
Ketogenic Diet Improves Semen Quality and Restores Gonadal Hormonal Balance Following Monosodium Glutamate-Induced Testicular Dysfunction in Rats
Damilare E. Rotimi, Omowumi T. Kayode, Tomilola D. Olaolu, and Oluyomi Stephen Adeyemi*Published Date : 2022-10-18
DOI : https://doi.org/10.12982/CMUJNS.2022.055
Journal Issues : Number 4, October-December 2022
Abstract This study aims to investigate the effects of semen quality and gonadal hormonal balance in monosodium glutamate-induced testicular dysfunction in rats. Two groups of twenty-three animals each were treated (control and monosodium glutamate (MSG) group) orally for 28 days to establish testicular dysfunction. After testicular dysfunction was successfully confirmed, the animals from the two treatment groups were further subdivided (post-treatment) into six groups and treated for 42 days as follows; Control (Untreated); Ketogenic diet; Curcumin; MSG group only; MSG + Ketogenic diet; MSG + Curcumin. In MSG treatment, the administration of MSG significantly (P <0.05) decreased the level of serum testosterone in the rats. The treatment with MSG significantly (P <0.05) reduced the sperm count, sperm motility, sperm viability, and sperm morphology compared to the control rats. In the post-MSG treatment, ketogenic diet treatment significantly (P <0.05) increased the levels of testosterone and luteinizing hormone, while curcumin treatment increased only luteinizing hormone. The group fed with curcumin showed a significant increase in sperm morphology and sperm count compared to the control. This study revealed that MSG administration might have led to a disruption of the gonadal hormonal balance and sperm quality.
Keywords: Ketogenic diet, Medicinal biochemistry, Monosodium glutamate, Sperm, Reproductive function, Testosterone
Citation: Rotimi, D.E., Kayode, O.T., Olaolu, T.D., Adeyemi, O.S. 2022. Ketogenic diet improves semen quality and restores gonadal hormonal balance following monosodium glutamate-induced testicular dysfunction in rats. CMUJ. Nat. Sci. 21(4): e2022055.
INTRODUCTION
The testis is an oval-shaped organ that synthesizes male gametes via hormonal secretion and spermatogenesis. The hypothalamo-pituitary-testicular axis regulates reproductive functions through the action of hormones. In males, luteinizing hormone (LH) activates the synthesis of testosterone in Leydig cells, which subsequently stimulates sperm synthesis in the Sertoli cells. Follicle stimulating hormone (FSH) activates Sertoli cells by facilitating testosterone passage leading to androgen production (Olaolu et al., 2018). Testosterone is a crucial male sex hormone required for spermatogenesis and it enhances the growth and secretory activity of the testes (Rotimi et al., 2022). There has been a decline in male reproductive health worldwide as a result of several factors, which includes hormonal disorders, drugs, genital infections, smoking, environmental pollution, alcohol, genetic defects, reactive oxygen species, illnesses and access to toxicants. Exposure to certain toxicants such as monosodium glutamate (MSG) could alter the semen quality and gonadal function leading to reproductive dysfunction. MSG has been reported to cause deterioration of sperm cell structure and production and it also triggers hemorrhage in the testis (Alalwani, 2014).
MSG is a food additive found basically in many food substances, especially in Asia and Western Africa (Kayode et al., 2020a). MSG widely improves food palatability and as preservatives, but it may come with harmful effects on different systems of the body. There are reported abuses of MSG because it is cheap and readily available. The LD50 (Medium lethal dose) of MSG is between 15 – 18 g/kg in rats and at high consumption symptoms like dizziness, headache, numbness, weakness, and muscle pain have been observed (Kayode et al., 2020b). Frequent MSG consumption has been associated with the triggering of asthma, cancer, obesity, diabetes, and oxidative stress (Rim, 2017). MSG intake initiates other deleterious effects including genotoxicity, renal toxicity, hepatotoxicity, and reproductive toxicity (Abd-Ella and Mohammed, 2016).
Available treatment for reproductive alterations includes hormonal supplementation, surgical operations, phytomedicine, drugs, and diet. Castro et al. (2018) revealed that ketogenic diet, which is a low carbohydrate/high fat diet, ameliorates sexual dysfunction, especially in obese patients. The diet helps to metabolize fats as an alternative fuel for the body due to lesser carbohydrate metabolism (Vidali et al., 2015). Therefore, this study intends to investigate the effect of ketogenic diet ingestion on semen quality and gonadal hormonal levels in MSG-induced testicular dysfunction rats.
MATERIALS AND METHODS
Materials
Chemicals
Analytical grade, monosodium glutamate (MSG) and Curcumin are a product of Sigma chemical (St. Loius, USA) reagent was acquired. All chemicals and reagents used were of analytical grade (Sigma chemical) and they were prepared in glasswares and stored at 25°C.
Experimental animals
Healthy male rats (8 – 10 weeks old) were obtained from the animal unit, Department of Biochemistry, University of Ilorin, Kwara State. The rats were allowed to acclimatize for fourteen days in a well-aerated room and fed ad libitum with chow and drinking water. They were maintained at humidity (45-55%), constant temperature (25-27°C), photoperiod (12 h cycle). The care, use, and treatment of the rats were carried out as described and approved by the Landmark University Ethical Committee with ethical number LUAC/2020/0047B.
A total of 46 rats were used in this study with a weight range of 180 ± 40 g, and rats were randomized into 2 groups containing twenty-three rats each; the control group (untreated) and induced group (4 mg/kg bw MSG) were treated orally for 28 days to establish testicular dysfunction (23 rats per group). To confirm that testicular dysfunction was successfully induced by MSG in rats, 5 rats were randomly selected and necropsied from each of the control and MSG treatment groups.
After a confirmation of successful induction of testicular dysfunction in rats, the remaining animals from the two groups (control and MSG) were subdivided into three groups each and treated for another 42 days. The control group was subdivided into control group (fed distilled water), ketogenic diet group, and curcumin group (15 mg/kg bw in distilled water), while the MSG group was divided into MSG only (untreated) group, ketogenic diet group and curcumin group (6 rats per group). All rats were sacrificed after the last day of treatment.
The ketogenic diet feed formulation used in the study was as previously reported by Kayode et al. (2020b). Briefly, this diet consisted of: fiber (50%) obtained from coconut meal, protein (15%) from crayfish, oil (20%) from coconut oil, vitamix (10%) and food binder (5%) from pysllum husk. The ketogenic diet components were commercially sourced.
Preparation of serum
The rats were sacrificed and rat serum was prepared as described by Yakubu et al. (2005). This was carried out twenty-four hours after the last day of each treatment. Briefly, under diethyl ether anesthesia, a sterile scalpel blade was used to cut veins of the rats. The collected blood was left to clot and then centrifuged using a Uniscope Laboratory centrifuge (Model SM800B) at 5,000 x g for 10 min. The supernatants were obtained and stored for hormonal analyses.
Assays
Hormonal assays
The rat serum testosterone, luteinizing hormone (LH), and follicle stimulating hormone (FSH) levels were determined quantitatively using the enzyme immunoassay (EIA) kit (Monobind Inc. Lake Forest USA).
Semen analysis
The epididymis was carefully removed from the testis and were exposed and incised using a surgical blade. The semen was squeezed out of the caudal epididymis into a sterile clean petri dish with phosphate buffer (0.1 M). This was swirled gently for 10 min at 37°C to ensure homogeneity and the semen was used for sperm analysis.
Sperm counts were carried out by briefly diluting the semen. The diluted semen was dropped on an improved Neubauer counting chamber with an overlaid slip and further observed under a microscope to count the sperm cells in the central square.
The sperm morphology was determined by using a prewarmed slide containing semen. A drop of nigrosin/Eosin stain and semen were thoroughly mixed on the slides to form an even smear and then allowed to be dried. The stained slide was then observed at × 400 magnification under a light microscope. Random slide fields were selected to ascertain their morphological features. The sperm morphology was expressed as percentage incidence.
For sperm motility, the semen was added to drops of 2.9% sodium citrate and placed on a prewarmed microscope slide at 27°C. A warm cover slip was used to cover the slide before examining it under the microscope at × 400 magnification. The microscope fields were selected randomly, and the sperm motility was evaluated from the field and were categorised as motile, immotile, or sluggish (Saalu et al., 2010).
Statistical analysis
Values were expressed as mean ± standard error of mean (SEM) and analyzed with one-way ANOVA and student t-test on a GraphPad Prism 6 (GraphPad Software Inc., San Diego, California, USA). Tukey’s post-hoc test was used to compare group mean values and P < 0.05 was considered statistically significant.
RESULTS
Serum hormones
In MSG treatment, the administration of MSG significantly (P <0.05) decreased the level of serum testosterone in the rats. A nonsignificant decrease was observed in the level of rat serum luteinizing hormone and follicle stimulating hormone (Figures 1-3).
In the post-MSG treatment, Ketogenic diet treatment significantly (P <0.05) increased the levels of testosterone and luteinizing hormone (Figures 1-2). Moreover, curcumin treatment increased significantly (P <0.05) the level of serum luteinizing hormone (Figure 2). However, there was a nonsignificant increase observed in the hormonal levels for the group fed with MSG + ketogenic diet in the rats.
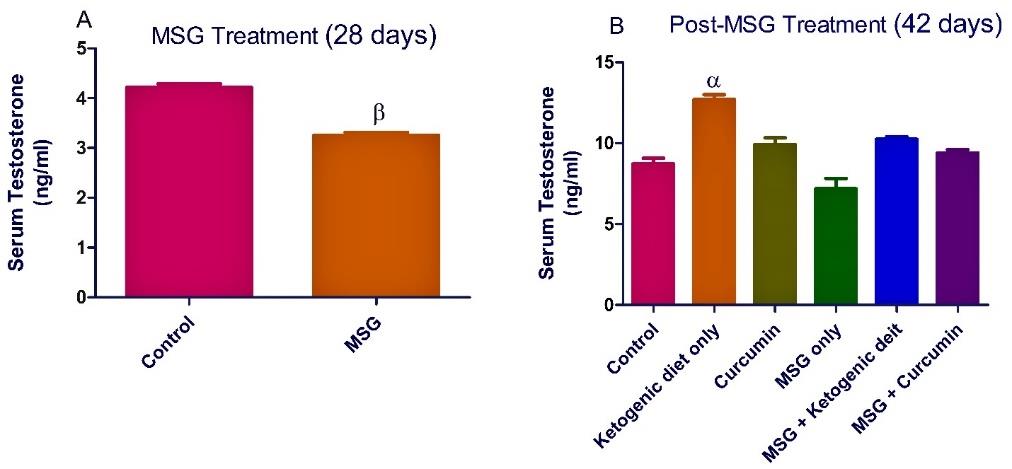
Figure 1. Ketogenic diet mitigates the level of serum testosterone in monosodium glutamate (MSG) treated rats. Data are presented as mean ± SEM of 6 replicates. β: values differ significantly from control at P < 0.01 in the pretreatment. α: values differ significantly from control at P < 0.05 in the post-treatment
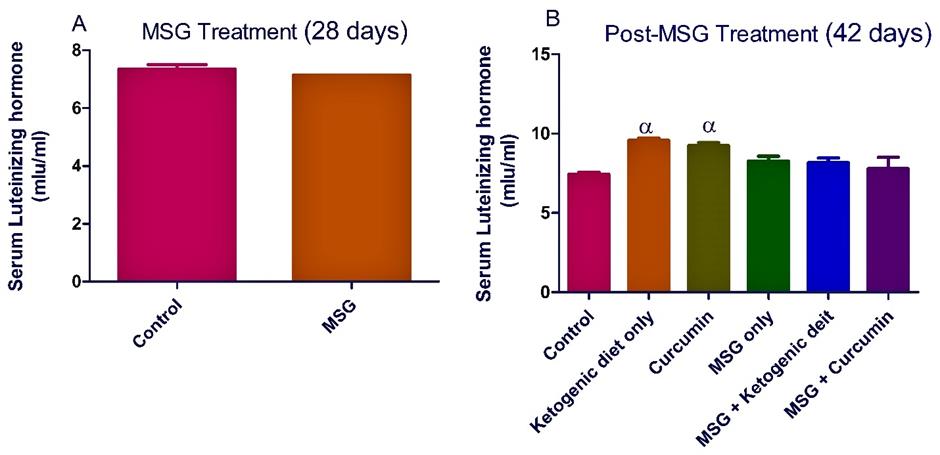
Figure 2. Ketogenic diet mitigates the level of serum luteinizing hormone in monosodium glutamate (MSG) treated rats. Data are presented as mean ± SEM of 6 replicates. α: values differ significantly from control at P < 0.05 in the post-treatment.
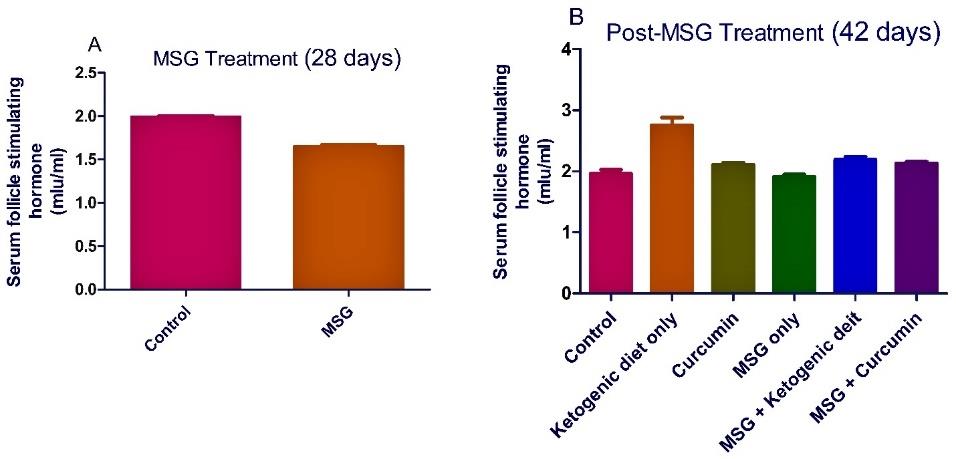
Figure 3. Ketogenic diet mitigates the level of serum follicle stimulating hormone in monosodium glutamate (MSG) treated rats. Data are presented as mean ± SEM of 6 replicates.
Sperm analysis
The treatment with MSG significantly (P <0.05) reduced the sperm parameters compared to the control rats (Figures 4-7).
Post-MSG treatment with ketogenic diet led to a significant (P <0.05) improvement in all measured sperm parameters (Figures 4-7). The group fed with curcumin showed a significant increase in the sperm morphology and sperm count compared to the control. For MSG + ketogenic diet as well as MSG + curcumin, there was a significant decrease in rat sperm parameters when compared with the control.
A scheme summarizing the probable mode of action of MSG in initiating reproductive dysfunction and the ameliorative path of ketogenic diet is presented in Figure 8.

Figure 4. Ketogenic diet mitigates sperm motility in monosodium glutamate (MSG) treated rats. Data are presented as mean ± SEM of 6 replicates. α: values differ significantly from control at P < 0.05; β at P < 0.01 in the pretreatment. α: values differ significantly from control at P < 0.05; β at P < 0.01; γ at P < 0.001 in the post-treatment.
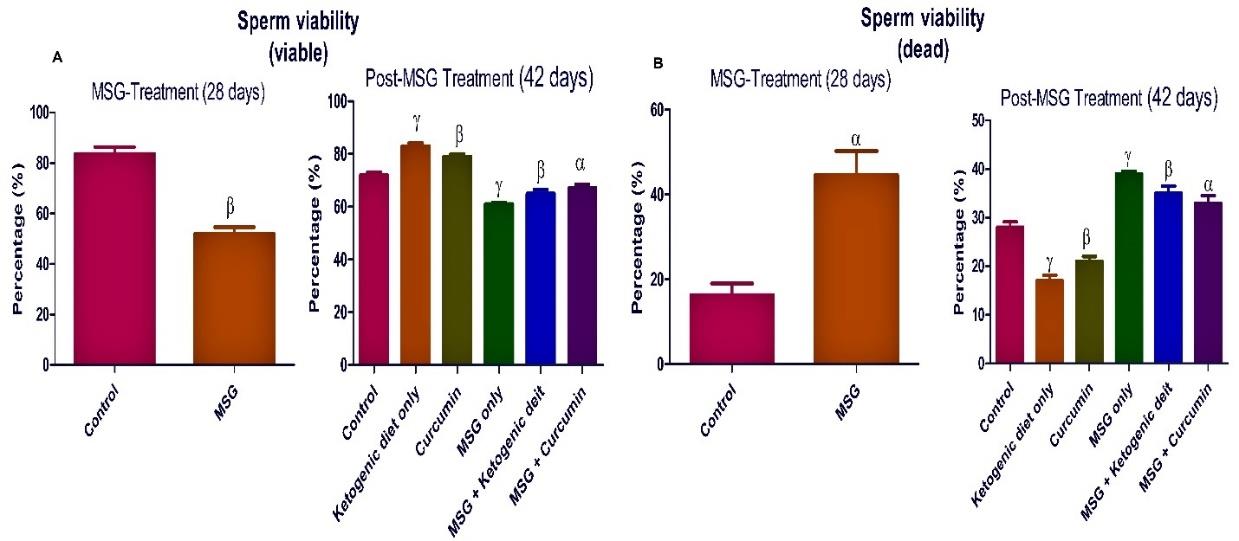
Figure 5. Ketogenic diet mitigates sperm viability in monosodium glutamate (MSG) treated rats. Data are presented as mean ± SEM of 6 replicates. α: values differ significantly from control at P < 0.05; β at P < 0.01 in the pretreatment. α: values differ significantly from control at P < 0.05; β at P < 0.01; γ at P < 0.001 in the post-treatment.
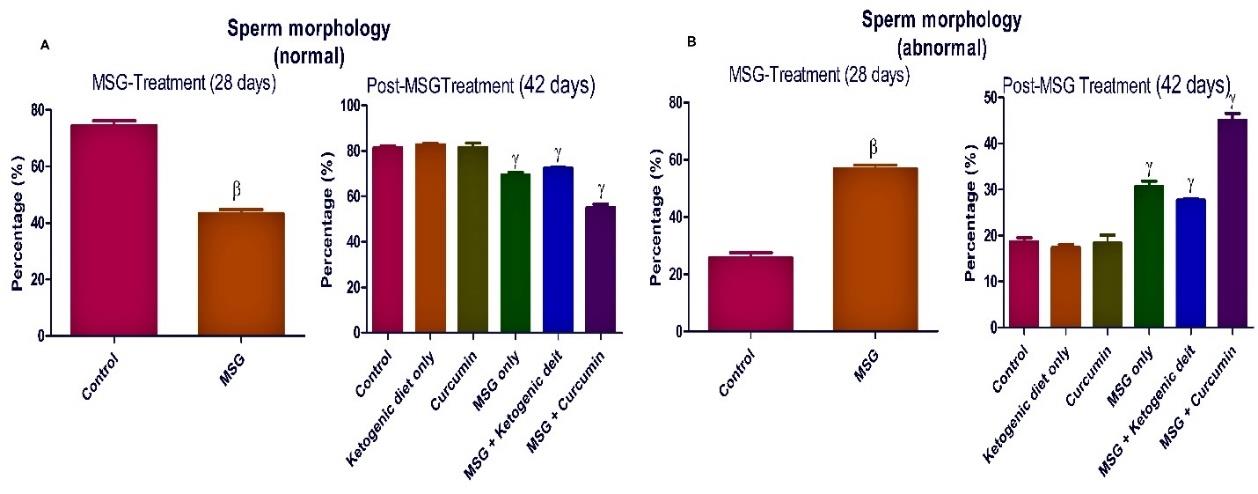
Figure 6. Ketogenic diet mitigates sperm morphology in monosodium glutamate (MSG) treated rats. Data are presented as mean ± SEM of 6 replicates. β: values differ significantly from control at P < 0.01 in the pretreatment. γ: values differ significantly from control at P < 0.001 in the post-treatment.
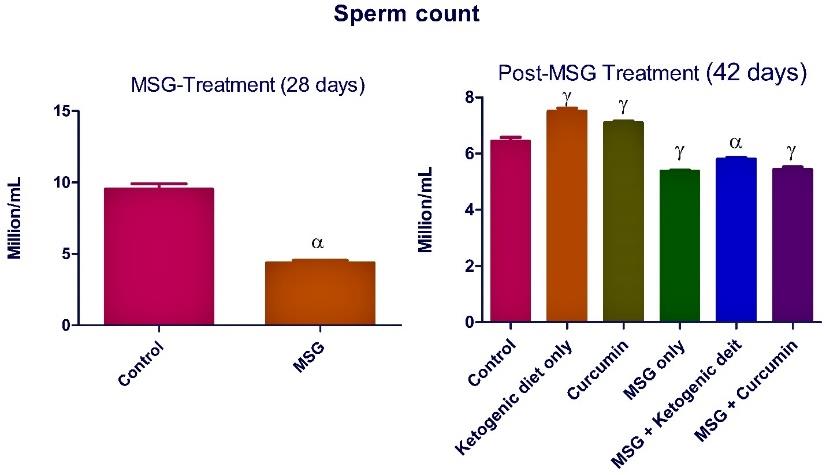
Figure 7. Ketogenic diet mitigates sperm count in monosodium glutamate (MSG) treated rats. Data are presented as mean ± SEM of 6 replicates. α: values differ significantly from control at P < 0.01 in the pretreatment. α: values differ significantly from control at P < 0.05; γ at P < 0.001 in the post-treatment.
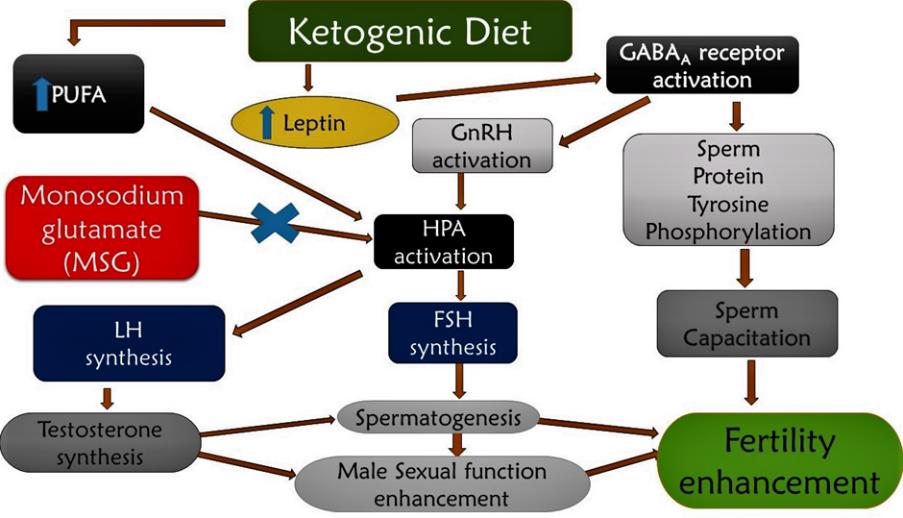
Figure 7. Ketogenic diet mitigates sperm count in monosodium glutamate (MSG) treated rats. Data are presented as mean ± SEM of 6 replicates. α: values differ significantly from control at P < 0.01 in the pretreatment. α: values differ significantly from control at P < 0.05; γ at P < 0.001 in the post-treatment.
DISCUSSION
In this study, we show evidence that MSG administration led to diminished levels of testosterone and gonadotropins, hence affirming a state of reduced fertility in the animal subjects. Previously, MSG excitotoxicity to neuronal cells has been established through its ability to disrupt the hypothalamic-pituitary adrenal axis (HPA) pathway (Hanipah et al., 2018). It achieves this by allowing a large influx of calcium into the cells, initiating hyper-excitatory action and perhaps death of the neurons. Thus, HPA disruption leads to the reduction in the level of sex hormones and gonadotropins, hence altering reproductive agility in animals (Iyiola et al., 2019; Kayode et al., 2020b). However, in the present study, ketogenic diet treatment led to a significant improvement (P <0.05) in gonadotropins and testosterone levels in rats. This finding may indicate improved rat testicular function and thus suggest an enhancement of fertility potential. Possibly, the ketogenic diet increases the levels of polyunsaturated fatty acids (PUFA), which are known inhibitors of neuronal sodium and calcium ion channels (Dashti et al., 2007; Tigerholm et al., 2012). Therefore, ketogenic diet may facilitate blocking neuronal membrane excitability, leading to a balancing of the HPA system and upregulation of gonadotropins. To further support the fact that ketogenic diet administration might have restored the disruption of the HPA system was evidenced by the increased production of testosterone, the major sex hormone for male sexual activation (Kayode and Yakubu, 2017; Olaolu and Rotimi, 2017). The ketogenic diet similarly improved the production of FSH, which is required for the progression of spermatogenesis and subsequent spermatozoa production for fertilization of the matured ovaries. Furthermore, the sperm characteristics and morphology support the potential of the ketogenic diet to significantly (P <0.05) boost the semen quality.
The activation of gamma amino butyric acid (GABA) synthesis by ketogenic diet has been well established (Dashti et al., 2007). In this case, activation of GABA is expected to enhance GnRH function and thereby facilitate fertility. Although GABA is mostly an inhibitory neurotransmitter for adult neurons, it is interestingly excitatory for GnRH neurons (Sullivan et al., 2003). GABA similarly enhances tyrosine phosphorylation of sperm protein, which enhances the motility of sperm through the fallopian tube, a condition required for natural fertilization to proceed in animals (Ritta et al., 2004). Taken together, findings from our study support that ketogenic diet may not only possess fertility enhancement potential, but that it holds prospects for the treatment of testicular dysfunction.
CONCLUSION
This study revealed that MSG administration might have led to a disruption of the gonadal hormonal balance and to a larger extent, spermatogenesis in rats. However, ketogenic diet therapy not only improved semen quality, but restored gonadal hormonal balance, thereby mitigating MSG-induced testicular dysfunction in rats.
ACKNOWLEDGMENTS
The authors wish to thank Dr. Raji of the Department of Veterinary Theriogenology and Production, University of Ilorin, Kwara State for his assistance in semen analysis and laboratory staff in the Biochemistry Department, Landmark University, Omu-Aran, Nigeria.
AUTHOR CONTRIBUTIONS
Damilare Rotimi designed and conducted all experiments and wrote the manuscript. Tomilola Olaolu, Omowumi Kayode, and Oluyomi Adeyemi assisted in conducting the experiments, performed the statistical analysis and data visualization and wrote the manuscript. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests
REFERENCES
Abd-Ella, E.M.M. and Mohammed, A.M. 2016. Attenuation of monosodium glutamate-induced hepatic and testicular toxicity in albino rats by Annona muricata linn. (annonaceae) leaf extract. IOSR Journal of Pharmacy and Biological Sciences. 11: 61-69.
Alalwani, A.D. 2014. Monosodium glutamate induced testicular lesions in rats (histological study). Middle East Fertility Society Journal. 19: 274-280.
Castro, A.I., Gomez-Arbelaez, D., Crujeiras, A.B., Granero, R., Aguera, Z., Jimenez-Murcia, S., Sajoux, I., Lopez-Jaramillo, P., Fernandez-Aranda, F., and Casanueva, F.F. 2018. Effect of a very low-calorie ketogenic diet on food and alcohol cravings, physical and sexual activity, sleep disturbances, and quality of life in obese patients. Nutrients. 10: 1348.
Dashti, H.M., Mathew, T.C., Khadada, M., Al-Mousawi, M., Talib, H., Asfar, S.K., Behbahani, A.I., and Al-Zaid, N.S. 2007. Beneficial effects of ketogenic diet in obese diabetic subjects. Molecular and Cellular Biochemistry. 302: 249-256.
Hanipah, E.N., Yahya, N.J., Ajik, E.M., Yusoff, N.A., and Taib, I. 2018. Monosodium glutamate induced oxidative stress in accessory reproductive organs of male Sprague-Dawley Rats. Jurnal Sains Kesihatan Malaysia. 16: 67-73.
Iyiola, O.A., Sulaiman, A.F., Sulaiman, A.A., Anifowoshe, A.T., Akolade, J.O., Adisa, M.J., Otohinoyi, D.A., Rotimi, D.E., Batiha, G.E., Maimako, R.F., and Adeyemi, O.S. 2019. Cypermethrin and chlorpyrifos raises serum urea level and causes abnormal sperm morphology in Wistar rats. Biointerface Research in Applied Chemistry. 9: 3969-3973
Kayode, O.T., Rotimi, D.E., Kayode, A.A.A., Olaolu, T.D., and Adeyemi, O.S. 2020a Monosodium glutamate (MSG)‐induced male reproductive dysfunction: a mini review. Toxics. 8: 7.
Kayode, O.T., Rotimi, D.E., Olaolu, T.D., and Adeyemi, O.S. 2020b. Ketogenic diet improves and restores redox status and biochemical indices in monosodium glutamate-induced rat testicular toxicity. Biomedicine & Pharmacotherapy. 127: 110227.
Kayode, O.T. and Yakubu, M.T. 2017. Parquetina nigrescens leaves: chemical profile and influence on the physical and biochemical indices of sexual activity of male Wistar rats. Journal of Integrative Medicine. 15: 64-76.
Olaolu, T.D. and Rotimi, D.E. 2017. Evaluation of the effect of Cissus populnea aqueous root infusion on some testicular function indices and serum hormones of male rats. Nigerian Journal of Biotechnology. 34: 105-116
Olaolu, T.D., Rotimi, D.E., and Olaolu, A.P. 2018. Effect of alcohol infusion of Cissus populnea root on testicular function and serum hormone of male Wistar rats. Asian Pacific Journal of Reproduction. 7: 117-122.
Rim, K.T. 2017. Toxicological evaluation of MSG for the manufacturing workers’ health: A literature review. Toxicology and Environmental Health Sciences. 9: 1-11.
Ritta, M.N., Bas, D.E., and Tartaglione, C.M. 2004. In vitro effect of gamma‐aminobutyric acid on bovine spermatozoa capacitation. Molecular Reproduction and Development. 67: 478-486
Rotimi, D.E., Ojo, O.A., Olaolu, T.D. and Adeyemi, O.S., 2022. Exploring Nrf2 as a therapeutic target in testicular dysfunction. Cell and Tissue Research, pp.1-11.
Saalu, L.C., Kpela, T., Benebo, A.S., Oyewopo, A.O., Anifowope, E.O., and Oguntola, J.A. 2010. The dose-dependent testiculoprotective and testiculotoxic potentials of telfairia occidentalis hook f. Leaves extract in rat. International Journal of Applied Research in Natural Products. 3: 27-38.
Sullivan, S.D., DeFazio, R.A., and Moenter, S.M. 2003. Metabolic regulation of fertility through presynaptic and postsynaptic signaling to gonadotropin-releasing hormone neurons. Journal of Neuroscience. 23: 8578-8585.
Tigerholm, J., Börjesson, S.I., Lundberg, L., Elinder, F., and Fransén, E. 2012. Dampening of hyperexcitability in CA1 pyramidal neurons by polyunsaturated fatty acids acting on voltage-gated ion channels. PLoS One. 7: e44388.
Vidali, S., Aminzadeha, S., Lambert, B., Rutherford, T., Sperl, W., Kofler, B., and Feichtingera, R. 2015. Mitochondria: The ketogenic diet-A metabolism-based therapy. International Journal of Biochemistry & Cell Biology. 63: 55–59.
Yakubu, M.T., Akanji, M.A., and Oladiji, A.T. 2005. Aphrodisiac potentials of the aqueous extract of Fadogia agrestis (Schweinf. Ex Hiern) stem in male albino rats. Asian Journal of Andrology. 7: 399-404.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand.
https://cmuj.cmu.ac.th
Damilare E. Rotimi1, Omowumi T. Kayode2, Tomilola D. Olaolu1, and Oluyomi Stephen Adeyemi1, *
1 Department of Biochemistry, Landmark University, PMB 1001, Omu-Aran – 251101, Nigeria
2 Biochemistry Unit, Department of Biological Sciences, Mountain Top University, Prayer city, Ogun State, Nigeria
Corresponding author: Adeyemi, Oluyomi Stephen, E-mail: yomibowa@yahoo.com
Total Article Views
Editor: Korakot Nganvongpanit,
Chiang Mai University, Thailand
Article history:
Received: February 22, 2022;
Revised: July 26, 2022;
Accepted: August 11, 2022;
Published online: August 19, 2022

