
In Vitro Preparation and Evaluation of Chitosan/Pluronic F-127 Hydrogel as a Local Delivery of Crude Extract of Phycocyanin for Treating Gingivitis
Mingxing Li#, Patipat Kamdenlek# , Panwong Kuntanawat, Komgrit Eawsakul, Thantrira Porntaveetus, Thanaphum Osathanon, and Chawan Manaspon*Published Date : 2022-10-18
DOI : https://doi.org/10.12982/CMUJNS.2022.052
Journal Issues : Number 4, October-December 2022
Abstract The phycocyanin (PC) in the crude extract is one of the main active compounds that has significant anti-inflammatory properties and the potential to treat gingival inflammation, the common oral disease. This work reports the preparation and characterization of chitosan/pluronic F-127 hydrogels entrapping the crude extracts of PC from Arthrospira platensis (C005H and C005L) as a local drug delivery system aiming to prolong PC release for the treatment of gingivitis. The results showed that the total phenolic content (TPC) values of the crude extracts were in the range of 2 to 5 μg GAE/100g and presented above 85% inhibition in the protein denaturation test and over 65% using a lipoxygenase (LOX) inhibition test. The hydrogels incorporating the crude extracts from C005H and C005L were perfectly prepared via the electrostatic interaction between chitosan and pluronic F-127 with very high encapsulation efficiency. The crude extracts of PC C005H and C005L were released over 70% from the loaded hydrogel within 6 hours under artificial saliva conditions. The anti-inflammatory activity of the released supernatant from the hydrogels after 6 hours was around 32-47% by LOX inhibition. The hydrogel vehicles and loaded-hydrogels did not show any cytotoxic effects against mouse fibroblast cell lines (L929) and human gingival fibroblast cells. Our current work shows that the crude extract PC-loaded chitosan/pluronic F-127 hydrogel is biocompatibility with human cells and shows prolonged crude extract release properties, suggesting it as an alternative treatment approach for gingivitis.
Keywords: Phycocyanin, Gingivitis, Hydrogel, Chitosan, Pluronic F-127
Funding: This work was supported by The Junior Researcher Fund, Chiang Mai University (R0000022723). TP is supported by Health Systems Research Institute (65-139) and National Research Council of Thailand (NRCT)(N42A650229).
Citation: Li, M., Kamdenlek,P., Kuntanawat, P., Eawsakul, K., Porntaveetus, T., Osathanon, T., and Manaspon, C. 2022. In vitro preparation and evaluation of chitosan/pluronic F-127 hydrogel as a local delivery of crude extract of phycocyanin for treating gingivitis. CMUJ. Nat. Sci. 21(4): e2022052.
INTRODUCTION
Gingivitis is one of the common oral diseases which mainly caused by the accumulation of dental plaque, leading to gingival inflammation. The progress of this symptom is caused by periodontal bacteria (gram negative), which is involved to the surface of the tooth surface and gums. According to the statistical data, around 8.52% of adults aged 20 to 64 face gingivitis (Eke et al., 2018). The traditional treatment for gingivitis is the removal of dental plaque and tartars together with the infected bacteria from the tooth surfaces and gums. Local application of antibiotic agents such as chlorhexidine mouthwash is applied as adjuvant therapy.
Phycocyanin (PC) is one of the major pigment constituents of cyanobacteria, it is nutritional and therapeutic values have been very well documented around the world. PC can be directly extracted from the cyanobacteria using different techniques such as the acid extraction method and ultrasonic-associated extraction method (Pagels et al., 2021). It shows an excellent anti-inflammatory potential, which is a series of multi-site effects such as removing various reactive oxygen species (ROS) and inhibiting the protein denaturation or enzyme (Prabakaran et al., 2020), resulting in the recovery of the tissue. Moreover, the toxicity of the PC is low or nontoxic as an orally active compound. Its lethal dose (LD50) in rats and mice has been reported at concentrations greater than 2 g/kg (Grover et al., 2021).
The local drug delivery system, which makes the drug or compound reach its site of action with a higher concentration than the original dosage form, is an emerging research field with minimal to no side-effect vehicles for drugs or compounds used in humans (Rajeshwari et al., 2019). Using delivery systems, the drug or compound is protected by the carrier from biological environments such as saliva washout, temperature, or other secreted biological solutions. Therefore, the local drug delivery system optimizes the therapeutic properties of the drug in terms of safety, effectiveness, and reliability.
Chitosan is a cationic polysaccharide obtained by deacetylation of chitin. It is a low-toxic, inexpensive, hydrophilic, biodegradable, & biocompatible biopolymer. Thus, chitosan polymer is widely used in tissue engineering and drug delivery application research (Mohebbi et al., 2019). Liu et al. (2022) reported the various preparation of chitosan-based hydrogels such as cross-linked networks ionic complexes or self-assembling vehicles for drug delivery of a therapeutic payload. Here, the developed chitosan hydrogels could control its release to high bioavailability at the site of action (Liu et al., 2022). Wei et al. (2022) developed a crosslinking-based hydrogel from carboxymethyl chitosan (CMCS) and polyethylene glycol (PEG) as a wound dressing. Based on CMCS backbone of the hydrogel, antioxidant and antibacterial agents were also confirmed the grafting using Fourier Transform Infrared Spectroscopy (FTIR) and 1H NMR. Pluronic F-127 also called Poloxamer 407, is a hydrophilic polymer. Pluronic F-127 has been approved by the US Food and Drug Administration (FDA) for use in humans and has been widely used as a drug carrier and tissue engineering (PJ Jaquilin et al., 2022). Turabee et al. (2019) fabricated docetaxel-loaded hydrogels from pluronic F127 and N,N,N-trimethyl chitosan to treat brain tumors. They performed the hydrogels under different pH to sustain the release of docetaxel. The released docetaxel significantly inhibited the glioblastoma (U87MG) xenograft tumor growth in mice (Turabee et al., 2019). García-Couce et al. (2022) developed chitosan/pluronic F-127 hydrogels-based using TPP (triphosphate) as a crosslinking agent. This hydrogel was used to entrap dexamethasone (DMT) for osteoarthritis treatment. The mixture was transformed into the gel by raising the temperature above 37°C. This hydrogel enhanced drug permeation and prolonged the retention, especially the formulation with TPP. The cumulative DMT release was around 65% after 170 hours. Curcumin-loaded quaternized chitosan/benzaldehyde-terminated pluronic® F127 (QCS/PF1.0) hydrogel was prepared and investigated its gelation, antibacterial activity, and antioxidant property by Qu et al. 2018. The results showed excellent biocompatibility with L929 cell line, over 90% killing ratio with E. coli and S. aureus, and over 80% scavenging efficiency by DPPH antioxidant test. Curcumin-QCS/PF1.0 hydrogels also promoted fibroblast migration and 4-folds upregulated vascular endothelial growth factor (VEGF) resulting in an assisting as a wound dressing in Kunming mouse.
In this work, we reported hydrogel preparation from chitosan and pluronic F-127. The hydrogel was used to encapsulate the crude extracts of phycocyanin (PC) from 2 strains of cyanobacterial (Arthrospira platensis strain C005H and strain C005L). Several essential properties such as protein denaturation inhibition, lipoxygenase (LOX) inhibition activity, total phenolic compound (TPC), and in vitro release studies were carried out. The characteristic properties of the hydrogel carrier were evaluated as well as its biocompatibility using fibroblast cell line (L929) and gingival fibroblast cells (GF).
MATERIAL AND METHODS
Materials
Two crude extracts of phycocyanins (PC) were prepared at the Institute of Agricultural Technology, Suranaree University of Technology. Herein, the crude extracts PC were prepared from Arthrospira platensis stain C005H (wild-type spirulina, helical trichomes) and Arthrospira platensis stain C005L (straight trichomes) by ultrasonic-assisted extraction method (Chaiyasitdhi et al., 2018). C005H strain was generously provided by Applied Algal Research Laboratory, Faculty of Science, Chiang Mai University. After a prolong period of time, straight trichomes were found immerged from the C005H culture. A single trichrome was then reisolated and renamed as C005L. Bovine serum albumin (BSA), phosphate buffer saline (PBS: tablet form), 3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyltetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), and triton X-100 were obtained from Amresco (Washington, USA). Pluronic F-127, Folin & Ciocalteu’s phenol reagent, lipoxygenase (LOX) enzyme, linoleic acid, and quercetin were obtained from Sigma-Aldrich (Missouri, USA). Chitosan powder was obtained from Ta Ming Enterprises CO. LTD (Samut Sakon, Thailand). Acetic acid and sodium chloride (NaCl) were obtained from Merck (New Jersey, USA). Sodium carbonate (Na2CO3) was obtained from Ajax Finechem (Sydney, Australia). Kanolone® was obtained from L.B.S. Laboratory LTD., PART. (Bangkok, Thailand). Boric acid was obtained from RCI-Labscan LTD (Bangkok, Thailand).
In vitro crude extracted characterizations
Total phenolic compound (TPC) of the crude extract of PC.
The total phenolic compound (TPC) of the crude extracts of PC (C005H and C005L) was determined using the Folin-Ciocalteu reagent (Hidayati et al., 2020). Briefly, 100 µl of the crude extract of PC was dissolved in deionized (DI) water and mixed with 100 µl of 1N of Folin-Ciocalteu reagent. The mixture was then left for 5 min at room temperature, followed by adding 80 µl of 5% (w/v) Na2CO3 solution. The reaction was plated in the dark for 1 hour. Absorbance was measured using a microplate reader spectrophotometer (HiPo MPP-96, BioSan, Latvia) at a wavelength of 568 nm. The standard gallic acid solutions (10, 25, 50, 100, 175, 250 µg/ml) were prepared and used to express all PCs in the unit of gram gallic acid equivalent (µg GAE/100g) using the following equation:
![]()
Protein denaturation inhibition
The crude extract of PCs at 50 µg/ml was mixed with 1% (w/v) of bovine serum albumin (BSA). The mixture at 1 ml was incubated at 37°C for 15 min, then heated at 70°C for 5 min (Gunathilake et al., 2018; Leelaprakash and Dass 2011). After cooling down, the clear solution of the sample was measured the absorbance at 660 nm using a UV/VIS spectrometer (GenesysTM 10s UV-Vis, Thermo Scientific, USA). PBS was used as the control group. The inhibition of protein denaturation was calculated by using the following equation.
![]()
LOX inhibition activity
The crude extract of PCs was investigated its LOX inhibitory potential by dissolving in DMSO at 50 µg/ml. The crude extract of PCs (25 µl) solution was mixed with 975 µl of LOX enzyme, 400 U/ml in 0.2 M, pH 9 borate buffer. One milliliter of the substrate (linoleic acid) at 250 µM was then added to the mixture (Marathe SJ et al., 2022). The absorbance of the reaction mixture was measured at 234 nm at 5 min after adding the substrate using a microplate reader (TECAN, Infinite 200 Pro M Plex, Männedorf, Switzerland). DMSO was used as a control group, whereas quercetin and gallic acid were used as reference active compounds. The percentage of LOX inhibition activity was calculated by using the following equation.
![]()
Hydrogels preparation and encapsulation of the crude extract PC
Chitosan (1 g) was dissolved in 100 ml of acetic acid solution (2% v/v). The solution was then dialyzed in DI water for 2 days and normal saline for another day to normalize the pH at the natural condition. Dialyzed chitosan solution (10 ml) was mixed with 2 g of pluronic F-127. The solution was mixed and kept at 4°C overnight and at room temperature for 1 day. After formulation, the hydrogels were kept at room temperature for up to 8 weeks with weekly observation for a stability investigation.
To prepare the crude extract of PC-loaded hydrogel, the crude extract of PC (5 mg) was dissolved in 1 ml DMSO solution (0.1% (v/v)). This DMSO solution was added to the chitosan solution. Pluronic F-127 powder was then added and prepared the hydrogel from the previous section.
Characterization of PC-loaded hydrogels
Release profile
The crude extract of PC-loaded hydrogels (C005H- and C005L-loaded hydrogels) at 0.1 g was plated on a membrane filter (Whatman®) and left on the top of a 24-well plate containing 2 ml of artificial saliva (0.75 g KCl, 0.07 g MgCl2, 0.199 g CaCl2, 0.965 g K2HPO4, 0.435 g KH2PO4, 36 g sorbitol, 2.4 g sodium Benzoate, and 1,200 ml DI water, pH 7.4). At determination time points (30 min, 1, 2, 3, and 6 hours), the artificial saliva solutions were collected and refreshed with equal volume (Gupta et al., 2014; Pelegrino et al., 2018). The harvested solutions were then measured the absorbance at 620 nm and calculated the release profile using the following equation.
![]()
Hence, the released samples at 3 and 6 hours-incubation were then collected by centrifuge. The supernatants were used to investigate the protein denaturation inhibition and the LOX inhibition activity using artificial saliva solution as a control group. The procedures were mentioned in the previous section.
In vitro biocompatibility testing
L929 (Mouse fibroblast cell) cell line was obtained from the Japanese Collection of Research Bioresources Cell Bank (JCRB Cell Bank). Cells were grown in Dulbecco’s Modified Eagle Medium (DMEM, Gibco, USA). The medium was supplemented with 10% (v/v) of fetal bovine serum (FBS, Gibco, USA) and 100 U/ml of penicillin/100 µg/ml of streptomycin (Gibco, USA). For gingival fibroblast cells (GF), cells were isolated from healthy patients using the approved protocol by the Human Research Ethics Committee, Faculty of Dentistry, Chulalongkorn University (No. 049/2020). The GF cells were cultured with the same culture medium as the L929 cell. Those cells were incubated and grown at 37°C in a humidified atmosphere with 5% CO2.
Cytotoxicity of the crude extracts of PC
Cells (L929 or GF) were plated into 96 well plates with a density of 10,000 cells per well at least 1 day before the experiment. Serum-free culture medium was used to prepare the series dilution/various concentrations of the crude extracts of PC in the range of 0 – 1,000 µg/ml. Cells were treated with several concentrations of the extracts at different periods, including 1 and 3 days at 37°C (Hernandez et al., 2017). Fresh serum-free DMEM and 1% Triton X-100 were used as negative control and positive control conditions, respectively. The cell viability of the study was evaluated by MTT assay.
Cytotoxicity of Chitosan/Pluronic F-127 hydrogels
The chitosan/pluronic F-127 hydrogels (with or without the crude extracts) were prepared by adding 1 ml of serum-free DMEM medium to 0.1 g of the hydrogels (following ISO 10993 Part 12). The extracted medium was collected after 24 hours-incubation at 37°C. The extracted medium was prepared at 100% and 50% by diluting with the fresh culture medium. These test media were plated to the cells (L929 and GF) which were plated into 96 well plates with a density of 10,000 cells per well at least 1 day before the experiment. All experiments were evaluated the number of cells by MTT assay after 1 day of incubation. In this study, fresh serum-free DMEM and 1% Triton X-100 were used as control and negative control group.
Wound healing assay
GF cells were plated into 12 well plates with a density of 50,000 cells per well and incubated for at least 1 day to fulfil cell confluence in the well. The culture media were refreshed with the serum-free DMEM before the experiment for at least another 1 day. The cells were scraped in a perpendicular straight line using a 10 μl pipette tip (Kominato et al., 2022). The migration of the GF cells was investigated by taking a series of bright-field images at the initial time and 1 day of incubation. All images were exported as 16-bit TIFF files and processed with the ImageJ (Version 1.53) program (National Institutes of Health) using a plugin “MRI_Wound_Healing_Tool.ijm” as a calculation tool.
Statistical analysis
All studies were investigated at least triplicate and presented as the mean ± standard deviation for all assays tested. A student t-test was performed to compare all data. The differences were considered to be significant at a level of P < 0.05.
RESULTS
Total phenolic compound (TPC), protein denaturation inhibition, and LOX inhibition activity
The total phenolic content (TPC) of the crude extracts of PC from Arthrospira platensis strain C005H and Arthrospira platensis strain C005L was evaluated using the Folin-Ciocalteu assay. The investigated concentration of the crude from both strains was 50 µg/ml. The TPC value of C005H and C005L were 4.9 ± 0.6 and 3.6 ± 0.9 µg GAE/100g, respectively. Moreover, the protein denaturation of the crude extracts (C005H and C005L) was investigated using the heat shock with the mock protein, bovine serum albumin (BSA). In this study, the crude extracts were prepared at 50 µg/ml with 1% (w/v) of BSA. The result showed that the extract from C005H and C005L strain referred significantly higher than the control group at 91.0 ± 5.2 and 86.4 ± 9.8% inhibition, whereas the control group (PBS) was 64.8 ± 13.0%. For lipoxygenase enzyme or LOX, this enzyme plays a role in the synthesis of mediators like leukotrienes. The study of inhibition of LOX could refer to the anti-inflammatory effect. In this test, the crude extract samples at the same concentration as the protein denaturation study (50 µg/ml) were prepared and investigated using quercetin (49.7 ± 2.7%) and gallic acid (37.5 ± 2.5%) as reference active compounds. The extract from C005H was 75.0 ± 2.5%, and 86.4 ± 9.8% for the crude from C005L.
Preparation and Characterization of the crude extract PC-loaded Hydrogel
The mixture of chitosan solution (1% w/v) with 20% (w/v) of pluronic F-127 was successfully formed as a gel. The blank hydrogel and the crude extract of PC-loaded hydrogels images were shown in Figure 1. The visible result of the blank hydrogels appeared as a white matrix, Figure 1a. Interestingly, there slightly changed by losing their humidity during the two months at room temperature. The addition of the crude extract resulted in a different color of the mixture. C005H- and C005L-loaded hydrogels were cleanly fabricated and shown in Figures 1b and 1c.
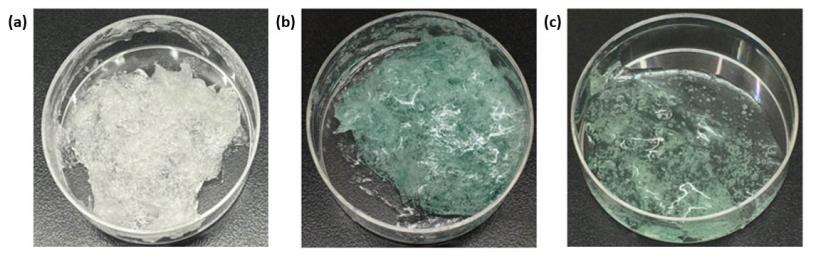
Figure 1. Photos of (a) blank hydrogel (b) C005H-loaded hydrogel (c) C005L-loaded hydrogel.
The relationship between the cumulative release vs time for C005H-loaded hydrogel and C005L-loaded hydrogel was compared as shown in Figure 2. In this study, the cumulative release was increasing gradually over time. The cumulative release of the crude extracts of PC from C005H-loaded hydrogel and C005L-loaded hydrogel at 6 h was 81.5% and 72.6%, respectively. There was no different release profile between those loaded hydrogels.
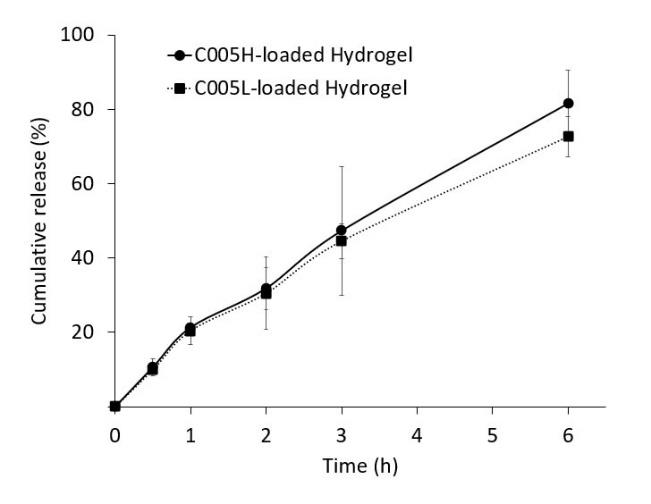
Figure 2. The release profile of the crude extract of phycocyanin (PC) from the hydrogels.
Herein, the released samples at 3 and 6 hours-incubation were collected and investigated their anti-inflammatory potential. The results of % inhibition of LOX activity showed that released samples prepared from C005H-loaded hydrogel at 3 and 6 hours-incubation were increasing from 26.4 + 4.0 to 32.5 + 2.7%. The inhibitions of protein denaturation were noted as 11.2 + 1.1 and 20.0 + 10.4% at 3- and 6-hours incubation time, respectively. The LOX inhibition activity of the samples prepared from C005L-loaded hydrogel showed significantly higher C005H-loaded hydrogel samples at 39.6 + 2.7 and 47.5 + 1.5% at 3 and 6 hours-incubation. But, the results from protein denaturation were not significantly different from the C005H-loaded hydrogel samples at 10.7 + 6.0 and 15.5 + 4.7% (3- and 6 hours-incubation samples).
In vitro biocompatibility testing
Cytotoxicity of the extracted against L929 and GF
Cytotoxicity results of the extracted PC on L929 and GF cells were shown in Figure 3. For both L929 and GF cells, the MTT results from 1 day-incubation showed that the relative cell survival of C005H and C005L at different concentrations was basically above 70%. Increasing the incubation time to 3 days, overall cell survival was still greater than 80%, except the crude extract of PC from C005L showed the toxic above 100 µg/ml. Herein, the IC50 (Inhibitory Concentration) of the C005L crude extract was approximately 194 µg/ml.
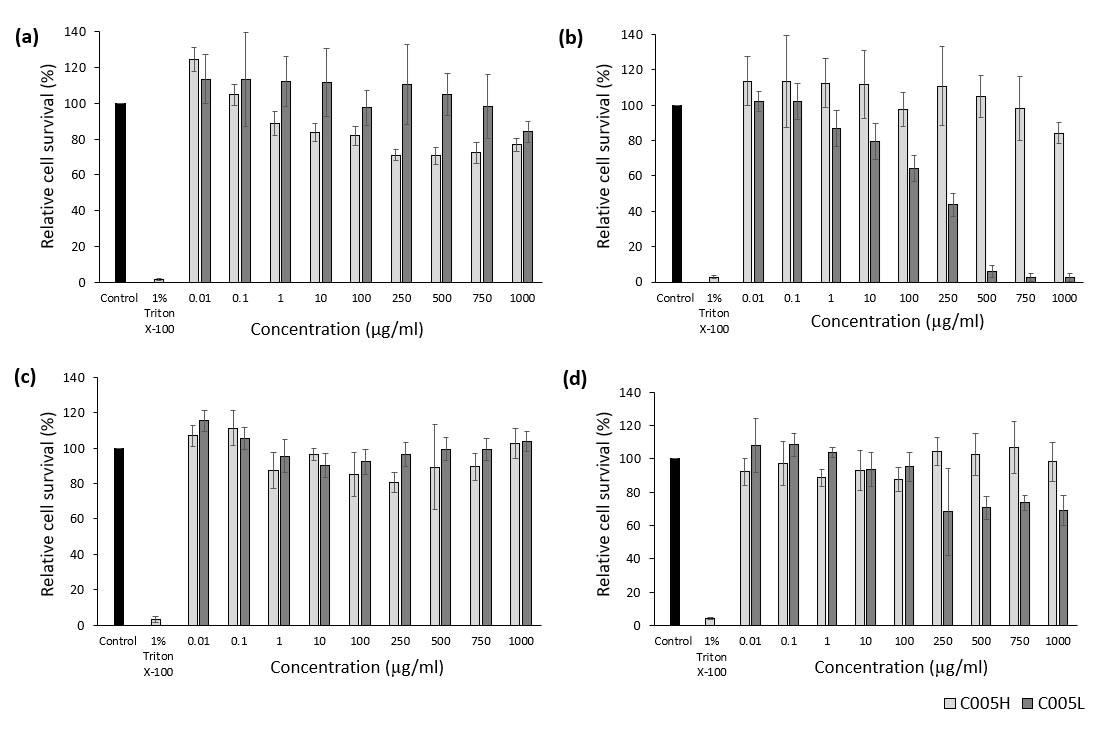
Figure 3. In vitro cytotoxicity of the crude extract from 2 strains: Arthrospira platensis strain C005H and Arthrospira platensis stain C005L. Graphs showed the relationship between cell viability (%) and concentration of the crude extract: (a) 1-day incubation with L929 cells day, (b) 3-day incubation with L929 cells, (c) 1-day incubation with GF cells, and (d) 3-day incubation with GF cells. (n = 6).
Cytotoxicity of Chitosan/Pluronic F-127 hydrogel
Cytotoxicity results of the hydrogels and cell morphological observation were shown in Figures 4 and 5. Both L929 and GF cells were treated with the extracted medium prepared from the blank hydrogel, C005H-, and C005L-loaded hydrogels. Overall results of the blank and extracted PC-loaded hydrogel showed over 90% cell survival, Figures 4e and 5e. There were no significantly toxic to L929 and GF cells after treating those cells with 100% extracted medium, Figure 4c-d, and Figure 5c-d. Meanwhile, treated cells with 1% (v/v) Triton X-100 had altered cell shapes and below 3% cell viability, Figure 4b and Figure 5b. Herein, a commercial product (Kanolone®) was used to compare. There was no cellular toxicity after treated L929 and GF cells using the same procedure as mentioned above.
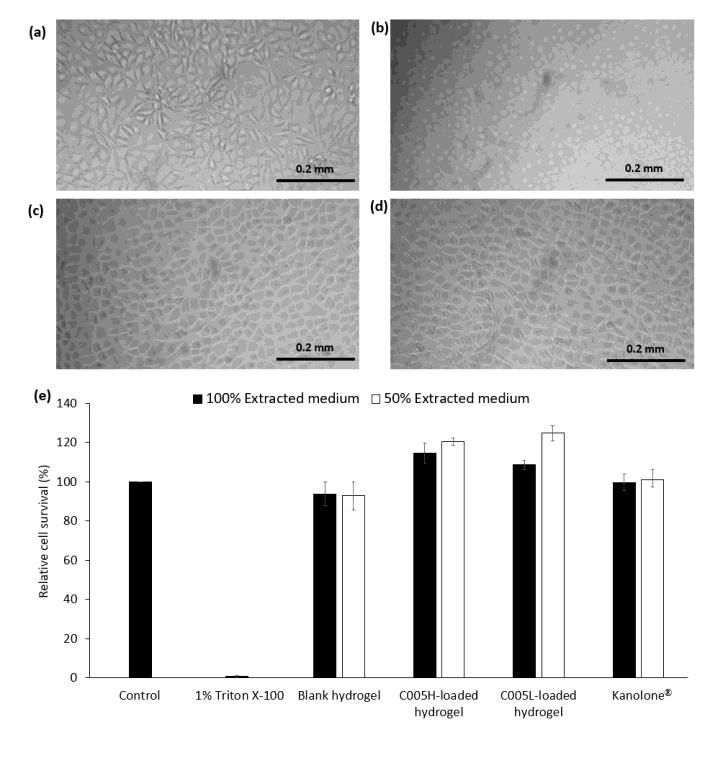
Figure 4. Morphology and viability of L929 cells: L929 cells were treated with (a) fresh culture medium (control), (b) 1% Triton X-100, 100% extracted medium prepared from (c) C005H-loaded hydrogel, and (d) C005L-loaded hydrogel. (e) L929 cell viability after 1 day treated with extracted culture medium (100% and 50%) prepared from chitosan/pluronic F-127 hydrogel and crude extract PC-loaded hydrogel on L929 cells. The scale bar is 0.2 mm.
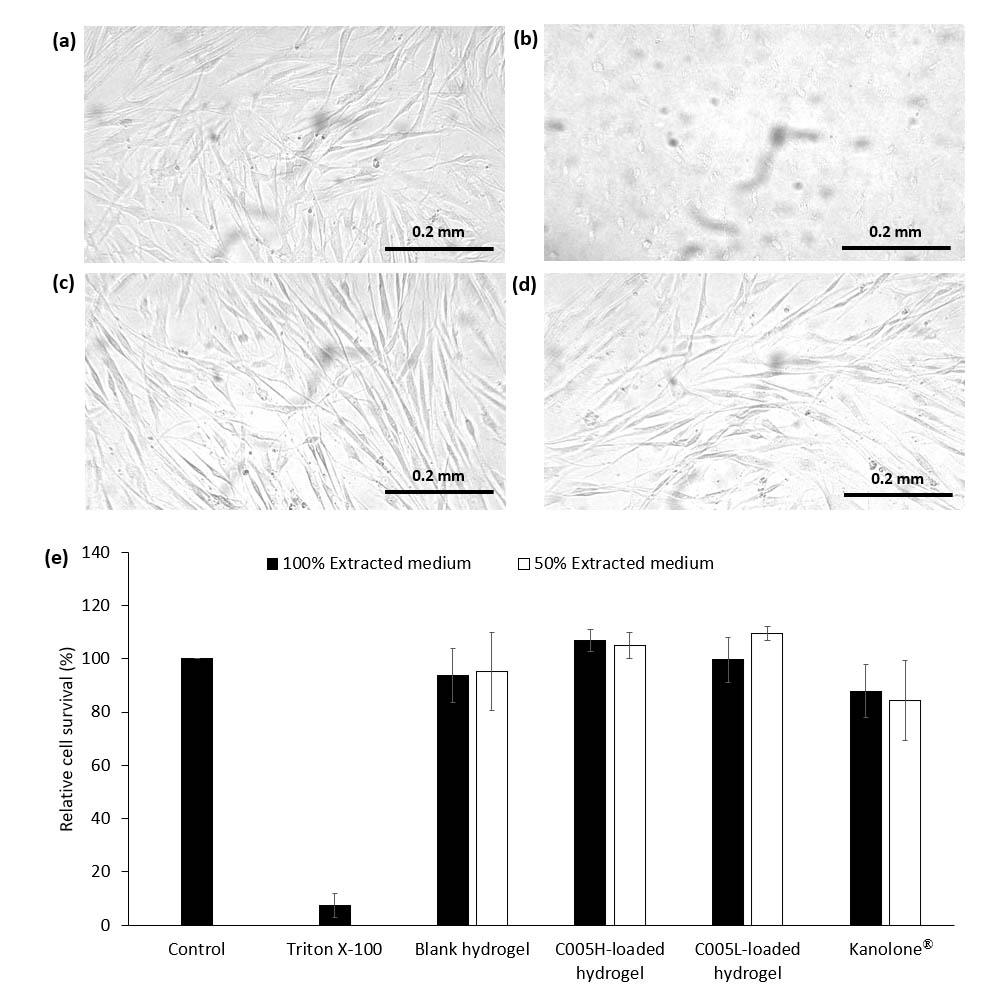
Figure 5. Morphology and viability of GF cells: GF cells were treated with (a) fresh culture medium (control), (b) 1% Triton X-100, 100% extracted medium prepared from (c) C005H-loaded hydrogel, and (d) C005L-loaded hydrogel. (e) GF cell viability after 1 day treated with extracted culture medium (100% and 50%) prepared from chitosan/pluronic F-127 hydrogel and crude extract PC-loaded hydrogel on GF cells. The scale bar is 0.2 mm.
Wound healing assay
The wound-healing assay of GF cells was evaluated by measuring the recovery area after 24 hours of scratching the monolayer of GF cells. The GF cells were cultured under different conditions of the culture medium as shown in Figure 6. The recovery area of the GF cells treated with serum-free medium and the extracted medium prepared from Kanolone® were 38.5% and 53.1%. In contrast, the testing medium prepared from blank hydrogel, C005H-, and C005L-loaded hydrogel were 0.2%, 20.5%, and 13.4%, respectively.
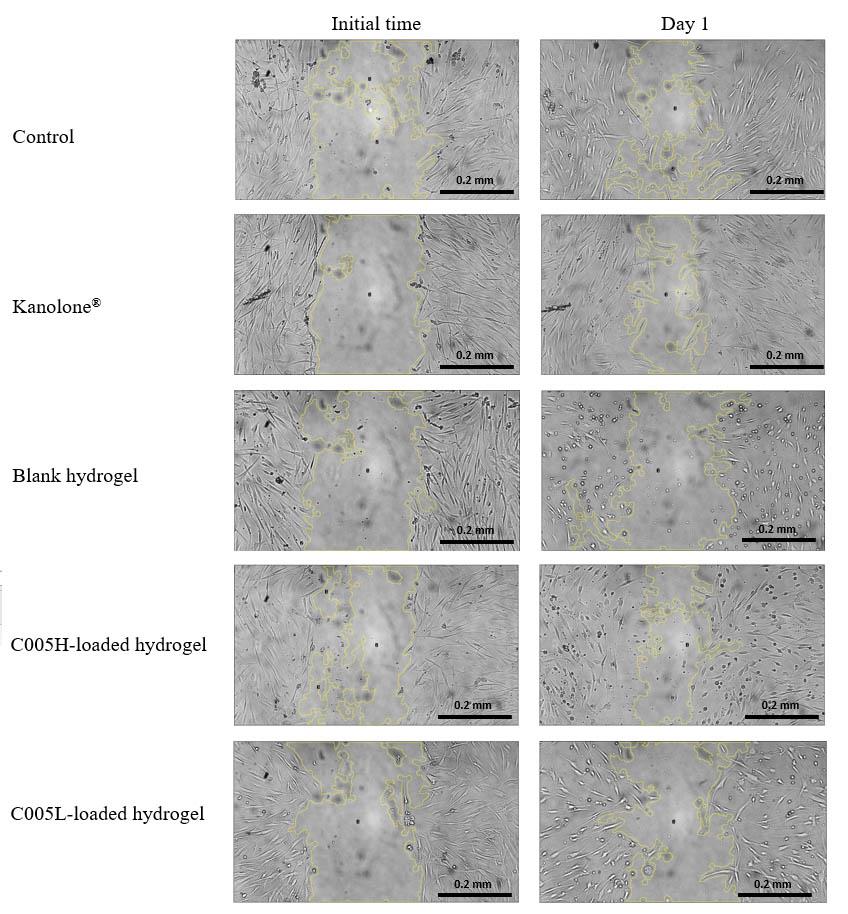
Figure 6. The wound-healing assay area of the GF cells was analyzed by using the Image J software after scratching. The recovery area was presented after 24 hours of incubation.
DISCUSSION
PC is one of the main phenolic compounds found in the crude extract from Arthrospira platensis. The phenolic compounds exhibit free radical inhibition, peroxide decomposition, and protection against oxidative disease burden in biological systems (Tayag et al., 2010). The phenolic compounds act as good electron donors because their hydroxyl groups can directly contribute to antioxidant effects. The free radical scavenging ability of the extract was related to its total phenolic content (TPC) concentration. Generally, the crude extract from spirulina has been used as food additives mainly in Asia-Pacific where the market is grown at a Compound Annual Growth Rate (CAGR) of 6.1% by 2026 reported by Mordor Intelligence on the website, May 2022.
For this study, the wild-type strain of spirulina (C005H) and the straight trichomes stain (C005L) did not show different numbers of the TPC value. However, the crude extracts had studied the antioxidant capacity like protein denaturation. Compared with the case without the crude extract (64.8%), the addition of 2 crude extracts from both C005H and C005L significantly increased the percentage of the inhibition of the mock protein (BSA) degradation at high temperature. Herein, the LOX inhibition activity was used to explain the anti-inflammatory potential. The activity of the crude extracts of PCs were significantly greater than our reference active compound (quercetin and gallic acid) at least 2 times. These results indicated that the crude extract of PC from both stains had a strong resistance to inflammatory reactions. However, the limitations of this work were in vitro experiments and the low purity of the PC. The recovery and purification process of the PC might require cost and time. It might interrupt the activity level of the extracted compound. For biocompatibility testing, the cell viability results of the crude extract did not show any significant severe toxicity against L929 and GF cells. Both L929 and GF cells appeared normal under a microscope. Herein, we recommended using both crude extracts of PC below 100 µg/ml for future studies. This present study employed the benefit of the use of the direct crude extract of PC from the spirulina C005H and C005L stains for future development. The extract could be increased the value by incorporating the hydrogel carriers.
The chitosan/pluronic F-127 hydrogel was spontaneously formed when pluronic F-127 was added to the chitosan solution. Pluronic F-127 acted as a non-toxic cross-linker between chitosan cationic polymers using an electrostatic interaction between different polar of those polymers (Mohamed et al., 2022). Our finding has shown that over 15% (w/v) of pluronic F-127 in the mixture could form the hydrogel. In addition to stability improvement, higher pluronic F-127 contained in the mixture could form the hydrogel for up to 2 months at room temperature as mentioned in PF127-TMC/DTX hydrogels by Turabee et al. (2019). Unlike the curcumin-QCS/PF1.0 hydrogel by Qu et al. (2018), our hydrogels did not provide a bond like a methyl bond by glycidytrimethylammonium chloride (GTMAC). Thus, gelation time would be increased up to a day. Swelling and gel recovery were not studied by stretching/compression and releasing the hydrogels. Although C005H and C005L compound did not link to the backbone of the hydrogel matrix, the release of the crude extract of PC (C005H and C005L) could be controlled and did not provide as a burst release within the first 2 hours. Because the electrostatic and gelation processes are random, this was a simple process that could entrap the crude extracts of PC into the gels using pluronic F-127 as a challenging matter as the polyplex nanoparticles caused by the charges of dsRNA and chitosan (Lichtenberg et al., 2019). As expected, the amount of the crude extracts of PC resulted in almost 100% encapsulation efficiency. The photos of C005H- and C005L-loaded hydrogel showed in green color due to the presence of those pigments. Based on the calculation, the loading efficiency of the crude extract of PC (both C005H and C005L) into the chitosan/pluronic F-127 was approximately 0.5 %. This condition was selected for further studies.
In a real application, the non-strong cross-linker, pluronic F-127, can be easily disrupted by the biological solution. Over 70% of released-crude extract of PC showed a prolonged period (6 hours) at the site and kept the concentration of the PC within the therapeutic index. Compared with QCS/PF1.0 hydrogels, the release of curcumin was approximately 20% within 100 hours due to the conjugation bond requiring an acidic condition to cleave (Qu et al, 2018). Moreover, the anti-inflammatory activity of the released samples showed the potential of the use of these hydrogels for gingivitis treatment. It should be noted that all conditions of the PC-loaded hydrogels were no toxic effect on L929 and GF cells. In the part of wound healing, we compared the area of the cell treated with the extract medium from the PC-load hydrogels with the control and commercial product, Kanolone®. The effectiveness of wound healing of the PC-loaded hydrogels was rather lower than Kanolone® due to this study used a 50% concentration of the extracted medium and was exposed to the GF cells for 24 hours. The lower purity of PC might show an ineffective compound as a limitation of this study. Furthermore, this study did not perform over 24 hours due to the previous report by Afrasiabi et al. (2021). The toxicity of chitosan hydrogel to human gingival fibroblast cells (HGFs) was found if the concentration of the hydrogel was over 156.2 μg/ml with 72-hour-incubation (Afrasiabi et al., 2021). Thus, we found that the extracted medium prepared from the blank hydrogel did not increase the recovery area as the extracted medium from PC-loaded hydrogels.
A major advantage of this preparation is simply because of a spontaneous method. Consequently, the hydrogels are likely to be non-toxic and biocompatible. The hydrogels can be easily degraded in the presence of water to release the crude extract of PC. We believe that this preparation is promising as an oral delivery vehicle for the treatment of gingivitis.
CONCLUSION
The present study described the anti-inflammatory capacity and cytotoxicity of PC, as well as the hydrogel preparation, PC release profile, and cytotoxicity of PC-containing chitosan/pluronic F-127 hydrogels. The crude extract of PC from both stains showed potential in inhibiting protein degradation and LOX inhibition activity with no cytotoxicity at concentrations below 100 μg/ml. These crude extracts of PC were completely encapsulated into the chitosan/pluronic F-127 hydrogels. In addition, the system was free of cross-linking agents and increased the biocompatibility of the hydrogel as there were no possible toxic by-products. The hydrogels were found to prolong-release the crude extracts within 6 hours with effective anti-inflammatory activity. Thus, PC-loaded chitosan/pluronic F-127 hydrogel from this study might be an alternative treatment for human gingivitis.
ACKNOWLEDGMENTS
The authors would like to acknowledge Applied Algal Research Laboratory, Chiang Mai University especially for providing the Arthrospira platensis starter stain (C005H). This study was supported by the Junior Researcher, Chiang Mai University (R000022723). TP was supported by Health Systems Research Institute (65-139) and National Research Council of Thailand (NRCT)(N42A650229).
AUTHOR CONTRIBUTIONS
ML and PK (Kamdenlek) contributed to experimental design, data acquisition, statistical analysis, data interpretation, and manuscript drafting. PK (Kuntanawat) provided the extract samples, information, and contributed to the experimental design and data interpretation. KE, TP, and TO contributed to experimental design and data interpretation. CM contributed to study conceptualization, experimental design, and data interpretation. All authors critically revised the manuscript and gave final approval for publication.
CONFLICT OF INTEREST
All authors declare no competing interests.
REFERENCES
Afrasiabi, S., Bahador A., and Partoazar A. 2021. Combinatorial therapy of chitosan hydrogel-based zinc oxide nanocomposite attenuates the virulence of Streptococcus mutans. BMC Microbiology. 21: 62.
Chaiyasitdhi, A., Miphonpanyatawichok W., Riehle M.O., Phatthanakun R., Surareungchai W., Kundhikanjana W., and Kuntanawat P. 2018. The biomechanical role of overall-shape transformation in a primitive multicellular organism: A case study of dimorphism in the filamentous cyanobacterium Arthrospira platensis. PLoS ONE. 13: e0196383.
Eke, P.I., Thornton-Evans G.O., Wei L., Borgnakke W.S., Dye B.A., and Genco R.J. 2018. Periodontitis in us adults: National health and nutrition examination survey 2009-2014. The Journal of the American Dental Association. 149: 576-588.
García-Couce, J., Tomás M., Fuentes G., Que I., Almirall A., and Cruz L.J. 2022. Chitosan/Pluronic F127 thermosensitive hydrogel as an injectable dexamethasone delivery carrier. Gels. 8: 44.
Grover, P., Bhatnagar. A., Kumari. N., Bhatt. A.N., Nishad. D.K., and Purkayastha. J. 2021. C-phycocyanin-a novel protein from Spirulina platensis-In vivo toxicity, antioxidant and immunomodulatory studies. Saudi Journal of Biological Sciences. 28: 1853-1859.
Gunathilake, K., Ranaweera K., and Rupasinghe H. 2018. In vitro anti-inflammatory properties of selected green leafy vegetables. Biomedicines. 6: 107.
Gupta, H., Sharma A., and Shrivastava B. 2014. Pluronic and chitosan based in situ gel system for periodontal application. Asian Journal of Pharmaceutics. 3: 94-96.
Hernandez, F.Y.F., Khandual S., and López I.G.R. 2017. Cytotoxic effect of Spirulina platensis extracts on human acute leukemia kasumi-1 and chronic myelogenous leukemia k-562 cell lines. Asian Pacific Journal of Tropical Biomedicine. 7: 14-19.
Hidayati, J.R., Yudiati E., Pringgenies D., Oktaviyanti D.T., and Kusuma A.P. 2020. Comparative study on antioxidant activities, total phenolic compound and pigment contents of tropical Spirulina platensis, Gracilaria arcuata and Ulva lactuca extracted in different solvents polarity. E3S Web of Conferences. 147: 03012.
Kominato, H., Takeda K., Mizutani K., Mikami R., Kido D., Buranasin P., Saito N., Takemura S., Nakagawa K., Nagasawa T., and Iwata T. 2022. Metformin accelerates wound healing by Akt phosphorylation of gingival fibroblasts in insulin‐resistant prediabetes mice. Journal of Periodontology. 93: 258-70.
Leelaprakash, G. and Dass S.M. 2011. In vitro anti-inflammatory activity of methanol extract of Enicostemma axillare. International Journal of Drug Development and Research. 3: 189-196.
Lichtenberg, S.S., Tsyusko O.V., Palli S.R., and Unrine J.M. 2019. Uptake and bioactivity of chitosan/double-stranded RNA polyplex nanoparticles in Caenorhabditis elegans. Environmental Science & Technology. 53: 3832-3840.
Liu, Z., Wang K., Peng X., and Zhang L. 2022. Chitosan-based drug delivery systems: current strategic design and potential application in human hard tissue repair. European Polymer Journal. 166: 110979.
Marathe, S.J., Hamzi W., Bashein A.M., Deska J., Seppänen-Laakso T., Singhal R.S., and Shamekh S. 2022. Anti-angiogenic effect of Cantharellus cibarius extracts, its correlation with lipoxygenase inhibition, and role of the bioactives therein. Nutrition and Cancer. 74: 724-734.
Mohamed, H.B., Attia Shafie M.A., and Mekkawy A.I. 2022. Chitosan nanoparticles for meloxicam ocular delivery: Development, in vitro characterization, and in vivo evaluation in a rabbit eye model. Pharmaceutics. 14: 893.
Mohebbi, S., Nezhad M.N., Zarrintaj P., Jafari S.H., Gholizadeh S.S., Saeb M.R., and Mozafari M. 2019. Chitosan in biomedical engineering: a critical review. Current Stem Cell Research & Therapy. 14: 93-116.
Pagels, F., Pereira R.N., Vicente A.A., and Guedes AC. 2021. Extraction of pigments from microalgae and cyanobacteria—A review on current methodologies. Applied Sciences. 11: 5187.
Pelegrino, M.T., de Araujo Lima B., Do Nascimento M.H., Lombello C.B., Brocchi M., and Seabra A.B. 2018. Biocompatible and antibacterial nitric oxide-releasing pluronic F-127/chitosan hydrogel for topical applications. Polymers. 10: 452.
PJ Jaquilin, R., Oluwafemi O.S., Thomas S., and Oyedeji A. 2022. Recent advances in drug delivery nanocarriers incorporated in temperature-sensitive Pluronic F-127–A critical review. Journal of Drug Delivery Science and Technology. 72: 103390.
Prabakaran, G., Sampathkumar. P., Kavisri. M., and Moovendhan. M. 2020. Extraction and characterization of phycocyanin from Spirulina platensis and evaluation of its anticancer, antidiabetic and antiinflammatory effect. International Journal of Biological Macromolecules. 153: 256-263.
Qu, J., Zhao X., Liang Y., Zhang T., Ma P.X., Guo B. 2018. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials. 183:185-199.
Rajeshwari, H., Dhamecha D., Jagwani S., Rao M., Jadhav K., Shaikh S., Puzhankara L., and Jalalpure S. 2019. Local drug delivery systems in the management of periodontitis: A scientific review. Journal of Controlled Release. 307: 393-409.
Tayag, C.M., Lin Y.-C., Li C.-C., Liou C.-H., and Chen J.-C. 2010. Administration of the hot-water extract of Spirulina platensis enhanced the immune response of white shrimp Litopenaeus vannamei and its resistance against vibrio alginolyticus. Fish & Shellfish Immunology. 28: 764-773.
Turabee, M.H., Jeong T.H., Ramalingam P., Kang J.H., and Ko, Y.T. 2019. N, N, N-trimethyl chitosan embedded in situ pluronic F127 hydrogel for the treatment of brain tumor. Carbohydrate Polymers. 203: 302-309.
Wei, Q., Wang Y., Wang H., Qiao L., Jiang Y., Ma G., Zhang W., and Hu Z. 2022. Photo-induced adhesive carboxymethyl chitosan-based hydrogels with antibacterial and antioxidant properties for accelerating wound healing. Carbohydrate Polymers. 278: 119000.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand.
https://cmuj.cmu.ac.th
Mingxing Li1, #, Patipat Kamdenlek1, #, Panwong Kuntanawat2, Komgrit Eawsakul3, Thantrira Porntaveetus4, Thanaphum Osathanon5, and Chawan Manaspon1,*
1 Biomedical Engineering Institute, Chiang Mai University, Chiang Mai, 50200, Thailand.
2 School of Biotechnology, Institute of Agricultural Technology, Suranaree University of Technology, Nakhon Ratchasima, 30000, Thailand.
3 School of Medicine, Walailak University, Nakhon-Si-Thammarat, 80160, Thailand.
4 Center of Excellence in Genomics and Precision Dentistry, Department of Physiology, Faculty of Dentistry, Chulalongkorn University, Bangkok, 10330, Thailand.
5 Dental Stem Cell Biology Research Unit, Department of Anatomy, Faculty of Dentistry, Chulalongkorn University, Bangkok, 10330, Thailand.
# contributed equally
Corresponding author: Chawan Manaspon, E-mail: chawan.m@cmu.ac.th
Total Article Views
Editor: Wasu Pathum-Aree,
Chiang Mai University, Thailand
Article history:
Received: May 10, 2022;
Revised: June 2, 2022;
Accepted: July 6, 2022;
Published online: July 18, 2022

