
Root Yield and Starch Synthase Type IV Gene Activity under Different Micro Nutrient Fertilizer and Harvest Ages on Cassava (Manihot esculenta Crantz)
Kukuh Setiawan*, Paul Benyamin Timotiwu, Agustiansyah, Muhammad Syamsoel Hadi, Muhammad Kamal, Ardian, and Wawan Abdullah Setiawan
Published Date : 2023-01-05
DOI : https://doi.org/10.12982/NLSC.2023.002
Journal Issues : Number 1, January-March 2023
Abstract The important part of cassava root is starch which is probably controlled by starch synthase type IV (SSIV) gene. The information of micro nutrient and harvest age related to the activity starch synthase type IV (SSIV) gene is still very rare. The objectives of this study were to evaluate root fresh weight of cassava, to compare yield of storage root, and to evaluate the activity of starch synthase type IV (SSIV) gene by real-time quantitative polymerase chain reaction (RT-PCR) applied by micro nutrient fertilizer. Treatments were arranged by factorial (3 × 3) in randomized complete block design (RCBD) with three replications used as block. The first factor was three different dosages of micro nutrient fertilizer as 0, 20, and 40 kg/ha. The second factor was harvest ages as 7, 8, and 10 months after planting (MAP). The micro nutrient fertilizer mainly contents of 5,888 ppm Fe and 1,368 ppm Zn. Variables were leaf number (LN), leaf fresh weight (LFW), leaf dry weight (LDW), stem fresh weight (SFW), stem dry weight (SDW), root fresh weight (RFW), root dry weight (RDW), skin root fresh weight (SRFW), skin root dry weight (SRDW), starch content, and activity of SSIV gene. The result showed that RDW of cassava applied by 40 kg micro nutrient/ha was significantly increased at 10 MAP. The increase in RDW was due to mainly high SSIV gene activity. Additionally, the SSIV gene activity caused by 20 kg micro nutrient/ha treatment showed almost as twice as those by 40 kg micro nutrient/ha.
Keywords: Real-time PCR, Root dry weight, Root yield, Starch, Stem dry weight
Funding: The authors are grateful for the research funding provided by Institute for Research and Community Service, University of Lampung, Lampung, Indonesia.
Citation: Janthakhin, Y., Kingtong, S., Aphibanthammakit, C., and Juntapremjit, S. 2023. Metformin Setiawan, K., Timotiwu, P.B., Agustiansyah, Hadi, M.S., Kamal, M., Ardian, and Setiawan, W.A. 2023. Root yield and starch synthase type IV gene activity under different micro nutrient fertilizer and harvest ages on cassava (Manihot esculenta Crantz). Nat. Life Sci. Commun. 22(1): e2023002.
INTRODUCTION
Cassava (Manihot esculenta Crantz) is widely distributed in Lampung Province of Indonesia. It seems that Lampung is the central production of cassava in Indonesia. In 2020 the harvest area for planting cassava was around 250 thousand ha (BPS, 2021) where the most (> 85%) of areal cassava belonged to small holder of farmers and the rest (around 15%) belonged to private company. It is well known that fresh cassava root contains starch. According to Apea-Bah et al. (2011) the optimum starch content of cassava will be achieved at 10 or 12 months after planting (MAP), as 71-75%. Unfortunately, based on the habitual Indonesian farmers, they harvest cassava at 7-8 MAP then sell to the tapioca industry of private company. The main reason for this condition was unstable price of fresh cassava root. Young cassava root could absolutely reduce both fresh root weight and starch content (Prammanee et al., 2010; Baafi and Safo-Kantanka, 2007), contain low protein (Sagrilo et al., 2003), and low amylose (Apea-Bah et al., 2011). On the other hand, the starch granule size would be small when harvested at early time in cassava (Sriroth et al., 1999) and in potato (Noda et al., 2004; Perry, 2000). Chatakanonda et al. (2003) informed that the granule quality tended to be low due to lost packing of amylose and amylopectin. It means that late harvested cassava root would increase starch granule size lead to the higher starch content.
The recommendation for harvesting time of cassava root is around 10 or 12 MAP to get the optimum starch content, approximately 69-78 % (based on root dry weight) or 23-26 % (based on root fresh weight) (Li et al., 2016; Cock et.al., 1979; Kayode, 1983; Apea-Bah et al., 2011; Agbaje and Akinlosotu, 2004). In amylose content, Janket et al. (2018) reported that late harvest time produced amylose content of 21.2 % while at harvest produced time amylose content of 22.5%. According to Zhu (2014) cassava starch has finer surface than potato starch and the more age cassava to be harvested the more granule would clearly distribute. Consequently, the proper harvest time would be at 10-12 MAP because starch granule would evenly distribute in the root resulted in increasing starch content.
The other problem is that the farmers or even private company frequently fertilized macro nutrient as N, P, and K but rarely put micro nutrient in the field. Moreover, in Thailand and Vietnam, the application of fertilizer for cassava tended to be more P than N and K (Howeler, 2001). The information regarding micro nutrient fertilizer for enhancing cassava starch yield is apparently rare. In Indonesia, Hadi (2010) gave information that application of micro nutrient could increase fresh weight of storage root (yield increment of 0.47 kg) when early harvested at 210 DAP. This means that low yield of cassava root and starch harvested at early time could be improved by adding micro nutrient fertilizer. Application of Zn could increase cassava fresh root weight and starch content in Thailand up to 30 % and 29 %, respectively (Panitnok et al., 2013) and sorghum grain yield (Kumar, 2013). Setiawan et al. (2017) showed their research result that the application of micro nutrient containing 5,888 ppm Fe and 1,368 ppm Zn could increase fresh root weight and starch granule size from 660 g to 870 g/plant and starch granule size from 16 µm to 26 µm, respectively. In addition, Dos Santos et al. (2014) concluded that absorption of micro nutrient would be influenced by cassava age and N content in plant. However, absorption of Fe showed high concentration in leaf and storage root, as 0.45 and 0.38 kg/ha, respectively in cassava plant without fertilizer application (Howeler, 1981 and 2001). Fageria (2009) reported that absorption of Fe would be high in the condition of low pH. Panitnok et al. (2013) reported that combination of Zn, Mg and S did not show the effect on storage root production but would be significant in the production of starch content. It would be 28.5% when applied by Zn but 24.9 % when applied no Zn (only Mg and S). Based on this, Li et al. (2016) reported that four factors could influence starch accumulation in root, as a) strong capacity of transport from stem part, b) low efficient of starch degradation, c) transportation of glucose from stem, and d) the ability of root to accumulate starch. Additionally, Guan et al. (2016) informed that one of the main key enzymes for starch biosynthesis was starch synthase.
Miao et al. (2014) conducted the study of starch synthase (GSS) gene on banana. They concluded that activity of GSS would increase gradually as the growth of banana fruit was developing. It is well known that starch is synthesized through four main fractions namely, ADP-Glc pyrophosphorylase (AGPase), soluble starch synthase (SSs), starch-branching enzyme (SBE), and starch-debranching enzyme (SDBE). The expression of M. esculenta isoamylase1 gene (Meisa1) was dependent on plant parts and age. Moreover, Beyene et al. (2010) concluded that Meisa1 was highest expression at 9 MAP in root parts compared to leaf and stem parts. Besides, Baguma (2004) informed that the transcriptional activity of sbe, a starch synthesis gene in the storage root would increase as the plant aged. Consequently, duplication and adaptive selection of SSs gene and granule bound starch synthase (GBSS) were indicated in the development of cassava starch (Yang et al., 2013) and (Salehhuzzaman et al., 1993), respectively. Setiawan et al. (2017) showed that micro nutrient application could increase the expression of SSIV gene considerably as many 200 times as control of without micro nutrient application at 190 DAP harvest time. This means that early harvest of cassava could be improved by micro nutrient application.
The objectives of this study were to evaluate fresh root weight of cassava applied by micro nutrient fertilizer, to compare yield of storage root treated by micro nutrient fertilizer as 0, 20, and 40 kg/ha, and to evaluate the activity of starch synthase type IV (SSIV) gene by real-time quantitative PCR (RT-PCR).
MATERIALS AND METHODS
This experiment was conducted on dry land of Central Lampung, Lampung, Indonesia, from March 2019 to February 2020. The soil type is yellowish red podzolic with the pH around 4.8. The planting material was variety cassava of UJ3 (originally selected from Kasetsart cassava clone) with planting distance of 100 cm x 80 cm. Fertilizers used in this study were urea (46% N), SP36 (36% P2O5), and KCl (60% K2O) as, 200 kg/ha, 100 kg/ha, and 200 kg/ha, respectively. These fertilizers were applied at 30 days after planting (DAP), however urea was split into two parts of application, first was at 30 DAP or one month after planting (MAP) and second was 120 DAP or three MAP. The application of micro nutrient fertilizer containing 5,888 ppm Fe and 1,368 ppm Zn was at 120 DAP together with the application of second urea.
Experimental design and observation variables
Treatments were arranged by factorial (3 x 3) in RCBD with three replications that were used as block. First factor was three levels of micro nutrient fertilizer as 0, 20, and 40 kg/ha. Second factor was three harvest ages as 7, 8, and 10 MAP. The observed variables in this study were vegetative parts and root parts. Vegetative parts were leaf number (LN), leaf fresh weight (LFW), leaf dry weight (LDW), leaf greenness (LG), stem fresh weight (SFW), stem dry weight (SDW). The root parts were root fresh weight (RFW), root dry weight (RDW), skin root fresh weight (SRFW), skin root dry weight (SRDW), starch content, and the expression of starch synthase gene activity. All variables (except for the expression of starch synthase gene activity) were observed three ages as 7, 8, and 10 MAP. Leaf number was measured from total leaves fully open per plant. Total fresh leaves were weighed and then put into oven at 80°C for 3 days so called LFW and LDW, respectively. This procedure was the same as for SFW, SDW, RFW, and RDW. Leaf greenness of middle leaf was measured by SPAD equipment. However, the expression of starch synthase (SSIV) gene activity was observed from the sample of micro nutrient treatment taken at 10 MAP of fresh root cassava. The reasons for this are that (a) the starch content was similar at 7, 8, and 10 MAP and (b) according to some references that the optimum starch content of cassava was 10-12 MAP. Observation data were examined with Analysis of Variance (ANOVA). The treatments having significant effect would be further tested using the Least Significant Difference (LSD) at a 5 % significance level (Gomez and Gomez, 1984). Additionally, the values of correlation among variables were based on 54 observations (n=54).
Starch measurement
The content of starch at 7, 8, and 10 MAP with the micro nutrient application of 0, 20, and 40 kg/ha was determined using the modification of combined procedure of McCleary et al. (2018) and Moorthy and Padmaja (2002) based on dry weight. Approximately 100 g of ground fresh cassava root was dissolved in 50 mL of cold water and allowed to stand for 24 hours. After separating from water, starch was then heated in oven at temperature of 60°C for 24 hours. The dried starch sample was then analyzed according to the procedure of Moorthy and Padmaja (2002). The starch content was measured based on ratio between filtrate dry weight and root dry weight multiply by 100%. The sample for starch analysis which was so called technical replicates was based on composite of two plants taken from each block. Consequently, each treatment has one sample per block for starch analysis.
Measurement of SSIV gene
The activity of SSIV gene was analyzed according to modification of Livak and Schmittgen (2001). Every treatment of micro nutrient application has one sample taken from one cassava plant which shows optimum growth. Fresh storage root of cassava was sampled from 0, 20, and 40 kg micro nutrient fertilizer/ha based on quantity of transcription of SSIV gene by using real-time quantitative PCR (RT-PCR). Approximately 0.1 g of fresh storage root that was sampled from cassava root treated by 0, 20, and 40 kg micro nutrient fertilizer/ha was taken aseptically then put in the solution of 1 ml RNA-later in sterile micro tube (DNAse and RNAse free) to protect RNA from nuclease activity during the process of sampling. The 0.1 g sample of fresh cassava root was taken from destructive sample at 10 MAP. This sample was taken and then kept in cool box during in the field until processed in the laboratory. The extraction of RNA was conducted by the method of Pure ZOL RNA isolation reagent from Biorad. Such total RNA was then checked the quantity of concentration and purity by UV-Vis nanospectrophotometer from Implen (Table 1). After getting the RNA total, cDNA library was made following the procedure determined by iScriptTM cDNA Synthesis Kit from Biorad. The material volume ofiScript reverse transcriptase, iScript reaction mix, and total RNA in RNAse-free water are 1, 4, and 15 µl, respectively.
Table 1. Concentration and purity of total RNA from nanospectrophotometer.
|
No |
Sample |
Concentration of total RNA (ng/µl) |
Purity of total RNA (A260/A280) |
|
1 |
U0 |
184 |
1,415 |
|
2 |
U1 |
146 |
1,377 |
|
3 |
U2 |
194 |
1,644 |
Note: U0 = Sample of fresh root cassava treated by 0 kg micro nutrient/ha; U1 = Sample of fresh root cassava treated by 20 kg micro nutrient/ha; U2 = Sample of fresh root cassava treated by 40 kg micro nutrient/ha
The cDNA was verified by electrophoresis in 1% agarose gel with TAE 1x. The agarose gel of 0.4 g was added in 40 ml TAE 1x then heated until in the form of solution and added again by 0.4 µl gel red to be cooled. After that, the result of this cDNA was amplified by PCR (sensoquest thermocycler from Germany) to verify the amplification result. Result of this PCR was migrated to electrophoresis tool for 45 minutes with 100 v electric voltages using mupid-exu from Japan.
The marker used in this study was Marker PCR 100 bp, then there were three primers as primer starch synthase gene of Manihot esculenta (± 187 bp), primer beta tubulin gene of Hevea brasiliensis (± 215 bp), and primer gene of 18s Manihot esculenta (± 219 bp). The programs for optimizing PCR are as below a) pre-denaturation at 95°C for 5 minutes, b) denaturation at 95ºC for 0.5 minutes, c) annealing at 57ºC for 0.5 minutes, d) extension at 72ºC for 0.5 minutes, then step 2, 3, and 4 is repeated as many as 40 x, e) post-PCR at 72ºC for 5 minutes, and f) cooling at 20ºC for 10 minutes.
The primer designed for SSIV gene was based on Manihot esculenta starch synthase IV (SSIV) mRNA, complete cds, with accession number: KT033500.1. The reason for this is that the valid sequence is already published and also very closed to the taxon of cassava studied. The Primer is >SSSF for TCGACGTGGTTTCTGAGTCA and also >SSSR for GAGGTTGTTCAGGAGAGGCT. For the internal control, primer pair for 18s rRNA designed from Manihot esculenta gene for 18S ribosomal RNA, partial sequence with the accession number: AB233568.1. Again, the reason for this is the valid sequence already published and also very closes to the taxon of cassava studied. Primer is >18sSSF for TGAAAGACGAACAACTGCGAA and the >18sSSR for TTAAGTTTCAGCCTTGCGACC. Before quantifying by real-time PCR, Rotorgene-Q 5-plex HMR from QIAGEN, Germany, PCR setting for cDNA which is already achieved is optimized by Sensodirect PCR thermocycler from Sensoquest, Germany, with the primer already ordered. These primers were used for qRT-PCR.
Template cDNA with concentration of 75 ng/µl from each treatment (0, 20, and 40 kg micro nutrient/ha) was used for analyzing qRT-PCR by Rotor-Gene® SYBR® Green. Primer real-time PCR used in this analysis was primer SSIV and primer 18s rRNA (already designed previously) to be as reported gene. The mixed materials for analyzing RT-PCR of each template cDNA (M0, M20, and M40) and each primer were presented in Table 2.
Table 2. The mixed materials for RT-PCR reaction.
|
No |
Material |
Volume (µl) |
|
1 |
Rotor-Gene® SYBR® Green Master Mix 1x |
12,5 |
|
2 |
Primer 18s rRNA forward 1 µM |
1 |
|
3 |
Primer 18s rRNA reverse 1 µM |
1 |
|
4 |
Template cDNA 75 ng/µl |
1 |
|
5 |
RNAse-Free Water |
9,5 |
|
Volume Total |
25 |
|
The quantitative analysis of RT-PCR was determined by RotorGene Q 5-plex HRM from QIAGEN, Germany. The program of RT-PCR as followed: a) pre – denaturation at temperature of 95ºC for 5 minutes, b) denaturation at temperature of 95ºC for 30 second, and c) annealing and elongation at temperature of 57ºC for 1 minute, while step 2 and 3 was repeated as many as 40 cycles.
Statistical analysis
Data of leaves, stem, and root parts were analyzed by analysis of variance using Minitab ver.18. Starch content and the activity of SSIV gene were analyzed by average from samples then put in histogram. The close relation among variables observed was analyzed by simple correlation. The result of RT-PCR analysis was directly presented by Rotor-Gene Q Series Software 2.0.2 in the form of graphic as followed, then each quantity of SSIV gene was calculated. The value of ΔCt SS root is SSIV root - 18s then the value of Normalization (ΔΔCt) is (ΔCt SS root)i - ΔCt SS root(control). Consequently, the value of regulated expression is 2-n where n is normalization (ΔΔCt). The sample of cassava root treated by 0, 20, and 40 kg micro nutrient fertilizer/ha was taken aseptically for PCR analysis then put in the solution of 1 ml RNA-later in sterile micro tube (DNAse and RNAse free) to protect RNA from nuclease activity during the process of sampling. There is no technical replicate used in the qRT-PCR because the equipment used in this study has high sensitivity and precision.
RESULTS
Experimental design and observation variables
The observed variables as LN, LFW, LDW, LG, SFW, SDW, RFW, SRFW, and RDW showed high variation at different harvest age (Table 3). There is variation of LFW due to harvest age means that there will be high and low weight of fresh leaves. Moreover, the variables of LN, LFW, SFW, SDW, and RFW indicated high variation applied by micro nutrient. Yet, the interaction between micro nutrient and harvest age induced the variation of LN, LG, and RDW.
Table 3. Variable values of mean square of harvest age, block, micro nutrient, and interaction between harvest age and micro nutrient.
|
Variables |
Mean square |
|||
|
Block |
Harvest age |
Micro nutrient |
Harvest age ´ Micro nutrient |
|
|
LN |
716.16** |
2,275.10** |
1,586.76** |
434.72* |
|
LFW |
3,254.35 |
10,622.57** |
32,945.95** |
315.35 |
|
LDW |
316.97* |
1,028.22** |
2,927.48 |
174.65 |
|
LG |
5.81 |
248.68** |
10.55 |
24.18** |
|
SFW |
33,587.27* |
170,317.20** |
210,798.67** |
19,476.56 |
|
SDW |
6,034.59** |
10,683.16** |
25,454.61** |
2,972.91 |
|
RFW |
41,064.89 |
4,288,460.00** |
547,209.07* |
71,432.44 |
|
SRFW |
3,993.91 |
167,659.20** |
6,044,14 |
1,562.83 |
|
RDW |
4,230.19 |
16,926.79* |
13,031.23 |
17,888.45** |
Note: * and ** showing significant at 5 % and significant at 1 %, respectively.
Three of harvest age, as 7, 8, and 10 MAP showed variation of variables differently. LFW of 7 MAP was the same as that of 10 MAP yet the lowest LFW was at 8 MAP (Table 4). Moreover, the higher values of variable average, as LDW, SFW, SDW, RFW, and SRFW were shown at harvest age of 10 MAP. However, the lower average values of RFW and SRFW were shown at 7 MAP.
Table 4. Leaf fresh weight (LFW), leaf dry weight (LDW), stem fresh weight (SFW), stem dry weight (SDW), root fresh weight (RFW), and skin root fresh weight (SRFW) of cassava at different harvest age.
|
Harvest age |
LFW |
LDW |
SFW |
SDW |
RFW |
SRFW |
|
g/plant |
||||||
|
7 MAP |
110.20a |
26.40b |
215.70b |
59.00b |
128.40c |
20.30c |
|
8 MAP |
73.60b |
23.50b |
137.70c |
51.50b |
439.80b |
37.90b |
|
10 MAP |
109.80a |
36.00a |
306.00a |
91.20a |
824.70a |
144.70a |
|
LSD (0.05) |
31.20 |
4.23 |
71.20 |
8.02 |
95.30 |
12.50 |
Means followed by similar letters in the same column are not significantly different using LSD (0.05).
Table 5. Leaf fresh weight (LFW), leaf dry weight (LDW), stem fresh weight (SFW), stem dry weight (SDW), root fresh weight (RFW), and skin root fresh weight (SRFW) of cassava applied by different dosages of micro nutrient fertilizer.
|
Micro nutrient (kg/ha) |
LFW |
LDW |
SFW |
SDW |
RFW |
SRFW |
|
g/plant |
||||||
|
20 |
89.40b |
25.50b |
213.00b |
66.10b |
910.60b |
137.00a |
|
40 |
138.00a |
40.90a |
316.70a |
100.00a |
960.50a |
138.10a |
|
LSD (0.05) |
23.60 |
5.32 |
94.60 |
25.70 |
160.80 |
21.40 |
|
20 |
89.40b |
25.50b |
213.00b |
66.10b |
910.60b |
137.00a |
Note: Means followed by similar letters in the same column are not significantly different using LSD (0.05).
The variable variations shown in Table 3 due to micro nutrient treatments were analyzed by LSD at 5% level of differences to evaluate the effects of micro nutrient on LFW, LDW, SFW, SDW, RFW, and SRFW (Table 5). Without micro nutrient treatment, the variables of LFW, LDW, SFW, SDW, RFW were the lower. This result was contradictory with treatment of 40 kg micro nutrient/ha which showed the higher average values of LFW, LDW, SFW, SDW, RFW.
Table 6. Leave number (LN), leaf greenness (LG), and root dry weight (RDW) of cassava at different harvest age and applied by different dosages of micro nutrient fertilizer.
|
Harvest age |
Micro nutrient (kg/ha) |
LN (no. /plant) |
LG |
RDW (g/plant) |
|
7 MAP |
0.00 |
36.40f |
44.30bc |
85.10c |
|
|
20.00 |
51.80cd |
47.60a |
150.00b |
|
|
40.00 |
60.90b |
46.10ab |
106.80bc |
|
8 MAP |
0.00 |
48.10de |
47.00a |
143.70b |
|
|
20.00 |
56.10bc |
47.90a |
210.10a |
|
|
40.00 |
72.00a |
48.50a |
101.10bc |
|
10 MAP |
0.00 |
37.80f |
42.60c |
141.80b |
|
|
20.00 |
42.10ef |
43.40c |
144.10b |
|
|
40.00 |
38.00f |
43.80c |
216.50a |
|
LSD (0.05) |
|
6.50 |
2.41 |
55.40 |
Note: Means followed by similar letters in the same column are not significantly different using LSD (0.05).
The significant interaction between micro nutrient and harvest age treatments means that the effect of harvest age would synergize with that of micro nutrient on the variables of LN, LG, and RDW (Table 6). The application of 40 kg micro nutrient/ha could increase LN, LG, and RDW at 8 MAP. The high RDW as 216.5 g/plant due to 40 kg micro nutrient/ha at 10 MAP was the same as that of at 8 MAP. However, there was no correlation between LN, LG, and RDW (Table 7).
Table 7. Correlation values among variables, leaf number (LN), leaf fresh weight (LFW), leaf dry weight (LDW), leaf greenness (LG), stem fresh weight (SFW), stem dry weight (SDW), root fresh weight (RFW), and root dry weight (RDW) of cassava. The values of correlation are upper diagonal and the values of probability are below diagonal.
|
|
LN |
LFW |
LDW |
LG |
SFW |
SDW |
RFW |
RDW |
|
LN |
- |
0.30** |
0.49** |
-0.04 |
0.10 |
0.15 |
-0.08 |
-0.01 |
|
LFW |
0.01 |
- |
0.84** |
-0.06 |
0.80** |
0.71** |
0.18 |
0.05 |
|
LDW |
0.00 |
0.00 |
- |
-0.06 |
0.55** |
0.51** |
-0.11 |
-0.11 |
|
LG |
0.74 |
0.63 |
0.60 |
- |
-0.17 |
-0.18 |
-0.13 |
-0.16 |
|
SFW |
0.41 |
0.00 |
0.00 |
0.16 |
- |
0.95** |
0.40** |
0.25* |
|
SDW |
0.21 |
0.00 |
0.00 |
0.12 |
0.00 |
- |
0.39** |
0.27* |
|
RFW |
0.50 |
0.13 |
0.35 |
0.28 |
0.00 |
0.00 |
- |
0.68** |
|
RDW |
0.94 |
0.66 |
0.34 |
0.18 |
0.03 |
0.02 |
0.00 |
- |
Note: * indicates significant difference at 5% and ** indicates significant difference at 1%.
LN showed significant correlation with LFW (r = 0.30**) and LDW (r = 0.49**). Moreover, LDW would have significant correlation with SDW as r = 0.51** and SDW showed high correlation with RDW as r = 0.27**.
Starch measurement
Starch content harvested at the age of 7, 8, and 10 MAP showed the similar trend due to micro nutrient application. Without application of micro nutrient, starch content increased just a little bit from 7, 8, and 10 MAP, as approximately 18-19% (Figure 1). Furthermore, the increasing micro nutrient application could enhance the starch content yet the increase of starch content depended on harvest age. At harvest age of 7 MAP, the increase of starch content due to 20 and 40 kg micro nutrient/ha was approximately 3.8% and 4.7%, respectively. This condition was similar to the harvest age of 8 MAP due to 20 and 40 kg micro nutrient/ha. However, the increment of starch content would seem relatively high when harvest age was at 10 MAP, especially followed by 20 and 40 kg micro nutrient/ha, as 3.8% and 5.2%, respectively.
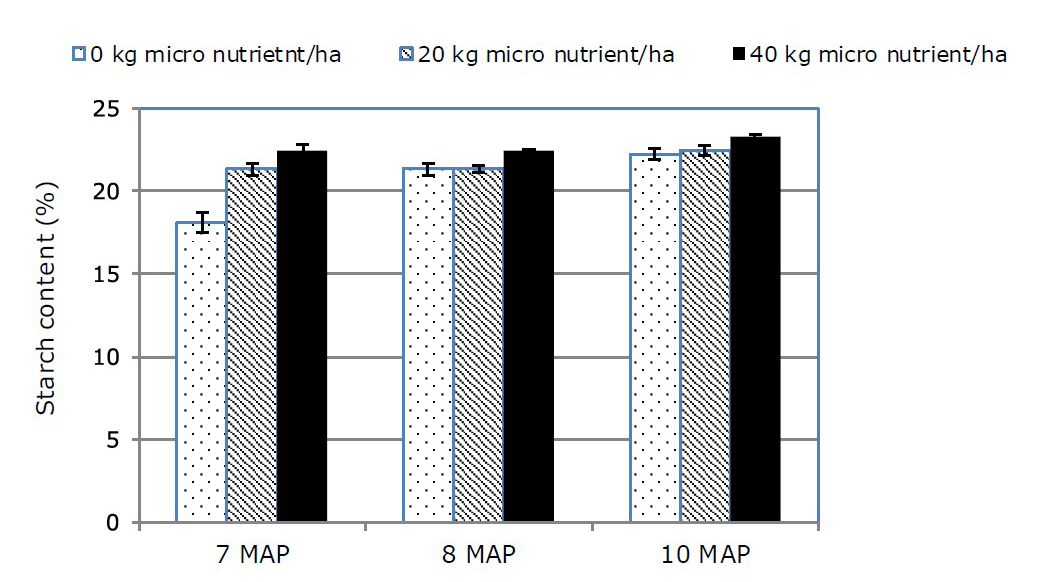
Figure 1. The starch content of cassava storage root at different harvest age under various micro nutrient application.
Measurement of SSIV gene
The result of this cDNA was amplified by using PCR (sensoquest thermocycler from Germany) to verify the amplification result. Result of this PCR was migrated to electrophoresis tool for 45 minutes with 100 v electric voltages (Figure 2) using mupid-exu from Japan. The result of optimizing PCR was close to 200 bp. After this, the result of RT-PCR analysis was directly presented by Rotor-Gene Q Series Software 2.0.2 in the form of graphic as followed, then each quantity of SSIV gene was calculated (Figure 3). The value of ΔCt SS root is SSIV root - 18s then the value of Normalization (ΔΔCt) is (ΔCt SS root)i - ΔCt SS root (control). Consequently, the value of regulated expression is 2-n where n is normalization (ΔΔCt).
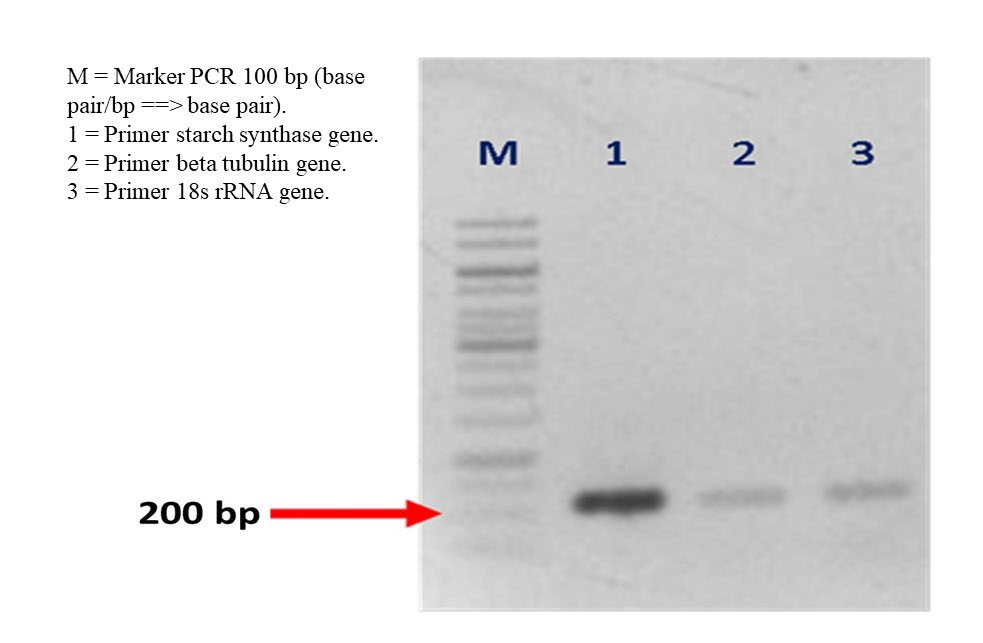
Figure 2. The result of optimizing PCR in close to 200 bp (red arrow sign) using template cDNA from control cassava plant.
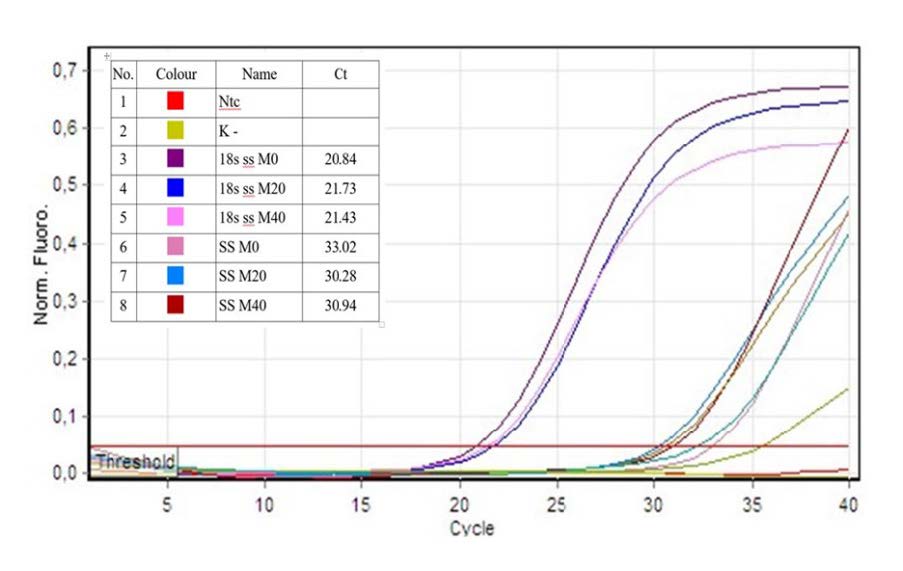
Figure 3. Quantitation data for Cycling A.Green.
The quantitated data for calculating the activity of SSIV gene was on Table 8. Without application of micro nutrient was regulated by 1 value. Based on this, the activity of SSIV gene applied by micro nutrient could be quantitatively identified (Figure 4).
Table 8. The result of calculation of SSIV root, ΔCt ss root, normalization (ΔΔCt), and regulated expression.
|
Micronutrient treatment |
18s |
SSIV Root |
ΔCt SS Root |
Normalization (ΔΔCt) |
Regulated Expression |
|
0 kg/ha |
20.84 |
33.02 |
12.18 |
0.00 |
1.00 |
|
20 kg/ha |
21.73 |
30.28 |
8.55 |
-3.63 |
12.38 |
|
40 kg/ha |
21.43 |
30.94 |
9.51 |
-2.67 |
6.36 |
The increase of SSIV gene activity was affected by micro nutrient application of 20 and 40 kg/ha. The application of 20 kg micro nutrient/ha was able to increase SSIV gene activity as many as 12.38 compared to that of without micro nutrient. Moreover, application of 40 kg micro nutrient/ha could enhance SSIV gene activity as 6.36 compared to that of without micro nutrient.
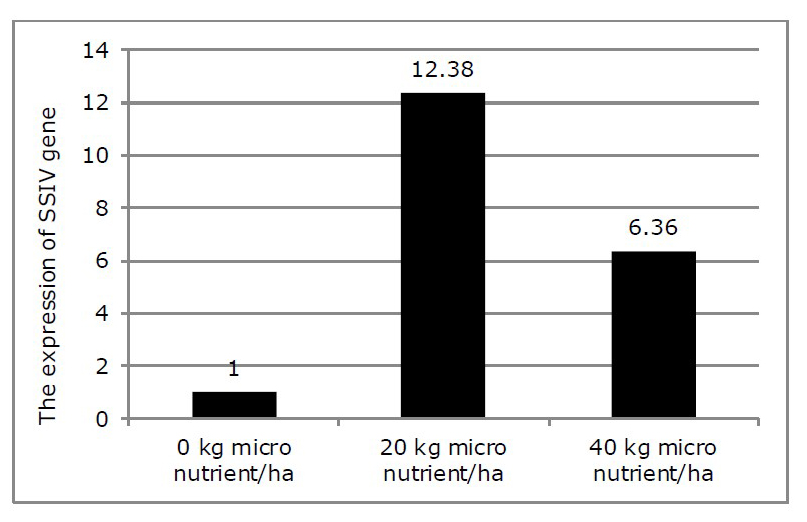
Figure 4. The expression of SSIV gene activity on fresh root of cassava under different micro nutrient fertilizers.
DISCUSSION
The variation of variables was significantly affected by different micro nutrient supplies except for LDW, LG, SRFW and RDW. This could be explained that micro nutrient application might induce the number of leaves and RFW. Moreover, the application of micro nutrient was able to stimulate the variation of water content in root resulted in no variation of RDW. SRFW on the other hand, was affected by harvest age because as the root enlarged according to the time, skin of root would follow the root. The spectacular theory is that SRFW showed high variation, it means that treatments of micro nutrient could affect the thickness of skin root. When cassava plant continuously grows, the roots will expendably develop. SRFW as a result, was affected by harvest age because as the root enlarged according to the time, skin of root would follow the root size. However, such theory in this study was not proven yet and needed further research. Moreover, the application of micro nutrient was able to stimulate the variation of water content in root resulted in no variation of RDW. Three observed variables displayed high variation at interaction between harvest age and micro nutrient application as LN, LG, and RDW. It means that both of harvest age and micro nutrient fertilizer synergy to each other to affect LG and RDW. Consequently, harvest age could influence the application of micro nutrient to induce LG and RDW showing variation. It could be explained that leaf greenness and high leaf number could directly affect photosynthesis resulted in high photoassimilate translocation from leaf parts to the root. Marcelis (1996) described a model of dry matter partitioning as source parts into sink parts (potential capacity to accumulate photoassimilate or assimilate). According to Polthanee et al. (2014) the increasing leaf area index (LAI) was concomitant with the increasing dry root yield of Rayong-72 compared to those of Huaybong-80. The cassava variety used in this study was UJ 3 that was originally selected from Rayong and Huaybong developed by Thai Tapioca Development Institute (TTDI). One of the ways to increase leaf area index is by reducing leaf losses after achieving the maturity stage. It means that the more leaf number the more metabolism system could support the photosynthesis activity at maturity stage. The other result showed that LN would closely relate to LDW and led to increase photoassimilate to develop fresh storage root yield (Poltanee et al., 2014; Richardson, 2011).
The other result showed that LN also decreased at 10 MAP although micro nutrient application increased. Additionally, this study, two main variables of leaves as LN and LG were low at 10 MAP. It is very clear that LN and LG were decreasing due probably to the plant age. According to the cassava growth period, Alves (2002) reported that maximum growth rate of leaves was achieved at 3-6 MAP. Additionally, photoassimilate partition from leaves to roots was accelerated. The application of 40 kg micro nutrient/ha, the decrease in LN and LG would associate with the yield as RDW to be higher at 10 MAP, as 216.5 kg/plant than at 7 and 8 MAP. Interestingly, application of 20 and 40 kg micro nutrient/ha would not affect the cassava yield yet at 7 and 8 MAP. This means that LN closely associated with LG that is very important for producing photoassimilate as source to be partitioned to sink part as roots (RDW). This result was concomitant with the result of Veltkamp (1986) who reported that remarkable leaf reduction after 8 MAP was associated with the low leaf area index (LAI). Such condition was supported by Edet et al. (2015) who informed that correlation between LAI and LN was r=0.77** but correlation between LN and RDW was low as r=0.02. It possibly means that as the cassava plant aged, the fall leaf would be high then the partition of photoassimilate would be to the root parts. Begum and Paul (2005) also explained that LAI increased from the beginning until reached certain peak and then decreased according to the cassava aged. In this study LDW drastically dropped from 36.0 g at 7 MAP to 23.5 g at 10 MAP.
The increase in RFW and SRFW of different harvest age could be explained that there was photoassimilate allocation from leaves part as source to the stem and root parts as sink. It could be explained that SRFW increased as the plant age increased. This also means that the optimum harvest time for cassava is 10 MAP. Additionally, it is true that translocation of photoassimilate produced in leaves would be distributed to the stem and then to the root. Such condition was supported by the result studied by Sagrilo et al. (2008) who concluded that photoassilimate partition in cassava happened as the age increased, dry weight of leaves would fell down yet weight of stem and root parts would increase. Harmens et al. (2000) and Begum and Paul (2005) concluded that leaf dry matter had closely related to photoassimilate. The photoassimilate would be transported from stem to the root part and this system would be controlled by starch synthase of root part (Li et al., 2016).
The effect of micro nutrient on LFW, LDW, SFW, SDW, RFW, and SRFW were found significantly different as the application of 40 kg micro nutrient/ha. It seems that under micro nutrient application, partition of photoassimilate in leaf and stem closely related to that in root yield as mentioned before. Moreover, the little allocation of photoassimilate was observed to the root skin of cassava. This means that there is a positive relation between shoot parts (leaves and stem) and root parts due mainly to micro nutrient application excepted for cassava root skin. This was proven LDW showed highly positive correlation with SDW (r = 0.51**). In this study, high application of micro nutrient tended to vastly increase fresh storage root yield from 677.6 g/plant (without micro nutrient) to 960.5 g/plant (40 kg micro nutrient/ha). It seems that micro nutrient fertilizer induces and increases the leaf characters leads to the enhancement of photoassimilate as a source. The application of micro nutrient in cassava studied by Setiawan et al. (2017) was able to increase fresh root weight. The other study was also conducted by Kumar (2013) who reported that the addition of Fe and Zn nutrient to sorghum could enhance the granule size.
According to Setiawan et al. (2017) roots with high starch content showed big granule size. They proved their result that the starch granule diameter was increased from 8.36 – 16.5 µm (without micro nutrient) to 20.30 – 26.79 µm (with 40 kg micro nutrient kg/ha) and led to increase in starch content. Janket et al. (2018 and 2020) supported this idea that most cassava genotypes which produced high starch content and starch yield would have large starch granules. In this study, according to harvest age, the content of starch at 7, 8, and 10 MAP was 21.4%, 21.5%, and 21.7%, respectively. Additionally, the starch content due to micro nutrient application was 19.4%, 22.0%, and 23.2% at 0, 20, and 40 kg/ha, respectively. The application of 20 and 40 kg micro nutrient/ha could increase starch content approximately 16% and 18%, respectively at 7, 8, and 10 MAP. The pattern of starch content enhancement due to micro nutrient applied at 7, 8, and 10 MAP was generally the same. It was clear that the increase in starch content would be affected by granule size. According to Jane (1994) the diameter size of cassava starch granule was in the range of 5-25 µm. Additionally, Lindebom et al. (2004) classified starch granule size into three groups, 0.5-5.0 µm categorized as very small size; 5.0-15 µm as small size; 15-36 µm as bimodal size. Perry (2000) and Sriroth et al. (1999) commented that starch granule size was not influenced by date but was affected by fertilizer condition or soil condition. They stated that starch granule size was progressively altering from very small size to bimodal size at late harvest age. The other information associated between the increase in starch granule size and amylose content was also explained by Boyer et al. (1976). It is well-known that starch is a glucose polymer and synthesized by starch synthase (SS) gene.
The expression of SSIV gene activity on the storage root of cassava treated by 20 kg micro nutrient/ha at 10 MAP showed as many as 12.4 times that of 0 kg micro nutrient/ha. Interestingly, the expression of SS gene activity on the storage root of cassava treated by 40 kg micro nutrient/ha at 10 MAP showed as many 6.36 times as that of 0 kg micro nutrient/ha (Figure 4). It means that the gene activity of SSIV was increasingly induced by 20 kg micro nutrient/ha higher than those by 40 kg micro nutrient/ha. The reason for this is still not clear yet. The application of 40 kg micro nutrient/ha seems to suppress the activity of SSIV gene. This could be recommended that the optimum application of micro nutrient would be 20 kg/ha. The reason is that first, the expression of SSIV gene is higher in 20 kg than 40 kg micro nutrient/ha and second, the increase in starch content is approximately 16 % with very small error bars. It means that application of 20 kg micro nutrient/ha would increase starch content more stable than that of 40 kg micro nutrient/ha. The low expression of SSIV gene applied by 20 kg micro nutrient in this study was due probably to sample of cassava fresh storage root taken at 10 MAP. This was supported by Munyikwa et al. (1997) who conducted research on enzyme for starch biosynthesis of AGPase that was proven to be the higher expression in young leaves than in old leaves. Such condition was also proven by Li et al. (2016) that the activity of starch synthase would be lower according to the development growth stage. Setiawan et al. (2017) proved that 20 kg micro nutrient fertilizer application absolutely affected the increase in root fresh weight and diameter of granule starch by increasing SSIV led possibly to high starch content. Based on these reason that the optimum application of micro nutrient for enhancing the starch content is 20 kg/ha. It is interesting that data of some observed variables in this study would support the proposed mechanism of micro nutrient role in developing root. The application of micro nutrient in the soil would be absorbed by cassava plant through roots that would stimulate SSIV gene to be active. High activity of SSIV gene would stimulate high starch content in root as a sink part. In the same time, it would effect photoassimilate production that was indicated by high number of leaves caused high dry matter as a source part. Such photoassimilate would fluently distribute to root parts as a sink through stem (indicated by high stem dry matter). Consequently, application of micro nutrient fertilizer increase the sink strength to pull photoassimilate as a source for the expression of starch in the root part.
CONCLUSION
Among three applications of micro nutrient, the application of 20 kg micro nutrient/ha could increase root dry weight of cassava at harvest age of 8 MAP. This application showed the same result as that of 40 kg micro nutrient/ha at harvest age of 10 MAP. At three harvest age, the increase in starch content of cassava showed the same trend by application of micro nutrient. The application of 20 kg micro nutrient/ha could induce the expression of high starch synthase type IV gene (SSIV) as many as 12.4 times. It seems that the application of 20 kg micro nutrient/ha could effect on heavier fresh storage root due mainly to higher starch content.
ACKNOWLEDGMENTS
We would like to thank to Institute for Research and Community Service, University of Lampung, Lampung, Indonesia for giving opportunity to conduct the valuable research to characterize and identify cassava starch both physically scanning electron-microscope (SEM) and genetically real time quantitative PCR (RT-PCR). Collaboration and cooperation with Biomolecular Division, Integrated Laboratory of University of Lampung (chairman and technicians) are also highly appreciated for the success of RT-PCR analysis.
AUTHOR CONTRIBUTIONS
Kukuh Setiawan contributed in breeding and genetics, Paul Benyamin Timotiwu executed the research in field and analyzes data, Agustiansyah is seed physiologist, Muhammad Syamsoel Hadi is agronomist, Muhammad Kamal is plant physiologist, Ardian managed the idea and supervised the analysis result, Wawan Abdullah Setiawan is biologist and laboratory analysist.
CONFLICT OF INTEREST
The authors declare there is no conflict of interest.
REFERENCES
Agbaje, G. O. and Akinlosotu, T. A. 2004. Influence of NPK fertilizer on tuber yield of early and late-planted cassava in a forest alfisol of south-western Nigeria. African Journal of Biotechnology 3: 547-551.
Alves, A. A. C. 2002. Cassava botany and physiology. In R.J. Hillocks, J.M. Thresh and A. C. Belloti (eds). Cassava: Biology, Production and utilization. Wallingford, UK: CABI Publishing.
Apea-Bah, F.B., Oduro, I., Ellis, W.O., and Kantanka, O. S. 2011. Factor analysis and age at harvest effect on the quality of flour from four cassava varieties. World Journal Dairy & Food Science 6: 43-54
Baafi E. and Kantanka, O.S. 2007. Effect of genotype, age, and location on cassava starch yield and quality. Journal of Agronomy 6: 581-585.
Baguma, Y. 2004. Regulation of starch synthesis in cassava. Doctoral thesis. University of Agricultural Sciences Uppsala, Swedish.
Begum, S. and Paul, N.K. 2005. Growth analysis of cassava (Manihot esculenta Crantz) varieties in relation to time of planting. Bangladesh Journal of Botany 34: 21-26.
Beyene, D., Baguma, Y.S., Mukasa, B., Sun, C., and Jansson, C. 2010. Characterization and role of Isoamylase1 (Meisa1) gene in cassava. African Crop Science Journal 18: 1 – 8.
Boyer, C. D., Shannon, J. C., Garwood, D. L, and Creech, R. G. 1976. Changes in starch granule size and amylose percentage during kernel development in several Zea mays L. genotypes. Cereal Chemistry 53: 327-337.
BPS. 2021. Produksi ubikayu menurut provinsi (ton), 2000-2020. Retrieved on January 05, 2022 from: https://www.bps.go.id/linkTableDinamis/view/id/880.
Chatakanonda P., Chinachoti, P., Sriroth, K., Piyachomkwan, K., Chotineeranat, Tang, S. H., and Hills, B. 2003. The influence of time and conditions of harvest on the functional behavior of cassava starch-a proton NMR relaxation study. Carbohydrate Polymer 53: 233-240.
Cock, J.H., Franklin, D., Sandoval, G., and Juri, P. 1979. The ideal cassava plant for maximum yield. Crop Science 19: 271-279.
Dos Santos, N.S., Alves, J. M. A., Uchôa, S.C.P., de Oliveira, N.T., de Anchieta, J., and de Albuquerque, A. 2014. Absorption of macronutrients by cassava in different harvest dates and dosages of nitrogen. Revista Ciência Agrononomica 45: 633-640.
Edet, M.A., Tijani-Eniola, H., Lagoke, S.T. O., and Tarawali, G. 2015. Relationship of cassava growth parameters with yield, yield related components and harvest time in Ibadan, Southwestern Nigeria. Journal of Natural Science Research 5: 87-92.
Fageria, N.K. 2009. The use of nutrients in crop plants. CRC Press Taylor and Francis Group, Boca Raton.
Gomez, K. A. and Gomez, A. A. (1984). Statistical procedures for agricultural research (2nd ed.). New Jersey, United States: John Wiley & Sons. Retrieved from https://scholar. google.co.id/scholar?cites=149579462351256 46981&as_sdt=2005&sciodt=0,5&hl= id&aut huser=3
Guan, Z., Chen, X., Xie, H., and W. Wang. 2016. Promoter regulatory domain identification of cassava starch synthase IIb gene in transgenic tobacco. Plant Physiology and Biochemistry 102: 92-96.
Hadi, M.S. 2010. Pengaruh frekuensi aplikasi hara mikro terhadap produksi ubi kayu di Blambangan, Way Kanan. In J. Hendri, S. D. Utomo, G. N. Susanto, D. Asmi, Warsono, Subekti, N. Sa’diyah, Muhartono, M. Riniati, M. I. Affandi, Sumaryo, W. Simanjuntak, Warji, and N. Nurcahyani (Eds.), Prosiding Seminar Nasional Sains MIPA dan Aplikasinya, Bandar Lampung 8-9 Dec 2010. Universitas Lampung.
Harmens, H., Stirling, C. M., Marshall, C., and Farrar, J. F. 2000. Is partitioning of dry weight and leaf area within Dactylis glomerata affected by N and CO2 Enrichment? Annals Botany 86: 833-839.
Howeler, R.H. 1981. Mineral nutrition and fertilization of cassava (Manihot esculenta Crantz). CIAT, Coli, Colombia.
Howeler, R.H. 2001. Nutrient inputs and losses in cassava-based cropping systems-examples from Vietnam and Thailand. International Workshop on Nutrient Balances for Sustainable Agricultural Production and Natural Resource Management in Southeast Asia. Bangkok, Thailand, 20-22 Feb 2001.
Jane, J. L., Kasemsuwan, T., Leas, S., Zobel, H., and Robyt, J. F. 1994. Anthology of starch granule morphology by scanning electron microscopy. Starch, 46: 121-129.
Janket, A., Vorasoot, N., Toomsan, B., Kaewpradit, W., Banterng, P., Theerakulpisut, P., and Jogloy, S. 2018. Seasonal variation in starch accumulation and starch granule size in cassava genotypes in a tropical savanna climate. Agronomy, 8: 2-16.
Janket, A., Vorasoot, N., Toomsan, B., Kaewpradit, W., Theerakulpisut, P., Holbrook, C.C., Kvien, C. K., Jogloy, S., and Banterng, P. 2020. Accumulation dynamics of starch and its granule size distribution of cassava genotypes at different growing seasons. Agriculture, 10. 380.
Kayode, G.O. 1983. Effects of various planting and harvesting times on the yield, HCN, dry-matter accumulation and starch content of four cassava varieties in a tropical rainforest region. Journal of Agriculture Science, 101: 633-636.
Kumar, A.A., Reddy, B.V.S., Ramaiah, B., Sahrawat, K.L., and Pfeiffer, W.H. 2011. Options for enhancing grain iron and zinc concentrations in sorghum. Presented at 3rd International Zinc Symposium, Improving Crop Production and Human Health. October 10-14, 2011, 2 pp. Hyderabad, India.
Li, Y. Z, Zhao, J. Y., Wu, S. M., Fan, X. W., Luo, X. L., and Chen, B. S. 2016. Characters related to higher starch accumulation in cassava storage roots. Scientific Reports 6: 19823.
Lindebom, N., Chang, P. R., and Tyler, R. T. 2004. Analytical, biochemical and physicochemical aspects of starch granule size, with emphasis on small granule starches: a review. Starch 56: 89-99.
Livak, K.J. and Schmittgen, T. D. 2001. Analysis of relative gene expression data using realtime quantitative PCR and the 2 -ΔΔTmethod. Methods 25: 402–408.
Marcelis, L.F.M. 1996. Sink strength as a determinant of dry matter partitioning in the whole plant. Journal of Experimental Botany 47: 1281-1291.
McCleary, B.V., Charmier, L.M.J., and McKie, V.A. 2018. Measurement of Starch: Critical Evaluation of Current Methodology. Megazyme. Published by WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Miao, H., Sun, P., Liu, W., Xu, B., and Jin, Z. 2014. Identification of genes encoding granule-bound starch synthase involved in amylose metabolism in banana fruit. Plos One 9.
Moorthy S. N. and Padmaja, G. 2002. A rapid titrimetric method for determination of starch content of cassava tubers. Journal of Food Crops 28: 30-37.
Munyikwa, T.R.I., Langeveld, S., Salehuzzaman, S.N.I.M., Jacobsen, E., and Visser, R.G.F. 1997. Cassava starch biosynthesis: new avenues for modifying starch quantity and quality. Euphytica 96: 65–75.
Noda T., Tsuda, S., Mori, M., and Yamauchi, H. 2004. The effect of harvest dates on the starch properties of various potato cultivars. Food Chemistry, 86: 199-125.
Panitnok, K., Chaisri, S., Sarobol, E., Ngamprasitthi, S., Chaisri, P., Changlek, P., and Thongluang, P. 2013. The combination effects of zinc, magnesium, sulphur foliar fertilizer management on cassava growth and yield grown on Map Bon, coarse-loamy variant Soil. Procedia – Social and Behavioral Sciences 91: 288 – 293.
Perry, L. 2000. Starch granule size and the domestication of manioc (Manihot esculenta) and sweet potato (Ipomoea batatas). Economical Botany 56: 335-349.
Polthanee A., Janthajam, C., and Promkhambut, A. 2014. Growth, yield, and starch content of cassava following rainfed lowland rice in northeast Thailand. International Journal of Agriculture Research 9: 319-324.
Prammanee, S., Kamprerasart, K., Salakan, S., and Sriroth, K. 2010. Growth and starch content evaluation on newly released cassava cultivars, Rayong 9, Rayong 7 and Rayong 80 at different harvest times. Kasetsart Journal of Natural Science 44: 558-563.
Richardson, K. V. A. 2011. Evaluation of three cassava varieties for tuber quality and yield. Crop Research Report 4: 1-12.
Sagrilo E., Filho, P. S. V., Pequeno, M. G., Scapim, C. A., Vidigal, M. C. G., de Souza Diniz, S. P. S., Modesto, E. C., and Kvitschal, M. V. 2003. Effect of harvest period on the quality of storage roots and protein content of the leaves in five cassava cultivars (Manihot esculenta Crantz). Brazilians Archives of Biology and Technology 46: 296-305.
Sagrilo, E., Filho, P. S. V., Pequeno, M. G., Gonçalves-Vidigaland, M. C., and Kvitschal, M. V. 2008. Dry matter production and distribution in three cassava (Manihot esculenta Crantz) cultivars during the secondvegetative plant cycle. Brazilians Archives of Biology and Technology 51: 1079-1087.
Salehuzzaman, S.N.I.M., Jacobsen, E., and Visser, R.G.F. 1993. Isolation and characterization of a cDNA encoding granule-bound starch synthase in cassava (Manihot esculenta Crantz) and its antisense expression in potato. Plant Molecular Biology 23: 947-962.
Setiawan, K., Timotiwu, P. B., Agustansyah, Hadi, M. S., Ardian, and Setiawan, W. A. 2017. Characterization of cassava starch and detection of starch synthase gene under different micro nutrient fertilizer levels by using scanning electronic microscopy (SEM) and real-time PCR. Retrieved on January 15, 2020 from https://www.researchgate.net/publication/321251757_Characterization_of_cassava_starch_and_detection_of_starch_synthase_gene_under_different_micro_nutrient_fertilizer_levels_by_using_scanning_electronic_microscopy_SEM_and_Real_Time_PCR.
Sriroth, K., Santisopasri, V., Petchalanuwat, C., and Oates, C. G. 1999. Cassava starch granule structure-function properties: Influence of time and conditions at harvest on four cultivars of cassava starch. Carbohydrate Polymer, 38: 161-170.
Veltkamp, H. J. 1986. Physiological causes of yield variation in cassava (Manihot esculenta Crantz). Agricultural University, Waginingen, Netherlands.
Yang, Z., Wang, Y., Xu, S., Xu, C., and Yan, C. 2013. Molecular evolution and functional divergence of soluble starch synthase genes in cassava (Manihot esculenta Crantz). Evolutionary Bioinformatics 9: 239-249
Zhu, F. 2014. Composition, structure, physicochemical properties, and modifications of cassava starch. Carbohydrate Polymers 122: 456–480.
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand.
Kukuh Setiawan1,*, Paul Benyamin Timotiwu1, Agustiansyah1, Muhammad Syamsoel Hadi1, Muhammad Kamal1, Ardian1, and Wawan Abdullah Setiawan2
1 Department of Agronomy and Horticulture, College of Agriculture University of Lampung, 35145, Indonesia.
2 Department of Biology, College of Mathematics and Natural Science University of Lampung, 35145, Indonesia.
Corresponding author: Kukuh Setiawan, E-mail: kukuhsetiawan38@gmail.com
Total Article Views
Editor: Tonapha Pusadee,
Chiang Mai University, Thailand
Article history:
Received: Mach 7, 2022;
Revised: July 21, 2022;
Accepted: September 30, 2022;
Published online: October 17, 2022

