafiltration. Journal of Modern Engineering Research, 5: 7-15.
Lin, T.M., Durance, D.T., and Scaman, C.H. 1998. Characterization of vacuum microwave, air and freeze dried carrot slices. Food Research International, 31: 111–117.
Kumer, A., Padmashree, A., Govindraj, T., Semwal, A.D. and Sharma, G.K. 2018. Comparison of conventional, microwave and infrared roasting methods on the changes in physico-chemical and antioxidant properties of peanuts (Arachis hypogaea). International Journal of Food and Fermentation Technology, 8: 161-169.
Kjeldahl, J.G.C.T. 1883. Neue methode zur bestimmung des stickstoffs in organischen korpern. Fresenius'. Journal of Analytical Chemistry, 22: 366-382.
Makishi, G.L.A., Lacerda, R.S., Mamani, H.N.C., Costa, P.A., Bittante, A.M.Q.B. and Sobral, P.J.A. 2014. Effect of alkaline agent and ph on the composition of freeze-dried proteins extracted from Castor bean (Ricinus communis L.) cake. Chemical Engineering, 37: 697-702.
Mariotti, F. Tome, D. and Mirand, P.P. 2008. Converting nitrogen into protein—Beyond 6.25 and Jones’ factors.Crit. Critical Reviews in Food Science and Nutrition, 48:177–184.
Mir, N. A., Riar, C. S. and Singh, S. 2019. Effect of pH and holding time on the characteristics of protein isolates from Chenopodium seeds and study of their amino acid profile and scoring. Food Chemistry, 272: 165–173.
Mullin, J. Microwave Processing. 1995. In New Methods of Food Preservation; Gould, G.W.; Ed.; Springer: Dordrecht, Netherlands, 112–134 p.
Murthy, G.S. and Prasad, S. 2005. A completely coupled model for microwave heating of foods in microwave oven heating. Paper presented at the 2005 ASAE Annual Meeting.
Nahimana, H. and Zhang, M. 2011. Shrinkage and color change during microwave vacuum drying of carrot. Drying Technology, 29: 836–847.
Nijhuis, H.H., Torringa, H.M., Muresan, S., Yuksel, D., Leguijt, C., and Kloek, W. 1998. Approaches to improving the quality of dried fruit and vegetables. Trends in Food Science & Technology, 9: 13–20.
Nisoa, M., Wattanasit, K., Tamman, A., Sirisathitkul, Y., and Sirisathitkul, C. 2021. Microwave drying for production of rehydrated foods: A case study of stink bean (Parkia speciose) seed. Applied Sciences, 11:2918.
Office of Agricultural Economics. 2021. The soybean quantity is imports and exports of thailand [Online]. Available: http:// http://www.oae.go.th/oae_report_export /import_result.php. (7 July 20121).
Orphanides, A., Goulas, V., and Gekas, V. 2016. Drying technologies: Vehicle to high-quality herbs. Food Engineering Reviews, 8: 164–80.
Paiboon, T. 1989. Food Processing. 1st ed. Odeon Store, Bangkok, 302 p.
Pays, M., Polaszczyk, S., Leszczynska, T. and Piatkowska, E. 2012. Effect of microwave field on trypsin inhibitors activity and protein quality of broad bean seeds (Vicia Fabavar. Major). Acta Scientiarum Polonorum Technologia Alimentaria, 1: 193-199.
Phongthai, S., Lim, S.T., and Rawdkuen, S. 2016. Optimization of microwave-assisted extraction of rice bran protein and its hydrolysates properties. Journal of Cereal Science, 70: 146–154.
Reynosa, A.A., Romani, A. Jasso, R.M.R., Aguilar, C.N., Garrote, G. and Ruiz, H.A. 2017. Microwave heating processing as alternative of pretreatment in second generation biorefinery: An overview. Energy Conversion and Management, 136: 50-65.
Sedani, S., Pardeshi, I.L., and Dorkar, A.R. 2021. Study on the effect of stepwise decreasing microwave power drying (SDMPD) of moth bean sprouts on its quality. [Online]. Available: https://onlinelibrary.wiley.com/doi/epdf/10.1002/leg3.84. (14 January 2022)
Somchai, P. 2015. Research and development on soybean. Research project report. Department of Agriculture. Ministry of Agriculture and Cooperatives of Thailand, Bangkok. 89 p.
Tassi, A.L.W., Bento, J.A.C., Ferreira, K.C., Caliari, M., Silva, V.S.N., Pacheco, M.T.B., Ida, E.I. and Junior, M.S.S. 2019. Roasting soybeans in a microwave for manufacturing chocolate dragées. Ciência Rural Santa Maria Journal, 1-10.
Vagadia, B.H., Vanga, S.K. Singh, A. Gariepy, Y. and Raghavan, V. 2018. Comparison of conventional and microwave treatment on soymilk for inactivation of trypsin inhibitors and in vitro protein digestibility. [Online]. Available: https://www.mdpi.com/2304-8158/7/1/6/htm. (16 September 2021).
Varghese, T. and Pare, A. 2019. Effect of microwave assisted extraction on yield and protein characteristics of soymilk. Journal of Food Engineering, 262: 92-99.
Wani, A.A., Sogi, D.S., Grover, L., and Saxena, D.C. 2006. Effect of temperature, alkali concentration, mixing time and meal/solvent ratio on the extraction of watermelon seed proteins-a response surface approach. Journal of Biosystems Engineering, 94: 67–73.
Xu, Y., Zhang, M., Mujumdar, A.S., Duan, X., and Jin-cai, S. 2006. A two-stage vacuum freeze and convective air drying method for strawberries. Drying Technology, 24: 1019–1023
Yanyang, X., Min, Z., Mujumdar, A.S., Le-qun, Z., and Jin-cai, S. 2004. Studies on hot air and microwave vacuum drying of wild cabbage. Drying Technology, 22:2201–2209.
Zhang, B., Cui, Y., Yin, G., Li, X. and Zhou, X. 2009. Alkaline extraction method of cottonseed protein isolate. Modern Applied Science, 3: 76-83.
Zhong, X., Dolan, K.D., and Almenar, E. 2015. Effect of steamable bag microwaving versus traditional cooking methods on nutritional preservation and physical properties of frozen vegetables: A case study on broccoli (Brassica oleracea). Innovative Food Science and Emerging Technologies, 31: 116–122.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand.
https://cmuj.cmu.ac.th
Pakorn Suwannasopon1, *, Viboon Changrue2, *, Tanachai Pankasemsuk3, Nattasak Krittigamas3 and Kunakorn Katsri4
1 Postharvest Technology Research Center, Faculty of Agriculture, Chiang Mai University, Chiang Mai 50200, Thailand.
2 Department of Mechanical Engineering, Faculty of Engineering, Chiang Mai University, Chiang Mai 50200, Thailand.
3 Department of Plant and Soil Sciences, Faculty of Agriculture, Chiang Mai University, Chiang Mai 50200, Thailand.
4 School of Agriculture and Natural Resources, University of Phayao, Phayao 56000, Thailand.
Corresponding author: Pakorn Suwannasopon, E-mail: pakornsuwannasopon@gmail.com;
Viboon Changrue, E-mail: viboon@eng.cmu.ac.th
Total Article Views
Editor: Pornchai Rachtanapun,
Chiang Mai University, Thailand
Article history:
Received: May 31, 2022;
Revised: August 31, 2022;
Accepted: September 7, 2022;
Published online: September 14, 2022

