
The Capacity of Heavy Metal Remediation by Cyperus alternifolius, Chrysopogon zizanioides (L.) Roberty, and Aloe vera (L.) Burm.f. under Industrial and Urban Wastewater Treatment
Sareh Ebrahimi Nokande, Seyed Mehdi Razavi* and Mansour Afshar MohammadianPublished Date : 2022-10-18
DOI : https://doi.org/10.12982/CMUJNS.2022.057
Journal Issues : Number 4, October-December 2022
Abstract Urban wastewater (UWW) and industrial wastewater (IWW) release lots of heavy metals into the environment, leading to various risks regarding agricultural production, food quality, human and animal health, and environmental safety. Phytoremediation is known as one of the best and most affordable strategies to eliminate or reduce heavy metals in different environments. This study examined the phytoremediation ability of three plant species (Cyperus alternifolius, Chrysopogon zizanioides (L.) Roberty, and Aloe vera (L.) Burm.f) to absorb heavy metals of Zn, Cr, Pb, Cu, Mn, Ni, and Mg from the contaminated soil with UWW and IWW for 14 months. The result showed that all three examined plants reduced heavy metal pollution of the soil using the phytostabilization method. The metal accumulation index (MAI) of the root in V. zizanioides was higher than C. alternifolius and A. vera. While MAI of the shoot in A. vera was higher than C. zizanioides and C. alternifolius. Based on the results, it is suggested to cultivate C. zizanioides and A. vera in the polluted areas to decrease the amount of soil contaminants and create a green belt.
Keywords: Heavy metal, Industrial and urban wastewater, Metal accumulation index, Phytoremediation, Phytostabilization
Funding: The authors are grateful for the research funding provided by the Faculty of Sciences, University of Mohaghegh Ardabili, Iran.
Citation: Nokande, S.E., Razavi, S.M., and Mohammadian, M.A. 2022. The capacity of heavy metal remediation by Cyperus alternifolius, Chrysopogon zizanioides (L.) Roberty, and Aloe vera (L.) Burm.f. under industrial and urban wastewater treatment. CMUJ. Nat. Sci. 21(4): e2022057.
INTRODUCTION
Increasing the levels of pollution caused by heavy metals is causing concern worldwide. Human activities such as the disposal of households waste, hazardous solid waste disposal, industrial activities, mining, fertilizers application, herbicides, pesticides, insecticides, the use of wastewater for irrigation, coal combustion residues, spillage of chemical or petrochemicals, atmospheric deposition, etc. mainly import heavy metals to the soil (European Commission, 2013; EPA, 2021). Any naturally occurring metal or metalloid with an atomic number of 20 or greater and an elemental density of more than 5 grams per cubic centimeter is considered heavy metal. Usually, these metals are highly persistent and are not biodegradable (Sarkar, 2002; Ali et al., 2013). Heavy metal pollution of soil can harm human life and the ecosystem through direct intake or contact with contaminated soil, drinking of contaminated water, and entering the food chain (soil-plant-human or soil-plant-animal-human). As a result of phytotoxicity with heavy metals, agricultural production ability and food quality (safety and marketability) are reduced which leads to food insecurity. There will be longterm consequences for human health when heavy metals enter the food chain, including skin, liver, renal, and bone damage, cardiovascular and gastrointestinal, neurological diseases, cancer, and reproductive and developmental problems (Ling et al., 2008; European Commission, 2013).
To remove or reduce the environmental pollutants, phytoremediation is regarded as a biological method employed by some plants to remove, stabilize and transfer contaminants in the soil or groundwater. There are four main types of phytoremediation including phytostabilization, phytoextraction, rhizodegradation, and phytodegradation (Gajic and Pavlovic, 2018). Although common methods for cleaning contaminated soil, such as ion exchange and ultra-filtration have proven to be efficient, they may not be economically feasible because of their relatively high cost, particularly when used for the removal of heavy metals at low concentrations (Torresday et al., 2005). Phytoremediation technology does not require high-tech equipment or materials and can operate in all media (aquatic sediment, soil, and atmosphere), and uses solar energy. Moreover, different types of many contaminations can be treated at one time and aesthetically is green. Using phytoremediation is a promising technique for separating pollutants and removing them from the environment at a low cost and aside from being environmentally friendly, the procedure can be done in situ and soil can be used promptly after treatment and following harvest, the metal-rich parts can be easily and safely processed by incinerating, smelting or composting (Pilon Smits, 2005; Dordio and Carvalho, 2013). A majority of phytoremediation studies have been conducted on only a single type of contaminant element, without taking into account the impact of other elements (Ng et al., 2020). Also heavy metals in industrial or urban solid wastes have been studied in terms of diversity, concentration, fractionation, and release by environmental scientists (Allue, 2014; Mohammad Hosseini et al., 2018; Taghipour and Jalali, 2019). Hence, it is necessary to study the content of heavy metals in wastewaters in any given area and also compare plants that can grow in polluted environments. Also, studying the ability of plants which considerably absorb heavy metals will help us to introduce suitable plants for phytoremediation in the polluted area.
The province of Guilan, located in the north of Iran, has a humid subtropical climate and is a fertile province in terms of agriculture. Urban population and industrial factories are increasing day by day in this province, and urban and industrial waste does not have a suitable place to dump so the leakage flows directly into the river or land fields. This problem is dangerous for the agricultural products of Guilan province and has created an important challenge. It seems that using plant with high phytoremediation ability and creating a green belt in two places, including the landfill site of urban wastewater of Saravan and the discharge site of wastewater of paper production factory, is the most feasible and low-cost method in situ. This method can decontaminate and beautify the polluted area in addition to stabilizing the lands against wind and water erosion. Considering the mentioned factors, in this study, we examined three plant species as candidates for phytoremediation, including Cyperus alternifolius from the family of Cyperaceae and Chrysopogon zizanioides (L.) Roberty from the family of Poaceae, and Aloe vera (L.) Burm.f. from the family of Asphodelaceae to remove heavy metals from the contaminated soil. All the three examined plants are evergreen with a relatively high environmental adaptability. They are easily found in different areas including Guilan province with no risk of livestock poisoning or invasion problem. These three plants have the ability to produce high biomass and are tolerant to adverse climatic conditions, and to grow well in the environmental and climatic conditions of Guilan province. On the other hand, the study of these three plants has not been done for soil remediation in wastewaters in Guilan province. The purposes of this study were to determine the heavy metals accumulation capacity of examined plants and to choose the most suitable plant to create a green belt, and compare these plants' potential to ad/absorb and remove heavy metals including Cr, Pb, Ni, Cu, Mn, Zn, and Mg from the soil treated with two wastewaters including urban wastewater (UWW) and industrial wastewater (IWW). IWW was used from the discharge site of wastewater of the paper production factory, and UWW from the landfill site of Saravan in Guilan province, Iran.
MATERIALS AND METHODS
Soil and wastewater
For this study, soil samples were collected at 0-30 cm depth from the Faculty of Science, the University of Guilan, Iran, located in latitude 37°15’43.49″ N, longitude 49°35’10.52″ E, and mixed with sand (3:1), and analyzed for Physico-chemical characteristics before planting. The analysis of the soil showed that the physical and chemical characteristics of primary soil (PS) consisted of Clay 14%, Sand 64%, Silt 22% (sandy loamy soil), organic carbon 3.19%, total N 0.28%, P 13.5, and K 131 (mg kg-1). The pH of PS was 6.64, and EC was 0.88 dSm-1. Total nitrogen and phosphorous content of the soil were examined using the method of Bremner and Mulvaney (1982) and Olsen et al., (1954) method (Cary 60 UV Spectrophotometer), respectively. Potassium and organic carbon content of the soil were measured using the methods of Richards (1954) (Corning Flame Photometer 410) and Walkley and Black (1934), respectively. Electrical conductivity (EC) and pH of the soil were measured using a conductivity meter (Metrohm Conductometer) and pH meter, respectively (Richards, 1954; Uba et al., 2009). Soil samples were dried in an oven at 30°C for 24 h and were then passed through a 2 mm sieve before analysis (Uba et al., 2009). 1 g of soil sample was digested with concentrated HCl (37%) and concentrated HNO3 (65%) in a ratio of 3:1. The mixture was left overnight in the digestion block without heating under the switch-on fume cupboard. The following day, this solution was heated for 15 min to 150°C (a gradual increase in temperature was used to prevent foaming). Then mixture was placed at room temperature to cool (the lid of the container was closed in all stages). Then it was filtered through a Whatman No.54 filter paper and made up to volume (100 mL) with deionized water in a volumetric flask (Salt et al., 1998; AL-Oud Saud, 2003; Subhashini et al., 2015). Then the clear solution was analyzed for heavy metals Zn, Pb, Cu, Cr, Mn, Ni, and Mg by Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES)- Model (ARCOS, Spectro, Germany).
Two types of wastewater were used for plant treatment including IWW from the discharge site of wastewater of a paper production factory, and UWW from a landfill site of urban wastewater in Guilan province, Iran. The discharge site of wastewater of the paper production factory is located in (latitude 37°33’03.21″ N, longitude 49°06’48.15″ E), and the Saravan landfill is located in (latitude 37°04’24.68″ N, longitude 49°37’43.68″ E).
Wastewaters were collected once a month and stored in special tanks in a shady place away from the sun and heat, and before treatment, the wastewater were stirred carefully using a plastic rod.
Plant cultivation and treatments
The study was a pot experiment conducted in glass roofed open space at the Faculty of Science, University of Guilan, at the period of 29 April 2019 to 27 June 2020. A factorial experiment with a completely randomized design with three replications was conducted in this study (Table 1). Experimental treatments included three groups: the first treatment was urban water as the control, the second treatment was UWW collected from the landfill site of Saravan and the third treatment was IWW collected from the discharge site of wastewater of the paper production factory. Each pot (diameter 40 cm, height 35 cm) was filled with 11 kg soil pre-sieved (4 mm sieve size). C. alternifolius, C. zizanioides and A. vera were gathered from the local nursery and were planted in the pots. To prevent the impact of stress on the plants and adapting the new condition, all experimental plants were irrigated with urban water (twice a week, each time 200 mL) for one month. After one month, control pots were irrigated with 300 mL of urban water and treatment pots with either 300 mL UWW or 300 mL IWW twice a week for 14 months, separately. Plants were allowed to grow under a glass shelter in normal environmental conditions. The average air temperature and humidity during the experiment were 18.25°C and 77.25%, respectively. Leaves shed during the growing season were collected and washed in running filter water and then distilled water and dried in an oven (50 ºC for 72 hours). Then they were stored in dry paper bags. Additionally, to determine the amount of heavy metals added to the soil from wastewaters and compare it to maximum standard levels, pots filled with 11 kg of soil without plants were considered and irrigated like pots containing plants.
Table 1. Overall experimental design of the study.
|
Soil (No Plant) |
|
C. alternifolius |
||||
|
6.IWW |
3.UWW |
8.UWW |
|
7.UWW |
1.Control |
9.IWW |
|
1.Control |
7.UWW |
2.Control |
|
5.Control |
4.IWW |
8.UWW |
|
5.IWW |
4.Control |
9.IWW |
|
2.IWW |
6.UWW |
3.Control |
|
C. zizanioides |
|
A. vera |
||||
|
1.Control |
3.UWW |
2.IWW |
|
9.Control |
1.IWW |
7.UWW |
|
8.IWW |
6.Control |
4.IWW |
|
6.UWW |
4.UWW |
2.IWW |
|
5.Control |
9.IWW |
7.UWW |
|
8.Control |
3.IWW |
5.Control |
Note: Control: Water UWW: Urban wastewater IWW: Industrial wastewater
Heavy metals assessment
A sampling of plants and soil to assess element accumulation was conducted 14 months after planting. Soil samples of each pot were homogenized and dried in an oven at 30°C for 24 hours and were passed through a 2 mm sieve and the EC, pH, and concentration of heavy metals were measured (Richards, 1954; Salt et al., 1998; Uba et al., 2009).
All freshly harvested roots and shoots were carefully washed in running filter water and then distilled water. Also, roots were placed in distilled water for 24 hours, and then gently washed with deionized water to remove any adhering soil particles. After washing, plant samples were dried in an oven (50ºC for 72 hours) until obtaining a constant dry weight, and then all dried plant tissues were ground into powder using an electric mill. Then the concentration of the metal elements (Zn, Cr, Pb, Cu, Mn, Ni, and Mg) in the plant samples was determined using ICP-OES according to the method of (Salt et al., 1998; AL-Oud Saud, 2003; Subhashini et al., 2015).
Data analysis
TF (Translocation factor), BCF (bioconcentration factor), BAC (biological accumulation coefficient), and MAI (metal elements accumulation index)
Metal accumulation in the roots and the removal of the heavy metals from the soil are represented by the bioconcentration factor (BCF) and calculated by dividing the heavy metal concentration (µg g-1) in the root by its concentration (µg g-1) in the soil (BCF = C Root/C Soil). The translocation factor (TF) is the ratio of metal concentration (µg g-1) in plant shoots to the roots (TF = C Shoot/C Root). The biological accumulation coefficient (BAC) of a plant for a particular metal equals the heavy metal concentration (µg g-1) of the shoot divided by the same metal concentration (µg g-1) in the soil (BAC = C Shoot/C Soil) (Qihang et al., 2011).
To determine the simultaneous accumulation of metals in plants, the metal elements accumulation index (MAI) was calculated using the following formula:
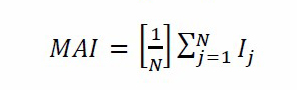
Based on this formula, N is the total number of analyzed metals and, Ij = x/δx is the sub-index for variable j, obtained by dividing the mean value (x) of each metal by its standard deviation (δx) (Liu et al., 2007).
Statistical analysis
The statistical analyses were carried out using a commercial statistical package SPSS 20.0 (SPSS Inc., Chicago, USA), and Microsoft Excel 2013. All the analyses were performed in three replications for each sample and expressed as mean ± SE. The significant difference in heavy metal (Zn, Cr, Pb, Cu, Mn, Ni, and Mg) concentration between wastewaters, species, and their interactions (wastewaters × species) were tested by two-way analysis of variance (ANOVA). The significant difference in pH and EC in soils was tested by one-way analysis of variance (ANOVA).
RESULTS
Physical and chemical parameters of soil
According to the current results, by adding wastewater to the soil, the amount of EC significantly increased, and the amount of pH decreased after 14 months of irrigation with UWW and IWW (Table 2). The highest EC (8.12 dSm-1) and lowest pH (5.43) were found in the final urban wastewater soil (UWS).
Table 2. EC and pH of the soil samples in different treatments.
|
Soil (no plant) |
EC dSm-1 |
pH |
|
PS |
0.88 ± 0.06 c |
6.64 ± 0.01 a |
|
FCS |
0.79 ± 0.05 c |
6.57 ± 0.08 a |
|
Final UWS |
8.12 ± 0.07 a |
5.43 ± 0.12 c |
|
Final IWS |
2.35 ± 0.05 b |
6.30 ± 0.06 b |
Note: PS: Primary soil, FCS: Final control (water) soil, UWS: Urban wastewater soil, IWS: Industrial wastewater soil. Mean values with the same letters are not significantly different at the 0.05 level according to the Duncan test. Data are means ± SE of three replications. (n=12).
Heavy metal changes in soil
In the current study, the initial amount of heavy metals in PS was 16, 14, 8, 20, 240, 9, 2400 µg g-1dry weight for Zn, Cr, Pb, Cu, Mn, Ni, and Mg, respectively. According to the result, after 14 months of adding urban and industrial wastewater, significant changes (P <0.05) were observed in the amount of heavy metals in all treated soil samples. The concentrations of heavy metals in the soil samples including FCS, UWS and IWS are shown in Table 3. The results showed that heavy metal concentrations in the soil with no plant treated with UWW and IWW were in the following order: Mg>Mn>Zn>Cu>Pb>Cr>Ni. Also, the amount of all metal elements except Cu was greater in the soil treated with UWW than in soil treated with IWW. The amount of all examined metal elements in the treated soil with UWW and IWW exceeded standard levels, except Cr and Ni (Table 3).
Table 3. The mean ± SE levels µg g-1 DW of heavy metals (Zn, Cr, Pb, Cu, Mn, Ni, and Mg) in the soil samples and the maximum standard levels.
|
Metals
|
FCS: Final Control Soil |
UWS: Urban Wastewater Soil |
IWS: Industrial Wastewater Soil |
Standard in soil |
|||
|
USEPA |
WHO |
Global Average |
|
||||
|
Zn |
30.00 ± 3.61 |
117.67 ± 6.23 |
99.67 ± 10.17 |
50 |
50-100 |
59.8 |
|
|
Cr |
12.33 ± 1.76 |
42.67 ± 3.28 |
38 ± 1.73 |
100 |
65 |
80 |
|
|
Pb |
9.00 ± 1.15 |
52.33 ± 1.86 |
45.67 ± 4.41 |
10 |
10-70 |
20 |
|
|
Cu |
29.00 ± 4.16 |
79.67 ± 2.96 |
92 ± 3.61 |
30 |
6-60 |
45 |
|
|
Mn |
264.00 ± 26.06 |
663.33 ± 21.86 |
630 ± 20.82 |
600 |
437 |
850 |
|
|
Ni |
8.33 ± 1.45 |
24 ± 1.15 |
20.67 ± 0.88 |
40 |
75-150 |
33.7 |
|
|
Mg |
2,933.33 ± 145.30 |
7,566.67 ± 338.30 |
6,900 ± 230.94 |
- |
- |
- |
|
Note: USEPA: United States Environmental Protection Agency, 2005. WHO: World Health Organization and encyclopedia environmental science, 2000. (n=9). In this table only FCS, UWS, and IWS are given to compare with standard levels.
Heavy metal concentration in different parts of the plants
The results showed that the effects of interaction between wastewater and plant species on the heavy metal contents in the root and shoot were significant at a 5% level for Zn, Cu, and Mn in the root, and Zn and Mg in the shoot. The amount of heavy metal (µg g-1 DW) in the root and shoot of C. alternifolius, C. zizanioides, and A. vera is shown in Tables 4 and 5. The concentration of the heavy metals in all three studied plants was as follow, respectively: in the roots: Mg>Mn>Cu>Zn>Pb>Cr>Ni and in the shoots: Mg>Mn>Zn>Cu>Pb>Cr. The amount of Ni in the shoots of the plants was not detected by the ICP device, because the amount was too low, and was not within the detection range of the device.
Table 4. The amount of heavy metal (µg g -1 DW) in the root of C. alternifolius, C. zizanioides, and A. vera under UWW and IWW treatment.
|
|
root (µg g-1 DW) |
Zn |
Cr |
Pb |
Cu |
|
|||
|
C. alternifolius |
UWW |
25.67 ± 2.03b |
6.00 ± 0. 58 ab |
9.00 ± 1.00 bc |
28.00 ± 2.08 c |
|
|||
|
IWW |
23 .00 ± 1.53 b |
4.33 ± 0.88 b |
9.33 ± 0.88 abc |
23.33 ± 1.20 c |
|
||||
|
C. zizanioides |
UWW |
36.00 ± 0.58 a |
7.33 ± 0.67 a |
13.33 ± 1.45 a |
37.00 ± 0.67 b |
|
|||
|
IWW |
26.00 ± 0.67 b |
6.67 ± 0.88 ab |
12.00 ± 2.08 ab |
44.67 ± 0.67 a |
|
||||
|
A. vera |
UWW |
22.33 ± 1.86 b |
5.67 ± 0.88 ab |
6.33 ± 0.67 c |
41.00 ± 2.19 ab |
|
|||
|
IWW |
23.67 ± 1.86 b |
6.00 ± 1.15 ab |
6.67 ± 0.88 c |
40.33 ± 3.53 ab |
|
||||
|
|
root (µg g-1 DW) |
Mn |
Ni |
Mg |
|
||||
|
C. alternifolius |
UWW |
49.67 ± 1.67 d |
2.67 ± 0.33 c |
1,866.67 ± 66.67 b |
|
||||
|
IWW |
41.67 ± 0.88 e |
3.00 ± 0.58 c |
1,800.00 ± 100.00 c |
|
|||||
|
C. zizanioides |
UWW |
81.00 ± 1.15 a |
6.33 ± 0.88 a |
2,766.67 ± 88.19 a |
|
||||
|
IWW |
76.33 ± 0.88 b |
5.00 ± 0.57 b |
2,200.00 ± 57.74 b |
|
|||||
|
A.vera |
UWW |
44.66 ± 1.45 e |
2.67 ± 0.33 c |
1,966. 67 ± 202.76 b |
|
||||
|
IWW |
58.33 ± 2.03 c |
2.33 ± 0.33 c |
1,933.33 ± 176.39 b |
|
|||||
Note: Mean values with the same letters are not significantly different at the 0.05 level according to the Duncan test. Data are means ± SE of three replications. (n=18). ND: not detected by ICP-OES.
Table 5. Heavy metals content (µg g -1 DW) in the shoot of C. alternifolius, C. zizanioides, and A. vera under UWW and IWW.
|
|
shoot (µg g-1 DW) |
Zn |
Cr |
Pb |
Cu |
|
||
|
C. alternifolius |
UWW |
23.00 ± 2.08 c |
3.00 ± 0.58 c |
2.33 ± 0.33 c |
19.33 ± 2.40 bc |
|
||
|
IWW |
21.67 ± 1.86 c |
2.33 ± 0.33 bc |
3.33 ± 0.67 bc |
15.00 ± 1.15 c |
|
|||
|
C. zizanioides |
UWW |
30.67 ± 3.18 ab |
4.33 ± 0.33 ab |
6.00 ± 0.58 a |
20.33 ± 1.76 abc |
|
||
|
IWW |
28.00 ± 2.65 bc |
4 ab ± 0.58 |
6.33 ± 0.33 a |
21.33 ± 1.86 ab |
|
|||
|
A. vera |
UWW |
39.00 ± 2.52 a |
5.33 ± 0.33 a |
5.33 ± 0.33 a |
25.00 ± 1.53 a |
|
||
|
IWW |
26.00 ± 1.00 bc |
4.67 ± 0.33 b |
4.67 ± 0.88 ab |
22.67 ± 0.67 ab |
|
|||
|
|
shoot (µg g-1 DW) |
Mn |
Ni |
Mg |
|
|||
|
C. alternifolius |
UWW |
112.67 ± 2.33 c |
ND |
2,333.33 ±240.37 c |
|
|||
|
IWW |
85.33 ± 4.84 d |
ND |
2100 ± 152.75 c |
|
||||
|
C. zizanioides |
UWW |
133 ± 3.51 b |
ND |
2,233.33 ± 133.33 c |
|
|||
|
IWW |
108 ± 4.73 c |
ND |
2,833.33 ± 66.67 b |
|
||||
|
A.vera |
UWW |
177.67 ± 2.33 a |
ND |
3,866.67 ± 88.19 a |
|
|||
|
IWW |
140 ± 5.51 b |
ND |
3,533.33 ± 66.67 a |
|
||||
Note: Mean values with the same letters are not significantly different at the 0.05 level according to the Duncan test. Data are means ± SE of three replications. (n=18). ND: not detected by ICP-OES.
Effect of wastewater on BCF, BAC, and TF of plants
The present study showed the range of TF for Zn, Cr, Pb, Cu, Mn, Ni, and Mg from 0.85 to 1.79, 0.50 to 0.99, 0.28 to 0.86, 0.48 to 0.70, 1.41 to 3.98 and 0.81 to 2, respectively (Table 6). The highest TF was for A. vera with a value of 3.98 for Mn, 2 for Mg, and 1.79 for Zn, under UWW treatment.
The value of BAC was significant (P <0.05) for Zn and Mn, and the highest value of BAC was related to Mg. In the current study the effect of interaction (treatment × species) on the value of BCF was significant (P <0.05) for Mn. According to Table 7, Cu and Mg had the highest BCF value of 0.7-1.31 and 0.65 to 1.02, respectively, which indicates Cu and Mg were better absorbed than other examined elements. Ni, Mn, and Cr had the lowest BCF values, 0.13-0.29, and 0.15 to 0.26 respectively while Zn and Pb had BCF values of 0.47-0.76 and 0.29 to 0.71, respectively, indicating moderate accumulation in the examined plants.
Table 6. TF of C. alternifolius, C. zizanioides, and A. vera in response to UWW and IWW treatment.
|
TF |
|
Zn |
Cr |
Pb |
Cu |
|
||
|
C. alternifolius |
UWW |
0.90 ± 0.07 b |
0.50 ± 0.08 c |
0.28 ± 0.08 d |
0.70 ± 0.10 a |
|
||
|
IWW |
0.94 ± 0.03 b |
0.56 ± 0.06 bc |
0.37 ± 0.09 cd |
0.65 ± 0.06 a |
|
|||
|
C. zizanioides |
UWW |
0.85 ± 0.08 b |
0.6 ± 0.05 abc |
0.45 ± 0.05 bcd |
0.55 ± 0.05 a |
|
||
|
IWW |
1.06 ± 0.08 b |
0.6 ± 0.02 abc |
0.56 ± 0.09 bc |
0.48 ± 0.05 a |
|
|||
|
A. vera |
UWW |
1.79 ± 0.24 a |
0.99 ± 0.14 a |
0.86 ± 0. 08 a |
0.61 ± 0.06 a |
|
||
|
IWW |
1.11 b ± 0.05 b |
0.86 ± 0.22 ab |
0.69 ± 0.08 ab |
0.57 ± 0.05 a |
|
|||
|
TF |
|
Mn |
Ni |
Mg |
|
|||
|
C. alternifolius |
UWW |
2.27 ±0.09 bc |
NA |
1.18 ± 0.12 b |
|
|||
|
IWW |
2.05 ± 0.09 c |
NA |
1.12 ± 0.13 bc |
|
||||
|
C. zizanioides |
UWW |
1.64 ± 0.05 d |
NA |
0.81 ± 0.05 c |
|
|||
|
IWW |
1.41 ± 0.06 e |
NA |
1.29 ± 0.05 b |
|
||||
|
A.vera |
UWW |
3.98 ± 0.08 a |
NA |
2.00 ± 0.17 a |
|
|||
|
IWW |
2.41 ± 0.12 b |
NA |
1.85 ± 0.14 a |
|
||||
Note: Mean values with the same letters are not significantly different at the 0.05 level according to the Duncan test. Data are means ± SE of three replications. (n=18).
NA: Not available, because the Ni concentration was not detectable in some treatments.
Table 7. BCF and BAC of C. alternifolius, C. zizanioides, and A. vera in response to UWW and IWW treatment.
|
Treatment |
Zn |
Cr |
Pb |
|
|||||||||||||||||||||||||||
|
|
BCF |
BAC |
BCF |
BAC |
BCF |
BAC |
|
||||||||||||||||||||||||
|
C. alternifolius |
UWW |
0.50 ± 0.03bc |
0.44 ± 0.01 c |
0.20 ± 0.03 a |
0.1 ± 0.02 bc |
0.50 ± 0.05 abc |
0.13 ± 0.33 c |
|
|||||||||||||||||||||||
|
IWW |
0.47 ± 0.06 c |
0.44 ± 0.05 c |
0.15 ± 0.03 a |
0.08 ± 0.01 c |
0.54 ± 0.01 ab |
0.20 ± 0.05 ab |
|
||||||||||||||||||||||||
|
C. zizanioides |
UWW |
0.76 ± 0.04 a |
0.65 ± 0.06 b |
0.26 ± 0.03 a |
0.15 ± 0.02 ab |
0.71 ± 0.05 a |
0.32 ± 0.02 a |
|
|||||||||||||||||||||||
|
IWW |
0.64 ± 0.05 ab |
0.69 ± 0.05 b |
0.23 ± 0.04 a |
0.14 ± 0.03 abc |
0.57 ± 0.12 ab |
0.30 ± 0.04 a |
|
||||||||||||||||||||||||
|
A. vera |
UWW |
0.51 ± 0.06 bc |
0.88 ± 0.05 a |
0.2 ± 0.03 a |
0.19 ± 0.01 a |
0.29 ± 0.04 c |
0.25 ± 0.02 ab |
|
|||||||||||||||||||||||
|
IWW |
0.55 ± 0.04 bc |
0.61 ± 0.05 b |
0.21 ± 0.05 a |
0.16 ± 0.01 ab |
0.38 ± 0.08 bc |
0.26 ± 0.05 ab |
|
||||||||||||||||||||||||
|
Treatment |
Cu |
Mn |
|
||||||||||||||||||||||||||||
|
|
BCF |
BAC |
BCF |
BAC |
|
|
|||||||||||||||||||||||||
|
C. alternifolius |
UWW |
0.70 ± 0.30 c |
0.48 ± 0.05 bc |
0.16 ± 0.01 c |
0.36 ± 0.01 c |
|
|
||||||||||||||||||||||||
|
IWW |
0.60 ± 0.05 c |
0.38 ± 0.02 c |
0.13 ± 0.01 d |
0.26 ± 0.02 d |
|
|
|||||||||||||||||||||||||
|
C. zizanioides |
UWW |
1.11 ± 0.08 ab |
0.60 ± 0.02 ab |
0.29 ± 0.03 a |
0.48 ± 0.04 b |
|
|
||||||||||||||||||||||||
|
IWW |
1.31 ± 0.01 a |
0.63 ± 0.07 a |
0.26 ± 0.02 a |
0.37 ± 0.03 c |
|
|
|||||||||||||||||||||||||
|
A. vera |
UWW |
0.99 ± 0.09 b |
0.60 ± 0.04 ab |
0.15 ± 0.01 cd |
0.60 ± 0.03 a |
|
|
||||||||||||||||||||||||
|
IWW |
1.08 ± 0.13 ab |
0.60 ± 0.01 ab |
0.20 ± 0.02 b |
0.50 ± 0.04 b |
|
|
|||||||||||||||||||||||||
|
Treatment |
Ni |
Mg |
|
|
|
||||||||||||||||||||||||||
|
BCF |
BAC |
BCF |
BAC |
|
|
|
|||||||||||||||||||||||||
|
C. alternifolius |
UWW |
0.15 ± 0.02 b |
NA |
0.65 ± 0.65 b |
0.83 ± 0.13 bc |
|
|
|
|||||||||||||||||||||||
|
IWW |
0.18 ± 0.04 b |
NA |
0.65 ± 0.64 b |
0.77 ± 0.07 c |
|
|
|
||||||||||||||||||||||||
|
C. zizanioides |
UWW |
0.36 ± 0.05 a |
NA |
1.02 ± 0.08 a |
0.83 ± 0.11 bc |
|
|
|
|||||||||||||||||||||||
|
IWW |
0.32 ± 0.07 a |
NA |
0.88 ±0.08 ab |
1.13 ± 0.08 bc |
|
|
|
||||||||||||||||||||||||
|
A. vera |
UWW |
0.14 ± 0.02 b |
NA |
0.82 ± 0.20 ab |
1.58 ± 0.27 a |
|
|
|
|||||||||||||||||||||||
|
IWW |
0.13 ± 0.02 b |
NA |
0.68 ± 0.05 b |
1. 25 ± 0.01 ab |
|
|
|
||||||||||||||||||||||||
Note: Mean values with the same letters are not significantly different at the 0.05 level according to the Duncan test. Data are means ± SE of three replications. (n=18).
NA: Not available, because the Ni concentration was not detectable in some treatments.
Metal accumulation index (MAI) in root and shoot of plants
To assess the overall performance of the examined plants, MAI values to accumulate metals in the roots and shoots of the plants are shown in Figure 1 and 2. The MAI of the root and shoot in the treatments were more than the control. The MAI of the root, in C. zizanioides, was higher than C. alternifolius and A. vera under both IWW and UWW treatments, while the MAI of the shoot in A. vera was higher than C. zizanioides and C. alternifolius under UWW and IWW treatments.
C. zizanioides had the highest MAI value for roots (33.95) under IWW and A. vera had the highest MAI value for shoots (24.07) under UWW treatment.
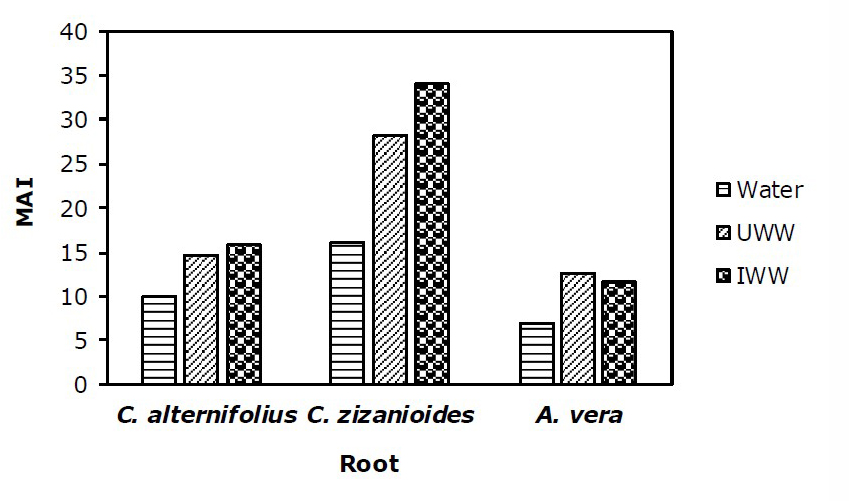
Figure 1. MAI value for (Zn, Cr, Pb, Cu, Mn, Ni, and Mg) in the roots of C. alternifolius, C. zizanioides, and A. vera under Water, UWW, and IWW treatment. (n=7).
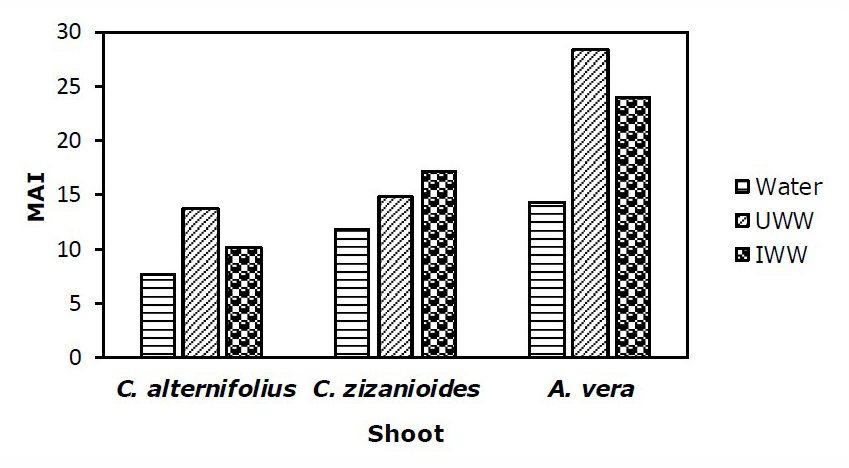
Figure 2. MAI value for (Zn, Cr, Pb, Cu, Mn, and Mg) in the shoots of C. alternifolius, C. zizanioides, and A. vera under Water, UWW, and IWW treatment. (n=6).
DISCUSSION
The availability of metals in soil is mainly affected by the nature of metal and the properties of soil. For example, soil pH and EC are important properties of soil for the bioavailability of metal. According to the results of this study, with decreasing the pH and increasing the salinity, the availability of metals in the plant increased (Table 2). However, the amount of adsorption and absorption is different based on the type and the amount of total heavy metals and the type of salt that causes salinity (Weggler et al., 2000; Acosta et al., 2011). It has been found that organic materials and high concentrations of some minerals such as calcium, magnesium, sodium, and chlorine can impact the EC and pH of the wastewater soil. In this study, according to the results of the physical and chemical parameters of the soil, it is most likely that the UWW contained some soluble salts (such as the cations of Ca+2, Mg+2, Na+, k+, and the anions of Cl-, SO4-2, NO3-, HCO3-) compared to IWW that caused an EC increase in final UWS compared to final IWS and FCS. Also, the pH may have been reduced as a result of organic acids released during the decomposition process (Abbaspour et al., 2008). The reason there is more metal in soil treated with UWW is probably due to more pollution in the UWW (Table 3). According to the Environmental Protection Agency (EPA, 2021), some of the remaining household products, such as paints, cleaners, oils, and batteries are considered hazardous household waste and emphasize that special care should be taken when disposed of.
In current study, according to Tables 4 and 5, there was a significant difference between the examined species regarding the heavy metals concentrations of the roots and shoots (except for Cr in roots). The most amounts of metal elements were observed in the roots and shoots treated with UWW. Heavy metals are absorbed by plants from the soil solution and the availability of the metals is dependent on the reaction of the soil and its organic matter content. It seems that the amounts of heavy metals absorbed by the examined plants and their concentrations in the soil have a linear relationship, and this is in agreement with the results of Brunner et al., (2008).
The distribution of metal elements in different parts of the plants depends on the species, its growth phase and the role microelements play in the metabolic processes (Bielecka and Krolak, 2019) and toxic ions can be absorbed by plants along with the beneficial ions due to their chemical similarity (Baker, 1981). Moreover, there are distribution and tolerance mechanisms in plant roots and leaves that impact the uptake and transport of metal elements. Plants respond to metal toxicity or deficiency by maintaining metal homeostasis in cells through the regulation of transporters involved in the absorption, efflux, translocation, and sequestration of the elements (Memon and Schroder, 2009).
The ability of plants in contaminated sites to uptake, transport, and tolerate metals can be determined by BCF and TF. These factors can be used to assess the phytoremediation potential and the survival strategy of the plants (Yoon et al., 2006). BCF provides data regarding the accumulation of heavy metals in roots as well as the relationship between metal concentration in roots and its concentration in the soil. When assessing the phytoremediation potential of a given species, BCF is often cited as one of the key factors (Zhao et al., 2003). TF illustrates the relationship between the metal content of roots and shoots. The efficiency of plants in transporting elements from the roots to the leaves can be determined by TF. When BCF and TF values are higher, there is a stronger ability to migrate heavy metals from the soil to the root and from the root to the shoot (Jung et al., 2017; Jalali and Hemati Matin, 2019).
According to Table 6, all treatments had TF value 1 is an indicator of a plant metal accumulator. TF value of greater than 1 in the metal elements accumulators and less than 1 in metal elements excluder species was observed (Kouhi and Moudi 2020).
According to table 7, Cu and Mg had the highest BCF compared to other elements. Based on Tables 6 and 7, the TF of Mg was ˃1, but the BCF value for Mg was < 1. Also, the TF of Mn was ˃1, but the BCF value was < 1. The element of Zn had TF value ˃1 in A. vera under both UWW and IWW treatments and in C. zizanioides under IWW treatment, but their BCF values were < 1. TF>1 and BCFC. zizanioides under IWW treatment had a good phytostabilization potential for Zn. Cr and Pb had TF
TFC. zizanioides, and in IWW treatments in A. vera, whiles in all of these conditions TF value were 1 and TF<1, have phytostabilization potential, and plants with BCF>1 and TF>1 are good accumulators and suitable for phytoextraction.
Previous studies have reported that plants with a combination of BCF1, BCF1 and TF
Commonly, by phytostabilization method can be reduced metals as mineral pollutants in the environment. Plants that are suitable for phytostabilization should have deep roots. They prevent wind erosion and increase the content of organic matter and improve physicochemical and biological conditions in contaminated soil. It has been reported that plants used for phytostabilization should show a low ability to accumulate pollutants in shoots and should be tolerant to variations in pH, soil moisture and salinity. Previous studies have reported that plants accumulate metals only in the roots for phytoremediation (Materac et al., 2015).
According to figures 1 and 2, simultaneous accumulation of metallic elements in roots and shoots in all three plants under UWW and IWW was more than water treatment (control). Also, C. zizanioides and C. alternifolius accumulated more heavy metals in their roots than shoots, but A. vera collected more heavy metals in its shoot than root. This difference in the simultaneous accumulation of metallic elements of roots and stems can be caused by the difference in the absorption of metals by the roots and shoots of the plant depending on plant species, metal type, and bioavailability (Nakbanpote et al., 2010). Some heavy metals are easily translocated to shoots but the majority of heavy metals are stored in the roots of the plants. Also, lateral roots accumulate more heavy metals than main roots, and also older leaves accumulate more heavy metals than younger leaves (Cheng, 2003).
Given that MAI displays the general performance of plants to simultaneously accumulate metal elements for its deviation in metal uptake (Liu et al., 2007), it seems that C. zizanioides and A. vera were more successful and had more simultaneously accumulated of Zn, Cr, Pb, Cu, Mn, Ni, and Mg under UWW and IWW treatments. Previous studies have reported that plant species with a high MAI value should be used as barriers between contaminated and vulnerable areas such as parks, and residential areas (Nadgorska–Socha et al., 2017).
CONCLUSION
In the current study, with increasing UWW and IWW irrigation, the amount of heavy metals and EC significantly increased, and the pH of the soil decreased, significantly. Following the increase of heavy metals in the soil, the amount of the examined elements increased in the roots and shoots of all experimented plants. According to the results of BCF>1 and TF>1, BCF1, BCF1 and TF<1, the comparative analysis of the three examined plants showed that all three plants species used the strategy of phytostabilization in heavy metal-contaminated soils. Based on the results of the metal elements accumulation index of the examined plants, MAI of the root was found to be in the order of C. zizanioides > C. alternifolius > A. vera under both IWW and UWW treatments, while MAI of the shoot was in the order of A. vera > C. zizanioides > C. alternifolius under UWW and IWW treatments. Generally, based on the results of this study, A. vera leaf and C. zizanioides root were better accumulators of the examined contaminants. According to the current results, it is suggested to cultivate C. zizanioides and A. vera in the landfill site of urban wastewater and discharge site of wastewater of paper production factory or any other similar polluted area to decrease the amount of the soil contaminants and create a green belt. However, since metal element accumulation in plant species varies depending on the element, species, and the location, more studies are needed in this area. This study was a pot experiment in glass-roofed open space, so, it is suggested to implement this plan in the field in a completely natural condition and in a period of more than 14 months to be able to choose the most suitable plant for contaminant remediation.
ACKNOWLEDGMENTS
The authors thank the University of Mohaghegh Ardabili and the University of Guilan for supporting this research, financially and instrumentally.
AUTHOR CONTRIBUTIONS
Seyed Mehdi Razavi designed the experiments. Mansour Afshar Mohammadian assisted and Sareh Ebrahimi Nokande conducted the experiments. All authors have read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Abbaspour, A., Kalbasi, M., Hajrasuliha S., and Fotovat, A. 2008. Effect of organic matter and salinity on ethylenediaminetetraacetic acid–extractable and solution species of cadmium and lead in three agricultural soils. Communications in Soil Science and Plant Analysis. 39: 983-1005.
Acosta, J. A., Jansen, B., Kalbitz, K., Faz, A., and Martinez, S. 2011. Salinity increases mobility of heavy metals in soils. Chemosphere. 85: 1318-1324.
Ali, H., Khan, E., Sajad, M. A. 2013. Phytoremediation of heavy metals concepts and applications. Chemosphere. 91: 869-881.
Allue, J., Garces, A. M., Bech, J., Barcelo J., and Poschenrieder, Ch. 2014. Fractionation of chromium in tannery sludge-amended soil and its availability to fenugreek plants. Journal of Soils and Sediments. 14: 697–702.
AL-Oud Saud, S. (2003) Heavy Metal Contents in Tea and Herb Leaves. Pakistan Journal of Biological Sciences. 6: 208-221.
Baker, A. J. M. 1981. Accumulation and excluders-strategies in the response of plants to heavy metals. Journal of Plant Nutrition. 1-4: 643–654.
Bielecka, A. and Krolak, E. 2019. The accumulation of Mn and Cu in the morphological parts of Solidago canadensis under different soil conditions. PeerJ. 7: e 8175.
Bremner, JM. and Mulvaney. CS. 1982. Nitrogen-Total. Methods of Soil Analysis. Chemical and Microbial properties. American Society of Agronomy and Soil Science Society of America. Inc. Madison, Wisconsin.
Brunner, I., Luster, J., Gunthardt-Goerg M. S., and Frey, B. 2008. Heavy metal accumulation and phytostabilisation potential of tree fine roots in a contaminated soil. Environmental Pollution. 152: 559-568.
Cheng, S. 2003. Heavy metals in plants and phytoremediation. International Journal of Environmental Science and Pollution Research. 10:335-340.
Dordio, A.V. and Carvalho A. J. P., 2013. Organic xenobiotics removal in constructed wetlands, with emphasis on the importance of the support matrix. Journal of Hazardous Materials. 252: 272–292.
European Commission. 2013. Soil Contamination: Impacts on Human Health. Science for Environmental Policy. Report produced for the European Commission DG Environment, September. Science Communication Unit, University of the West of England, Bristol.
Gajic, G., Mitrovic, M., and Pavlovic, P. 2020. Feasibility of Festuca rubra L. native grass in phytoremediation. Phytoremediation Potential of Perennial Grasses. 115-164.
Gajic, G., and Pavlovic, P. 2018. The role of vascular plants in the phytoremediation of fly ash deposits. Phytoremediation: Method. Management and Assessment. 151-236.
Jalali, M. and Hemati Matin, N. 2019. Nutritional status and risks of potentially toxic elements in some paddy soils and rice tissues. International Journal of Phytoremediation. 18: 1-9.
Jung, H., Lee, J., Chae, M., Kong, M. S., Lee, C. H., Kang, S. S., and Kim, Y. H. 2017. Growth inhibition patterns and transfer-factor profiles in arsenicstressed rice (Oryza sativa L.). Environmental Monitoring and Assessmen. 189: 638–649.
Kouhi, S. M. M., and Moudi, M. 2020. Assessment of phytoremediation potential of native plant species naturally growing in a heavy metal-polluted saline–sodic soil. Environmental Science and Pollution Research. 27: 10027-10038.
Ling, W., Shen, Q., Gao, Y., Gu, X., and Yang, Z. 2008. Use of bentonite to control the release of copper from contaminated soils. Soil Research. 45: 618-623.
Liu, J., Li, K., Xu J., Zhang, Z., Ma, T., Lu, X., and Zhu, Q. 2004. Lead toxicity, uptake and translocation in different rice cultivars. Plant Science. 165: 793-802.
Liu, Y. J., Zhu, Y. G., and Ding, H. 2007. Lead and cadmium in leaves of deciduous trees in Beijing, China: Development of a metal accumulation index (MAI). Environ. Pollut. 145: 387–390.
Materac, M., Wyrwicka, A., and Sobiecka, E. 2015. Phytoremediation techniques of wastewater treatment. Environmental biotechnology. 11: 10-13.
Memon, A. R. and Schroder, P. 2009. Implications of metal accumulation mechanisms to phytoremediation. Environmental Science and Pollution Research. 16: 162-175.
Mohammadhosseini, M., Bahmanpour, H. and Lotfi, S. 2018. Investigation and identification of types and amounts of heavy metals in soil of an industrial area. Journal of Chemical Health Risks. 4: 33-44.
Moreira, H., Marques A. P. G. C., Rangel, A. O. S. S., and Castro, P. M. L. 2011. Heavy metal accumulation in plant species indigenous to a contaminated Portuguese site: prospects for phytoremediation. Water Air Soil Pollution. 221: 377–389.
Nadgórska–Socha, A., Kandziora-Ciupa, M., Trzęsicki, M. and Barczyk, G. 2017. Air pollution tolerance index and heavy metal bioaccumulation in selected plant species from urban biotopes. Chemosphere. 183: 471-482.
Nakbanpote, W., Panitlertumpai, N., Sukadeetad, K., Meesungneon, O., amd Noisa-nguan, W. 2010. Advances in phytoremediation research: A case study of Gynura pseudochina (L.) DC. Advanced knowledge application in practice. Sciyo, Croatia. 353-378.
Ng, C. C., Boyce, A. N., Abas, M. R., Mahmood, N. Z. and Han, F. 2020. Evaluation of vetiver grass uptake efficiency in single and mixed heavy metal contaminated soil. Environmental Processes. 7: 207-226.
Olsen, C. R., Cole, C. V., Watanabe F. S., and Dean, L. A. 1954. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. Washington: United States Department of Agriculture.
Pilon Smits, E. 2005. Phytoremediation. Annual Review of Plant. Biol. 56: 15–39.
Qihang, W., Wang, S., Thangavel, P., Qingfei, L., Zheng, H., Jun, B., Qui, R. 2011. Phytostabilization of Jatropha curcas L. in polymetalic acid mine tailings. International Journal of Phytoremediation. 13: 788–804.
Richards, L. A. 1954. Agriculture, Handbook, US Department of Agriculture, Washington. phytoremediation with the hyperaccumulator Thlaspi caerulescens. Plant and Soil.
Subhashini, V. and Swamy, A. V. V. S. 2015. Phytoremediation of lead, cadmium and chromium contaminated soils using selected weed plants. Acta Biological Indica. 4: 205-212.
Salt, D. E., Smith, R. D., and Raskin, I. 1998. Phytoremediation: Annual Review of Plant Physiology and Plant Molecular Biology. 49: 643–668.
Sarkar, B. 2002. Heavy metals in the environment. CRC press. Mar 21.
Shanker, A. K., Cervantes, C., Loza-Tavera, H. and Avudainayagam, S. 2005. Chromium toxicity in plants. Environment International. 31: 739-753.
Singh, R., Singh, D. P., Kumar, N. S., Bhargava, K., and Barman, S. C. 2010. Accumulation and translocation of heavy metals in soil and plants from fly ash contaminated area. Journal of Environmental Biology. 31: 421-430.
Taghipour, M., and Jalali, M. 2019. Impact of some industrial solid wastes on the growth and heavy metal uptake of cucumber (Cucumis sativus L.) under salinity stress. Ecotoxicology and Environmental Safety. 182: 109347.
Torresday, J. l., Videa, J. R. P., Rosa, G. D., and Parsons, J. 2005. Phytoremediatoin of heavy metals and study of the metal coordination by X- ray absorption spectroscopy. Coordination Chemistry Reviews. 249:1797-1810.
Uba, S., Uzairu, A., and Okunola, O. J. 2009. Content of heavy metals in Lumbricus terrestris and associated soils in dump sites. International Journal of Environmental Research. 3(3): 353- 358.
United States Environmental Protection Agency (USEPA). 2005. United States of Environmental Protection Agency. Office of Water Regulations and Standards. EPA 440/5-86-001, p. 273.
United States Environmental Protection Agency (USEPA). 2021. http://www.epa.gov/hw/household-hazardous-waste-hhw.
Walkley, A. and Black, I. A. 1934. An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Science. 37: 29-38.
Weggler-Beaton, K., McLaughlin, M. J., and Graham, R. D. 2000. Salinity inceases cadmium uptake by wheat and Swiss chard from soil amended with biosolids. Australian Journal of Soil Research. 38: 37-45.
WHO. 2000. Safety evaluation of certain food additives and contaminants. International Programme on Chemical Safety. WHO Food Additive Series 52.
Yoon, J., Cao, X., Zhou, Q., and Ma, L. Q. 2006. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Science of the Total Environment. 368: 456-464.
Zhao, F. J., Lombi, E., and McGrath, S. P. 2003. Assessing the potential for zinc and cadmium phytoremediation with the hyperaccumulator Thlaspi caerulescens. Plant and Soil. 249: 37-43.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand.
https://cmuj.cmu.ac.th
Sareh Ebrahimi Nokande1, Seyed Mehdi Razavi1,* and Mansour Afshar Mohammadian2
1 Department of Biology, Faculty of Sciences, University of Mohaghegh Ardabili, Ardabil, Iran
2 Department of Biology, Faculty of Sciences, University of Guilan, Rasht. Iran.
Corresponding author: Seyed Mehdi Razavi, E-mail: razavi694@gmail.com
Total Article Views
Editor: Wasu Prathum-Aree,
Chiang Mai University, Thailand
Article history:
Received: May 3, 2022;
Revised: August 18, 2022;
Accepted: August 22, 2022;
Published online: August 29, 2022

