
Metformin Mitigates Memory Impairment of Diabetic Mice through Modulation of Plasma Pro-inflammatory Cytokines and Aβ1-42 Levels
Yoottana Janthakhin*, Sutin Kingtong, Chutima Aphibanthammakit, and Sirikran JuntapremjitPublished Date : 2023-01-05
DOI : https://doi.org/10.12982/NLSC.2023.001
Journal Issues : Number 1, January-March 2023
Abstract Experimental and clinical studies reported that type 2 diabetes mellitus (T2DM) is associated with cognitive dysfunction and promotes the onset of dementia. Metformin is an antihyperglycemic drug used for the treatment of T2DM. A growing number of evidence revealed neuroprotective, antioxidant, and anti-inflammation effects exerted by metformin. The present study aimed to investigate the effect of metformin on cognitive function, systemic proinflammatory cytokines and amyloid-beta 1-42 (Aβ1-42) which is a pathological hallmark of Alzheimer’s disease (AD) in diabetic mice. C57BL/6N mice were divided into the following experimental groups: normal control group (NC); diabetes mellitus group (DM) induced by a high-fat diet combined with streptozotocin (STZ) injection; diabetes mellitus treated with metformin 100 mg/kg (DM+Met). Cognitive performance was evaluated by the novel object recognition test (NORT). Systemic proinflammatory cytokines and Aβ1-42 were assessed by the enzyme-linked immunosorbant assay (ELISA) test. We found that diabetic mice exhibited cognitive impairment in NORT whereas the treatment with metformin restored the cognitive function of diabetic mice. Moreover, diabetic mice presented an increase in plasma IL-6 and TNF-α levels while Aβ1-42 was decreased when compared to NC mice. Nevertheless, the administration of metformin allowed the levels of plasma IL-6, TNF-α, and Aβ1-42 to normalize in diabetic mice. Taken together, our findings suggest that metformin improves the cognitive function of diabetic mice possibly via the modulation of plasma pro-inflammatory cytokines and Aβ1-42 levels. Metformin may potentially be used as a therapeutic agent for patients with T2DM who show cognitive deficits.
Keywords: Diabetes mellitus, Cognitive impairments, Pro-inflammatory cytokines, Amyloid-beta, Metformin
Funding: This research was supported by the Thailand Science Research and Innovation Fund and University of Burapha, grant number 64A108000005 (Y.J.), and a grant from Thai Traditional Medical Knowledge Fund, Department of Thai Traditional and Alternative Medicine (Y.J.).
Citation: Janthakhin, Y., Kingtong, S., Aphibanthammakit, C., and Juntapremjit, S. 2023. Metformin mitigates memory impairment of diabetic mice through modulation of plasma pro-inflammatory cytokines and Aβ1-42 levels. Nat. Life Sci. Commun. 22(1): e2023001.
INTRODUCTION
Diabetes mellitus (DM) has been considered as a common metabolic disorder that can cause many complications such as nephropathy, retinopathy, cardiovascular and cerebrovascular diseases. A growing body of evidence indicates that T2DM is associated with an increased risk to develop mild cognitive impairment (MCI) and dementia including Alzheimer’s disease (AD) (Arnold et al., 2018; Biessels et al., 2006). Previous studies demonstrated that patients with AD exhibit greater impairments in glucose and insulin metabolism when compared to individuals with normal cognitive function (Craft et al., 1992; Janson et al., 2004). High glucose levels increased the expression of pro-inflammatory cytokines including tumor necrosis factor-α (TNF-α), interleukin-1b (IL-1b), and interleukin-6 (IL-6) (Wang et al., 2012; Niu et al., 2016) and the generation of reactive oxygen species (ROS) (West, 2000). However, the molecular mechanisms underlying the relationship between T2DM and cognitive dysfunction remain unclear.
AD is a progressive neurodegenerative disorder characterized by the accumulation of senile plaques and neurofibrillary tangles (NFT) in the brain. Senile plaques containing amyloid-beta protein (Aβ) in the brain play a pivotal role in AD pathogenesis. In addition, the level of Aβ in the brain is determined by the balance between Aβ production and its clearance (Wang et al., 2006). Notably, it was reported that Aβ in the brain can be transported into the peripheral blood (Tarasoff-Conway et al., 2015; Xiang et al., 2015). The association between the cognitive impairment and plasma Aβ levels in the context of T2DM remains unknown and the effect of metformin treatment on plasma Aβ1-42 levels in diabetic mice have not been investigated.
Metformin is a drug for the treatment of T2DM, in which its antidiabetic mechanisms are exerted by decreasing hepatic glucose production and improving insulin sensitivity by increasing peripheral glucose uptake and utilization (Gong et al., 2012). Previous studies revealed the neuroprotective effect of metformin in some neurological disorders such as epilepsy, cerebral ischemia, and neurodegenerative diseases (Arbeláez-Quintero and Palacios, 2017; Bojja et al., 2021; Rotermund et al., 2018). However, the effect of metformin on neurocognitive function in the DM mouse model induced by a high-fat diet (HFD) combined with streptozotocin remains unknown. Furthermore, the effect of metformin administration on plasma proinflammatory cytokines and Aβ1-42 levels in diabetic mice has never been investigated.
In the present study, we aimed to investigate the effect of T2DM, induced by HFD feeding combined with STZ injections on cognitive function. Meanwhile, we tend to evaluate whether metformin treatment could prevent cognitive impairment and modulate pro-inflammatory cytokine and Aβ levels in the plasma of diabetic mice.
MATERIALS AND METHODS
Animals and treatment
Three-week-old male C57BL/6N mice were obtained from Nomura Siam International, Thailand. The animals were housed in a climate-controlled environment with an alternating 12h light–dark cycles. After one week of acclimatization, all animals were fed either a control diet (CD: n=10) offering a total of 3.04 kcal/g (containing 4.5% crude fat, 24% crude protein) (the National Animal Center, Salaya Campus, Mahidol University, Bangkok, Thailand) or a high-fat diet (HFD: n=20) supplemented with 25% sucrose (containing 14.4% crude fat, 24.3% crude protein) (Quick fat; CLEA, Japan) offering a total of 4.11 kcal/g for four weeks. The diabetic animal model was constructed by two intraperitoneal injections of STZ, dissolved in cold citrate buffer (0.01 M, pH 4.5) at the dose of 100 mg/kg with the injection volume of 10 mL/kg (Sigma-Aldrich), while the normal control (NC) group received the same volume injection of citrate buffer (vehicle). The second injection was performed 48h after the first injection. One week after the first injection of STZ, a fasting blood glucose (FBG) test was applied to assess the diabetic model. STZ-treated mice which met the criterion (FBG > 200 mg/dL) (Kang et al., 2017) were randomly separated into two groups as followed: diabetic group (DM group) and diabetic treated with metformin (DM+MET group). Three mice who did not meet the criterion, were excluded from the study. During the experiment, two mice from the DM+MET group were dead and excluded for the statistical analysis. The DM+MET group was received a daily oral gavage of metformin at the dose of 100 mg/kg. The DM group and DM+MET group were continued on HFD meanwhile the NC group was given a normal chow diet for additional seven weeks. The sample size was determined according to the previous study which recommended 5 to 7 animals per group will be required (Arifin and Zahiruddin, 2017). All experiments were guided in accordance with the Animal Care and Use Committee of Burapha University (IACUC Number 023/2562).
Novel object recognition test
Novel object recognition test (NORT) was appraised as described previously with some modifications (Juntapremjit and Janthakhin, 2021; Janthakhin et al., 2022). Briefly, the test consisted of three phases: habituation phase, training phase, and test phase. During the habituation phase, mice were exposed to the open-field box in the absence of object for five minutes. Mice were then returned to their home cage. On the following day (training phase), mice were placed in the same open-field with two identical objects and were allowed to freely explore the environment and objects for 10 minutes. Twenty-four hours later (testing phase), mice were placed back in the open-field and at this time, allowed to explore a novel object and a familiar one for 10 minutes. During the experiment, the open-field box and objects were cleaned using 70% alcohol to eliminate the olfactory cue. Time spent exploring each object was collected in the test phase in which the mouse’s nose was within 2 cm of the object. A percentage of time exploring the novel object versus the total object exploration time was presented.
Open-field test
Open-field test was used to measure the anxiety-like behavior. Briefly, each mouse was placed in the center of the open square box (40x40x25cm) made from white and non-porous plastic, and animals were allowed to freely explore the open-field box for five minutes. Time spent in the center zone and number of entries into the center of the open-field were recorded.
Measurement of body weight and blood glucose level
Body weight was monitored weekly across the experiment. The blood glucose levels were evaluated at one week after diabetic induction, three weeks, and six weeks after drug administration. After a 6-hour fast, blood samples were collected from a small incision at the end of the mice’s tails. A drop of blood was placed onto a glucose strip and blood glucose was measured with a glucose meter according to the user instructions guideline (ACCU-CHEK Guide, Roche, Thailand).
TNF-α, IL-6, and Aβ1-42 measurements
Plasma samples from each group of mice were collected and stored at -20°C until they were thawed for the assay. Plasma TNF-α, IL-6, and Aβ1-42 were detected using ELISA kits (ABclonal Technology, Wuhan, China) according to the instructions. The optical density (OD) value of the sample at 450 nm was collected by microplate reader (Thermo Fisher, U.S.A.). The sample concentration was calculated according to a corresponding standard curve.
Statistical analysis
All data are presented as mean ± standard error of mean (SEM). Shapiro-Wilk and Levene’s tests were used to test the normality and homogeneity of variance, respectively (Supplementary data Table 1.). One-way ANOVA was used to compare the means of parameters addressed among three groups. Tukey’s test was used as multiple comparison post hoc test when equality of variance was assumed and Dunnett T3 was applied when equality of variance was not assumed. A general linear model was used to analyze the body weight and the FBG levels among three groups at different time points. P value < 0.05 was considered as significant.
RESULTS
Effect of metformin on mouse body weight and blood glucose levels
A general linear model was used to analyze the body weight during 7 weeks of treatment. The Mauchly’s test of sphericity showed an unequal compound symmetry (Mauchly’ W = 0, P < 0.001). The Greenhouse-Geisser correction revealed a significance of time effect (F(1.50,32.99) = 12.80, P < 0.001), and an interaction between time and treatment effect (F(3.00,32.99) = 27.63, P < 0.001). At the end of the treatment (W7), the body weight in the DM group and DM+MET group was significantly lower than the NC group (P < 0.001). In addition, the body weight of the DM+MET group did not differ to that of the DM group (P > 0.05) (Figure 1A).
Regarding the effect of metformin on glucose metabolism, the FBG levels were evaluated at three weeks and six weeks after treatment. A general linear model was used to analyze the FBG levels. The box’s test of equality of covariance matrix showed a significant difference in covariance matrix (Box’ M = 62.107, F(12,19) = 4.09,
P < 0.001). The Mauchly’s test of sphericity showed an unequal compound symmetry (Mauchly’ W = 0.362, P < 0.001). The Greenhouse-Geisser correction revealed a significance of time effect (F(1.22,26.86) = 29.37, P < 0.001), and an interaction between treatment and time effect (F(2.442, 26.86) = 6.59, P < 0.001). Post hoc analysis indicated that both the DM group and the DM+MET group exhibited significant increases in the FBG levels when compared to the NC group at three time points (P < 0.001). However, the levels of FBG of the DM+MET group did not differ to those of the DM group at both three weeks and six weeks after treatment
(P > 0.05) (Figure 1B).
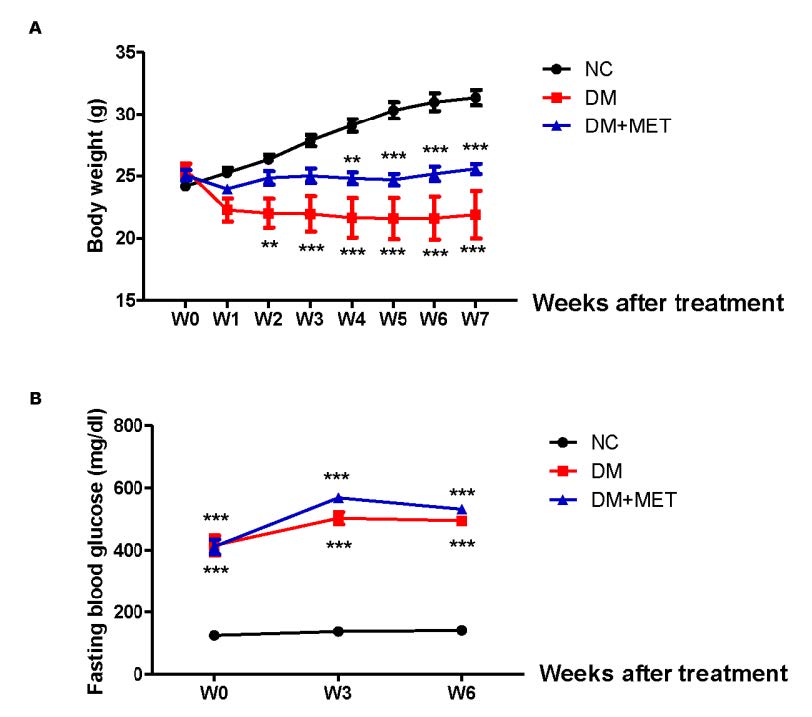
Figure 1. Effect of metformin on body weight (A) and blood glucose levels (B) in mice with type 2 diabetes mellitus. Data were presented as mean ± SEM. Normal control (NC) (n = 10), diabetic mice (DM) (n = 8), diabetic mice treated with metformin (DM+MET) (n = 7). ***P < 0.001 when compared with the normal control group.
Effect of metformin on cognitive functions in diabetic mice
The novel object recognition test was used to evaluate non-spatial memory in NC, DM, and DM+MET mice. One-way ANOVA showed a significant treatment effect for a percentage of recognition index for the testing phase (F(2,22) = 19.84, P < 0.001). A Tukey post doc test revealed that the percentage of recognition index of the DM group was significantly lower than that of the NC group (P < 0.001) indicating a cognitive deficit in DM mice. Interestingly, the DM+MET mice exhibited a higher percentage of recognition index than DM mice (P < 0.01) suggesting that metformin could reverse the cognitive deficit of DM mice (Figure 2A). However, the total time spent exploring the objects during testing did not differ among groups (F(2,22) = 0.37, P > 0.05) (Figure 2B).
The anxiety-like behavior was also evaluated in the open-field test. An ANOVA for the time spent in the center revealed a non-significant difference between groups (F(2,22) = 3.27, P > 0.05) (Figure 2C). Moreover, the number of entries into the center of the open-field arena did not differ among groups (F(2,22) = 1.95, P > 0.05) (Figure 2D) suggesting that diabetes mellitus did not affect anxiety-like behavior in these mice.
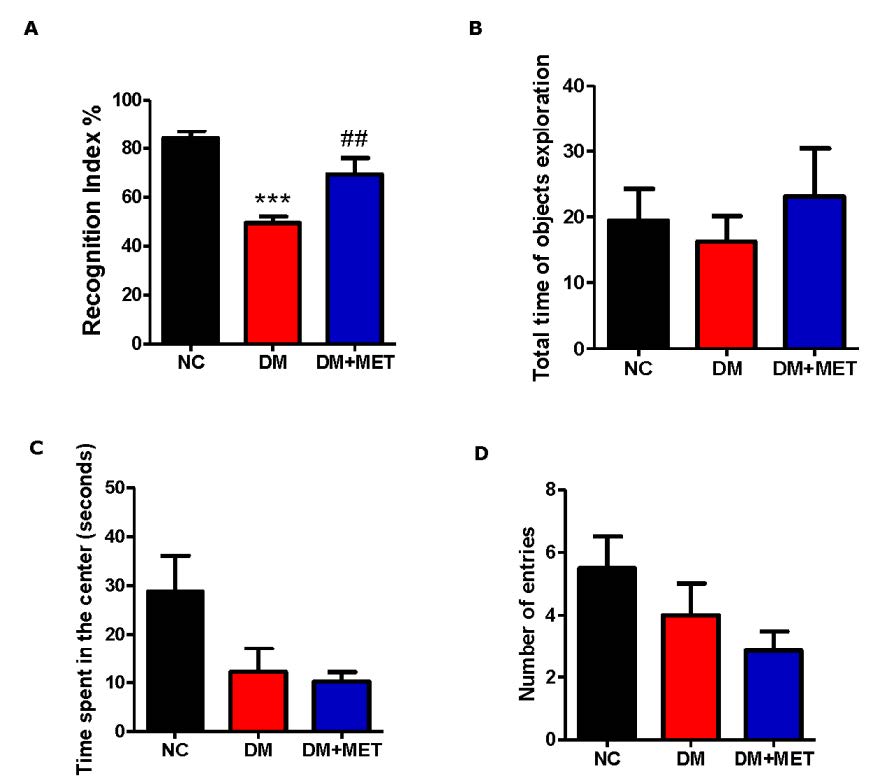
Figure 2. Effect of metformin on recognition index (A), the total time of objects exploration (B), time spent in the center (C) and number of entries into the center of the open-field (D) in diabetic mice. Data were presented as mean ± SEM. Normal control (NC) (n = 10), diabetic mice (DM) (n = 8), diabetic mice treated with metformin (DM+MET) (n = 7). ***P < 0.001 when compared with the normal control group, ##P < 0.01 when compared with the diabetic group.
Effect of metformin on plasma pro-inflammatory cytokines levels in diabetic mice
We further investigated the levels of plasma pro-inflammatory cytokines, i.e., IL-6, and TNF-α in diabetic mice and evaluated whether the metformin treatment could affect these plasma pro-inflammatory cytokines levels. An ANOVA for the plasma IL-6 level showed a significant difference between groups (F(2,22) = 23.15, P < 0.001). A Dunnett T3 post doc test revealed a significant increase in the plasma IL-6 level in the DM group when compared to that of the NC group (P < 0.001). Interestingly, the level of plasma IL-6 was lower in the DM+MET group than the DM group (P < 0.01) (Figure 3A). We also found a difference in the plasma TNF-α level among groups (F(2,22) = 3.95, P < 0.05). A Tukey post doc test revealed that the DM+MET group exhibited a decrease in the plasma TNF-α level when compared to the DM group (P < 0.05) (Figure 3B).
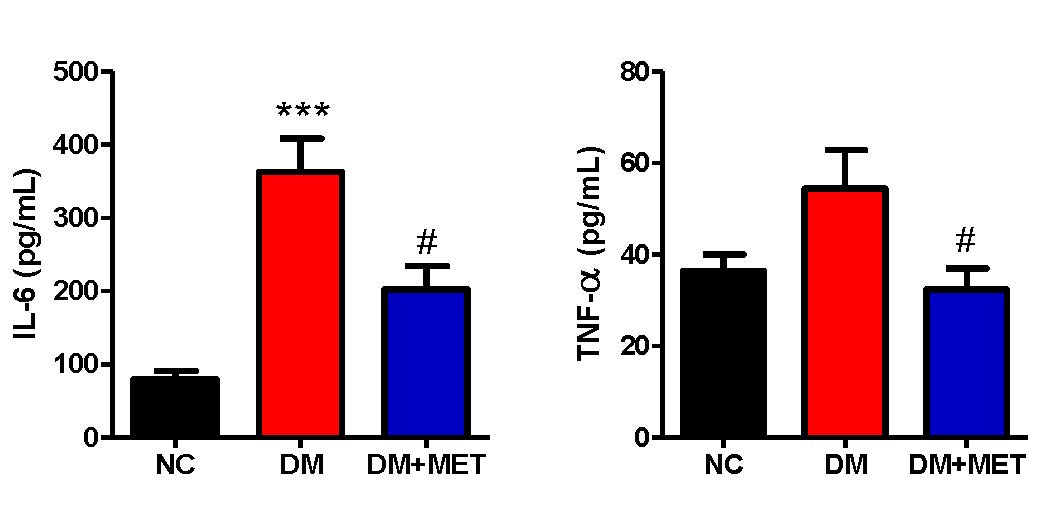
Figure 3. Effect of metformin on plasma IL-6 level (A) and plasma TNF-α level (B) in diabetic mice. Data were presented as mean ± SEM. Normal control (NC) (n = 10), diabetic mice (DM) (n = 8), diabetic mice treated with metformin (DM+MET) (n = 7). *** P < 0.001 when compared with the normal control group, # P < 0.05 when compared with the diabetic group.
Effect of metformin on plasma Aβ1-42 levels in diabetic mice
The association between type 2 diabetes and Alzheimer’s disease (AD) has epidemiologically been demonstrated, thus plasma Aβ has been used as a potential biomarker for AD diagnosis. Therefore, the level of plasma Aβ1-42 was investigated in our study. We found a difference in plasma Aβ1-42 level between groups (F(2,22) = 9.80, P < 0.001). A Tukey post doc analysis revealed that the level of plasma Aβ1-42 of the DM group was lower than that of the NC group (P < 0.001). Interestingly, the level of plasma Aβ1-42 of the DM+MET group was augmented when compared to the DM group (P < 0.05) (Figure 4).
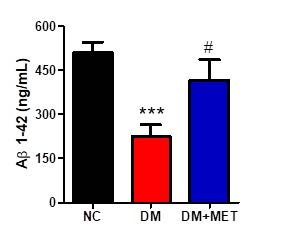
Figure 4. Effect of metformin on plasma Aβ1-42 levels in diabetic mice. Data were presented as mean ± SEM. Normal control (NC) (n = 10), diabetic mice (DM) (n = 8), diabetic mice treated with metformin (DM+MET) (n = 7). *** P < 0.001 when compared with the normal control group, # P < 0.05 when compared with the diabetic group.
Correlation between object recognition memory and plasma pro-inflammatory cytokines and Aβ1-42 levels
We further investigated the correlation between object recognition memory performance and plasma pro-inflammatory cytokines and Aβ1-42 levels. We found that the percentage of recognition index negatively correlated with plasma IL-6 level (r = -0.68, P < 0.001) (Figure 5A). Additionally, the percentage of recognition index was found to correlate positively with plasma Aβ1-42 level (r = 0.72, P < 0.001) (Figure 5C). However, the level of plasma TNF-α tended to correlate with the recognition index but it did not reach the level of statistical significance level (r = -0.30, P > 0.05) (Figure 5B).
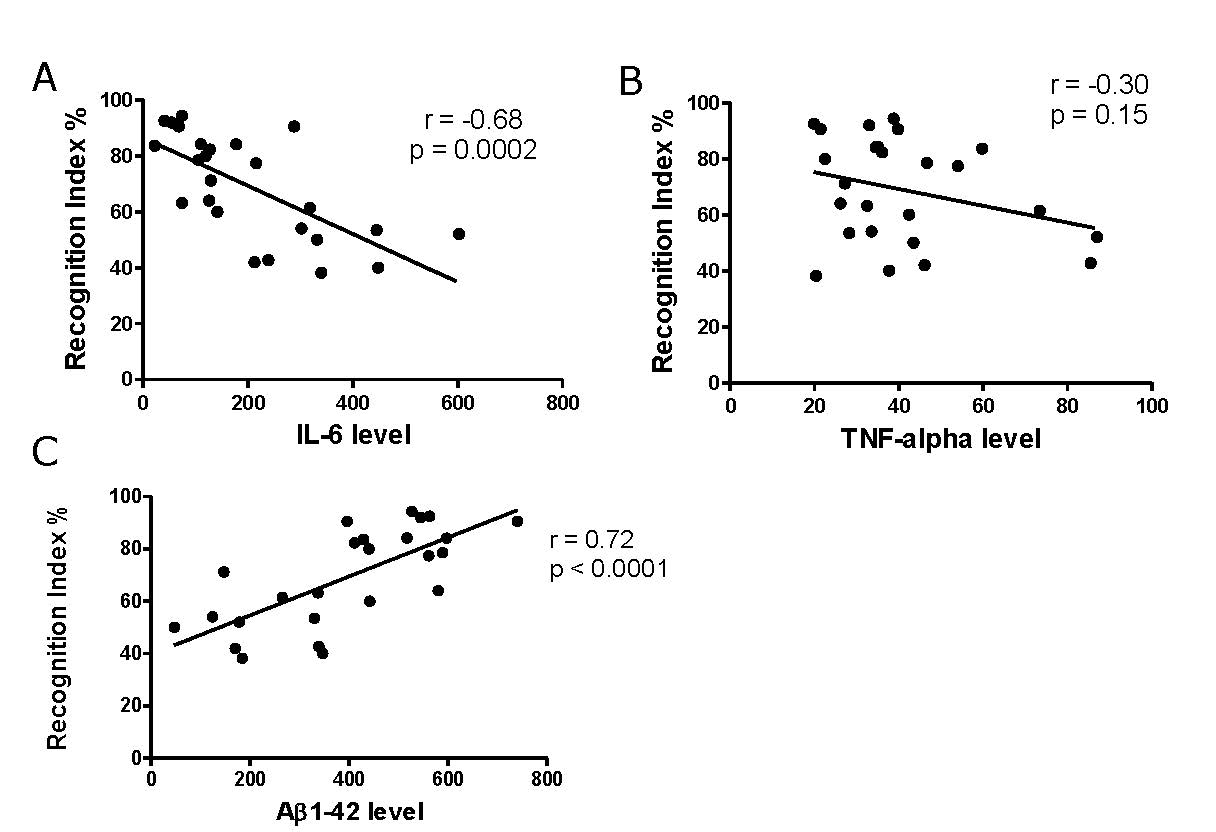
Figure 5. Correlation between object recognition memory and plasma pro-inflammatory cytokines and Aβ1-42 levels (n = 25).
DISCUSSION
The present study showed that diabetic mice exhibited non-spatial memory impairment evaluated by a novel object recognition test (NORT) and the treatment with metformin at the dose of 100 mg/kg/day for seven weeks prevented cognitive deficits in diabetic mice. We further found that diabetic mice presented increased plasma IL-6 and decreased Aβ1-42 levels compared to the control group. Interestingly, the treatment with metformin could normalize the levels of plasma IL-6 and Aβ1-42 of diabetic mice. In addition, we found a negative correlation between object recognition memory performance and plasma IL-6 level. Moreover, the positive correlation between object recognition memory performance and plasma Aβ1-42 level has been found.
In the present study, we used the combination of high-fat diet (HFD) consumption and streptozotocin (STZ) administration to induce type 2 diabetes mellitus (T2DM) model in mice and cognitive deficits were observed in the diabetic mouse model. In line with our results, previous studies revealed memory impairments in several cognitive tests in the T2DM animal model induced by HFD intake combined with STZ injection, including passive avoidance test (Cassano et al., 2020), novel object recognition test (Cassano et al., 2020), fear conditioning test (Chen et al., 2017), Morris water maze test (Datusalia and Sharma, 2014; Park et al., 2020; Ren et al., 2019) and in the Y-maze test (Esmaeili et al., 2020). In our study, we decided to use the novel object recognition paradigm to evaluate object recognition memory because this type of memory is thought to be an important aspect of human declarative memory (Brown and Aggleton, 2001). Additionally, clinical studies demonstrated that individuals with T2DM showed specific alterations in the declarative memory process (Bruehl et al., 2007; Yau et al., 2009). Although both clinical and experimental studies indicated that diabetes mellitus affected cognitive functions, the mechanisms underpinning diabetes-induced cognitive deficits remains unclear.
We showed that metformin treatment rescued cognitive deficits in T2DM mice induced by HFD feeding combined with STZ injection. This finding was consistent with previous studies revealing the protective effects of metformin against HFD/STZ-induced cognitive impairments in the animal model of T2DM (Salman et al., 2013; Jiang et al., 2021). Although the effect of metformin treatment per se on cognitive function in non-diabetic control animals was not investigated in the present study, Oliveira and colleagues reported that oral treatment with metformin at the dose of 200 mg/kg for 21 days had no effect on cognition (Oliveira et al., 2016).
Regarding the mechanistic effects exerted by metformin in improving cognition in diabetic mice, hyperglycemia is one of mechanisms that has been proposed to be involved in cognitive dysfunction induced by diabetes mellitus (Bernier et al., 2021; Rawlings et al., 2019; Whitmer et al., 2021). However, in our study, we found that the oral treatment with metformin could not reduce fasting blood glucose levels in diabetic mice. Our finding suggests that metformin could improve memory in HFD/STZ-induced diabetic mice independent of glycemic control.
Inflammatory markers have been proposed to contribute to cognitive decline in T2DM (Chornenkyy et al., 2019; Li et al., 2015). Increased levels of inflammatory markers and proinflammatory cytokines in blood circulation such as, tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) have been observed in people with T2DM (Donath, 2014; Liu et al., 2016; Mirza et al., 2012). In the present study, we found that diabetic mice exhibited an increase in plasma IL-6 and TNF-α levels when compared to a normal control group. Furthermore, the negative correlation between object recognition memory performance and plasma IL-6 level has been observed. Our results were consistent with the previous research reporting increased levels of circulating TNF-α, IL-1β, and IL-6 in STZ-induced diabetic mice (Niu et al., 2016; Sun et al., 2021; Zhang et al., 2016). Interestingly, we found that the treatment with metformin reduced systemic pro-inflammatory cytokines levels in diabetic mice. To the best of our knowledge, this is the first study to show that elevated proinflammatory cytokines, i.e., IL-6 and TNF-α levels participate in object recognition memory impairments in HFD/STZ-induced diabetic mice. In addition, metformin preserves the cognitive function of HFD/STZ-induced diabetic mice possibly via a reduction of IL-6 and TNF-α levels. Further study is still needed to evaluate the effect of IL-6 or TNF-α inhibitor on cognitive function in diabetic mice.
Previous studies reported that chronic hyperglycemia in the diabetic condition resulted in an elevation of serum IL-6 and MCP-1 levels. This increase in serum IL-6 and MCP-1 levels in diabetic mice originated from the increase in the macrophage infiltration (Niu et al., 2016). Moreover, it has been reported that hyperglycemia in diabetes caused the generation and accumulation of advanced glycation end products (AGE), that in turn, lead to the activation of pro-inflammatory cytokines being responsible for endothelial dysfunction and vascular inflammation (Basta et al., 2002). Thus, strategies aiming to reduce the production of AGE may become an important approach for preventing cognitive deficits in conjunction with reduced systemic inflammation in HFD/STZ-induced diabetic mice.
The mammalian target of rapamycin (mTOR) is a conserved protein kinase which plays a crucial role in keeping a balance between protein degradation and synthesis, involved in several biological processes such as cellular growth, survival and death (Laplante and Sabatini, 2012). Previous studies reported hyperactivation of mTOR signaling in the hippocampus associated with cognitive deficits in animals with type 2 diabetes mellitus (Sun et al., 2019; Wang et al., 2014). Therefore, it is interesting to investigate whether metformin treatment would affect the hippocampal mTOR signaling and restore cognitive function of HFD/STZ-induced diabetic mice.
Previous studies demonstrated the relationship between type 2 diabetes mellitus and Alzheimer’s disease (AD) as evidence by molecular, functional, and clinical data (Arnold et al., 2018; Biessels and Despa, 2018). Furthermore, it has been demonstrated that plasma Aβ1-42 levels were negatively correlated with the cognitive performance of individuals with mild cognitive impairment (MCI) and individuals with AD (Nakamura et al., 2018). However, studies of Aβ1-42 levels in people with diabetes or in an animal model of diabetes were inconclusive (Peters et al., 2017; Meakin et al., 2020; Peng et al., 2020). In our study, we found that diabetic mice exhibited a decrease in plasma Aβ1-42 levels when compared to control mice and the treatment with metformin normalized the levels of plasma Aβ1-42 in diabetic mice. Our findings suggest that the clearance of Aβ from the brain to the peripheral blood has been disturbed in diabetic mice. Therefore, it seems that the alteration of Aβ transport from the brain to the peripheral blood leading to a decrease in plasma Aβ1-42 level, may participate in cognitive deficits in diabetic mice.
CONCLUSION
The present study found that metformin can significantly reduce systemic inflammation and increase amyloid beta expression, which subsequently enhance improvements in object recognition memory of diabetic mice. In conclusion, metformin may be used as a therapeutic alternative for patients with type 2 diabetes mellitus who show cognitive deficits. Further research is needed to investigate the effect of metformin in combination with another drug such as glucagon-like peptide 1 receptor agonist (GLP-1 receptor agonist) or sodium-glucose transport protein 2 (SGT-2) inhibitor on cognitive impairment in diabetic mice.
ACKNOWLEDGMENTS
The authors thank the Faculty of Science, Burapha University, Animal Cognitive Neuroscience Lab (ACON), College of Research Methodology and Cognitive Science (RMCS), and Cognitive Science and Innovation Research Unit (CSIRU), Burapha University for providing instruments.
AUTHOR CONTRIBUTIONS
Yoottana Janthakhin: conceptualization, methodology, formal analysis, investigation, writing – original draft preparation, and writing – review and editing. Sutin Kingtong: investigation and writing – review and editing. Chutima Aphibanthammakit: investigation and writing – review and editing. Sirikran Juntapremjit: investigation and writing – review and editing. All authors have read and agreed to the published version of the manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Arbeláez-Quintero, I. and Palacios, M. 2017. To use or not to use metformin in cerebral ischemia: A review of the application of metformin in stroke rodents. Stroke Research and Treatment, 2017.
Arifin, W.N., and Zahiruddin, W.M. 2017. Sample size calculation in animal studies using resource equation approach. The Malaysian Journal of Medical Sciences: MJMS, 24: 101-105.
Arnold, S.E., Arvanitakis, Z., Macauley-Rambach, S.L., Koenig, A.M., Wang, H.Y., Ahima, R.S., Craft, S., Gandy, S., Buettner, C., et al. 2018. Brain insulin resistance in type 2 diabetes and Alzheimer disease: Concepts and conundrums. Nature Reviews Neurology, 14: 168-181.
Basta, G., Lazzerini, G., Massaro, M., Simoncini, T., Tanganelli, P., Fu, C., Kislinger, T., Stern, D.M., Schmidt, A.M., et al. 2002. Advanced glycation end products activate endothelium through signal-transduction receptor RAGE: A mechanism for amplification of inflammatory responses. Circulation, 105: 816-822.
Bernier, F., Ohno, K., Katsumata, N., Shimizu, T., and Xiao, J. 2021. Association of plasma hemoglobin A1c with improvement of cognitive functions by probiotic Bifidobacterium breve supplementation in healthy adults with mild cognitive impairment. Journal of Alzheimer's Disease, 81: 493-497.
Biessels, G.J., and Despa, F. 2018. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nature Reviews Endocrinology, 14: 591-604.
Biessels, G.J., Staekenborg, S., Brunner, E., Brayne, C., and Scheltens, P. 2006. Risk of dementia in diabetes mellitus: A systematic review. The Lancet Neurology, 5: 64-74.
Brown, M.W. and Aggleton, J.P. 2001. Recognition memory: What are the roles of the perirhinal cortex and hippocampus?. Nature Reviews Neuroscience, 2: 51-61.
Bruehl, H., Rueger, M., Dziobek, I., Sweat, V., Tirsi, A., Javier, E., Arentoft, A., Wolf, O.T., and Convit, A. 2007. Hypothalamic-pituitary-adrenal axis dysregulation and memory impairments in type 2 diabetes. The Journal of Clinical Endocrinology & Metabolism, 92: 2439-2445.
Bojja, S.L., Medhi, B., Anand, S., Bhatia, A., Joshi, R., and Minz, R.W. 2021. Metformin ameliorates the status epilepticus-induced hippocampal pathology through possible mtor modulation. Inflammopharmacology, 29: 137-151.
Cassano, V., Leo, A., Tallarico, M., Nesci, V., Cimellaro, A., Fiorentino, T.V., Citraro, R., Hribal, M.L., De Sarro, G.D., et al. 2020. Metabolic and cognitive effects of ranolazine in Type 2 diabetes mellitus: Data from an in vivo model. Nutrients, 12: 382-396.
Chen, Q., Mo, R., Wu, N., Zou, X., Shi, C., Gong, J., Li, J., Fang, K., Wang, D., et al. 2017. Berberine ameliorates diabetes-associated cognitive decline through modulation of aberrant inflammation response and insulin signaling pathway in DM rats. Frontiers in Pharmacology, 8: 334-348.
Chornenkyy, Y., Wang, W.X., Wei, A., and Nelson, P.T. 2019. Alzheimer’s disease and type 2 diabetes mellitus are distinct diseases with potential overlapping metabolic dysfunction upstream of observed cognitive decline. Brain Pathology, 29: 3-17.
Craft, S., Zallen, G., and Baker, L.D. 1992. Glucose and memory in mild senile dementia of the Alzheimer type. Journal of Clinical and Experimental Neuropsychology, 14: 253-267.
Datusalia, A.K., and Sharma, S.S. 2014. Amelioration of diabetes-induced cognitive deficits by GSK-3β inhibition is attributed to modulation of neurotransmitters and neuroinflammation. Molecular Neurobiology, 50: 390-405.
Donath, M.Y. 2014. Targeting inflammation in the treatment of type 2 diabetes: Time to start. Nature reviews Drug discovery, 13: 465-476.
Esmaeili, M.H., Enayati, M., Abkenar, F.K., Ebrahimian, F., and Salari, A.A. 2020. Glibenclamide mitigates cognitive impairment and hippocampal neuroinflammation in rats with type 2 diabetes and sporadic Alzheimer-like disease. Behavioural Brain Research, 379: 112359-112394.
Gong, L., Goswami, S., Giacomini, K.M., Altman, R.B., and Klein, T.E. 2012. Metformin pathways: Pharmacokinetics and pharmacodynamics. Pharmacogenetics and Genomics, 22: 820-827.
Janson, J., Laedtke, T., Parisi, J.E., O’Brien, P., Petersen, R.C., and Butler, P.C. 2004. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes, 53: 474-481.
Janthakhin, Y., Kingtong, S., and Juntapremjit, S. 2022. Inhibition of glucocorticoid synthesis alleviates cognitive impairment in high-fat diet-induced obese mice. Comprehensive Psychoneuroendocrinology, 10, 1-4.
Jiang, X., Xu, Q., Zhang, A., Liu, Y., Li, Z., Tang, H., Cao, D., and Zhang, D. 2021. Revealing the hypoglycemic effects and mechanism of GABA-rich germinated Adzuki beans on T2DM mice by untargeted serum metabolomics. Frontiers in Nutrition, 8: 1-14.
Juntapremjit, S. and Janthakhin, Y. 2021. Effects of Indian gooseberry fruit on anxiety-related behaviors and memory performance in high-fat diet-induced obese mice. Journal of Natural Sciences, 20: 1-11.
Kang, S., Kim, C.H., Jung, H., Kim, E., Song, H.T., and Lee, J.E. 2017. Agmatine ameliorates type 2 diabetes induced-Alzheimer's disease-like alterations in high-fat diet-fed mice via reactivation of blunted insulin signalling. Neuropharmacology, 113: 467-479.
Laplante, M. and Sabatini, D.M. 2012. mTOR signaling in growth control and disease. Cell, 149: 274-293.
Li, X., Song, D., and Leng, S. X. 2015. Link between type 2 diabetes and Alzheimer’s disease: From epidemiology to mechanism and treatment. Clinical Interventions in Aging, 10: 549-560.
Liu, C., Feng, X., Li, Q., Wang, Y., Li, Q., and Hua, M. 2016. Adiponectin, TNF-α and inflammatory cytokines and risk of type 2 diabetes: A systematic review and meta-analysis. Cytokine, 86: 100-109.
Meakin, P.J., Coull, B.M., Tuharska, Z., McCaffery, C., Akoumianakis, I., Antoniades, C., Jane Brown,J., Griffin, K.J., Platt, F., et al. 2020. Elevated circulating amyloid concentrations in obesity and diabetes promote vascular dysfunction. The Journal of Clinical Investigation, 130: 4104-4117.
Mirza, S., Hossain, M., Mathews, C., Martinez, P., Pino, P., Gay, J.L., Rentfro, A., McCormick,J.B., and Fisher-Hoch, S.P. 2012. Type 2-diabetes is associated with elevated levels of TNF-alpha, IL-6 and adiponectin and low levels of leptin in a population of Mexican Americans: A cross-sectional study. Cytokine, 57: 136-142.
Nakamura, A., Kaneko, N., Villemagne, V.L., Kato, T., Doecke, J., Doré, V., Fowler, C., Li, Q-X., Martins, R., Rowe, C., et al. 2018. High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature, 554: 249-254.
Niu, S., Bian, Z., Tremblay, A., Luo, Y., Kidder, K., Mansour, A., Zen, K., and Liu, Y. 2016. Broad infiltration of macrophages leads to a proinflammatory state in streptozotocin-induced hyperglycemic mice. The Journal of Immunology, 197: 3293-3301.
Oliveira, W.H., Nunes, A K., França, M.E.R., Santos, L.A., Los, D.B., Rocha, S.W., Barbosa, K.P., Rodrigues, G.B., and Peixoto, C.A. 2016. Effects of metformin on inflammation and short-term memory in streptozotocin-induced diabetic mice. Brain Research, 1644: 149-160.
Park, K.A., Jin, Z., Lee, J.Y., An, H.S., Choi, E.B., Kim, K. E., Shin, H.J., Jeong, E.A., Min, K.A., Shin, M.C., et al. 2020. Long-lasting exendin-4 fusion protein improves memory deficits in high-fat diet/streptozotocin-induced diabetic mice. Pharmaceutics, 12: 159.
Peng, X., Xu, Z., Mo, X., Guo, Q., Yin, J., Xu, M., Peng, Z., Sun, T., Zhou, L., Peng, X., et al. 2020. Association of plasma β-amyloid 40 and 42 concentration with type 2 diabetes among Chinese adults. Diabetologia, 63: 954-963.
Rawlings, A.M., Sharrett, A.R., Albert, M.S., Coresh, J., Windham, B.G., Power, M. C., Knopman, D.S., Walker, K., Burgard, S., Mosley, T.H., et al. 2019. The association of late-life diabetes status and hyperglycemia with incident mild cognitive impairment and dementia: The ARIC study. Diabetes Care, 42: 1248-1254.
Ren, T., Liu, J., Ge, Y., Zhuo, R., Peng, L., Liu, F., Jin, X., and Yang, L. 2019. Chronic oleoylethanolamide treatment attenuates diabetes-induced mice encephalopathy by triggering peroxisome proliferator-activated receptor alpha in the hippocampus. Neurochemistry International, 129: 104501.
Rotermund, C., Machetanz, G., and Fitzgerald, J.C. 2018. The therapeutic potential of metformin in neurodegenerative diseases. Frontiers in Endocrinology, 9: 400-426.
Salman, Z.K., Refaat, R., Selima, E., El Sarha, A., and Ismail, M.A. 2013. The combined effect of metformin and L-cysteine on inflammation, oxidative stress and insulin resistance in streptozotocin-induced type 2 diabetes in rats. European Journal of Pharmacology, 714: 448-455.
Sun, Q., Li, T., Li, Y., Wei, L., Zhang, M., and Deng, S. 2021. Bactericidal/Permeability-increasing protein improves cognitive impairment in diabetic mice via blockade of the LPS-LBP-TLR4 signaling pathway. Frontiers in Physiology, 11: 718-729.
Sun, Q., Wei, L.L., Zhang, M., Li, T.X., Yang, C., Deng, S.P., and Zeng, Q.C. 2019. Rapamycin inhibits activation of AMPK-mTOR signaling pathway-induced Alzheimer’s disease lesion in hippocampus of rats with type 2 diabetes mellitus. International Journal of Neuroscience, 129: 184-193.
Tarasoff-Conway, J.M., Carare, R.O., Osorio, R.S., Glodzik, L., Butler, T., Fieremans, E., Axel, L., Rusinek, H., Nicholson, C., Zlokovic, B.V., et al. 2015. Clearance systems in the brain—implications for Alzheimer disease. Nature Reviews Neurology, 11: 457-470.
Wang, J., Li, G., Wang, Z., Zhang, X., Yao, L., Wang, F., Liu, S., Yin, J., Ling, E-A., Wang, L., et al. 2012. High glucose-induced expression of inflammatory cytokines and reactive oxygen species in cultured astrocytes. Neuroscience, 202: 58-68.
Wang, S., Zhou, S.L., Min, F.Y., Ma, J.J., Shi, X.J., Bereczki, E., and Wu, J. 2014. mTOR-mediated hyperphosphorylation of tau in the hippocampus is involved in cognitive deficits in streptozotocin-induced diabetic mice. Metabolic Brain Disease, 29: 729-736.
Wang, Y.J., Zhou, H.D., and Zhou, X.F. 2006. Clearance of amyloid-beta in Alzheimer's disease: Progress, problems and perspectives. Drug Discovery Today, 11: 931-938.
West, I.C. 2000. Radicals and oxidative stress in diabetes. Diabetic Medicine, 17: 171-180.
Whitmer, R.A., Gilsanz, P., Quesenberry, C.P., Karter, A.J., and Lacy, M.E. 2021. Association of type 1 diabetes and hypoglycemic and hyperglycemic events and risk of dementia. Neurology, 97: 275-283.
Xiang, Y., Bu, X. L., Liu, Y.H., Zhu, C., Shen, L.L., Jiao, S.S., Zhu, X-Y., Giunta, B., Tan, J., Song, W-H., et al. 2015. Physiological amyloid-beta clearance in the periphery and its therapeutic potential for Alzheimer’s disease. Acta Neuropathologica, 130: 487-499.
Yau, P. L., Javier, D., Tsui, W., Sweat, V., Bruehl, H., Borod, J. C., and Convit, A. 2009. Emotional and neutral declarative memory impairments and associated white matter microstructural abnormalities in adults with type 2 diabetes. Psychiatry Research: Neuroimaging, 174: 223-230.
Zhang, Z., Li, Y., and Li, Y. 2016. Grape seed proanthocyanidin extracts prevent hyperglycemia-induced monocyte adhesion to aortic endothelial cells and ameliorates vascular inflammation in high-carbohydrate/high-fat diet and streptozotocin-induced diabetic rats. International Journal of Food Sciences and Nutrition, 67: 524-534.
Supplementary data
Table 1. The assumption of normality and homogeneity of variance.
|
Parameters addressed |
Shapiro-Wilk test for normality |
Levene’s test for homogeneity of variance |
Statistical analysis |
|
a percentage of recognition index during testing |
assumed |
assumed |
one-way ANOVA |
|
total time spent exploring the objects during testing |
assumed |
assumed |
one-way ANOVA |
|
time spent in the center |
assumed |
not assumed |
one-way ANOVA |
|
number of entries into the center of the open-field |
assumed |
assumed |
one-way ANOVA |
|
plasma IL-6 level |
assumed |
not assumed |
one-way ANOVA |
|
plasma TNF-α level |
assumed |
assumed |
one-way ANOVA |
|
plasma Aβ1-42 level |
assumed |
assumed |
one-way ANOVA |
OPEN access freely available online
Natural and Life Sciences Communications
Chiang Mai University, Thailand.
Yoottana Janthakhin1,*, Sutin Kingtong2, Chutima Aphibanthammakit3, and Sirikran Juntapremjit1
1 Cognitive Science and Innovation Research Unit (CSIRU), College of Research Methodology and Cognitive Science, Burapha University, Chonburi 20131, Thailand.
2 Department of Biology, Faculty of Science, Burapha University, Chonburi 20131, Thailand.
3 National Nanotechnology Center (NANOTEC), National Science and Technology Development Agency (NSTDA), Klong Luang, Pathumthani 10120, Thailand.
Corresponding author: Yoottana Janthakhin, E-mail: yoottana.ja@buu.ac.th
Total Article Views
Editor: Veerasak Punyapornwithaya,
Chiang Mai University, Thailand
Article history:
Received: June 2, 2022;
Revised: August 10, 2022;
Accepted: September 13, 2022;
Published online: September 28, 2022

