
Response Surface Methodology for Simple Non-Acid Ultrasonic-Assisted Extraction of Pectin from Taiwan’s Citrus depressa H. Peels
Wei-Jyun Chien, Dinar S. Saputri*, Sahri Yanti, and Dinesh Chandra AgrawalPublished Date : 2022-10-18
DOI : https://doi.org/10.12982/CMUJNS.2022.062
Journal Issues : Number 4, October-December 2022
Abstract Exploration of pectin from Citrus depressa H. peels (CD pectin) could add value to the waste arising from Citrus depressa H. beverage products. Preliminary analysis showed that temperature and acidity were insignificantly affect pectin yield, degree of esterification and DPPH scavenging activity. In order to optimize the ultrasonication time and solid: solvent ratio (SLR) of CD pectin extraction, response surface methodology central composite design (RSM-CCD) was used as the statistical tool. The optimum UAE conditions were: extraction time 39.96 minutes and solid: iquid ratio 1:20 (w/v). Under these conditions, the pectin yield was 16.07%, with 83.49% DPPH scavenging activity and 62.46% degree of esterification. The prediction model with a 0.922 desirability value was verified with adequate “lack of fit” values and confirmed consistent response in high and low prediction intervals (95%). Titration analysis, FTIR-ATR, NMR and XRD spectroscopy had been done to the dried pectin, and found that CD pectin is considered as high methoxylated (DE > 50%) with amorphous and crystalline structures. CD pectin peel also exhibited high DPPH scavenging activity (74.14 – 83.56%) that the food industry could potentially use as natural antioxidants.
Keywords: Ultrasonication, Pectin, Optimization, Structural, Functional
Citation: Chien, W-J., Saputri, D.S., Yanti, S., and Agrawal, D.C. 2022. Response surface methodology for simple non-acid ultrasonic-assisted extraction of pectin from taiwan’s citrus depressa H. peels. CMUJ. Nat. Sci. 21(4): e2022062.
INTRODUCTION
Citrus depressa Hayata is a popular citrus plant grown naturally in Japan and Taiwan (Shiu et al., 2016). The fruit is very sour and consumed fresh as a garnish or processed into seasoning, snacks, and juice. The textile industry also utilized the fruit as a stain remover and cleaner (Takenaka et al., 2007; Tamaki et al., 2008; Asikin et al., 2012; Yamamoto et al., 2017). Previous research reported that Citrus depressa H. contains high levels of health-promoting polymethoxy flavonoids such as nobiletin, tangeretin, sinensetin, and natsudaidain, which have antimutagenic, antiproliferative, anti-inflammatory, hypolipidemic, anti-obesity and anti-diabetic properties (Asikin et al., 2012; Upadhyay et al., 2014; Shiu et al., 2016; Chien et al., 2022).
Citrus is one of the most abundant fruits globally, with annual production exceeding 124 million tons. Approximately 70% of the worldwide production was processed for juice which led to a massive amount of peels, pulps, and seeds waste (Guo et al., 2017; Das and Arora, 2021; Liu et al., 2021). Aerobic bacteria cause environmental problems such as water pollution by decomposing the biodegradable organic matter from the waste into products such as carbon dioxide, nitrates, sulfates, and phosphates in water (Umaña et al., 2019; Das and Arora, 2021). However, these residues are rich in bioactive compounds such as phenolic, flavonoids, essential oils, pigments, and dietary fibers that could be valuable for the pharmaceutical, food, and cosmetics industries (Moorthy et al., 2015; S.-Y. Xu et al., 2018). Fruit by-product utilization to produce economically beneficial products promotes sustainable development and an eco-friendly environment (Mao et al., 2019; Picot-Allain et al., 2022). Mainly, citrus fruit pulps and peels are known as a good source of pectin (Tamaki et al., 2008; Rodsamran and Sothornvit, 2019).
Pectin is a native, complex and heterogeneous polysaccharide in the cell wall and middle lamellae of many land-growing plants such as fruits and vegetables. Pectin is a high molecular weight carbohydrate consisting of approximately 300–1000 units of α-(1,4) galacturonic acid backbone that is partially esterified with methyl alcohol or acetic acid (Xu et al., 2014; Adetunji et al., 2017; Freitas et al., 2020). The degree of esterification (DE) is one of the properties that determine the gelling nature of pectin. DE ranged from 56.74 to 84.72%, depending on the fruit type, maturation degree, extraction parameters, and extractors (Liew et al., 2014; Güzel and Akpınar, 2019). High methoxylated pectin (HMP) requires high sugar content and acidic condition for gel formation, while gelling in low methoxylated pectin (LMP) needs less sugar which is more preferred in the production of dietary products (Pereira et al., 2016).
The stabilizing, thickening and emulsifying properties are utilized in food industries such as fat replacer in spreads, ice cream, yoghurt, fruit juices, bakery glazing, meat products, and carbonated beverages. In non-food industries, pectin is utilized in cosmetics, personal care (paints, toothpaste, and shampoos), and pharmaceutical (gel caps) (Karbuz and Tugrul, 2021). Moreover, pectin was reported to exhibit several human health benefits, including anti-ulcer and cholesterol decreasing activity, wound healing, anti-tumor activity, antioxidant, prebiotic, improve the immune systems, and reducing lipase activity (Ciriminna et al., 2016; Naqash et al., 2017; Marić et al., 2018; Rahmani et al., 2020; Cui et al., 2021; Karbuz and Tugrul, 2021). The high availability, safety, low price, and functionality have put pectin in the biopolymers market, with annual demand approximately 40.000 tons worldwide (Valdés et al., 2015; Liu et al., 2021; Moslemi, 2021). The pectin market is expected to experience a Compound Annual Growth Rate of 7.6% during 2019 and 2026 due to its growing demand in diverse industries (Das and Arora, 2021).
Citrus and apple waste from juice manufacture are commonly used for commercial pectin production. Apple pomace contains 10-15%, while citrus peel contains 20-30% of pectin. Previous studies also reported several pectin sources such as apricot, cherries, eggplant, dragon fruit, and carrots (Srivastava and Malviya, 2011; Bayar et al., 2017; Nguyen and Pirak, 2019). Conventional commercial extraction in pectin production was using hot water (60 – 100°C) that was acidified to pH 1.5 – 3 with mineral or organic acid (sulfuric, phosphoric, nitric, hydrochloric, hydrochloric, or citric acid) for 0.5 – 6 hours. The process involved the disposal of acidic wastewater, which caused environmental problem. Furthermore, this process is time-consuming and leads to pectin degradation (Xu et al., 2014; Yang et al., 2018). Ultrasound-assisted extraction (UAE) is one of the environmentally friendly technologies to extract pectin from natural sources (Das and Arora, 2021). Adetunji et al. (2017) and Karbuz and Tugrul (2021) reported several UAE advantages in polysaccharide extraction, including shorter time, energy-saving, effective mixing, a lesser solvent used, reduced pectin degradation at high-temperature process, selective extraction, higher purity of the final product and increased production. Optimal operating conditions, such as temperature, pressure, particle size, moisture content, polarity, volatility and toxicity of the solvent, molecular weight, solid-liquid ratio are several factors to consider in choosing technologies for pectin extraction.
The current study aims to optimize the simple UAE method to extract pectin from Citrus depressa H. peels. Extraction time and sample/solvent ratio were the considered factors with pectin yield, degree of esterification, and DPPH scavenging activity of pectin as the response. The structural properties were also analyzed by spectroscopic studies.
Cell wall disruption by cavitation, particle size reduction, and mass transfer across cell membranes was the basic mechanism of ultrasound-assisted extraction. Cavitation bubbles were collapsed close to the cell walls could induce cell disruption and intensify solvent contact with the target compounds (Moorthy et al., 2015; Marić et al., 2018; Sabater et al., 2020).
MATERIALS AND METHODS
Materials
Citric acid (99%), hydrochloric acid (37%), sodium hydroxide (≥99%), DPPH (2,2-Diphenyl-1-Picrylhydrazyl), and standard galacturonic acid were bought from Merck Chemical Co. (Darmstadt, Germany), ethanol (96%) from Echo chemical (Taiwan), acetic acid, sodium acetate trihydrate, deuterium oxide from Sigma-Aldrich. Distilled water was purified using a Sartorius arium pro (Göttingen, Germany).
Methods
Citrus sample preparation
Citrus depressa Hayata from Pingtung region (North Taiwan) were purchased at ripe (yellow peels) and unripe (dark green peels) maturity stages. Distilled water were used to remove dirt, microflora, and pesticide residue on the fruits surface. Peels were separated manually and oven dried at 45°C until constant moisture content. Dried peels were pulverized by a domestic blender, sieved to 50 mesh, and stored in a container with desiccant until use.
Preliminary study of extraction parameters
Some factors in ultrasonication affect the yield and functional and chemical properties of pectin. The factors are pH, acid type, time, fruit ripeness, and sample: solvent ratio (SLR). Extraction was done using an ultrasonic water bath DC400H (CAE Tech. Co., Ltd.). The temperature during extraction was adjusted at 25°C with temperature control panel. Extraction times of 10 – 60 minutes, SLR of 1:10 to 1:100 g/mL, solvent pH, and acid type (citric acid and HCl) were also evaluated for extraction. The influence of each factor on the targeted yield in extracts was investigated using one-at-a-time method. Experiments were carried out in triplicate to ensure validity.
Extraction optimization using central composite design (CCD)
RSM is an experimental statistical technique applied for multiple regression analysis using quantitative data obtained from adequately designed experiments. Various parameters that influenced the extraction efficiency were optimized for the efficient extraction of pectin from Citrus depressa Hayata’s peels. After a preliminary study, time (X1) and SLR (X2) were selected as independent factors with pectin yield (%), DPPH scavenging activity (%), and degree of esterification (%) as the dependent factors to obtain the optimal extraction conditions by using central composite design. As shown in Table 1, the experimental design sets out the extraction time and SLR for testing with five center points and two replications.
Table 1. Independent variables and factors level of center composite design.
|
Independent variables |
Factors level |
||||
|
-1.41 |
-1 |
0 |
1 |
1.41 |
|
|
Time (min.) |
1.72 |
10 |
30 |
50 |
58.28 |
|
Sample:solvent |
0.04 |
0.05 |
0.08 |
0.1 |
0.11 |
The quadratic polynomial step-by-step regression method and data were analyzed using Design-Expert (version 13 trial, Stat-Ease, Inc., Minneapolis, MN, USA) software. The model given below was used to predict the response variables.
![]()
Statistical Analysis and Model Fitting
Analysis of variance (ANOVA) was utilized to evaluate the model that showed the interaction between process variables to the different responses: pectin yield, degree of esterification and DPPH scavenging activity. Software package Design-Expert ver. 13 (trial), Stat-Ease, Inc. (Minneapolis, USA) was used to generate regression analysis, develop equation on experimental parameters and the responses, and also to plot the contour and response surface graphs at the optimized condition. The outcome interaction between factors and responses were demonstrated in the three-dimensional surface plots and contours. The established model were validated through random extraction condition. Significance of the factors was computed at P-value less than 0.05, and insignificance of lack of fit tests in the same program. Furthermore, the suitability of the model to the data was evaluated by the determination of R2.
Pectin Extraction (Ultrasonic-assisted Extraction)
Dried peel powder was mixed with dH2O and ultrasonicated according to the designed variation parameters. After the extraction process, the solids were separated from the extracting solution by centrifugation at 6000 rpm for 10 minutes. Pectin precipitation was achieved with the addition of 98% ethanol to the liquid, with ethanol:solution ratio of 1:2. The solution is then left to precipitate overnight at 4°C. Precipitated pectin was recovered by centrifugation for 10 minutes at 6000 rpm. Isolated pectin pellets were dried at 40°C.
Pectin Characterization
Degree of esterification (DE)
Degree of esterification (%), as one of the most important properties of pectin, was determined for all pectin samples using the method described by Hosseini et al. (2019). Briefly, 100 mg of pectin sample was dissolved in 20 mL deionized water and 2 mL ethanol (96%). Adequate amount of phenolphthalein were added to the solution and titrated with NaOH solution (0.1 M) until the solution color changed to pink (V1). Afterward, 10 mL of NaOH solution (0.1M) was added, and the mixture was stirred for 20 min. HCl solution (10 mL, 0.1M) was added and stirred until the pink color disappeared. At last, the solution was again titrated with NaOH (0.1 M) (V2).
![]()
Equivalent weight (Ew)
Equivalent weights were analyzed using the method by Vellaisamy Singaram and Ganesan (2021). Briefly, 0.5 g dried pectin was moistened with 5 ml ethanol for determination EW in 250 ml conical flask. Adequate amount of phenol red indicator were added. Standardized NaOH (0.1N) was titrated slowly until the color of the solution changed to pink (pH 7.5) and kept for at least 30 seconds. The neutralized solution was used to determine methoxyl. The following equation was used to calculate the EW:
![]()
Methoxyl content (MeO)
The methoxyl (MeO) content was determined using the method by Vellaisamy Singaram and Ganesan (2021). Briefly add 25 ml of 0.1 N NaOH to the titrated solution from EW experiment, which was shaken thoroughly, and allowed to stand for 15 minutes at room temperature in the stoppered flask. 25 mL of 0.1 N HCl will be added and titrated to the same endpoint pink as before.
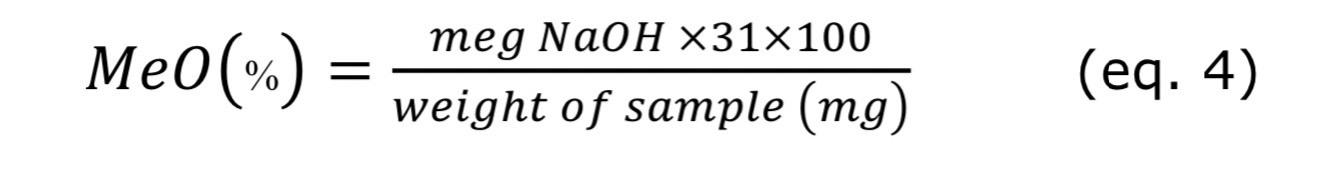
Where 31 is the molecular weight of the methoxyl group
Total anhydrouronic acid
The estimation of anhydrouronic acid (AUA) was also determined using the method by Vellaisamy Singaram and Ganesan (2021). This content was essential to determine the purity and degree of esterification (DE), by using EW and methoxyl content value. Total AUA of pectin was obtained by the following formula:
![]()
where molecular unit of AUA (1 U) = 176 g, z = ml of NaOH from equivalent weight determination, y = ml of NaOH from methoxyl content determination, w = weight of sample.
FTIR/ATR analysis
The FTIR spectra were recorded on a Perkin Elmer FTIR spectrometer equipped with a diamond crystal cell for attenuated total reflection (ATR) operation. The spectra were acquired in the range of 4000–600 cm-1 at 4 cm-1 resolution with 32 scans per sample. Spectra were corrected using background spectrum of air. The analysis was carried out at room temperature. Dried samples were placed on the surface of the ATR crystal for measurement. ATR crystal was regularly cleaned and checked spectrally before acquiring a spectrum from different samples.
NMR
The dried pectin was dissolved in D2O and analyzed by 1H NMR spectroscopy using the method by Kazemi et al. (2019) with modification. One dimensional proton NMR spectrum was recorded using Bruker AVANCE 500 MHz NMR spectrometer, BRUKER SpectroSpin 11.7 Tesla. The internal temperature was 298 °K, and 1024 scans were collected with a relaxation delay of 2 seconds. The pulse sequence used to suppress the solvent signal is p3919gp (O1 = 2347.88 Hz).
Pectin’s functional properties analysis
DPPH scavenging activity
Free radical-scavenging capacity of Citrus depressa Hayata pectin (CD pectin) was tested according to the reported method by Mokhtar et al. (2021). The maximum absorbance of color changing of the stable DPPH radical from deep violet to colorless were recorded by UV Vis spectrometer at 517 nm. A volume of 0.1 mL of the tested samples were added to 1 mL of DPPH. After incubation for 30 minutes in a dark room, the absorbance was measured at 517 nm. DPPH scavenging activity (%) was calculated by following equation:
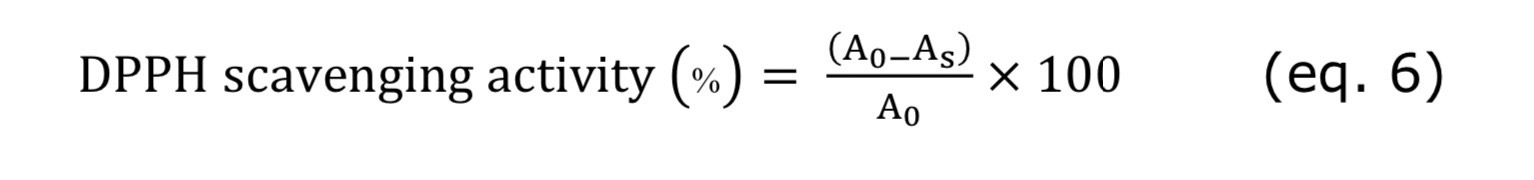
where A0 is the absorbance of the blank (methanol) and As is the absorbance of the sample.
XRD (x-ray diffraction) pattern analysis
The X-ray diffraction pattern of pectin was analyzed by the method by (Kazemi et al., 2019). The spectra were recorded using an X-ray diffractometer (PHILIPS, Amsterdam, Netherland). The pectin powder was scanned at a diffraction angle (2θ) from 10° to 65°. The step size and time per point were 0.05° (2θ) and 1 s, respectively.
Oil holding capacity (OHC) and water holding capacity (WHC)
OHC and WHC of CD pectin were determined using the method by Kazemi et al., (2019). In brief, 1 g of pectin was mixed into 10 ml of distilled water then the obtained mixture was vortexed vigorously for 1 min and centrifuged for 30 minutes at 6000 rpm. The supernatant was removed, and the remnants were weighted. The WHC was defined as gram of water held per gram of pectin. The OHC measurement was similar to WHC, except that corn oil (density of oil: 0.92 g/ml) was used instead of distilled water and OHC was defined as gram of oil held per gram of pectin.
RESULTS
Preliminary Study of Extraction Parameters
Preliminary experiments were conducted before optimization by response surface methodology. These factors should be evaluated to see which factor affects the most and set an appropriate range of variables for the extraction process. Factors that showed negative response to pectin yield, DPPH scavenging activity, and degree of esterification will be eliminated. In this current study of pectin extraction using ultrasonic bath, several evaluated factors were fruit ripeness, extraction time, SLR, and solvent acidity. Three replicates analyses were performed. SPSS version 16.0 for Windows (SPSS Inc., Chicago, IL) were utilized for the statistical analyses. Significant differences between groups of data were calculated by one-way ANOVA and Duncan’s post-test. Statistical significance consideration were obtained when p-value less than 0.05 (P < 0.05).
Effect of fruit ripeness and extraction time
The result showed a significant difference (α < 0.05) in pectin yield between peels from ripe fruit (yellow peels) and unripe fruit, where ripe peels contain 10 – 15% of pectin while unripe peels contain 6 – 8% (Figure 1).
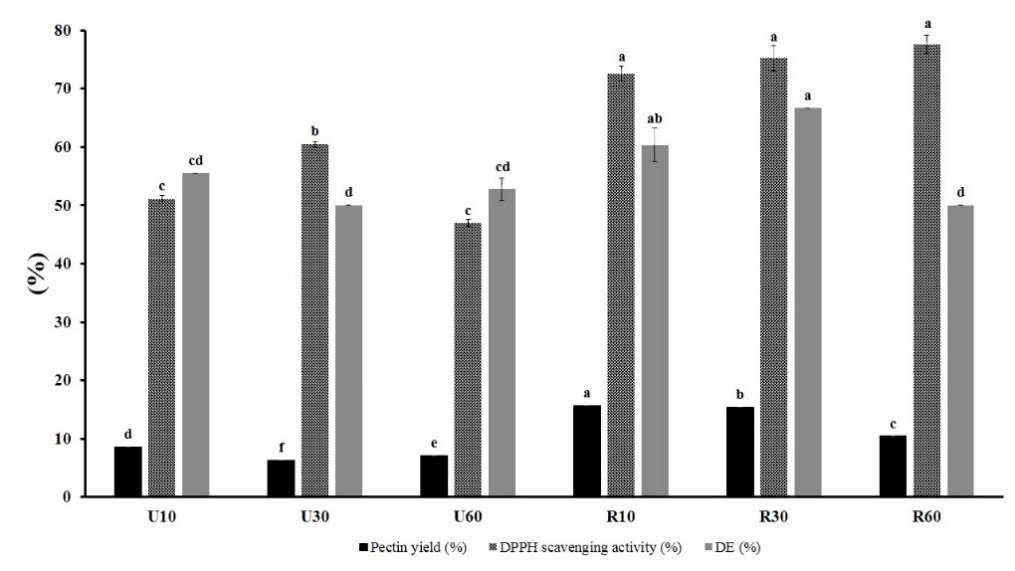
Figure 1. Evaluation of fruit ripeness and extraction time on pectin yield, DPPH scavenging activity, and degree of esterification (U: unripe peels; R: ripe peels; 10, 30, 60: time in minutes).
This result determined extraction times between 10 to 50 minutes for the optimization process. Moreover, since beverage products from Citrus depressa Hayata in Taiwan were mostly from ripe fruit that produces peels waste, ripe peels were selected as a sample for optimization analysis.
Effect of solvent ratio (SLR)
The result showed insignificant increase of pectin yield between SLR 1:10 and 1:20, but a significant decrease occurred at 1:30 and dropped more in 1:100 SLR (Figure 2).
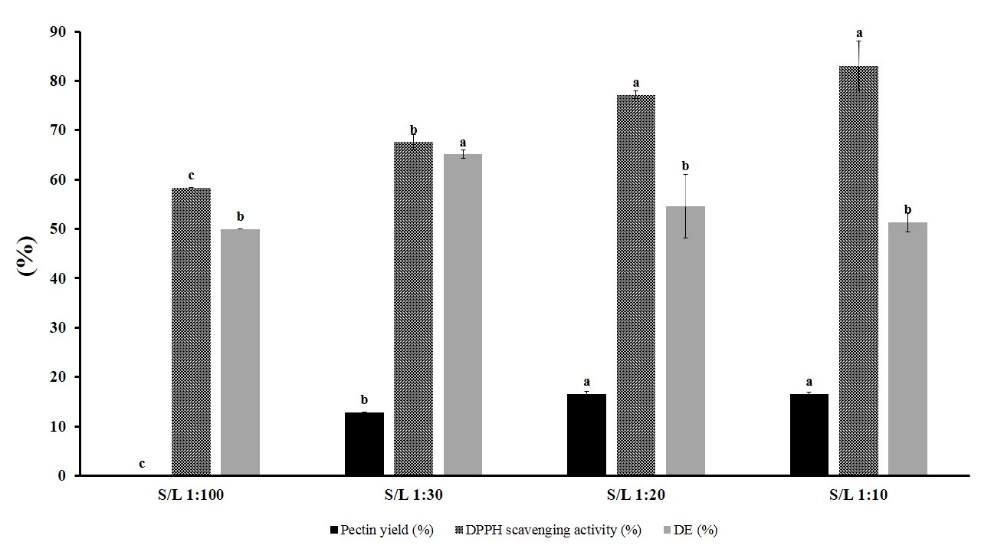
Figure 2. Evaluation of SLR on pectin yield, DPPH scavenging activity, and degree of esterification.
From this result, the selected SLR range for optimization analysis is 1:10 to 1:30.
Effect of solvent acidity
Current experiment was performed by ultrasonication for 28 minutes at room temperature with 1:20 SLR ratio. pH 1.8 was set by HCl and citric acid. The result showed an insignificant difference of pectin yield that were extracted with acid or neutral pH dH2O as solvent (Figure 3).
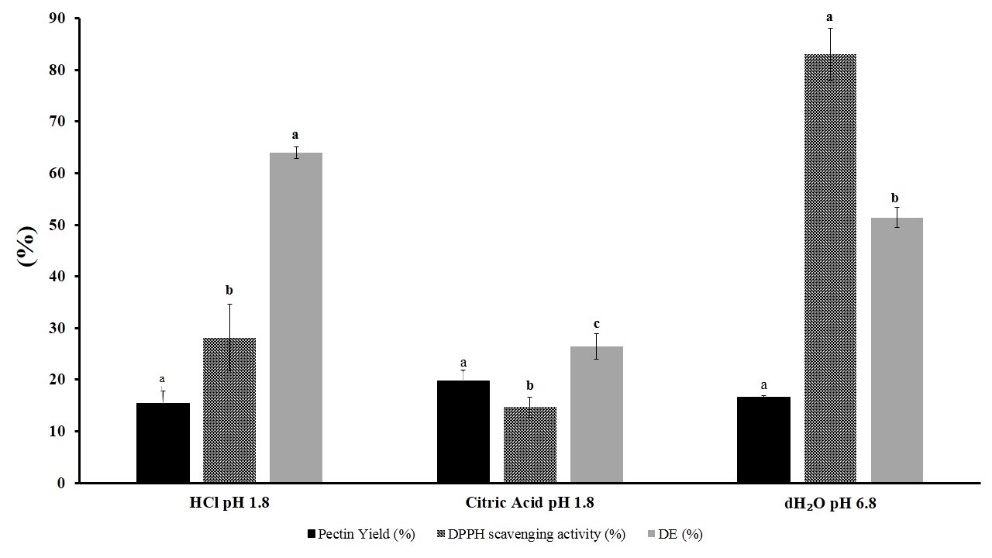
Figure 3. Evaluation of acidity on pectin yield, DPPH scavenging activity, and degree of esterification.
From this result, considering the insignificant difference in pectin yield, decreased DPPH scavenging activity, and the acid waste issue after using acid extraction, dH2O was selected for extraction optimization.
Model fitting and optimization process
Central-composite design (CCD) was utilized to optimize the production of pectin from Citrus depressa H. peels using ultrasonic-assisted extraction. The effect of extraction time (X1 = A, 10 – 50 minutes) and SLR (X2 = B, 0.05 – 0.1) on pectin yield, DPPH scavenging activity, and degree of esterification were evaluated. The experiment matrix and responses are shown in Table 2.
Table 2. Experimental design and response of pectin extraction using ultrasonic-assisted method.
|
No. |
Level |
Factor A |
Factor B |
Response 1 |
Response 2 |
Response 3 |
|
|
Time |
SLR |
Time(min.) |
SLR(W/V) |
Pectin Yield (%) |
DPPH Scavenging Activity(%) |
Degree of Esterification (%) |
|
|
1 |
-1.00 |
-1.00 |
10.00 |
0.05 |
11.40 |
78.91 |
59.33 |
|
2 |
-1.00 |
-1.00 |
10.00 |
0.05 |
12.01 |
78.81 |
59.67 |
|
3 |
0.00 |
-1.41 |
30.00 |
0.04 |
15.90 |
83.42 |
62.50 |
|
4 |
0.00 |
0.00 |
30.00 |
0.075 |
14.82 |
83.13 |
66.23 |
|
5 |
0.00 |
0.00 |
30.00 |
0.075 |
14.57 |
83.11 |
65.80 |
|
6 |
-1.00 |
1.00 |
10.00 |
0.10 |
12.19 |
77.70 |
62.44 |
|
7 |
1.00 |
1.00 |
50.00 |
0.10 |
13.57 |
80.11 |
58.27 |
|
8 |
0.00 |
0.00 |
30.00 |
0.075 |
13.52 |
82.93 |
65.21 |
|
9 |
0.00 |
0.00 |
30.00 |
0.075 |
12.72 |
83.23 |
66.33 |
|
10 |
-1.41 |
0.00 |
1.72 |
0.075 |
8.63 |
74.14 |
57.14 |
|
11 |
0.00 |
0.00 |
30.00 |
0.075 |
13.85 |
83.12 |
65.80 |
|
12 |
0.00 |
0.00 |
30.00 |
0.075 |
13.74 |
83.03 |
65.80 |
|
13 |
-1.41 |
0.00 |
1.72 |
0.075 |
8.05 |
74.14 |
57.33 |
|
14 |
0.00 |
1.41 |
30.00 |
0.11 |
14.53 |
81.45 |
64.57 |
|
15 |
0.00 |
0.00 |
30.00 |
0.075 |
14.00 |
82.88 |
65.80 |
|
16 |
1.41 |
0.00 |
58.28 |
0.075 |
14.05 |
78.84 |
55.00 |
|
17 |
1.41 |
0.00 |
58.28 |
0.075 |
13.54 |
78.76 |
54.78 |
|
18 |
0.00 |
0.00 |
30.00 |
0.075 |
13.89 |
83.03 |
66.00 |
|
19 |
0.00 |
0.00 |
30.00 |
0.075 |
14.00 |
83.05 |
65.11 |
|
20 |
1.00 |
-1.00 |
50.00 |
0.05 |
16.93 |
81.79 |
59.16 |
|
21 |
1.00 |
1.00 |
50.00 |
0.10 |
12.89 |
82.20 |
58.94 |
|
22 |
0.00 |
0.00 |
30.00 |
0.075 |
14.33 |
83.18 |
65.67 |
|
23 |
1.00 |
-1.00 |
50.00 |
0.05 |
15.15 |
82.20 |
58.77 |
|
24 |
-1.00 |
1.00 |
10.00 |
0.10 |
10.99 |
77.80 |
62.89 |
|
25 |
0.00 |
1.41 |
30.00 |
0.11 |
13.56 |
81.35 |
64.71 |
|
26 |
0.00 |
-1.41 |
30.00 |
0.04 |
16.21 |
83.56 |
61.00 |
Lack of fit test were used to verify the suitability of the model to fit the experimental data. The results of ANOVA (Table 3) for the models of extraction yield (eq. 7), DPPH scavenging activity (eq. 8), and degree of esterification (eq. 9) are listed in Table 2. The high F-value, high regression, and low P-value (< 0.001) for both factors and insignificant lack-of-fit indicated that the predicted models are significant on the respective response variables.
Table 3. ANOVA analysis of model for optimization of pectin extraction in Citrus depressa Hayata.
|
Source |
Sum of squares |
Df |
Mean |
F value |
P-value |
|
Pectin Yield (Y1) |
|||||
|
Model |
94.911 |
5 |
18.982 |
51.564 |
< 0.001 |
|
X1-time |
46.877 |
1 |
46.877 |
127.339 |
< 0.001 |
|
X2-SLR |
8.311 |
1 |
8.311 |
22.577 |
< 0.001 |
|
X1 X2 |
3.647 |
1 |
3.647 |
9.906 |
0.005 |
|
X1² |
27.945 |
1 |
27.945 |
75.911 |
< 0.001 |
|
X2² |
4.569 |
1 |
4.569 |
12.412 |
0.002 |
|
Residual |
7.363 |
20 |
0.368 |
|
|
|
Lack of Fit |
0.781 |
3 |
0.260 |
0.672 |
0.581 |
|
Total |
102.273 |
25 |
|
|
|
|
R² (model) |
0.928 |
|
|
|
|
|
DPPH (Y2) |
|||||
|
Model |
190.364 |
5 |
38.073 |
212.332 |
< 0.001 |
|
X1-time |
43.088 |
1 |
43.088 |
240.304 |
< 0.001 |
|
X2-SLR |
6.027 |
1 |
6.027 |
33.612 |
< 0.001 |
|
X1 X2 |
0.037 |
1 |
0.037 |
0.205 |
0.655 |
|
X1² |
140.518 |
1 |
140.518 |
783.673 |
< 0.001 |
|
X2² |
0.519 |
1 |
0.519 |
2.895 |
0.104 |
|
Residual |
3.586 |
20 |
0.179 |
|
|
|
Lack of Fit |
1.186 |
3 |
0.395 |
2.799 |
0.071 |
|
Total |
193.950 |
25 |
|
|
|
|
R² (model) |
0.982 |
|
|
|
|
|
DE (Y3) |
|||||
|
Model |
356.568 |
5 |
71.314 |
341.949 |
< 0.001 |
|
X1-time |
15.669 |
1 |
15.669 |
75.133 |
< 0.001 |
|
X2-SLR |
11.891 |
1 |
11.891 |
57.015 |
< 0.001 |
|
X1 X2 |
6.196 |
1 |
6.196 |
29.710 |
< 0.001 |
|
X1² |
317.889 |
1 |
317.889 |
1524.278 |
< 0.001 |
|
X2² |
20.481 |
1 |
20.481 |
98.205 |
< 0.001 |
|
Residual |
4.171 |
20 |
0.209 |
|
|
|
Lack of Fit |
1.191 |
3 |
0.397 |
2.265 |
0.118 |
|
Total |
360.739 |
25 |
|
|
|
|
R² (model) |
0.988 |
|
|
|
|
Effect of extraction time and solvent ratio on pectin yield
Response surface analysis of the experimental data demonstrates that both extraction time and SLR have quadratic effect on pectin yield ability with a good regression coefficient (R2 = 0.928), as shown in Table 2. The relationship between extraction factors and pectin yield is shown in eq. 7 as follows:
![]()
Figure 4 and the equation model showed that pectin yield was significantly (P < 0.01) affected by extraction period, while SLR shows a significant negative linear effect (P < 0.01).
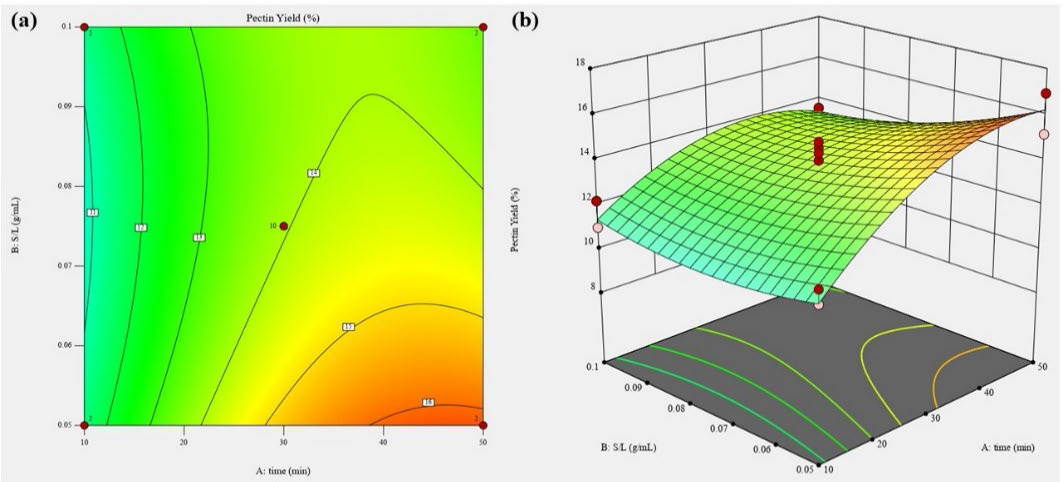
Figure 4. Contour plot (a) and response surface plot (b) of pectin yield as a function of extraction time and SLR
Effect of extraction factors on dpph scavenging activity
Response surface analysis of DPPH scavenging activity shown in Table 2 demonstrated that the relationship between DPPH scavenging activity and extraction parameters is quadratic with a good regression coefficient (R2 = 0.982), and eq. 8 shows the relationship as follows:
![]()
Equation model showed that extraction time and SLR both had significant linear effects (P < 0.01) and no significant interaction effect (P > 0.05) observed (Table 3). DPPH scavenging activity of 1 mg/mL Citrus depressa H. peels pectin ranged from 74.14 to 83.56%. The maximal DPPH scavenging activity predicted by response surface analysis was 83.69 %, with the optimum extraction time of 34.96 minutes and 1:20 SLR
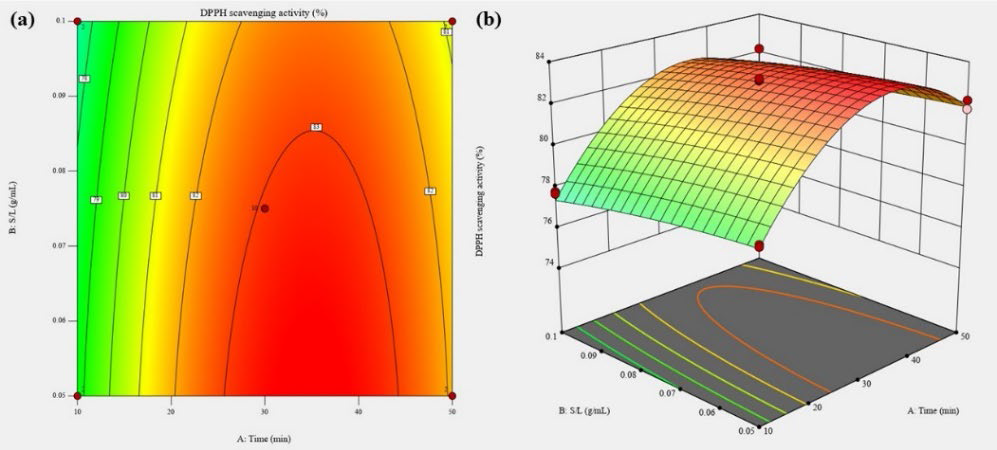
Figure 5. Contour plot (a) and response surface plot (b) of DPPH scavenging activity as a function of extraction time and SLR.
Effect of extraction factors on degree of esterification
Quadratic relationship with a good regression coefficient (R2=0.98) between extraction parameters and degree of esterification were obtained through the response surface analysis. The relationship were shown in Eq. 9 below:
![]()
Extraction time and SLR both exhibit significant negative quadratic effect (P < 0.01) on the DE value. Significant negative interaction effects between extraction time and SLR (P < 0.01) were also observed (Table 3).
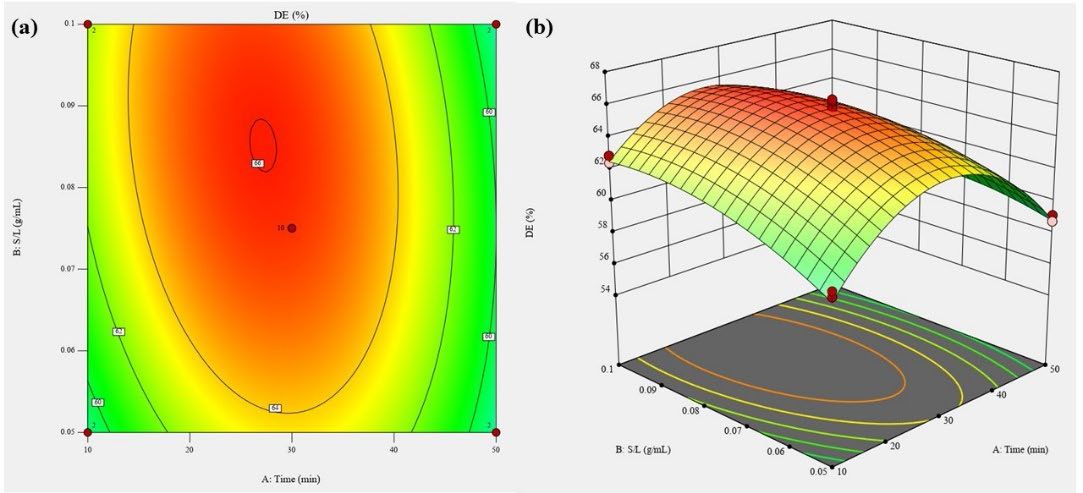
Figure 6. Contour plot (a) and response surface plot (b) of degree of esterification (DE) as a function of extraction time and SLR.
Figure 6 shows the contour and response surface plot of DE with the effect of extraction time and SLR variation.
Pectin Characterization
Equivalent weight, methoxyl content, and total anhydrouronic acid
Table 4 showed methoxyl content, equivalent weight, and anhydrouronic acid (AUA) content of Citrus depressa H. pectin extracted under optimum ultrasonication compared to commercial pectin and Citrus depressa H. pectin extracted using ultrasonication in citric acid pH 1.8.
Table 4. Chemical and functional properties of Citrus depressa H. pectin extracted under optimum ultrasonication conditions compared to commercial pectin.
|
|
UAE pectin |
Commercial pectin |
|
Methoxyl content (%) |
7.63 ± 1.53b |
5.51 ± 0.74a |
|
Equivalent weight (g) |
671.88 ± 2.41b |
629.17 ± 1.28a |
|
AUA (%) |
60.56 ± 1.24a |
66.59 ± 0.84b |
|
OHC (g/g) |
5.11 ± 1.12b |
2.17 ± 0.07a |
|
WHC (g/g) |
6.89 ± 0.55a |
9.72 ± 0.33b |
Current methoxyl content results showed that pectin from Citrus depressa H. extracted by ultrasonication is high methoxylated (> 7%), while commercial pectin resulted in low methoxylated pectin (< 7%).
Structural properties of Citrus depressa H. pectin
According to the FTIR spectrum in Figure 7, the broad characteristic peaks ranging from 3,000-3,500 cm−1 were due to stretching vibration of hydroxyl groups related to the galacturonic acid units (X. Yang et al., 2018; Y. Yang et al., 2018; Wathoni et al., 2019). The weak band between 2,850 and 3,000 cm-1 was attributed to CH vibrational modes of galacturonic acid units, including CH, CH2, and CH3 stretching and bending vibrations (Xu et al., 2018; Güzel and Akpınar, 2019; Rahmani et al., 2020). The signal at around 1,736 cm-1 was caused by the C=O stretching vibration of methyl esterified carboxyl groups (Wang et al., 2016; Umaña et al., 2019). While the absorption at around 1,600 cm-1 was caused by stretching vibration of the esterified and unesterified carboxyl groups (-COOR) of galacturonic acid polymer (Kazemi et al., 2019; Sabater et al., 2020). The peak region between 1,350 and 1,500 cm-1 represented aromatic amines, alkanes, the presence of a CH3 group, uronic acids, and other nitro compounds (Bayar et al., 2017; Muthusamy et al., 2019; Karbuz and Tugrul, 2021). The absorption band at 1233 cm-1 was from the cyclic C–C bond (-CH3CO stretching) in the ring structure of pectin (Pereira et al., 2016; Muñoz-Almagro et al., 2021). Intense peaks related to polygalacturonic acid as pectin polysaccharides characteristics appeared at 1223, 1156, 1099, 1024, and 962 cm-1, assigned to C=O, C-O, C-C, C-H stretching, and C-O bending, respectively (Rodsamran and Sothornvit, 2019). Furthermore, absorption peaks between 1,101 and 1,018 cm-1 indicate the potential presence of pyranose ring (X. Yang et al., 2018), while the characteristic bands at 919 to 829 cm-1 were attributed to the rocking mode of CH3 and CCH and COH bending at the C-6 position (Muñoz-Almagro et al., 2021).
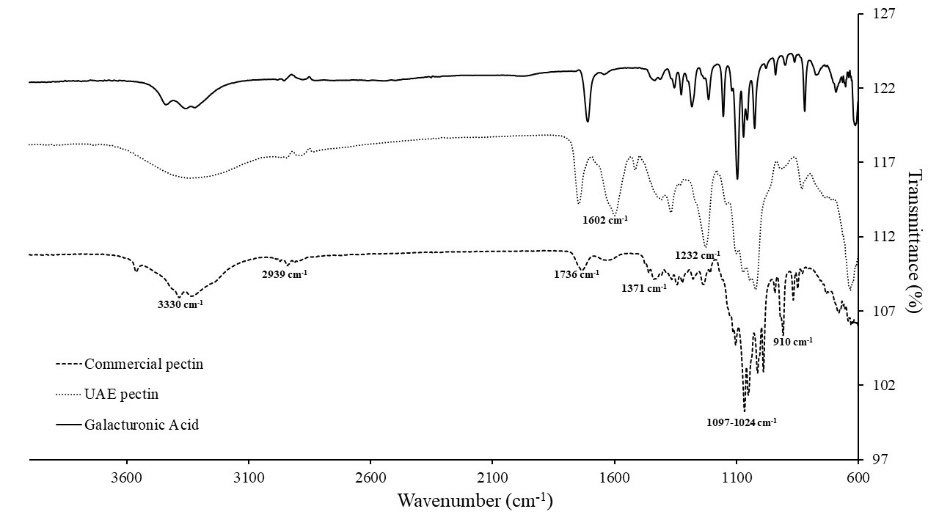
Figure 7. FTIR-ATR spectra of CD pectin compared to commercial pectin and galacturonic acid standard.
The 1H NMR spectrum of CD pectin compared to commercial citrus pectin is shown in Figure 8. In the case of CD pectin spectrum, signals appeared between 1 and 5.7 ppm, while commercial citrus pectin showed signals in the range of 3.2 to 5.4 ppm. Signals at around 1.1 to 1.2 ppm in the high-field region of the 1H NMR spectrum of CD pectin were certainly caused by the methyl group (C-6) (Wu et al., 2020) or methoxyl groups of O-2 and O-2,4 linked of rhamnose residues (Wang et al., 2016). Two strong characteristic signals at 3.7 and 2.1 ppm were derived from methyl and acetyl groups of the esterified galacturonic acid units in the structure of CD pectin. Signals of protons from arabinose residue were only observed in CD pectin at H-1 around 5.0 ppm, H-2 4.2 ppm, H-3 4.0 ppm, H-4 4.1 ppm, and H-5 3.8 ppm (Wang et al., 2016). Indication of multiple types of sugars in CD pectin was shown in proton signals at the regions between 4.9 – 5.7 ppm. Strong proton peaks at 5.02–5.71 ppm were only observed in CD pectin, indicating the presence of α-glycosidical linkage (Wu et al., 2020). Chemical shifts at 4.0–5.0 ppm indicate that the pectin contained β-glycosidical configuration (X. Yang et al., 2018). Both pectins exhibited typical distribution signals of polysaccharides at 3.0–5.3 ppm. Five major signals were assigned as the proton of C-1, C-2, C-3, C-4 and C-5 of the esterified D-galacturonic acid units. The signals for H-1 have appeared at 4.8 – 5.1 ppm; H-2, 3.4 – 3.8 ppm; H-3, 3.9 ppm; H-4, 4.1 – 4.4 ppm and H-5, 4.5 – 4.9 ppm (Tamaki et al., 2008; Wang et al., 2016; Kazemi et al., 2019;).
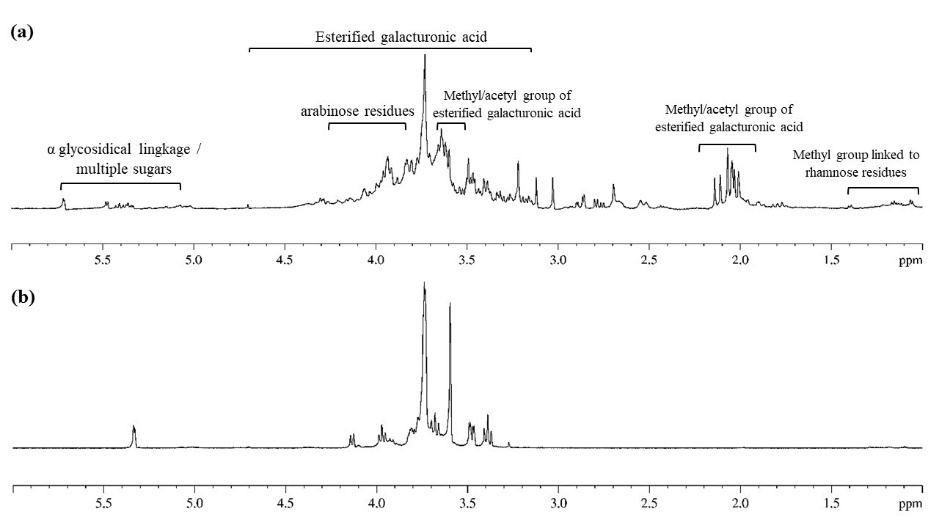
Figure 8. NMR spectra of pectin extracted under optimal time and SLR condition (a) and commercial citrus pectin (b)
The XRD patterns of CD pectin extracted under optimum conditions are shown in Figure 9.
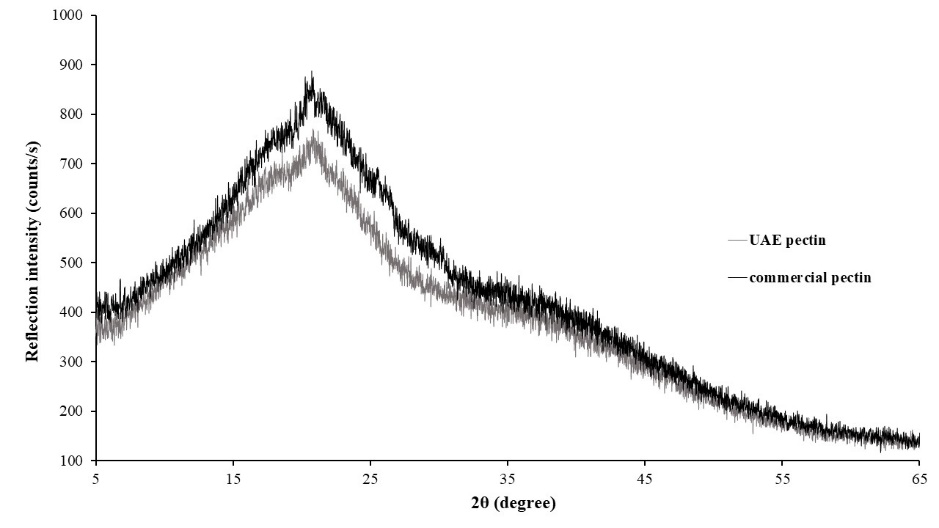
Figure 9. XRD diffractogram of CD pectin extracted under optimal time and SLR.
As can be seen, CD pectin showed several intense peaks at 16.06, 18.92, and 21.19o which represent some crystalline structure. Diffractogram pattern were similar compared to commercial pectin, only that commercial pectin showed intense peaks at 20.84, 25.59 and 30.3o. However, narrow diffraction peaks that represent amorphous structure also observed in both pectins spectrum.
Functional properties (WHC and OHC)
Water holding capacity (WHC) is the quantity of bound water per gram of sample, while oil holding capacity (OHC) is the amount of oil fixed by one gram of the sample after homogenization, incubation, and centrifugation. WHC and OHC values compared to commercial pectin were presented in Table 5. In this study, WHC of CD pectin extracted in optimum ultrasonic time and SLR is 6.89 ± 0.55 g water/g pectin which is lower than commercial citrus pectin (10.35 g/g) reported by (Asgari et al., 2020). This result is higher than several WHC of non-commercial pectin from different sources. OHC of CD pectin extracted in optimum ultrasonic time and SLR is 5.11 ± 1.12 g water/g pectin.
DISCUSSION
Preliminary study of extraction parameters
Effect of fruit ripeness and extraction time to the yield of pectin
Current result showed that mature Citrus depressa fruit peels contain higher amount of pectin than immature fruit peels. This result is consistent with a previous report on grapefruit pectin by Ferguson et al. (2021), where pectin yields on a dry weight basis of mature fruit (14.8%) were higher than immature fruit (12.9%). The galacturonic acid levels of fruit peel were increased due to the insoluble carbohydrates as fruit matures. Lekhuleni et al. (2021) also reported a higher pectin content in mature South African prickly pear than the immature fruit. However, Sirisakulwat et al. (2008) and Azad et al. (2014) researched on pectin from mango and lemon peels showed that pectin content were depend more on the plant source and extraction condition than fruit ripeness.
Pectin from ripe fruit peels showed higher DPPH scavenging activity than pectin from unripe peels. This result is in accordance to previous report by Chien et al. (2022) on antioxidant and phenolic compounds in Citrus depressa Hayata dried peels that showed unripe peels contain more polymethoxyflavonoid (PMF), which could decrease the antioxidant activity.
The degree of esterification showed insignificant difference between samples with different maturity (50 – 60%), which is considered as high methoxyl pectin. This result is consistent with several research reports on pectin’s functional properties, where extraction process and pectin source were more responsible to the value of DE (Ferguson et al., 2021; Lekhuleni et al., 2021; Sirisakulwat et al., 2008).
Furthermore, extraction time also affects the pectin yield and functional properties. Karbuz and Tugrul (2021) reported that the recovery of pectin was increased as the UAE time increased from 15 to 45 minutes. Ultrasound waves generate cavitational effect in the solvent medium that intensify the solvent penetration into the fruit peels. The release of pectin into the solvent were accelerated by the amount of waves and turbulent vibration that increase solvent-solid interfaces. However, cell wall disruption could release soluble and insoluble molecules that saturate the surrounding medium. Heating effect of ultrasonication caused modification and fragmentation of pectin’s structures that reduce pectin recovery (Muthusamy et al., 2019). Pectin yield produced from lemon peel using UAE in acidic solvent was 10.11%, while mandarin and kiwi peels were 11.29 and 17.30%, respectively (Karbuz and Tugrul, 2021).
Effect of solvent ratio (SLR) on pectin yield
The solvent quantity is also an important factor that affects pectin yield. SLR is a typical parameter that significantly influences the extraction efficiency. Current research evaluated 1:10, 1:20, 1:30 and 1:100 SLR for pectin extraction with dH2O as the solvent. The ultrasonication were performed for 28 minutes (Moorthy et al., 2015).
Lower SLR caused higher imposed ultrasound intensity to the average vegetal tissue which contribute to the fragmentation of raw material. Excessive material swelling caused by large solvent volume resulted in the disruption of cell wall, which resulted in the release of pectin to the medium. Polysaccharide concentration in the solution affect the extraction rate. Low concentration of raw material gives less protection to the dissolved pectin, facilitating pectin degradation, while high concentration also negatively affect the mass transfer and supress the transfer of desired compound to the solution that could reduce pectin yield (Prakash Maran et al., 2013; Xu et al., 2014; Saberian et al., 2018). DPPH scavenging activity increased with the increase of SLR. Meanwhile, the DE of high and low SLR showed insignificant difference.
Effect of solvent acidity
Since pectin is trapped in the protopectin, high concentration of hydrogen ions in low pH solvent is essential to spilt soluble pectin and cellulose. Pectin extraction is commonly performed in aqueous medium pH 1.5 – 3 at 75 – 100°C for 1 – 3 hours with continuous stirring (Xu et al., 2018).
Chemical extraction methods using mineral or organic acids are commonly used in pectin production. Hot acid method requires high production costs, and the disposal of acid wastewater can cause serious environmental problems (Yeoh et al., 2008; Adetunji et al., 2017; Yang et al., 2018; Belkheiri et al., 2021). Higher and neutral pH could cause pectin degradation and accumulate pectin that prevents its release from plant material (Adetunji et al., 2017; Lekhuleni et al., 2021).
However, the result showed an insignificant (α > 0.05) difference of pectin yield that were extracted with acid or neutral pH dH2O as solvent (Figure 3). This result was similar to the pectin extraction of orange peels in previous study by Yeoh et al., (2008). A report by He et al., (2021) in the optimization of pectin extraction from clementine peel stated that the effect of extraction pH was minimal at low temperatures, but it diverged widely at high temperatures. The neutral extraction condition also showed higher DPPH (82.93 ± 5.05%) scavenging activity than the acid condition (14.68 ± 1.94 – 28.14 ± 6.46%). Bayar et al. (2017) found a negative effect of pH in pectin extraction by ultrasound waves. Ultrasonication could affect the structure of protopectin, making it soluble in water even in slight acidic pH conditions.
Pectin extracted using HCl showed higher degree of esterification (DE) than citric acid and neutral pH. Kurita et al. (2008), Oliveira et al. (2016), Rodsamran and Sothornvit (2019); Sayah et al. (2016), and Yapo (2009) reported the opposite result where pectin that was extracted using organic acid (citric or acetic acid) showed higher DE value of pectin that was extracted by using mineral acid. Organic acids exhibit lower dissociation content that causes lower hydrolysis reaction, polymerization, and fragmentation ability than mineral acids. However, this result is consistent with the previous research on acid type effect to degree of esterification by Li et al. (2019) that showed the DE of sugar beet pulp pectin extracted using mineral acid was higher than DE of pectin extracted by organic acid. As a weak acid, citric acid could provide more protons to improve the hydrolysis of methyl ester than mineral acid under the same pH. (Yapo, 2009) stated that the degree of esterification of isolated pectin could be slightly overestimated because of the low acetyl groups in acid-extracted pectin from citrus peel.
Model fitting and optimization process
Temperature was excluded from the analyzed factors because, although it is considered as one of the most influential factors in pectin extraction, a report by Das and Arora (2021) showed that temperature combined with ultrasonication could induce the degradation of the compound.
According to the results, pectin yield extracted from Citrus depressa H. peels ranged from 8.05 to 16.93%, with DPPH scavenging activity from 74.14 to 83.56% and degree of esterification from 54.78 to 66.33%. Moreover, at the optimum extraction point (39.96 minutes of ultrasonication and 1:20 SLR), the pectin yield of 16.07% with 83.49% DPPH scavenging activity and 62.46% degree of esterification was achieved using the predicted model for Citrus depressa H. pectin extraction with 0.922 desirability.
The models were evaluated by three replicates on randomly selected time and SLR conditions (30 minutes and 1:20 SLR) to ensure that the prediction model for extraction process was in high accuracy. From the selected condition, obtained pectin yield, DPPH scavenging activity, and degree of esterification were 15.98 %, 82.89% and 62.36%. These results are in the range of 95 % predicted interval (PI) low and 95% PI high (Table 5), which means that the formula is consistent.
Table 5. Confirmation of predicted extraction parameters at the random point.
|
Response |
Predicted mean |
Data Mean |
95% PI Low |
95% PI High |
|
Pectin Yield (%) |
16.07 |
15.98 |
15.18 |
16.96 |
|
DPPH scavenging activity (%) |
83.49 |
82.89 |
82.86 |
84.11 |
|
DE (%) |
62.46 |
62.36 |
61.79 |
63.14 |
Effect of extraction factors on pectin yield
Current result shows that the pectin yield was increased by decreasing SLR and increasing extraction time. Extraction time and SLR also exhibit significant interaction effect (Table 3). The amount of pectin yield was the lowest when samples were ultrasonicated for 1.72 minutes (8.05 – 8.63%), but then increased to maximum at 50 minutes (16.93%) and decreased at further prolonging ultrasound time beyond 50 minutes. The maximum pectin yield predicted by response surface analysis was 16.24 %, with the optimum extraction time of 46.85 minutes and 1:20 SLR (Figure 4). Solvent penetration into the samples was increased through the cavitational effect of ultrasound waves. This phenomenon consists of the formation, growth, and implosion of gas nano/microbubbles into the liquid due to pressure fluctuations generated by the ultrasonic waves. The collapse of cavitation bubbles near the surface of the plant material increases the pressure and temperature, causing the deterioration of plant cell walls and enhanced solvent entrance into the cells, and intensify the mass transfer. Modification and fragmentation of the polysaccharides at prolonged ultrasonication time that leads to decreased pectin recovery, lack of energy distribution were the disadvantages of ultrasonication (Adetunji et al., 2017; Marić et al., 2018; Umaña et al., 2019; Karbuz and Tugrul, 2021). This finding was most likely because ultrasonic cavitation could accelerate the expansion and hydration of plant materials in the early stage of extraction. Furthermore, as solvent permeated into the substrate, the dissolution of pectin content from plant material was improved, resulting in more time for pectin mass transfer from solid particles into solution and thereby improving the extraction performance. (Kazemi et al., 2019).
Low SLR (1:20) showed more pectin yield than high SLR. The ratio between solid material to extractant solvent, known as liquid-solid (L/S) or solid-liquid ratio (S/L), is also important and should be optimized. SLR is typically represented in mass units per volume (g/ml) for most investigations. Increasing solvent percentage could improve the concentration gradient and reduce the viscosity of the solution which increase the contact surface area between plant matrix and solvent that generate higher extraction rate and pectin yield (Adetunji et al., 2017; Saberian et al., 2018). A lower sample concentration could dissolve more polysaccharide molecules in the solvent and decrease the yield because of the increasing degradation rate of polymers. Moreover, excessive SLR would require higher energy and cost more in wastewater disposal. However, when the ratio is less than 1:10, the amount of solvent is inadequate to dissolve and extract the pectin, and hydrolysis could occur due to solution over dilution (Adetunji et al., 2017; Belkheiri et al., 2021).
Reports on pectin extraction of Citrus depressa H. peels were rarely found. Tamaki et al. (2008) reported 4.1% polysaccharide yield from Citrus depressa H. endocarp extracted using hot acid method (pH 4.5, 85°C, 1 h). Pectin yield of Citrus depressa H. peels extracted by dH2O and ultrasonication in the current study were comparable to other reports on different citrus or other fruit peels pectin extraction that uses hot acid and longer extraction time. Ferguson et al. (2021) extracted 10.8 – 14.8% pectin from grapefruit peels using hot acid for 3 hours. Pasandide et al. (2017) extracted 5.94 – 20.00% pectin from Citrus medica using hot water (70 -90°C) for 1 – 3 hours. Y. Yang et al. (2018) were using hot nitric acid solution (pH 1.7, 86°C) for 80 minutes to extract 8.5% pectin from pomegranate peels. Commercial pectin is extracted from citrus (lemon, lime, orange) peels (85.5%), while a small proportion comes from apple pomace (14.0%) and sugar beet pulp (0.5%) (Picot-Allain et al., 2022). Pectin yield extracted from Citrullus alain using a microwave for 60 – 180 minutes in pH 1 – 1.5 were 13.09 – 24.18% (Prakash Maran et al., 2014).
Effect of extraction factors on DPPH scavenging activity
DPPH• free radicals (purple) would shift to the non-radical form (yellow) when scavenged by the samples (Bayar et al., 2017; Xu et al., 2018).
Figure 5 showed that DPPH scavenging activity of Citrus depressa H. peels pectin was increased at prolonged extraction time (30 to 40 minutes) yet decreased at higher SLR (1:10). This result is consistent to DPPH scavenging activity result of Citrus depressa H. peels extract reported by Chien et al. (2022) where the highest antioxidant activity were achieved by ultrasonication of samples from 30 to 40 minutes.
Current DPPH scavenging activity results of pectin from Citrus depressa H. are comparable to different samples such as DPPH scavenging activity of 1 mg/mL polysaccharides extracted from Citrus maxima Merr. (Wu et al., 2020), extract of jackfruit peels (Xu et al., 2018) showed 60 to 70% activity. Meanwhile, pectin from Opuntia ficus indica cladodes needs a higher concentration (20 mg/mL) to get 80% DPPH scavenging activity (Bayar et al., 2017). The free radical scavenging activities were probably generated by pectin structures or its interaction with phenolic compounds. Dietary fiber with different molecular weights might exhibit different antioxidant capacities. Ramification type such as carboxymethylcellulose, hydroxyethylcellulose, hydroxypropyl methylcellulose and methylcellulose of pectin also influences antioxidant capacity. High numbers of active hydroxyl and carboxyl groups on galacturonic acid residues of pectin are vitally important to its antioxidant activities. Phenolic compounds were found in the structure of pectin from the peels of dragon fruit and showed 81.91% antioxidant capacity. The type and amount of phenolic compounds present in the polysaccharide matrix influence the antioxidant capacity. Consequently, the dietary fiber protects the phenolic compounds from oxidation, favoring an excellent antioxidant capacity (Cui et al., 2019; Mercado-Mercado et al., 2020).
Effect of extraction factors on degree of esterification
The complex pectin molecules contain D-galacturonic acid linked by α-1,4 galacturonosyl linkages. This poly-galacturonic acid is interfered by L-rhamnose or xylose units as side chains. Carboxyl groups on the galacturonic acid backbone chain were esterified by methyl groups that cause the variation in the degree of methyl esterification (DE or DM). Pectin could be classified as high methyl-esterified (DM>50%) and low methyl-esterified (DM<50%). Moreover, the degree of methylation of the extracted pectin depends on the type of plant and degree of maturation (particularly for fruits). The degree of esterification of pectin is important to the functional properties in the plant cell wall and also affects its application as gelling and thickening agent (Pereira et al., 2016; Adetunji et al., 2017; Marić et al., 2018; S.-Y. Xu et al., 2018; Umaña et al., 2019; Belkheiri et al., 2021; Rahmani et al., 2020).
DE values of pectin from Citrus depressa H. extracted using ultrasonication ranged from 54.78 to 66.23%, which considered as high methoxylated pectin. The degree of esterification was observed to increase at moderate extraction time (30 minutes) and SLR (1:13). An increase of DE was observed with prolonged ultrasonication time from 20 to 30 minutes, and SLR below 1:14 decreases the DE value. The maximal DE predicted by response surface analysis was 64.79%, with the optimum extraction time of 26.87 minutes and 1:17 SLR.
The pectin extracted by ultrasound wave and microwave showed similar high levels of esterification (> 50.51%) that require both high concentration of sugar (usually >55%) and acidic conditions (pH < 3.5) to stabilize the hydrophobic interactions between methoxyl groups when used as gelling agent or stabilizer in food products such as dessert fillings, fruit juices, ice creams, yoghurt, and dairy drinks (Talekar et al., 2020; Karbuz and Tugrul, 2021; Ciriminna et al., 2022). The values of the DE of pectin extracted from lime and orange peel were in the range of 70 – 91.58% (Rodsamran and Sothornvit, 2019; Liu et al., 2021). Orange peels pectin extracted by varying green extraction methods showed 60 – 80% DE values (Benassi et al., 2021). Pectin mainly present in nature as high methoxylated pectin depends on the type and extraction condition. Extracted pectin from several other fruit peels also showed high DE values, such as mango peels (67%), cubiu fruit peels (62%), ponkan peels (85.7%), pomegranate peels (75%), citron peels (77%) and kiwi fruit (82 – 90%) (Umaña et al., 2019; Picot-Allain et al., 2022). Furthermore, (Karbuz and Tugrul, 2021) reported that ultrasonication did not have a significant effect (P > 0.05) on the DE value.
Pectin characterization
Equivalent weight, methoxyl content, and total anhydrouronic acid.
Methoxyl content represents the amount of moles of methyl alcohol in 100 mol galacturonic acid. Methoxyl content value determine the ability of pectin solution to form gels, sensitivity to metal ions and gel setting times. Higher methoxyl content increases the spreading quality and sugar-binding capacity of pectin. Methoxyl content of pectin varies from 0.2% to 14%, depending on the source and mode of extraction. Methoxyl content above 7% was considered as high methoxyl pectins (HMP) with degrees of esterification between 50 and 80%, while pectins with methoxyl contents below 7%, corresponding to a DE below 50%, are regarded as low methoxyl pectins (LMP) (Pereira et al., 2016; Nguyen and Pirak, 2019; Lekhuleni et al., 2021).
The low methoxyl content obtained may be attributed to the low pH and medium extraction power that depolymerized galacturonan chains into shorter polygalacturonic acid chains (Lekhuleni et al., 2021). Rodsamran and Sothornvit (2019) also reported high methoxyl content of pectin from lime peels. Meanwhile, pectin from sweet lemon peels, dragon fruit peels, and all colors of prickly pear fruit was grouped as low methoxyl pectin (Devi et al., 2014; Nguyen and Pirak, 2019; Lekhuleni et al., 2021).
Equivalent weight is the total content of non-esterified galacturonic acid in the pectin molecular chains. High equivalent weight would have high gel formation, whereas low equivalent weight would have low gel formation because the pectin would be highly degraded. Pectin with the equivalent or combining weight in the range of 250–350 will form the gel in the presence of calcium or magnesium ions regardless of the presence of sugar (Nguyen and Pirak, 2019; Lekhuleni et al., 2021). Current result showed a high equivalent weight (671.88 ± 2.41 g) in pectin extracted from Citrus depressa H. using optimized UAE compared to acid extracted (206.29 ± 1.77 g) and commercial pectin (629.17 ± 1.28 g). (Devi et al., 2014) reported equivalent weight value of pectin extracted from sweet lemon peel ranged from 312.5 to 833.33. (Altaf et al., 2015) also reported 455.1 to 912.17 equivalent weight of pectin from papaya.
Meanwhile, dragon fruit peels and all color prickly pear fruit showed low equivalent weight value (119.73 – 446.41 g) (Nguyen and Pirak, 2019; Lekhuleni et al., 2021). This parameter as reported in the literature varies widely depending on the method and the nature of the citrus fruits used for extraction. A high equivalent weight would have higher gel-forming effect, and the lower equivalent weight could be higher partial pectin degradation. The increased or decreased of the equivalent weight might be also dependent upon the amount of free acid (Nguyen and Pirak, 2019).
The total anhydrouronic (AUA) content determines the purity and DE. It also evaluates the physical characteristics of extracted pectin, and it should be not less than 65% as suggested by Food Chemicals Codex 2016. Generally, the AUA content obtained was less than 65% which points out that the pectin may not be adequately pure because of the existence of proteins, starch, and sugars in the precipitated pectins (Devi et al., 2014; Nguyen and Pirak, 2019; Lekhuleni et al., 2021). Since the anhydrouronic acid (AUA) content express the purity of the extracted pectin, AUA value not less than 65% were recommended for pectin utilization as food additives or pharmaceutical. This requirement has limited the potential sources of food and pharmaceutical pectins pectin (Khamsucharit et al., 2018). Current result showed AUA value of pectin from Citrus depressa H. was less than 65% (60.56 ± 1.24%) which means that the extracted pectin was less pure than commercial pectin (66.59 ± 0.84%). Similar results were also reported in previous pectin studies. The average AUA content for pectin extracted from orange, purple, and green prickly pear pectin was 25.58, 25.93, and 38.84% (Lekhuleni et al., 2021). Moreover, the AUA contents of different banana peel pectins ranged from 34.56 to 69.10 % (Khamsucharit et al., 2018). Ismail et al. (2012) reported AUA content of pectin from dragon fruit pectin were 45.3–52.2%.
Structural properties of Citrus depressa H. pectin
The structural features of Citrus depressa H. pectin extracted under optimal time and SLR conditions were indicated using FTIR, H-NMR, and XRD spectroscopy. The FTIR, NMR, and XRD spectra were compared to commercial pectin from citrus peels. In this part, FTIR, H-NMR, and XRD spectroscopies were used to illuminate the structural information of CD pectin.
FTIR-ATR spectra showed a similar spectra pattern between the samples and galacturonic acid, only that galacturonic acid and commercial pectin showed narrower peaks. This result indicates that the extracted pectin composed of galacturonic acid as the major compound that formed the pectin backbone, but not adequately pure.
NMR signal of CD pectin showed strong characteristic signals of esterified galacturonic acid. The higher intensity of these signals shows a higher degree of esterification in the pectin samples, which consistent to high degree of esterification result. The intensity of these peaks represents a higher amount of methyl esterified than acetyl esterified galacturonic acid units (X. Yang et al., 2018; Kazemi et al., 2019; Asgari et al., 2020; Rahmani et al., 2020). CD pectin also exhibit signal of rhamnose, arabinose, multiple sugar, α- and β-glycosidical configuration. This spectrum indicate that CD pectin is a galacturonic acid polymer comprising homogalacturonan, rhamnogalacturonan and arabinogalacturonan arranged in α- and β-glycosidical configuration. Commercial citrus pectin showed more simple NMR signals. De-esterification of carboxyl groups galacturonic acid contributed to the complexity of the NMR spectra. This result is similar to previous research by Tamaki et al. (2008) on pectin from the endocarp of Citrus depressa, where the signal at 2.1 ppm that represent acetyl groups were disappeared after de-esterification treatment.
XRD (X-ray diffraction) spectroscopy was applied to investigate the crystalline structure and assess the compatibility of CD pectin (Nisar et al., 2019; Asgari et al., 2020). XRD diffractograms showed a series of sharp peaks for crystalline material, while amorphous product produces a broad background pattern (Güzel and Akpınar, 2019). Previous studies reported that pectin has several characteristic XRD diffractogram peaks representing the pure pectin crystallinity at 12.70, 16.30, 18.40, 25.32, and 40.14o in the citrus sample (Nisar et al., 2019). Walnut pectin showed sharp peaks at 14.61, 17.19, 20.86, 32.36 and 36.66° of 2θ (Asgari et al., 2020), sweet lemon pectin at 12.36, 13.96, 14.91, 19.61, 18.91, 21.36, 32.46 and 36.66° (Rahmani et al., 2020) while sweet orange pectin showed peaks at 17, 18, 19, 21, 24, 29, 31 and 36° (Hosseini et al., 2019).
The obtained diffractogram pattern suggested both crystalline and amorphous structures for CD pectin. Pectin with crystalline and amorphous structures was similar to several reports on pectin from different resources (Güzel and Akpınar, 2019; Hosseini et al., 2019; Rahmani et al., 2020). XRD diffractogram of standard pectin reported by (Wathoni et al., 2019) showed an amorphous structure. (Kazemi et al., 2019) stated that raw material source and extraction procedure affect the crystallinity of pectin.
Functional properties
Water holding capacity is the tendency of powder to absorb and retain water. This property is crucial to select the proper treatment such as drying process and the selection of suitable packaging. The differences between these values could be due to various intrinsic factors such as pectin source, extraction process, chemical composition, and concentration of free hydroxyl groups in the pectin structure (Bayar et al., 2017; Asgari et al., 2020; Muñoz-Almagro et al., 2021).
Hosseini et al. (2019) reported 3.10 ± 0.12 g water/g pectin WHC value of sour orange peel pectin. Eggplant peel, pistachio green hull, Opuntia ficus, walnut, and chayote (Sechium edule) pectin which exhibited 6.22, 4.21, 4.84, 5.84, and 3.14 g water/g pectin (Bayar et al., 2017; Kazemi et al., 2019, 2020; Asgari et al., 2020; Ke et al., 2020). WHC could lock up moisture that improves textural properties, increase the bulk volume of foods, improve sensorial and textural properties effectively and avoid syneresis problems in some food products such as yogurt. Dietary fiber with high WHC could induce intestinal peristalsis and accelerate the release of toxins (Asgari et al., 2020; Kazemi et al., 2020; Ke et al., 2020; Huang
et al., 2021).
The OHC is a parameter having high importance in food industries and it can be influenced by various factors such as the overall surface charge density and the hydrophilic nature of constituents related to the chemical composition of compounds as well as their affinity to oil (Bayar et al., 2017; Hosseini et al., 2019; Kazemi et al., 2019; Asgari et al., 2020; Muñoz-Almagro et al., 2021).
The OHC value is in accordance to pectin sample from berries extracted using enzymatic (cellulose) and ultrasonication reported by Muñoz-Almagro et al., (2021) that showed OHC value in the range of 4.6 to 17.8 g oil/g pectin. Moreover, this result is significantly higher than commercial and non-commercial pectin reported in previous research. Sweet orange peel, Opuntia ficus indica, eggplant peel pectin, commercial apple pectin and commercial citrus pectin which exhibited 1.32, 1.01, 2.12, 2.22, 2.59 g oil/g pectin OHC (Bayar et al., 2017; Hosseini et al., 2019; Kazemi et al., 2019; Asgari et al., 2020). OHC is an important property and essential characteristic of hydrocolloids that can allow the stabilization of high-fat emulsions and food products (Asgari et al., 2020; Kazemi et al., 2020; Muñoz-Almagro et al., 2021).
CONCLUSION
The current study aims to optimize the simple ultrasonication method to extract pectin from Citrus depressa Hayata’s peels. The result showed that optimum time was 39.96 minutes with 1:20 SLR could extract comparable amount of pectin than other methods that use hot acid and longer extraction time. The prediction formula for time and SLR was also confirmed to be consistent. UAE pectin from Citrus depressa H. was high methoxylated pectin and exhibited DPPH scavenging activity. Profound research on the application and functional properties of CD pectin needs to be done.
ACKNOWLEDGMENTS
Authors would like to thanks to Chaoyang University of Technology for the research support.
AUTHOR CONTRIBUTIONS
- Wei-Jyun Chien: funding acquisition, supervision, resources, conceptualization, reviewing
- Dinar Suksmayu Saputri: conceptualization, methodology, data curation, formal analysis, writing-original draft
- Sahri Yanti: methodology, data curation
- Dinesh Chandra Agrawal: supervision, resources
All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Adetunji, L.R., Adekunle, A., Orsat, V., and Raghavan, V. 2017. Advances in the pectin production process using novel extraction techniques: A review. Food Hydrocolloids, 62: 239–250.
Altaf, U., Immanuel, G., and Iftikhar, F. 2015. Extraction and characterization of pectin derived from papaya (Carica papaya Linn.) peel. International Journal of Science, Engineering and Technology, 3: 970–974.
Asgari, K., Labbafi, M., Khodaiyan, F., Kazemi, M., and Hosseini, S.S. 2020. High-methylated pectin from walnut processing wastes as a potential resource: Ultrasound assisted extraction and physicochemical, structural and functional analysis. International Journal of Biological Macromolecules, 152: 1274–1282.
Asikin, Y., Taira, I., Inafuku-Teramoto, S., Sumi, H., Ohta, H., Takara, K., and Wada, K. 2012. The composition of volatile aroma components, flavanones, and polymethoxylated flavones in Shiikuwasha (Citrus depressa Hayata) peels of different cultivation lines. Journal of Agricultural and Food Chemistry, 60: 7973–7980.
Azad, A.K.M., Ali, M.A., Akter, Mst. S., Rahman, Md. J., and Ahmed, M. 2014. Isolation and characterization of pectin extracted from lemon pomace during ripening. Journal of Food and Nutrition Sciences, 2: 30.
Bayar, N., Bouallegue, T., Achour, M., Kriaa, M., Bougatef, A., and Kammoun, R. 2017. Ultrasonic extraction of pectin from Opuntia ficus indica cladodes after mucilage removal: Optimization of experimental conditions and evaluation of chemical and functional properties. Food Chemistry, 235: 275–282.
Belkheiri, A., Forouhar, A., Ursu, A.V., Dubessay, P., Pierre, G., Delattre, C., Djelveh, G., Abdelkafi, S., Hamdami, N., and Michaud, P. 2021. Extraction, characterization, and applications of pectins from plant by-products. Applied Sciences, 11: 6596.
Benassi, L., Alessandri, I., and Vassalini, I. 2021. Assessing green methods for pectin extraction from waste orange peels. Molecules, 26: 1766.
Chien, W.-J., Saputri, D.S., and Lin, H.-Y. 2022. Valorization of Taiwan’s Citrus depressa Hayata peels as a source of nobiletin and tangeretin using simple ultrasonic-assisted extraction. Current Research in Food Science, 5: 278–287.
Ciriminna, R., Fidalgo, A., Delisi, R., Ilharco, L.M., and Pagliaro, M. 2016. Pectin production and global market. Agro FOOD Industry Hi Tech, 27: 17–20.
Ciriminna, R., Fidalgo, A., Scurria, A., Ilharco, L.M., and Pagliaro, M. 2022. Pectin: New science and forthcoming applications of the most valued hydrocolloid. Food Hydrocolloids, 127: 107483.
Cui, M., Wu, D., Bao, K., Wen, Z., Hao, Y., and Luo, L. 2019. Dynamic changes of phenolic compounds during artificial aging of soybean seeds identified by high-performance liquid chromatography coupled with transcript analysis. Analytical and Bioanalytical Chemistry, 411: 091–3101.
Das, I., and Arora, A. 2021. Kinetics and mechanistic models of solid-liquid extraction of pectin using advance green techniques- a review. Food Hydrocolloids, 120: 106931.
Devi, W.E., Shukla, R.N., Bala, K.L., Kumar, A., Mishra, A.A., and Yadav, K.C. 2014. Extraction of pectin from citrus fruit peel and its utilization in preparation of jelly. International Journal of Engineering Research and Technology, 3: 1925–1932.
Ferguson, K., da Cruz, M.A., Ferrarezi, R., Dorado, C., Bai, J., and Cameron, R.G. 2021. Impact of Huanglongbing (HLB) on grapefruit pectin yield and quality during grapefruit maturation. Food Hydrocolloids, 113: 106553.
Freitas, C.M.P., Sousa, R.C.S., Dias, M.M.S., and Coimbra, J.S.R. 2020. Extraction of Pectin from Passion Fruit Peel. Food Engineering Reviews, 12: 460–472.
Guo, X., Zhao, W., Liao, X., Hu, X., Wu, J., and Wang, X. 2017. Extraction of pectin from the peels of pomelo by high-speed shearing homogenization and its characteristics. LWT - Food Science and Technology, 79: 640–646.
Güzel, M. and Akpınar, Ö. 2019. Valorisation of fruit by-products: Production characterization of pectins from fruit peels. Food and Bioproducts Processing, 115: 126–133.
He, C., Sampers, I., and Raes, K. 2021. Isolation of pectin from clementine peel: a new approach based on green extracting agents of citric acid/sodium citrate solutions. ACS Sustainable Chemistry and Engineering, 9: 833–843.
Hosseini, S.S., Khodaiyan, F., Kazemi, M., and Najari, Z. 2019. Optimization and characterization of pectin extracted from sour orange peel by ultrasound assisted method. International Journal of Biological Macromolecules, 125: 621–629.
Huang, J., Hu, Z., Hu, L., Li, G., Yao, Q., and Hu, Y. 2021. Pectin-based active packaging: A critical review on preparation, physical properties and novel application in food preservation. Trends in Food Science and Technology, 118: 167–178.
Ismail, N.S. Mohd., Ramli, N., Hani, N. Mohd., and Meon, Z. 2012. Extraction and characterization of pectin from dragon fruit (Hylocereus polyrhizus) using various extraction conditions. Sains Malaysiana, 41: 41–45.
Karbuz, P. and Tugrul, N. 2021. Microwave and ultrasound assisted extraction of pectin from various fruits peel. Journal of Food Science and Technology, 58: 641–650.
Kazemi, M., Khodaiyan, F., and Hosseini, S.S. 2019. Eggplant peel as a high potential source of high methylated pectin: Ultrasonic extraction optimization and characterization. LWT, 105: 182–189.
Kazemi, M., Khodaiyan, F., Labbafi, M., and Hosseini, S.S. 2020. Ultrasonic and heating extraction of pistachio by-product pectin: Physicochemical, structural characterization and functional measurement. Journal of Food Measurement and Characterization, 14: 679–693.
Ke, J., Jiang, G., Shen, G., Wu, H., Liu, Y., and Zhang, Z. 2020. Optimization, characterization and rheological behavior study of pectin extracted from chayote (Sechium edule) using ultrasound assisted method. International Journal of Biological Macromolecules, 147: 688–698.
Khamsucharit, P., Laohaphatanalert, K., Gavinlertvatana, P., Sriroth, K., and Sangseethong, K. 2018. Characterization of pectin extracted from banana peels of different varieties. Food Science and Biotechnology, 27: 623–629.
Kurita, O., Fujiwara, T., and Yamazaki, E. 2008. Characterization of the pectin extracted from citrus peel in the presence of citric acid. Carbohydrate Polymers, 74: 725–730.
Lekhuleni, I.L.G., Kgatla, T.E., Mashau, M.E., and Jideani, A.I.O. 2021. Physicochemical properties of South African prickly pear fruit and peel: Extraction and characterisation of pectin from the peel. Open Agriculture, 6: 178–191.
Li, J., Zhang, L., Li, J., and Li, D. 2019. Comparative studies of combined influence of variables on the esterification degree of pectin extracted by sulfuric acid and citric acid. Advances in Polymer Technology, 2019: 1–8.
Liew, S.Q., Chin, N.L., and Yusof, Y.A. 2014. Extraction and characterization of pectin from passion fruit peels. Agriculture and Agricultural Science Procedia, 2: 231–236.
Liu, N., Li, X., Zhao, P., Zhang, X., Qiao, O., Huang, L., Guo, L., and Gao, W. 2021. A review of chemical constituents and health-promoting effects of citrus peels. Food Chemistry, 365: 130585.
Mao, G., Wu, D., Wei, C., Tao, W., Ye, X., Linhardt, R.J., Orfila, C., and Chen, S. 2019. Reconsidering conventional and innovative methods for pectin extraction from fruit and vegetable waste: Targeting rhamnogalacturonan I. Trends in Food Science and Technology, 94: 65–78.
Marić, M., Grassino, A.N., Zhu, Z., Barba, F.J., Brnčić, M., and Rimac Brnčić, S. 2018. An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: Ultrasound-, microwaves-, and enzyme-assisted extraction. Trends in Food Science and Technology, 76: 28–37.
Mercado-Mercado, G., de la Rosa, L.A., and Alvarez-Parrilla, E. 2020. Effect of pectin on the interactions among phenolic compounds determined by antioxidant capacity. Journal of Molecular Structure, 1199: 126967.
Mokhtar, M., Bouamar, S., Di Lorenzo, A., Temporini, C., Daglia, M., and Riazi, A. 2021. The influence of ripeness on the phenolic content, antioxidant and antimicrobial activities of pumpkins (Cucurbita moschata duchesne). Molecules, 26: 3623.
Moorthy, I.G., Maran, J.P., Surya, S.M. @, Naganyashree, S., and Shivamathi, C. S. 2015. Response surface optimization of ultrasound assisted extraction of pectin from pomegranate peel. International Journal of Biological Macromolecules, 72: 1323–1328.
Moslemi, M. 2021. Reviewing the recent advances in application of pectin for technical and health promotion purposes: From laboratory to market. Carbohydrate Polymers, 254: 117324.
Muñoz-Almagro, N., Ruiz-Torralba, A., Méndez-Albiñana, P., Guerra-Hernández, E., García-Villanova, B., Moreno, R., Villamiel, M., and Montilla, A. 2021. Berry fruits as source of pectin: Conventional and non-conventional extraction techniques. International Journal of Biological Macromolecules, 186: 962–974.
Muthusamy, S., Manickam, L.P., Murugesan, V., Muthukumaran, C., and Pugazhendhi, A. 2019. Pectin extraction from Helianthus annuus (sunflower) heads using RSM and ANN modelling by a genetic algorithm approach. International Journal of Biological Macromolecules, 124: 750–758.
Naqash, F., Masoodi, F.A., Rather, S.A., Wani, S.M., and Gani, A. 2017. Emerging concepts in the nutraceutical and functional properties of pectin—A Review. Carbohydrate Polymers, 168: 227–239.
Nguyen, B.M.N. and Pirak, T. 2019. Physicochemical properties and antioxidant activities of white dragon fruit peel pectin extracted with conventional and ultrasound-assisted extraction. Cogent Food and Agriculture, 5: 1633076.
Nisar, T., Wang, Z.-C., Alim, A., Iqbal, M., Yang, X., Sun, L., and Guo, Y. 2019. Citrus pectin films enriched with thinned young apple polyphenols for potential use as bio-based active packaging. CyTA - Journal of Food, 17: 695–705.
Oliveira, T.Í.S., Rosa, M.F., Cavalcante, F.L., Pereira, P.H.F., Moates, G.K., Wellner, N., Mazzetto, S.E., Waldron, K.W., and Azeredo, H.M.C. 2016. Optimization of pectin extraction from banana peels with citric acid by using response surface methodology. Food Chemistry, 198: 113–118.
Pasandide, B., Khodaiyan, F., Mousavi, Z.E., and Hosseini, S.S. 2017. Optimization of aqueous pectin extraction from Citrus medica peel. Carbohydrate Polymers, 178: 27–33.
Pereira, P.H.F., Oliveira, T.Í.S., Rosa, M.F., Cavalcante, F.L., Moates, G.K., Wellner, N., Waldron, K.W., and Azeredo, H.M.C. (2016). Pectin extraction from pomegranate peels with citric acid. International Journal of Biological Macromolecules, 88: 373–379.
Picot-Allain, M.C.N., Ramasawmy, B., and Emmambux, M.N. 2022. Extraction, characterisation, and application of pectin from tropical and sub-tropical fruits: A review. Food Reviews International, 38: 282–312.
Prakash Maran, J., Sivakumar, V., Thirugnanasambandham, K., and Sridhar, R. 2013. Optimization of microwave assisted extraction of pectin from orange peel. Carbohydrate Polymers, 97: 703–709.
Prakash Maran, J., Sivakumar, V., Thirugnanasambandham, K., and Sridhar, R. 2014. Microwave assisted extraction of pectin from waste Citrullus lanatus fruit rinds. Carbohydrate Polymers, 101: 786–791.
Rahmani, Z., Khodaiyan, F., Kazemi, M., and Sharifan, A. 2020. Optimization of microwave-assisted extraction and structural characterization of pectin from sweet lemon peel. International Journal of Biological Macromolecules, 147: 1107–1115.
Rodsamran, P. and Sothornvit, R. 2019. Extraction of phenolic compounds from lime peel waste using ultrasonic-assisted and microwave-assisted extractions. Food Bioscience, 28: 66–73.
Sabater, C., Sabater, V., Olano, A., Montilla, A., and Corzo, N. 2020. Ultrasound-assisted extraction of pectin from artichoke by-products. An artificial neural network approach to pectin characterisation. Food Hydrocolloids, 98: 105238.
Saberian, H., Hamidi‐Esfahani, Z., Ahmadi Gavlighi, H., Banakar, A., and Barzegar, M. 2018. The potential of ohmic heating for pectin extraction from orange waste. Journal of Food Processing and Preservation, 42: e13458.
Sayah, M. Y., Chabir, R., Benyahia, H., Rodi Kandri, Y., Ouazzani Chahdi, F., Touzani, H., and Errachidi, F. 2016. Yield, esterification degree and molecular weight evaluation of pectins isolated from orange and grapefruit peels under different conditions. PLoS One, 11: e0161751.
Shiu, Y.-L., Lin, H.-L., Chi, C.-C., Yeh, S.-P., and Liu, C.-H. 2016. Effects of hirami lemon, Citrus depressa Hayata, leaf meal in diets on the immune response and disease resistance of juvenile barramundi, Lates calcarifer (Bloch), against Aeromonas hydrophila. Fish and Shellfish Immunology, 55: 332–338.
Sirisakulwat, S., Nagel, A., Sruamsiri, P., Carle, R., and Neidhart, S. (2008). Yield and quality of pectins extractable from the peels of thai mango cultivars depending on fruit ripeness. Journal of Agricultural and Food Chemistry, 56: 10727–10738.
Srivastava, P. and Malviya, R. 2011. Sources of pectin, extraction and its applications in pharmaceutical industry − An overview. Indian Journal of Natural Products and Resources, 2: 10–18.
Takenaka, M., Nanayama, K., Isobe, S., Ozaki, K., Miyagi, K., Sumi, H., Toume, Y., Morine, S., and Ohta, H. 2007. Effect of extraction method on yield and quality of Citrus depressa juice. Food Science and Technology Research, 13: 281–285.
Talekar, S., Vijayraghavan, R., Arora, A., and Patti, A.F. 2020. Greener production of low methoxyl pectin via recyclable enzymatic de-esterification using pectin methylesterase cross-linked enzyme aggregates captured from citrus peels. Food Hydrocolloids, 108, 105786.
Tamaki, Y., Konishi, T., Fukuta, M., and Tako, M. 2008. Isolation and structural characterisation of pectin from endocarp of Citrus depressa. Food Chemistry, 107: 352–361.
Umaña, M.M., Dalmau, M.E., Eim, V.S., Femenia, A., and Rosselló, C. 2019. Effects of acoustic power and pH on pectin‐enriched extracts obtained from citrus by‐products. Modelling of the extraction process. Journal of the Science of Food and Agriculture, 99: 6893–6902.
Upadhyay, A., Tuenter, E., Amin, A., Exarchou, V., Hermans, N., Apers, S., and Pieters, L. 2014. 5-O-Demethylnobiletin, a polymethoxylated flavonoid, from Citrus depressa Hayata peel prevents protein glycation. Journal of Functional Foods, 11: 243–249.
Valdés, A., Burgos, N., Jiménez, A., and Garrigós, M. 2015. Natural pectin polysaccharides as edible coatings. Coatings, 5: 865–886.
Vellaisamy Singaram, A. J. and Ganesan, N. D. 2021. Modeling the influence of extraction parameters on the yield and chemical characteristics of microwave extracted mango (Mangifera indica L.) peel pectin by response surface methodology. Preparative Biochemistry and Biotechnology, 1–13.
Wang, W., Ma, X., Jiang, P., Hu, L., Zhi, Z., Chen, J., Ding, T., Ye, X., and Liu, D. 2016. Characterization of pectin from grapefruit peel: A comparison of ultrasound-assisted and conventional heating extractions. Food Hydrocolloids, 61: 730–739.
Wathoni, N., Yuan Shan, C., Yi Shan, W., Rostinawati, T., Indradi, R. B., Pratiwi, R., and Muchtaridi, M. 2019. Characterization and antioxidant activity of pectin from Indonesian mangosteen (Garcinia mangostana L.) rind. Heliyon, 5: e02299.
Wu, Z., Li, H., Luo, Y., Chen, G., Li, J., Wang, Y., Yang, Y., and Tan, H. 2020. Insights into the structural characterisations, bioactivities and their correlations with water‐soluble polysaccharides extracted from different pomelo (Citrus maxima Merr.) tissues. International Journal of Food Science and Technology, 55: 3091–3103.
Xu, S.-Y., Liu, J.-P., Huang, X., Du, L.-P., Shi, F.-L., Dong, R., Huang, X.-T., Zheng, K., Liu, Y., and Cheong, K.-L. 2018. Ultrasonic-microwave assisted extraction, characterization and biological activity of pectin from jackfruit peel. LWT, 90: 577–582.
Xu, Y., Zhang, L., Bailina, Y., Ge, Z., Ding, T., Ye, X., and Liu, D. 2014. Effects of ultrasound and/or heating on the extraction of pectin from grapefruit peel. Journal of Food Engineering, 126: 72–81.
Yamamoto, M., Takakura, A., Tanabe, A., Teramoto, S., and Kita, M. 2017. Diversity of Citrus depressa Hayata (Shiikuwasha) revealed by DNA analysis. Genetic Resources and Crop Evolution, 64: 805–814.
Yang, X., Nisar, T., Hou, Y., Gou, X., Sun, L., and Guo, Y. 2018. Pomegranate peel pectin can be used as an effective emulsifier. Food Hydrocolloids, 85: 30–38.
Yang, Y., Wang, Z., Hu, D., Xiao, K., and Wu, J.-Y. 2018. Efficient extraction of pectin from sisal waste by combined enzymatic and ultrasonic process. Food Hydrocolloids. 79: 189–196.
Yapo, B. M. 2009. Biochemical characteristics and gelling capacity of pectin from yellow passion fruit rind as affected by acid extractant nature. Journal of Agricultural and Food Chemistry. 57: 1572–1578.
Yeoh, S., Shi, J., and Langrish, T.A.G. 2008. Comparisons between different techniques for water-based extraction of pectin from orange peels. Desalination. 218: 229–237.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand.
https://cmuj.cmu.ac.th
Wei-Jyun Chien, Dinar S. Saputri*, Sahri Yanti, and Dinesh Chandra Agrawal
Department of Applied Chemistry, Chaoyang University of Technology, Wufeng District, Taichung, 41349 Taiwan, R.O.C.
Corresponding author: Dinar S. Saputri, E-mail: s10832905@gm.cyut.edu.tw
Total Article Views
Editor: Pornchai Rachtanapun,
Chiang Mai University, Thailand
Article history:
Received: May 31, 2022;
Revised: August 31, 2022;
Accepted: September 30, 2022;
Published online: September 12, 2022

